Highlights
-
•
ARID5A facilitates exhaustion of CD8+ CAR-T Cells in the tumor microenvironment.
-
•
ARID5A functions as an RNA-binding protein, stabilizing the presence of IDO1 mRNA by binding to the 3′ untranslated regions (UTRs).
-
•
IDO1 enhanced by ARID5A is essential for CD8+ T cell exhaustion.
-
•
ARID5A promotes the exhausted phenotype in CD8+ T lymphocytes from cancer patients by augmenting tryptophan-kynurenine metabolism.
Keywords: CAR-T cell therapy, ARID5A, IDO1, AhR, CD8+ T exhaustion
Abstract
Resistance to chimeric antigen receptor (CAR) T-cell therapy remains a significant challenge in the treatment of solid tumors. This resistance is attributed to various factors, including antigen loss, immunosuppressive tumor microenvironment, and upregulated checkpoint molecules. Indoleamine 2,3-dioxygenase 1 (IDO1) is an immunosuppressive enzyme that promotes immune escape in tumors. In this study, we investigated the role of ARID5A (AT-rich interactive domain 5A) in resistance to CAR-T cell therapy. Our findings revealed that ARID5A upregulation in tumor cells induces T cell exhaustion and immune evasion. Mechanistically, ARID5A plays a crucial role in resistance to CAR-T cell therapy by stabilizing IDO1 mRNA, leading to upregulation of IDO1 expression. Elevated IDO1 expression facilitates the conversion of tryptophan to kynurenine, which contributes to CAR-T cell exhaustion. Moreover, kynurenine accumulation within CAR-T cells activates the aryl hydrocarbon receptor (AhR), further exacerbating the exhaustion phenotype. Importantly, we demonstrated that targeting the ARID5A-IDO1-AhR axis using AhR or IDO1 inhibitors effectively alleviated T cell exhaustion induced by ARID5A. These findings suggest that modulating the ARID5A-IDO1-AhR axis may represent a promising therapeutic strategy to overcome CAR T-cell therapy resistance in solid tumors and enhance treatment efficacy.
Introduction
Chimeric antigen receptor (CAR) T-cell therapy is a promising immunotherapy that genetically modifies patient T-cells to target and eliminate tumors [1]. While FDA-approved therapies, such as Kymriah and Yescarta, have shown significant efficacy in B-cell malignancies and relapsed lymphoma, resistance remains a challenge in solid cancers, such as colon and gastric cancers [2,3]. Resistance mechanisms involve both intrinsic and extrinsic factors [3], [4], [5]. Tumor cells can develop resistance through mutations or loss of the CD19 antigen, which is targeted by CAR-T cells [6]. Within the tumor microenvironment (TME), factors like reduced CAR T cell persistence, increased exhaustion, upregulated death receptors, the presence of myeloid-derived suppressor cells, trans-differentiation profiles, and overexpression of checkpoint molecules (TIM3, LAG3, and PD-1) contribute to resistance [3]. Overcoming these resistance mechanisms is crucial for improving the effectiveness of CAR T-cell therapy in solid cancers.
Indoleamine 2,3-dioxygenase 1 (IDO1), IDO2, and tryptophan 2,3-dioxygenase (TDO) are key enzymes in tryptophan metabolism that convert tryptophan to kynurenine (Kyn) and 3-hydroxyanthranilic acid [7], [8], [9], [10]. Tryptophan is essential for the proper functioning of dendritic and T cells [11,12]. High IDO1 expression in cancer is associated with a poor prognosis and reduced T-cell infiltration [13,14]. IFN-γ, produced by cytotoxic T cells, enhances tumor cell susceptibility to cytolytic killing by increasing MHC-I expression and antigen presentation [15,16]. However, cancer cells can upregulate immunosuppressive molecules like PD-L1 and IDO1 in response to IFN-γ, evading immune elimination [16]. Understanding the role of IDO1 and its interaction with CD8+ T cells is crucial to overcoming immunosuppression in cancer.
In this study, analysis of the TCGA database in CRC revealed a positive correlation between ARID5A expression and the exhaustion phenotype of T cells. This finding was further confirmed in two CAR-T animal models, suggesting the potential involvement of ARID5A in CAR-T cell exhaustion. Additional experiments, such as RIP-qPCR, demonstrated that ARID5A could bind to IDO1 and stabilize its mRNA. Furthermore, the secretion of Kyn by IDO1 activated the aryl hydrocarbon receptor (AhR), promoting CAR-T cell exhaustion. These findings were validated in patient models, indicating that targeting the IDO1-AhR axis in patients with high ARID5A expression could be a promising therapeutic strategy.
Method and materials
Cell lines and reagents
HCT116 cell line (CCL-247) and HT29 (HTB-38) cell lines were obtained from the American Type Culture Collection, United States. The cell lines were cultured in DMEM (10829018; Thermo Fisher Scientific, Canoga Park, CA, USA) supplemented with 10 % Gibco™ fetal bovine serum (10270106, Thermo Fisher Scientific, Australia), 1 % L-glutamine (25030081, Thermo Fisher Scientific, USA), 1 % Gibco™ MEM non-essential amino acids (11140050, Thermo Fisher Scientific, USA), 100 μg/mL streptomycin (15140122, Thermo Fisher Scientific, USA), and 100 U/mL penicillin (P8294, Sigma-Aldrich, USA) under standard conditions of 37 °C and 5 % CO2. The identity of these cell lines was confirmed using STR typing, and PCR was performed to detect mycoplasma contamination. SR-1 (S2858, Selleck, USA) was purchased from Sigma-Aldrich (USA).
Patients and ethics
Following surgical resection, tissue samples from 20 patients with colon cancer were collected at the First Affiliated Hospital of Jinzhou Medical University and immediately frozen in liquid nitrogen. This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Jinzhou Medical University (No. 202030), and all patients provided signed consent. Colon cancer specimens were pathologically staged according to the seventh edition of the American Joint Committee on Cancer staging method (AJCC-7). None of the enrolled patients had received radiation or chemotherapy before surgery.
Measurement of Kyn concentrations
The amount of Kyn in the cell culture or tissue samples was determined using high-performance liquid chromatography (HPLC). Proteins were precipitated from samples using trichloroacetic acid. Purified samples were separated using the reverse-phase C18 column and eluted with 0.015 M sodium acetate buffer (pH 6.4) with 2.7 % acetonitrile as the mobile phase. The HPLC system used in this study was equipped with a C18 column (Waters, 186003018, USA) and a UV detector (Waters, 2487). Kyn levels were assessed by measuring the UV absorbance at 279 and 360 nm. Calibration curves prepared using known concentrations of Kyn standards (Sigma-Aldrich, K8625, UK) were used to quantify the Kyn levels in the samples.
Animals
Experimental animals (NSG mice aged 6 weeks) were obtained from the Shanghai Model Organisms Center. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the First Affiliated Hospital of Jinzhou Medical University (No. 202030) and complied with the Declaration of Helsinki. All measures were taken to minimize animal suffering. The maximum tumor diameter was 20 mm, which was approved by our ethics committee, and the maximum tumor size was not exceeded.
Human T cells purification and adoptive transfer
Isolation of CD8+ T cells from the splenocyte suspension was performed using a human CD8+ T cell enrichment kit (STEMCELL, 19053, Canada), according to the manufacturer's protocol. The lentiviral supernatant containing the CAR construct was generated using the 293T packaging cell line, following established methods described in the literature. Briefly, 293T cells at 95 % confluency in 10-cm plates were co-transfected with 12 μg of CAR plasmids, 6 μg of pMD2.G, and 6 μg of psPAX2 packaging plasmid DNA using Lipofectamine 2000 (11668019, Thermo Fisher Scientific). The viral supernatant was collected at 48 and 72 h post-transfection, followed by ultracentrifugation (Millipore) at 4000 rpm for 1 h. The concentrated virus was subsequently stored at -80 °C for future use in experimental procedures.
Cell transfection and generation of IDO1 or ARID5A knockout using CRISPR-Cas9
Lentiviral sgRNA (Hanbio, China) was used to block the target gene expression. HT29 and HCT116 cells in logarithmic phase were seeded in 24-well plates at a density of 1 × 105 cells/well. Once the cells reached 60 % confluence, they were transfected with retroviruses coated with 8 µg/mL polybrene (Sigma Aldrich). After 48 hr, the cells were collected to assess the effectiveness of the target gene knockout. The following primers were used: SGGFP: 5′-CACCGGGGCGAGGAGCTGTTCACCG-3′; ARID5A-SG1:5′- CTTCCTGTTCCCTTTGACAG-3′; ARID5A -SG2:5′- GAACAGGAAGCAGTCCACGG-3′; IDO1-SG1:5′- TTACTGCCTTCTCTTACCTG-3′; and IDO1-SG2:5′- TACCATATTGATGAAGAAGT-3′
In vivo tumor model
In this study, we established subcutaneous mouse models of colon cancer by implanting 1 × 106 HCT116 cells transduced with a non-targeting control (NC) or IDO/ARID5A -shRNA. CAR-T cells (5 × 106) were administered intratumorally or intravenously twice at one-week intervals. Tumor growth was measured every 5 days using an electronic caliper, and tumor volume was calculated using the following formula: shortest diameter2 × longest diameter/2. Once the tumor size exceeded 2000 mm3, euthanasia was performed to minimize animal suffering.
Isolation of tumor infiltrating lymphocytes (TIL)
Tumor-infiltrating lymphocytes (TILs) were extracted from single-cell suspensions using a human tumor dissociation kit (Miltenyi, Germany) following the manufacturer's protocol. Briefly, tumors from patients were mechanically separated to obtain single-cell suspensions, following which the cells were treated with a mixture of enzymes to dissociate the cells. The resulting TILs were used for flow cytometric analysis and sorting.
Apoptosis assay
Flow cytometry was used to detect apoptosis using an APC Annexin V Apoptosis Detection Kit (BioLegend).
Organoid culture and growth
Colon tumors measuring 10–20 mm were removed from patients, manually dissociated with a scalpel, and enzymatically digested on a shaker (150 rpm, 37 °C, 1 h) in the presence of a collagenase solution containing DMEM-F12 (Thermo Fisher Scientific), 2 mg/mL collagenase (Sigma, United Kingdom), 2 mg/mL trypsin (Thermo Fisher Scientific), 5 % FBS (Thermo Fisher Scientific), 5 μg/mL insulin (Thermo Fisher Scientific), and 50 μg/mL gentamicin (Solarbio, China). Colon cancer organoids were grown as three-dimensional spheroid cultures in Matrigel and kept at 37 °C and cultured in GlutaMAX (Thermo Fisher Scientific, USA) and human complete medium (advanced DMEM/Ham's F-12 supplemented with penicillin/streptomycin). Matrigel drops were prepared using minimal basal media supplemented with 1 × B27 (Thermo Fisher Scientific, USA) and 10 μM Y-27632 (Selleck Chemicals, Houston, TX, USA). Following removal of the media, the Matrigel was broken using PBS to pass the organoids. Following a 90-s trypsinization at 37 °C in a water bath with 1 × TrypLE (Thermo Fisher Scientific), the organoids were plated in drops ranging from 10 to 15 μL. Photographs were captured every 24 h and examined. The relative growth of the cells was measured. Following the manufacturer's instructions, human CD8+ Isolation kit was used to isolate human T cell from the peripheral blood mononuclear cell (PBMC) of colon cancer patients for organoid-T cell co-culture.
Flow cytometry and antibody
CD8 + T cells from tumor-infiltrating lymphocytes were isolated using flow cytometry-assisted cell sorting. The purity of the sorted cells was >95 %. To detect cytokine production, lymphocytes were stimulated for 2 h in the presence of a cell-stimulating mixture (eBioscience, USA, 00-4975-93) and then stained with the surface markers anti-LAG-3 (BioLegend, USA, Cat. No. 125214) and anti-PD-1 (BioLegend, Cat. No. 135206), and CTLA4 (BioLegend, Cat. No. 106308) at a 1:100 dilution in PBS containing 1 % FBS. Following a 30 min incubation on ice, a cell fixation/permeabilization kit (BD Biosciences, USA, Cat. No. 554714) and a transcription factor fixation/permeabilization buffer (BioLegend, Cat. No. 424401) were employed for intracellular cytokine and nuclear transcription factor labeling, respectively. Anti-IFN-γ (BioLegend, USA, Cat. No. 505810), anti-TNF-α (BioLegend, Cat. No. 506306), and anti-TOX (Miltenyi Biotec, Germany, Cat. No. 130-120-337) were diluted 1:50 in permeabilization buffer and incubated overnight at 4 °C in the presence of anti-IFN-γ and anti-TNF-α(BioLegend, USA). FlowJo software (Tree Star, USA) was used to analyze FACSCelesta (BD, USA) flow cytometry data. All analyses were performed using GraphPad Prism 8 (GraphPad Software, USA) and t-tests. P < 0.05 was deemed statistically significant.
Western blot
Total proteins were extracted from frozen tumor tissues using RIPA buffer (R0010, Solarbio, China) with protease inhibitor cocktail Set VI (539133, Solarbio) for 30 min on ice, followed by centrifugation at 14,000 × g for 15 min. The protein concentration of the obtained supernatant was measured using the BCA assay (P0009, Beyotime, China). Protein samples (50 ug) were denatured using SDS-PAGE Sample Loading Buffer (P0015L, Beyotime) to linearize the peptide chains. Next, the samples were loaded and separated by SDS-PAGE on 8–12 % tris-glycine gels before being transferred onto nitrocellulose membranes. The membranes were then incubated overnight at 4 °C with primary antibodies against mouse IDO1 (1:1000 dilution, Cell Signaling Technology) and mouse GAPDH (1:5000 dilution, Cell Signaling Technology) diluted in 5 % skim milk. The primary antibody was conjugated to secondary horseradish peroxidase-coupled antibodies (A0208; Beyotime) and used at a concentration of 1:3000. Enhanced chemiluminescence was recorded according to the manufacturer's instructions (BeyoECL Moon P0018FS; Beyotime).
RIP assays
A Magna RIP kit was used to conduct RIP experiments (Millipore, USA). ARID5A-SG and OE-ARID5A-MC38/HCT116 cells were then generated. Immunoprecipitated RNA was treated for 15 min at room temperature with 10 U of RNase, following the pulldown of cell lysates with anti-ARID5A [immunoglobulin G (IgG)] as the negative control. RT-PCR was used to analyze the undigested RNA fragments.
Dual luciferase reporter assay
A Dual-LumiTM II Luciferase Reporter Gene Assay Kit (RG089S, Beyotime) was used in a Modulus II Microplate Multimode Reader, and a dual luciferase reporter assay was conducted (Turner Biosystems). After transfection, cells were lysed and collected 48 h later. Briefly, a dual-Glo luciferase substrate was added to each well of a 96-well plate and incubated for 10 min at room temperature. The firefly illumination signal was captured using a multimode reader. The second Stop-Glo substrate was added for 10 min, and the Renilla luciferase signal was captured. Values were calculated and standardized data expressed relative to the control wells.
Statistical analysis
Prior to treatment, all mice to be included in the experiments were randomly assigned and grouped. Five to ten animals were used per experiment to ensure acceptable statistical power. GraphPad Prism 8 was used to calculate the statistical significance using paired two-sided t-tests and one-way analysis of variance (ANOVA) with Tukey's post-hoc test for more than two groups. The relationship analysis was conducted using SPSS for Windows (version 23.0; SPSS Inc., Chicago, IL, USA).
Results
ARID5A expression facilitated CD8+ CAR-T cell exhaustion in tumor microenvironments in colorectal carcinoma
To further investigate whether ARID5A participates in the regulation of CAR-T CD8+ T cell exhaustion, we first examined the relevance of ARID5A to T cell exhaustion signature genes, as reported previously, in human colorectal carcinoma samples [17], [18], [19], [20]. Using RNA sequencing (RNA-seq) data from The Cancer Genome Atlas (TCGA) database, we discovered that ARID5A expression showed a high positive correlation with the T-cell exhaustion signature in patients with colorectal carcinoma (Fig. 1A). We found that ARID5A expression did not correlate with the signature genes in normal tissues (Fig. 1B). These bioinformatics findings revealed that ARID5A may increase the generation of the exhausted CD8+ T cell phenotype, leading us to investigate the effect of ARID5A on tumor-infiltrating CAR-T cell exhaustion in vivo. Exhausted CD8+ CAR-T cells often lose their effector capability by expressing several inhibitory receptors (IRs) and decreasing effector cytokines [21,22]. Adoptively transferred tumor HER2–specific CAR-T CD8+ T cells gradually entered an enhanced exhausted state in tumor microenvironments in a HCT116-ARID5A overexpression CRC NSG mouse model, as evidenced by upregulation of IRs (PD-1, TIM-3, LAG-3) and decreased release of effector cytokines IFN-γ and tumor necrosis factor (TNF) as compared with the HCT116-vector group (Figs. 1C, D and S1A, B). In contrast, disruption of ARID5A expression by the CRISPR/Cas9 system effectively inhibited tumor HER2–specific CAR-T cell exhaustion in these mice (Figs. 1E, F and S1C). In adoptively transferred CAR-T cell animal models, mice with ARID5A OE tumor cells exhibited significantly enhanced tumor growth compared to mice with vector tumor cells (Fig. 1G). In the ARID5A sgRNA group, mice showed a marked reduction in tumor volume compared with mice with sgNC tumor cells (Fig. 1H). Similar results were observed for survival (Fig. 1G, H). The same results were validated in a mouse model of CEA-specific CAR-T cells (Fig. S1D, E). Furthermore, we observed that knocking out or overexpressing ARID5A affected the growth of tumor cells in directly implanted tumor-bearing mice without subsequent adoptive transfer of CAR-T cells (Fig. S1F, G). Collectively, these results suggest that the expression of ARID5A increases CD8+ CAR T cell exhaustion in the tumor microenvironment of colorectal carcinoma.
Fig. 1.
ARID5A expression facilitated CD8+ CAR-T cell exhaustion in tumor microenvironments in colorectal carcinoma. A, B, TCGA database RNA-seq analysis of the correlation between the expression of ARID5A and exhausted T cell signature genes in individuals with colon cancer (A) or normal tissue (B). FPKM, fragments per kilobase of transcript per million reads mapped. C, D, HER2-specific CAR-T were adoptively transferred into NSG mice bearing 5 × 5 mm HCT116-ARID5A OE or Vec tumor for the indicated time periods. CAR-T-transferred cells were isolated from the tumors for flow cytometric analysis of the expression of IRs (PD-1, LAG-3, and TIM-3) (C) or isolated CAR-T cells were stimulated for 4 h with phorbol myristate acetate and ionomycin, and the expression of TNF and IFN-γ (D) was measured by flow cytometry. E, F, the same as (C, D), except that the mice were adoptively transferred with CAR-T cells into mice bearing HCT116-ARID5A KO tumor (5 × 5 mm) and vector tumor. G, H, the same as C–F. Tumor growth and mouse survival were analyzed. In C–H, n = 6 mice; one-way analysis of variance (ANOVA) followed by Bonferroni's test (C–H) or Pearson's correlation test (A, B). Data represent mean ± s.d. *p < 0.05, **p < 0.01, ***p < 0.001.
ARID5A as a RNA-binding protein that stabilized IDO1 mRNAs via binding their 3′ UTRs
IDO1 catalyzes the rate-limiting step in tryptophan metabolism along the Kyn pathway, which can promote the establishment of an immunosuppressive TME through the induction of T regulatory cells or the upregulation of PD-1 in CD8+ cytotoxic T lymphocytes [23]. Previous reports indicated that ARID5A plays a role in stabilizing multiple mRNAs, thereby exerting biological effects [24]. ARID5A promotes IDO1 mRNA stability in colon cancer; however, this remains largely unexplored. Interestingly, IDO1 expression was higher in tissues from the HCT116-ARID5A overexpression CRC murine model than vector group (Fig. 2A). However, IDO1 expression was lower in tissues from the HCT116-ARID5A KO CRC murine model (Fig. 2B). Next, we verified that IDO1 is a target of ARID5A in CRC cell lines using RIP qPCR IDO1, which was enriched by ARID5A, and further validated this in HCT116 and HT29 cells. The results demonstrated that IDO1 was specifically enriched by the anti-ARID5A antibody in HCT116 and HT29 cells, and ARID5A overexpression enhanced this enrichment effect (Fig. 2C). The qRT-PCR results showed that IDO1 was upregulated or downregulated in HCT116 and HT29 cells after overexpressing or knockout ARID5A (Fig. 2D). Moreover, we established a psi-Check2 vector carrying the IDO1 3′ UTR sequence, and luciferase activity assays revealed that overexpression of ARID5A dramatically increased firefly luciferase activity, suggesting that ARID5A stabilized IDO1 mRNA by binding to its 3′ UTR (Fig. 2E). After 6 h of actinomycin D treatment, the rate of IDO1 mRNA degradation was considerably slower in the ARID5A-OE group than in the vector group and faster in the ARID5A-sg group than in the sgNC group (Fig. 2F, G).
Fig. 2.
ARID5A as a RNA-binding protein that stabilized IDO1 mRNAs via binding their 3′ UTRs. A, B, IDO1 expression was quantified in tissues from HCT116-ARID5A overexpression or tissues (A) from the HCT116-ARID5A KO group (B). C, RIP–qPCR analysis was performed with ARID5A antibody and IDO1 3′UTR-specific primers in HCT116-ARID5A vector or OE cell. D, IDO1 expression was quantified in cells from the HCT116-ARID5A overexpression or HCT116-ARID5A KO group. E, Luciferase analysis was performed on HCT116-ARID5A KO or OE cells. F, G, HCT116 or HT29 cells expressing ARID5A sgRNA or ARID5A OE at the indicated times in the presence of actinomycin D (1 μg ml−1). The remaining IDO1 mRNA levels were determined. C–G, n = 3 independent experiments; one-way analysis of variance (ANOVA) followed by Bonferroni's test (C–G). Data represent mean ± s.d. *p < 0.05, **p < 0.01, ***p < 0.001.
IDO1 enhanced by ARID5A is required for CD8+ T cell exhaustion
Next, we used sgRNAs to knock out IDO1 expression in ARID5A-OE-HCT116 cells and verified its knockdown effectiveness by western blotting (Fig. 3A). When co-cultured with HER2-specific CAR-T cells for 3 days, suppression of IDO1 activity by knocking out IDO1 in ARID5A-OE-HCT116 cells resulted in increased tumor cell apoptosis (Fig. 3B). In the in vivo mouse model, adoptively transferred HER2-specific CAR-T cells gradually entered an exhausted state in tumor microenvironments, but inhibition of IDO1 in ARID5A-OE-HCT116 led to a decrease in exhaustion-associated surface marker expression in tumor-infiltrating CD8+ HER2-specific CAR-T cells (Fig. 3C), but an increase in IFN-γ and TNF-α (Fig. 3D). The same results were validated in a model of CEA-specific CAR-T cells (Fig. 3E, F). Furthermore, when IDO1 was knocked out by sgRNA in the tumor model, a significant reduction in tumor size and improvement in overall survival were observed (Fig. 3G, H). Furthermore, knocking out or overexpressing IDO1 in ARID5Ahigh tumors did not affect the growth of tumor cells in NSG mice (Fig. S1H). This suggests that targeted inhibition of IDO in ARID5Ahigh tumors has therapeutic effects in inhibiting tumor growth and enhancing the antitumor immune response of CAR-T cells.
Fig. 3.
IDO1 enhanced by ARID5A is required for CD8+ T cell exhaustion. A, Western blot assay of IDO1 expression in ARID5A-OE-HCT116--IDO1-sgNC, ARID5A-OE-HCT116‐IDO1-sg1, ARID5A-OE-HCT116‐IDO1-sg2 cell. B–D, after treatment with CAR-T (E: T= 0.1:1) for 72 h, ARID5A-OE-HCT116--IDO1-sgNC, ARID5A-OE-HCT116‐IDO1-sg1, ARID5A-OE-HCT116‐IDO1-sg2 cell were isolated, stained with Annexin V and PI, and analyzed by flow cytometry for apoptosis detection (B) HER2 CAR-T transferred cells were isolated from tumor tissue for flow cytometric analysis of the expression of IRs (PD-1, LAG-3, TIM-3) (C) or isolated CAR-T cells were stimulated for 4 h with PMA and ionomycin and the expression of TNF and IFN-γ (D) was detected by flow cytometry. E, F, same as in (C-D), except that the tumor was treated with CEA-specific CAR-T cells. G, H, the mice were adoptively transferred with CAR-T cells into NSG mice bearing ARID5A-OE-HCT116--IDO1-sgNC, ARID5A-OE-HCT116‐IDO1-sg1, ARID5A-OE-HCT116‐IDO1-sg2 tumor (5 × 5 mm). Tumor growth (G) and mouse survival (H) were analyzed (n = 6). B, n = 3 independent experiments; C–G, n = 6 mice; one-way analysis of variance (ANOVA) followed by Bonferroni's test (C–G) or log-rank survival analysis (H). Data represent mean ± s.d. *p < 0.05, **p < 0.01, ***p < 0.001.
ARID5A promotes CD8+ T cell exhaustion by augmenting tryptophan-Kyn metabolism
Intriguingly, we observed significantly higher concentrations of Kyn in the supernatants of the ARID5A-overexpressing HCT116-T cell co-culture system than in the Vec-HCT116-T cell co-culture system (Fig. 4A). Furthermore, ARID5A overexpression increased Kyn levels in tumor tissues from HCT116-bearing mice (Fig. 4B). To investigate the enhanced exhausted CD8+ CAR T cell phenotype induced by Kyn, we co-cultured CAR T cells with ARID5A-overexpressing HCT116 cells in tryptophan-free medium. We found that tryptophan deprivation reduced the expression of exhaustion-associated surface markers while increasing the expression of IFN-γ and TNF-α in T lymphocytes (Fig. 4 C, D). Additionally, this deprivation enhanced apoptosis caused by CAR-T cells in ARID5A-overexpressing HCT116 cells (Fig. 4E). The fact that AhR activation by the IDO1 product Kyn results in the development of immune-tolerant dendritic cells and regulatory T cells, which foster a tumor immunological microenvironment that is incapable of recognizing and eliminating tumor cells [25,26] is widely believed. Our previous study demonstrated that IDO1 activates the key transcription factor TOX to promote T-cell exhaustion [21]. Here, we investigated whether the ARID5A-IDO-Kyn axis could promote T cell exhaustion through AhR activation. Interestingly, we found that ARID5A overexpression significantly enhanced AhR entry into the nuclei of CD8+ T cells (Fig. 4F). Furthermore, this phenomenon was reversed in tryptophan-free culture medium (Fig. 4F). Cytotoxic lymphoid T cells treated with the AhR inhibitor SR-1 exhibited not only greater cytotoxic activity against ARID5A-overexpressing tumor cells (Fig. 4G), but also a lower exhaustion-associated pattern than CAR-T cells treated with PBS (Fig. 4H). Collectively, these results suggest that Kyn produced by ARID5A-overexpressing HCT116 cells contributes to the increased exhausted CD8+ T-cell phenotype.
Fig. 4.
ARID5A promotes CD8+ T cell exhaustion by augmenting tryptophan-Kyn metabolism. A, HPLC analysis of the secretion of Kyn in ARID5A-OE-HCT116 or vector-cells (n = 3). B, the same as (A), except that the tumor tissues were from ARID5A-OE-HCT116 or vector cells (n = 3). C–E, after treatment with CAR-T (E:T = 0.1:1) for 72 h, HER2 CAR-T cells cultured in normal or tryptophan-free medium were isolated from the co-culture system for flow cytometric analysis for the expression of IRs (PD-1, LAG-3, TIM-3) (C) or isolated CAR-T cells were stimulated for 4 h with phorbol myristate acetate and ionomycin, and the expression of TNF-α and IFN-γ (D) was detected by flow cytometry (n = 3). Tumor cells cultured in normal or tryptophan-free medium were isolated, stained with Annexin V and PI, and analyzed by flow cytometry for detection of apoptosis (E) (n = 3). F, the same as (C-E), except that the isolated CAR-T cells were sorted out to capture the nuclear localization of AHR. G, H, the same as (C–E), except that the CAR-T cells were treated with PBS or SR-1 (1 uM). In A-H, n = 3 independent experiments. Two-tailed Student's t-test (A, B, D, E) or one-way analysis of variance (ANOVA) followed by Bonferroni's test (C, F–H). Data represent mean ± s.d. *p < 0.05, **p < 0.01, ***p < 0.001.
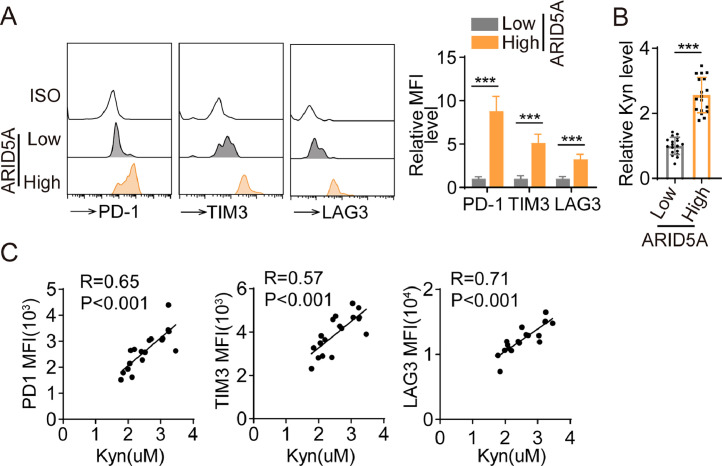
ARID5A promotes the exhausted phenotype in CD8+ T lymphocytes from cancer patients
In conclusion, we validated our findings using clinical samples. Flow cytometry analysis showed that tumor-infiltrating CD8+ T cells from colon cancer patients with high ARID5A expression exhibited increased expression of exhaustion-associated surface markers compared to those with low ARID5A expression (Fig. 5A). Concurrently, Kyn levels in tumor tissues were significantly elevated in cancer patients with high ARID5A levels compared with those with low ARID5A levels (Fig. 5B). Interestingly, Kyn levels were strongly associated with the expression of exhaustion-associated surface markers in patients with colon cancer but not in healthy individuals (Fig. 5C). To further corroborate our in vivo findings, we generated patient-derived organoids (PDOs) (Fig. S2A). Remarkably, we found that colon cancer-patient-derived organoids with high ARID5A expression displayed resistance to CAR-T cell cytotoxicity (Fig. 6A). However, blocking IDO1 activity with the inhibitor 1-methyl-L-tryptophan (1-MT) in high ARID5A-expressing PDOs led to increased tumor apoptosis when co-cultured with patient-derived CAR-T CD8+ T cells (Fig. 6A), which was consistent with the results observed in PDOs infected with ARID5A overexpression lentivirus (Fig. 6B). Upon treating CAR-T cells with SR-1 derived from patients, we observed an expected decrease in the expression of exhaustion-related surface markers and dramatically enhanced CAR-T cytotoxic function (Fig. 6C, D). Moreover, based on TCGA or GEO datasets, the expression of ARID5A increased as colorectal cancer progressed (Fig. 6E, F). To further investigate the role of ARID5A in CRC, overall and disease-free survival analyses were conducted. In the GEO dataset, increased ARID5A expression was associated with a shortened OS for COAD (p = 0.036; Fig. 6G). Consistent with the overall survival analyses, the relapse-free survival analyses demonstrated the same trend in COAD (p = 0.00065; Fig. 6H).
Fig. 5.
ARID5A promotes the exhausted phenotype in CD8+ T lymphocytes from cancer patients. A, Tumor-infiltrating CD8+ T cells were isolated from colon cancer patients with high ARID5A expression for flow cytometric analysis of the expression of IRs (PD-1, LAG-3, TIM-3) by flow cytometry (n = 6). B, HPLC analysis of Kyn secretion in tumor tissues from cancer patients with high or low ARID5A levels (n = 20). C, Percentage of PD-1, TIM-3, and LAG3 expression in peripheral CD8+ T cells was measured by flow cytometry and correlated with Kyn levels in patients with CRC (n = 20). Two-tailed Student's t-test (B), one-way analysis of variance (ANOVA), followed by Bonferroni's test (A) or Pearson's correlation test (C). Data represent mean ± s.d. *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 6.
ARID5A promotes the exhausted phenotype in CD8+ T lymphocytes from cancer patients. A, After treatment with CAR-T cells (E:T = 0.1:1) for 72 h, HER2 CAR-T cells co-cultured with PDOs were treated with PBS or 1-MT and analyzed by flow cytometry for apoptosis detection (n = 3). B, as in (A), except that the PDOs were infected with ARID5A overexpression lentivirus. C, D, after treatment with CAR-T (E:T = 0.1:1) for 72 h, HER2 CAR-T cells co-cultured with PDOs were treated with PBS or SR-1 (1 uM) and detected by flow cytometry for exhaustive phenotype (C) and apoptosis detection (D) (n = 3). E Comparison of ARID5A expression among GC samples (T1–T4) based on the GSE41258 dataset (n = 390). F, Comparison of ARID5A expression between GC samples (M0 and M1) based on GSE21510 dataset (n = 148). G, H, Survival analysis of ARID5A based on the GSE39582 (G, n = 585) or GSE72970 (H, n = 124) databases. Two-tailed Student's t-test (D), one-way analysis of variance (ANOVA), followed by Bonferroni's test (A–C, E, F), or the log-rank test (G, H). Data represent mean ± s.d. *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
CAR-T cell immunotherapy has demonstrated remarkable success in the treatment of hematological malignancies and is being actively explored for the treatment of solid tumors [27], [28], [29]. However, the immunosuppressive tumor microenvironment poses significant challenges to the efficacy of CAR-T cells in solid tumor [30]. The complex interplay between cancer cells and CAR-T cells within the tumor microenvironment influences disease progression and clinical outcomes [1,3]. IDO1 is a crucial immunomodulatory enzyme that plays a pivotal role in regulating T cell function under various physiological and pathological conditions [23]. IDO1 catalyzes the conversion of tryptophan to kyn, which subsequently modulates T cell activation, differentiation, and survival [7], [8], [9], [10]. This enzyme contributes to the immunosuppressive environment by promoting regulatory T cell (Treg) differentiation, inhibiting T helper 1 (Th1) and Th17 cell development, and inducing T cell apoptosis [23].
Recent studies have identified AT-rich interactive domain-containing protein 5A (ARID5A) as a potential regulator of IDO1 stability [31]. We investigated the role of ARID5A in IDO1 stabilization and its effect on CAR T cell exhaustion via Kyn release. We observed a positive correlation between ARID5A and IDO1 expression in cancer cell lines and primary tumor samples. Manipulation of ARID5A levels in cancer cells leads to changes in IDO1 mRNA levels. We found that ARID5A directly interacts with IDO1 mRNA, preventing its degradation. Our study revealed increased Kyn production and an enhanced CAR-T cell exhaustion phenotype in cancer cells with high ARID5A expression. Importantly, inhibiting Kyn production or blocking the signaling pathway partially reversed CAR T cell exhaustion, highlighting the critical role of ARID5A-stabilized IDO1 in promoting immune evasion.
Previous studies have suggested that combination therapy along with immune checkpoints and other drugs may benefit patients [32]. Targeting the ARID5A-IDO1 axis in CAR-T cell immunotherapy may lead to more effective treatment by disrupting the immunosuppressive effects of IDO1, allowing CAR-T cells to better eradicate cancer cells and improve patient outcomes. Several strategies can be employed to target the ARID5A-IDO1 axis, such as the genetic modification of CAR-T cells or combination therapies with small-molecule inhibitors or immune checkpoint inhibitors.
Our study has some limitations. First, we only assessed the effect of SR-1, an AHR inhibitor, on CAR-T cell immunotherapy and did not expand our analysis to other immunotherapeutic approaches, such as checkpoint blockade therapies or post-transplant immunotherapy [33], which may have significant benefits for patients with colorectal cancer [34]. Second, previous studies have shown that sex can significantly influence the effectiveness of immunotherapy [35]. However, we did not further evaluate the effect of SR-1 combined with CAR-T cell treatment in different sexes.
In conclusion, our findings provide novel insights into the molecular mechanism by which ARID5A stabilizes IDO1, contributing to an immunosuppressive tumor microenvironment through Kyn release and T cell exhaustion (Fig. 7). Targeting the ARID5A-IDO1 axis may represent a promising therapeutic strategy for enhancing anti-tumor immunity and improving cancer treatment outcomes.
Fig. 7.
Graphical abstract.
Funding information
This study was supported by the Applied Basic Research Program Project of Liaoning (Grant No.: 2023JH2/101300069).
Ethics statements
The experiments were performed in accordance with the ethical guidelines of the Declaration of Helsinki. Approval of the Research Protocol by the Institutional Review Board: This study was approved by the First Affiliated Hospital of Jinzhou Medical University (202357). All the patients provided written informed consent to participate in this study.
Informed consent
N/A.
Registry and the registration no. of the study/trial
N/A.
Animal studies
All animal experiments were performed following a protocol approved by the Institutional Animal Care and Use Committee of Jinzhou Medical University (202030).
CRediT authorship contribution statement
Dandan Wu: Investigation, Funding acquisition, Conceptualization, Formal analysis, Writing – review & editing, Writing – original draft. Guijun Wang: Methodology, Formal analysis, Investigation, Writing – review & editing. Shuang Wen: Methodology, Writing – review & editing. Xian Liu: Methodology, Investigation, Writing – review & editing. Qiang He: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare no conflict of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2024.101900.
Appendix. Supplementary materials
References
- 1.Wang Z., Wu Z., Liu Y., Han W. New development in CAR-T cell therapy. J. Hematol. Oncol. 2017;10(1):53. doi: 10.1186/s13045-017-0423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu J., Jiang G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer. 2022;21(1):194. doi: 10.1186/s12943-022-01663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N.N., Fry T.J. Mechanisms of resistance to CAR T cell therapy. Nat. Rev. Clin. Oncol. 2019;16(6):372–385. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majzner R.G., Mackall C.L. Tumor antigen escape from CAR T-cell therapy. Cancer Discov. 2018;8(10):1219–1226. doi: 10.1158/2159-8290.Cd-18-0442. [DOI] [PubMed] [Google Scholar]
- 5.van de Donk N., Usmani S.Z., Yong K. CAR T-cell therapy for multiple myeloma: state of the art and prospects. Lancet Haematol. 2021;8(6):e446–e461. doi: 10.1016/s2352-3026(21)00057-0. [DOI] [PubMed] [Google Scholar]
- 6.Furqan F., Shah N.N. Multispecific CAR T cells deprive lymphomas of escape via antigen loss. Annu. Rev. Med. 2023;74:279–291. doi: 10.1146/annurev-med-042921-024719. [DOI] [PubMed] [Google Scholar]
- 7.Fiore A., Murray P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021;70:7–14. doi: 10.1016/j.coi.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Modoux M., Rolhion N., Mani S., Sokol H. Tryptophan metabolism as a pharmacological target. Trends Pharmacol. Sci. 2021;42(1):60–73. doi: 10.1016/j.tips.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Platten M., Friedrich M., Wainwright D.A., Panitz V., Opitz C.A. Tryptophan metabolism in brain tumors - IDO and beyond. Curr. Opin. Immunol. 2021;70:57–66. doi: 10.1016/j.coi.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Platten M., Nollen E.A.A., Röhrig U.F., Fallarino F., Opitz C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019;18(5):379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 11.Mulder K., et al. Cross-tissue single-cell landscape of human monocytes and macrophages in health and disease. Immunity. 2021;54(8):1883–1900. doi: 10.1016/j.immuni.2021.07.007. .e1885. [DOI] [PubMed] [Google Scholar]
- 12.Bender M.J., et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell. 2023;186(9):1846–1862. doi: 10.1016/j.cell.2023.03.011. .e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munn D.H., Mellor A.L. IDO in the tumor microenvironment: inflammation, counter-regulation, and tolerance. Trends Immunol. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiyozumi Y., et al. IDO1 expression is associated with immune tolerance and poor prognosis in patients with surgically resected esophageal cancer. Ann. Surg. 2019;269(6):1101–1108. doi: 10.1097/sla.0000000000002754. [DOI] [PubMed] [Google Scholar]
- 15.Lv J., et al. Epigenetic modification of CSDE1 locus dictates immune recognition of nascent tumorigenic cells. Sci. Transl. Med. 2023;15(681):eabq6024. doi: 10.1126/scitranslmed.abq6024. [DOI] [PubMed] [Google Scholar]
- 16.Boehm U., Klamp T., Groot M., Howard J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L., et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature. 2018;564(7735):268–272. doi: 10.1038/s41586-018-0694-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Q., et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179(4):829–845. doi: 10.1016/j.cell.2019.10.003. e820. [DOI] [PubMed] [Google Scholar]
- 19.Zheng C., et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356. doi: 10.1016/j.cell.2017.05.035. e1316. [DOI] [PubMed] [Google Scholar]
- 20.Zheng L., et al. Pan-cancer single-cell landscape of tumor-infiltrating T cells. Science. 2021;374(6574):abe6474. doi: 10.1126/science.abe6474. (New York, NY) [DOI] [PubMed] [Google Scholar]
- 21.Wu D., Zhu Y. Role of kynurenine in promoting the generation of exhausted CD8(+) T cells in colorectal cancer. Am. J. Transl. Res. 2021;13(3):1535–1547. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., et al. IL-2 regulates tumor-reactive CD8(+) T cell exhaustion by activating the aryl hydrocarbon receptor. Nat. Immunol. 2021;22(3):358–369. doi: 10.1038/s41590-020-00850-9. [DOI] [PubMed] [Google Scholar]
- 23.Cheong J.E., Sun L. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy - challenges and opportunities. Trends Pharmacol. Sci. 2018;39(3):307–325. doi: 10.1016/j.tips.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Nyati K.K., Kishimoto T. Recent advances in the role of Arid5a in immune diseases and cancer. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.827611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen N.T., et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc. Natl. Acad. Sci. U S. A. 2010;107(46):19961–19966. doi: 10.1073/pnas.1014465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vuerich M., et al. Altered aryl-hydrocarbon-receptor signalling affects regulatory and effector cell immunity in autoimmune hepatitis. J. Hepatol. 2021;74(1):48–57. doi: 10.1016/j.jhep.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin H., Cheng J., Mu W., Zhou J., Zhu L. Advances in universal CAR-T cell therapy. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.744823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sermer D., Brentjens R. CAR T-cell therapy: full speed ahead. Hematol. Oncol. 2019;37(Suppl 1):95–100. doi: 10.1002/hon.2591. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz A., Cui H., Caligiuri M.A., Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020;13(1):168. doi: 10.1186/s13045-020-00998-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G., Rui W., Zhao X., Lin X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell Mol. Immunol. 2021;18(5):1085–1095. doi: 10.1038/s41423-021-00655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parajuli G., et al. Arid5a promotes immune evasion by augmenting tryptophan metabolism and chemokine expression. Cancer Immunol. Res. 2021;9(8):862–876. doi: 10.1158/2326-6066.Cir-21-0014. [DOI] [PubMed] [Google Scholar]
- 32.Santoni M., et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol. Immunother. CII. 2023;72(6):1365–1379. doi: 10.1007/s00262-022-03349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandi G., et al. Is post-transplant chemotherapy feasible in liver transplantation for colorectal cancer liver metastases? Cancer Commun. 2020;40(9):461–464. doi: 10.1002/cac2.12072. (Lond) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzo A., et al. Dose reduction and discontinuation of standard-dose regorafenib associated with adverse drug events in cancer patients: a systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020;12 doi: 10.1177/1758835920936932. 1758835920936932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santoni M., et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit. Rev. Oncol. Hematol. 2022;170 doi: 10.1016/j.critrevonc.2022.103596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.