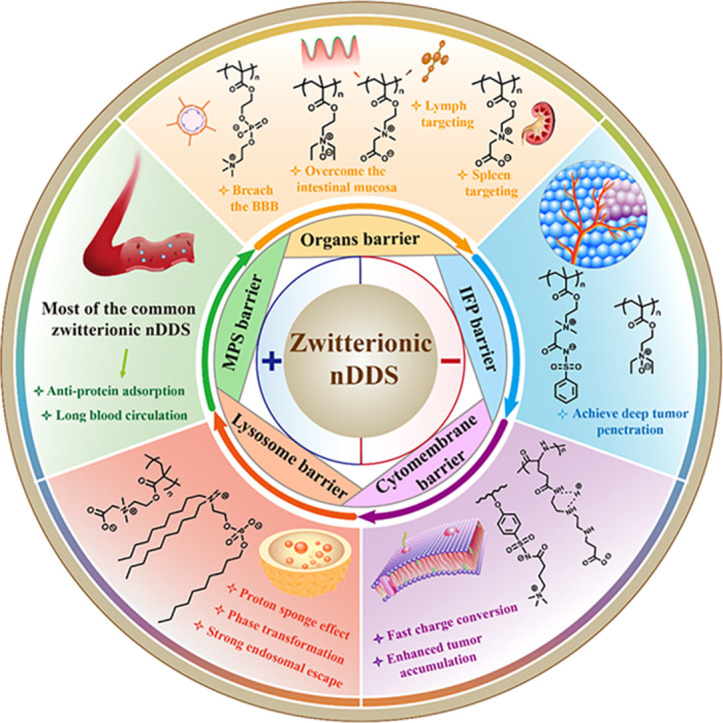
Abstract
Nanoscale drug delivery systems (nDDS) have been employed widely in enhancing the therapeutic efficacy of drugs against diseases with reduced side effects. Although several nDDS have been successfully approved for clinical use up to now, biological barriers between the administration site and the target site hinder the wider clinical adoption of nDDS in disease treatment. Polyethylene glycol (PEG)-modification (or PEGylation) has been regarded as the gold standard for stabilising nDDS in complex biological environment. However, the accelerated blood clearance (ABC) of PEGylated nDDS after repeated injections becomes great challenges for their clinical applications. Zwitterionic polymer, a novel family of anti-fouling materials, have evolved as an alternative to PEG due to their super-hydrophilicity and biocompatibility. Zwitterionic nDDS could avoid the generation of ABC phenomenon and exhibit longer blood circulation time than the PEGylated analogues. More impressively, zwitterionic nDDS have recently been shown to overcome multiple biological barriers such as nonspecific organ distribution, pressure gradients, impermeable cell membranes and lysosomal degradation without the need of any complex chemical modifications. The realization of overcoming multiple biological barriers by zwitterionic nDDS may simplify the current overly complex design of nDDS, which could facilitate their better clinical translation. Herein, we summarise the recent progress of zwitterionic nDDS at overcoming various biological barriers and analyse their underlying mechanisms. Finally, prospects and challenges are introduced to guide the rational design of zwitterionic nDDS for disease treatment.
Keywords: Zwitterionic polymer, Nano drug delivery system, Biological barrier, Targeting delivery, Disease treatment
Graphical abstract
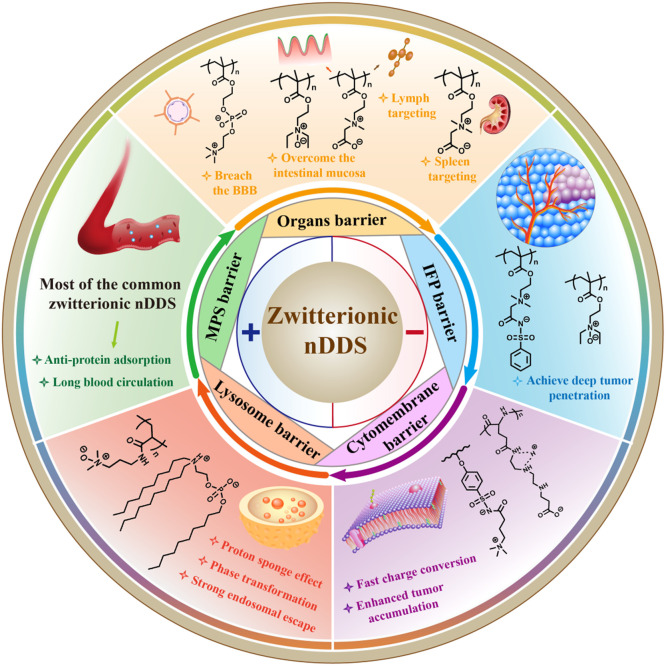
The scheme of zwitterionic nanoscale drug delivery system (nDDS) to overcome multiple biological barriers including mononuclear phagocytic system (MPS) barrier, organs barrier, interstitial fluid pressure (IFP), cytomembrane barrier and lysosome barrier.
1. Introduction
Systemic drug administration is commonly used to treat diseases; however, the treatment efficacy remains low because free drugs are usually distributed non-specifically in vivo after administration with unwanted side effects [1,2]. To combat these shortcomings, nanoscale drug delivery systems (nDDS) have been developed to transport free drugs to diseased locations precisely, which enhances the therapeutic effect and minimize the adverse effects in the meantime [3], [4], [5], [6], [7]. nDDS could increase the water solubility of free drugs and improves their biodistribution in vivo [8], [9], [10]. Since PEGylated liposomal doxorubicin, known as Doxil®, was originally licensed by the US Food and Drug Administration (FDA) in 1995, several nDDS have been developed and utilised in clinics worldwide [11], [12], [13]. For example, the small interfering ribonucleic acid (siRNA)-based lipid nanoparticles (LNPs) known as Onpattro was approved for the treatment of multiple neuropathies caused by hereditary transthyroxine protein mediated amyloidosis in adult patients in 2018, which paves a way for the treatment of rare genetic diseases by siRNA therapy [14,15]. Recently, messenger ribonucleic acid (mRNA)-based LNPs were developed and approved as the vaccine to combat severe acute respiratory syndrome coronavirus disease, enabling future clinical applications of mRNA therapy [16,17]. Despite the progress, the therapeutic efficacy of nDDS in diseases such as cancers is limited owing to the multiple biological barriers between the administration site and tumor tissue [18], [19], [20]. Although diverse strategies have been exploited to achieve specific organ targeting, deep tissue penetration, enhanced cellular uptake or endo-lysosomal escape of nDDS, complex and multi-stage preparation and modification are needed which greatly impede the wide application of nDDS in the clinic [21,22]. More powerful yet simple and facile nDDS must be fabricated to overcome these biological barriers and maintain sufficient levels of drugs at the diseased site.
Polyethylene glycol (PEG) has been widely used as a gold standard to modify nDDS and improve their stability in vivo [23,24]. The stealth properties of nDDS improves after PEGylation due to the strong hydrogen bonds between PEG and water, which could generate a water swelling layer, rendering the nDDS less vulnerable to the uptake by the mononuclear phagocytic system (MPS) [25,26]. In spite of the advantages, PEGylation has been demonstrated to induce anti-PEG antibodies in vivo after repeated injection, leading to the generation of accelerated blood clearance (ABC) phenomenon [27,28]. As an alternative to PEG, zwitterionic polymers with equal numbers of anionic and cationic groups could bind with water molecules more strongly than PEG, which enhances the anti-fouling ability and blood circulation time of zwitterionic nDDS compared to PEGylated nDDS [29], [30], [31]. Furthermore, zwitterionic nDDS retain similar pharmacokinetic (PK) behavior and do not induce the ABC phenomenon after repeated injections [32,33]. Therefore, zwitterionic nDDS have garnered much attention to treat various diseases as nanocarriers in recent years [34], [35], [36], [37]. Since there are multiple biological barriers in the drug delivery process, overcoming only a single barrier such as blood clearance is difficult to realize optimized drug delivery efficiency [38]. Therefore, it is of great demand to design simple and facile nDDS which could overcome most of the biological barriers to promote the bench-to-bedside of nDDS [39]. Recently, zwitterionic nDDS were able to overcome diverse biological barriers such as MPS uptake in blood circulation, nonspecific organ distribution, elevated interstitial fluid pressure (IFP), impermeable cell membranes and lysosome traps without additional structural modification, which provides a promising platform for nDDS [40], [41], [42], [43]. This review summarises the recent advances of zwitterionic nDDS to overcome biological barriers as well as their underlying mechanisms to guide the rational design of zwitterionic nDDS, as shown in Fig. 1. The prospects and challenges of zwitterionic nDDS are further discussed to facilitate its clinical adoption in the future.
Fig. 1.
The scheme of zwitterionic nDDS to overcome multiple biological barriers including MPS barrier, organs barrier, IFP barrier, cytomembrane barrier and lysosome barrier.
2. The features of biological barriers in vivo
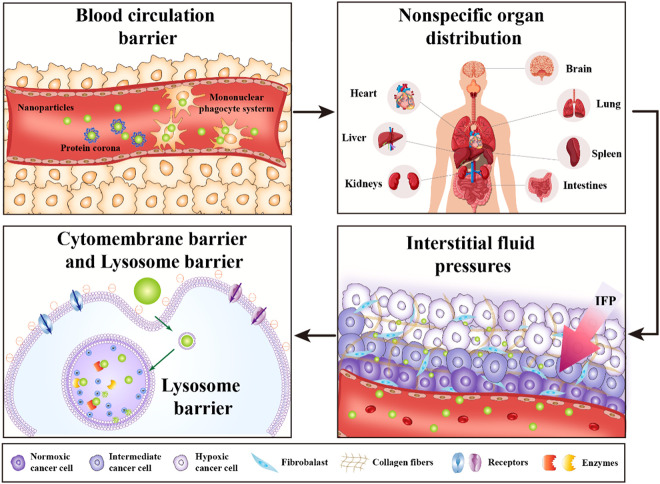
It has been widely recognized that multiple biological barriers in the drug delivery process severely hinders the therapeutic efficiency of nDDS [44,45]. After administration, the nDDS may undergo opsonisation and subsequent sequestration by the MPS, nonspecific organ distribution, pressure gradients in diseased tissue, cytomembrane barrier and lysosome barrier [46], [47], [48], as illustrated in Fig. 2. The overall delivery efficiency of nDDS is the sum of the efficiencies across each biological barrier, which requires the developed nDDS with the ability to overcome all or most of the biological barriers at the same time [49]. Apart from the substantial challenges presented by the biological barriers, it is worth noting that the drug delivery efficiency is also relevant to the route of drug administration, type of disease, state of disease progression and so on [50]. Therefore, it is of great importance to identify the characteristics of each biological barrier carefully to achieve the optimized drug delivery efficiency.
Fig. 2.
The scheme of multiple biological barriers in drug delivery process.
2.1. Opsonisation and sequestration by the MPS
The immune system of human body has evolved to recognise foreign nDDS as invaders and rapidly eliminate them from blood circulation rapidly [51]. After entering the bloodstream, plasma proteins such as serum albumin, apolipoproteins, complement components or immunoglobulins are prone to adsorb onto the surface of nDDS, generating a protein corona [52]. The physicochemical parameters such as surface charge, hydrophilicity, size and surface chemistry of nDDS are crucial to the formation of protein corona on the surface [53]. Following the generation of protein corona, nDDS are easily recognised and ingested by macrophages, leading to the shortened time in blood circulation. Furthermore, targeting groups such as transferrin (Tf), arginine-glycine-aspartic acid (RGD) and folic acid may be sheltered by the protein corona, which can impair the ability of nDDS to target the diseased site [54].
Thus far, there are already a plethora of strategies available to breach the blood barriers. Firstly, the nDDS is often coated with hydrophilic polymers like PEG, which can form strong hydrogen bonds with water molecules and generate a hydrating layer around nDDS, leading to the resistance of protein adsorption and subsequent clearance by the MPS [55]. In addition, cell membrane coating strategies have been developed in recent years to provide a biomimetic surface that prolongs in vivo circulation because of the ‘do not eat me’ marker CD47 on cell membranes [56]. Moreover, zwitterionic polymers represent a novel family of anti-fouling materials which exhibit stronger resistance to protein adsorption and uptake by MPS than PEG [57,58].
2.2. Nonspecific organ distribution
After in vivo administration, current nDDS are prone to nonspecific distribution and thus suboptimal accumulation in targeted tissues. Indeed, most nDDS are enriched in the liver because liver macrophages preferably interact with circulating nDDS [59]. For instance, most mRNA-based LNPs accumulate in liver tissue, thereby hindering the application of mRNA therapy to extrahepatic diseases that affect organs such as the brain, lung and spleen [60,61]. Different organs usually have different physical structures and cell types. The conventional approach is to modify nDDS with specific targeting groups for enhanced uptake by targeting cells through receptor-mediated interactions. However, the modification of ligands increases the complexity of the preparation process, which may delay the clinical translation of nDDS. Notably, precise regulation of physicochemical parameters of nDDS such as acidity coefficient and hydrophilicity could produce the specific protein corona in vivo, thereby ensuring accumulation of nDDS in target organs [62,63].
2.3. Elevated IFP in diseased tissue
IFP arises in diseased tissue such as tumours due to rapidly proliferating cancer cells, poor lymphatic drainage and extensive fibrosis [64]. As a result, nDDS struggle to penetrate within deep tumour tissues regardless of the well-known enhanced penetration and retention effect. In addition, the suboptimal therapeutic effect of nDDS on deep cancers amidst IFP often causes drug resistance and recurrence of cancers [65]. Several strategies have been proposed to overcome elevated IFP, such as normalisation of the tumour vasculature, reduction of collagen density in tumour tissue and size or charge conversions of nDDS for deep penetration [66]. For example, Yuan et al. developed nDDS for sustained release of vandetanib, an anti-VEGFR2 inhibitor, leading to the reversal of hypoxia and heterogeneity in the tumour microenvironment (TME) and inhibition of tumour progression [67]. Furthermore, Schroeder et al. fabricated a liposome encapsulating collagenase that degraded collagen in the extracellular matrix of pancreatic ductal adenocarcinoma, resulting in enhanced tissue permeability and drug accumulation [68]. Moreover, Lu et al. designed a self-splittable nanoraspberry that disassembles into smaller nanoparticles within the acidic TME, thereby overcoming IFP and enabling deep penetration of nDDS for photo-immunometabolic cancer therapy [69].
2.4. Cytomembrane barrier
During disease treatment, nDDS are usually required to overcome cellular membrane barriers because most drug targets are located inside organelles such as cell nucleus, ribosome and mitochondria. Although most of the hydrophobic drugs with low molecular weight can diffuse through the lipid bilayer membrane, biological agents such as peptides, nucleic acids and proteins with large molecule weight and negative charge are difficult to across the cytomembrane barrier, which requires the assisstance of nDDS [70]. The surface charge of nDDS greatly determines the cellular internalisation because negatively charged cell membranes are prone to interacting with positively charged nDDS through electrostatic interactions [71,72]. For example, Wang et al. developed a type of zwitterionic nDDS which could shed their anionic component in tumor acidic environment, resulting in the positive surface charge of nDDS and enhanced tumour cellular uptake [42]. Furthermore, modifying the surface of nDDS with targeting groups can enhance the uptake capacity of tumour cells due to the interaction between membrane receptors and targeting groups. For instance, nDDS are often modified with RGD, Tf and folic acid to strengthen cellular uptake through receptor-mediated endocytosis [73].
2.5. Lysosome barrier
Endosomal vesicles are derived from a basic innate defence mechanism of cells in that any foreign matter invading the membrane is first entrapped in an endosome and transformed into a late endosome (pH 4.5-5.5), which finally merges with the degradative lysosomes (pH∼5) [74]. The endocytosed matter is degraded in the lysosomal compartment due to acidic pH and digestive enzymes, which obstruct intracellular delivery and warrant unique strategies that circumvent endosomal escape and improve the cytosolic bioavailability of bioactive molecules [75]. Many approaches have been employed to overcome the lysosomal barriers such as proton sponge effect and osmotic lysis, membrane fusion, pore formation, membrane destabilisation/disruption, vesicle budding and collapse [76]. For example, polycations such as polyethylenimine (PEI) with an excellent buffering capacity near the physiological pH could serve as ‘proton sponges’ to prevent endosomal acidification [77]. Endosomes swell osmotically due to the transport of chloride ions, which neutralizes the positive charge inside the vesicles and eventually causes the endosomal membranes to collapse. Moreover, the viral membrane prefers to fusion with the endosomal membrane during endosomal uptake, permitting the viral capsid to enter the cytosol. Additionally, some liposomes with a lipid bilayer can also fuse with endosomal membranes, thereby releasing their vesicular contents into the cytosol.
3. Zwitterionic nDDS to overcome biological barriers
3.1. Zwitterionic nDDS to achieve long blood circulation
For systemic drug delivery, long blood circulation time has been considered the prerequisite for high accumulation of nDDS at the diseased site [78]. Nevertheless, the circulation half-life of the current nDDS is still far from satisfactory. The body has evolved defence mechanisms to easily recognise nanoparticles as foreign invaders and rapidly clear them from the circulation [79]. Upon entering the blood, nanoparticles are prone to absorbing serum proteins, resulting in protein corona and subsequent recognition and clearance by the MPS [80]. Therefore, the design of nDDS should reduce the nonspecific protein adsorption and elude the MPS to prolong the blood circulation half-life. As a super-hydrophilic material, zwitterionic polymers have been widely used to shield nanoparticles from protein adsorption due to their stronger binding affinity with water molecules compared to the PEG-based analogues [81,82].
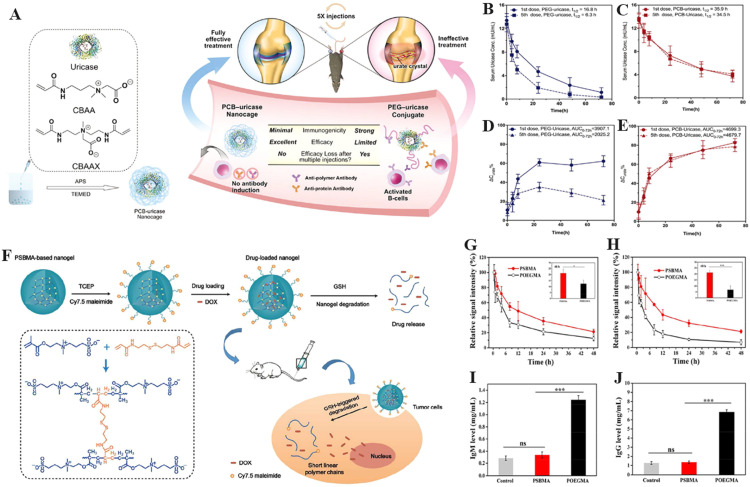
Polycarboxybetaine (PCB), polysulfobetaine (PSB) and polyphosphorylcholine (PMPC) have emerged as the most widely used anti-fouling zwitterionic polymers in recent years [83]. In the past decade, PCB has been proved to have excellent anti-protein adsorption properties in complex in vivo environments. Jiang's group dedicated a series of innovative works in the field of PCB-based nDDS for biomedical applications [84,85]. For example, Jiang et al. showed that proteins or gold nanoparticles coated with PCB evaded nonspecific protein adsorption and immune responses in vivo, which significantly prolonged the blood circulation time of the coated nanoparticles [86,87]. However, covalently binding polymers to proteins was found to reduce the reactivity of the antibodies to proteins and functional efficacy. To solve this problem, Jiang et al. continued to use a redox reaction to fabricate PCB nanocages composed of carboxybetaine monomer, zwitterionic crosslinker and uricase without covalent binding (Fig. 3A) [88]. The PCB nanocage-coated uricase not only retained its secondary structure without sacrificing its bioactivity but also enhanced its stability at high temperature. Repetitive intravenous injection of native uricase or PEGylated uricase (PEG-uricase) elicited anti-uricase antibody responses whereas PCB-uricase didn't in the same conditions. Furthermore, the circulation time of PEGylated uricase declined after multiple administration while PCB-uricase maintained an excellent circulation half-life of 34.5 h even after five repeated intravenous injections, which demonstrates the superior ability of PCB-based nDDS to overcome blood circulation barriers (Fig. 3B–3D).
Fig. 3.
Zwitterionic PCB and PSB based nDDS to overcome the blood circulation barriers. (A) Encapsulation of uricase with PCB nDDS. The PK of PEGylated uricase (B) and uricase encapsulated by PCB nDDS (C) after the first and fifth injections. The urate-eliminating ability of PEGylated uricase (D) and uricase encapsulated by PCB nDDS (E) after the first and fifth injections (Reproduced with permission from [88]. Copyright 2018 WILEY-VCH Verlag GmbH & Co. KGaA). (F) A schematic of DOX-loaded biodegradable zwitterionic PSB nanogels. PK profiles of PEGylated nanogels and PSB nanogels after the first (G) and second (H) injection in BALB/c mice (1 mg/ml). (I) Blood levels of immune globulin M (IgM) 5 d after the initial nanogel injection (n=5). (J) Blood levels of immune globulin G (IgG) 5 d post the second nanogel injection (n=5). The ns means that there is no significance (*P < 0.05, **P < 0.01, ***P < 0.001). (Reproduced with permission from [93]. Copyright 2018 American Chemical Society).
The difference performances in anti-fouling ability and pharmacokinetics between PCB and PEG could be attributed to their varied hydration ability and hydrophobic interactions [89]. Firstly, the hydration free energy of PCB is much lower than that of PEG, indicating that zwitterionic materials exhibit stronger hydration. Previous research revealed that water molecules near the surface of PCB showed lower mobility and wider dipole orientation distribution than those near the PEG [90]. Furthermore, water molecules near PCB surfaces are less oriented than those near PEG. The decreased mobility makes it harder to repel the water layer near PCB, which demonstrated the higher repulsive forces against nonspecific protein adsorption of PCB compared to PEG [91]. Secondly, PCB and PEG showed distinctive effects on the hydrophobic interactions in aqueous solutions. PEG exhibited amphiphilic feature, which made PEG preferentially cover the hydrophobic domains of proteins in aqueous solution, thereby inducing the anti-PEG responses after multiple drug administration. On the contrary, PCB was super-hydrophilic with highly charged groups which avoid interactions with hydrophobic substrates [92].
In addition to PCB, PSB has also been used to fabricate nDDS that overcome the blood circulation barrier. For instance, Yang et al. developed one-step reflux precipitation polymerisation to fabricate biodegradable PSB nanogels with disulfide bonds (Fig. 3E) [93]. The PSB nanogels showed slow drug release (7% release in 24 h) in a physiological environment but rapid drug release (85% release in 8 h) in cytoplasmic reductive environment. It's worth noting that the blood circulation time of PSB nanogels were superior than that of PEGylated analogues without inducing significant ABC phenomenon (Fig. 3F-3J). Indeed, DOX-loaded PSB nanogels exhibited stronger tumour inhibition effect with negligible side effects compared to other treatment groups in human hypopharyngeal carcinoma-bearing mouse models. Furthermore, PMPC has been regarded as the membrane-mimicking structure which exhibited long blood circulation. For example, Lu et al. recently developed a hypoxia-degradable PMPC nanogel which presented a blood circulation half-life of 44.2 h without inducing ABC phenomenon after repeated administration [94]. Due to the azo bond structure in the crosslinker, PMPC nanogels rapidly degraded into oligomers of low molecular weight in tumour hypoxic environment, thereby effectively releasing loaded drug and inhibiting tumours in vivo. It's worth noting that several PMPC-derived biomedical products have been approved by the FDA in the field of contact lenses and cosmetics. The first example is the ProclearⓇ 1-d daily disposable contact lens under the Coopervision brand which contains with MPC polymers. MPC polymers allow moisture to collect on the surface of the lens, which improves the water wettability and biocompatibility of the contact lenses [95]. In addition, the NOF CORPORATION is the first company to industrialize MPC polymer, and its product LipidureⓇ-PMB containing with MPC polymer has been used in basic make-up for a long time. Furthermore, it has been reported that an MPC polymer named as PMB30 with an MPC unit composition of 30% was evaluated as the blood-compatible material which approved by the FDA under Master Access File LIPIDURE-CM5206 [35].
Although zwitterionic nDDS exhibited the ability to overcome the blood circulation barriers, their properties varied according to the different zwitterionic chemical structure [31]. Among zwitterionic polymers, PCB contains a cationic trimethyl ammonium group and an anionic carboxylic group, while PSB contains cationic trimethyl ammonium group and an anionic sulfonate group. Different molecular structures of anion group and the distance between charged groups make an impact on the anti-fouling properties. Firstly, the difference in charge density between carboxylic group (-5.3 e/nm3) and trimethyl ammonium group (-3.0 e/nm3) is greater than that between sulfonate group (-4.5 e/nm3) and trimethyl ammonium group (-3.0 e/nm3), which leads to the stronger self-association effect of PSB than PCB [96]. The self-association effect weakens the hydrophily of the zwitterionic polymers, leading to the stronger anti-fouling property and longer blood circulation of PCB-based mDDS than the PSB-based analogues. Secondly, the distance between charged groups also exert influence on the hydration properties of zwitterionic polymers. For example, molecular dynamics simulation showed that the water molecules in the hydration shell of the carboxylic group with one methylene group between charged groups have lower residence time and wider dipole-orientation distribution than those in the hydration shell of the carboxylic group with three methylene groups, which leads to the superior anti-fouling properties of PCB with one methylene group between charged groups than that with three methylene groups between charged groups [97]. Lastly, PMPC shows a positive charge at the end of molecular chain and a negative charge in the middle of the structure, which is different from the PCB and PSB. Our previous research revealed that the blood circulation time of PMPC-coated Fe3O4 is shorter than the PCB or PSB coated analogues, which may be attributed to the interaction between negative biomacromolecules and positive charge at the end of the PMPC chains [98].
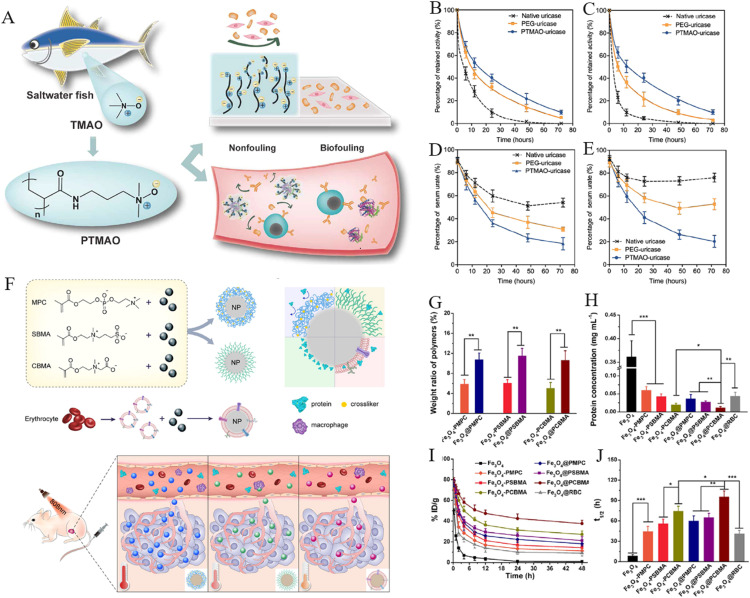
In addition to the traditional zwitterionic polymers, Jiang et al. developed a new class of poly (trimethylamine N-oxide) (PTMAO) anti-fouling biomaterials based on trimethylamine N-oxide (TMAO), a small organic osmolyte found in saltwater fishes [99] (Fig. 4A). Compared to the one methylene groups between PCB charge moieties, the opposing charge moieties of TMAO are directly connected (Me3N+-O−) with methylene between two charges, which may bring about stronger hydration capacity. Therefore, uricase was encapsulated by PTMAO to investigate whether it extends the blood circulation time in vivo. The in vitro experiments indicated that PTMAO-coated uricase (PTMO-uricase) overwhelmingly alleviated protein adsorption in non-diluted serum. The blood circulation half-life of PTMO-uricase was calculated as 19.1 h after the first dose and 18.2 h after the third dose, which was significantly superior than those of native uricase and PEG-coated uricase (Fig. 4B-4C). Fig. 4D and 4E exhibited that PTMO-uricase decreased urate levels in the blood of mice by over 80% and this reduction persisted after three administrations, demonstrating the superior performance of PTMO-uricase in systemic circulation and enhancing urate metabolism. Inspired by the long blood circulation of nDDS given by natural cell membrane, Yang et al. developed a membrane coating strategy to endow nanoparticles with a variety of zwitterionic polymer membranes [98]. The thermogravimetric study uncovered that the structure of the zwitterionic polymer membranes are denser than that of zwitterionic polymer brushes with the same hydrodynamic size, leading to the stronger resistance of protein adsorption and macrophages uptake of the zwitterionic polymer membrane-coated Fe3O4 (Fig. 4G-4H). PK experiments showed that the circulation half-life of PCB membrane-coated Fe3O4 was as long as 96.0 h, which was superior to that of PCB brush-coated Fe3O4 or RBC membranes-coated Fe3O4 (Fig. 4I-4J). Moreover, PCB membrane-coated Fe3O4 displayed longer circulation time than PSB or PMPC membrane-coated Fe3O4, which might be attributed to the shorter alkyl chain length between the anions and cations in PCB. Notably, 24 h after intravenous injection, PCB membrane-coated Fe3O4 nanoparticles showed 2.6-fold higher blood retention than RBC membrane-coated Fe3O4 nanoparticles, indicating that zwitterionic membranes are superior to natural cell membranes in overcoming systemic circulation barrier.
Fig. 4.
Zwitterionic PTMAO and polymer membrane-based nDDS to overcome the blood circulation barriers. (A) The PTMAO with excellent anti-fouling properties was originally identified in saltwater fish. Following the first (B) and third (C) intravenous injection, mice sera were used to determine the PK profiles of each sample. The urate level in mice sera was assessed following the first (D) and third (E) intravenous injections (Reproduced with permission from [99]. Copyright 2019 The Authors). (F) A schematic depicting of the fabrication of zwitterionic polymer membranes-coated Fe3O4 and their biological properties. (G) The mass proportion of zwitterionic polymer membranes to zwitterionic polymer brushes coated on Fe3O4. (H) The protein adsorption of nanoparticles following a 30-min incubation in mouse blood. (I) The PK of nanoparticles upon intravenous administration. (J) The blood circulation half-life of nanoparticles in the bloodstream (Reproduced with permission from [98]. Copyright 2019 Elsevier Ltd.) (*P < 0.05, **P < 0.01 and ***P < 0.001)
To extend the blood circulation time of peptide and protein drugs, Chilkoti et al. inserted a zwitterionic sequence into polypeptides with varying numbers of cationic and anionic residues and chain lengths [100]. They discovered that the zwitterionic polypeptides increased blood retention and circulation half-life by almost two-fold relative to the pure polypeptides. In addition to zwitterionic polymers, mixed-charge nDDS also show the potential to overcome blood circulation barriers. For instance, Ji et al. developed gold nanoparticles (AuNPs) coated with equal numbers of quaternary ammonium and sulfonic groups [101]. Indeed, AuNPs coated with mixed-charge zwitterions exhibited significantly longer blood circulation (≈4.6 fold) with less accumulation in liver and spleen than PEG-coated AuNPs. Moreover, AuNPs coated with mixed-charge zwitterions showed decreased uptake and distinct aggregation states in liver Kupffer cells and spleen macrophages compared with their PEG-coated counterparts.
3.2. Zwitterionic nDDS to achieve organ targeting
Targeted drug delivery aims to improve the accumulation of drugs at the diseased site [102,103]. However, most nDDS are taken up by the liver after systemic administration, which limited the application of nDDS to treat extrahepatic disease [6,104]. Impressively, zwitterionic nDDS have been shown to target diverse organs such as brain, intestine, lymphatics and spleen without the demand of specific targeting groups. Therefore, zwitterionic nDDS provides a facile platform to deliver drugs to extrahepatic organs which is difficult to achieve by the common nDDS.
3.2.1. Zwitterionic nDDS to achieve brain targeting
The blood-brain barrier (BBB) is a significant obstacle to achieve the effective treatments for a wide range of diseases affecting the central nervous system, including Parkinson's disease, Alzheimer's disease, epilepsy, stroke and primary and metastatic brain tumour [105,106]. As a result, drug concentration in the brain is generally 1,000-fold lower than that in the bloodstream. It has been calculated that over 98% of small-molecule therapeutic candidates and virtually all macromolecular drugs cannot penetrate into the brain [107,108]. To overcome the BBB, many methods, including receptor-mediated transport, transporter-mediated transport, adsorptive-mediated transport and temporary opening of the BBB with chemical compounds or focused ultrasound, have been developed as potential means to circumvent the BBB [109], [110], [111], [112], [113]. Notably, Tf receptor, insulin receptor, low-density lipoprotein (LDL) receptor-related protein, nicotinic acetylcholine receptor, insulin-like growth factor receptor, diphtheria toxin receptor, scavenger receptor call B type, leptin receptor and the neonatal Fc receptor are a few of the many receptors that are over-expressed on the BBB [114], [115], [116], [117], [118]. These receptors can bind to matching ligands or ligand-modified nDDS to enhance the uptake by endothelial cells, which mediates transport through the BBB.
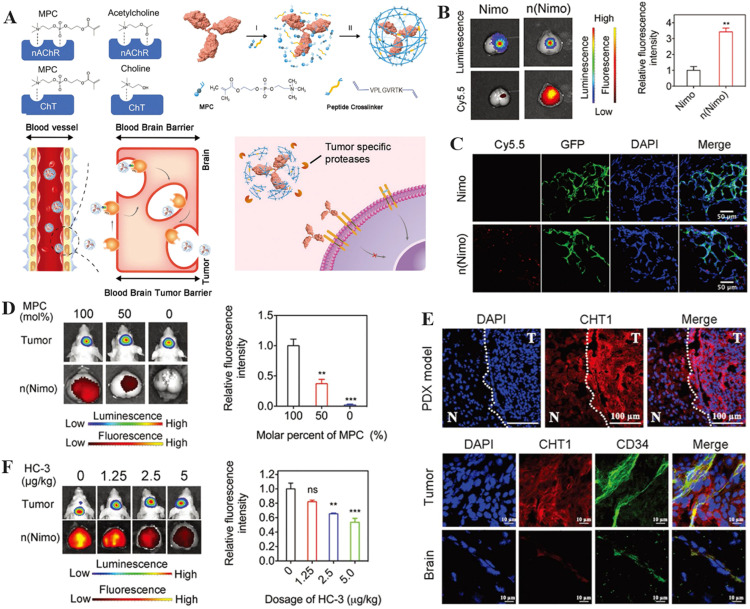
Recently, PMPC-based nDDS have received a lot of attention not only in the field of long-circulating materials, but also in the breakthrough of the BBB to deliver drugs to the brain [32,[119], [120], [121], [122]]. For instance, Lu et al. encapsulated monoclonal antibodies (mAbs) with PMPC shell and a peptide crosslinker to generate n(Nimo) through in situ polymerisation (Fig. 5A) [123]. Ex vivo fluorescence imaging revealed that the intensity of brain fluorescence among mice treated with n(Nimo) was approximately 3.5-fold higher than in mice treated with native nimotuzumab (Fig. 5B). The colocalization of cyanine 5.5 (Cy 5.5) signal from n(Nimo) and the green fluorescent protein expressed by the tumour was showed in the confocal pictures of the tumour tissues, which indicated that n(Nimo) was successfully delivered to the brain tumour (Fig. 5C). Furthermore, both in vivo and ex vivo bioluminescence images revealed that a higher molar percentage of PMPC in nDDS enhanced their accumulation in the brain, suggesting that the PMPC moiety is responsible for effective drug delivery (Fig. 5D). The mechanism by which PMPC-based nDDS can breach the BBB has also been revealed. PMPC contains the phosphorylcholine structure which exhibits properties similar to choline groups. Increased expression of choline transporter 1 (ChT) on brain tumor endothelial cells was demonstrated, suggesting that this may facilitate efficient delivery of n(Nimo) to the brain tumour (Fig. 5E). Furthermore, a ChT inhibitor, hemicholinium-3 (HC-3), was intraperitoneally injected into the glioma-bearing mice. It was found that increasing the dose of HC-3 significantly reduced the fluorescence intensity of the glioma-bearing brain, indicating that ChT promoted n(Nimo) transfer into brain (Fig. 5F). In addition, the peptide crosslinker could be cleaved by the over-expressed protease in the tumour to ensure the controlled release of the encapsulated mAbs, thereby inhibiting tumour growth while prolonging the survival time of the mice. Yu et al. also fabricated hypoxia-degradable zwitterionic phosphorylcholine nanogels (HPMPC) to improve the treatment of glioblastoma. The in vivo experiments demonstrated that the nanogel was able to effectively cross the BBB and accumulate in glioblastoma tissue. The azobenzene-contained crosslinker allowed the HPMPC nanogels to degrade in a hypoxic environment, resulting in rapid drug release within the tumour. Finally, the drug-loaded HPMPC nanogels effectively inhibited tumours in an in vivo glioblastoma model [40].
Fig. 5.
The illustration of zwitterionic nDDS to achieve brain targeting. (A) Schematic of mAb encapsulation with PMPC nanocapsules for glioma treatment. (B) Fluorescence and bioluminescence images taken 10 d after injecting Cy5.5-labeled n(Nimo) into brain tissues dissected from patients with gliomas. The histogram summarizes the relative fluorescence intensity of tumor-bearing brain tissue. (C) The colocalization of n(Nimo) with the tumor tissue. (D) Photographs of brain ex vivo fluorescence and bioluminescence obtained 4 h after a single injection of Cy5.5-labeled n(Nimo) (5 mg/kg) in orthotopic U87-EGFRwt glioma xenograft mice. (E) Images of human tumor tissue xenograft (PDX) in mouse brain taken with confocal microscopy (separated by a dashed line, T indicates tumor and N indicates normal tissue). (F) Orthotopic U87-EGFRwt glioma xenograft mice bioluminescence and ex vivo fluorescence images of the dissected brain 4 h after a single injection of Cy5.5-labeled n(Nimo) (5 mg/kg) (Reproduced with permission from [123]. Copyright 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim). (*P < 0.05, **P < 0.01 and ***P < 0.001)
In addition to brain tumours, PMPC has also been used to target other CNS diseases such as stroke or neural diseases. For instance, a PMPC-coated nanocapsule containing nerve growth factor (NGF) was developed which could effectively penetrate the BBB in healthy mice and nonhuman primates [124]. Due to the hydrolysis of the polylactic acid diacrylate, the nanocapsule could achieve the sustained release of NGF in acidic TME. The in vivo experiments revealed that the delivery of NGF enabled neural regeneration, tissue remodeling and functional recovery in mice with spinal cord injury. Likewise, the treatment of ischaemic stroke is often hindered by the BBB and off-target effects. Accordingly, Kang et al. selectively delivered intravenous immune globulin (IVIg) to ischaemic regions using PMPC-nanocapsules that penetrated the BBB and thus enabled the accumulation of IVIg at the diseased site [125]. In a model of middle cerebral artery occlusion, neurological deficits and mortality were decreased with early administration of low-dose IVIg encapsulated by PMPC-nanocapsules.
3.2.2. Zwitterionic nDDS to achieve intestinal targeting
Oral administration is a technique applied for both local and systemic delivery of many drugs and remains the preferred route of administration due to the higher patient compliance and lower manufacturing costs [126,127]. However, oral drug delivery also faces some drawbacks, including rapid degradation in the gastrointestinal tract and poor penetration of the mucosal barrier, resulting in low bioavailability [128]. Therefore, nDDS have been employed to reduce dose-related toxicity and increase the oral bioavailability of therapeutic molecules [129], [130], [131]. Generally, surface-functionalised or ligand-grafted nDDS are required to increase the uptake of the nDDS by diverse intestinal cells such as enterocytes, mucus-secreting goblet cells or dendritic cells. Recently, researchers have shown that zwitterionic nDDS can overcome the intestinal barrier without opening tight junctions through receptor-mediated endocytosis, which provided an attractive approach to achieve intestinal drug delivery.
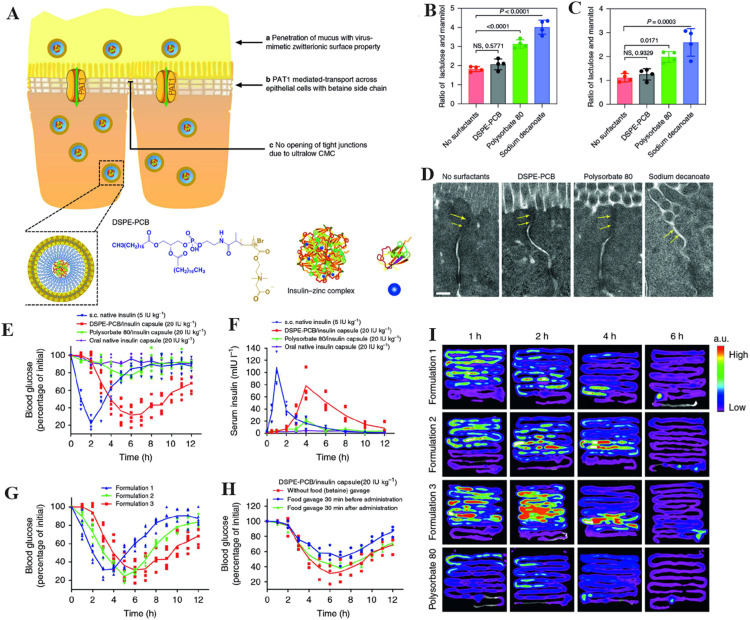
To overcome the poor penetration of protein drugs, Cao et al. fabricated virus-mimetic PCB micelles to deliver insulin orally (Fig. 6A) [132]. An intestinal fluid penetration test showed that the ensemble-averaged geometric mean square displacement of the zwitterionic PCB micelle was approximately 6.7 times higher than that of the PEG micelle, indicating much faster diffusion of the PCB micelle through mucus. Intestinal permeability and mucosal barrier integrity are evaluated by studying the excretion ratio of lactulose to mannitol (L/M) in urine. The L/M ratio did not change visibly in the PCB micelle-treated group, but it increased dramatically in the polysorbate 80- and sodium decanoate-treated groups (Fig. 6B -6C). Tight junctions were observed to be intact in control and PCB micelle-treated tissue, but to be compromised in polysorbate 80 and sodium decanoate-treated tissue, as seen in transmission electron microscopy (Fig. 6D). In comparison to the polysorbate 80/insulin capsule, which only had a pharmacological activity of 8.56% based on the glucose-lowering profile, the PCB/insulin capsule exhibited a much higher 43.4% pharmacological activity (Fig. 6E). Meanwhile, compared to polysorbate 80/insulin, the PCB/insulin capsule showed a bioavailability of 42.6%. (Fig. 6F). Modifying the Zn2+ content in the formulation would allow for fine-tuning of the blood glucose-lowering profile (Fig. 6G). Fig. 6H demonstrated that potential food interference such as the intake of betaine could be minimised by administering the oral insulin capsule 30 min before meal consumption. Compared to polysorbate 80/insulin, the ex vivo fluorescence pictures revealed that PCB/insulin was better retained and absorbed by the small intestine (jejunum and ileum). PCB micelle was found to penetrate across the epithelial cell layer through the mechanism mediated by the pathway of proton-assisted amino acid transporter 1 (PAT1). In vitro assays displayed significantly higher-level uptake of PCB micelles by PAT1 over-expressed cells (Caco-2) and notable inhibitory uptake in the presence of PAT1 substrates, such as betaine and l-tryptophan. Therefore, this work revealed a simple yet robust zwitterionic PCB micelle to overcome the intestinal barrier without opening the tight junctions.
Fig. 6.
The illustration of zwitterionic nDDS to achieve intestinal targeting. (A) The scheme of oral administration of insulin using DSPE-PCB micelles. Diabetic mice (B) and healthy mice (C) underwent intestinal permeability tests by evaluating the ratio of lactulose and mannitol concentration in urine collected 1 h after co-administering lactulose, mannitol and surfactants through the ileum. (D) Images captured by TEM of representative epithelial tissues 1 h after injection into ileum with various surfactants. Arrows indicate tight junctions. Scale bar indicates 0.2 µm. (E) Oral gavage of a DSPE-PCB/insulin capsule produced hypoglycemic effects in diabetic rats. (F) Oral gavage of a DSPE-PCB/insulin capsule into diabetic rats and the resulting serum insulin concentration (bioavailability). (G) Oral gavage testing of the hypoglycemic effectiveness of different DSPE-PCB/insulin capsule formulations in diabetic rats. Formulations 1, 2 and 3 were fed with insulin/ZnCl2 at ratios of 50:1, 20:1 and 2.5:1 (weight ratio) during encapsulation, respectively. (H) The impact of food on the DSPE-PCB/insulin capsule's ability to lower blood sugar levels. (I) Oral insulin absorption locations and kinetics. After fasting for 12 h, healthy rats received either DSPE-PCB/Cy7-insulin enteric capsules (formulations 1-3) or polysorbate 80/Cy7-insulin enteric capsules (20 IU/kg) via oral gavage. The ns indicates no statistical significance(*P < 0.05, **P < 0.01, ***P < 0.001). (Reproduced with permission from [132]. Copyright 2020, The Authors.)
In addition to PCB, Chen et al. designed a pH-triggered zwitterionic PSB hydrogel-coated metal-organic framework (MOF) nanoparticles for the treatment of hyperglycemia [133]. Exendin-4 was encapsulated into the framework of the MOF with a loading percent greater than 40%. In the acidic gastric environment, the pH-responsive capsules maintained their shape, thereby protecting exendin-4 from destruction. The bubble-generating reaction by sodium bicarbonate and citric acid facilitated nanoparticle transport in the mucus solution. More importantly, both Caco-2 and E12 cells were better able to internalize the MOF nanoparticles with a zwitterionic PSB hydrogel shell. Plasma levels of extendin-4 significantly increased after oral administration of capsules in a rat model of diabetes, which promoted the endogenous insulin secretion with a relative pharmacological availability of 17.26%. Moreover, Shen et al. fabricated a poly[2-(N-oxide-N,N-diethylamino)ethyl methacrylate] (OPDEA) micelle, which was highly non-fouling to blood proteins and other biomacromolecules but weakly binded to phospholipids. It was found that the OPDEA micelle could travel through the viscous mucus and bound to villi. More impressively, orally administration of OPDEA micelles loaded with paclitaxel (PTX) hindered the growth of patient-derived hepatocellular carcinoma xenografts and triple-negative breast tumours, which was more effectively than PEG-based equivalents or free PTX by intravenous administration [134].
3.2.3. Zwitterionic nDDS to achieve lymphatic and spleen targeting
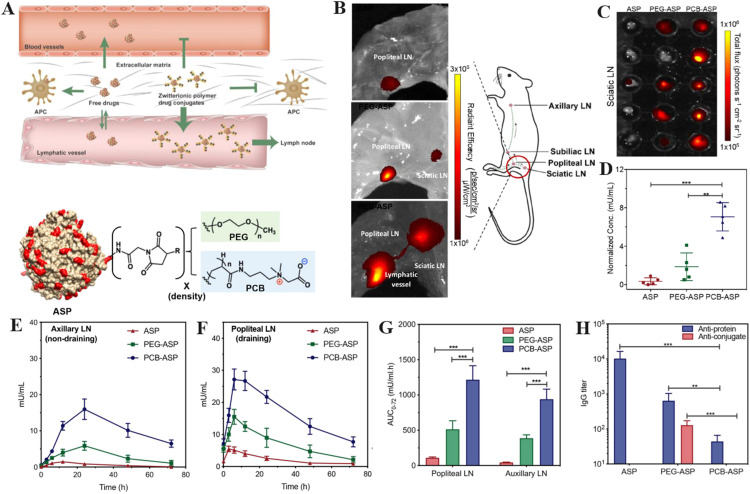
The lymphatic system consists of a network of capillaries and nodes that enable the proliferation and progression of diseases such as viral infections and cancer [135]. Unfortunately, most drugs cannot penetrate lymphatic tissues due to the biological boundaries in the interstitial space [136]. Recently, Jiang et al. conjugated zwitterionic PCB onto L-asparaginase (ASP) to achieve the lymphatic targeting drug delivery (Fig. 7A) [137]. Significant near-infrared ray fluorescence was observed in the popliteal and sciatic lymph nodes (LNs) after intravenous administration of ASP modified with PCB (PCB-ASP), whereas relatively weaker fluorescence intensity was found for pure ASP and ASP modified with PEG (PEG-ASP) (Fig. 7B). The mouse sciatic LNs were also photographed and the total fluorescence signal flux of every LN was converted into the ASP concentration for quantitative comparison. As shown in Fig. 7C-7D, PCB conjugation facilitated transport of ASP to the lymphatics with the higher ASP concentration at the LNs on the end of the ischium. Notably, PCB-ASP exhibited superior penetration ability through the LN barriers with approximately 3.5-fold higher than PEG-ASP. Fig. 7E and F showed that PCB-ASP resulted in significantly greater ASP concentrations in draining popliteal LNs (27 mU/ml) and non-draining axillary LNs (15 mU/ml) than PEG-ASP and native ASP. Therefore, PCB conjugation not only permitted ASP accumulation in LNs but also enhanced ASP transportation inside the lymphatic system. Fig. 7G confirmed that PCB-ASP displayed significantly higher bioavailability in popliteal and axillary LNs compared to pure ASP and PEG-ASP. ELISA showed that PCB-ASP mitigated the production of anti-protein IgG and anti-conjugate IgG, indicating its low immunogenicity (Fig. 7H). The possible mechanism why PCB-ASP can effectively target lymph nodes lies in their unique super-hydrophilicity, which leads to negligible interaction between PCB-ASP and the interstitial environment. On the contrary, PEG-ASP is easily trapped in the lymphatic space environment due to the hydrophobic interaction with proteins. Therefore, PCB conjugation offers promise for the treatment of lymph-based illnesses by overcoming lymphatic barrier.
Fig. 7.
The illustration of zwitterionic nDDS to achieve lymphatic and spleen targeting. (A) Intravascular distribution of zwitterionic and PEGylated nDDS is depicted schematically. NIR fluorescence pictures of (B) skin-removed ventral left hind leg muscle tissue and (C) the sciatic LNs at 180 min after subcutaneous foot injection of native L-ASP, PEG-ASP, and PCB-ASP conjugates. (D) The relative abundance of ASP in sciatic LNs was measured against the total flux of fluorescent signal. Mice were given native ASP, PEG-ASP, and PCB-ASP conjugates through subcutaneous injection once weekly for three weeks. Non-draining axillary LNs (E and draining popliteal LNs (F) from mice were obtained at various time intervals after the third injection for lymph PK investigation. (G) The bioavailability of each sample after third dosage in axillary and popliteal LNs. (H) Antibody titers were discovered in the popliteal LNs 72 h following the third injection. (**P<0.01; ***P<0.001). (Reproduced with permission from [137]. Copyright 2020 American Chemical Society).
In recent years, mRNA-based therapy has shown potential for vaccine development and treatment of genetic diseases [138,139]. Indeed, pairing mRNA with nDDS such as LPNs could overcome the biological barriers and enhance bioavailability in targeted cells, not unlike the anti-COVID-19 vaccine [140,141]. However, mRNA delivered by LPNs often accumulates in the liver, limiting wider applications of mRNA therapy [142]. Recently, Siegwart et al. reported a simple and facile method to synthesise zwitterionic phospholipidated polymers (ZPPs) by zwitterionic modification of cationic polymers, allowing splenic-targeting and LNs-targeting delivery of mRNA [143]. By fractionally conjugating several alkylated dioxaphospholane oxide molecules to 15 distinct cationic polymers, a library of 420 ZPPs were synthesized with vastly increased serum stability and endosomal escape. Moreover, ZPPs targeted the spleen much better than their cationic counterparts. Interestingly, hydrophilicity-hydrophobicity balance is an important factor for successful in vivo mRNA delivery. The alkyl length of amines in ZPPs exhibited a significant effect on spleen mRNA expression. Furthermore, there was also evidence that ZPPs with particular zwitterionic structures and quantities could transfect LNs. ZPPs containing four zwitterions and four long alkyl tails, which were designed rationally to balance amphoteric and amphiphilic functions, were highly efficient at transporting mRNA to far-flung LNs. The spleen-targeting of ZPPs was ascribed to the introduction of negative phosphate and reduced electrostatic interaction toward mRNA, which was verified by the study that incorporation of negatively charged lipids into LNPs facilitated spleen tropism [62].
3.3. Zwitterionic nDDS to achieve deep tumour penetration
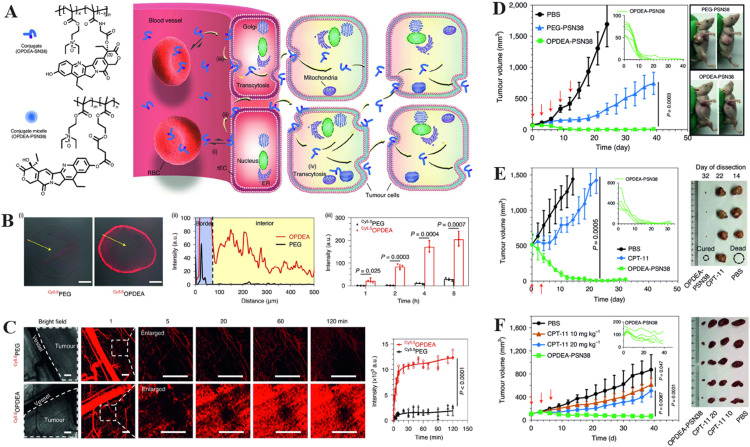
Heterogeneous vascular networks and IFP hinder anti-tumour drugs from permeating solid tumours deeply [144,145]. To overcome the IFP barrier, the size, surface charge and shape of nDDS could be adjusted, which can be achieved through programmed delivery strategies [10,146,147]. Recently, zwitterionic chemistry realised the deep tumour penetration of nDDS without the need of complex modifications [148]. For instance, Shen et al. developed a zwitterionic OPDEA polymer, whose anti-fouling properties and weak interactions with phospholipids enabled long circulation times and reversible interactions with cell membranes (Fig. 8A) [149]. The fluorescent surface and interior of multicellular tumour spheroids treated with OPDEA were 15-fold brighter than the PEG-treated analogue after incubation for 4 h, indicating deep penetration of OPDEA-based nDDS (Fig. 8B). In vivo real-time imaging of tumour blood vessels showed that OPDEA rapidly diffused from the vasculature into the tumour and dispersed across the area after 120 min and its intensity was approximately 6-fold higher than that of PEG (Fig. 8C). Small tumours (∼70 mm3) were first used to evaluate the anti-tumour effect. Five out of six mice exhibited no evidence of tumours after the fifth treatment with 7-ethyl-10-hydroxycamptothecin conjugated with OPEDA (OPEDA-PSN38) (Fig. 8D). Furthermore, OPEDA-PSN38 also had a remarkable therapeutic index among large tumours (∼500 mm3) and human tumours in a mouse model (Fig. 8E-8F). Therefore, OPEDA represents simple zwitterionic polymer that can achieve prolonged circulation time, deep tumour penetration and enhanced cellular uptake. It is speculated that the above excellent performance of OPDEA is strongly related to its molecular structure, where the opposite charge groups in OPDEA are directly linked through N-oxide, leading to its lack of stickiness to proteins, which facilitates diffusive transport and further deep penetration under high IFP without any complex modifications.
Fig. 8.
The illustration of zwitterionic nDDS to overcome IFP barriers. (A) Intracellular processes following the combination of the anticancer drug SN38 with OPDEA-based conjugates or block copolymers. (B) Penetration of Cy5.5OPDEA and Cy5.5PEG (shown in red) in HepG2 MTSs. (i) Representative images of MTS slices after 4 h incubation. Scale bars = 200 μm. (ii) The intensity of fluorescence is plotted from the MTS surface to the center along arbitrary lines (yellow arrow). (iii) Cy5.5 integrated fluorescence intensity of MTSs at different times. (C) Fluorescence in vivo image of Cy5.5OPDEA or Cy5.5PEG (red) extravasation and distribution in a subcutaneous 4T1 tumour in mice following injection of the polymer via tail vein. Representative images and zoomed photographs of the area chosen at random (dotted outline) is shown graphically (left) along with the average total fluorescence intensity of the selected area was plotted (right). Scale bars indicate 500 µm. (D) Subcutaneous HepG2 xenograft tumours are effectively inhibited by OPDEA-PSN38 micelles. (E) OPDEA-PSN38 micelles exhibited stronger antitumor effect than CPT-11 against big subcutaneous HepG2 tumors. (F) Antitumor efficacy of OPDEA-PSN38 micelles in a PDX model of liver cancer. (*P < 0.05, **P < 0.01, ***P < 0.001). (Reproduced with permission from [149]. Copyright 2021, The Author(s), under exclusive licence to Springer Nature Limited.)
Likewise, subtle molecular structure changes may lead to different properties, such as hydration and thermal response. For instance, a zwitterionic sulfamide-based nanogel, named as poly (2-((2-(methacryloyloxy)ethyl) dimethylammonio)acetyl) (phenylsulfonyl) amide (PMEDAPA), was developed by Lu et al for microwave-augmented deep tumour therapy [150]. The insertion of hydrophobic benzene groups into the zwitterionic structure weakened the hydration and enhanced the thermal response of the nanogel. By adjusting the degree of crosslinking, the volume phase transition temperature of PMEDAPA nanogel was modulated to physiological temperature. After modification with targeting group Tf, the PMEDAPA-Tf nanogel shielded Tf under physiological temperature but exposed it under mild microwave heating, enabling superior penetration in 3D tumour spheroids and rapid release of the loaded drugs. In vivo experiments uncovered that the drug-loaded PMEDAPA-Tf nanogel significantly inhibited the growth of tumours without adverse effects in a humanised orthotropic liver cancer model.
3.4. Zwitterionic nDDS to enhance cellular uptake
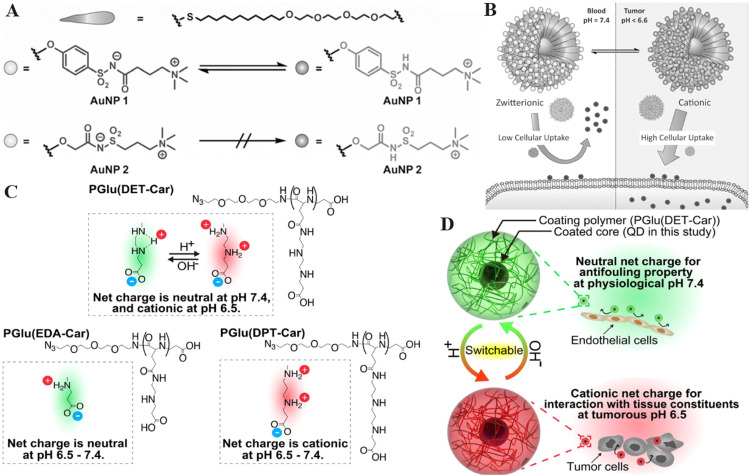
As a class of super-hydrophilic polymers, zwitterionic nDDS tend to generate water-swelling layers in aqueous solution that weaken interactions between zwitterionic nDDS and cell membranes [71]. In addition, the surface of zwitterionic nDDS is usually electrically neutral, leading to negligible electrostatic interactions between zwitterionic nDDS and negatively charged cell membrane [151,152]. Generally, nDDS with positive surface charge are easily taken up by the cell membrane through strong electrostatic interactions [153]. Therefore, the development of zwitterionic nDDS which could remain neutral charge in normal tissue whereas convert to positive charge in diseased tissue may achieve long blood circulation and enhanced uptake by targeting cells in the meantime. For instance, Wang et al. established a zwitterionic polymer-based nDDS that was modified with 2,3-dimethylmaleic anhydride (DMA) for enhanced drug delivery to tumours. The nDDS were neutral charged under physiological conditions and rapidly transformed to cationic charged upon entering the TME owing to the shedding of DMA in the acidic environment [42]. Therefore, the designed nDDS delivered DOX into the tumor cells with high efficiency which greatly restricted the growth of tumors in vivo. In addition, Rotello et al. developed alkoxyphenyl acylsulfonamide ligands that remained zwitterionic state at neutral pH but became cationic state in acidic environments due to the protonation of acylsulfonamide group (Fig. 9A) [154]. The Au NPs modified with the alkoxyphenyl acylsulfonamide ligands (AALs) exhibited low cellular uptake at pH 7.4 and improved cellular uptake when the pH was below 6.6 due to the conversion from zwitterion to positive charge (Fig. 9B). Au NPs modified with the AALs showed significant toxicity to tumour cells, whereas Au NPs modified with the alkyl analogue exhibited negligible toxicity. Moreover, Nishiyama et al. developed an ethylenediamine-based betaine structure whose neutral net charge became cationic in the TME, which improved the accumulation of the coated particles in tumours [155]. The developed polyzwitterion contained ethylenediamine groups which realised gradual protonation and initiated di-protonation below the pH of 6.5, leading to the charge-conversion of nDDS in acidic TME (Fig. 9C-9D). Quantum dots coated with the developed polyzwitterion demonstrated anti-fouling ability in neutral environment and enhanced tumour cellular uptake at the same time, which supported the potential of charge-switching polyzwitterions to achieve specific tissue targeting.
Fig. 9.
The illustration of zwitterionic nDDS to overcome cell membrane barriers. (A) Chemical structure of the zwitterionic acylsulfonamide monolayer-protected gold nanoparticles. (B) The strategy for pH-responsive zwitterionic acylsulfonamide delivery system into tumors. (Reproduced with permission from [154]. Copyright 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.) (C) Illustration of the charge-conversion mechanism of PGlu(DET-Car) in different pH. (D) Illustration of tumor cellular uptake of PGlu(DET-Car) controlled by the pH value (Reproduced with permission from [155]. Copyright 2018Wiley-VCHVerlagGmbH&Co. KGaA,Weinheim).
3.5. Zwitterionic nDDS to achieve endosome/lysosome escape
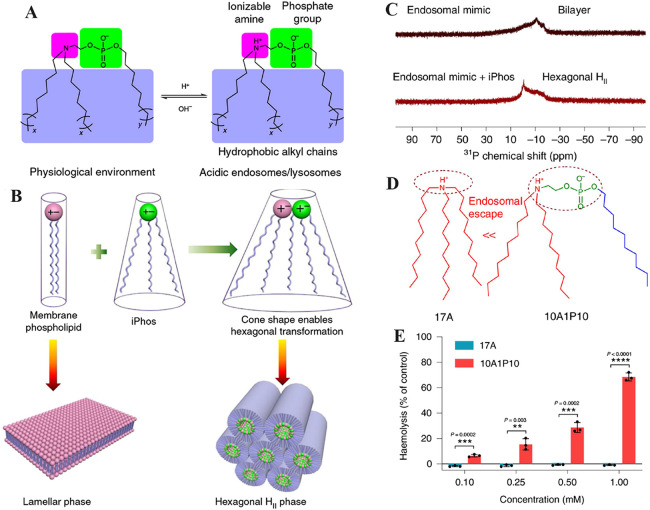
In recent years, gene therapy has sparked new hope for the treatment of refractory diseases [156,157]. For example, siRNA therapy promises a powerful tool to treat genetic diseases [158]. mRNA vaccines also alleviate the burden of infectious diseases worldwide [159]. Despite the progress, endosomal and lysosomal traps greatly hinder the wider application of gene therapy due to the rapid degradation of biomacromolecules in the environment of endosome or lysosome [160]. The development of nDDS that can overcome the endosome/lysosome barriers may broaden applications of gene therapy. Recently, Siegwart et al. designed a new class of ionisable phospholipids (iPhos) with one pH-switchable zwitterion and three hydrophobic tails (Fig. 10A) [161]. When the protonated tertiary amine entered the acidic endosomes/lysosomes, it induced a zwitterionic head group of iPhos, transforming the endosomal membrane phase from lamellar to hexagonal to allow nDDS to escape endosomes and lysosomes (Fig. 10B). 31P NMR spectroscopy showed that the asymmetry of the peak was reversed, and the liposomes were converted to a hexagonal phase after mixing with iPhos lipids (Fig. 10C). Compared to the commonly used 17A lipid, the zwitterionic 10A1P10 lipid escaped the endosome much more effectively according to haemolysis experiments in acidic environments (Fig. 10D-10E). Therefore, functional zwitterionic phospholipids are prime candidates to overcome endosomal barriers for enhanced gene therapy. Phase transformation, however, is not the only strategy and the proton sponge effect offers another route for zwitterionic polymers to escape the endosome/lysosome barriers. For instance, Zhang et al. fabricated zwitterionic PCB-based liposomes to deliver siRNA for tumour therapy [162]. Compared to PEGylated liposomes, PCB-based liposomes showed extended circulation time without inducing the ABC phenomenon. Moreover, endosome/lysosome escape and efficacy are enhanced since carboxyl groups can be protonated in an acidic endosomal environment, converting the surface charge of PCB-based liposomes to cations. In addition, Zhang et al. developed a peroxisomal mimic by in situ synthesis of CeO2 and Pt nanoparticles with monolayer of cross-linked zwitterionic vesicles (cZVs) [43]. The cZVs exhibited pH-dependent charge conversion from +7.9 mV (pH 7.4) to +27.1 mV (pH 5.0), which enhanced the escape from endosomes through the proton sponge effect. Promisingly, the cZVs alleviated reactive oxygen species-induced ear inflammation in vivo.
Fig. 10.
The illustration of zwitterionic nDDS to overcome endosome/lysosome barriers. (A) iPhos lipids had one ionizable amine, one phosphate group, and three hydrophobic alkyl tails. Protonation of the tertiary amine in acidic endosomes/lysosomes resulted in a zwitterionic head group that could easily insert into membranes. (B) The tiny ion pair head and numerous hydrophobic tails of iPhos lipids formed a cone shape that allowed for hexagonal transformation as the lipids were combined and inserted into the membranes of endosomes and lysosomes. (C) Analysis of endosomal mimic and endosomal mimic-iPhos 9A1P9 mixtures by 31P NMR spectroscopy. Membrane hexagonal HII transformation was triggered by iPhos lipid mixing. (D) Endosomal escape scheme for lipid 17A and lipid 10A1P10. (E) Lipid 17A and 10A1P10 haemolysis at pH 5.5. (Reproduced with permission from [161]. Copyright 2021, The Author(s), under exclusive licence to Springer Nature Limited.)
4. Summary and prospect
Over the past three decades, nDDS have been applied to treat various diseases with high efficiency and reduce side effects. Despite this progress, the clinical transformation of nDDS remains limited and unsatisfactory compared to the number of research articles published, which may be attributed to the diverse biological barriers in drug delivery. This review introduced the potential of zwitterionic nDDS to overcome diverse biological barriers without additional modification with targeting groups. Overall, zwitterionic nDDS were demonstrated to overcome the following obstacles: blood circulation barrier, nonspecific organ distribution, IFP, cell membrane barriers and lysosomal barriers. The advantages and limitations of zwitterionic nDDS were introduced below.
First, as an alternative to PEG, zwitterionic nDDS shows excellent anti-biological adhesion properties and significantly prolonged circulation times, which are conducive to intravenous drug delivery. Moreover, PEG induces the production of IgM and IgG after repeated injections, leading to the generation of ABC phenomenon. Impressively, zwitterion nDDS elicit negligible immune response in vivo owing to the stronger hydration and lower hydrophobic interactions compared with PEGylated nDDS. Second, traditional nDDS require ligand modifications or the incorporation of stimuli-responsive groups to overcome biological barriers, which increases the complexity of the whole formulations and weakens the batch stability. Alternatively, zwitterionic nDDS pass through the biological barriers without the additional modifications or reactions, keeping the chemical structure as simple as possible. In addition, one type of zwitterionic nDDS may overcome several biological barriers simultaneously. For instance, PMPC-based nDDS can not only overcome blood barriers but also target specific organs such as brain, spleen or LN. Third, the biosafety and biocompatibility of nDDS are prerequisites for clinical application. As a compound that mimics cell membrane phospholipids, PMPC have been used in the field of contact lenses and cosmetics. Therefore, zwitterionic nDDS have the potential to be a reliable drug carrier for disease treatment.
Despite the advantages mentioned above, zwitterionic nDDS still face some limitations before the clinical use as nanocarriers. Due to their super-hydrophilicity, most zwitterionic materials are insoluble in organic solvents, which significantly increases the difficulty in synthesizing zwitterionic block polymers or lipids in organic solvents. Some zwitterionic polymers such as PSB are susceptible to high salt concentrations owing to the self-association effect, which may bring about challenges for the use in complex environment in vivo. Many zwitterionic ligands or polymers also lack reactive groups such as sulfhydryl, amino or maleic anhydride groups compared to PEG, limiting the ability to modify them to proteins or nucleic acids. Lastly, the mechanisms of zwitterionic nDDS to achieve biological barriers such as organ targeting remain unclear. In the face of above challenges, the following strategies need to be implemented. Firstly, it is essential to develop new technology to synthesise zwitterionic polymers in aqueous solutions, which could not only solve the problem of zwitterionic polymer insoluble in organic solvent but also make the preparation process safer and more environmentally friendly. Secondly, it is necessary to study the relationship between zwitterion polymer structure and self-association effect. In some cases, self-association effect could be utilized in drug delivery by exploiting salt-responsive zwitterionic nDDs which could respond to disease microenvironment with a high salt concentration. Third, zwitterionic polymers with different functional groups and precise molecular weights need to be developed and marketed for laboratory use before the clinical applications. Zwitterionic nDDS will have a better chance of clinical application when more types of zwitterionic polymers are available in market. Last but not least, the mechanism of zwitterionic nDDS to achieve specific organ targeting should be exploited in-depth in the future. The relationship between organ targeting and the charge types (N, P, S, O) or function group position of zwitterionic polymers should be clarified to make it easier to promote zwitterionic polymers to practical use.
In summary, this review highlights the recent progress of zwitterionic nDDS in the perspective of overcoming multiple biological barriers zwitterionic nDDS have become the research hotspot due to the outstanding abilities to pass through biological barriers with brief structures. Although zwitterionic nDDS have not been approved in clinical use yet, they show great promise as an alternative to PEG and exhibit great potentials in the field of LNPs as new charge-converting lipid. Overall, the successful development of zwitterionic nDDS in clinics depends on the combined efforts of chemists, physicists, biologists, engineers and medical professionals, (Table 1).
Table 1.
Summary of zwitterionic nDDS to overcome biological barriers.
| Biological barriers | Zwitterionic type | Nanostructure | Loaded drug | Diseases/Applications | Ref |
|---|---|---|---|---|---|
| Blood circulation barrier | |||||
| MPS clearance | PCB | Nanogel | / | Anti-inflammatory | [84] |
| Croslinked network | Uricase | Gouty | [86] | ||
| Linear polymer | Au | / | [87] | ||
| Nanocage | Uricase | Gouty | [88] | ||
| Crosslinked membrane | Fe3O4 | A549 tumour | [98] | ||
| PSB | Nanogel | DOX | FaDu tumour | [93] | |
| PMPC | Nanogel | DOX | Liver cancer | [94] | |
| PTMAO | Linear polymer | Uricase | / | [99] | |
| ZIPPs | Linear polymer | GLP1 | Diabetes | [100] | |
| Mixed-charge zwitterions | Ligands | Au | KB xenografts tumour | [101] | |
| Nonspecific organ distribution | |||||
| BBB | PMPC | Nanocapsule | BSA, HRF, RTX, NGF | CNS delivery | [120] |
| Nanoparticle | RTX | CNS lymphomas | [122] | ||
| n(Nimo), n(Tras) | Glioma | [123] | |||
| NGF | Neural regeneration | [124] | |||
| IVIg | Stroke | [125] | |||
| Inherbin3/cMBP | TMZ-resistant gliomas | [121] | |||
| Nanogel | DOX | Glioblastoma | [40] | ||
| Intestinal barrier | PCB | Micelle | Insulin | Diabetes | [132] |
| PSB | Nanocomposite | Exendin-4 | Diabetes | [133] | |
| OPDEA | Micelle | PTX | Hepatocellular carcinoma xenografts, triple-negative breast tumors | [134] | |
| Lymphatic and spleen barrier | PCB | Polymer conjugation | ASP | Lymph-resident disease | [137] |
| ZPPs | Micelle | mRNA | Delivery to the spleen and lymph nodes | [143] | |
| IFP barrier | |||||
| OPDEA | Micelle | PSN38 | HepG2 tumour | [149] | |
| PMEDAPA | Nanogel | DOX | HepG2 tumour | [150] | |
| Cellular membrane barrier | |||||
| zwitterionic polymer modified with DMA | Micelle | DOX | MDA-MB-231 breast cancer | [42] | |
| AALs | Ligands | Au nanoparticle | / | [154] | |
| PGlu(DET-Car) | Linear polymer | Quantum dot | CT26 tumour | [155] | |
| Lysosome barrier | |||||
| iPhos | Liposome | mRNA | CRISPR-Cas9 gene editing | [161] | |
| PCB | Liposome | siRNA | Hela tumour | [162] | |
| PCB | Cross-linked vesicles | Pt, CeO2 | Anti-inflammatory | [43] | |
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (grant no. 8217070298), Guangdong Basic and Applied Basic Research Foundation (grant no. 2020A1515110770, 2021A1515220011, 2022A1515010335).
Peer review under responsibility of Shenyang Pharmaceutical University.
Contributor Information
Ke Zheng, Email: zhengke@dgut.edu.cn.
Zhiqing Pang, Email: zqpang@fudan.edu.cn.
Shaojun Peng, Email: shaojunpeng@ext.jnu.edu.cn.
References
- 1.Tu Z, Zhong Y, Hu H, Shao D, Haag R, Schirner M, et al. Design of therapeutic biomaterials to control inflammation. Nat Rev Mater. 2022;7(7):557–574. doi: 10.1038/s41578-022-00426-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong XY, Cheng R, Wang J, Fang Y, Hwang KC. Nanomedicines inhibiting tumor metastasis and recurrence and their clinical applications. Nano Today. 2021;36 [Google Scholar]
- 3.Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6(9):1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu QY, Sun WJ, Wang C, Gu Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv Drug Deliv Rev. 2016;98:19–34. doi: 10.1016/j.addr.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae YH, Park K. Advanced drug delivery 2020 and beyond: perspectives on the future. Adv Drug Deliv Rev. 2020;158:4–16. doi: 10.1016/j.addr.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu R, Luo C, Pang ZQ, Zhang JM, Ruan SB, Wu MY, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chinese Chem Lett. 2023;34(2) [Google Scholar]
- 7.Wong KH, Yang D, Chen S, He C, Chen M. Development of nanoscale drug delivery systems of dihydroartemisinin for cancer therapy: a review. Asian J Pharm Sci. 2022;17(4):475–490. doi: 10.1016/j.ajps.2022.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu WQ, Liu R, Zhou Y, Gao HL. Size-tunable strategies for a tumor targeted drug delivery system. ACS Cent Sci. 2020;6(2):100–116. doi: 10.1021/acscentsci.9b01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He QY, Chen J, Yan JH, Cai SD, Xiong HJ, Liu YF, et al. Tumor microenvironment responsive drug delivery systems. Asian J Pharm Sci. 2020;15(4):416–448. doi: 10.1016/j.ajps.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang HY, Dong JS, Li ZM, Feng XR, Xu WG, Tulinao CMS, et al. Biointerface engineering nanoplatforms for cancer-targeted drug delivery. Asian J Pharm Sci. 2020;15(4):397–415. doi: 10.1016/j.ajps.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao HH, Yan LS, Dempsey EM, Song WT, Qi RG, Li WL, et al. Recent progress in polymer-based platinum drug delivery systems. Prog Polym Sci. 2018;87:70–106. [Google Scholar]
- 12.Park H, Otte A, Park K. Evolution of drug delivery systems: from 1950 to 2020 and beyond. J Control Release. 2022;342:53–65. doi: 10.1016/j.jconrel.2021.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng SJ, Xiao FF, Chen M, Gao HL. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv Sci. 2022;9(1) doi: 10.1002/advs.202103836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, et al. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med. 2013;369(9):819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 15.Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 16.Khurana A, Allawadhi P, Khurana I, Allwadhi S, Weiskirchen R, Banothu AK, et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38 doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain A, Yang H, Zhang M, Liu Q, Alotaibi G, Irfan M, et al. mRNA vaccines for COVID-19 and diverse diseases. J Control Release. 2022;345:314–333. doi: 10.1016/j.jconrel.2022.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat Biotechnol. 2015;33(9):941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SM, Faix PH, Schnitzer JE. Overcoming key biological barriers to cancer drug delivery and efficacy. J Control Release. 2017;267:15–30. doi: 10.1016/j.jconrel.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Q, Deng YY, Chen XH, Ji J. Rational design of cancer nanomedicine for simultaneous stealth surface and enhanced cellular uptake. ACS Nano. 2019;13(2):954–977. doi: 10.1021/acsnano.8b07746. [DOI] [PubMed] [Google Scholar]
- 22.Wan MM, Chen H, Da Wang Z, Liu ZY, Yu YQ, Li L, et al. Nitric oxide-driven nanomotor for deep tissue penetration and multidrug resistance reversal in cancer therapy. Adv Sci. 2021;8(3) doi: 10.1002/advs.202002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 24.Wang JL, Du XJ, Yang JX, Shen S, Li HJ, Luo YL, et al. The effect of surface poly(ethylene glycol) length on in vivo drug delivery behaviors of polymeric nanoparticles. Biomaterials. 2018;182:104–113. doi: 10.1016/j.biomaterials.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Kolate A, Baradia D, Patil S, Vhora I, Kore G, Misra A. PEG-a versatile conjugating ligand for drugs and drug delivery systems. J Control Release. 2014;192:67–81. doi: 10.1016/j.jconrel.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 26.Grossen P, Witzigmann D, Sieber S, Huwyler J. PEG-PCL-based nanomedicines: a biodegradable drug delivery system and its application. J Control Release. 2017;260:46–60. doi: 10.1016/j.jconrel.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Abu Lila AS, Kiwada H, Ishida T. The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J Control Release. 2013;172(1):38–47. doi: 10.1016/j.jconrel.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 28.Zhang P, Sun F, Liu SJ, Jiang SY. Anti-PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244:184–193. doi: 10.1016/j.jconrel.2016.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao ZQ, Jiang SY. Super-hydrophilic zwitterionic poly(carboxybetaine) and amphiphilic non-ionic poly(ethylene glycol) for stealth nanoparticles. Nano Today. 2012;7(5):404–413. [Google Scholar]
- 30.Liu SJ, Jiang SY. Zwitterionic polymer-protein conjugates reduce polymer-specific antibody response. Nano Today. 2016;11(3):285–291. [Google Scholar]
- 31.Shao Q, Jiang SY. Molecular understanding and design of zwitterionic materials. Adv Mater. 2015;27(1):15–26. doi: 10.1002/adma.201404059. [DOI] [PubMed] [Google Scholar]
- 32.Xie RH, Yang P, Peng SJ, Cao YB, Yao XX, Guo SD, et al. A phosphorylcholine-based zwitterionic copolymer coated ZIF-8 nanodrug with a long circulation time and charged conversion for enhanced chemotherapy. J Mater Chem B. 2020;8(28):6128–6138. doi: 10.1039/d0tb00193g. [DOI] [PubMed] [Google Scholar]
- 33.Yao XX, Ma SP, Peng SJ, Zhou GX, Xie RH, Jiang Q, et al. Zwitterionic polymer coating of sulfur dioxide-releasing nanosystem augments tumor accumulation and treatment efficacy. Adv Healthc Mater. 2020;9(5) doi: 10.1002/adhm.201901582. [DOI] [PubMed] [Google Scholar]
- 34.Qian HL, Wang K, Lv MT, Zhao CS, Wang H, Wen SC, et al. Recent advances on next generation of polyzwitterion-based nano-vectors for targeted drug delivery. J Control Release. 2022;343:492–505. doi: 10.1016/j.jconrel.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Li QS, Wen CY, Yang J, Zhou XC, Zhu YN, Zheng J, et al. Zwitterionic biomaterials. Chem Rev. 2022;122(23):17073–17154. doi: 10.1021/acs.chemrev.2c00344. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Yu P, Xie J, Li JS. Recent advances of zwitterionic-based topological polymers for biomedical applications. J Mater Chem B. 2022;10(14):2338–2356. doi: 10.1039/d1tb02323c. [DOI] [PubMed] [Google Scholar]
- 37.Zhang M, Peng X, Ding Y, Ke X, Ren K, Xin QW, et al. A cyclic brush zwitterionic polymer based pH-responsive nanocarrier-mediated dual drug delivery system with lubrication maintenance for osteoarthritis treatment. Mater Horiz. 2023;10:2554–2567. doi: 10.1039/d3mh00218g. [DOI] [PubMed] [Google Scholar]
- 38.Sun QH, Zhou ZX, Qiu NS, Shen YQ. Rational design of cancer nanomedicine: nanoproperty integration and synchronization. Adv Mater. 2017;29(14) doi: 10.1002/adma.201606628. [DOI] [PubMed] [Google Scholar]
- 39.Manzari MT, Shamay Y, Kiguchi H, Rosen N, Scaltriti M, Heller DA. Targeted drug delivery strategies for precision medicines. Nat Rev Mater. 2021;6(4):351–370. doi: 10.1038/s41578-020-00269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.She DJ, Huang HH, Li JM, Peng SJ, Wang H, Yu XR. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem Eng J. 2021;408 [Google Scholar]
- 41.Jackson MA, Werfel TA, Curvino EJ, Yu F, Kavanaugh TE, Sarett SM, et al. Zwitterionic nanocarrier surface chemistry improves siRNA tumor delivery and silencing activity relative to polyethylene glycol. ACS Nano. 2017;11(6):5680–5696. doi: 10.1021/acsnano.7b01110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan YY, Mao CQ, Du XJ, Du JZ, Wang F, Wang J. Surface charge switchable nanoparticles based on zwitterionic polymer for enhanced drug delivery to tumor. Adv Mater. 2012;24(40):5476–5480. doi: 10.1002/adma.201202296. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y, Tan JB, Zhang Q, Xin T, Yu YL, Nie Y, et al. Artificial organelles based on cross-linked zwitterionic vesicles. Nano Lett. 2020;20(9):6548–6555. doi: 10.1021/acs.nanolett.0c02298. [DOI] [PubMed] [Google Scholar]
- 44.Finbloom JA, Sousa F, Stevens MM, Desai TA. Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv Drug Deliv Rev. 2020;167:89–108. doi: 10.1016/j.addr.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Chen XC, Cao J, Gao HL. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J Mater Chem B. 2020;8(31):6765–6781. doi: 10.1039/d0tb00649a. [DOI] [PubMed] [Google Scholar]
- 46.Tang YX, Wang XY, Li J, Nie Y, Liao GJ, Yu Y, et al. Overcoming the reticuloendothelial system barrier to drug delivery with a “Don’t-Eat-Us” strategy. ACS Nano. 2019;13(11):13015–13026. doi: 10.1021/acsnano.9b05679. [DOI] [PubMed] [Google Scholar]
- 47.Liu YK, Zhao ZH, Li M. Overcoming the cellular barriers and beyond: recent progress on cell penetrating peptide modified nanomedicine in combating physiological and pathological barriers. Asian J Pharm Sci. 2022;17(4):523–543. doi: 10.1016/j.ajps.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai S, Zhang Y, Li DF, Shi XX, Lin G, Liu G. Gain an advantage from both sides: smart size-shrinkable drug delivery nanosystems for high accumulation and deep penetration. Nano Today. 2021;36 [Google Scholar]
- 49.Ruan SB, Zhou Y, Jiang XG, Gao HL. Rethinking CRITID procedure of brain targeting drug delivery: circulation, blood brain barrier recognition, intracellular transport, diseased cell targeting, internalization, and drug release. Adv Sci. 2021;8(9) doi: 10.1002/advs.202004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Sawy HS, Al-Abd AM, Ahmed TA, El-Say KM, Torchilin VP. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: past, present, and future perspectives. ACS Nano. 2018;12(11):10636–10664. doi: 10.1021/acsnano.8b06104. [DOI] [PubMed] [Google Scholar]
- 51.Lazarovits J, Chen YY, Sykes EA, Chan WC. Nanoparticle-blood interactions: the implications on solid tumour targeting. ChemComm. 2015;51(14):2756–2767. doi: 10.1039/c4cc07644c. [DOI] [PubMed] [Google Scholar]
- 52.Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–781. doi: 10.1038/nnano.2013.181. [DOI] [PubMed] [Google Scholar]
- 53.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 54.Oh JY, Kim HS, Palanikumar L, Go EM, Jana B, Park SA, et al. Cloaking nanoparticles with protein corona shield for targeted drug delivery. Nat Commun. 2018;9(1):4548. doi: 10.1038/s41467-018-06979-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 56.Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci USA. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asha AB, Chen Y, Narain R. Bioinspired dopamine and zwitterionic polymers for non-fouling surface engineering. Chem Soc Rev. 2021;50(20):11668–11683. doi: 10.1039/d1cs00658d. [DOI] [PubMed] [Google Scholar]
- 58.Zhao CS, Wen SC, Pan JF, Wang K, Ji YC, Huang DC, et al. Robust construction of supersmall zwitterionic micelles based on hyperbranched polycarbonates mediates high tumor accumulation. ACS Appl Mater Interfaces. 2023;15(2):2725–2736. doi: 10.1021/acsami.2c20056. [DOI] [PubMed] [Google Scholar]
- 59.Ngo W, Ahmed S, Blackadar C, Bussin B, Ji Q, Mladjenovic SM, et al. Why nanoparticles prefer liver macrophage cell uptake in vivo. Adv Drug Deliv Rev. 2022;185 doi: 10.1016/j.addr.2022.114238. [DOI] [PubMed] [Google Scholar]
- 60.Hu B, Li B, Li K, Liu YY, Li CH, Zheng LL, et al. Thermostable ionizable lipid-like nanoparticle (iLAND) for RNAi treatment of hyperlipidemia. Sci Adv. 2022;8(7):eabm1418. doi: 10.1126/sciadv.abm1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qiu M, Tang Y, Chen JJ, Muriph R, Ye ZF, Huang CF, et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci USA. 2022;119(8) doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15(4):313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci USA. 2021;118(52) doi: 10.1073/pnas.2109256118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khawar IA, Kim JH, Kuh HJ. Improving drug delivery to solid tumors: priming the tumor microenvironment. J Control Release. 2015;201:78–89. doi: 10.1016/j.jconrel.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 65.Souri M, Soltani M, Moradi Kashkooli F, Kiani Shahvandi M. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J Control Release. 2022;341:227–246. doi: 10.1016/j.jconrel.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 66.Hu J, Yuan XW, Wang F, Gao HL, Liu XL, Zhang W. The progress and perspective of strategies to improve tumor penetration of nanomedicines. Chinese Chem Lett. 2021;32(4):1341–1347. [Google Scholar]
- 67.Shen RY, Peng LJ, Zhou WT, Wang D, Jiang Q, Ji J, et al. Anti-angiogenic nano-delivery system promotes tumor vascular normalizing and micro-environment reprogramming in solid tumor. J Control Release. 2022;349:550–564. doi: 10.1016/j.jconrel.2022.07.015. [DOI] [PubMed] [Google Scholar]
- 68.Zinger A, Koren L, Adir O, Poley M, Alyan M, Yaari Z, et al. Collagenase nanoparticles enhance the penetration of drugs into pancreatic tumors. ACS Nano. 2019;13(10):11008–11021. doi: 10.1021/acsnano.9b02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L, Jiang W, Su YH, Zhan MX, Peng SJ, Liu H, et al. Self-splittable transcytosis nanoraspberry for NIR-II photo-immunometabolic cancer therapy in deep tumor tissue. Adv Sci. 2022;9(32) doi: 10.1002/advs.202204067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang NJ, Hinner MJ. Getting across the cell membrane: an overview for small molecules, peptides, and proteins. Methods Mol Biol. 2015;1266:29–53. doi: 10.1007/978-1-4939-2272-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng SJ, Men YZ, Xie RH, Tian YF, Yang WL. Biodegradable phosphorylcholine-based zwitterionic polymer nanogels with smart charge-conversion ability for efficient inhibition of tumor cells. J Colloid Interface Sci. 2019;539:19–29. doi: 10.1016/j.jcis.2018.12.035. [DOI] [PubMed] [Google Scholar]
- 72.Li Y, Yang HY, Thambi T, Park JH, Lee DS. Charge-convertible polymers for improved tumor targeting and enhanced therapy. Biomaterials. 2019;217 doi: 10.1016/j.biomaterials.2019.119299. [DOI] [PubMed] [Google Scholar]
- 73.Dutta B, Barick KC, Hassan PA. Recent advances in active targeting of nanomaterials for anticancer drug delivery. Adv Colloid Interface Sci. 2021;296 doi: 10.1016/j.cis.2021.102509. [DOI] [PubMed] [Google Scholar]
- 74.Pei DH, Buyanova M. Overcoming endosomal entrapment in drug delivery. Bioconjug Chem. 2019;30(2):273–283. doi: 10.1021/acs.bioconjchem.8b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ma D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale. 2014;6(12):6415–6425. doi: 10.1039/c4nr00018h. [DOI] [PubMed] [Google Scholar]
- 76.Shete HK, Prabhu RH, Patravale VB. Endosomal escape: a bottleneck in intracellular delivery. J Nanosci Nanotechnol. 2014;14(1):460–474. doi: 10.1166/jnn.2014.9082. [DOI] [PubMed] [Google Scholar]
- 77.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Stolnik S, Illum L, Davis SS. Long circulating microparticulate drug carriers. Adv Drug Deliv Rev. 2012;64:290–301. [Google Scholar]
- 79.Oroojalian F, Beygi M, Baradaran B, Mokhtarzadeh A, Shahbazi MA. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small. 2021;17(12) doi: 10.1002/smll.202006484. [DOI] [PubMed] [Google Scholar]
- 80.Ge CC, Tian J, Zhao YL, Chen CY, Zhou RH, Chai ZF. Towards understanding of nanoparticle-protein corona. Arch Toxicol. 2015;89(4):519–539. doi: 10.1007/s00204-015-1458-0. [DOI] [PubMed] [Google Scholar]
- 81.Jiang SY, Cao ZQ. Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications. Adv Mater. 2010;22(9):920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 82.Keefe AJ, Jiang SY. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nat Chem. 2011;4(1):59–63. doi: 10.1038/nchem.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blackman LD, Gunatillake PA, Cass P, Locock KES. An introduction to zwitterionic polymer behavior and applications in solution and at surfaces. Chem Soc Rev. 2019;48(3):757–770. doi: 10.1039/c8cs00508g. [DOI] [PubMed] [Google Scholar]
- 84.Zhang L, Cao ZQ, Bai T, Carr L, Ella-Menye JR, Irvin C, et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat Biotechnol. 2013;31(6):553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 85.Mi L, Jiang SY. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew Chem Int Ed Engl. 2014;53(7):1746–1754. doi: 10.1002/anie.201304060. [DOI] [PubMed] [Google Scholar]
- 86.Zhang P, Sun F, Tsao C, Liu SJ, Jain P, Sinclair A, et al. Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proc Natl Acad Sci USA. 2015;112(39):12046–12051. doi: 10.1073/pnas.1512465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang W, Liu SJ, Bai T, Keefe AJ, Zhang L, Ella-Menye JR, et al. Poly(carboxybetaine) nanomaterials enable long circulation and prevent polymer-specific antibody production. Nano Today. 2014;9(1):10–16. [Google Scholar]
- 88.Li BW, Yuan ZF, Zhang P, Sinclair A, Jain P, Wu K, et al. Zwitterionic nanocages overcome the efficacy loss of biologic drugs. Adv Mater. 2018;30(14) doi: 10.1002/adma.201705728. [DOI] [PubMed] [Google Scholar]
- 89.Shao Q, He Y, White AD, Jiang SY. Difference in hydration between carboxybetaine and sulfobetaine. J Phys Chem B. 2010;114(49):16625–16631. doi: 10.1021/jp107272n. [DOI] [PubMed] [Google Scholar]
- 90.He Y, Hower J, Chen SF, Bernards MT, Chang Y, Jiang SY. Molecular simulation studies of protein interactions with zwitterionic phosphorylcholine self-assembled monolayers in the presence of water. Langmuir. 2008;24(18):10358–10364. doi: 10.1021/la8013046. [DOI] [PubMed] [Google Scholar]
- 91.Kondo T, Nomura K, Gemmei-Ide M, Kitano H, Noguchi H, Uosaki K, et al. Structure of water at zwitterionic copolymer film-liquid water interfaces as examined by the sum frequency generation method. Colloids Surf B Biointerfaces. 2014;113:361–367. doi: 10.1016/j.colsurfb.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 92.Kane RS, Deschatelets P, Whitesides GM. Kosmotropes form the basis of protein-resistant surfaces. Langmuir. 2003;19(6):2388–2391. [Google Scholar]
- 93.Men YZ, Peng SJ, Yang P, Jiang Q, Zhang YH, Shen B, et al. Biodegradable zwitterionic nanogels with long circulation for antitumor drug delivery. ACS Appl Mater Interfaces. 2018;10(28):23509–23521. doi: 10.1021/acsami.8b03943. [DOI] [PubMed] [Google Scholar]
- 94.Peng SJ, Ouyang BS, Xin YJ, Zhao W, Shen S, Zhan MX, et al. Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm Sin B. 2021;11(2):560–571. doi: 10.1016/j.apsb.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ishihara K. Blood-compatible surfaces with phosphorylcholine-based polymers for cardiovascular medical devices. Langmuir. 2019;35(5):1778–1787. doi: 10.1021/acs.langmuir.8b01565. [DOI] [PubMed] [Google Scholar]
- 96.Shao Q, Mi L, Han X, Bai T, Liu SJ, Li YT, et al. Differences in cationic and anionic charge densities dictate zwitterionic associations and stimuli responses. J Phys Chem B. 2014;118(24):6956–6962. doi: 10.1021/jp503473u. [DOI] [PubMed] [Google Scholar]
- 97.Shao Q, Jiang SY. Effect of carbon spacer length on zwitterionic carboxybetaines. J Phys Chem B. 2013;117(5):1357–1366. doi: 10.1021/jp3094534. [DOI] [PubMed] [Google Scholar]
- 98.Peng SJ, Ouyang BS, Men YZ, Du Y, Cao YB, Xie RH, et al. Biodegradable zwitterionic polymer membrane coating endowing nanoparticles with ultra-long circulation and enhanced tumor photothermal therapy. Biomaterials. 2020;231 doi: 10.1016/j.biomaterials.2019.119680. [DOI] [PubMed] [Google Scholar]
- 99.Li B, Jain P, Ma J, Smith JK, Yuan Z, Hung HC, et al. Trimethylamine N-oxide-derived zwitterionic polymers: a new class of ultralow fouling bioinspired materials. Sci Adv. 2019;5(6):eaaw9562. doi: 10.1126/sciadv.aaw9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banskota S, Yousefpour P, Kirmani N, Li XH, Chilkoti A. Long circulating genetically encoded intrinsically disordered zwitterionic polypeptides for drug delivery. Biomaterials. 2019;192:475–485. doi: 10.1016/j.biomaterials.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu XS, Li H, Chen YJ, Jin Q, Ren KF, Ji J. Mixed-charge nanoparticles for long circulation, low reticuloendothelial system clearance, and high tumor accumulation. Adv Healthc Mater. 2014;3(9):1439–1447. doi: 10.1002/adhm.201300617. [DOI] [PubMed] [Google Scholar]
- 102.Nejati S, Vadeghani EM, Khorshidi S, Karkhaneh A. Role of particle shape on efficient and organ-based drug delivery. Eur Polym J. 2020;122:38961–38976. [Google Scholar]
- 103.Liu XL, Zhong X, Li C. Challenges in cell membrane-camouflaged drug delivery systems: development strategies and future prospects. Chinese Chem Lett. 2021;32(8):2347–2358. [Google Scholar]
- 104.Li AN, Zhao JW, Fu JR, Cai J, Zhang P. Recent advances of biomimetic nano-systems in the diagnosis and treatment of tumor. Asian J Pharm Sci. 2021;16(2):161–174. doi: 10.1016/j.ajps.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discov. 2016;15(4):275–292. doi: 10.1038/nrd.2015.21. [DOI] [PubMed] [Google Scholar]
- 106.Han L, Jiang C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm Sin B. 2021;11(8):2306–2325. doi: 10.1016/j.apsb.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li J, Zheng M, Shimoni O, Banks WA, Bush AI, Gamble JR, et al. Development of novel therapeutics targeting the blood-brain barrier: from barrier to carrier. Adv Sci. 2021;8(16) doi: 10.1002/advs.202101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Niu XQ, Chen JJ, Gao JQ. Nanocarriers as a powerful vehicle to overcome blood-brain barrier in treating neurodegenerative diseases: focus on recent advances. Asian J Pharm Sci. 2019;14(5):480–496. doi: 10.1016/j.ajps.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pang ZQ, Gao HL, Yu Y, Guo LR, Chen J, Pan SQ, et al. Enhanced intracellular delivery and chemotherapy for glioma rats by transferrin-conjugated biodegradable polymersomes loaded with doxorubicin. Bioconjug Chem. 2011;22(6):1171–1180. doi: 10.1021/bc200062q. [DOI] [PubMed] [Google Scholar]
- 110.Gaillard PJ, Appeldoorn CC, Rip J, Dorland R, van der Pol SM, Kooij G, et al. Enhanced brain delivery of liposomal methylprednisolone improved therapeutic efficacy in a model of neuroinflammation. J Control Release. 2012;164(3):364–369. doi: 10.1016/j.jconrel.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 111.Lu W, Wan J, She ZJ, Jiang XG. Brain delivery property and accelerated blood clearance of cationic albumin conjugated pegylated nanoparticle. J Control Release. 2007;118(1):38–53. doi: 10.1016/j.jconrel.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 112.Toman P, Lien CF, Ahmad Z, Dietrich S, Smith JR, An Q, et al. Nanoparticles of alkylglyceryl-dextran-graft-poly(lactic acid) for drug delivery to the brain: Preparation and in vitro investigation. Acta Biomater. 2015;23:250–262. doi: 10.1016/j.actbio.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 113.Aryal M, Vykhodtseva N, Zhang YZ, Park J, McDannold N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169(1-2):103–111. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang TT, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci. 2016;4(2):219–229. doi: 10.1039/c5bm00383k. [DOI] [PubMed] [Google Scholar]
- 115.Guo LR, Ren JF, Jiang XG. Perspectives on brain-targeting drug delivery systems. Curr Pharm Biotechnol. 2012;13(12):2310–2318. doi: 10.2174/138920112803341770. [DOI] [PubMed] [Google Scholar]
- 116.Qiao RR, Jia QJ, Huwel S, Xia R, Liu T, Gao FB, et al. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano. 2012;6(4):3304–3310. doi: 10.1021/nn300240p. [DOI] [PubMed] [Google Scholar]
- 117.Dixit S, Novak T, Miller K, Zhu Y, Kenney ME, Broome AM. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale. 2015;7(5):1782–1790. doi: 10.1039/c4nr04853a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang XY, Xin HL, Ren QY, Gu JJ, Zhu LJ, Du FY, et al. Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials. 2014;35(1):518–529. doi: 10.1016/j.biomaterials.2013.09.094. [DOI] [PubMed] [Google Scholar]
- 119.Liang S, Liu Y, Jin X, Liu G, Wen J, Zhang LL, et al. Phosphorylcholine polymer nanocapsules prolong the circulation time and reduce the immunogenicity of therapeutic proteins. Nano Res. 2016;9(4):1022–1031. [Google Scholar]
- 120.Wu D, Qin M, Xu D, Wang L, Liu CY, Ren J, et al. A bioinspired platform for effective delivery of protein therapeutics to the central nervous system. Adv Mater. 2019;31(18) doi: 10.1002/adma.201807557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meng XQ, Zhao Y, Han B, Zha CJ, Zhang YG, Li ZW, et al. Dual functionalized brain-targeting nanoinhibitors restrain temozolomide-resistant glioma via attenuating EGFR and MET signaling pathways. Nat Commun. 2020;11(1):594. doi: 10.1038/s41467-019-14036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wen J, Wu D, Qin M, Liu CY, Wang L, Xu D, et al. Sustained delivery and molecular targeting of a therapeutic monoclonal antibody to metastases in the central nervous system of mice. Nat Biomed Eng. 2019;3(9):706–716. doi: 10.1038/s41551-019-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han L, Liu CY, Qi HZ, Zhou JH, Wen J, Wu D, et al. Systemic delivery of monoclonal antibodies to the central nervous system for brain tumor therapy. Adv Mater. 2019;31(19) doi: 10.1002/adma.201805697. [DOI] [PubMed] [Google Scholar]
- 124.Xu D, Wu D, Qin M, Nih LR, Liu CY, Cao Z, et al. Efficient delivery of nerve growth factors to the central nervous system for neural regeneration. Adv Mater. 2019;31(33) doi: 10.1002/adma.201900727. [DOI] [PubMed] [Google Scholar]
- 125.Jin WL, Wu Y, Chen N, Wang QX, Wang YF, Li YS, et al. Early administration of MPCn(IVIg) selectively accumulates in ischemic areas to protect inflammation-induced brain damage from ischemic stroke. Theranostics. 2021;11(17):8197–8217. doi: 10.7150/thno.58947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu Y, Shrestha N, Preat V, Beloqui A. Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. J Control Release. 2020;322:486–508. doi: 10.1016/j.jconrel.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 127.Wang G, Zhao LC, Jiang QK, Sun YX, Zhao DY, Sun MC, et al. Intestinal OCTN2- and MCT1-targeted drug delivery to improve oral bioavailability. Asian J Pharm Sci. 2020;15(2):158–173. doi: 10.1016/j.ajps.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Filipski KJ, Varma MV, El-Kattan AF, Ambler CM, Ruggeri RB, Goosen TC, et al. Intestinal targeting of drugs: rational design approaches and challenges. Curr Top Med Chem. 2013;13(7):776–802. doi: 10.2174/1568026611313070002. [DOI] [PubMed] [Google Scholar]
- 129.Collnot EM, Ali H, Lehr CM. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 2012;161(2):235–246. doi: 10.1016/j.jconrel.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 130.Zhang C, Li JX, Xiao M, Wang D, Qu Y, Zou L, et al. Oral colon-targeted mucoadhesive micelles with enzyme-responsive controlled release of curcumin for ulcerative colitis therapy. Chinese Chem Lett. 2022;33(11):4924–4929. [Google Scholar]
- 131.Hu SS, Yang ZX, Wang S, Wang LP, He QQ, Tang H, et al. Zwitterionic polydopamine modified nanoparticles as an efficient nanoplatform to overcome both the mucus and epithelial barriers. Chem Eng J. 2022;428 [Google Scholar]
- 132.Han XF, Lu Y, Xie JB, Zhang E, Zhu H, Du H, et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat Nanotechnol. 2020;15(7):605–614. doi: 10.1038/s41565-020-0693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhou YH, Chen XZ, Zhao D, Li D, He CL, Chen XS. A pH-triggered self-unpacking capsule containing zwitterionic hydrogel-coated MOF nanoparticles for efficient oral Exendin-4 delivery. Adv Mater. 2021;33(32) doi: 10.1002/adma.202102044. [DOI] [PubMed] [Google Scholar]
- 134.Fan WF, Wei QY, Xiang JJ, Tang YS, Zhou Q, Geng Y, et al. Mucus penetrating and cell-binding polyzwitterionic micelles as potent oral nanomedicine for cancer drug delivery. Adv Mater. 2022;34(16) doi: 10.1002/adma.202109189. [DOI] [PubMed] [Google Scholar]
- 135.Xie YM, Bagby TR, Cohen MS, Forrest ML. Drug delivery to the lymphatic system: 5importance in future cancer diagnosis and therapies. Expert Opin Drug Deliv. 2009;6(8):785–792. doi: 10.1517/17425240903085128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA. 2014;111(6):2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li BW, Yuan ZF, He YW, Hung HC, Jiang SY. Zwitterionic nanoconjugate enables safe and efficient lymphatic drug delivery. Nano Lett. 2020;20(6):4693–4699. doi: 10.1021/acs.nanolett.0c01713. [DOI] [PubMed] [Google Scholar]
- 138.Castillo-Hair SM, Seelig G. Machine learning for designing next-generation mRNA therapeutics. ACC Chem Res. 2022;55(1):24–34. doi: 10.1021/acs.accounts.1c00621. [DOI] [PubMed] [Google Scholar]
- 139.Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics. 2020;12(2):102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomas SJ, Moreira ED, Kitchin N, Jr., J Absalon, A Gurtman, S Lockhart, et al. Safety and efficacy of the bnt162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Witzigmann D, Kulkarni JA, Leung J, Chen S, Cullis PR, van der Meel R. Lipid nanoparticle technology for therapeutic gene regulation in the liver. Adv Drug Deliv Rev. 2020;159:344–363. doi: 10.1016/j.addr.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu S, Wang X, Yu XL, Cheng Q, Johnson LT, Chatterjee S, et al. Zwitterionic phospholipidation of cationic polymers facilitates systemic mRNA delivery to spleen and lymph nodes. J Am Chem Soc. 2021;143(50):21321–21330. doi: 10.1021/jacs.1c09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li ZM, Shan XT, Chen ZD, Gao NS, Zeng WF, Zeng XW, et al. Applications of surface modification technologies in nanomedicine for deep tumor penetration. Adv Sci. 2020;8(1) doi: 10.1002/advs.202002589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang ZW, Wang H, Tan T, Li J, Wang ZW, Li YP. Rational design of nanoparticles with deep tumor penetration for effective treatment of tumor metastasis. Adv Funct Mater. 2018;28(40) [Google Scholar]
- 146.Zhang YJ, Chen WZ, Yang CC, Fan QL, Wu W, Jiang XQ. Enhancing tumor penetration and targeting using size-minimized and zwitterionic nanomedicines. J Control Release. 2016;237:115–124. doi: 10.1016/j.jconrel.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 147.Hu J, Yuan XW, Wang F, Gao HL, XL L, Zang W. The progress and perspective of strategies to improve tumor penetration of nanomedicines. Chinese Chem Lett. 2021;32(4):1341–1347. [Google Scholar]
- 148.Zhao Z, Feng Y, Xiang J, Liu J, Piao Y, Shao S, et al. Screening of zwitterionic liposomes with red blood cell-hitchhiking and tumor cell-active transporting capability for efficient tumor entrance. Adv Funct Mater. 2023;33(16) [Google Scholar]
- 149.Chen SQ, Zhong Y, Fan WF, Xiang JJ, Wang GW, Zhou Q, et al. Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity. Nat Biomed Eng. 2021;5(9):1019–1037. doi: 10.1038/s41551-021-00701-4. [DOI] [PubMed] [Google Scholar]
- 150.Peng SJ, Wang H, Zhao W, Xin YJ, Liu Y, Yu XR, et al. Zwitterionic polysulfamide drug nanogels with microwave augmented tumor accumulation and on-demand drug release for enhanced cancer therapy. Adv Funct Mater. 2020;30(23) [Google Scholar]
- 151.Ou HL, Cheng TJ, Zhang YM, Liu JJ, Ding YX, Zhen JR, et al. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2018;65:339–348. doi: 10.1016/j.actbio.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 152.Wang S, Zhang FW, Yu GC, Wang ZT, Jacobson O, Ma Y, et al. Zwitterionic-to-cationic charge conversion polyprodrug nanomedicine for enhanced drug delivery. Theranostics. 2020;10(15):6629–6637. doi: 10.7150/thno.47849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.He CB, Hu YP, Yin LC, Tang C, Yin CH. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 154.Mizuhara T, Saha K, Moyano DF, Kim CS, Yan B, Kim YK, et al. Acylsulfonamide-functionalized zwitterionic gold nanoparticles for enhanced cellular uptake at tumor pH. Angew Chem Int Ed Engl. 2015;54(22):6567–6570. doi: 10.1002/anie.201411615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ranneh AH, Takemoto H, Sakuma S, Awaad A, Nomoto T, Mochida K, et al. An ethylenediamine-base sswitch to render the polyzwitterion cationic at tumorous pH for effective tumor accumulation of coated nanomaterials. Angew Chem Int Ed. 2018;57:5057–5061. doi: 10.1002/anie.201801641. [DOI] [PubMed] [Google Scholar]
- 156.Ma CC, Wang ZL, Xu T, He ZY, Wei YQ. The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv. 2020;40 doi: 10.1016/j.biotechadv.2019.107502. [DOI] [PubMed] [Google Scholar]
- 157.Dong Y, Siegwart DJ, Anderson DG. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev. 2019;144:133–147. doi: 10.1016/j.addr.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pan XH, Veroniaina H, Su N, Sha K, Jiang FL, Wu ZH, et al. Applications and developments of gene therapy drug delivery systems for genetic diseases. Asian J Pharm Sci. 2021;16(6):687–703. doi: 10.1016/j.ajps.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Verbeke R, Lentacker I, De Smedt SC, Dewitte H. The dawn of mRNA vaccines: the COVID-19 case. J Control Release. 2021;333:511–520. doi: 10.1016/j.jconrel.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ye L, Liu HM, Fei X, Ma D, He XZ, Tang QY, et al. Enhanced endosomal escape of dendrigraft poly-L-lysine polymers for the efficient gene therapy of breast cancer. Nano Res. 2022;15(2):1135–1144. [Google Scholar]
- 161.Liu S, Cheng Q, Wei T, Yu XL, Johnson LT, Farbiak L, et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR-Cas gene editing. Nat Mater. 2021;20(5):701–710. doi: 10.1038/s41563-020-00886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Y, Liu RY, Shi YJ, Zhang ZZ, Zhang X. Zwitterionic poly(carboxybetaine)-based cationic liposomes for effective delivery of small interfering RNA therapeutics without accelerated blood clearance phenomenon. Theranostics. 2015;5(6):583–596. doi: 10.7150/thno.11234. [DOI] [PMC free article] [PubMed] [Google Scholar]