Abstract
We previously reported that bakuchiol, a phenolic isoprenoid anticancer compound, and its analogs exert anti-influenza activity. However, the proteins targeted by bakuchiol remain unclear. Here, we investigated the chemical structures responsible for the anti-influenza activity of bakuchiol and found that all functional groups and C6 chirality of bakuchiol were required for its anti-influenza activity. Based on these results, we synthesized a molecular probe containing a biotin tag bound to the C1 position of bakuchiol. With this probe, we performed a pulldown assay for Madin-Darby canine kidney cell lysates and purified the specific bakuchiol-binding proteins with SDS-PAGE. Using nanoLC-MS/MS analysis, we identified prohibitin (PHB) 2, voltage-dependent anion channel (VDAC) 1, and VDAC2 as binding proteins of bakuchiol. We confirmed the binding of bakuchiol to PHB1, PHB2, and VDAC2 in vitro using Western blot analysis. Immunofluorescence analysis showed that bakuchiol was bound to PHBs and VDAC2 in cells and colocalized in the mitochondria. The knockdown of PHBs or VDAC2 by transfection with specific siRNAs, along with bakuchiol cotreatment, led to significantly reduced influenza nucleoprotein expression levels and viral titers in the conditioned medium of virus-infected Madin-Darby canine kidney cells, compared to the levels observed with transfection or treatment alone. These findings indicate that reducing PHBs or VDAC2 protein, combined with bakuchiol treatment, additively suppressed the growth of influenza virus. Our findings indicate that bakuchiol exerts anti-influenza activity via a novel mechanism involving these mitochondrial proteins, providing new insight for developing anti-influenza agents.
Keywords: bakuchiol, influenza A virus, prohibitin, voltage-dependent anion channel, target protein
Seasonal influenza is an acute respiratory disease caused by infection with influenza viruses; the World Health Organization has reported that annual influenza epidemics causes approximately 290,000 to 650,000 mortalities worldwide (https://www.who.int/news-room/fact-sheets/detail/influenza-[seasonal]). Thus, the prevention and control of influenza virus infections remain global public health challenges. Many antiviral drugs targeting influenza viral proteins, including the M2-ion channel, neuraminidase, and cap-dependent endonucleases have been approved for treating influenza infection (1, 2). However, almost all influenza strains are highly resistant to M2-ion channel inhibitors; oseltamivir-resistant strains have also been detected among influenza A H1N1, H3N2, and H5N1 viruses (1, 3, 4, 5, 6, 7). Further, cap-dependent endonuclease inhibitor-resistant influenza H1N1 and H3N2 viruses with I38T/M/F-substituted polymerase acidic protein have been reported, with records of human-to-human transmission of a polymerase acidic–I38 mutant H3N2 virus (8, 9). Therefore, anti-influenza drugs with novel mechanisms of action that target host factors associated with the viral life cycle, but not those of influenza viral proteins need to be urgently developed to prevent and control broad-spectrum influenza viruses, including antiviral drug-resistant viruses.
(+)-(S)-bakuchiol (1) (Fig. 1A) is a phenolic isoprenoid with a chiral tetra-alkylated (all-carbon) quaternary center that was first isolated from the seeds of Psoralea corylifolia Linn. by Mehta et al. (10, 11). 1 can be chemically synthesized from (E)-geranic acid in four steps (12). 1 and its derivatives have been reported to possess a range of biological and pharmacological activities, including anticancer (13, 14, 15), anti-inflammation (16, 17), antibacterial (18, 19, 20), and antioxidant (21, 22) activities. Among these, inhibition of oxidative stress by 1 is well known to be effective; further, many studies have reported that 1 prevents mitochondrial lipid peroxidation and protects other enzymes from oxidative stress (21, 23, 24). 1 activates the nuclear factor erythroid 2–related factor 2 (Nrf2) signaling pathway for protection from oxidative stress by reactive oxygen species (ROS) production (25, 26). We previously reported that 1 and its analogs induce enantiospecific anti-influenza A virus activity involving Nrf2 activation (27, 28). Thus, these properties suggest that 1 has potential as a therapeutic agent against various diseases.
Figure 1.
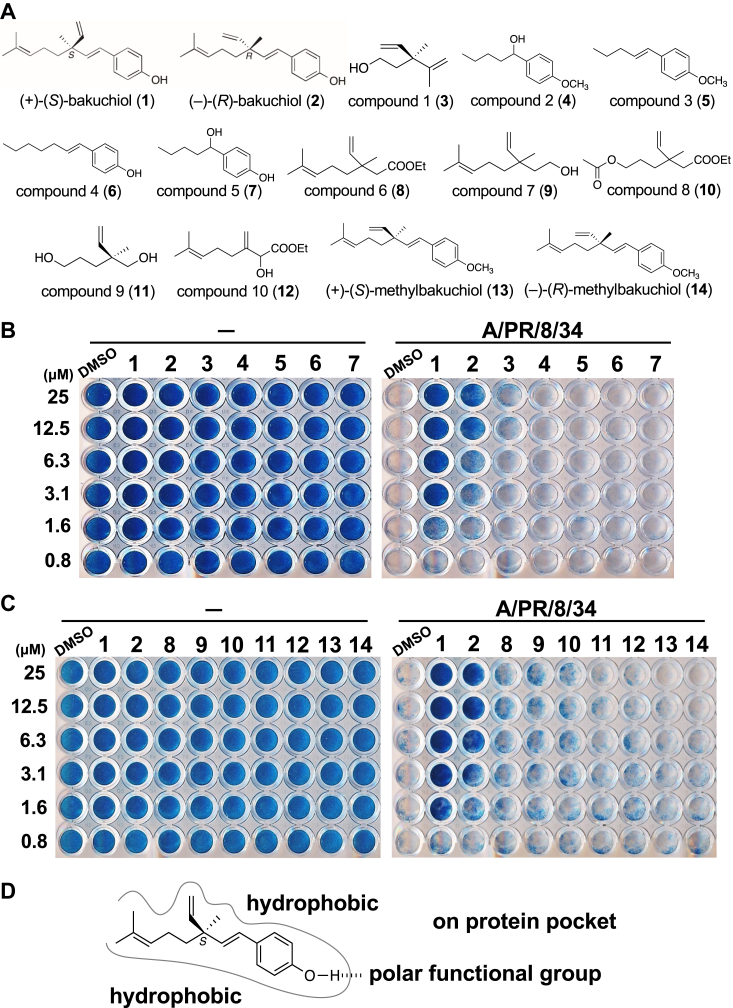
Effects of bakuchiol derivatives with various deletions or methylation on cell survival upon infection with influenza A virus.A, chemical structures of (+)-(S)-bakuchiol (1), (−)-(R)-bakuchiol (2), bakuchiol derivatives with various deletions, compounds 1 to 10 (3–12), (+)-(S)-O-methylbakuchiol (13), and (−)-(R)-O-methylbakuchiol (14) according to synthetic routes of Fig. S1 and our previous report (12). B and C, the indicated concentrations of 3 to 14 in DMSO were mixed without virus (−) (B and C, left panels) or with A/PR/8/34 virus (MOI; 10) (B and C, right panels) and added to MDCK cells. DMSO or 1 and 2 were used as negative or positive controls, respectively. MDCK cell survival after 72 h postinfection and treatment with 3 to 7 (B) or 8 to 14 (C) was determined using NB staining. Data are representative of four independent experiments, and the results were reproducible. D, image of the binding site of target molecules with 1. DMSO, dimethyl sulfoxide; MDCK, Madin-Darby canine kidney; MOI, multiplicity of infection; NB, naphthol blue-black.
Limited studies have reported the different molecular targets of 1, and Krishna et al. have reviewed these (29). Molecular docking analysis of 1 with different targets has revealed that 1 binds to hematopoietic cell kinase and p38 mitogen-activated protein kinase (MAPK) to exert anticancer effects (15); with tyrosinase inhibitors (30), LasR, and Rh1R to exert antibacterial effects (19); with estrogen receptor 1 alpha to exert effects on cell proliferation (31); and with daboxin P, phospholipase A2 enzyme from the venom of Indian Daboia russelii (32). Further, Long et al. reported that PI3K-AKT and MAPK are involved in 1-induced apoptosis of gastric cancer cells (33). Nrf2-upstream effector proteins, such as hematopoietic cell kinase, p38 MAPK, and PI3K-AKT, targeted for the anticancer activity of 1 could also be considered targets of 1 in the inhibitory effect on oxidative stress and anti-influenza A virus activity. However, the direct target factors of 1 for these activities remain to be fully evaluated.
The avidin–biotin affinity approach is widely used to identify the molecular targets of bioactive small molecules from whole-cell lysates. This is based on strong noncovalent interactions between biotin and avidin. Sato et al. reported a PEG rod approach with the biotin–avidin complex (34). A molecular probe used in this approach was designed for an eight PEG linker between a small molecule and biotin tag to permit its interaction with the protein targets. This approach can isolate a high density of binding proteins (34). Further, PEG, which possesses suitable hydrophilicity for solubility in water, was selected as the linker, and the length of the linker was determined as eight PEG units, which are widely used and commercially available. Therefore, we hypothesized that the direct target proteins of 1 can be isolated with high density using the PEG rod approach and subsequently identified using nanoLC-MS/MS analysis.
In this study, to determine the binding site of eight PEG linkers with a biotin tag for 1, we investigated the structure-activity relationship in its anti-influenza activity. Based on these results, we synthesized a molecular probe with eight PEG linkers and a biotin tag bound to a C1 position of 1. Subsequently, we performed a pulldown assay using this biotin-linker molecular probe and streptavidin beads; the binding proteins of 1 were then analyzed using nanoLC-MS/MS. We identified prohibitin (PHB) 2, voltage-dependent anion channel (VDAC) 1, and VDAC2 as the binding partners of 1. We confirmed the binding of 1 to PHB1, PHB2, and VDAC2 in vitro and in cells and further evaluated the involvement of PHBs or VDAC2 in the anti-influenza activity of 1 by transfecting their specific siRNAs. Our findings provide new insights into the development of antiviral agents with novel mechanisms.
Results
Identification of the structure-activity relationship in the anti-influenza activity of (+)-(S)-bakuchiol
We used the PEG rod approach with the biotin–avidin complex, as previously reported (34) to isolate protein targets of 1 from whole-cell lysates. Thus, to determine the binding site of eight PEG linkers with a biotin tag (biotin linker) for 1 while retaining its anti-influenza activity, we investigated the structure-activity relationship of its anti-influenza activity. To identify the chemical structures responsible for the anti-influenza activity of 1, we synthesized bakuchiol derivatives with various deletions of the phenol group and/or tetraalkyl quaternary carbon in 1, compounds 1 to 10 (3–12), (+)-(S)-O-methylbakuchiol (13), and (−)-(R)-O-methylbakuchiol (14), according to the synthetic routes described in Fig. S1 and in our previous report (12) (Fig. 1A). Subsequently, the effect of 3 to 14 on the survival of Madin-Darby canine kidney (MDCK) cells infected with A/Puerto Rico/8/34 (A/PR/8/34) virus was examined using naphthol blue-black staining. Dimethyl sulfoxide (DMSO) or 1 and (−)-(R)-bakuchiol (2) (Fig. 1A) were used as negative or positive controls, respectively. Samples without viruses added were stained blue at all indicated concentrations (Fig. 1, B and C, left panels), indicating that the concentrations used in this experiment did not induce cytotoxicity. Exposure of viruses to 3 to 14 reduced the survival of cells infected with virus similar to DMSO compared to that with 1 and 2 (Fig. 1, B and C, right panels), suggesting that all functional groups and C6 chirality of 1 were required to express the anti-influenza activity. It is also well known that the phenolic hydroxy groups interact with the polar functional groups of various biomolecules, such as proteins, via hydrogen bonding (29); thus, the phenolic hydroxy group of 1 might be the most important functional group to interact with the target molecule (Fig. 1D). Therefore, these results suggest that the biotin linker should be bound to the C1 position of 1, which is the farthest position from the phenolic hydroxy group, via the peptide bond to retain its anti-influenza activity.
Isolation and identification of (+)-(S)-bakuchiol–binding proteins using a biotin-linker-(+)-(S)-bakuchiol probe and nanoLC-MS/MS analysis
Based on the above results, we synthesized three probes, as described in Figure 2A, according to the synthetic routes shown in Fig. S2. As the acetyl group is much smaller than 1 and the 5-phenylpentanoyl group lacks the phenolic hydroxy group for hydrogen bonding, they would be expected to have no influence on binding to target biomolecules. Thus, the biotin-linker attached acetyl and 5-phenylpentanoyl, acetamide (15) (Fig. 2A, top) or 5-phenylpentanylamide (16) (Fig. 2A, middle) were synthesized as negative controls (NCs) according to the synthetic routes in Fig. S2A or S2B. The biotin-linker bound to the C1 position in 1 (17) (Fig. 2A, bottom) was synthesized for isolating its binding proteins according to the synthetic routes shown in Fig. S2C.
Figure 2.
Isolation and identification of (+)-(S)-bakuchiol–binding proteins.A, the chemical structures of molecular probes for isolating 1–binding proteins. As negative controls for the experiments, a biotin-linker attached acetyl and 5-phenylpentanoyl, acetamide (15) (top) or 5-phenylpentanylamide (16) (middle) were synthesized. The biotin-linker bound to a C1 position in 1 (17) was synthesized for isolating its binding proteins (bottom). B and C, the probes 15, 16, or 17 were tested for the ability to isolate the binding proteins in a pulldown assay. Their binding proteins from the lysates of MDCK cells infected without virus (−) (B) or with A/PR/8/34 virus (MOI; 5) (C) were analyzed on a silver-stained SDS gel and the specific bands in 17-sample lanes of cell lysates infected without/with A/PR/8/34 virus were found in the 25 to 37 kDa region (B and C, black arrows). D, the specific band in the 17-sample lane and its same position in the 16-sample lane were analyzed using nanoLC-MS/MS. Data were also analyzed using the UniProt dog reference proteome database. The peptide identification confidence for the dataset was evaluated based on the FDR. The number of peptides identified was counted for each protein. FDR, false discovery rate; MDCK, Madin-Darby canine kidney; MOI, multiplicity of infection.
These compounds were conjugated with streptavidin magnetic beads using the biotin–streptavidin interaction and tested for their ability to isolate binding proteins from the lysate of MDCK cells infected without/with A/PR/8/34 virus using a pulldown assay. When their bead-binding proteins were separated by analysis on a silver-stained SDS gel, we found specific bands in the 17-sample lanes of cell lysates infected without/with A/PR/8/34 virus at the 25 to 37 kDa region compared with those of 15 and 16 (Fig. 2, B and C, black arrows). NanoLC-MS/MS analysis of the 17-specific band revealed various proteins and the high number of peptides as compared with those of 16 at the same position of 17-sample lane, 160, 20, or 16 peptides were matched to PHB2, VDAC2, or VDAC1, respectively, using the UniProt dog reference proteome database (Fig. 2D). PHB2, VDAC2, or VDAC1 have never been reported as binding proteins of 1 in previous studies.
PHB2 is an evolutionarily conserved ∼34 kDa protein that is ubiquitously expressed in eukaryotic cells with a highly homologous member, PHB1 (35). PHB2 localizes to the inner mitochondrial membrane by forming heterodimeric complexes with PHB1 and has various functions within the mitochondria (36). VDAC, a ∼33 kDa protein localizes to the outer mitochondrial membrane (OMM) and has three isoforms, VDAC1, VDAC2, and VDAC3, in mammalian cells; these regulate the entry and exit of mitochondrial metabolites between mitochondria and other cellular compartments (37). Influenza A viral titers in culture supernatants have been reported to be significantly reduced when PHB2, VDAC1, or VDAC2 are silenced using their specific siRNAs (38, 39), suggesting that these host factors are required for virus production. Therefore, PHB2, VDAC1, or VDAC2 may be target proteins of 1 for its anti-influenza activity.
Binding of (+)-(S)-bakuchiol to PHBs or VDACs in vitro and in cells
To evaluate the presence of PHBs and VDACs in beads and supernatant samples after the pulldown assay using 17-conjugated streptavidin beads, we performed Western blot (WB) analysis using their specific antibodies. The 15- and 16-conjugated streptavidin beads were used as NCs. WB analysis confirmed the presence of PHB1, PHB2, and VDAC2 in the 17-conjugated bead samples from the lysates of MDCK (Fig. 3A), A549 (Fig. 3B), or HeLa (Fig. 3C) cells infected without/with A/PR/8/34 virus, but not those of 15 and 16. Interestingly, the presence of PHB1 and PHB2, but not VDAC2, in supernatant samples of 17-conjugated beads from MDCK cell lysates was reduced relative to that in the samples of 15 and 16 (Fig. 3A), suggesting that 1 was strongly bound to PHBs relative to VDAC2. Further, to confirm the binding of 1 to PHB proteins, we examined its interaction with overexpressed PHB1 or PHB2 harboring a FLAG tag at its C terminus (PHB1- or PHB2-FLAG). Expression plasmids for PHB1- or PHB2-FLAG, driven by a cytomegalovirus (CMV) promoter (pCMV-PHB1- or pCMV-PHB2-FLAG), were transfected into 293T cells. pCMV-FLAG, excluding the PHB gene (pCMV-empty), was used as a NC. The supernatant of each transfectant was used for the pulldown assay. The WB analysis revealed the presence of overexpressed PHB1- and PHB2-FLAG proteins similar to that of PHB1 and PHB2 in the pulldown samples of 17-conjugated beads (Fig. 3D). The protein levels of PHB1- (∗p < 0.05) (Fig. 3E) and PHB2-FLAG (∗∗p < 0.01) (Fig. 3F) in the 17-conjugated bead samples were significantly increased relative to those with the 15 and 16 samples. Therefore, these results indicated that 1 binds to PHBs and VDAC2 in vitro.
Figure 3.
Western blot analysis with pulldown assay for the binding of (+)-(S)-bakuchiol to PHBs and VDACs in the cell extracts.A–C, the presence of PHB1, PHB2, VDAC1, and VDAC2 in the beads or supernatant samples of the pulldown assay using 17-conjugated with streptavidin beads from lysates of MDCK (A), A549 (B), or HeLa (C) cells infected with/without A/PR/8/34 virus was analyzed by Western blotting using the specific antibodies in Table S1. 15- and 16-conjugated with streptavidin beads were used as negative controls. Data are representative of three independent experiments, and the results were reproducible. D–F, expression plasmids of PHB1 or PHB2 harboring a FLAG tag at its C terminus, driven by a CMV (pCMV-PHB1-FLAG or pCMV-PHB2-FLAG) were transfected into 293T cells. pCMV-FLAG excluding the PHB gene (pCMV-empty) was used as a negative control. Each supernatant in the transfectant was used for a pulldown assay. The presence of overexpressed PHB1-FLAG and PHB2-FLAG proteins similar to PHB1 and PHB2 in the pulldown samples of 15-, 16-, or 17-conjugated beads or supernatants was analyzed by Western blotting using the specific antibodies in Table S1 (D). Signal intensities were measured using ImageJ software, and the protein levels of PHB1- (n = 4 each) (E) and PHB2-FLAG (n = 4 each) (F) in the 15-, 16-, or 17-conjugated beads were calculated relative to those of pCMV-empty-transfected samples. Data represent the mean ± SEM and are representative of four independent experiments, and the results were reproducible. ∗p < 0.05 or ∗∗p < 0.01 denote the significance levels for the indicated comparisons determined through one-way ANOVA, followed by the post hoc Tukey’s test. CMV, cytomegalovirus; MDCK, Madin-Darby canine kidney; PHB, prohibitin; VDAC, voltage-dependent anion channel.
Next, to investigate the binding of 1 to PHBs and VDAC2 in cells, we analyzed their intracellular distribution using confocal fluorescence microscopy. MDCK, A549, or HeLa cells were treated with 15, 16, or 17 (25 μM). Biotin-linker compounds were detected using Alexa Fluor 488–conjugated streptavidin (green). PHB1, PHB2, VDAC2, or ATP synthase beta (ATP5B), a mitochondrial marker, were detected using their specific antibodies (red). Cell nuclei were stained with diamidino-2-phenylindole (blue). The images are shown in Figure 4. Imaging analysis revealed colocalization of 17 and PHB1 (Fig. 4A), PHB2 (Fig. 4B), or VDAC2 (Fig. 4C) in MDCK, A549, and HeLa cells (yellow color and white arrows in images of 17-treated cells). 17 was also colocalized with ATB5B (Fig. 4D, yellow color and white arrows), indicating that 17 is localized in the mitochondria. Therefore, these results indicated that 1 was bound to PHB1, PHB2, and VDAC2 in cells and was also localized in the mitochondria.
Figure 4.
Immunofluorescence analysis for intracellular interactions with (+)-(S)-bakuchiol and PHB1, PHB2, VDAC2, or mitochondria. MDCK, A549, or HeLa cells were seeded on glass bottom wells at a density of 5 × 103 cells. The cells were fixed and subsequently permeabilized. The cells were blocked for endogenous biotin and then treated with 25 μM 15, 16, or 17 for 24 h. Biotin-linker compounds was detected with Alexa Fluor 488–conjugated streptavidin (green). PHB1 (A), PHB2 (B), VDAC2 (C), or ATP5B (D), a mitochondrial maker, were detected using their specific antibodies (red). Cell nuclei were stained with DAPI (blue). Images were captured on a confocal laser-scanning fluorescence microscope. The indicated images were the merged images of three colors (green, red, and blue). The white scale bar in each image represents 50 μm. The white squares in images of 17-treated cells were enlarged, and white arrows indicate regions of colocalization, as indicated by the yellow color. The white scale bar in the enlarged image represents 10 μm. Data are representative of three or four independent experiments, and the results were reproducible. DAPI, diamidino-2-phenylindole; MDCK, Madin-Darby canine kidney; PHB, prohibitin; VDAC, voltage-dependent anion channel.
Involvement of PHBs or VDAC2 in the anti-influenza activity of (+)-(S)-bakuchiol
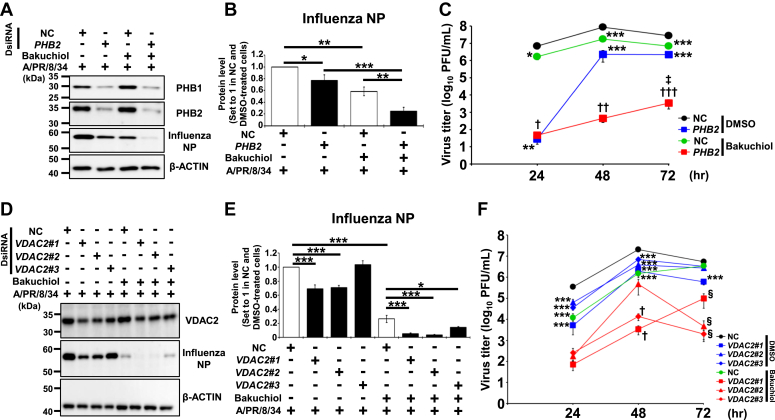
To further evaluate the roles of PHBs or VDAC2 in the anti-influenza activity of 1, we examined its effects on influenza viral growth in the PHB- or VDAC2-reduced MDCK cells transfected with their specific siRNAs. Human PHB2 or VDAC2, described in #1, 2, or 3, and dicer-substrate siRNA (DsiRNA) were used for downregulating PHBs or VDAC2 in MDCK cells. NC DsiRNA was used as a NC. WB analysis revealed that PHB2 DsiRNA transfection reduced the expression levels of PHB1 and PHB2 proteins in MDCK cells (Fig. 5A). The influenza nucleoprotein (NP) expression level (Fig. 5, A and B) and viral titers of conditioned media (Fig. 5C) in MDCK cells infected with A/PR/8/34 virus were significantly reduced upon transfection with PHB2 DsiRNA (∗p < 0.05 in Fig. 5B or ∗∗p < 0.01 at 24 h and ∗∗∗p < 0.001 at 48–72 h in Fig. 5C) or treatment with 1 (∗∗p < 0.01 in Fig. 5B or ∗p < 0.05 at 24 h and ∗∗∗p < 0.001 at 48–72 h in Fig. 5C). Both PHB2 DsiRNA transfection and treatment with 1 significantly reduced the influenza NP expression levels (Fig. 5, A and B) and viral titers of conditioned media (Fig. 5C) in virus-infected MDCK cells compared to the observations made when each treatment was used alone (∗∗p < 0.01 and ∗∗∗p < 0.001 in Fig. 5B or †p < 0.05 at 24 h, ††p < 0.01 at 48 h, †††p < 0.001 at 72 h, and ‡p < 0.05 at 72 h in Fig. 5C).
Figure 5.
Effects of PHB2 or VDAC2 knockdown on the inhibition of influenza viral growth by (+)-(S)-bakuchiol.A, B, D, and E, MDCK cells were transfected with the dicer-substrate siRNA (DsiRNA) of 10 nM human PHB2 or 30 nM human VDAC2#1, #2, or #3 using reverse transfection and incubated for 48 h. Further, 10 or 30 nM negative control (NC) DsiRNA was used as a negative control. The DsiRNA-treated cells were incubated with 12.5 μM 1 and with 0.1 MOI of A/PR/8/34 virus for 24 h, and 0.125% DMSO was used as a negative control. The cells were lysed in a SDS sample buffer, and the presence of PHB1, PHB2, and influenza NP (A) or VDAC2 and influenza NP (D) was analyzed using Western blotting, respectively. Signal intensities were measured using ImageJ software, and the protein levels of influenza NP in PHB2 DsiRNA- (n = 4 each) (B) or VDAC2#1 to 3 DsiRNA- (n = 3 each) (E) transfected cells were analyzed relative to those in the NC and DMSO-treated cells. The indicated protein levels were normalized to those of β-actin. Data represent the mean ± SEM and are representative of four or three independent experiments, and the results were reproducible. ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001 denote the significance levels for the indicated comparisons through one-way ANOVA, followed by the post-hoc Tukey’s test. (C and F) MDCK cells (1 × 105 cells/well) were transfected with 10 nM human PHB2 or 30 nM human VDAC2#1, 2, or 3 using reverse transfection and incubated for 24 h. Furthermore, 10 or 30 nM DsiRNA was used as the negative control (NC). The DsiRNA-treated cells were infected with 0.0001 MOI of A/PR/8/34 virus for 1 h. Subsequently, 12.5 μM 1 was added to the cells in the medium supplemented with 3 μg/ml L-tosylamido-2-phenyl ethyl chloromethyl ketone–treated trypsin. DMSO (0.125%) was used as the negative control. The cells were incubated for 24, 48, or 72 h. At specified time points, culture-conditioned media were collected from each well and viral titers in the conditioned media were determined in NC- or PHB2 DsiRNA- (n = 11–15 each) transfected and virus-infected cells (C), or NC- or VDAC2#1 to 3 DsiRNA- (n = 7–9 each) (F) transfected and virus-infected cells. The data represent the mean ± SEM and are representative of five or three independent experiments, and the results were reproducible. ∗p < 0.05, ∗∗p < 0.01, or ∗∗∗p < 0.001 was considered statistically significant for the comparisons of both NC transfection and DMSO treatment by one-way ANOVA, followed by the post hoc Tukey’s test. †p < 0.05, ††p < 0.01, or †††p < 0.001 was considered statistically significant for the comparison of both NC transfection and bakuchiol treatment by Student’s t test. ‡p < 0.05 was considered statistically significant for the comparisons of both PHB2 transfection and bakuchiol treatment by Student’s t test. §p < 0.05 was considered statistically significant for the comparison of both NC transfection and bakuchiol treatment by one-way ANOVA with the post hoc Tukey’s test. DMSO, dimethyl sulfoxide; MDCK, Madin-Darby canine kidney; MOI, multiplicity of infection; NP, NP, nucleoprotein; PHB, prohibitin; VDAC, voltage-dependent anion channel.
VDAC2#1 or VDAC2#2 DsiRNA transfection reduced the VDAC2 protein expression in MDCK cells, whereas the effect of VDAC2#3 DsiRNA was weak (Fig. 5D). The influenza NP expression level (Fig. 5, D and E) and viral titers of conditioned media (Fig. 5F) in virus-infected MDCK cells were reduced upon transfecting VDAC2#1 (∗∗∗p < 0.001 in Fig. 5E or ∗∗∗p < 0.001 at 24–72 h in Fig. 5F), VDAC2#2 (∗∗∗p < 0.001 in Fig. 5E or ∗∗∗p < 0.001 at 24–48 h in Fig. 5F), VDAC2#3 (∗∗∗p < 0.001 at 24–48 h in Fig. 5F) DsiRNA, or treatment with 1 (∗∗∗p < 0.001 in Fig. 5E or ∗∗∗p < 0.001 at 24–48 h in Fig. 5F). Both VDAC2#1 (∗∗∗p < 0.001 in Fig. 5E or †p < 0.05 at 48 h and §p < 0.05 at 72 h in Fig. 5F), VDAC2#2 (∗∗∗p < 0.001 in Fig. 5E or §p < 0.05 at 72 h in Fig. 5F), or VDAC2#3 (∗p < 0.05 in Fig. 5E or †p < 0.05 at 48 h and §p < 0.05 at 72 h in Fig. 5F) DsiRNA transfection and treatment with 1 significantly reduced the influenza NP expression levels (Fig. 5, D and E) and viral titers of conditioned media (Fig. 5F) in virus-infected MDCK cells compared to the observations made when each treatment was used alone. Therefore, these results indicated that the additive effects of PHBs or VDAC2 protein reduction and 1 treatment suppressed influenza viral growth more effectively than either treatment alone.
Discussion
In this study, we investigated the chemical structures responsible for the anti-influenza activity of 1 using bakuchiol derivatives with various deletions and found that all functional groups and C6 chirality of bakuchiol were required to express its anti-influenza activity (Fig. 1D). Based on these results, we synthesized a molecular probe of the biotin-linker bound to the C1 position of 1 (Fig. 2A) and subsequently performed a pulldown assay and nanoLC-MS/MS analysis. We identified PHB2, VDAC1, and VDAC2 as the binding proteins of 1 from lysates of MDCK cells (Fig. 2D) and confirmed the binding of 1 to PHBs or VDAC2 in vitro (Fig. 3) and in MDCK, A549, and HeLa cells (Fig. 4). In addition, we indicated that the additive effects of PHBs or VDAC2 protein reductions and 1 treatment suppressed influenza viral growth more effectively than each treatment alone in MDCK cells (Fig. 5).
Chemical structures responsible for the anti-influenza activity of (+)-(S)-bakuchiol
(+)-(S)-bakuchiol (1), (4-(3-ethenyl-3,7-dimethyl-1,6-octadienyl)-phenol), is structurally a phenol unit conjugated with terpene and has a partly phenolic and terpene nature. This chemical structure consists of a single hydroxyl group on the aromatic ring, an unsaturated hydrocarbon chain with three olefinic linkages and one tetra-alkylated (all-carbon) quaternary stereocenter (29). Krishna et al. reviewed that the biological activity of 1 significantly depends on its chemical structure, such as the phenolic hydroxyl group, vinyl groups, stereoisomeric, and terpenic chains as described in previous studies (29). Chemical modification of the phenolic hydroxyl group, phenolic C–H bonds, vinyl, and isopropylidene groups of 1 is reported to enhances its antiproliferative activity (40, 41, 42, 43) or anti-microbial activity (20, 40), suggesting that these structures are important in the interactions between 1 and its target proteins. The phenolic hydroxyl group and terpenoid of 1 are also considered to be strongly related to its antioxidant activity (21, 23, 24). We found that bakuchiol derivatives with various deletions of the phenol group and/or tetraalkyl quaternary carbon in 1 (3–12) and the O-methylated bakuchiols (13 and 14) strongly reduced the survival of virus-infected cells (Fig. 1, B and C, right panels), indicating that all functional groups and the C6 chirality of 1 were required to express the anti-influenza activity. The results of structure-activity relationship indicated that three points are important for the binding of bakuchiol to the target protein: (1) hydrogen bonding, (2) hydrophobic interactions, and (3) stereo-recognition. Considering that cyclobakuchiol A demonstrated activity comparable to that of 1 (28), it is suggested that bakuchiol binds to the target protein in a bent conformation from the 3-position. Concerning the hydrophobic binding site, a decrease in activity was observed for the stereoisomers, cyclobakuchiols B–D (28), suggesting that the steric conformation of 1 is recognized to some extent. Above all, the phenolic hydroxy group of 1 was considered the most important functional group. Our results on the structure-activity relationship of the anti-influenza activity of 1 match those of previous studies on its other biological activities.
Development of a novel molecular probe for target proteins of (+)-(S)-bakuchiol using the biotin-conjugated eight PEGs linker
Based on the results described in Figure 1, to isolate more target proteins of 1 while retaining its anti-influenza activity, we bound the biotin-linker to the C1 position of 1, which is the farthest position from the phenolic hydroxy group, through the peptide bond (Fig. 2A, bottom). Our molecular probes of 1 (17) were used to isolate specific proteins from lysates of MDCK cells infected without/with A/PR/8/34 virus (Fig. 2, B and C) as compared with those of the control probes and to identify the target proteins of 1 as PHBs and VDAC2, which has never been reported previously (Fig. 2D). 17 can isolate the target proteins of 1 from the lysates of MDCK cells (Fig. 3A) as well as human cells such as A549 (Fig. 3B), HeLa (Fig. 3C), and 293T (Fig. 3D) cells; it can also reveal the intracellular distribution of 1 in confocal fluorescence microscopy (Fig. 4). The biological and pharmacological effects of 1 have been shown in various bacterial strains (18, 19, 20) and cell types, including mouse epidermal cells (15), microglia (16), myoblasts (44), macrophages (17); rat chondrocytes (31); canine kidney cells (27, 28); and human keratinocyte (15), hepatoma (45), lung adenocarcinoma (13), colorectal carcinoma (14) and cervical adenocarcinoma (46) cells. Many of these reports have not shown the target factors and intracellular distribution of 1. Our novel molecular probes for 1 will thus be very useful to identify the target proteins of 1 and elucidate the mechanisms of its effects.
The roles of PHBs and VDAC2 as target proteins of (+)-(S)-bakuchiol on the oxidative stress response in mitochondria
PHB2 localizes in the inner mitochondrial membrane by forming heterodimeric complexes with PHB1. PHBs interact with various host cell factors, such as mitochondrial proteins, transcription factors, nuclear factors, and signaling proteins, resulting in their involvement in many cell physiologies (47). Above all, PHBs have various functions within mitochondria, including mitochondrial structural stability and dynamics (36). In mitochondrial structural stability, PHBs maintain the cristae structure and mitochondrial integrity to provide a place for supplying ATP. They can also stabilize and promote ATP production (48). In mitochondrial dynamics, PHBs regulate molecules related to mitochondrial division and fusion, such as optic atrophy 1 and dynamin-related protein 1 (48). Downregulation of PHBs also impairs the formation of mitochondrial respiratory super complexes and increases the generation of ROS in mitochondria, resulting in the activation of mitochondrial flash, which is the dynamic activity of mitochondria associated with the production of large amounts of metabolites and free radicals to supply energy to cells (49).
VDAC is localized to the OMM and has three isoforms, VDAC1, VDAC2, and VDAC3, in mammalian cells, which regulate the entry and exit of mitochondrial metabolites between mitochondria and other cellular compartments (37). Although VDACs are ubiquitously expressed in mammalian cells, they are not equally abundant, with VDAC1 being 10 and 100 times more abundant than VDAC2 and VDAC3, respectively (50). VDACs regulate the exchange of ATP, ADP, phosphate, and Ca2+ between the mitochondria and cytoplasm. Although mitochondrial function and ATP production are strongly dependent on mitochondrial Ca2+ concentrations (51), VDACs play important roles in regulating mitochondrial Ca2+ homeostasis to transport ATP and Ca2+ through the OMM (52, 53). Feno et al. reviewed the crosstalk between Ca2+ and ROS in various disorders and found that the overload of Ca2+ uptake into the mitochondria through VDACs promotes mitochondrial ROS production in pathological conditions (54). Thus, VDACs are a molecular link between Ca2+ and ROS in the mitochondria. Therefore, PHBs and VDAC2 are strongly involved in oxidative stress via ROS production.
(+)-(S)-bakuchiol (1) inhibits oxidative stress by increasing ROS production, prevents mitochondrial lipid peroxidation, and protects other enzymes from oxidative stress (21, 23, 24). 1 activates the Nrf2 signaling pathway and the antioxidant response to protect against oxidative stress by ROS production (25, 26, 27). In this study, we identified PHB2, VDAC1, and VDAC2 as new target proteins of 1 from MDCK cell lysates using 17-conjugated streptavidin beads (Fig. 2, B–D) and confirmed the binding 1 to PHB1, PHB2, and VDAC2 in lysates of MDCK cells (Fig. 3A), A549 (Fig. 3B), or HeLa (Fig. 3C) cells. We also demonstrated that 1 interacted with PHB1 and PHB2 in vitro using overexpression (Fig. 3, D and E) or with PHB1, PHB2, and VDAC2 in cells using immunofluorescence staining (Fig. 4, A–C). We further found that 1 was localized in the mitochondria (Fig. 4D). Taken together, 1 may activate the Nrf2 signaling pathway via oxidative stress of ROS production by targeting PHBs and VDAC2 in the mitochondria.
The roles of PHBs and VDAC2 as target proteins of (+)-(S)-bakuchiol in its anti-influenza activity
PHBs and VDACs in mitochondria or other compartments are associated with the infection and growth of various human viruses, including human immunodeficiency virus type 1 (55, 56), human hepatitis C virus (57, 58), dengue virus (59, 60), severe acute respiratory syndrome coronavirus (61, 62), and influenza virus (38, 39, 63). In influenza A virus, Liu et al. reported that the expression of PHB protein increases in A549 cells infected with avian influenza A H5N1 virus and that downregulation of PHB by its specific siRNA reduces viral titers in the culture supernatants of virus-infected A549 cells (38). Watanabe et al. reported that siRNA-mediated downregulation of PHB causes influenza A H1N1 viral NP accumulation in the nucleus of virus-infected HEK293 cells, and downregulation of PHBs or VDACs reduces viral titers in the culture supernatants of virus-infected cells (39). Thus, PHBs and VDACs may be attractive drug targets for controlling influenza virus infections. We confirmed that the DsiRNA-mediated downregulation of PHBs or VDAC2 reduced the influenza NP expression levels (Fig. 5, A, B, D, and E) and viral titers in conditioned media (Fig. 5, C and F) of virus-infected MDCK cells. We also found that both downregulation of PHBs and VDAC2 and treatment with 1, reduced the influenza NP expression levels (Fig. 5, A, B, D, and E) and viral titers in conditioned media (Fig. 5, C and F) compared to the observations made when each treatment was used alone. Bakuchiol binds to PHBs and VDAC2 proteins, which are believed to activate mitochondria under stress conditions (Figs. S3 and S4, panel B). This action potentially leads to the release of ROS, activation of the Nrf2 pathway, and the exertion of anti-influenza activity as observed in a previous study (27) and this study. Reducing PHBs or VDAC2 proteins through DsiRNA transfection can induce mitochondria into stressed conditions and subsequently releases ROS (64). This process activates the Nrf2 pathway and exerts anti-influenza activity (Figs. S3 and S4, panel C). Bakuchiol treatment and PHB2 or VDAC2 DsiRNA transfection induced mitochondria to adapt to more stressful conditions. Consequently, this leads to an increased release of ROS, activation of the Nrf2 pathway, and a more robust anti-influenza activity (Figs. S3 and S4, panel D). Therefore, these results indicate that the combined effects of PHBs or VDAC2 protein reduction and treatment with 1 suppress influenza viral growth. This suggests that 1 may exhibit anti-influenza activity by targeting both PHBs and VDAC2 proteins. However, further studies are needed to elucidate the roles of PHBs and VDAC2 as target proteins of 1 in its anti-influenza activity.
Conclusion
Based on the findings of this study and our previous report, as described in Figure 6, bakuchiol targets PHBs/VDAC2 and activates the Nrf2 signaling pathway, resulting in anti-influenza activity. In conclusion, the findings from this study demonstrate that bakuchiol binds to PHBs/VDAC2 in vitro and in cells. Furthermore, the additive effects of reducing PHBs or VDAC2 protein alongside bakuchiol treatment can suppress influenza viral growth. Our findings provide new insights into the development of antiviral agents and will contribute to elucidate the mechanisms of various other biological and pharmacological effects exerted by bakuchiol.
Figure 6.
Schematic showing the mechanisms underlying the anti-influenza virus activity of bakuchiol by targeting PHBs and VDAC2. This scheme includes the results and conclusions of the present study. Information of the tertiary structures of PHB2 (PDB ID: 6IQE) and VDAC2 (PDB ID: 4BUM) were obtained from a protein data bank. The tertiary structures of PHBs dimer and VDAC2 were visualized by PyMOL (https://pymol.org/2/) molecular graphics system (Schrödinger, LLC.). VDAC2 is pore-like structure and localized in the outer-membrane of mitochondria. PHBs are α-helical dimer and mainly localized in the inner membrane there. PHB, prohibitin; VDAC, voltage-dependent anion channel.
Experimental procedures
Preparation of (+)-(S)-bakuchiol and (−)-(R)-bakuchiol
(+)-(S)-Bakuchiol (1) and (−)-(R)-bakuchiol (2) were chemically synthesized from (E)-geranic acid and purified using previously reported methods (12). Their chemical structures are shown in Figure 1A. Stock solutions (10 mM) were prepared by dissolving these compounds in DMSO.
Preparation of bakuchiol derivatives, compounds 1 to 10, and (+/−)-(S/R)-O-methylbakuchiols
The chemical structures of bakuchiol derivatives, compounds 1 to 10 (3–12), (+)-(S)-O-methylbakuchiol (13), and (−)-(R)-O-methylbakuchiol (14) are shown in Figure 1A.
We have described the full details of the synthesis of bakuchiol derivatives with various deletions of the phenol group and/or tetraalkyl quaternary carbon in 1, compounds 1 to 10 (3–12) in the Supporting information.
(+)-(S)-O-methylbakuchiol (13) and (−)-(R)-O-methylbakuchiol (14) were synthesized according to our previous report (12).
Stock solutions (10 mM) were prepared by dissolving the bakuchiol derivatives 3 to 14 in DMSO.
Cells
MDCK, A549, HeLa, and 293T cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Wako) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 100 units/ml penicillin, 100 μg/ml streptomycin (P/S; Thermo Fisher Scientific), and 4 mM l-glutamine at 37 °C with 5% CO2.
Strain of influenza A virus
The A/PR/8/34 virus strain provided by Takahashi and Kido (65) was used for the experiments. Viral titers were determined by immunostaining for influenza NP, as previously described (27, 66, 67).
Analysis of the effects of influenza A virus on MDCK viability using naphthol blue black
MDCK cells were seeded in a 96-well plate (1 × 104 cells/well). Bakuchiols and their derivatives (1–14) (0.8–25 μM) were mixed with A/PR/8/34 virus in 10% fetal bovine serum–supplemented growth medium at a multiplicity of infection (MOI) of 10. DMSO (0.008–0.25%) or 1 and 2 (0.8–25 μM) (27) were used as negative or positive controls, respectively. The resulting mixture was added to the cells after incubation for 30 min at 37 °C with 5% CO2 and then for 72 h at 37 °C with 5% CO2. After incubation, the cells were stained with naphthol blue-black, as previously described (27, 67, 68). Viable cells in each well stained blue, whereas dead cells remained unstained.
Preparation of biotin-linker-(+)-(S)-bakuchiol
The chemical structures of biotin-linker molecular probes 15, 16, and 17 were shown in Figure 2, A. Full details of the synthesis of the three probes are described in the Supporting information. Stock solutions (10 mM) were prepared by dissolving 15, 16, and 17 in DMSO.
Binding of biotin-linker compounds to streptavidin magnetic beads
Five microliters of 10 mM 15, 16, or 17 was rotated with 195 μl of 1 mg streptavidin-magnetic Ferrite poly Glycidyl methacrylate beads (Tamagawa Seiki) in PBS (total 250 μM compounds in 200 μl) for 1 h at 4 °C using a microtube mixer. Each biotin-like compound was conjugated with streptavidin-magnetic Ferrite poly Glycidyl methacrylate beads via biotin–streptavidin interaction. The biotin-linker compound-conjugated streptavidin beads were washed thrice with wash buffer (10 mM Hepes-NaOH [pH 7.9], 50 mM KCl, 1 mM EDTA, and 10% glycerol) and then suspended in 200 μl of cell lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 1 mM EDTA, and complete ULTRA Tablets, Mini, EDTA-free, protease inhibitor cocktail [Roche]).
Pulldown assay
MDCK, A549, or HeLa cells, seeded in a 6-well plate (1 × 106 cells/well), were infected with the A/PR/8/34 virus at five MOI in the infection medium. This medium comprised high-glucose DMEM (WAKO), supplemented with 1% globulin-free bovine serum albumin (BSA) (WAKO), P/S (Thermo Fisher Scientific), and 4 mM l-glutamine. The cells were incubated at 37 °C with 5% CO2. After incubation for 24 h, the cells were lysed in cell lysis buffer. The cell lysates without/with virus were centrifuged at 15,000 rpm at 4 °C for 10 min, and the supernatant was collected. Then, 100 μl of the biotin-linker compound-conjugated streptavidin beads were rotated with 400 μl of cell lysates with/without the virus (final: approximately 25 μM compounds in 0.1 mg beads) at 4 °C overnight using a microtube mixer. After collecting the supernatants, the proteins bound to the beads were washed thrice with cell lysis buffer and suspended in 100 μl of cell lysis buffer.
Silver staining
Proteins bound to the beads in the pulldown assay were added to a SDS sample buffer (125 mM Tris–HCl [pH 6.8], 5% SDS, 25% glycerol, 0.1% bromophenol blue, and 10% β-mercaptoethanol), boiled at 95 °C for 5 min, and then separated using SDS-PAGE. The gel was stained using the Silver Stain MS Kit (WAKO) according to the manufacturer’s instructions.
Identification of (+)-(S)-bakuchiol–binding protein using nanoLC-MS/MS analysis
To identify (+)-(S)-bakuchiol–binding proteins, proteins bound to the beads in the pulldown assay were subjected to nanoLC-MS/MS analysis as described previously (69). Protein bands were excised from the silver-stained gel of proteins bound to the beads in the pulldown assay, and the gel pieces cut into cubes were transferred to a 1.5 ml tube. Gel pieces were incubated in a fixing solution containing 50% methanol and 5% acetic acid for 10 min and then dehydrated with acetonitrile. Proteins in the gel pieces were reduced and alkylated with 10 mM DTT and 50 mM iodoacetamide and were digested overnight with trypsin at 37 °C. Tryptic peptides were extracted from gel pieces using 30% acetonitrile containing 3% trifluoroacetic acid. After drying, the peptides were resuspended in 1% trifluoroacetic acid and purified using SDB-XC StageTip (GL Sciences) according to previous reports (70, 71). The peptides were injected into a nanoLC-MS/MS system with a TripleTOF 5600 (Sciex) equipped with a Dionex Ultimate 3000 RSLCnano (Thermo Fisher Scientific). The injection volume was 5 μl, and the flow rate was 300 nl/min. Data were acquired in the data-dependent acquisition mode and analyzed using ProteinPilot 4.5 (https://sciex.com/products/software/proteinpilot-software) (Sciex) using the UniProt dog reference proteome database. The peptide identification confidence for the dataset was evaluated based on the false discovery rate. The number of peptides identified in each protein was counted.
Western blot
Protein samples were added to SDS sample buffer, boiled for 5 min, and separated using SDS-PAGE. The proteins were transferred to polyvinylidene fluoride microporous membranes (Millipore). Respective proteins were detected using primary and secondary antibodies, as described in Table S1. The signals were detected using the Immobilon Western Chemiluminescent Horse Radish Peroxidase Substrate (Millipore). β-actin expression was used as an internal control. Signal intensities were measured using ImageJ software (https://imagej.net/ij/), and the indicated protein levels were normalized to those of β-actin.
Overexpression of PHB1- or PHB2-FLAG proteins for detecting the binding to (+)-(S)-bakuchiol
The expression plasmid of PHB1 (NM_002634, restriction site: SgfI-MluI) harboring a FLAG tag at its C terminus, which was driven by a CMV promoter (pCMV-PHB1-FLAG), was purchased from Origene Inc. (#RC201229). PHB2 cDNA was amplified by PCR using the primer set forward:5′-TTTGCGATCGCCATGGCCCAGAACTTGAAG-3′ (underline, SgfI restriction site as the forward primer) and reverse:5′- TTTACGCGTTTTCTTACCCTTGATGAGGCTG-3′ (underline, MluI restriction site as the reverse primer), and PrimeSTAR HS DNA polymerase (TaKaRa). The template for this PCR was the product of reverse transcription of total RNA from HeLa cells using SuperScript IV Reverse Transcriptase according to the manufacturer's instructions. pCMV-PHB1-FLAG was digested with SgfI and MluI to excise PHB1, and the SgfI-MluI digested PCR product of PHB2 cDNA was ligated using T4 ligase (Promega), resulting in the construction of the pCMV-PHB2-FLAG plasmid. SgfI and MluI sites in the PHB1-excised pCMV-PHB1-FLAG were blunted with a Quick Blunting Kit (New England Biolabs), and this plasmid was ligated using T4 ligase (Promega), resulting in construction of the pCMV-FLAG plasmid (pCMV-empty). The pCMV-empty plasmid was used as a NC.
The plasmids pCMV-empty, pCMV-PHB1-FLAG, or pCMV-PHB2-FLAG (10 μg each) were transfected into 293T cells grown to 70 to 90% confluency in 10 cm cell culture dishes in the presence of Lipofectamine 2000 reagent (Thermo Fisher Scientific), according to the manufacturer's instructions. Transfected cells were incubated at 37 °C with 5% CO2 for 24 h and then lysed in a cell lysis buffer. The cell lysates were centrifuged at 15,000 rpm at 4 °C for 10 min, and the supernatant was collected. Each supernatant in the transfectant was used for the pulldown assay of biotin-linker compound-conjugated streptavidin beads, as mentioned above. The supernatants and bead samples were analyzed by WB.
Immunofluorescence staining for detecting intracellular interactions with (+)-(S)-bakuchiol and PHBs or VDAC2 proteins
MDCK, A549, or HeLa cells were seeded on 35 mm glass bottom dishes with 14 mm microwells (5 × 103 cells/well). After incubation at 37 °C with 5% CO2 for 24 h, the cells were fixed with 4% paraformaldehyde in PBS for 30 min at 4 °C and subsequently permeabilized by adding 0.3% Triton X-100 for 20 min at 25 °C. The cells were blocked for the endogenous biotin using Endogenous Biotin-Blocking Kit (Thermo Fisher Scientific) according to the manufacturer's instructions and then treated with 25 μM of 15, 16, or 17 in 1% BSA/PBS-Tween 20 (1% BSA/PBS-T) at 4 °C for 24 h. After washing with PBS-T, primary antibodies were used to detect PHB1, PHB2, VDAC2, or ATP5B proteins, as described in Table S1. Alexa Fluor 488–conjugated streptavidin or Alexa Fluor 546–conjugated antibodies were used as secondary antibodies. Cell nuclei were stained with diamidino-2-phenylindole (Thermo Fisher Scientific). Images were captured using a confocal laser-scanning fluorescence microscope (Nikon A1, Nikon instruments).
Assessment of anti-influenza activity of (+)-(S)-bakuchiol in PHB2 or VDAC2 DsiRNA-treated MDCK cells by analysis of influenza NP expression levels or influenza viral growth assay
Analysis of influenza NP expression levels
MDCK cells (1 × 105 cells/well) were transfected with 10 nM human PHB2 DsiRNA (Design ID: hs.Ri.PHB2.13.2; integrated DNA technologies [IDT]) or 30 nM human VDAC2#1, 2, or 3 DsiRNA (Design ID: #1, hs.Ri.VDAC2.13.1; #2, hs.Ri.VDAC2.13.2; #3, hs.Ri.VDAC2.13.3, IDT) in a 24-well plate using Lipofectamine RNAiMAX reagent (Thermo Fisher Scientific) through reverse transfection following the manufacturer's instructions. The cells were then incubated at 37 °C with 5% CO2 for 48 h. Further, 10 or 30 nM NC DsiRNA (#51-01-14-03, IDT) was used as a NC. The DsiRNA-transfected cells were incubated with 12.5 μM 1 and 0.1 MOI of A/PR/8/34 virus in the infection medium at 37 °C with 5% CO2 for 24 h. Additionally, 0.125% DMSO was used as a NC. The WB analysis was performed as described previously, and the expression levels of influenza NP were analyzed.
Influenza viral growth assay
MDCK cells (1 × 105 cells/well) were transfected with 10 nM human PHB2 DsiRNA (hs.Ri.PHB2.13.2; IDT) or 30 nM human VDAC2#1, 2, or 3 DsiRNA (#1, hs.Ri.VDAC2.13.1; #2, hs.Ri.VDAC2.13.2; #3, hs.Ri.VDAC2.13.3; IDT) in a 24-well plate via reverse transfection, following the previously described procedure, and were incubated at 37 °C with 5% CO2 for 24 h. Further, 10 or 30 nM NC DsiRNA (#51-01-14-03; IDT) was used as a NC. The DsiRNA-transfected cells were infected with 0.0001 MOI of A/PR/8/34 virus in the infection medium at 37 °C with 5% CO2 for 1 h. The infected cells were washed with DMEM, and the infection medium containing 12.5 μM 1, supplemented with 3 μg/ml L-tosylamido-2-phenyl ethyl chloromethyl ketone-treated trypsin (Sigma-Aldrich) was added to the infected cells. DMSO (0.125%) was added to the cells in the infection medium and was used as the NC. The cells were incubated for 24, 48, or 72 h at 37 °C under 5% CO2. At the specified time points, culture-conditioned media were collected from each well, and the viral titers in the conditioned media were determined using immunostaining for influenza NP, as previously described (27, 66, 67).
Statistical analysis
All results are presented as the mean ± SEM. The statistical significance between two groups was analyzed using Student’s t test, while comparisons involving more than two groups were analyzed through one-way ANOVA, followed by the post hoc Tukey’s test. Results were considered statistically significant at p < 0.05.
Data availability
All data generated or analyzed during this study are included in this article (and its Supplementary information files).
Supporting information
This article contains Supporting information (27, 64, 72, 73).
Conflict of interest
The authors declare that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Author contributions
M. S. and T. K. conceptualization; M. S. and T. K. project administration; M. S., T. E., T. M., N. T., R. P., H. S., M. W., and S. O. investigation; M. S. and T. K. visualization; M. S. data curation; M. S. and T. K. writing-original draft; M. S. funding acquisition; T. E., M. W., E. T., and H. K. resources; T. M. and S. O. formal analysis; M. S., T. E., T. M., N. T., R. O., H. S., M. W., E. T., H. K., S. O., and T. K. writing-reviewing and editing; T. K. supervision.
Funding and additional information
This work was supported by a Grant from Japan Society for the Promotion of Science (JSPS) KAKENHI (18K15173) (to M. S.). This work was also supported in part by a Grant from Tokushima Bunri University for Educational Reform and Collaborative Research (No. TBU2020-2-1 and TBU2022-2-2), and JSPS KAKENHI (20K07457) (to M. S.). This work was also supported by a grant from Tokushima Bunri University. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reviewed by members of the JBC Editorial Board. Edited by Craig Cameron
Contributor Information
Masaki Shoji, Email: masaki-shoji@ph.bunri-u.ac.jp.
Takashi Kuzuhara, Email: kuzuhara@ph.bunri-u.ac.jp.
Supporting information
References
- 1.Fiore A.E., Fry A., Shay D., Gubareva L., Bresee J.S., Uyeki T.M., et al. Antiviral agents for the treatment and chemoprophylaxis of influenza --- recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm. Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 2.Hayden F.G., Sugaya N., Hirotsu N., Lee N., de Jong M.D., Hurt A.C., et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N. Engl. J. Med. 2018;379:913–923. doi: 10.1056/NEJMoa1716197. [DOI] [PubMed] [Google Scholar]
- 3.de Jong M.D., Tran T.T., Truong H.K., Vo M.H., Smith G.J., Nguyen V.C., et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N. Engl. J. Med. 2005;353:2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 4.Le Q.M., Kiso M., Someya K., Sakai Y.T., Nguyen T.H., Nguyen K.H., et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 5.Hatakeyama S., Sugaya N., Ito M., Yamazaki M., Ichikawa M., Kimura K., et al. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA. 2007;297:1435–1442. doi: 10.1001/jama.297.13.1435. [DOI] [PubMed] [Google Scholar]
- 6.Hurt A.C., Ho H.T., Barr I. Resistance to anti-influenza drugs: adamantanes and neuraminidase inhibitors. Expert Rev. Anti Infect. Ther. 2006;4:795–805. doi: 10.1586/14787210.4.5.795. [DOI] [PubMed] [Google Scholar]
- 7.Poland G.A., Jacobson R.M., Ovsyannikova I.G. Influenza virus resistance to antiviral agents: a plea for rational use. Clin. Infect. Dis. 2009;48:1254–1256. doi: 10.1086/598989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takashita E., Ichikawa M., Morita H., Ogawa R., Fujisaki S., Shirakura M., et al. Human-to-Human transmission of influenza A(H3N2) virus with reduced susceptibility to Baloxavir, Japan, February 2019. Emerg. Infect. Dis. 2019;25:2108–2111. doi: 10.3201/eid2511.190757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Checkmahomed L., M'Hamdi Z., Carbonneau J., Venable M.C., Baz M., Abed Y., et al. Impact of the Baloxavir-resistant polymerase acid I38T substitution on the fitness of contemporary influenza A(H1N1)pdm09 and A(H3N2) strains. J. Infect. Dis. 2020;221:63–70. doi: 10.1093/infdis/jiz418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta G., Nayak U.R., Dev S. Bakuchiol, a novel monoterpenoid. Tetrahedron Lett. 1966;7:4561–4567. [Google Scholar]
- 11.Mehta G., Nayak U.R., Dev S. Meroterpenoids—I Psoralea corylifolia Linn.—1. Bakuchiol, a novel monoterpene phenol. Tetrahedron. 1973;29:1119–1125. [Google Scholar]
- 12.Esumi T., Yamamoto C., Fukuyama Y. A short synthesis of (+)-Bakuchiol. Synlett. 2013;24:1845–1847. [Google Scholar]
- 13.Chen Z., Jin K., Gao L., Lou G., Jin Y., Yu Y., et al. Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line. Eur. J. Pharmacol. 2010;643:170–179. doi: 10.1016/j.ejphar.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 14.Park M.H., Kim J.H., Chung Y.H., Lee S.H. Bakuchiol sensitizes cancer cells to TRAIL through ROS- and JNK-mediated upregulation of death receptors and downregulation of survival proteins. Biochem. Biophys. Res. Commun. 2016;473:586–592. doi: 10.1016/j.bbrc.2016.03.127. [DOI] [PubMed] [Google Scholar]
- 15.Kim J.E., Kim J.H., Lee Y., Yang H., Heo Y.S., Bode A.M., et al. Bakuchiol suppresses proliferation of skin cancer cells by directly targeting Hck, Blk, and p38 MAP kinase. Oncotarget. 2016;7:14616–14627. doi: 10.18632/oncotarget.7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim H.S., Kim Y.J., Kim B.Y., Jeong S.J. Bakuchiol suppresses inflammatory responses via the downregulation of the p38 MAPK/ERK signaling pathway. Int. J. Mol. Sci. 2019;20:3574. doi: 10.3390/ijms20143574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q., Bian M., Gong G., Bai C., Liu C., Wei C., et al. Synthesis and evaluation of bakuchiol derivatives as potent anti-inflammatory agents in Vitro and in Vivo. J. Nat. Prod. 2022;85:15–24. doi: 10.1021/acs.jnatprod.1c00377. [DOI] [PubMed] [Google Scholar]
- 18.Katsura H., Tsukiyama R.I., Suzuki A., Kobayashi M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001;45:3009–3013. doi: 10.1128/AAC.45.11.3009-3013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain F.M., Ahmad I., Khan F.I., Al-Shabib N.A., Baig M.H., Hussain A., et al. Seed extract of Psoralea corylifolia and its constituent bakuchiol impairs AHL-based quorum sensing and biofilm formation in food- and human-related pathogens. Front. Cell Infect. Microbiol. 2018;8:351. doi: 10.3389/fcimb.2018.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Liu J., Liu C.F., Li H., Luo J., Fang S., et al. Design, synthesis, and biological evaluation of membrane-active bakuchiol derivatives as effective broad-spectrum antibacterial agents. J. Med. Chem. 2021;64:5603–5619. doi: 10.1021/acs.jmedchem.0c02059. [DOI] [PubMed] [Google Scholar]
- 21.Haraguchi H., Inoue J., Tamura Y., Mizutani K. Inhibition of mitochondrial lipid peroxidation by Bakuchiol, a meroterpene from Psoralea corylifolia. Planta Med. 2000;66:569–571. doi: 10.1055/s-2000-8605. [DOI] [PubMed] [Google Scholar]
- 22.Adhikari S., Joshi R., Patro B.S., Ghanty T.K., Chintalwar G.J., Sharma A., et al. Antioxidant activity of bakuchiol: experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain. Chem. Res. Toxicol. 2003;16:1062–1069. doi: 10.1021/tx034082r. [DOI] [PubMed] [Google Scholar]
- 23.Kim K.A., Shim S.H., Ahn H.R., Jung S.H. Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage. Toxicol. Appl. Pharmacol. 2013;269:109–120. doi: 10.1016/j.taap.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Liu H., Guo W., Guo H., Zhao L., Yue L., Li X., et al. Bakuchiol attenuates oxidative stress and neuron damage by regulating Trx1/TXNIP and the phosphorylation of AMPK after subarachnoid hemorrhage in mice. Front. Pharmacol. 2020;11:712. doi: 10.3389/fphar.2020.00712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma W., Guo W., Shang F., Li Y., Li W., Liu J., et al. Bakuchiol alleviates hyperglycemia-induced diabetic cardiomyopathy by reducing myocardial oxidative stress via activating the SIRT1/nrf2 signaling pathway. Oxid. Med. Cell Longev. 2020;2020 doi: 10.1155/2020/3732718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y., Gao X., Wang L., Yang M., Xie R. Bakuchiol ameliorates cerebral ischemia-reperfusion injury by modulating NLRP3 inflammasome and Nrf2 signaling. Respir. Physiol. Neurobiol. 2021;292 doi: 10.1016/j.resp.2021.103707. [DOI] [PubMed] [Google Scholar]
- 27.Shoji M., Arakaki Y., Esumi T., Kohnomi S., Yamamoto C., Suzuki Y., et al. Bakuchiol is a phenolic isoprenoid with novel enantiomer-selective anti-influenza A virus activity involving Nrf2 activation. J. Biol. Chem. 2015;290:28001–28017. doi: 10.1074/jbc.M115.669465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoji M., Esumi T., Tanaka N., Takeuchi M., Yamaji S., Watanabe M., et al. Organic synthesis and anti-influenza A virus activity of cyclobakuchiols A, B, C, and D. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna T.P.A., Edachery B., Athalathil S. Correction: bakuchiol - a natural meroterpenoid: structure, isolation, synthesis and functionalization approaches. RSC Adv. 2022;12 doi: 10.1039/d1ra08771a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng M., Chen Z. Screening of tyrosinase inhibitors by capillary electrophoresis with immobilized enzyme microreactor and molecular docking. Electrophoresis. 2017;38:486–493. doi: 10.1002/elps.201600367. [DOI] [PubMed] [Google Scholar]
- 31.Xu K., Sha Y., Wang S., Chi Q., Liu Y., Wang C., et al. Effects of Bakuchiol on chondrocyte proliferation via the PI3K-Akt and ERK1/2 pathways mediated by the estrogen receptor for promotion of the regeneration of knee articular cartilage defects. Cell Prolif. 2019;52 doi: 10.1111/cpr.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devi A., Namsa N.D., Doley R. In silico and in vitro neutralization of PLA(2) activity of Daboxin P by butein, mimosine and bakuchiol. Int. J. Biol. Macromol. 2020;165:1066–1078. doi: 10.1016/j.ijbiomac.2020.09.223. [DOI] [PubMed] [Google Scholar]
- 33.Long Lv B.L. Anti-tumor effects of bakuchiol on human gastric carcinoma cell lines are mediated through PI3K/AKT and MAPK signaling pathways. Mol. Med. Rep. 2017;16:8977–8982. doi: 10.3892/mmr.2017.7696. [DOI] [PubMed] [Google Scholar]
- 34.Sato S., Kwon Y., Kamisuki S., Srivastava N., Mao Q., Kawazoe Y., et al. Polyproline-rod approach to isolating protein targets of bioactive small molecules: isolation of a new target of indomethacin. J. Am. Chem. Soc. 2007;129:873–880. doi: 10.1021/ja0655643. [DOI] [PubMed] [Google Scholar]
- 35.Merkwirth C., Langer T. Prohibitin function within mitochondria: essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta. 2009;1793:27–32. doi: 10.1016/j.bbamcr.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Oyang L., Li J., Jiang X., Lin J., Xia L., Yang L., et al. The function of prohibitins in mitochondria and the clinical potentials. Cancer Cell Int. 2022;22:343. doi: 10.1186/s12935-022-02765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoshan-Barmatz V., De Pinto V., Zweckstetter M., Raviv Z., Keinan N., Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Aspects Med. 2010;31:227–285. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Liu C., Zhang A., Guo J., Yang J., Zhou H., Chen H., et al. Identification of human host proteins contributing to H5N1 influenza virus propagation by membrane proteomics. J. Proteome Res. 2012;11:5396–5405. doi: 10.1021/pr3006342. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe T., Kawakami E., Shoemaker J.E., Lopes T.J., Matsuoka Y., Tomita Y., et al. Influenza virus-host interactome screen as a platform for antiviral drug development. Cell Host Microbe. 2014;16:795–805. doi: 10.1016/j.chom.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majeed R., Reddy M.V., Chinthakindi P.K., Sangwan P.L., Hamid A., Chashoo G., et al. Bakuchiol derivatives as novel and potent cytotoxic agents: a report. Eur. J. Med. Chem. 2012;49:55–67. doi: 10.1016/j.ejmech.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 41.Gautam L.N., Ling T., Lang W., Rivas F. Anti-proliferative evaluation of monoterpene derivatives against leukemia. Eur. J. Med. Chem. 2016;113:75–80. doi: 10.1016/j.ejmech.2016.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Cha M.-R., Choi C.-W., Lee J.-Y., Kim Y.-S., Yon G.-H., Choi S.-U., et al. Anti-proliferative effect of synthesized bakuchiol analogues on cultured human tumor cell lines. Bull. Korean Chem. Soc. 2012;33:2378–2380. [Google Scholar]
- 43.Gupta N., Sharma S., Raina A., Dangroo N.A., Bhushan S., Sangwan P.L. Synthesis and anti-proliferative evaluation of novel 3,4-dihydro-2H-1,3-oxazine derivatives of bakuchiol. RSC Adv. 2016;6:106150–106159. [Google Scholar]
- 44.Lee S.J., Yoo M., Go G.Y., Kim D.H., Choi H., Leem Y.E., et al. Bakuchiol augments MyoD activation leading to enhanced myoblast differentiation. Chem. Biol. Interact. 2016;248:60–67. doi: 10.1016/j.cbi.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Guo Z., Li P., Wang C., Kang Q., Tu C., Jiang B., et al. Five constituents contributed to the psoraleae fructus-induced hepatotoxicity via mitochondrial dysfunction and apoptosis. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.682823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu C.Z., Hong S.S., Cai X.F., Dat N.T., Nan J.X., Hwang B.Y., et al. Hypoxia-inducible factor-1 and nuclear factor-kappaB inhibitory meroterpene analogues of bakuchiol, a constituent of the seeds of Psoralea corylifolia. Bioorg. Med. Chem. Lett. 2008;18:2619–2623. doi: 10.1016/j.bmcl.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 47.Thuaud F., Ribeiro N., Nebigil C.G., Desaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem. Biol. 2013;20:316–331. doi: 10.1016/j.chembiol.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshinaka T., Kosako H., Yoshizumi T., Furukawa R., Hirano Y., Kuge O., et al. Structural basis of mitochondrial scaffolds by prohibitin complexes: insight into a role of the coiled-coil region. iScience. 2019;19:1065–1078. doi: 10.1016/j.isci.2019.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jian C., Xu F., Hou T., Sun T., Li J., Cheng H., et al. Deficiency of PHB complex impairs respiratory supercomplex formation and activates mitochondrial flashes. J. Cell Sci. 2017;130:2620–2630. doi: 10.1242/jcs.198523. [DOI] [PubMed] [Google Scholar]
- 50.De Pinto V., Guarino F., Guarnera A., Messina A., Reina S., Tomasello F.M., et al. Characterization of human VDAC isoforms: a peculiar function for VDAC3? Biochim. Biophys. Acta. 2010;1797:1268–1275. doi: 10.1016/j.bbabio.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 51.Gunter T.E., Sheu S.S. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim. Biophys. Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shoshan-Barmatz V., Krelin Y., Shteinfer-Kuzmine A. VDAC1 functions in Ca(2+) homeostasis and cell life and death in health and disease. Cell Calcium. 2018;69:81–100. doi: 10.1016/j.ceca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Wagner S., De Bortoli S., Schwarzlander M., Szabo I. Regulation of mitochondrial calcium in plants versus animals. J. Exp. Bot. 2016;67:3809–3829. doi: 10.1093/jxb/erw100. [DOI] [PubMed] [Google Scholar]
- 54.Feno S., Butera G., Vecellio Reane D., Rizzuto R., Raffaello A. Crosstalk between calcium and ROS in pathophysiological conditions. Oxid. Med. Cell Longev. 2019;2019 doi: 10.1155/2019/9324018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Emerson V., Holtkotte D., Pfeiffer T., Wang I.H., Schnolzer M., Kempf T., et al. Identification of the cellular prohibitin 1/prohibitin 2 heterodimer as an interaction partner of the C-terminal cytoplasmic domain of the HIV-1 glycoprotein. J. Virol. 2010;84:1355–1365. doi: 10.1128/JVI.01641-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiao H., McMillan J.R. Gelsolin segment 5 inhibits HIV-induced T-cell apoptosis via Vpr-binding to VDAC. FEBS Lett. 2007;581:535–540. doi: 10.1016/j.febslet.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 57.Liu S., Wang W., Brown L.E., Qiu C., Lajkiewicz N., Zhao T., et al. A novel class of small molecule compounds that inhibit hepatitis C virus infection by targeting the prohibitin-CRaf pathway. EBioMedicine. 2015;2:1600–1606. doi: 10.1016/j.ebiom.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duponchel S., Monnier L., Molle J., Bendridi N., Alam M.R., Gaballah A., et al. Hepatitis C virus replication requires integrity of mitochondria-associated ER membranes. JHEP Rep. 2023;5 doi: 10.1016/j.jhepr.2022.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharma A., Vasanthapuram R., M M.V., Desai A. Prohibitin 1/2 mediates Dengue-3 entry into human neuroblastoma (SH-SY5Y) and microglia (CHME-3) cells. J. Biomed. Sci. 2020;27:55. doi: 10.1186/s12929-020-00639-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jitobaom K., Tongluan N., Smith D.R. Involvement of voltage-dependent anion channel (VDAC) in dengue infection. Sci. Rep. 2016;6 doi: 10.1038/srep35753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornillez-Ty C.T., Liao L., Yates J.R., 3rd, Kuhn P., Buchmeier M.J. Severe acute respiratory syndrome coronavirus nonstructural protein 2 interacts with a host protein complex involved in mitochondrial biogenesis and intracellular signaling. J. Virol. 2009;83:10314–10318. doi: 10.1128/JVI.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thompson E.A., Cascino K., Ordonez A.A., Zhou W., Vaghasia A., Hamacher-Brady A., et al. Metabolic programs define dysfunctional immune responses in severe COVID-19 patients. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2021.108863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zamarin D., Garcia-Sastre A., Xiao X., Wang R., Palese P. Influenza virus PB1-F2 protein induces cell death through mitochondrial ANT3 and VDAC1. PLoS Pathog. 2005;1:e4. doi: 10.1371/journal.ppat.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang H., Zhang S., Li Y., Liu Z., Mi L., Cai Y., et al. Suppression of mitochondrial ROS by prohibitin drives glioblastoma progression and therapeutic resistance. Nat. Commun. 2021;12:3720. doi: 10.1038/s41467-021-24108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mizuno D., Kimoto T., Sakai S., Takahashi E., Kim H., Kido H. Induction of systemic and mucosal immunity and maintenance of its memory against influenza A virus by nasal vaccination using a new mucosal adjuvant SF-10 derived from pulmonary surfactant in young cynomolgus monkeys. Vaccine. 2016;34:1881–1888. doi: 10.1016/j.vaccine.2016.02.061. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi E., Kataoka K., Indalao I.L., Konoha K., Fujii K., Chida J., et al. Oral clarithromycin enhances airway immunoglobulin A (IgA) immunity through induction of IgA class switching recombination and B-cell-activating factor of the tumor necrosis factor family molecule on mucosal dendritic cells in mice infected with influenza A virus. J. Virol. 2012;86:10924–10934. doi: 10.1128/JVI.01207-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoji M., Woo S.Y., Masuda A., Win N.N., Ngwe H., Takahashi E., et al. Anti-influenza virus activity of extracts from the stems of Jatropha multifida Linn. collected in Myanmar. BMC Complement. Altern. Med. 2017;17:96. doi: 10.1186/s12906-017-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shoji M., Takahashi E., Hatakeyama D., Iwai Y., Morita Y., Shirayama R., et al. Anti-influenza activity of c60 fullerene derivatives. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hatakeyama D., Shoji M., Yamayoshi S., Yoh R., Ohmi N., Takenaka S., et al. Influenza A virus nucleoprotein is acetylated by histone acetyltransferases PCAF and GCN5. J. Biol. Chem. 2018;293:7126–7138. doi: 10.1074/jbc.RA117.001683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rappsilber J., Ishihama Y., Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 2003;75:663–670. doi: 10.1021/ac026117i. [DOI] [PubMed] [Google Scholar]
- 71.Rappsilber J., Mann M., Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007;2:1896–1906. doi: 10.1038/nprot.2007.261. [DOI] [PubMed] [Google Scholar]
- 72.Salmela M., Sanmark H., Efimova E., Efimov A., Hytönen V.P., Lamminmäki U., et al. Molecular tools for selective recovery and detection of lignin-derived molecules. Green Chem. 2018;20:2829–2839. [Google Scholar]
- 73.Kasai S., Shimizu S., Tatara Y., Mimura J., Itoh K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;10:320. doi: 10.3390/biom10020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article (and its Supplementary information files).