Abstract
Background:
Keratoconus (KCN) is an ectatic disorder of the cornea characterized by stromal weakness and apical protrusion of the cornea, and is associated with a gradual and painless reduction in visual acuity. KCN in pediatric patients has certain important characteristics, such as a progressive and aggressive nature. We aimed to analyze the visual, refractive, and topographic outcomes of implanting a single 210° arc-length Keraring segment according to a novel, objective, Q value-based nomogram (Q-N) for the treatment of pediatric versus adult KCN.
Methods:
This prospective, multicenter, non-randomized, open-label trial included 47 eyes of 47 patients who were allocated to one of two groups. The adult group included 33 eyes of patients ≥ 18 years of age, whereas the pediatric group included 14 eyes of patients aged 14 – 17 years. All patients underwent femtosecond laser-assisted implantation of a single 210° arc-length Keraring segment according to the Q-N and were followed up for 6 months. All eyes underwent visual acuity measurement, cycloplegic refraction, and corneal topography at baseline and 6 months after surgery.
Results:
The study groups were comparable in terms of sex proportions and KCN grades (both P > 0.05). The adult group exhibited significant postoperative improvements in mean uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), sphere, cylinder, spherical equivalent (SE), and Kmax (all P < 0.001) with a mean change of -0.56 logarithm of the minimal angle of resolution (logMAR), - 0.40 logMAR, 3.07 diopters (D), 0.70 D, 3.42 D, and - 5.26 D, respectively. The pediatric group exhibited significant postoperative improvements in mean UDVA, CDVA, sphere, SE, and Kmax (all P < 0.05) with a mean change of - 0.62 logMAR, - 0.34 logMAR, 3.18 D, 3.67 D, and - 5.37 D, respectively. There were no significant differences between the groups in terms of the mean change in visual, refractive, and topographic variables (all P > 0.05). No postoperative complications were observed in either group.
Conclusions:
Use of the objective Q-N was efficient in the treatment of pediatric KCN, with postoperative improvements in the mean visual, refractive, and topographic parameters, comparable to outcomes in adult keratoconus. Q-N achieved good corneal remodeling with subsequent improvements in visual, refractive, and topographic outcomes in both adult and pediatric patients with keratoconus. To verify our preliminary findings, we recommend further multicenter randomized clinical trials using the Q-N nomogram in a larger sample of pediatric patients with KCN as an adjunct treatment before or after CXL.
Key Words: Keratoconus, pediatric patients, intracorneal ring segments, KeraRing, nomogram, Q value-based nomogram, corneal topographies, ocular refraction, visual acuities
INTRODUCTION
Keratoconus (KCN) is an ectatic disorder of the cornea characterized by stromal weakness and apical protrusion of the cornea associated with a gradual and painless reduction in visual acuity [1, 2]. Its etiology is complex and multifactorial, and as recently reported, it seems to involve a cascade of pro-inflammatory factors [1, 2].
The disease usually manifests in late childhood or adolescence and tends to stabilize in adulthood [1]. KCN in pediatric patients has certain important characteristics such as a progressive and aggressive nature [2, 3]. The most important risk factors associated with its onset and progression include family history, comorbidities such as vernal keratoconjunctivitis, environmental factors such as chronic eye rubbing and nocturnal eye compression during sleep (which have been given increasing recognition), hormonal changes, hereditary factors, and the dynamic nature of pediatric corneas [1-8]. Corneal cross-linking (CXL), which has been effective in the medium and long term in various studies, has a higher risk of failure in children than in adults, particularly if the transepithelial approach is used [2-7].
The Q value in normal eyes is simply a coefficient of corneal asphericity with a mean (standard deviation [SD]) of - 0.26 (0.18) and ranges from - 0.88 to + 0.50. Prolateness increases in KCN, thus creating an inverse relationship between its severity and the Q value [9, 10]. Ferrara and Torquetti [11] revealed that targeting a normal Q value could improve intracorneal ring segment (ICRS) nomograms. Iqbal et al. [12] provided a novel Q value-based nomogram (Q-N) for early grades of KCN to improve the postoperative outcomes of Keraring implantation and to avoid the unpredictable outcomes of the manufacturer’s standard nomogram (S-N). They demonstrated the superiority of the Q-N to the S-N for the treatment of KCN in adult patients with a mean age of 28 years [12].
In the current study, our primary objective was to investigate the efficacy of the Q-N nomogram in pediatric versus adult KCN by analyzing short-term visual, refractive, and topographic outcomes.
METHODS
This prospective, multicenter, nonrandomized, open-label trial was approved by the Ethics Committee of the Faculty of Medicine, Sohag University, Egypt. The study obtained a clinical trial registry number from the Pan-African Clinical Trial Registry (PACTR201811652174046) and followed the tenets of the Declaration of Helsinki. All patients were recruited and followed up in two homogeneous private centers in Sohag (Future Eye Center and Al-Mashriq Eye Center); however, all surgeries were performed in the Future Eye Center in Sohag City, Egypt. The nature of KCN, its treatment pathways, and the potential operative consequences were carefully explained to all adult patients and the parents or legal guardians of the pediatric patients, and written informed consent was obtained before surgery. All parents of the pediatric patients were informed and agreed that the children in this study would undergo accelerated epithelium-off CXL after the end of the study’s 6-month follow-up, as an additional therapeutic alternative aimed at stabilizing the postoperative Q-N outcomes and following several recommendations in the literature, including ours [3-6]. The CXL results were not included in this study.
This study included 47 eyes of 47 patients who were allocated to one of two groups. The adult group (aged ≥ 18 years) included 33 eyes of 33 patients with KCN, and the pediatric group (aged 14 – 17 years) included 14 eyes of 14 patients with KCN. All patients underwent femtosecond laser-assisted implantation of a single 210° Keraring segment (Keraring ICRS; Mediphacos Ltd., Belo Horizonte, Brazil) according to the novel Q value-based nomogram (Table 1) [12].
Table 1.
The novel Q value-based nomogram [12]
| Q-anterior |
CTO < 450µm
Degree/µm |
CTO 450–500µm
Degree/µm |
CTO > 500µm
Degree/µm |
|---|---|---|---|
| More than - 0.50 | 210/150 | 210/150 | 210/150 |
| - 0.50 to > - 1 | 210/150 | 210/200 | 210/250 |
| - 1 to - 1.50 | 210/200 | 210/250 | 210/300 |
| Less than - 1.50 | 210/250 | 210/250 | 210/300 |
Abbreviations: CTO, corneal thickness at the optical zone; µm, micrometers. Note: The values are indicated as Keraring segment (Keraring ICRS, Mediphacos Ltd., Belo Horizonte, Brazil) arc length in degrees/thickness in µm; Q-anterior, asphericity of the anterior corneal surface; “more than” and “less than” should be considered with respect to mathematical signs.
Eligible participants underwent complete ophthalmological examination, measurement of uncorrected and corrected distance visual acuities (UDVA and CDVA) using a Snellen chart (Auto Chart Projector SMART CP 11; MEDIZS, Daejeon, Korea) with values converted to logarithm of the minimal angle of resolution (logMAR), intraocular pressure measurement using the Goldmann applanation tonometer (AT900; Haag-Streit, Koeniz, Switzerland), a detailed undilated and dilated slit-lamp biomicroscopy examination (SL-450; Nidek Co., Ltd., Gamagori, Japan) of the anterior and posterior segments, and cycloplegic refraction.
Adult group included patients aged ≥ 18 years who presented to the Cornea Outpatient Clinic with intolerance to spectacles and gas permeable contact lenses (GPCL), stable KCN for at least one year (Kmax remained < 1 diopter [D]), grade 1 and 2 Amsler – Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13]. The cone asymmetry was type 1 and type 2 (100% and 80% of the cones were on one side of the steepest meridian, respectively). The thinnest corneal thickness (TCT) should be > 380 μm.
Pediatric group included patients aged 14 – 17 years who presented to the Cornea Outpatient Clinic with intolerance to spectacles and GPCL, grade 1 and 2 A-K classification [13], type 1 and 2 cone asymmetry, TCT > 380 μm, and spherical equivalent of the refractive error (SE) worse than - 4 D. The exclusion criteria for both groups were previous CXL treatment, dry eye disease, previous corneal or eye surgery, concomitant eye pathology, and infection.
Our primary outcome measures were UDVA and CDVA in logMAR; keratometry values including front keratometry in flat meridian (K1) and steep meridian (K2), front mean keratometry (Kaverage), and front maximum keratometry (Kmax); TCT; the spherical component of the refractive error (sphere) in D, the cylindrical component of the refractive error (cylinder) in diopter cylinder (DC), and SE in D calculated as sphere + 1/2 cylinder; and the anterior and posterior Q values representing corneal asphericity on corneal surfaces (Q-ant and Q-post, respectively).
Regarding SE measurement, all eyes underwent cycloplegic refraction at baseline and 6 months post-treatment. Cycloplegia was induced in each eye by instilling 1% cyclopentolate hydrochloride (Cyclophrine 1%; Kahira Pharmaceuticals and Chemical Industries Company, Cairo, Egypt) three times at 5-min intervals. Cycloplegic refraction was performed 30 min after the third instillation using a Welch Allyn 3.5v streak retinoscope (Welch Allyn, Inc., NY, USA) in a dimly lit room. Each refraction was recorded as the sphere in D, the cylinder in DC, and its axis direction.
The Sirius corneal topography device (Costruzioni Strumenti Oftalmici, Florence, Italy) was used to document corneal topography, and an iFS advanced femtosecond laser (Abbott Laboratories Inc., Abbott Park, IL, USA) was used to create corneal tunnels for Keraring implantation. All patients underwent Keraring implantation with a single 210° arc segment according to the novel Q-N, with a segment thickness of nearly 60% of the actual corneal thickness in the optical zone (CTO) at the site of segment implantation (Table 1). We implanted an SI-5 model of the Keraring segment, which features a triangular cross-sectional design with an optical zone of 5 mm.
We instilled two drops of the topical anesthetic benoxinate hydrochloride 0.4% (BENOX Sterile Ophthalmic Solution; EIPICO, Tenth of Ramadan City, Egypt) into the eyes 10 min before surgery. The 0 – 180° axis was marked on the slit lamp, while the steepest corneal meridian was marked on the table just before the application of a suction ring into the eye. The patients fixated on a flashing light while the corneal center was marked. The suction ring was introduced into the eye while carefully centralizing the cornea within the ring, thus fixing the eye during the corneal tunneling process. We then introduced a spatula inside the corneal tunnel to check its patency. Thereafter, a single 210° arc-length Keraring segment was cautiously implanted into the corneal tunnel according to the Q-N (Table 1). Finally, a soft-bandage contact lens (CooperVision; Cooper Companies, Inc., Pleasanton, CA, USA) was placed on the cornea.
We used the following iFS parameters for corneal tunneling: 5 mm inner diameter, 5.9 mm outer diameter, 1 mm incision axis according to steepest corneal meridian, 1.4 mm cut thickness, and 1.95 microjoules for both ring and entry cut energies while the depth of the corneal tunnel was fixed at 75% depth at the thinnest point along the tunnel.
All patients were instructed to instill a combination of antibiotic and steroid eye drops (tobramycin 3 mg and dexamethasone 1 mg, Tobradex® Suspension; Alcon Laboratories, Inc., Fort Worth, TX, USA) on an hourly basis on the first postoperative day, then four times daily for one week, and tapering to twice daily for another week. Artificial tear substitute eye drops (Systane® Ultra, Alcon Laboratories, Inc.) were also prescribed four times daily for two weeks. All medications were discontinued after the second postoperative week. The soft-bandage contact lens was removed on postoperative day one. All patients were examined at 1 day, 1 week, and 1 month postoperatively. They were also instructed to follow up at 3 and 6 months.
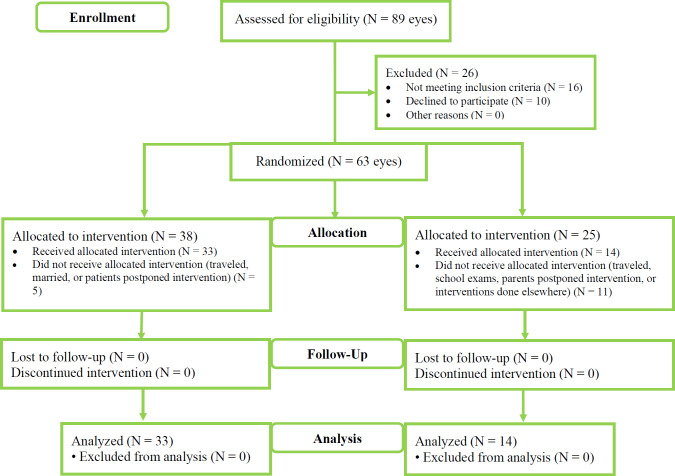
All participants were followed up for 6 months. All pediatric eyes underwent CXL after the end of the study, as described in detail elsewhere [6, 12]; however, the accelerated epithelium-off CXL outcomes were not included in this study. All adult and pediatric participants were instructed to continue follow-up at the Cornea Outpatient Clinic at the end of the study. Figure 1 shows the CONSORT flow diagram.
Figure 1.
Patient allocation into adult or pediatric group based on their age. Abbreviations: N, number of eyes; KCN, Keratoconus. Note: Adult group included patients aged ≥ 18 years with intolerance to spectacles and gas permeable contact lenses (GPCL), stable KCN for at least one year (Kmax remained < 1 diopter [D]), grade 1 and 2 Amsler – Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13]. The cone asymmetry was type 1 and type 2 (100% and 80% of the cones were on one side of the steepest meridian, respectively), and the thinnest corneal thickness (TCT) should be > 380 μm; Pediatric group included patients aged 14 – 17 years with intolerance to spectacles and GPCL, grade 1 and 2 A-K classification [13], type 1 and 2 cone asymmetry, TCT > 380 μm, and the spherical equivalent of the refractive error worse than - 4 D
Data were analyzed using STATA version 14.2 (Stata Corp., College Station, TX, USA). The Shapiro – Wilk test was used to assess the normality of data distribution. Quantitative data are presented as mean (standard deviation [SD]) or median (range). Data were analyzed using Student’s t-test to compare the means of the two groups. When the data were not normally distributed, the Mann – Whitney U test was used. Preoperative and postoperative data were compared using either a paired t-test if data were normally distributed or a Wilcoxon matched-pairs signed-rank test if the data were not normally distributed. Qualitative data are presented as numbers and percentages and were compared using the chi-square test. Graphs were produced using STATA software. A P-value was considered statistically significant if less than 0.05.
RESULTS
Table 2 lists the characteristics of the study participants. We included 47 eyes of 47 patients (25 male and 22 female) with mean (SD) ages of 29.97 (5.84) and 16.36 (0.93) years in adult and pediatric groups, respectively (P < 0.001).
Table 2.
Patient characteristics of the study groups
| Variable | Adult group (n = 33 eyes) | Pediatric group (n = 14 eyes) | P -value |
|---|---|---|---|
|
Age (y), Mean ± SD
Median (Range) |
29.97 ± 5.84 31 (20 to 41) |
16.36 ± 0.93 17 (14 to 17) |
< 0.001 |
| Sex (Male / Female), n (%) | 18 (54.5) / 15 (45.5) | 7 (50.0) / 7 (50.0%) | 0.78 |
|
Preoperative A-K KCN grading
Grade 1 (24 eyes) / Grade 2 (23 eyes), n (%) |
18 (54.5) / 15 (45.5) | 6 (42.9) / 8 (57.1) | 0.46 |
Abbreviations: n, numbers; y, years; SD, standard deviation; KCN, keratoconus; µm, micrometers. Note: Adult group included patients aged ≥ 18 years with intolerance to spectacles and gas permeable contact lenses (GPCL), stable KCN for at least one year (Kmax remained < 1 diopter [D]), grade 1 and 2 Amsler – Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13]. The cone asymmetry was type 1 and type 2 (100% and 80% of the cones were on one side of the steepest meridian, respectively), and the thinnest corneal thickness (TCT) should be > 380 μm; Pediatric group included patients aged 14 – 17 years with intolerance to spectacles and GPCL, grade 1 and 2 A-K classification [13], type 1 and 2 cone asymmetry, TCT > 380 μm, and the spherical equivalent of the refractive error worse than - 4 D.
Table 3 lists the visual, refractive, and topographic outcomes of adult group. We observed statistically significant postoperative improvements in the mean UDVA, CDVA, sphere, cylinder, SE, and all keratometry readings (all P < 0.05) (Table 3). A statistically significant postoperative improvement with a reduction in negativity of the Q-ant (P < 0.001) was observed; however, no significant postoperative changes in the Q-post (P > 0.05) were detected (Table 3).
Table 3.
Outcomes of the Q value-based nomogram in adult group with keratoconus
| Variable | Preoperative | Six month postoperative | Difference (post - pre) | P -value |
|---|---|---|---|---|
|
Mean ± SD
Median (Range) |
Mean ± SD
Median (Range) |
Mean (95 % CI) | ||
| UDVA | 1.11 ± 0.31 1.1 (0.6 to 1.7) |
0.55 ± 0.21 0.5 (0.2 to 1.1) |
-0.56 (-0.66 – -0.46) | < 0.001 |
| CDVA | 0.57 ± 0.17 0.5 (0.3 to 0.9) |
0.17 ± 0.14 0.1 (0.0 to 0.7) |
-0.40 (-0.46 – -0.34) | < 0.001 |
| Sphere | -4.12 ± 1.33 -3.5 (-6.8 to -2.0) |
-1.05 ± 0.75 -1 (-3.3 to -0.3) |
3.07 (2.69 – 3.45) | < 0.001 |
| Cylinder | -3.15 ± 1.78 -3.0 (-7.8 to -0.50) |
-2.45 ± 1.34 -2 (-6.5 to -0.5) |
0.70 (0.23 – 1.18) | 0.004 |
| SE | -5.69 ± 1.57 -4.9 (-8.0 to -2.5) |
-2.28 ± 1.03 -2.3 (-4.9 to -0.9) |
3.42 (2.96 – 3.88) | < 0.001 |
| K1 | 46.20 ± 2.26 45.6 (43.2 to 52.4) |
43.06 ± 2.43 42.9 (36.5 to 50.2) |
-3.14 (-3.60 – -2.67) | < 0.001 |
| K2 | 49.49 ± 2.84 44.9 (40.4 to 51.2) |
45.49 ± 2.84 44.9 (40.4 to 51.2) |
-4.05 (-4.67 – -3.43) | < 0.001 |
| Kaverage | 47.87 ± 2.36 47.1 (44.5 to 53.4) |
44.27 ± 2.52 44.2 (38.6 to 50.7) |
-3.60 (-4.06 – -3.13) | < 0.001 |
| Kmax | 55.07 ± 4.38 54.4 (46.7 to 65.6) |
49.82 ± 4.36 49.6 (42.8 to 61.5) |
-5.26 (-6.57 – -3.95) | < 0.001 |
| TCT | 439.30 ± 34.48 439.0 (384.0 to 516.0) |
443.06 ± 33.12 439.0 (386.0 to 509.0) |
3.75 (-0.18 – 7.69) | 0.06 |
| Q-ant | -0.96 ± 0.31 -0.9 (-1.5 to -0.6) |
-0.41 ± 0.33 0.4 (-1.3 to -0.0) |
0.55 (-0.42 – 0.67) | < 0.001 |
| Q-post | -1.16 ± 0.31 -1.2 (-1.8 to -0.5) |
-1.13 ± 0.37 -1.2 (-1.8 to -0.2) |
0.03 (-0.05 – 0.11) | 0.44 |
Abbreviations: Post, postoperative values at 6 months; Pre, preoperative values; SD, standard deviation; CI, confidence interval; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; Sphere, spherical component of refractive error; Cylinder, cylindrical component of refractive error; SE, spherical equivalent of refractive error calculated as sphere + 1/2 cylinder; K1, front keratometry in flat meridian; K2, front keratometry in steep meridian; Kaverage, front mean keratometry; Kmax, front maximum keratometry; TCT, thinnest corneal thickness; Q-ant, asphericity of the anterior corneal surface; Q-post, asphericity of the posterior corneal surface. Note: P-values < 0.05 are shown in bold; Adult group included patients aged ≥ 18 years with intolerance to spectacles and gas permeable contact lenses, stable keratoconus for at least one year (Kmax remained < 1 diopter [D]), grade 1 and 2 Amsler– Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13]. The cone asymmetry was type 1 and type 2 (100% and 80% of the cones were on one side of the steepest meridian, respectively), and the TCT should be > 380 μm.
Table 4 lists the visual, refractive, and topographic outcomes of pediatric group. We observed statistically significant postoperative improvements in the mean UDVA, CDVA, sphere, SE, and all keratometry values (all P < 0.05), with a borderline reduction in cylinder (P = 0.051) (Table 4). Moreover, there was a statistically significant postoperative improvement with a reduction in negativity of the Q-ant (P < 0.05), yet there were no significant postoperative changes in the Q-post (P > 0.05). The TCT remained unchanged in both groups (P > 0.05) (Table 4).
Table 4.
Outcomes of the Q value-based nomogram in pediatric group with keratoconus
| Variable | Preoperative | Six month postoperative | Difference (post - pre) | P -value |
|---|---|---|---|---|
|
Mean ± SD
Median (Range) |
Mean ± SD
Median (Range) |
Mean (95 % CI) | ||
| UDVA | 1.09 ± 0.27 1.1 (0.8 to 1.7) |
0.47 ± 0.22 0.4 (0.2 to 0.9) |
-0.62 (-0.82 – -0.42) | 0.001 |
| CDVA | 0.53 ± 0.14 0.5 (0.4 to 0.8) |
0.19 ± 0.22 0.1 (0.0 to 0.8) |
-0.34 (-0.44 – -0.24) | 0.001 |
| Sphere | -4.48 ± 1.27 -4.37 (-6.5 to -2.5) |
-1.30 ± 0.88 -1.0 (-3.0 to -0.3) |
3.18 (2.44 – 3.92) | 0.001 |
| Cylinder | -3.14 ± -1.47 -3.3 (-6.5 to -1.0) |
-2.16 ± 0.87 -2.0 (-3.8 to -1.0) |
0.98 (0.001 – 1.96) | 0.051 |
| SE | -6.05 ± 1.26 -6.3 (-8.0 to -4.4) |
-2.38 ± 0.97 -2.3 (-4.3 to -1.0) |
3.67 (2.68 – 4.66) | 0.001 |
| K1 | 46.41 ± 1.51 46.3 (43.9 to 49.1) |
43.00 ± 2.06 43.5 (39.2 to 45.2) |
-3.41 (-4.70 – -2.12) | < 0.001 |
| K2 | 49.65 ± 1.83 49.7 (46.4 to 53.4) |
45.28 ± 1.46 45.8 (42.1 to 46.5) |
-4.37 (5.65 – -3.10) | < 0.001 |
| Kaverage | 48.03 ± 1.45 48.2 (45.4 to 50.1) |
44.14 ± 1.71 44.7 (40.7 to 45.8) |
-3.89 (-5.05 – -2.73) | < 0.001 |
| Kmax | 56.14 ± 4.08 56.2 (48.7 to 62.7) |
50.8 ± 3.5 50.8 (44.2 to 56.5) |
-5.37 (-6.37 – -4.36) | < 0.001 |
| TCT | 457.29 ± 30.31 458.0 (396.0 to 494.0) |
452.21 ± 33.93 449.0 (392.0 to 505.0) |
-5.07 (-11.12 – 0.98) | 0.09 |
| Q-anterior | -1.17 ± 0.40 -1.3 (-1.7 to -0.3) |
-0.53 ± 0.33 -0.5 (-1.2 to -0.02) |
0.62 (0.39 – 0.89) | 0.002 |
| Q-posterior | -1.26 ± 0.51 -1.3 (-2.0 to -0.1) |
-1.27 ± 0.47 -1.3 (-1.9 to -0.1) |
-0.01 (-0.14 – 0.12) | 0.89 |
Abbreviations: Post, postoperative values at 6 months; Pre, preoperative values; SD, standard deviation; CI, confidence interval; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; Sphere, spherical component of refractive error; Cylinder, cylindrical component of refractive error; SE, spherical equivalent of refractive error calculated as sphere + 1/2 cylinder; K1, front keratometry in flat meridian; K2, front keratometry in steep meridian; Kaverage, front mean keratometry; Kmax, front maximum keratometry; TCT, thinnest corneal thickness; Q-ant, asphericity of the anterior corneal surface; Q-post, asphericity of the posterior corneal surface. Note: P-values < 0.05 are shown in bold; Pediatric group included patients aged 14 – 17 years with intolerance to spectacles and gas permeable contact lenses, grade 1 and 2 Amsler – Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13], type 1 and 2 cone asymmetry, TCT > 380 μm, and SE worse than - 4 D.
Table 5 compares the mean change in visual, refractive, and topographic outcomes between the two groups. We found no significant differences between the groups in any of the outcomes (all P > 0.05), except for TCT (P < 0.05) (Table 5). We observed no intraoperative or postoperative complications or KCN progression in either group during the 6-month follow-up.
Table 5.
Comparison of adult and pediatric groups outcomes
|
Final differences
(Six-month post – pre) |
Adult group
(n = 33 eyes) |
Pediatric group
(n = 14 eyes) |
Differences
(Adult – Pediatric) |
P -value |
|---|---|---|---|---|
|
Mean ± SD
Median (Range) |
Mean ± SD
Median (Range) |
Mean (95% CI) | ||
| UDVA | -0.56 ± 0.28 0.5 (-1.3 to -0.2) |
-0.62 ± 0.34 -0.6 (-1.3 to 0.0) |
0.06 (-0.67 – -0.49) | 0.42 |
| CDVA | -0.4 ± 0.17 -0.4 (-0.7 to -0.1) |
-0.34 ± 0.17 -0.4 (-0.7 to 0.0) |
-0.06 (-0.17 – 0.05) | 0.68 |
| Sphere | 3.07 ± 1.07 3.0 (0.8 to 5.3) |
3.18 ± 1.28 3.5 (1.0 to 5.0) |
-0.10 (-0.84 – 0.62) | 0.61 |
| Cylinder | 0.70 ± 1.35 0.8 (-2.5 to 4.3) |
0.98 ± 1.70 0.9 (-1.3 to 5.0) |
-0.28 (-1.22 – 0.66) | 0.61 |
| SE | 3.42 ± 1.31 3.5 (0.3 to 6.6) |
3.67 ± 1.72 3.7 (0.5 to 7.0) |
-0.25 (-1.17 – 0.68) | 0.59 |
| K1 | -3.14 ± 1.31 -2.98 (-6.8 to -1.2) |
-3.41 ± 2.24 -3.2 (-7.6 to -0.5) |
0.27 (-0.78 – 1.31) | 0.75 |
| K2 | -4.05 ± 1.76 -3.8 (-8.3 to -0.4) |
-4.37 ± 2.21 -4.6 (-7.5 to 0.2) |
0.32 (-0.89 – 1.54) | 0.42 |
| Kaverage | -3.60 ± 1.30 -3.3 (-7.5 to -1.0) |
-3.89 ± 2.02 -4.1 (-7.5 to -0.2) |
0.30 (-0.70 – 1.29) | 0.55 |
| Kmax | -5.26 ± 3.69 -4.4 (-21.5 to 0.04) |
-5.37 ± 1.74 -5.0 (-7.4 to -0.8) |
0.11 (-1.98 – 2.20) | 0.24 |
| TCT | 3.75 ± 11.09 2.0 (-21.0 to 37.0) |
-5.07 ± 10.48 -8.0 (-19.0 to 17.0) |
8.23 (1.81 – 15.84) | 0.004 |
| Q-ant | -0.55 ± -0.35 0.6 (-0.7 to 1.2) |
0.62 ± 0.43 0.7 (-0.5 to 1.3) |
-0.10 (-0.34 – 0.14) | 0.24 |
| Q-post | 0.03 ± 0.23 0.04 (-0.56 to 0.58) |
-0.008 ± 0.22 0.0 (-0.48 to 0.34) |
0.04 (-0.11 – 0.19) | 0.54 |
Abbreviations: Post, postoperative values at 6 months; Pre, preoperative values; SD, standard deviation; CI, confidence interval; n, number of eyes; UDVA, uncorrected distance visual acuity; CDVA, corrected distance visual acuity; Sphere, spherical component of refractive error; Cylinder, cylindrical component of refractive error; SE, spherical equivalent of refractive error calculated as sphere + 1/2 cylinder; K1, front keratometry in flat meridian; K2, front keratometry in steep meridian; Kaverage, front mean keratometry; Kmax, front maximum keratometry; TCT, thinnest corneal thickness; Q-ant, asphericity of the anterior corneal surface; Q-post, asphericity of the posterior corneal surface. Note: P-values < 0.05 are shown in bold; Adult group included patients aged ≥ 18 years with intolerance to spectacles and gas permeable contact lenses (GPCL), stable keratoconus for at least one year (Kmax remained < 1 diopter [D]), grade 1 and 2 Amsler – Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13]. The cone asymmetry was type 1 and type 2 (100% and 80% of the cones were on one side of the steepest meridian, respectively) and the thinnest corneal thickness (TCT) should be > 380 μm; Pediatric group included patients aged 14 – 17 years with intolerance to spectacles and GPCL, grade 1 and 2 A-K classification [13], type 1 and 2 cone asymmetry, TCT > 380 μm, and SE worse than - 4 D.
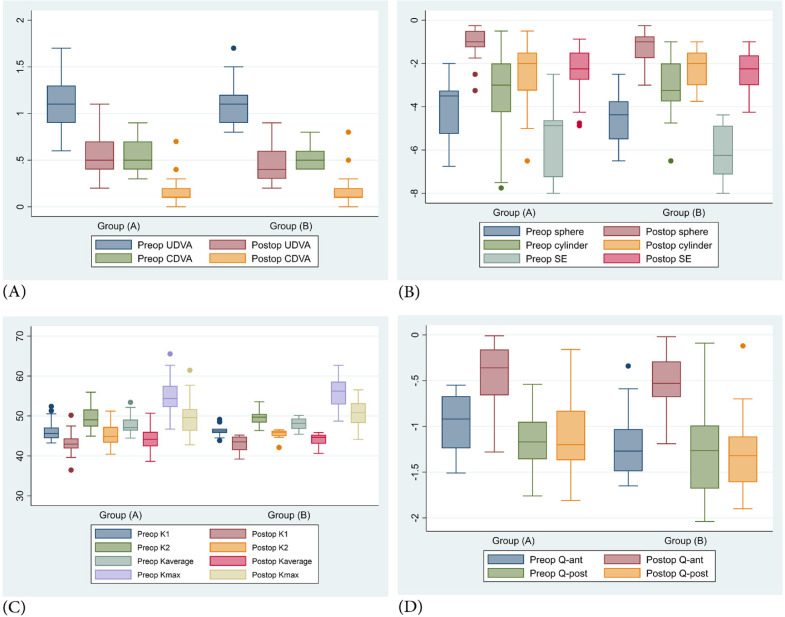
Figure 2 compares the postoperative outcomes between adult and pediatric groups at the end of the study. Figures 3 and 4 show the preoperative and postoperative corneal topographies of two pediatric patients with type 2 asymmetric cones (80% of the cones were on one side of the steepest meridian) and A-K grades 1 (Kaverage < 48 D) (Figure 3) and 2 (Kaverage 48 – 53 D) (Figure 4).
Figure 2.
Comparison of postoperative outcomes between adult (Group A) and pediatric (Group B) groups after 6 months, including (A) uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA); (B) sphere, cylinder, and SE; (C) keratometry readings (K1, K2, Kaverage, and Kmax); and (D) Q-ant and Q-post. Abbreviations: Postop, postoperative at 6 months; Preop, preoperative values; Sphere, spherical component of refractive error; Cylinder, cylindrical component of refractive error; SE, spherical equivalent of refractive error calculated as sphere + 1/2 cylinder; K1, front keratometry in flat meridian; K2, front keratometry in steep meridian; Kaverage, front mean keratometry; Kmax, front maximum keratometry; Q-ant, asphericity of the anterior corneal surface; Q-post, asphericity of the posterior corneal surface. Note: Group (A), Adult group included patients aged ≥ 18 years with intolerance to spectacles and gas permeable contact lenses (GPCL), stable keratoconus for at least one year (Kmax remained < 1 diopter [D]), grade 1 and 2 Amsler – Krumeich classification (A-K; Kaverage ≤ 48 D with < - 5 D myopia and astigmatism, 48 – 53 D with 5 – 8 D myopia and astigmatism, respectively) [13]. The cone asymmetry was type 1 and type 2 (100% and 80% of the cones were on one side of the steepest meridian, respectively), and the thinnest corneal thickness (TCT) should be > 380 μm; Group (B), Pediatric group included patients aged 14 – 17 years with intolerance to spectacles and GPCL, grade 1 and 2 A-K classification [13], type 1 and 2 cone asymmetry, TCT > 380 μm, and SE worse than - 4 D
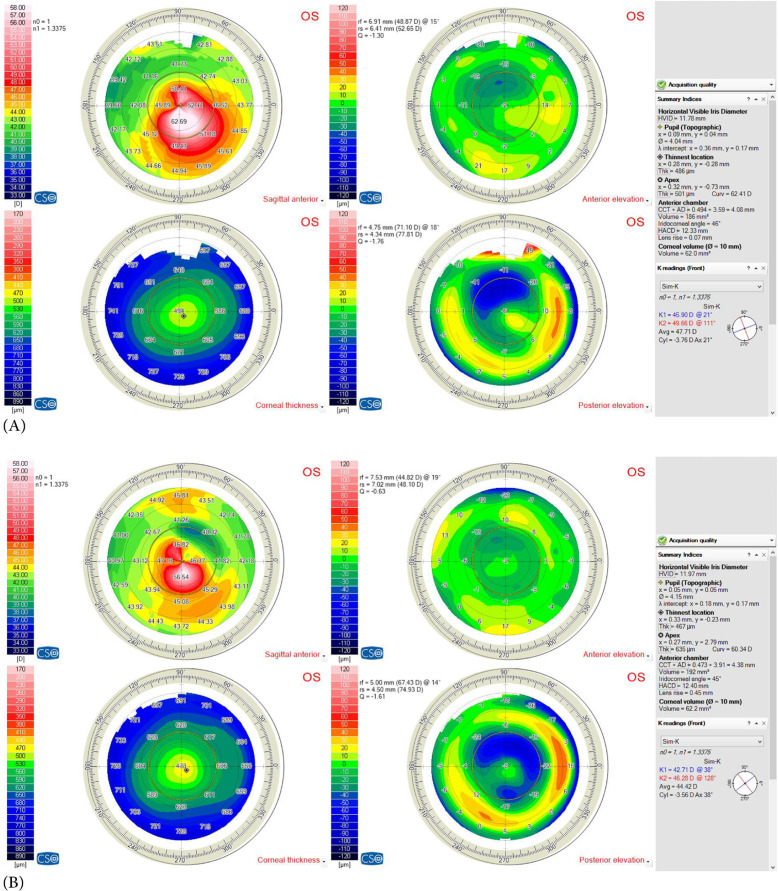
Figure 3.
(A) Preoperative and (B) postoperative corneal topographies (Sirius corneal topography device; Costruzioni Strumenti Oftalmici, Florence, Italy) of a pediatric patient with type 2 asymmetry cone (80% of the cone was on one side of steepest meridian) and Amsler – Krumeich [13] grade 1 (Kaverage < 48 D)
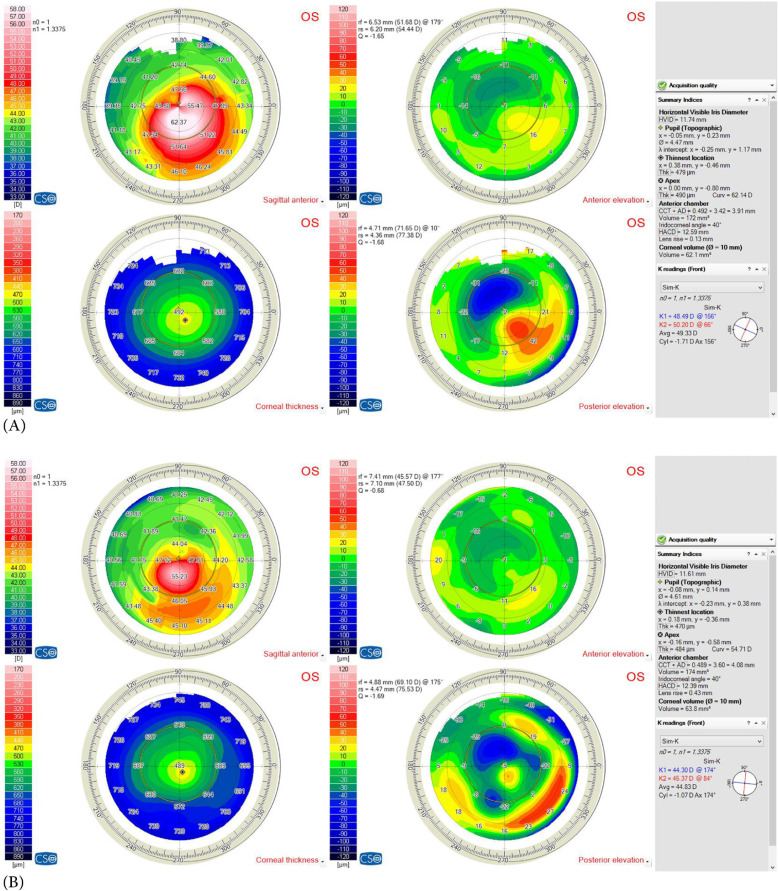
Figure 4.
(A) Preoperative and (B) postoperative corneal topographies (Sirius corneal topography device; Costruzioni Strumenti Oftalmici, Florence, Italy) of a pediatric patient with type 2 asymmetry cone (80% of the cone was on one side of steepest meridian) and Amsler – Krumeich [13] grade 2 (Kaverage 48 – 53 D)
DISCUSSION
We included adult and pediatric patients with KCN who underwent a single 210° arc-length femtosecond laser-assisted Keraring segment implantation. We found no significant differences between the groups after 6 months in terms of refractive, visual, and topographic outcomes. In comparing the efficacy of the Q-N in pediatric patients to that in adults, we observed efficacy of the Q-N in the treatment of both adult and pediatric patients with KCN. We observed no intra- or postoperative complications. The main aim of the Q-N is to normalize corneal asphericity. We believe that a major characteristic of the Q-N is its objective restoration of a normal Q value.
Pediatric KCN has unique characteristics that differ greatly from those of adult KCN [1-4, 14]. The most important characteristic is its aggressive nature and rapidly progressive course. Because of this, we expect higher rates of CXL treatment failure than in adult KCN, especially with transepithelial CXL treatments [2-7, 14-24]. Pediatric KCN is usually diagnosed at an advanced grade or stage owing to its rapidly progressive behavior, and it is often associated with the deterioration of refractive components and visual performance [2, 3, 14-25]. Furthermore, pediatric KCN has less favorable long-term postoperative outcomes, with a high probability of retreatment and further surgical intervention, especially following transepithelial CXL treatments [2, 3, 14-18, 26]. Our outcomes revealed the refractive and visual advantages of adding ICRSs as an adjunctive modality to CXL for managing KCN in adult and pediatric age groups.
To our knowledge, Iqbal et al. [12] published the first and only study comparing the short-term efficacy of the novel objective Q-N versus the manufacturer’s subjective S-N in the treatment of KCN [12]. The outcomes of our study revealed the efficacy of Q-N in both pediatric and adult patients with KCN. As in the current study, they [12] investigated 104 eyes of 104 patients with A-K grades 1 and 2 [13] with type 1 and 2 asymmetry cones. However, in the current study, we compared the short-term efficacy of the novel objective Q-N in the treatment of adult versus pediatric KCN and found no statistically significant differences between the two age groups. Both the present and previous [12] studies have revealed that Q-N resulted in smoother corneal remodeling and greater postoperative visual and refractive improvements. The major difference between these studies was the inclusion criteria.
Lisa et al. [27] reported the 3-year outcomes of inferior implantation of a single 210° arc-length Ferrara ring segment (AFR5; AJL Ophthalmic S.A., Vitoria-Gasteiz, Spain) in central KCN with a Q value ≥ - 1.00. They reported significant postoperative improvements in the mean UDVA, CDVA, SE, and Q values, which remained stable during the 36 postoperative follow-up months [27]. Their outcomes are similar to ours; however, the main difference is their use of the Ferrara ring nomogram (AJL Ophthalmic) and our use of the novel Q-N [12]. Another major difference is that they operated on KCN with central cone ectasia [27], whereas we operated on KCN with inferior decentered cone ectasia type 1 and 2 asymmetry cones. Finally, they reported long-term stability outcomes in adult KCN [27] as opposed to our short-term stability outcomes in pediatric and adult patients with KCN.
In a retrospective case series of 10 patients aged 23 – 35 years, Seleet et al. [28] examined the outcomes of using a modified Keraring nomogram by implanting a single 160° arc-length femtosecond laser-assisted Keraring segment. Their modification targeted postoperative improvements in CDVA by implanting the thickest possible 160° Keraring segment at a lower level than that described in the S-N. They observed statistically significant postoperative improvements in the mean CDVA, UDVA, and Q values [28]. Our study outcomes correlated with theirs, yet the main difference between the two study nomograms was that we targeted the Q value to restore normal corneal asphericity, while they [28] targeted CDVA to achieve maximal postoperative patient satisfaction. Another major difference was that we documented greater improvements in mean UDVA than in mean CDVA and greater postoperative improvements in the mean Q value in both groups, with better outcomes than those reported by Seleet et al. [28].
Utine et al. [29] analyzed the visual, refractive, and corneal asphericity outcomes following Keraring implantation and concluded that ICRS implantation helps restore near-normal Q values on the anterior corneal surface, thus improving both refractive and visual performance [29]. Their outcomes were consistent with ours, as we demonstrated that targeting normal Q values via Q-N yielded significant postoperative visual, refractive, and topographic improvements.
To our knowledge, this study is the first to evaluate the efficacy of Q-N in pediatric patients with KCN and to compare the pediatric outcomes of using this nomogram with those of adult patients with KCN, revealing a significant improvement in visual, refractive, and topographic outcomes in both groups. However, the study was limited by the small sample size in both groups, especially in the pediatric group, differences in the number of enrolled eyes in the adult versus the pediatric group, and the short-term follow-up period. To verify our preliminary findings, we recommend further multicenter randomized clinical trials using the Q-N nomogram in a larger sample of pediatric patients with KCN as an adjunct treatment before or after CXL.
CONCLUSIONS
Our results reaffirm the Q-N as an effective innovative nomogram that yields favorable results with significant short-term postoperative visual, refractive, and topographic improvements. Our short-term findings demonstrated that the objective Q-N was efficient in the treatment of pediatric KCN, with postoperative improvements comparable to the outcomes in adult KCN. Furthermore, the Q-N achieved reasonable corneal remodeling by restoring normal Q values. Therefore, an improvement in the Q value was accompanied by improvements in visual, refractive, and topographic outcomes in both adult and pediatric patients with KCN. In the present study, we performed the ICRS implantation in children 6 months before CXL (the outcomes of which were not part of this study). We believe that ICRS implantation in pediatric KCN ( ≥ 14 years) applying the Q-N should precede or be performed simultaneously with CXL treatment, although evidence is lacking to support this treatment sequence. Further studies are required to clarify this topic.
ETHICAL DECLARATIONS
Ethical approval:
This study was approved by the Ethics Committee of the Faculty of Medicine, Sohag University, Egypt. This study obtained a clinical trial registry number from the Pan-African Clinical Trial Registry (PACTR201811652174046) and followed the tenets of the Declaration of Helsinki. The nature of KCN, its treatment pathways, and the potential operative consequences were carefully explained to all adult patients and the parents or legal guardians of the pediatric patients, and written informed consent was obtained before surgery.
Conflict of interest:
None.
FUNDING
None.
ACKNOWLEDGMENTS
None.
References
- 1.Rabinowitz YS, Galvis V, Tello A, Rueda D, García JD. Genetics vs chronic corneal mechanical trauma in the etiology of keratoconus. Exp Eye Res. 2021;202:108328. doi: 10.1016/j.exer.2020.108328. [DOI] [PubMed] [Google Scholar]
- 2.Mukhtar S, Ambati BK. Pediatric keratoconus: a review of the literature. Int Ophthalmol. 2018;38(5):2257–2266. doi: 10.1007/s10792-017-0699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iqbal M, Elmassry A, Saad H, Am Gad A, Ibrahim O, Hamed N, et al. Standard cross-linking protocol versus accelerated and transepithelial cross-linking protocols for treatment of paediatric keratoconus: a 2-year comparative study. Acta Ophthalmol. 2020;98(3):e352–e362. doi: 10.1111/aos.14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iqbal M, Gad A, Kotb A, Abdelhalim M. Analysis of the outcomes of three different cross-linking protocols for treatment of paediatric keratoconus: A multicentre randomized controlled trial. Acta Ophthalmol. 2024;102(1):e105–e116. doi: 10.1111/aos.15686. [DOI] [PubMed] [Google Scholar]
- 5.Chatzis N, Hafezi F. Progression of keratoconus and efficacy of pediatric [corrected] corneal collagen cross-linking in children and adolescents. J Refract Surg. 2012;28(11):753–8. doi: 10.3928/1081597X-20121011-01. [DOI] [PubMed] [Google Scholar]
- 6.Iqbal M, Elmassry A, Tawfik A, Abou Samra W, Elgharieb M, Elzembely H, et al. Analysis of the Outcomes of Combined Cross-Linking with Intracorneal Ring Segment Implantation for the Treatment of Pediatric Keratoconus. Curr Eye Res. 2019;44(2):125–134. doi: 10.1080/02713683.2018.1540706. [DOI] [PubMed] [Google Scholar]
- 7.Gupta Y, Sharma N, Maharana PK, Saxena R, Sinha R, Agarwal T, et al. Pediatric Keratoconus: Topographic, Biomechanical and Aberrometric Characteristics. Am J Ophthalmol. 2021;225:69–75. doi: 10.1016/j.ajo.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 8.El-Massry A, Doheim MF, Iqbal M, Fawzy O, Said OM, Yousif MO, et al. Association Between Keratoconus and Thyroid Gland Dysfunction: A Cross-Sectional Case-Control Study. J Refract Surg. 2020;36(4):253–257. doi: 10.3928/1081597X-20200226-03. [DOI] [PubMed] [Google Scholar]
- 9.Gatinel D, Haouat M, Hoang-Xuan T. Etude des paramètres permettant la description mathématique de l’asphéricite cornéenne [A review of mathematical descriptors of corneal asphericity] J Fr Ophtalmol. 2002;25(1):81–90. [PubMed] [Google Scholar]
- 10.Xiong Y, Li J, Wang N, Liu X, Wang Z, Tsai FF, et al. The analysis of corneal asphericity (Q value) and its related factors of 1,683 Chinese eyes older than 30 years. PLoS One. 2017;12(5):e0176913. doi: 10.1371/journal.pone.0176913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara P, Torquetti L. The new Ferrara ring nomogram: the importance of corneal asphericity in ring selection. The Pan-American Journal of Ophthalmology. 2010;9(3):92–5. [Google Scholar]
- 12.Iqbal M, Elmassry A, Mounir A, Ibrahim O, Soliman A. A novel Q-value-based nomogram for single intracorneal ring segment implantation versus standard manufacturer’s nomogram combined with accelerated cross-linking for treatment of keratoconus: a randomized controlled trial. Acta Ophthalmol. 2021;99(4):e501–e511. doi: 10.1111/aos.14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colin J, Velou S. Current surgical options for keratoconus. J Cataract Refract Surg. 2003;29(2):379–86. doi: 10.1016/s0886-3350(02)01968-5. [DOI] [PubMed] [Google Scholar]
- 14.Mazzotta C, Traversi C, Baiocchi S, Bagaglia S, Caporossi O, Villano A, et al. Corneal Collagen Cross-Linking With Riboflavin and Ultraviolet A Light for Pediatric Keratoconus: Ten-Year Results. Cornea. 2018;37(5):560–566. doi: 10.1097/ICO.0000000000001505. [DOI] [PubMed] [Google Scholar]
- 15.Hed S, Matlov Kormas R, Shashar S, Malyugin BE, Boyko M, Knyazer B. Corneal Cross-Linking as Treatment in Pediatric Keratoconus: Comparison of Two Protocols. J Ophthalmol. 2021;2021:2659828. doi: 10.1155/2021/2659828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal M, Mounir A, Abd-Elaziz K, Said OM. Long-Term Visual, Refractive and Topographic Outcomes of KeraRings Combined with Accelerated Transepithelial Crosslinking for Management of Different Grades of Progressive Keratoconus: A Retrospective Cohort Study. The Open Ophthalmology Journal. 2021;15(1):54–69. [Google Scholar]
- 17.Iqbal M, Elmassry A, Badawi AE, Gharieb HM, Said OM. Visual and Refractive Long-Term Outcomes Following Standard Cross-Linking in Progressive Keratoconus Management. Clin Ophthalmol. 2019;13:2477–2488. doi: 10.2147/OPTH.S232954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soeters N, Wisse RP, Godefrooij DA, Imhof SM, Tahzib NG. Transepithelial versus epithelium-off corneal cross-linking for the treatment of progressive keratoconus: a randomized controlled trial. Am J Ophthalmol. 2015;159(5):821–8. doi: 10.1016/j.ajo.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal M, Elmassry A, Tawfik A, Elgharieb M, Nagy K, Soliman A, et al. Standard cross-linking versus photorefractive keratectomy combined with accelerated cross-linking for keratoconus management: a comparative study. Acta Ophthalmol. 2019;97(4):e623–e631. doi: 10.1111/aos.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padmanabhan P, Rachapalle Reddi S, Rajagopal R, Natarajan R, Iyer G, Srinivasan B, et al. Corneal Collagen Cross-Linking for Keratoconus in Pediatric Patients-Long-Term Results. Cornea. 2017;36(2):138–143. doi: 10.1097/ICO.0000000000001102. [DOI] [PubMed] [Google Scholar]
- 21.Cinar Y, Han CC, Sahin A, Syed ZA. Long term results of accelerated corneal collagen cross-linking in pediatric keratoconus. European Journal of Ophthalmology. 2021;31(6):3494–9. doi: 10.1177/11206721211018362. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal M, Elmassry A, Tawfik A, Elgharieb ME, El Deen Al Nahrawy OM, Soliman AH, et al. Evaluation of the Effectiveness of Cross-Linking Combined With Photorefractive Keratectomy for Treatment of Keratoconus. Cornea. 2018;37(9):1143–1150. doi: 10.1097/ICO.0000000000001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abou Samra W, Mokbel T, Elwan M, Saleh S, Elwehidy A, Iqbal M, et al. Two-stage procedure in the management of selected cases of keratoconus: clear lens extraction with aspherical IOL implantation followed by WFG-PRK. Int J Ophthalmol. 2018;11(11):1761–1767. doi: 10.18240/ijo.2018.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed Saleem MH. Combined cross-linking with femtosecond laser myoring implantation versus combined cross-linking with femtosecond laser keraring implantation in the treatment of keratoconus. Journal of the Egyptian Ophthalmological Society. 2015;108:140–147. [Google Scholar]
- 25.Saleem MIH, Ibrahim Elzembely HA, AboZaid MA, Elagouz M, Saeed AM, Mohammed OA, et al. Three-Year Outcomes of Cross-Linking PLUS (Combined Cross-Linking with Femtosecond Laser Intracorneal Ring Segments Implantation) for Management of Keratoconus. J Ophthalmol. 2018;2018:6907573. doi: 10.1155/2018/6907573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzzonetti L, Petrocelli G. Transepithelial corneal cross-linking in pediatric patients: early results. J Refract Surg. 2012;28(11):763–7. doi: 10.3928/1081597X-20121011-03. [DOI] [PubMed] [Google Scholar]
- 27.Lisa C, Fernández-Vega Cueto L, Poo-López A, Madrid-Costa D, Alfonso JF. Long-Term Follow-up of Intrastromal Corneal Ring Segments (210-Degree Arc Length) in Central Keratoconus With High Corneal Asphericity. Cornea. 2017;36(11):1325–1330. doi: 10.1097/ICO.0000000000001339. [DOI] [PubMed] [Google Scholar]
- 28.Seleet MM, Soliman AH, Alaaeldin OM. Femtosecond laser intracorneal ring segment implantation based on a nomogram modification in type 1 and type 2 ectasia. Journal of the Egyptian Ophthalmological Society. 2015;108(1):1–5. [Google Scholar]
- 29.Utine CA, Ayhan Z, Durmaz Engin C. Effect of intracorneal ring segment implantation on corneal asphericity. Int J Ophthalmol. 2018;11(8):1303–1307. doi: 10.18240/ijo.2018.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]