Abstract
Cytokines play a significant role in the regulation of Toxoplasma gondii in the central nervous system. Cytokine-activated microglia are important host defense cells in central nervous system infections. Recent evidence indicates that astrocytes can also be activated by cytokines to inhibit intracellular pathogens. In this study, we examined the effect of gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-1 on the growth of T. gondii in a primary murine astrocyte culture. Pretreatment of astrocytes with IFN-γ resulted in 65% inhibition of T. gondii growth. Neither TNF-α, IL-1, nor IL-6 alone had any effect on T. gondii growth. IFN-γ in combination with either TNF-α, IL-1, or IL-6 caused a 75 to 80% inhibition of growth. While nitric oxide was produced by astrocytes treated with these cytokines, inhibition of T. gondii growth was not reversed by the addition of the nitric oxide synthase inhibitor NG-monomethyl-l-arginine. Furthermore, IFN-γ in combination with IL-1, IL-6, or TNF-α also induced inhibition in astrocytes derived from syngeneic mice deficient in the enzyme inducible nitric oxide synthase. This finding suggests that the mechanism of cytokine inhibition is not nitric oxide mediated. Similarly, the addition of tryptophan had no effect on inhibition, indicating that the mechanism was not mediated via induction of the enzyme indoleamine 2,3-dioxygenase. The mechanism of inhibition remains to be elucidated. Results from this study demonstrate that cytokine-activated astrocytes are capable of significantly inhibiting the growth of T. gondii. These data indicate that astrocytes may be important host defense cells in controlling toxoplasmosis in the brain.
Toxoplasma gondii is an important pathogen in the central nervous system and causes a severe encephalitis in patients with AIDS. Cytokines play an important role in the regulation of T. gondii replication in the central nervous system (17). Gamma interferon (IFN-γ) has been shown to prevent reactivation of Toxoplasma encephalitis in mice (30). Tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and interleukin-6 (IL-6) are up-regulated in the brains of mice with chronic toxoplasmosis (9, 15, 16). Studies indicate that IFN-γ, TNF-α, IL-1, and IL-6 may control the growth of T. gondii in the brain via activation of microglia (4, 5). Studies of mice indicate that cytokines induce anti-Toxoplasma activity in microglia via a nitric oxide (NO)-mediated mechanism (10).
Recent evidence indicates that cytokines can also activate astrocytes to inhibit growth of T. gondii (6, 8, 25, 27). For example, IFN-γ has been shown to inhibit growth of T. gondii in the glioblastoma cell line 86HG39 (6). Inhibition was shown to be via induction of indoleamine 2,3-dioxygenase (IDO), resulting in the degradation of intracellular tryptophan (8). Pelloux et al. found that in the astrocytoma cell line GHE, TNF-α inhibited, IL-1 stimulated, and IFN-γ and IL-6 had no effect on growth of T. gondii (25). Finally, in primary human astrocytes, IFN-γ and IL-1 in combination have been shown to inhibit growth of T. gondii via the production of NO (27). Interpretation of these results is difficult due to variability found in astrocyte cell lines and differences between tumor cells and primary astrocytes.
For this reason, we have chosen to study the effects of cytokines on growth of T. gondii in a primary astrocyte culture. In this study, we evaluated the effects of IFN-γ, TNF-α, IL-1, and IL-6 on the replication of T. gondii ME49 in a primary murine astrocyte culture. The effects of these cytokines individually, and the effects of IFN-γ in combination with IL-1, TNF-α, and IL-6, on the growth of T. gondii were examined. The ability of each of these cytokines and cytokine combinations to induce nitric oxide production was assessed by using the Griess reagent. A nitric oxide-mediated mechanism of cytokine inhibition of T. gondii growth was addressed by using NG-monomethyl-l-arginine (NMMA), a nitric oxide inhibitor, and by using astrocytes derived from syngeneic mice deficient in the enzyme inducible nitric oxide synthase (iNOS). The IDO mechanism of inhibition was investigated via the addition of exogenous tryptophan. The aim of this study was to clarify the effect of cytokines on T. gondii replication in astrocytes and increase our understanding of the role of astrocytes in the host defense against T. gondii in the central nervous system.
MATERIALS AND METHODS
Primary astrocyte culture.
Murine astrocytes from C57BL/6 × SV129 mice or syngeneic mice, deficient in iNOS (iNOS−/−; gift of C. Nathan) (22), were cultivated from the brains of neonatal (less than 24 h old) mice. Murine pups were sacrificed, the brain was removed from the cranium, the forebrain was dissected and the meninges were removed. The tissue was minced and incubated in 0.25% trypsin for 5 min at 37°C. After 5 min, the trypsin was inactivated with a solution containing DNase and soybean trypsinase inhibitors, and the tissue was further disrupted by trituration in a 20-ml pipette. The dissociated cells were filtered through a 74-μm-pore-size Nitex mesh, centrifuged at 200 × g, suspended in growth medium at a concentration of 106 cells/ml, and plated onto poly-l-lysine-coated dishes. Astrocytes were maintained in endotoxin-free minimal essential medium (BRL-GIBCO, Gaithersburg, Md.) supplemented with 20% fetal bovine serum (BRL-GIBCO), 5% glucose, and 100 U of penicillin and streptomycin (BRL-GIBCO) per ml. The growth medium was changed every 3 days. After 7 days in vitro, a confluent layer of 1 × 104 to 2 × 104 cells/cm2 of astrocytes is reached. By this method, cells were found to be >95% astrocytes, as judged by positive staining for glial fibrillary acidic protein. Cultures contained <5% microglia, as identified by staining with the lectin BS1-B4 (catalog no. L-2895; Sigma, St. Louis, Mo.). Astrocytes were dissociated in trypsin-EDTA, replated onto poly-l-lysine-coated coverslips or 24-well plates at 104 cells/cm, and cultured for 7 to 10 days after replating. These astrocytes were then infected with T. gondii ME49 as described below.
Culture of T. gondii.
Tachyzoites from T. gondii ME49 were obtained by in vitro culture in Vero cells. Parasites were harvested after 4 to 5 days in culture, resuspended in minimal essential medium supplemented with 10% fetal bovine serum, and used for infection of murine astrocyte cultures.
Chemicals and cytokines.
Murine recombinant IFN-γ, IL-1β, TNF-α, and IL-6 were purchased from Genzyme (Cambridge, Mass.). NMMA and l-tryptophan were obtained from Sigma. [3H]uracil and [3H]tryptophan were purchased from Amersham Pharmacia Biotech (Arlington Heights, Ill.). The Griess reagent kit (catalog no. G-7921) was obtained from Molecular Probes (Eugene, Oreg.).
Cytokine treatments.
Murine astrocytes were stimulated with IFN-γ, TNF-α, IL-1β (each at 100 U/ml), or IL-6 (1 ng/ml), alone or in combination, for 72 h prior to infection, and supernatants were removed for determination of nitric oxide production. Cultures were then infected with T. gondii and incubated for 48 h without cytokines, and growth was determined by the [3H]uracil method described below. The percentage of infected astrocytes for each condition was determined by counting the number of infected cells per 500 cells under both phase and immunofluorescence microscopy. Testing for each condition was performed in triplicate. Immunofluorescence microscopy was performed with a 1:50 dilution of a commercial polyclonal rabbit anti-Toxoplasma antibody (DAKO, Carpenteria, Calif.) followed by detection with anti-rabbit fluorescein immunoglobulin G (Boehringer Mannheim, Indianapolis, Ind.) as previously described (13). All cultures were incubated in endotoxin-free medium, and no endotoxin contamination was detected in any of the experimental cultures.
Determination of Toxoplasma growth.
Cultures were infected with 105 T. gondii tachyzoites per well (a 5:1 tachyzoite/host cell ratio) for 2 h. The monolayer was then extensively washed to remove extracellular tachyzoites and [3H]uracil (2.5 μCi per well) was added. T. gondii growth was determined 48 h later by the [3H]uracil incorporation method described below. Before cell harvesting, the appearance of the culture was checked to verify that T. gondii-induced cell lysis had not begun. The monolayer was washed three times with phosphate-buffered saline to remove any nonincorporated [3H]uracil. The astrocyte monolayer was then lysed by incubation in 0.1% sodium dodecyl sulfate for 15 min at room temperature. Nucleic acids were precipitated by the addition of 3 M trichloroacetic acid. The contents of the wells were deposited on Whatman glass filters and washed extensively with 0.1 M trichloroacetic acid, and then radioactivity was determined with a liquid scintillation counter (21). For each experiment, controls included murine astrocytes cultured in the absence of T. gondii. The radioactivity of these samples was always near background levels, thus confirming that [3H]uracil incorporation was specific to the parasite.
Measurement of nitric oxide.
Supernatants from astrocyte cultures were collected after 72 h of incubation in cytokines, and nitrite was assayed with the Griess reagent kit (Molecular Probes). Briefly, a 150-μl aliquot of culture supernatant was mixed with 50 μl of the Griess reagent [0.05% N-(1-naphthyl)ethylenediamine dihydrochloride–0.5% sulfanilic acid in phosphoric acid] and diluted with 1.3 ml of water, and absorbance was measured at 548 nm in a spectrophotometer. The amount of nitrite in the sample was calculated from a sodium nitrite standard curve.
Effect of NMMA or tryptophan on cytokine inhibition.
Murine astrocytes were cultured as described above except that in some experiments, either NMMA (final concentration of 400 μM) or tryptophan (final concentration of 100 μg/ml) was added to the culture at the time of cytokine addition. Tryptophan uptake by astrocytes was measured by monitoring [3H]tryptophan uptake as described by Pfefferkorn (28). IDO activity was measured photometrically at 490 nm, by using the Ehrlich reagent to monitor the degradation of tryptophan to kynurenine as described by Däubener et al. (7).
Statistics.
Within each experiment, all conditions were repeated in triplicate, and each experiment was replicated two to three times. Data were analyzed by nonparametric (Wilcoxon signed-rank test) and/or parametric (Student t test and analysis of variance) methods, using Sigma Stat version 1.0 (Jandel Scientific, San Rafael, Calif.).
RESULTS
Effect of IFN-γ, TNF-α, IL-1, and IL-6 on growth of T. gondii in murine astrocytes.
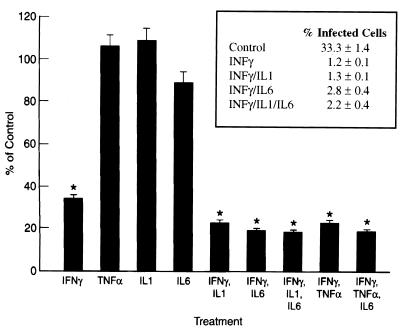
Astrocytes were pretreated with cytokines and then infected with T. gondii, and growth was measured 48 h after infection. The effect of treating astrocytes with IFN-γ, TNF-α, IL-1, and IL-6 alone and in combination is presented in Fig. 1. IFN-γ inhibited the growth of T. gondii by approximately 65% (P < 0.05), but there was no effect from IL-6, TNF-α, or IL-1 alone. IFN-γ in combination with either IL-6, IL-1, or TNF-α inhibited the growth of T. gondii in murine astrocytes by approximately 75 to 80% (P < 0.05), a level 10 to 15% greater than that observed after treatment with IFN-γ alone (P < 0.05). The addition of IL-6 did not reverse the inhibition of growth induced by either IFN-γ–IL-1 or IFN-γ–TNF-α in astrocytes. No inhibition of T. gondii growth was seen with either IFN-γ alone or any of the IFN-γ cytokine combinations when cytokines were added at the time of infection or 24 h prior to infection (data not shown).
FIG. 1.
Effect of cytokines on growth of T. gondii in mouse astrocytes. Cells were incubated with the cytokines IFN-γ (100 U/ml), IL1 (1 ng/ml), IL-6 (100 U/ml), and TNF-α (100 U/ml) for 72 h before infection. Control cultures were incubated in medium alone. [3H]uracil (2.5 μCi/ml) was added 2 h after infection, and cells were harvested 48 h later. Results are averages of three separate experiments. Bars indicate ± standard error of the mean. ∗, significance at the P < 0.05 level versus control as calculated by Student’s t test. (Insert) Percentages of cells (mean ± standard deviation) infected with T. gondii ME49 as determined by microscopy (multiplicity of infection, 5:1). The difference between each cytokine treatment and the control was significant at P < 0.05; there was no significant difference between any of the various cytokine treatments.
Cultures pretreated with IFN-γ alone and in combination with each of TNF-α, IL-1, and IL-6 were also assessed microscopically. The level of infection of cultures treated with cytokines was found to be <5%, compared to 30 to 35% in the controls (Fig. 1, inset), which correlated with the results of the uracil uptake assay. The percent infected cells approximately doubled when IL-6 was added to other cytokines (i.e., 1.2% infected with IFN-γ and 2.8% infected with IFN-γ–IL-6). While not statistically significant, this finding raises the possibility that in astrocytes, as found for macrophages (2), IL-6 may reverse part of the activation due to other cytokines.
NO production in cytokine-treated astrocytes.
NO production after treatment with each of the cytokine combinations is presented in Table 1. NO levels in the controls (i.e., no cytokines added) were between 2 and 3 μM, and no increase in NO was seen with IFN-γ, TNF-α, IL-1, or IL-6 treatment alone. All cytokine combinations resulted in a statistically significant elevation in NO above the control level (P < 0.05): IFN-γ–IL-1, 9.9 μM; IFN-γ–TNF-α, 15.2 μM; and IFN-γ–IL-6, 5 μM. The addition of IL-6 to IFN-γ–IL-1 decreased the NO levels induced by IFN-γ–IL-1 slightly, while the addition of IL-6 to IFN-γ–TNF-α had no effect on NO production.
TABLE 1.
Effect of cytokines on NO production in mouse astrocytes
| Treatment | Nitrite concn (μM)a |
|---|---|
| Control | 2.0 ± 1.2 |
| IFN-γ | 1.9 ± 0.8 |
| TNF-α | 1.3 ± 0.5 |
| IL-1 | 1.4 ± 0.6 |
| IL-6 | 0.7 ± 0.7 |
| IFN-γ, IL-1 | 9.9 ± 2.3b |
| IFN-γ, IL-6 | 5.0 ± 0.8b |
| IFN-γ, IL-1, IL-6 | 6.3 ± 1.7b |
| IFN-γ, TNF-α | 15.2 ± 1.0b |
| IFN-γ, TNF-α, IL-6 | 13.8 ± 0.7b |
| IFN-γ, IL-1, NMMA | 2.3 ± 1.7 |
| IFN-γ, IL-6, NMMA | 2.1 ± 1.7 |
| IFN-γ, IL-1, IL-6, NMMA | 1.8 ± 1.1 |
| IFN-γ, TNF-α, NMMA | 2.2 ± 1.6 |
| IFN-γ, TNF-α, IL-6, NMMA | 2.6 ± 1.6 |
Mean ± standard deviation of three separate experiments for NO in the supernatants of astrocyte cultures treated with IFN-γ (100 U/ml), IL-1 (1 ng/ml), IL-6 (100 U/ml), and TNF-α (100 U/ml), in the presence or absence of NMMA (400 μM); control cultures were incubated in medium alone. Cytokines were added to cultures 72 h prior to infection, and NMMA was added at the time of cytokine addition. 0 = level of detection (<1.0 μM).
P < 0.05 from the value of the control group as calculated by Student’s t test.
Effect of NMMA on cytokine inhibition of T. gondii growth in murine astrocytes.
Addition of NMMA decreased nitrite levels in all cytokine combinations to background levels (<2 μM) in all cultures (Table 1). The presence or absence of NMMA during the 72-h pretreatment with cytokines prior to infection of astrocytes with T. gondii did not affect the cytokine-mediated inhibition of T. gondii growth due to IFN-γ–IL-1, IFN-γ–IL-6, IFN-γ–IL-1–IL-6, IFN-γ–TNF-α, or IFN-γ–TNF-α–IL-6 (Table 2) or to the individual cytokines (data not shown).
TABLE 2.
Effect of NMMA on cytokine inhibition of T. gondii growth in murine astrocytes
| Treatment | Inhibition of T. gondii growth (% of control)a
|
|
|---|---|---|
| Medium only | Cytokines + NMMA | |
| IFN-γ, IL-1 | 22.4 ± 3.1 | 24.7 ± 1.1 |
| IFN-γ, IL-6 | 20.4 ± 2.3 | 21.0 ± 0.8 |
| IFN-γ, IL-1, IL-6 | 18.2 ± 1.5 | 15.2 ± 4.1 |
| IFN-γ, TNF-α | 23.3 ± 3.7 | 16.6 ± 2.5 |
| IFN-γ, TNF-α, IL-6 | 14.3 ± 2.9 | 16.8 ± 2.5 |
Mean ± standard deviation of three separate experiments for astrocyte cultures treated with IFN-γ (100 U/ml), IL-1 (1 ng/ml), IL-6 (100 U/ml), and TNF-α (100 U/ml), in the presence or absence of NMMA (400 μM), and for control cultures incubated in medium alone. Cells were incubated with cytokines for 72 h prior to infection, and NMMA was added at the time of cytokine addition; [3H]uracil (2.5 μCi/ml) was added 2 h after infection, and cells were harvested 48 h later. No endotoxin contamination was detected in any of the cultures.
Effect of cytokines on growth of T. gondii in iNOS−/− murine astrocytes.
Treatment of iNOS−/− astrocytes with IFN-γ–IL-1, IFN-γ–IL-6, IFN-γ–IL-1 plus IL-6, IFN-γ–TNF-α, or IFN-γ–TNF-α plus IL-6 inhibited the growth of T. gondii significantly (75 to 80%) (Table 3), as was seen with syngeneic control murine astrocytes. In addition, as found for control astrocytes, the addition of IL-6 had little or no effect on the degree of inhibition caused by either IFN-γ–IL-1 or IFN-γ–TNF-α. As expected, no nitric oxide was detected in the supernatants from the iNOS−/− astrocytes after treatment with any of the cytokine combinations (Table 3).
TABLE 3.
Effect of cytokines on growth of T. gondii in iNOS−/− murine astrocytes
| Treatment | Inhibition of T. gondii growth (% of control)a |
|---|---|
| IFN-γ, IL-1 | 22.8 ± 2.3 |
| IFN-γ, IL-6 | 21.1 ± 0.8 |
| IFN-γ, IL-1, IL-6 | 23.3 ± 3.1 |
| IFN-γ, TNF-α | 22.2 ± 1.8 |
| IFN-γ, TNF-α, IL-6 | 19.2 ± 5.9 |
Mean ± standard deviation of two separate experiments for astrocyte cultures treated with IFN-γ (100 U/ml), IL-1 (1 ng/ml), IL-6 (100 U/ml), and TNF-α (100 U/ml). Cells were incubated with cytokines for 72 h prior to infection; [3H]uracil (2.5 μCi/ml) was added 2 h after infection, and cells were harvested 48 h later. Control cultures were incubated in medium alone. All values are significantly different (P < 0.05) from the control group as calculated by Student’s t test. Nitrite was not detectable (i.e., <1.0 μM) in any sample.
Effect of tryptophan on cytokine inhibition of T. gondii growth.
Tryptophan (100 μg/ml) added to cultures stimulated with cytokines did not reverse inhibition of T. gondii growth caused by any of the cytokine treatments in normal or in iNOS−/− astrocytes (Table 4). Tryptophan uptake by astrocytes was verified by [3H]tryptophan incorporation (28). No IDO activity was detected in astrocytes with or without cytokine stimulation.
TABLE 4.
Effect of tryptophan on cytokine inhibition of T. gondii growth
| Treatment | Inhibition of T. gondii growth (% of control)a
|
|||
|---|---|---|---|---|
| Control astrocytes
|
iNOS−/− astrocytes
|
|||
| Medium | Cytokines + tryptophan | Medium | Cytokines + tryptophan | |
| IFN-γ | 35.5 ± 2.3 | 38.7 ± 3.4 | 32.3 ± 2.3 | 31.0 ± 2.1 |
| IFN-γ, IL-1 | 28.4 ± 3.9 | 33.3 ± 3.5 | 21.1 ± 1.1 | 15.6 ± 2.0 |
| IFN-γ, IL-6 | 21.5 ± 2.9 | 29.6 ± 1.4 | 20.5 ± 1.7 | 17.0 ± 4.1 |
| IFN-γ, IL-1, IL-6 | 20.6 ± 2.1 | 22.8 ± 5.4 | 25.5 ± 4.9 | 19.5 ± 1.7 |
| IFN-γ, TNF-α | 23.5 ± 3.5 | 22.5 ± 5.1 | 23.4 ± 0.7 | 16.8 ± 5.6 |
| IFN-γ, TNF-α, IL-6 | 21.0 ± 2.2 | 23.7 ± 1.1 | 15.0 ± 3.8 | 12.5 ± 1.6 |
Mean ± SD of two separate experiments for astrocyte cultures treated with IFN-γ (100 U/ml), IL-1 (1 ng/ml), IL-6 (100 U/ml), and TNF-α (100 U/ml) in the presence and absence of tryptophan (100 μg/ml) and for control cultures incubated with medium alone. Cells were incubated with cytokines for 72 h prior to infection, and tryptophan was added at the time of cytokine addition; [3H]uracil (2.5 μCi/ml) was added 2 h after infection, and cells were harvested 48 h later. No statistically significant difference was detected between any of the cultures as determined by Student’s t test and analysis of variance.
DISCUSSION
The cytokines IFN-γ, TNF-α, IL-1, and IL-6 are known to be important in controlling the replication of T. gondii in the brain. The importance of cytokine activation of microglia in regulating T. gondii infection in the brain is well established, but the role of astrocytes is less well understood. Variable effects on the growth of T. gondii have been demonstrated in different astrocyte tumor lines with IFN-γ, TNF-α, IL-1, and/or IL-6 treatment. In the glioblastoma cell line 86HG39, IFN-γ alone has been shown to inhibit the growth of T. gondii, while in the glioblastoma cell line 87HG31, the combination of TNF-α and IFN-γ was required to inhibit growth (6, 8); in GHE astrocytoma cells, TNF-α but not IFN-γ inhibited the growth of T. gondii (25). Additionally, in astrocytoma cells, IL-1 was found to stimulate growth and IL-6 had no effect on T. gondii growth (25).
In the present study using primary murine astrocytes, we have demonstrated that pretreatment with IFN-γ alone or in combination with IL-1, IL-6, or TNF-α but not IL-1, IL-6, or TNF-α alone significantly inhibited the growth of T. gondii in these cells. This result is consistent with findings for glioblastoma cell line 86HG39 (6) but in contrast to those for astrocytoma cell line GHE (25). The absence of IFN-γ-induced inhibition in GHE astrocytoma cells may be due to the fact that in this study, cells were pretreated with IFN-γ for only 24 h. Peterson et al. (26) found that pretreatment with IFN-γ for 24 h did not activate murine astrocytes to inhibit T. gondii, which is consistent with our observations. We found that murine astrocytes needed to be pretreated with IFN-γ for 48 to 72 h to induce inhibition. Similarly, Däubener et al. (6, 8) found optimal inhibition with IFN-γ when glioblastoma cells were pretreated for 72 h. Treatment of astrocytes with cytokines after infection did not induce inhibition. Thus, priming of astrocytes by IFN-γ is required for anti-Toxoplasma activity. This phenomenon has also been reported for monocytes (3).
IFN-γ in combination with either IL-1, TNF-α, or IL-6 inhibited T. gondii growth in murine astrocytes. The effect of adding either IL-1, IL-6, or TNF-α to IFN-γ significantly (by 15 to 20%) increased inhibition of growth induced by IFN-γ alone. Similarly, in microglia, IFN-γ activation is enhanced by TNF-α, IL-1, or IL-6 (4, 5). In macrophages, IL-6 has been reported to reverse the inhibition caused by IFN-γ–IL-1 (2). In our study, IL-6 did not reverse the inhibition caused by either IFN-γ–IL-1 or IFN-γ–TNF-α in murine astrocytes. The effect of IL-6 is of interest due to the implication that IL-6 plays an important role in the immunopathogenesis of Toxoplasma encephalitis (31).
IFN-γ in combination with IL-1 and other cytokines has been shown to stimulate nitric oxide production via the enzyme iNOS in both human and murine astrocytes (14, 19). Treatment of primary murine astrocytes with IFN-γ in combination with other cytokines also resulted in the production of nitric oxide. However, inhibition of T. gondii growth was found to be nitric oxide independent, as demonstrated by the inability of NMMA to reverse the inhibition and the ability of cytokines to inhibit T. gondii growth in iNOS−/− astrocytes.
IFN-γ has been shown to inhibit Toxoplasma growth via induction of the enzyme IDO, which results in the degradation of tryptophan in human fibroblasts, glioblastoma cells, retinal epithelial cells, and macrophages (8, 12, 23, 24, 29). Additionally, the IDO pathway has been shown to be activated by IFN-γ and TNF-α in some glioblastoma cells and native human astrocytes (7). In our study, the addition of tryptophan did not reverse the inhibition caused by the IFN-γ alone or IFN-γ in combination with either TNF-α, IL-1, or IL-6, and no increase in IDO activity could be detected in astrocytes following cytokine treatment. These data suggest that in murine astrocytes, cytokines inhibit T. gondii via an IDO-independent pathway.
IFN-γ-induced inhibition of T. gondii in endothelial cells has been shown to be mediated by an IDO-independent mechanism and was also demonstrated not to be mediated via nitric oxide or reactive oxygen intermediates (32). The presence of some other mechanism(s) induced by cytokines is not surprising given the many diverse effects that cytokines have on astrocyte functions (1, 20). For example, IL-1 induces a reactive astrocyte phenotype characterized by the expression of TNF-α, IL-6, and colony-stimulating factor in astrocytes (20). IFN-γ also induces many changes in cell physiology, including metabolic and cytoskeletal changes. The cytokine-induced inhibition of T. gondii in astrocytes may be due to some of these generalized effects on host cell function. It is possible that iron starvation (11) or other reactive oxygen intermediates can be induced by IFN-γ alone or in combination with other cytokines and that these mechanisms, which are the focus of our current investigations, are responsible for the cytokine-mediated inhibition of T. gondii growth in murine astrocytes. Whatever the mechanism(s) involved, this study clearly demonstrates that cytokine-activated astrocytes induce significant anti-Toxoplasma activity and that IFN-γ is the primary cytokine mediating this inhibition.
The ability of cytokines to activate astrocytes to inhibit replication of T. gondii may also be involved in the mechanism of reactivated Toxoplasma infections in AIDS. For instance, evidence suggests that in patients with AIDS, a shift from a Th1 to a Th2 cytokine profile occurs in the brain (18). The presence of the Th1 cytokines IFN-γ, TNF-α, and IL-1 in the brain would presumably activate astrocytes to exert anti-Toxoplasma activity. Concomitantly, a shift to a Th2 cytokine profile, which is accompanied by a decrease in IFN-γ and subsequent decreases in TNF-α and IL-1, might then promote growth of T. gondii in astrocytes. The effect of Th2 cytokines on T. gondii in astrocytes is not known, but data from our previous study (13) showed that astrocytes, when unstimulated by IFN-γ or other cytokines, serve as excellent host cells for T. gondii, supporting extensive replication resulting in the lysis and continual reinfection of astrocytes. This finding, in conjunction with the Th1/Th2 shift hypothesis, suggests that astrocytes may play a pivotal role in the pathophysiology of Toxoplasma encephalitis in the brains of patients with AIDS.
In conclusion, we found in murine astrocytes, IFN-γ alone or in combination with IL-1, TNF-α, or IL-6 significantly inhibited growth of T. gondii. Although IFN-γ–IL-1 and IFN-γ–TNF-α induced NO production, inhibition was not found to be via an NO-mediated mechanism. Cytokine-mediated inhibition was also not due to induction of IDO. The NO/IDO-independent pathway responsible for inhibition of T. gondii growth is currently under investigation in our laboratory. Given that IFN-γ has been shown to be the main cytokine controlling growth of T. gondii in the brain and that TNF-α, IL-1, and IL-6 are up-regulated in the brains of mice with chronic toxoplasmosis, results from the present study indicate astrocytes are most likely an important effector cell in limiting T. gondii replication in the brain.
ACKNOWLEDGMENTS
Sandra K. Halonen is an Aaron Diamond Foundation Fellow. This work was supported in part by a grant from The Aaron Diamond Foundation and in part by PHS grant AI 39454.
REFERENCES
- 1.Aloisi F, Borsellino G, Caré A, Testa U, Gallo P, Russo G, Peschle C, Levi G. Cytokine regulation of astrocyte function: in vitro studies using cells from the human brain. Int J Dev Neurosci. 1995;13:265–274. doi: 10.1016/0736-5748(94)00071-a. [DOI] [PubMed] [Google Scholar]
- 2.Beaman M H, Hunter C A, Remington J S. Enhancement of intracellular replication of Toxoplasma gondii by IL-6. Interactions with IFNγ and TNFα. J Immunol. 1994;153:4583–4587. [PubMed] [Google Scholar]
- 3.Channon J Y, Kasper L H. Toxoplasma gondii-induced immune suppression by human peripheral blood monocytes: role of gamma interferon. Infect Immun. 1996;64:1181–1189. doi: 10.1128/iai.64.4.1181-1189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao C C, Hu S, Gekker G, Novick W J, Jr, Remington J S, Peterson P K. Effects of cytokines on multiplication of Toxoplasma gondii in microglial cells. J Immunol. 1993;150:3404–3410. [PubMed] [Google Scholar]
- 5.Chao C C, Gekker G, Hu S, Peterson P K. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 6.Däubener W, Pilz K, Zennati S S, Bilzer T, Fischer H G, Hadding U. Induction of toxoplasmostasis in a human glioblastoma by interferon γ. J Neuroimmunol. 1993;43:31–38. doi: 10.1016/0165-5728(93)90072-7. [DOI] [PubMed] [Google Scholar]
- 7.Däubener W, Wanagat N, Pilz K, Seghrouchni S, Fischer H G, Hadding U. A new simple, bioassay for human IFN-γ. J Immunol Methods. 1994;168:39–47. doi: 10.1016/0022-1759(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 8.Däubener W C, Remscheid L, Nockemann S, Pilz K, Seghrouchni S, Mackenzie C, Hadding U. Anti-parasitic effector mechanisms in human brain tumor cells: role of interferon γ and tumor necrosis factor α. Eur J Immunol. 1996;26:487–492. doi: 10.1002/eji.1830260231. [DOI] [PubMed] [Google Scholar]
- 9.Deckert-Schlüter M, Albrecht S, Hof H, Wiestler O D, Schlüter D. Dynamics of the intracerebral and splenic cytokine mRNA production in Toxoplasma gondii-resistant and -susceptible congenital strains of mice. Immunology. 1995;85:408–418. [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Eltoum I, Wynn T A, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNFα and correlates with the down regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- 11.Gebran S J, Yamamoto Y, Newton C, Klein T W, Friedman H. Inhibition of Legionella pneumophila growth by gamma interferon in permissive A/J mouse macrophages: role of reactive oxygen species, nitric oxide, tryptophan and iron(III) Infect Immun. 1994;62:3197–3205. doi: 10.1128/iai.62.8.3197-3205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S L, Carlin J M, Pyati P, Dai W, Pfefferkorn E R, Murphy M J., Jr Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halonen S K, Lyman W D, Chiu F C. Growth and development of Toxoplasma gondii in humans neurons and astrocytes. J Neuropathol Exp Neurol. 1996;55:1150–1156. doi: 10.1097/00005072-199611000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hewett S J, Corbett J A, McDaniel M L, Choi D W. Interferon-γ and interleukin-1β induce nitric oxide formation from primary mouse astrocytes. Neurosci Lett. 1993;164:229–232. doi: 10.1016/0304-3940(93)90898-u. [DOI] [PubMed] [Google Scholar]
- 15.Hunter C A, Roberts C W, Murray M, Alexander J. Kinetics of cytokine mRNA production in the brains of mice with progressive toxoplasmic encephalitis. Eur J Immunol. 1992;22:2317–2322. doi: 10.1002/eji.1830220921. [DOI] [PubMed] [Google Scholar]
- 16.Hunter C A, Litton M J, Remington J S, Abrams J S. Immunocytochemical detection of cytokines in the lymph nodes and brains of mice resistant or susceptible to Toxoplasmic encephalitis. J Infect Dis. 1994;170:939–945. doi: 10.1093/infdis/170.4.939. [DOI] [PubMed] [Google Scholar]
- 17.Hunter C A, Remington J S. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis. 1994;170:1057–1067. doi: 10.1093/infdis/170.5.1057. [DOI] [PubMed] [Google Scholar]
- 18.Klein S A, Dobmeyer J M, Dobmeyer T S, Pape M, Ottmann O G, Helm E B, Hoelzer D, Rossol R. Demonstration of the Th1 to Th2 cytokine shift during the course of HIV-1 infection using cytoplasmic cytokine detection on single cell level by flow cytometry. AIDS. 1997;11:1111–1118. doi: 10.1097/00002030-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Lee S C, Dickson D W, Liu W, Brosnan C F. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993;46:19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- 20.Lee S C, Dickson D W, Brosnan C F. Interleukin-1, nitric oxide and reactive astrocytes. Brain Behav Immun. 1995;9:345–354. doi: 10.1006/brbi.1995.1032. [DOI] [PubMed] [Google Scholar]
- 21.Mack D G, McLeod R. New micromethod to study the effect of antimicrobial agents on Toxoplasma gondii: comparison of sulfadoxine and sulfadiazine individually and in combination with pyrimethamine and study of clindamycin, metronidazole, and cyclosporin A. Antimicrob Agents Chemother. 1984;26:26–30. doi: 10.1128/aac.26.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMicking J D, Nathan C, Hom G, Chartrain N, Fletcher D S, Trumbauer M, Stevens K, Xie Q W, Sokol K, Hutchison N, Chen H, Mudgett J S. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 23.Murray H W, Szuro-Sudol A, Wellner D, Oca M J, Granger A M, Libby D M, Rothermel C D, Rubin B Y. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagineni C N, Pardhasaradhi K, Martins M C, Detrick B, Hooks J J. Mechanisms of interferon-induced inhibition of Toxoplasma gondii replication in human retinal pigment epithelial cells. Infect Immun. 1996;64:4188–4196. doi: 10.1128/iai.64.10.4188-4196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelloux H, Pernod G, Polack B, Coursange E, Richard J, Verna J M, Ambroise-Thomas P. Influence of cytokines on Toxoplasma gondii growth in human astrocytoma-derived cells. Parasitol Res. 1996;82:598–603. doi: 10.1007/s004360050171. [DOI] [PubMed] [Google Scholar]
- 26.Peterson P K, Gekker G, Hu S, Chao C C. Intracellular survival and multiplication of Toxoplasma gondii in astrocytes. J Infect Dis. 1993;168:1472–1478. doi: 10.1093/infdis/168.6.1472. [DOI] [PubMed] [Google Scholar]
- 27.Peterson P K, Gekker G, Hu S, Chao C C. Human astrocytes inhibit intracellular multiplication of Toxoplasma by a nitric oxide-mediated mechanism. J Infect Dis. 1995;171:516–518. doi: 10.1093/infdis/171.2.516. [DOI] [PubMed] [Google Scholar]
- 28.Pfefferkorn E R. Interferon γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfefferkorn E R, Eckel M, Rebhun S. Interferon-γ suppresses the growth of Toxoplasma gondii in human fibroblasts through starvation for tryptophan. Mol Biochem Parasitol. 1986;20:215–224. doi: 10.1016/0166-6851(86)90101-5. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Conley F K, Remington J S. Importance of endogenous interferon-gamma in prevention of toxoplasmic encephalitis in mice. J Immunol. 1989;145:4185–4191. [PubMed] [Google Scholar]
- 31.Suzuki Y, Yang Q, Conley F K, Abrams J S, Remington J S. Antibody against interleukin-6 reduces inflammation and numbers of cysts in brains of mice with toxoplasmic encephalitis. Infect Immun. 1994;62:2773–2778. doi: 10.1128/iai.62.7.2773-2778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodman J P, Dimier I H, Bowl D T. Human endothelial cells are activated by IFN-γ to inhibit Toxoplasma gondii replication: inhibition is due to a different mechanism from that existing in mouse macrophages and human fibroblasts. J Immunol. 1991;147:2017–2023. [PubMed] [Google Scholar]