Abstract
Malignant pleural disease represents a growing healthcare burden. Malignant pleural effusion affects approximately 1 million people globally per year, causes disabling breathlessness and indicates a shortened life expectancy. Timely diagnosis is imperative to relieve symptoms and optimise quality of life, and should give consideration to individual patient factors. This review aims to provide an overview of epidemiology, pathogenesis and suggested diagnostic pathways in malignant pleural disease, to outline management options for malignant pleural effusion and malignant pleural mesothelioma, highlighting the need for a holistic approach, and to discuss potential challenges including non-expandable lung and septated effusions.
Shareable abstract
Malignant pleural disease represents a growing healthcare burden and is associated with disabling symptoms and limited life expectancy. This review gives an overview of epidemiology, pathogenesis, diagnosis and key considerations for management. https://bit.ly/3HdzT3L
Introduction
Malignant pleural disease (MPD) arises from direct extension of an adjacent tumour, pleural metastases of distant tumours or a primary pleural neoplasm, most commonly malignant pleural mesothelioma (MPM) [1]. MPD can manifest as solid disease and/or malignant pleural effusion (MPE), both associated with high morbidity and mortality [2].
MPE, defined by the accumulation of pleural fluid accompanied by malignant cells in the pleural space, can complicate any cancer [3, 4]. MPE compromises quality of life (QoL) and causes debilitating symptoms, including breathlessness, cough and pain [3, 5, 6]. Lung and breast cancer are the leading causes for MPE in men and women, respectively, accounting for 50–65% of all MPE combined [4]. MPM is associated with MPE in 90% of cases [3].
Despite recent treatment advances, MPE remains incurable, with a median survival of 3–12 months [7], and management is palliative. Given the heterogeneity within the MPE patient cohort, estimating prognosis and survival is challenging; previously reported mortality rates are as high as 37% at 30 days and 77% at 1 year [2]. This high morbidity and mortality mandate a focus on expedited diagnostic work-up in suspected cases and careful consideration of patient and disease factors in management of the debilitating symptoms for this cohort with a limited life expectancy. Minimising patient symptoms, optimising QoL, reducing hospital days and maximising time at home are key aspects to personalised management strategies [8].
Epidemiology
MPD incidence rates and associated healthcare costs are expected to rise, given increasing international cancer rates, improved diagnostics and advances in cancer therapies that improve life expectancy.
Pleural effusion affects up to 1.5 million people in the USA annually [9]. Accounting for one third of exudative effusions, MPE affects 150 000 patients in the USA and 50 000 in the UK each year [7, 10, 11]. In Europe, 100 000 effusions occur each year due to lung cancer alone [12]. This, in turn, leads to significant healthcare resource usage and hospital admission rates; median length of stay is 5.5 days and estimated inpatient charges are USD 5 billion per year in the USA alone [13].
Global incidence of MPM continues to rise worldwide. The World Health Organization (WHO) predicts an exponential rise in MPM in developing countries where asbestos use is not strictly regulated [14–20]. Despite regulations controlling asbestos use in the UK, the reduction in incidence has been only 7%, although a further projected fall over the next 20 years is anticipated [21]. An estimated 2700 new mesothelioma cases are diagnosed in the UK annually, with peak incidence in people aged 85–89 years [22].
Pathogenesis
Pleural fluid accumulates when production outweighs absorption. Post mortem studies of patients with MPD suggest the majority of cases occur secondary to haematogenous spread, initially invading the visceral pleura [23, 24]. Parietal disease in the absence of visceral pleural disease is exceptionally rare.
Key mechanisms in MPE accumulation are complex tumour–mesothelial interactions resulting in pleural inflammation, tumour angiogenesis and vascular hyperpermeability with subsequent plasma extravasation into the pleural space. Many host- and tumour-derived factors, including vasoactive mediators such as tumour-derived vascular endothelial growth factor (VEGF), participate in this pathway [25]. VEGF is a potent initiator of vasodilation and increased endothelial fenestration, resulting in increased permeability to protein, in turn leading to fluid exudation into the pleural space. Protective host-derived molecules including endostatin, an endogenous inhibitor of angiogenesis and tumour growth, play a role [26]. The balance between pleural levels of angiogenic and anti-angiogenic mediators is a major determinant of effusion development [25].
Multiple tumour-derived pro-inflammatory molecules have also been implicated, including tumour necrosis factor-α, monocyte chemotactic protein-1 and osteopontin [27–29]. Host and tumour-derived osteopontin work in a synergistic fashion to stimulate macrophage recruitment and tumour angiogenesis while protecting tumour cells from apoptosis. Both directly promote vascular hyperpermeability independently of VEGF [30]. Host-derived interleukin (IL)-5 recruits eosinophils and myeloid suppressor cells that facilitate tumour cell survival in the pleural space and enhance vascular permeability [31]. Mast cells increase pleural vasculature permeability through the release of mediators (tryptase AB1 and IL-1β) and trigger NF-κB activation in pleural tumour cells, promoting fluid accumulation and tumour proliferation [32].
For the excess fluid to remain in the pleural space, impaired removal is also required and may be the predominant factor in MPE development [33]. Parietal pleural invasion can lead to obstruction of stomata, preventing exit of the effusion via their lymphatic lacunae [33]. Downstream lymphatic invasion also plays a major role, and the presence of an effusion has been demonstrated to correlate better with nodal involvement than the extent of pleural disease [24, 33].
Diagnosis
A suggested diagnostic algorithm when MPD is suspected is shown in figure 1.
FIGURE 1.
Summary of diagnostic work-up in suspected malignant pleural disease (MPD). US: ultrasound; CT: computed tomography; PET: positron emission tomography; MT: medical thoracoscopy; VATS: video-assisted thoracoscopic surgery.
Clinical presentation
The most common presenting symptom of MPE is breathlessness, which is often disabling. Chest discomfort and cough occur less commonly and 15–25% are asymptomatic at presentation [34]. Symptom severity correlates poorly with effusion size [5, 35]; however, breathlessness that appears disproportionate to the volume of fluid present should prompt consideration of comorbid conditions, e.g. pulmonary embolism. Effusion is present at diagnosis in >90% of MPM cases, and chest pain is often prominent. Both MPE and MPM are associated with constitutional symptoms, including anorexia, weight loss and night sweats.
Breathlessness in MPE is multifactorial. Pleural effusions cause abnormalities in both gas exchange and respiratory mechanics [36] but post-drainage increases in lung volume correlate poorly with the volume of effusion removed [35, 37, 38] and changes in physiological parameters are minimal [39, 40]. The hypothesis that diaphragmatic dysfunction plays a leading role has been confirmed in studies using advanced ultrasound techniques [41–44]. Abnormal hemi-diaphragm movement pre-drainage was associated with a four-fold increased likelihood of breathlessness improvement post-drainage [41]. Combining data from five randomised controlled trials (RCTs), Mishra et al. [45] demonstrated that worse breathlessness at baseline is predictive of shorter survival.
Radiology
Radiography
Chest radiography can detect effusion volumes as low as 200 mL in the posteroanterior view but remains poorly sensitive up to 500 mL [46]. Chest radiography can demonstrate other features of MPD, such as pleural thickening or pleural plaques, indicating prior asbestos exposure [47].
Thoracic ultrasound
Thoracic ultrasound (TUS) is more sensitive than chest radiography in detecting the presence of pleural effusion [48]. TUS can assess both effusion volume and character; anechoic effusions can be transudative or exudative, but echogenicity is indicative of the latter [49, 50]. Pleural thickening >1 cm, pleural nodularity and diaphragmatic thickening >7 mm are suggestive of MPD [49]. The sonographic finding of nodularity of the parietal, visceral or diaphragmatic pleura in the presence of an effusion has a specificity of 96.9% for MPE [51]. Use of TUS is essential with any pleural intervention, due to an abundance of data illustrating reduced complication rates including pneumothorax [52]. Furthermore, periprocedural colour Doppler ultrasound can be used to identify and avoid intercostal arteries and collaterals [53, 54].
Computed tomography
Computed tomography (CT) is highly sensitive in identifying the presence of pleural fluid, although septations are better visualised on TUS [47, 55]. Delayed-phase contrast optimises visualisation of the pleura [56]. CT features that increase suspicion of malignant disease include parietal pleural thickening that is circumferential, nodular, >1 cm or affecting the mediastinal pleura [57]. A 2015 retrospective review demonstrated that the positive predictive value of a malignant CT report was 80%, with a negative predictive value of 65%, highlighting the need to pursue further investigation if there is a high clinical suspicion [58]. CT can also identify extra-pleural features suggestive of malignancy, such as a lung mass, pericardial effusion, lymphadenopathy, chest wall invasion or rib destruction.
Positron emission tomography
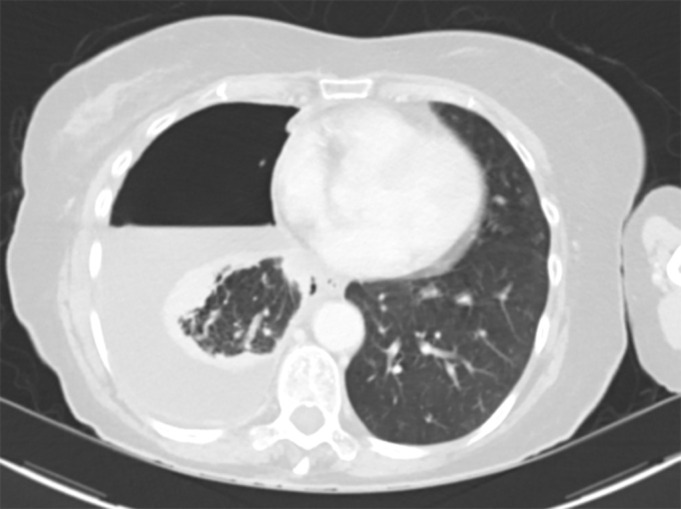
Positron emission tomography (PET)/CT has a sensitivity of 81% and specificity of 74% in discriminating benign pleural effusion from MPE [59]. Malignant pleural thickening is typically 2-fluoro-2-deoxy-d-glucose (FDG)-avid but infection and prior talc pleurodesis can demonstrate a similar appearance (figure 2) [60]. PET/CT has significant value in identifying the presence of nodal or extrathoracic metastases and determining optimal site for tissue biopsy [61]. In the imaging of MPM, PET/CT has been shown to have sensitivity of 95–100% and specificity of 78–92% [62].
FIGURE 2.
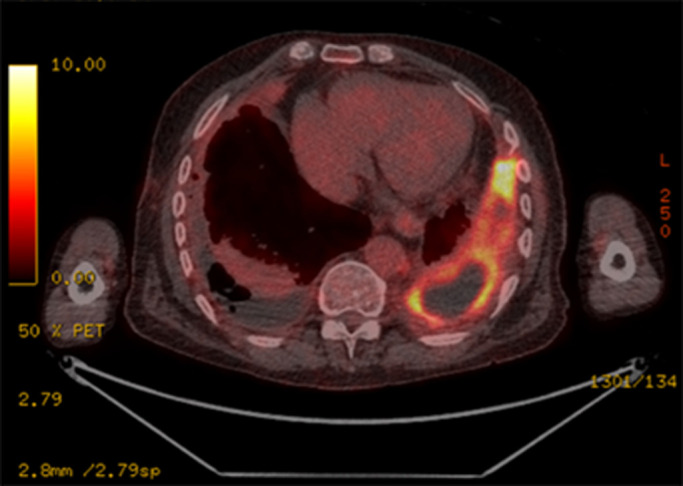
2-Fluoro-2-deoxy-d-glucose (FDG)-avid pleural thickening in non-malignant pleural disease following video-assisted thoracoscopic surgery biopsy and talc pleurodesis.
Pleural fluid
Biochemical analysis of MPE is generally consistent with an exudative effusion, although 5–10% are transudative by Light's criteria [63–65]. Pleural fluid pH and glucose have been found to correlate inversely with the extent of pleural disease, number of malignant cells in the fluid, cytology positivity and success of pleurodesis [66–68].
The minimum volume of pleural fluid required for cytopathological examination is 60–75 mL [69–71]. At least 20 mL are required to facilitate molecular testing for targetable mutations in MPE secondary to primary lung adenocarcinoma; however, specimen cellularity and particularly tumour cell proportion (tumour to non-tumour ratio >20%) are more important determinants of sample adequacy than fluid volume [72].
Sensitivity for pleural fluid cytology in the diagnosis of MPE is 50–60% [73–75]. Tumour type is an important determinant of diagnostic yield, with higher sensitivity (80%) in adenocarcinoma while haematological malignancies have a lower yield (<50%) [74]. With an average sensitivity of 60%, if the first sample is negative, a second sample can increase sensitivity by 27%, but further samples are unlikely to be useful [73, 76, 77]. Of note, cytological sensitivity for mesothelioma has been reported as low as 6% [74] but recent advances have improved this and reduced the need for pleural biopsy in many cases [78].
Given the limited sensitivity of cytological testing, research into novel pleural fluid biomarkers that may enhance diagnostic yield is ongoing. Cancer ratio, defined as the ratio of serum lactate dehydrogenase to pleural fluid adenosine deaminase, has demonstrated high sensitivity and specificity (84–94% and 92–98%, respectively) as an additional tool to differentiate between MPE and benign effusions [79, 80].
Prior meta-analyses have demonstrated significantly elevated levels of VEGF in MPE compared to benign pleural effusion, but with moderate sensitivity and specificity of 75% and 72%, respectively [81, 82]. Multiple studies have investigated the diagnostic accuracy of conventional cancer biomarkers including carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), carbohydrate antigens 125 (CA-125), 19-9 (CA19-9) and 15-3 (CA15-3), and a fragment of cytokeratin 19 (CYFRA 21-1). Overall, these have failed to demonstrate significant clinical utility, with high specificity but sensitivity of ∼50% [83]. A 2017 meta-analysis investigated the diagnostic accuracy of the combinations of positive pleural CEA+CA19-9 and CEA+CA15-3, demonstrating an extremely high specificity for MPE of ∼99%, although again sensitivity was low at 65% [84].
Pleural biopsy
In suspected MPD where pleural fluid cytology is negative for malignancy, pleural biopsy may be indicated. Closed or “blind” pleural biopsy is no longer recommended, where resources allow, due to inferior diagnostic yield and higher rates of complications [52]. Both ultrasound- and CT-guided biopsies demonstrate excellent diagnostic yield (93% and 84%, respectively) and are safe, with a low rate of adverse events (7% and 3%, respectively) [85]. In comparison to CT, ultrasound has advantages including lack of ionising radiation and real-time observation of the biopsy needle (figure 3). Choice of modality depends largely on local availability and operator experience.
FIGURE 3.
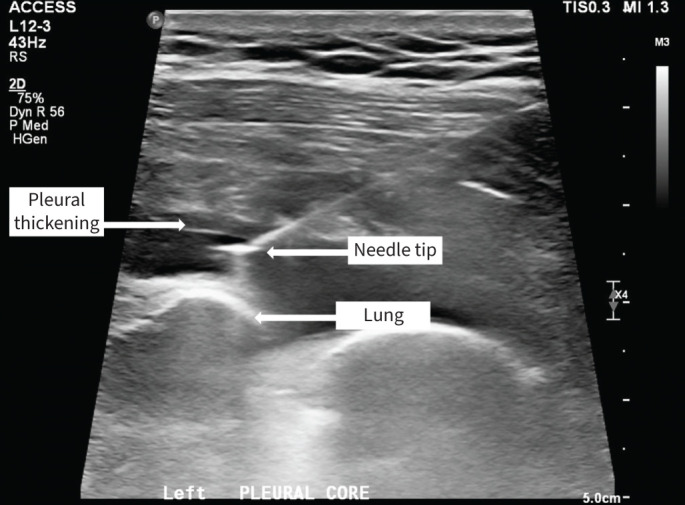
Real-time ultrasound-guided pleural biopsy.
In lung biopsies, the use of PET/CT guidance to identify targetable areas of increased metabolic activity reduces inconclusive results and the need for repeat sampling versus CT alone [86]. An ongoing RCT aims to examine the diagnostic yield of PET/CT- versus CT-guided biopsy in suspected MPD and may change best practice where resources allow [87].
Medical thoracoscopy (MT) has a 92.6% sensitivity in the diagnosis of MPD [10]. MT can provide a combined diagnostic and therapeutic procedure, allowing for large volume thoracentesis, direct visualisation, and biopsy of abnormal areas. Talc poudrage can be performed, although this requires certainty about the diagnosis and quality of the biopsy as successful pleurodesis may limit future investigations. MT has been shown to be safe, with a mortality rate of 0.34% and major complication rate of 1.8% [10]. Absolute contraindications to the procedure include severe respiratory distress or uncontrolled cough (causing procedural safety concerns) and a lack of pleural space resulting from adhesions of the pleural layer (e.g. pleural infection, pleural fibrosis or prior pleurodesis) [10, 88]. Video-assisted thoracoscopic surgery (VATS) shares many of the advantages of MT and has consistently shown high sensitivity of >90% in the diagnosis of MPD [89, 90]. VATS requires general anaesthesia and single-lung ventilation and is often unsuitable for this cohort with advanced malignancy.
Management
Prognosis is a key consideration in choosing the most appropriate management strategy. Prognostic scoring tools such as the LENT (pleural fluid lactate dehydrogenase, Eastern Cooperative Oncology Group performance score, neutrophil-to-lymphocyte ratio and tumour type) and PROMISE scores may aid in risk-stratifying and clinical decision-making, but remain imprecise for individual patients [91, 92]. Treatment strategies for MPEs can be largely divided into systemic and procedural categories. Procedural treatments are summarised in figure 4. Challenges that may arise during management, such as non-expandable lung (NEL) and septated effusions, will also be discussed in this section.
FIGURE 4.
Overview of management options for symptomatic malignant pleural effusion. IPC: indwelling pleural catheter; VATS: video-assisted thoracoscopic surgery.
Procedural treatment
Presence of MPE inherently signifies incurable disease; therefore, the primary focus of management is palliative, aiming to improve and maintain QoL. This is usually achieved through drainage of the effusion, ideally with the least number of minimally invasive interventions, delivered in an affordable manner and with as few hospital days as possible. Drainage options and their advantages and disadvantages should be discussed with the patient and an individualised decision should be made based on patient preference, probability of response to cancer-directed therapy, performance status, home supports, dexterity, comorbidities and available resources.
Therapeutic thoracentesis
Therapeutic thoracentesis involves removal of a large volume (≥1 L) of fluid. Maximum fluid removal is disputed, although current guidelines would recommend 1–1.5 L drainage at any one time, due to risk of re-expansion pulmonary oedema (RPO) [7, 93]. RPO is a rare, potentially fatal complication that occurs after rapid re-expansion of the lung from a collapsed state secondary to pneumothorax or pleural effusion [94]. Clinical signs of RPO include anterior chest discomfort, dyspnoea and desaturation. Recognised risk factors include rapid removal of fluid over a short period of time, younger age and large pneumothorax or massive effusion causing pulmonary collapse for a duration of >1 week [95, 96].
Therapeutic thoracentesis can 1) aid diagnosis, 2) confirm fluid drainage relieves breathlessness/cough (up to 25% do not improve post-drainage [41]), 3) monitor rate of re-accumulation [3, 7], and 4) assess for NEL post-drainage. Thoracentesis using ultrasound guidance is a safe procedure, with low complication rates, that can be done repeatedly as an inpatient or outpatient management option in patients with poor performance status and prognosis [97]. Fluid re-accumulation occurs in >85% and a definitive procedure is often required for long-term control [98].
Pleurodesis
Chemical pleurodesis involves the fusion of parietal and visceral pleura to prevent fluid re-accumulation. Intrapleural administration of sclerosant generates adhesions and seals the pleural space. Talc is a safe and effective sclerosing agent [99], provided graded-size talc preparation is used to minimise risk of respiratory complications associated with small-particle talc [100, 101]. Visceral and parietal pleural apposition is required to achieve pleurodesis, rendering it an unsuitable intervention where visceral pleural thickening causes incomplete lung re-expansion.
Pleurodesis is performed by administering talc slurry (suspension) via chest tube or by talc poudrage (atomised form) at thoracoscopy. Published pleurodesis success rates vary but in general are <80% across randomised studies [102–105]. The largest RCT on talc pleurodesis in MPE reported a success rate of ∼75% at 1 month, but progressively reduced to 50% at 6 months [104]. Ability to distribute talc throughout the pleural space at thoracoscopy is often considered an advantage; however, multiple studies have shown that there is no significant difference in successful pleurodesis rates, effusion recurrence or complication rates when comparing talc poudrage to talc slurry instillation [104, 106]. Talc poudrage is therefore only warranted if performing a thoracoscopy for another indication, e.g. pleural biopsy. Optimal chest tube size is often disputed; however, if talc slurry pleurodesis is intended, a chest tube size >12 F is recommended, given reduced pleurodesis success seen when directly comparing 12 and 24 F tubes [103]. Pleurodesis success is significantly lower in patients with MPM (73% versus 85%, p=0.002) [107]. This may reflect higher rates of NEL or significant tumour bulk preventing chemical adhesive success.
Fever and pain are the most common pleurodesis-related adverse events. There is no significant difference in pain or pleurodesis efficacy when utilising non-steroidal anti-inflammatory medication versus opiates [103]. Expert consensus suggests that corticosteroids should be reduced or withheld, if possible, in advance of pleurodesis.
Implementation of lung sliding scores using TUS after talc pleurodesis should be considered. When compared to regular British Thoracic Society standard guidelines (using plain film radiograph and monitoring fluid output), TUS-guided protocols reduce inpatient stay, with most patients discharged home within a day of pleurodesis. This potential pathway is also cost-effective and, infrastructure and staffing permitting, may replace current standard practice [108].
Indwelling pleural catheter
An indwelling pleural catheter (IPC) is a tunnelled ambulatory drainage device that can remain in situ long term (figure 5). The latest evidence-based guideline on MPE from the American Thoracic Society in 2018 recommended IPC as first-line therapy for NEL (previously estimated as >30% of patients) and, together with chest tube/talc pleurodesis, recommended these as equally acceptable first-line definitive therapies in patients with expandable lungs [52]. IPC has been demonstrated to improve both breathlessness and QoL at least as well as talc pleurodesis and reduced re-intervention rates from 22% to 4–6% [105, 109]. Immediate (peri-procedural) hospitalisation days were significantly reduced in the IPC arm versus chemical pleurodesis [105, 110]. Furthermore, lifetime hospitalisation days were reduced by 3.6 days per patient in the IPC arm versus chest tube/talc slurry pleurodesis [109]. On average, there is a modest reduction in hospital stay in the IPC group, but this may be significant in everyday practice for a patient group with limited life expectancy. Importantly, several prospective case series have demonstrated that IPC can provide effective palliation in the presence of NEL [111–114]. Spontaneous pleurodesis (most commonly defined as drainage of <50 mL on three consecutive drainage attempts) occurs in 30–60% of patients [105, 109, 110].
FIGURE 5.
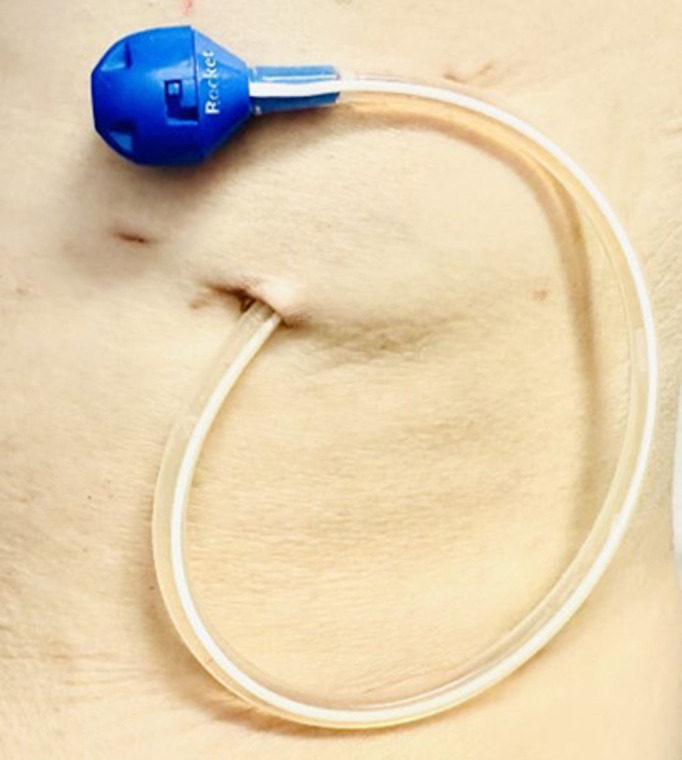
Indwelling pleural catheter in situ. The catheter is kept covered by a dressing in between drainages.
Daily fluid drainage after IPC insertion improved QoL as measured by the EQ-5D-5L questionnaire and improved rates of spontaneous pleurodesis over symptom-guided drainage [115, 116]. Furthermore, IPC and talc pleurodesis are not mutually exclusive. In the IPC-Plus RCT, instillation of talc slurry via IPC followed by intermittent drainage was shown to be feasible and safe and to accelerate pleurodesis [117]. If pleurodesis and drain removal is the priority, talc via IPC and daily drainage should be considered. If, however, reducing medical interactions is preferred, symptom-guided drainage is reasonable. Finally, IPC has been shown to be cost-effective in those with a shorter lifespan and, as a minimally invasive procedure, has very few exclusion criteria [118–120].
With these advantages comes the risk of IPC-specific complications as highlighted in table 1, necessitating a balanced discussion and individualised management, bearing in mind that patients with IPC in situ will require ongoing clinic appointments and home drainages. On average, a patient with an IPC for MPE in situ will self-drain three times per week [137].
TABLE 1.
Summary of indwelling pleural catheter (IPC)-associated complications
| Management notes | Incidence | References | |
| Common complications | |||
| Pain | Pre-procedural local anaesthetic instillation Pre-emptive use of analgesia Optimise rate of drainage (shearing force on pleura secondary to negative pressure can occur with accelerated drainage) Rarely leads to need for IPC removal (0.4%) Investigate as necessary for other complications including infection, bleeding, tumour progression or invasion of chest wall |
3–10% (mild) 0.4% (severe) |
[105, 109, 121–125] |
| Loculation | Can occur by nature of MPE disease process or as complication of infection Use of intrapleural fibrinolysis is safe and effective |
5–14% | [126] |
| Infection | Include pleural infection, empyema, wound site or skin tract infection Assess for systemic signs of infection Pleural fluid for culture (note bacterial colonisation is common), skin/wound swab Biochemical fluid markers are not overly helpful as malignant fluid may exhibit low pH, low glucose and/or high LDH levels with or without infection but change from baseline may be indicative Broad-spectrum antimicrobial cover Adequate fluid drainage (may require inpatient admission and attachment to underwater seal) Removal is usually unnecessary with above measures Intrapleural tPA/DNase via the IPC is safe and improves drainage in loculated IPC-related pleural infection |
0.4–1.3% | [127, 128] |
| Blockage | Sterile saline flushes to ensure patency Consider use of fibrinolytics |
1.5–3.7% | [122, 129] |
| Fluid leakage | Can occur secondary to rapid fluid accumulation and/or poor wound healing Limit tract size during insertion and avoid cuff placement near exit site to minimise risk Optimise adequate drainage and wound healing (appropriate dressings, dietary intake, etc.) |
0.6% | [122, 129, 130] |
| Catheter tract metastasis | Outgrowth of the pleural tumour to the subcutaneous tissue can occur subsequent to IPC insertion Regular monitoring of symptoms and IPC site inspection is recommended Benefit from analgesia and targeted radiotherapy as required |
14–42% (mesothelioma) 0.4–4.6% (other) |
[121, 131, 132] |
| Less common complications | |||
| Dislodgement | Can be associated with poor tract healing and cuff location Consider re-imaging and repositioning as appropriate |
1.2–18% | [130, 133, 134] |
| Fractured drain and retained cuffs | IPCs have adhesive cuff to anchor the catheter subcutaneously Fracture of the IPC and/or retained cuffs can occur on removing IPC No significant long-term complications in patients who had retained fractions of IPC (no surgical intervention necessary in most cases) |
9.8–23.5% | [126, 135, 136] |
MPE: malignant pleural effusion; LDH: lactate dehydrogenase; tPA: tissue plasminogen activator.
IPC complications include pain on insertion/drainage, symptomatic loculation (failure of fluid drainage due to formation of adhesions), infection (soft tissue, tract and pleural infection), catheter tract metastases and dislodgement [121]. Most complications can be managed conservatively and rarely require IPC removal [138]. Intrapleural fibrinolytic therapy can be administered for the symptomatic loculations that occur in up to 14% of IPC patients, the most commonly used agents being a single dose of alteplase (4–10 mg) or urokinase (100 000 IU) administered via the IPC [126, 139]. IPC infection is usually manageable with antibiotics alone [128]. If refractory loculated infection occurs, the IPC can be used as a conduit for administration of tissue plasminogen activator and DNase according to the MIST-2 protocol (Second Multicentre Intrapleural Sepsis Trial; alteplase 2.5–10 mg and dornase alpha 5 mg via the IPC 12-hourly, up to six doses) [127, 140]. Catheter tract metastases, more common in MPM than MPE, can be managed safely with targeted radiotherapy [141].
Video-assisted thoracoscopic surgery
VATS remains commonplace in the management of MPE worldwide [142], but evidence for its benefits and its reported success rates of 68–100% are largely supported by retrospective or single-centre series [143–146]. Three randomised studies comparing VATS to chest tube and talc pleurodesis did not demonstrate any significant benefits with VATS [104, 147, 148]. Complications of VATS include fever, pneumonia, prolonged air leak and post-VATS neuralgia, which can affect 25% of patients [104, 149–152]. No prospective studies have compared VATS versus IPC to date, but retrospective data suggest a reduced re-intervention rate and fewer hospital days with IPC [143]. The currently recruiting Australasian Malignant PLeural Effusion (AMPLE)-3 trial will provide urgently needed evidence to assist with the decision between VATS and IPC [153].
Challenges in management
Non-expandable lung
NEL arises following formation of a fibrous visceral pleural peel, malignant visceral pleural thickening or numerous visceral metastatic nodules, preventing full lung re-expansion and apposition of the visceral and parietal pleura (figure 6) [154]. NEL affects >30% of MPE patients [104], significantly limiting potential for long-lasting effusion control with conventional pleurodesis methods [155].
FIGURE 6.
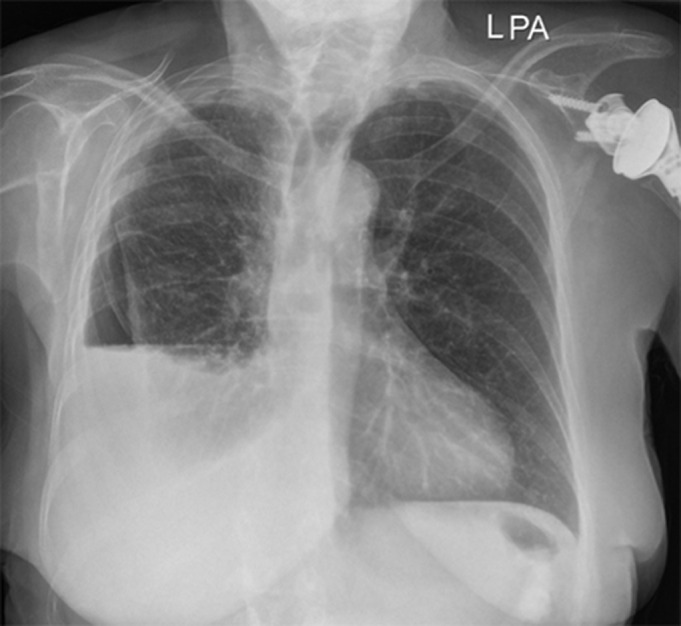
Non-expandable lung on chest radiography with re-accumulation of pleural fluid post-drainage leading to air–fluid level in the pleural space.
NEL frequently becomes apparent only after fluid drainage. Thoracentesis with concurrent pleural manometry can identify NEL [156]. Decreased lung compliance in the presence of NEL leads to more pronounced changes in pleural pressure with increased pleural elastance [157]. The superiority of manometry over clinical assessment for NEL (chest tightness during drainage, post-procedure radiograph) and utility of identifying NEL mid-procedure is debatable. Studies have failed to demonstrate lower rates of RPO or chest pain with concurrent use of pleural manometry during thoracentesis [158, 159].
Conversely, identifying NEL prior to definitive intervention would be of benefit as first-line IPC is the optimal choice in these cases. Noninvasive techniques that can forecast its probability include TUS identification of an absent sinusoid sign (a dynamic sonographic M-mode finding indicating motion of atelectatic lung during respiration within pleural fluid) and reduced motion of the atelectatic lung [160–162].
IPCs are now the mainstay of treatment in this group [3, 52]. Almost 30% of patients with NEL in the AMPLE-2 trial achieved spontaneous pleurodesis at 6 months after IPC insertion, with higher rates seen in the daily-drainage arm versus symptom-guided [115].
In select patients, there may be a role for surgical management. VATS pleurectomy and decortication allows surgical excision of the visceral pleural rind with reported success [163]. However, surgical attempts made to free the lung at thoracoscopy come at the cost of significantly increased procedure duration and high likelihood of persistent air leak post-operatively [146, 150].
Septated MPE
Septations within an MPE can impair drainage via chest tube or IPC. The residual fluid in the pleural cavity can cause persistent breathlessness and limits opportunities for achieving pleurodesis by preventing visceral and parietal pleural apposition. Adhesiolysis can be performed at thoracoscopy but, as discussed, MPE patients are frequently frail and comorbid, and surgery may be overly invasive. Intrapleural fibrinolytics alone have been unsuccessful in the management of pleural infection but have shown some benefit in improving drain output in MPE.
Although the largest RCT to date did not identify a statistically significant improvement in any outcome (breathlessness, re-intervention rates or pleurodesis success), intrapleural fibrinolytics (urokinase) in patients with residual septated effusion did reduce hospital days [164]. When combining these data with two previous smaller RCTs [165, 166], a recent meta-analysis in the British Thoracic Society guidelines demonstrated some improvement in each of these measurements with intrapleural fibrinolytics [167]. Intrapleural fibrinolytics improve drainage and may improve breathlessness in septated effusions with IPC in situ [126].
Systemic treatment
Systemic therapy (chemotherapy, immunotherapy and targeted therapy, or a combination) treatment decisions in advanced cancer should be based on patient suitability, histology, molecular profile and biomarkers.
Chemotherapy
Systemic chemotherapy for MPE depends on patient performance status, tolerability and overall suitability. Cisplatin is a potent anticancer drug used to treat a broad spectrum of malignancies and acts primarily by interfering with DNA replication. In advanced lung cancer, cisplatin is generally combined with a third-generation cytotoxic agent such as pemetrexed or paclitaxel, depending on the histological subtyping [168]. Cisplatin exerts its effect on MPE by inhibiting primary focal tumour, metastasis and fluid accumulation within the pleura via circulation through pleural vasculature [169].
Immunotherapy
Immunotherapy targets checkpoint receptors expressed by immune cells that act on the tumour microenvironment and improve T-cell functionality. Programmed cell death protein-1 (PD-1) and its primary ligand (PD-L1) are expressed on T-cells, and on tumour cells and tumour-infiltrating myeloid cells, respectively [170]. Immune checkpoint inhibitors (ICIs) that target PD-1/PD-L1 and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) are the two best understood pathways. The inhibition of these pathways by ICIs amplifies the anti-tumour effects of cytotoxic T-cells. Over the last decade, the innovation of these therapies has revolutionised the treatment of lung cancer, producing durable long-term responses not previously seen in advanced stage disease.
The KEYNOTE-189 phase III RCT demonstrated that the addition of pembrolizumab, a monoclonal antibody to PD-1, to standard-of-care chemotherapy improved overall survival and progression-free survival in patients without epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutations [171]. The level of PD-L1 expression reflects the likely degree of therapeutic benefit from PD-1/PD-L1 checkpoint inhibitors, with high expressors being eligible for single agent ICI without the addition of chemotherapy [172]. Correlation of PD-L1 expression in histological specimens of primary tumour in nonsmall cell lung cancer (NSCLC) and matched pleural fluid samples is high [173]; however, a number of studies have suggested that the response to ICIs is reduced in the presence of MPE [174, 175]. Utilisation of other anti-PD-1 agents and combinations is under evaluation across a number of clinical trials.
Anti-angiogenic therapy
Preliminary data suggest that bevacizumab, a VEGF-targeted recombinant monoclonal antibody that has an impact on tumour angiogenesis and further proliferation, in combination with carboplatin and pemetrexed chemotherapy, is likely to be efficacious in selected cases with MPE, with improved overall survival and progression-free survival reported [176, 177].
Ramucirumab, a VEGF receptor-2 antibody that inhibits tumour angiogenesis, has demonstrated activity in lung cancer patients and a phase II trial combined with docetaxel chemotherapy is currently recruiting patients with MPE [178].
Endostatin is a broad-spectrum anti-angiogenic therapy with reported therapeutic benefits, including tumour hypoxia when combined with chemotherapy in MPE patients, although further validation through prospective RCTs is required [169, 179–182].
Targeted therapy
Given the reported success of combination chemotherapy and ICI or anti-angiogenic regimens, a variety of trials have demonstrated improved survival for targeted therapies of recognised mutations, including EGFR tyrosine kinase inhibitors (such as osimertinib), ALK inhibitors (alectinib and lorlatinib) and ROS1 inhibitors (lorlatinib) [183, 184]. However, most patients with EGFR mutations will eventually develop resistance to therapy. Obtaining further pleural fluid is useful for molecularly profiling tumours, mandating change in therapy [185]. Newer oncogenic drivers have been identified, including the KRAS12C mutation, targeted by sotorasib and adagrasib, both recently approved by the United States Food and Drug Administration for treatment in patients who have progressed on at least one prior line of systemic treatment [186, 187].
Intrapleural agents
Intrapleural chemotherapy or alternate systemic therapy may offer localised cytotoxic effect, thus minimising systemic absorption and insult. A meta-analysis of intrapleural bevacizumab in addition to chemotherapy compared to intrapleural chemotherapy alone demonstrated improved rates of complete remission in the experimental (bevacizumab) arm, with only minor increased risk of adverse events [188]. An RCT comparing intrapleural versus intravenous bevacizumab in NSCLC with MPE demonstrated an increased rate of complete response, partial response and duration of response in the intrapleural arm, with lower rates of adverse events, although the positive results were not statistically significant [189]. The addition of intrapleural endostatin to standard chemotherapy has shown improved overall response in MPE patients [190]. Preliminary phase I and II trials with intrapleural chemotherapy have shown short-term partial or full response of MPE [191–196]. However, these options have not yet to our knowledge been directly compared to standard therapy and further randomised trials are needed.
Mesothelioma treatment
Molecular biomarkers BRCA-associated protein-1 (BAP1) or cyclin-dependent kinase inhibitor 2A (CDKN2A) may aid diagnosis and management strategies in MPM; however, validation is required [197–200]. Systemic chemotherapy has a proven survival benefit in MPM, with the combination of cisplatin and pemetrexed increasing median overall survival by 2.8 months when compared to cisplatin single therapy [201]. The CheckMate 743 RCT has demonstrated the ICI combination of nivolumab and ipilimumab improves response rates and overall survival compared to chemotherapy and is now the standard of care for patients with advanced mesothelioma [202].
Surgical management of MPM is advocated by some groups but neither of the main surgical procedures (extrapleural pneumonectomy and lung parenchyma preserving pleurectomy/decortication) has been shown to offer survival benefit in a prospective RCT [203]. In fact, both are associated with substantial morbidity and mortality rates, as high as 31% in extrapleural pneumonectomy [204]. Surgery as a standalone treatment, therefore, cannot be recommended in MPM, but novel approaches using multimodality intervention (surgery, radiotherapy, chemotherapy and immunotherapy) remain under investigation [205].
Multidisciplinary approach
Non-interventional adjunct therapies are being explored to improve symptom palliation of MPE patients [206]. Baseline nutritional status and body composition in MPM are associated with reduced activity levels and poorer QoL [207]. Utilising dietary interventions to improve outcomes is an untapped resource. MPM patients lose muscle mass over time, a finding associated with reduced activity levels and poorer survival [208]. Exercise has shown great promise in improving QoL but remains under-utilised and studies have been heterogeneous [209, 210]. QoL questionnaires and visual analogue scales for pain and breathlessness are inherently subjective. Objective functional assessment with actigraphy, using a tri-axial accelerometer worn at regular intervals, has been shown to be well tolerated by MPE patients and is an exciting development in the assessment of outcomes post-intervention [211, 212].
Key points
MPE affects an estimated 1 million people globally per year.
Minimising patient symptoms, optimising QoL and reducing hospital days are key aspects to personalised management strategies.
Diagnostic work-up should include full clinical history, examination, radiological investigation and pleural fluid analysis, with or without pleural biopsy.
Management plans include both procedural (pleurodesis, IPC and VATS) and systemic options (chemotherapy and immunotherapy).
Considerations when choosing a management plan are probability of response to cancer-directed therapy, performance status, home supports, dexterity, comorbidities and available resources.
Self-evaluation questions
- A 62-year-old smoker, with Eastern Cooperative Oncology Group performance score 3, is diagnosed with PD-L1-positive NSCLC on pleural fluid cytology. Her respiratory symptoms improved after drainage of 1 L. CT is performed after initial drainage (figure 7). 2 days later the patient describes worsening shortness of breath and chest radiography confirms re-accumulation of right-sided MPE. What is the most appropriate management for symptomatic relief?
- Repeat therapeutic thoracentesis
- Insert 12-F chest tube and administer talc slurry
- Start pembrolizumab
- Insert IPC
- Perform VATS decortication
Is the following statement true or false? Negative pleural fluid cytology definitively excludes a diagnosis of MPE.
- Which of the following patient factors are important when considering appropriate management of MPD?
- Performance status
- Patient preference
- Probability of response to cancer-directed therapy
- Overall prognosis
- All the above
- Which of the following statements regarding MPM is not true?
- Cytological sensitivity for mesothelioma has been reported as high as 60%
- Global incidence of MPM continues to rise worldwide
- Systemic therapy is the only modality that has proven survival benefit in MPM
- Incidence of catheter tract metastases is higher in mesothelioma than other cancers
FIGURE 7.
Computed tomography image to accompany self-evaluation question 1.
FIGURE 7.

Computed tomography image to accompany self-evaluation question 1.
Suggested answers
1. d.
2. False.
3. e.
4. a.
Footnotes
Author contributions: D.B. Fitzgerald is the guarantor of the article and takes responsibility for the integrity and content of the manuscript. All authors contributed to the study conception and design, and clinical interpretation of the data, as well as to the writing of the manuscript and its final approval.
Conflict of interest: All authors have reported that no potential conflicts of interest exist with any companies/organisations whose products or services may be discussed in this article.
References
- 1.Bonomo L, Feragalli B, Sacco R, et al. . Malignant pleural disease. Eur J Radiol 2000; 34: 98–118. doi: 10.1016/S0720-048X(00)00168-6 [DOI] [PubMed] [Google Scholar]
- 2.DeBiasi EM, Pisani MA, Murphy TE, et al. . Mortality among patients with pleural effusion undergoing thoracentesis. Eur Respir J 2015; 46: 495–502. doi: 10.1183/09031936.00217114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts ME, Neville E, Berrisford RG, et al. . Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65: Suppl. 2, ii32–ii40. doi: 10.1136/thx.2010.136994 [DOI] [PubMed] [Google Scholar]
- 4.Psallidas I, Kalomenidis I, Porcel JM, et al. . Malignant pleural effusion: from bench to bedside. Eur Respir Rev 2016; 25: 189–198. doi: 10.1183/16000617.0019-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977; 63: 695–702. doi: 10.1016/0002-9343(77)90154-1 [DOI] [PubMed] [Google Scholar]
- 6.Haas AR, Sterman DH, Musani AI. Malignant pleural effusions: management options with consideration of coding, billing, and a decision approach. Chest 2007; 132: 1036–1041. doi: 10.1378/chest.06-1757 [DOI] [PubMed] [Google Scholar]
- 7.Antony VB, Loddenkemper R, Astoul P, et al. . Management of malignant pleural effusions. Eur Respir J 2001; 18: 402–419. doi: 10.1183/09031936.01.00225601 [DOI] [PubMed] [Google Scholar]
- 8.Koegelenberg CFN, Shaw JA, Irusen EM, et al. . Contemporary best practice in the management of malignant pleural effusion. Ther Adv Respir Dis 2018; 12: 1753466618785098. doi: 10.1177/1753466618785098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Light RW. Pleural effusions. Med Clin North Am 2011; 95: 1055–1070. doi: 10.1016/j.mcna.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 10.Rahman NM, Ali NJ, Brown G, et al. . Local anaesthetic thoracoscopy: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65: Suppl. 2, ii54–ii60. [DOI] [PubMed] [Google Scholar]
- 11.Miller RJ, Chrissian AA, Lee YCG, et al. . AABIP evidence-informed guidelines and expert panel report for the management of indwelling pleural catheters. J Bronchology Interv Pulmonol 2020; 27: 229–245. doi: 10.1097/LBR.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 12.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. . Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013; 49: 1374–1403. doi: 10.1016/j.ejca.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 13.Taghizadeh N, Fortin M, Tremblay A. US hospitalizations for malignant pleural effusions: data from the 2012 National Inpatient Sample. Chest 2017; 151: 845–854. doi: 10.1016/j.chest.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 14.Park EK, Takahashi K, Hoshuyama T, et al. . Global magnitude of reported and unreported mesothelioma. Environ Health Perspect 2011; 119: 514–518. doi: 10.1289/ehp.1002845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le GV, Takahashi K, Park EK, et al. . Asbestos use and asbestos-related diseases in Asia: past, present and future. Respirology 2011; 16: 767–775. doi: 10.1111/j.1440-1843.2011.01975.x [DOI] [PubMed] [Google Scholar]
- 16.Keshava HB, Tang A, Siddiqui HU, et al. . Largely unchanged annual incidence and overall survival of pleural mesothelioma in the USA. World J Surg 2019; 43: 3239–3247. doi: 10.1007/s00268-019-05132-6 [DOI] [PubMed] [Google Scholar]
- 17.Kerger BD. Longevity and pleural mesothelioma: age-period-cohort analysis of incidence data from the Surveillance, Epidemiology, and End Results (SEER) Program, 1973–2013. BMC Res Notes 2018; 11: 337. doi: 10.1186/s13104-018-3436-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furuya S, Chimed-Ochir O, Takahashi K, et al. . Global asbestos disaster. Int J Environ Res Public Health 2018; 15: 1000. doi: 10.3390/ijerph15051000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diandini R, Takahashi K, Park EK, et al. . Potential years of life lost (PYLL) caused by asbestos-related diseases in the world. Am J Ind Med 2013; 56: 993–1000. doi: 10.1002/ajim.22206 [DOI] [PubMed] [Google Scholar]
- 20.Delgermaa V, Takahashi K, Park EK, et al. . Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011; 89: 716–724C. doi: 10.2471/BLT.11.086678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Chan SC, Ko S, et al. . Global incidence, mortality, risk factors and trends of melanoma: a systematic analysis of registries. Am J Clin Dermatol 2023; 24: 965–975. doi: 10.1007/s40257-023-00795-3 [DOI] [PubMed] [Google Scholar]
- 22.Cancer Research UK . Mesothelioma statistics: mesothelioma incidence. Date last accessed: July 2023. www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/mesothelioma#heading-Zero
- 23.Rodriguez-Panadero F, Borderas Naranjo F, Lopez Mejias J. Pleural metastatic tumours and effusions. Frequency and pathogenic mechanisms in a post-mortem series. Eur Respir J 1989; 2: 366–369. doi: 10.1183/09031936.93.02040366 [DOI] [PubMed] [Google Scholar]
- 24.Meyer PC. Metastatic carcinoma of the pleura. Thorax 1966; 21: 437–443. doi: 10.1136/thx.21.5.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stathopoulos GT, Kalomenidis I. Malignant pleural effusion: tumor-host interactions unleashed. Am J Respir Crit Care Med 2012; 186: 487–492. doi: 10.1164/rccm.201203-0465PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nasreen N, Mohammed KA, Sanders K, et al. . Pleural mesothelial cell (PMC) defense mechanisms against malignancy. Oncol Res 2003; 14: 155–161. doi: 10.3727/000000003771013053 [DOI] [PubMed] [Google Scholar]
- 27.Cui R, Takahashi F, Ohashi R, et al. . Osteopontin is involved in the formation of malignant pleural effusion in lung cancer. Lung Cancer 2009; 63: 368–374. doi: 10.1016/j.lungcan.2008.06.020 [DOI] [PubMed] [Google Scholar]
- 28.Stathopoulos GT, Kollintza A, Moschos C, et al. . Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer Res 2007; 67: 9825–9834. doi: 10.1158/0008-5472.CAN-07-1064 [DOI] [PubMed] [Google Scholar]
- 29.Stathopoulos GT, Psallidas I, Moustaki A, et al. . A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst 2008; 100: 1464–1476. doi: 10.1093/jnci/djn325 [DOI] [PubMed] [Google Scholar]
- 30.Psallidas I, Stathopoulos GT, Maniatis NA, et al. . Secreted phosphoprotein-1 directly provokes vascular leakage to foster malignant pleural effusion. Oncogene 2013; 32: 528–535. doi: 10.1038/onc.2012.57 [DOI] [PubMed] [Google Scholar]
- 31.Stathopoulos GT, Sherrill TP, Karabela SP, et al. . Host-derived interleukin-5 promotes adenocarcinoma-induced malignant pleural effusion. Am J Respir Crit Care Med 2010; 182: 1273–1281. doi: 10.1164/rccm.201001-0001OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giannou AD, Marazioti A, Spella M, et al. . Mast cells mediate malignant pleural effusion formation. J Clin Invest 2015; 125: 2317–2334. doi: 10.1172/JCI79840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahn SA. Pleural diseases related to metastatic malignancies. Eur Respir J 1997; 10: 1907–1913. doi: 10.1183/09031936.97.10081907 [DOI] [PubMed] [Google Scholar]
- 34.Kapp CM, Lee HJ. Malignant pleural effusions. Clin Chest Med 2021; 42: 687–696. doi: 10.1016/j.ccm.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 35.Estenne M, Yernault JC, De Troyer A. Mechanism of relief of dyspnea after thoracocentesis in patients with large pleural effusions. Am J Med 1983; 74: 813–819. doi: 10.1016/0002-9343(83)91072-0 [DOI] [PubMed] [Google Scholar]
- 36.Thomas R, Jenkins S, Eastwood PR, et al. . Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med 2015; 21: 338–345. doi: 10.1097/MCP.0000000000000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cartaxo AM, Vargas FS, Salge JM, et al. . Improvements in the 6-min walk test and spirometry following thoracentesis for symptomatic pleural effusions. Chest 2011; 139: 1424–1429. doi: 10.1378/chest.10-1679 [DOI] [PubMed] [Google Scholar]
- 38.Wang JS, Tseng CH. Changes in pulmonary mechanics and gas exchange after thoracentesis on patients with inversion of a hemidiaphragm secondary to large pleural effusion. Chest 1995; 107: 1610–1614. doi: 10.1378/chest.107.6.1610 [DOI] [PubMed] [Google Scholar]
- 39.Altschule MD, Zamcheck N. The effects of pleural effusion on respiration and circulation in man. J Clin Invest 1944; 23: 325–331. doi: 10.1172/JCI101498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perpina M, Benlloch E, Marco V, et al. . Effect of thoracentesis on pulmonary gas exchange. Thorax 1983; 38: 747–750. doi: 10.1136/thx.38.10.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muruganandan S, Azzopardi M, Thomas R, et al. . The Pleural Effusion And Symptom Evaluation (PLEASE) study of breathlessness in patients with a symptomatic pleural effusion. Eur Respir J 2020; 55: 1900980. doi: 10.1183/13993003.00980-2019 [DOI] [PubMed] [Google Scholar]
- 42.Garske LA, Kunarajah K, Zimmerman PV, et al. . In patients with unilateral pleural effusion, restricted lung inflation is the principal predictor of increased dyspnoea. PLoS One 2018; 13: e0202621. doi: 10.1371/journal.pone.0202621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aguilera Garcia Y, Palkar A, Koenig SJ, et al. . Assessment of diaphragm function and pleural pressures during thoracentesis. Chest 2020; 157: 205–211. doi: 10.1016/j.chest.2019.07.019 [DOI] [PubMed] [Google Scholar]
- 44.Umbrello M, Mistraletti G, Galimberti A, et al. . Drainage of pleural effusion improves diaphragmatic function in mechanically ventilated patients. Crit Care Resusc 2017; 19: 64–70. [PubMed] [Google Scholar]
- 45.Mishra EK, Muruganandan S, Clark A, et al. . Breathlessness predicts survival in patients with malignant pleural effusions: meta-analysis of individual patient data from five randomized controlled trials. Chest 2021; 160: 351–357. doi: 10.1016/j.chest.2021.02.052 [DOI] [PubMed] [Google Scholar]
- 46.Blackmore CC, Black WC, Dallas RV, et al. . Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol 1996; 3: 103–109. doi: 10.1016/S1076-6332(05)80373-3 [DOI] [PubMed] [Google Scholar]
- 47.Tsakok M, Hallifax R. Updates in pleural imaging. Clin Chest Med 2021; 42: 577–590. doi: 10.1016/j.ccm.2021.07.001 [DOI] [PubMed] [Google Scholar]
- 48.Yousefifard M, Baikpour M, Ghelichkhani P, et al. . Screening performance characteristic of ultrasonography and radiography in detection of pleural effusion; a meta-analysis. Emerg (Tehran) 2016; 4: 1–10. [PMC free article] [PubMed] [Google Scholar]
- 49.Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009; 64: 139–143. doi: 10.1136/thx.2008.100545 [DOI] [PubMed] [Google Scholar]
- 50.Yang PC, Luh KT, Chang DB, et al. . Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol 1992; 159: 29–33. doi: 10.2214/ajr.159.1.1609716 [DOI] [PubMed] [Google Scholar]
- 51.Shiroshita A, Nozaki S, Tanaka Y, et al. . Thoracic ultrasound for malignant pleural effusion: a systematic review and meta-analysis. ERJ Open Res 2020; 6: 00464-2020. doi: 10.1183/23120541.00464-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feller-Kopman DJ, Reddy CB, DeCamp MM, et al. . Management of malignant pleural effusions. An official ATS/STS/STR clinical practice guideline. Am J Respir Crit Care Med 2018; 198: 839–849. doi: 10.1164/rccm.201807-1415ST [DOI] [PubMed] [Google Scholar]
- 53.Salamonsen M, Dobeli K, McGrath D, et al. . Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology 2013; 18: 942–947. doi: 10.1111/resp.12088 [DOI] [PubMed] [Google Scholar]
- 54.Görg C, Bert T, Görg K, et al. . Colour Doppler ultrasound mapping of chest wall lesions. Br J Radiol 2005; 78: 303–307. doi: 10.1259/bjr/28232950 [DOI] [PubMed] [Google Scholar]
- 55.Kearney SE, Davies CW, Davies RJ, et al. . Computed tomography and ultrasound in parapneumonic effusions and empyema. Clin Radiol 2000; 55: 542–547. doi: 10.1053/crad.1999.0480 [DOI] [PubMed] [Google Scholar]
- 56.Arenas-Jiménez JJ, García-Garrigós E, Escudero-Fresneda C, et al. . Early and delayed phases of contrast-enhanced CT for evaluating patients with malignant pleural effusion. Results of pairwise comparison by multiple observers. Br J Radiol 2018; 91: 20180254. doi: 10.1259/bjr.20180254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leung AN, Muller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990; 154: 487–492. doi: 10.2214/ajr.154.3.2106209 [DOI] [PubMed] [Google Scholar]
- 58.Hallifax RJ, Haris M, Corcoran JP, et al. . Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015; 70: 192–193. doi: 10.1136/thoraxjnl-2014-206054 [DOI] [PubMed] [Google Scholar]
- 59.Porcel JM, Hernández P, Martínez-Alonso M, et al. . Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest 2015; 147: 502–512. doi: 10.1378/chest.14-0820 [DOI] [PubMed] [Google Scholar]
- 60.Nguyen NC, Tran I, Hueser CN, et al. . F-18 FDG PET/CT characterization of talc pleurodesis-induced pleural changes over time: a retrospective study. Clin Nucl Med 2009; 34: 886–890. doi: 10.1097/RLU.0b013e3181bece11 [DOI] [PubMed] [Google Scholar]
- 61.Sinha S, Swift AJ, Kamil MA, et al. . The role of imaging in malignant pleural mesothelioma: an update after the 2018 BTS guidelines. Clin Radiol 2020; 75: 423–432. doi: 10.1016/j.crad.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 62.Kruse M, Sherry SJ, Paidpally V, et al. . FDG PET/CT in the management of primary pleural tumors and pleural metastases. AJR Am J Roentgenol 2013; 201: W215–W226. doi: 10.2214/AJR.13.10572 [DOI] [PubMed] [Google Scholar]
- 63.Sahn SA. State of the art. The pleura. Am Rev Respir Dis 1988; 138: 184–234. doi: 10.1164/ajrccm/138.1.184 [DOI] [PubMed] [Google Scholar]
- 64.Light RW, MacGregor MI, Luchsinger PC, et al. . Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972; 77: 507–513. doi: 10.7326/0003-4819-77-4-507 [DOI] [PubMed] [Google Scholar]
- 65.Ashchi M, Golish J, Eng P, et al. . Transudative malignant pleural effusions: prevalence and mechanisms. South Med J 1998; 91: 23–26. doi: 10.1097/00007611-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 66.Clarkson B. Relationship between cell type, glucose concentration, and response to treatment in neoplastic effusions. Cancer 1964; 17: 914–928. doi: [DOI] [PubMed] [Google Scholar]
- 67.Sahn SA, Good JT Jr. Pleural fluid pH in malignant effusions. Diagnostic, prognostic, and therapeutic implications. Ann Intern Med 1988; 108: 345–349. doi: 10.7326/0003-4819-108-3-345 [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Panadero F, Lopez Mejias J. Low glucose and pH levels in malignant pleural effusions. Diagnostic significance and prognostic value in respect to pleurodesis. Am Rev Respir Dis 1989; 139: 663–667. doi: 10.1164/ajrccm/139.3.663 [DOI] [PubMed] [Google Scholar]
- 69.Beg S, Zanettini C, Queiroz L, et al. . Optimal fluid volume for detecting malignancy in serous effusions: a single institution experience. J Am Soc Cytopathol 2023; 12: 415–422. doi: 10.1016/j.jasc.2023.06.003 [DOI] [PubMed] [Google Scholar]
- 70.Rooper LM, Ali SZ, Olson MT. A minimum fluid volume of 75 mL is needed to ensure adequacy in a pleural effusion: a retrospective analysis of 2540 cases. Cancer Cytopathol 2014; 122: 657–665. doi: 10.1002/cncy.21452 [DOI] [PubMed] [Google Scholar]
- 71.Swiderek J, Morcos S, Donthireddy V, et al. . Prospective study to determine the volume of pleural fluid required to diagnose malignancy. Chest 2010; 137: 68–73. doi: 10.1378/chest.09-0641 [DOI] [PubMed] [Google Scholar]
- 72.Dalvi SD, Chau K, Sajjan S, et al. . Adequacy of pleural fluid cytology for comprehensive molecular analysis of lung adenocarcinoma: experience of a large health-care system. Cytojournal 2022; 19: 7. doi: 10.25259/Cytojournal_18_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hooper C, Lee YCG, Maskell N, et al. . Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010; 65: Suppl. 2, ii4–ii17. doi: 10.1136/thx.2010.136978 [DOI] [PubMed] [Google Scholar]
- 74.Arnold DT, De Fonseka D, Perry S, et al. . Investigating unilateral pleural effusions: the role of cytology. Eur Respir J 2018; 52: 1801254. doi: 10.1183/13993003.01254-2018 [DOI] [PubMed] [Google Scholar]
- 75.Hsu C. Cytologic detection of malignancy in pleural effusion: a review of 5,255 samples from 3,811 patients. Diagn Cytopathol 1987; 3: 8–12. doi: 10.1002/dc.2840030103 [DOI] [PubMed] [Google Scholar]
- 76.Johnston WW. The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 1985; 56: 905–909. doi: [DOI] [PubMed] [Google Scholar]
- 77.Garcia LW, Ducatman BS, Wang HH. The value of multiple fluid specimens in the cytological diagnosis of malignancy. Mod Pathol 1994; 7: 665–668. [PubMed] [Google Scholar]
- 78.Chai SM, Van Vliet C. Cytological diagnosis of malignant pleural mesothelioma. Curr Pulmonol Rep 2017; 6: 1–8. doi: 10.1007/s13665-017-0159-y [DOI] [Google Scholar]
- 79.Cavaco MJ, Mateus L, Nunes A, et al. . Diagnostic value of the cancer ratio in pleural effusion – a retrospective study. Eur Respir J 2022; 60: Suppl. 66, 3127. doi: 10.1183/13993003.congress-2022.3127 [DOI] [Google Scholar]
- 80.Verma A, Abisheganaden J, Light RW. Identifying malignant pleural effusion by a cancer ratio (serum LDH: pleural fluid ADA ratio). Lung 2016; 194: 147–153. doi: 10.1007/s00408-015-9831-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fafliora E, Hatzoglou C, Gourgoulianis KI, et al. . Systematic review and meta-analysis of vascular endothelial growth factor as a biomarker for malignant pleural effusions. Physiol Rep 2016; 4: e12978. doi: 10.14814/phy2.12978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen YC, Liu MQ, Wan C, et al. . Diagnostic accuracy of vascular endothelial growth factor for malignant pleural effusion: a meta-analysis. Exp Ther Med 2012; 3: 1072–1076. doi: 10.3892/etm.2012.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang M, Yan L, Lippi G, et al. . Pleural biomarkers in diagnostics of malignant pleural effusion: a narrative review. Transl Lung Cancer Res 2021; 10: 1557–1570. doi: 10.21037/tlcr-20-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y, Liu YL, Shi HZ. Diagnostic accuracy of combinations of tumor markers for malignant pleural effusion: an updated meta-analysis. Respiration 2017; 94: 62–69. doi: 10.1159/000468545 [DOI] [PubMed] [Google Scholar]
- 85.Mei F, Bonifazi M, Rota M, et al. . Diagnostic yield and safety of image-guided pleural biopsy: a systematic review and meta-analysis. Respiration 2021; 100: 77–87. doi: 10.1159/000511626 [DOI] [PubMed] [Google Scholar]
- 86.Cerci JJ, Bogoni M, Cerci RJ, et al. . PET/CT-guided biopsy of suspected lung lesions requires less rebiopsy than CT-guided biopsy due to inconclusive results. J Nucl Med 2021; 62: 1057–1061. doi: 10.2967/jnumed.120.252403 [DOI] [PubMed] [Google Scholar]
- 87.de Fonseka D, Underwood W, Stadon L, et al. . Randomised controlled trial to compare the diagnostic yield of positron emission tomography CT (PET-CT) TARGETed pleural biopsy versus CT-guided pleural biopsy in suspected pleural malignancy (TARGET trial). BMJ Open Respir Res 2018; 5: e000270. doi: 10.1136/bmjresp-2017-000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loddenkemper R, Lee P, Noppen M, et al. . Medical thoracoscopy/pleuroscopy: step by step. Breathe 2011; 8: 156–167. doi: 10.1183/20734735.011611 [DOI] [Google Scholar]
- 89.Shojaee S, Lee HJ. Thoracoscopy: medical versus surgical – in the management of pleural diseases. J Thorac Dis 2015; 7: Suppl. 4, S339–S351. doi: 10.3978/j.issn.2072-1439.2015.11.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fitzgerald DB, Koegelenberg CFN, Yasufuku K, et al. . Surgical and non-surgical management of malignant pleural effusions. Expert Rev Respir Med 2018; 12: 15–26. doi: 10.1080/17476348.2018.1398085 [DOI] [PubMed] [Google Scholar]
- 91.Clive AO, Kahan BC, Hooper CE, et al. . Predicting survival in malignant pleural effusion: development and validation of the LENT prognostic score. Thorax 2014; 69: 1098–1104. doi: 10.1136/thoraxjnl-2014-205285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Psallidas I, Kanellakis NI, Gerry S, et al. . Development and validation of response markers to predict survival and pleurodesis success in patients with malignant pleural effusion (PROMISE): a multicohort analysis. Lancet Oncol 2018; 19: 930–939. doi: 10.1016/S1470-2045(18)30294-8 [DOI] [PubMed] [Google Scholar]
- 93.Ault MJ, Rosen BT, Scher J, et al. . Thoracentesis outcomes: a 12-year experience. Thorax 2015; 70: 127–132. doi: 10.1136/thoraxjnl-2014-206114 [DOI] [PubMed] [Google Scholar]
- 94.Stawicki SP, Prosciak MP. The pulmonary artery catheter in 2008 – a (finally) maturing modality? Int J Crit Illn Inj Sci 2017; 7: 172–176. doi: 10.4103/IJCIIS.IJCIIS_57_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kasmani R, Irani F, Okoli K, et al. . Re-expansion pulmonary edema following thoracentesis. CMAJ 2010; 182: 2000–2002. doi: 10.1503/cmaj.090672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petiot A, Tawk S, Ghaye B. Re-expansion pulmonary oedema. Lancet 2018; 392: 507. doi: 10.1016/S0140-6736(18)31722-7 [DOI] [PubMed] [Google Scholar]
- 97.Cavanna L, Mordenti P, Bertè R, et al. . Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol 2014; 12: 139. doi: 10.1186/1477-7819-12-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson CB, Philpott GW, Ferguson TB. The treatment of malignant pleural effusions. Cancer 1974; 33: 916–922. doi: [DOI] [PubMed] [Google Scholar]
- 99.Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev 2004; 1: CD002916. doi: 10.1002/14651858.CD002916.pub2 [DOI] [PubMed] [Google Scholar]
- 100.Maskell NA, Lee YC, Gleeson FV, et al. . Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med 2004; 170: 377–382. doi: 10.1164/rccm.200311-1579OC [DOI] [PubMed] [Google Scholar]
- 101.Janssen JP, Collier G, Astoul P, et al. . Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet 2007; 369: 1535–1539. doi: 10.1016/S0140-6736(07)60708-9 [DOI] [PubMed] [Google Scholar]
- 102.Rintoul RC, Ritchie AJ, Edwards JG, et al. . Efficacy and cost of video-assisted thoracoscopic partial pleurectomy versus talc pleurodesis in patients with malignant pleural mesothelioma (MesoVATS): an open-label, randomised, controlled trial. Lancet 2014; 384: 1118–1127. doi: 10.1016/S0140-6736(14)60418-9 [DOI] [PubMed] [Google Scholar]
- 103.Rahman NM, Pepperell J, Rehal S, et al. . Effect of opioids vs NSAIDs and larger vs smaller chest tube size on pain control and pleurodesis efficacy among patients with malignant pleural effusion: the TIME1 randomized clinical trial. JAMA 2015; 314: 2641–2653. doi: 10.1001/jama.2015.16840 [DOI] [PubMed] [Google Scholar]
- 104.Dresler CM, Olak J, Herndon JE, et al. . Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005; 127: 909–915. doi: 10.1378/chest.127.3.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davies HE, Mishra EK, Kahan BC, et al. . Effect of an indwelling pleural catheter vs chest tube and talc pleurodesis for relieving dyspnea in patients with malignant pleural effusion: the TIME2 randomized controlled trial. JAMA 2012; 307: 2383–2389. doi: 10.1001/jama.2012.5535 [DOI] [PubMed] [Google Scholar]
- 106.Bhatnagar R, Piotrowska HEG, Laskawiec-Szkonter M, et al. . Effect of thoracoscopic talc poudrage vs talc slurry via chest tube on pleurodesis failure rate among patients with malignant pleural effusions: a randomized clinical trial. JAMA 2020; 323: 60–69. doi: 10.1001/jama.2019.19997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mercer RM, Macready J, Jeffries H, et al. . Clinically important associations of pleurodesis success in malignant pleural effusion: analysis of the TIME1 data set. Respirology 2020; 25: 750–755. doi: 10.1111/resp.13755 [DOI] [PubMed] [Google Scholar]
- 108.Psallidas I, Hassan M, Yousuf A, et al. . Role of thoracic ultrasonography in pleurodesis pathways for malignant pleural effusions (SIMPLE): an open-label, randomised controlled trial. Lancet Respir Med 2022; 10: 139–148. doi: 10.1016/S2213-2600(21)00353-2 [DOI] [PubMed] [Google Scholar]
- 109.Thomas R, Fysh ETH, Smith NA, et al. . Effect of an indwelling pleural catheter vs talc pleurodesis on hospitalization days in patients with malignant pleural effusion: the AMPLE randomized clinical trial. JAMA 2017; 318: 1903–1912. doi: 10.1001/jama.2017.17426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Putnam JB Jr, Light RW, Rodriguez RM, et al. . A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer 1999; 86: 1992–1999. doi: [DOI] [PubMed] [Google Scholar]
- 111.Efthymiou CA, Masudi T, Thorpe JAC, et al. . Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg 2009; 9: 961–964. doi: 10.1510/icvts.2009.211516 [DOI] [PubMed] [Google Scholar]
- 112.Qureshi RA, Collinson SL, Powell RJ, et al. . Management of malignant pleural effusion associated with trapped lung syndrome. Asian Cardiovasc Thorac Ann 2008; 16: 120–123. doi: 10.1177/021849230801600208 [DOI] [PubMed] [Google Scholar]
- 113.Pien GW, Gant MJ, Washam CL, et al. . Use of an implantable pleural catheter for trapped lung syndrome in patients with malignant pleural effusion. Chest 2001; 119: 1641–1646. doi: 10.1378/chest.119.6.1641 [DOI] [PubMed] [Google Scholar]
- 114.Bazerbashi S, Villaquiran J, Awan MY, et al. . Ambulatory intercostal drainage for the management of malignant pleural effusion: a single center experience. Ann Surg Oncol 2009; 16: 3482–3487. doi: 10.1245/s10434-009-0691-2 [DOI] [PubMed] [Google Scholar]
- 115.Muruganandan S, Azzopardi M, Fitzgerald DB, et al. . Aggressive versus symptom-guided drainage of malignant pleural effusion via indwelling pleural catheters (AMPLE-2): an open-label randomised trial. Lancet Respir Med 2018; 6: 671–680. doi: 10.1016/S2213-2600(18)30288-1 [DOI] [PubMed] [Google Scholar]
- 116.Wahidi MM, Reddy C, Yarmus L, et al. . Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions. The ASAP Trial. Am J Respir Crit Care Med 2017; 195: 1050–1057. doi: 10.1164/rccm.201607-1404OC [DOI] [PubMed] [Google Scholar]
- 117.Bhatnagar R, Keenan EK, Morley AJ, et al. . Outpatient talc administration by indwelling pleural catheter for malignant effusion. N Engl J Med 2018; 378: 1313–1322. doi: 10.1056/NEJMoa1716883 [DOI] [PubMed] [Google Scholar]
- 118.Puri V, Pyrdeck TL, Crabtree TD, et al. . Treatment of malignant pleural effusion: a cost-effectiveness analysis. Ann Thorac Surg 2012; 94: 374–380. doi: 10.1016/j.athoracsur.2012.02.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olfert JA, Penz ED, Manns BJ, et al. . Cost-effectiveness of indwelling pleural catheter compared with talc in malignant pleural effusion. Respirology 2017; 22: 764–770. doi: 10.1111/resp.12962 [DOI] [PubMed] [Google Scholar]
- 120.Penz ED, Mishra EK, Davies HE, et al. . Comparing cost of indwelling pleural catheter vs talc pleurodesis for malignant pleural effusion. Chest 2014; 146: 991–1000. doi: 10.1378/chest.13-2481 [DOI] [PubMed] [Google Scholar]
- 121.Tremblay A, Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 2006; 129: 362–368. doi: 10.1378/chest.129.2.362 [DOI] [PubMed] [Google Scholar]
- 122.Van Meter MEM, McKee KY, Kohlwes RJ. Efficacy and safety of tunneled pleural catheters in adults with malignant pleural effusions: a systematic review. J Gen Intern Med 2011; 26: 70–76. doi: 10.1007/s11606-010-1472-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ost DE, Jimenez CA, Lei X, et al. . Quality-adjusted survival following treatment of malignant pleural effusions with indwelling pleural catheters. Chest 2014; 145: 1347–1356. doi: 10.1378/chest.13-1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miyazaki T, Sakai T, Yamasaki N, et al. . Chest tube insertion is one important factor leading to intercostal nerve impairment in thoracic surgery. Gen Thorac Cardiovasc Surg 2014; 62: 58–63. doi: 10.1007/s11748-013-0328-z [DOI] [PubMed] [Google Scholar]
- 125.Messeder SJ, Thomson MC, Hu MK, et al. . Indwelling pleural catheters: an overview and real-life experience. QJM 2019; 112: 599–604. doi: 10.1093/qjmed/hcz116 [DOI] [PubMed] [Google Scholar]
- 126.Thomas R, Piccolo F, Miller D, et al. . Intrapleural fibrinolysis for the treatment of indwelling pleural catheter-related symptomatic loculations: a multicenter observational study. Chest 2015; 148: 746–751. doi: 10.1378/chest.14-2401 [DOI] [PubMed] [Google Scholar]
- 127.Fitzgerald DB, Muruganandan S, Tsim S, et al. . Intrapleural fibrinolytics and deoxyribonuclease for treatment of indwelling pleural catheter-related pleural infection: a multi-center observational study. Respiration 2021; 100: 452–460. doi: 10.1159/000514643 [DOI] [PubMed] [Google Scholar]
- 128.Fysh ETH, Tremblay A, Feller-Kopman D, et al. . Clinical outcomes of indwelling pleural catheter-related pleural infections: an international multicenter study. Chest 2013; 144: 1597–1602. doi: 10.1378/chest.12-3103 [DOI] [PubMed] [Google Scholar]
- 129.Wang S, Zhang R, Wan C, et al. . Incidence of complications from indwelling pleural catheter for pleural effusion: a meta-analysis. Clin Transl Sci 2023; 16: 104–117. doi: 10.1111/cts.13430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nasim F, Folch E, Majid A. Tunneled pleural catheter dysfunction: case report and review of complications. J Bronchology Interv Pulmonol 2012; 19: 149–152. doi: 10.1097/LBR.0b013e3182537d05 [DOI] [PubMed] [Google Scholar]
- 131.Thomas R, Budgeon CA, Kuok YJ, et al. . Catheter tract metastasis associated with indwelling pleural catheters. Chest 2014; 146: 557–562. doi: 10.1378/chest.13-3057 [DOI] [PubMed] [Google Scholar]
- 132.Mitchell MA, Li P, Pease C, et al. . Catheter tract metastasis in mesothelioma patients with indwelling pleural catheters: a retrospective cohort study. Respiration 2019; 97: 428–435. doi: 10.1159/000494500 [DOI] [PubMed] [Google Scholar]
- 133.Faiz SA, Pathania P, Song J, et al. . Indwelling pleural catheters for patients with hematologic malignancies. A 14-year, single-center experience. Ann Am Thorac Soc 2017; 14: 976–985. doi: 10.1513/AnnalsATS.201610-785OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhatnagar R, Reid ED, Corcoran JP, et al. . Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax 2014; 69: 959–961. doi: 10.1136/thoraxjnl-2013-204563 [DOI] [PubMed] [Google Scholar]
- 135.Matus I, Colt H. Pleural catheter fracture during IPC removal: an under-reported complication. J Bronchology Interv Pulmonol 2021; 28: e1–e3. doi: 10.1097/LBR.0000000000000684 [DOI] [PubMed] [Google Scholar]
- 136.Fysh ETH, Wrightson JM, Lee YCG, et al. . Fractured indwelling pleural catheters. Chest 2012; 141: 1090–1094. doi: 10.1378/chest.11-0724 [DOI] [PubMed] [Google Scholar]
- 137.Bhatnagar R, Kahan BC, Morley AJ, et al. . The efficacy of indwelling pleural catheter placement versus placement plus talc sclerosant in patients with malignant pleural effusions managed exclusively as outpatients (IPC-PLUS): study protocol for a randomised controlled trial. Trials 2015; 16: 48. doi: 10.1186/s13063-015-0563-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sidhu C, Davies HE, Muruganandan S, et al. . Indwelling pleural catheter: management of complications. Semin Respir Crit Care Med 2023; 44: 454–461. doi: 10.1055/s-0043-1769093 [DOI] [PubMed] [Google Scholar]
- 139.Lui MMS, Thomas R, Lee YCG. Complications of indwelling pleural catheter use and their management. BMJ Open Respir Res 2016; 3: e000123. doi: 10.1136/bmjresp-2015-000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rahman NM, Maskell NA, West A, et al. . Intrapleural use of tissue plasminogen activator and DNase in pleural infection. N Engl J Med 2011; 365: 518–526. doi: 10.1056/NEJMoa1012740 [DOI] [PubMed] [Google Scholar]
- 141.Janes SM, Rahman NM, Davies RJ, et al. . Catheter-tract metastases associated with chronic indwelling pleural catheters. Chest 2007; 131: 1232–1234. doi: 10.1378/chest.06-2353 [DOI] [PubMed] [Google Scholar]
- 142.Scarci M, Caruana E, Bertolaccini L, et al. . Current practices in the management of malignant pleural effusions: a survey among members of the European Society of Thoracic Surgeons. Interact Cardiovasc Thorac Surg 2017; 24: 414–417. doi: 10.1093/icvts/ivw373 [DOI] [PubMed] [Google Scholar]
- 143.Hunt BM, Farivar AS, Vallières E, et al. . Thoracoscopic talc versus tunneled pleural catheters for palliation of malignant pleural effusions. Ann Thorac Surg 2012; 94: 1053–1057. doi: 10.1016/j.athoracsur.2012.01.103 [DOI] [PubMed] [Google Scholar]
- 144.Schulze M, Boehle AS, Kurdow R, et al. . Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg 2001; 71: 1809–1812. doi: 10.1016/S0003-4975(01)02586-3 [DOI] [PubMed] [Google Scholar]
- 145.Barbetakis N, Asteriou C, Papadopoulou F, et al. . Early and late morbidity and mortality and life expectancy following thoracoscopic talc insufflation for control of malignant pleural effusions: a review of 400 cases. J Cardiothorac Surg 2010; 5: 27. doi: 10.1186/1749-8090-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Trotter D, Aly A, Siu L, et al. . Video-assisted thoracoscopic (VATS) pleurodesis for malignant effusion: an Australian teaching hospital's experience. Heart Lung Circ 2005; 14: 93–97. doi: 10.1016/j.hlc.2005.02.004 [DOI] [PubMed] [Google Scholar]
- 147.Terra RM, Junqueira JJM, Teixeira LR, et al. . Is full postpleurodesis lung expansion a determinant of a successful outcome after talc pleurodesis? Chest 2009; 136: 361–368. doi: 10.1378/chest.08-2448 [DOI] [PubMed] [Google Scholar]
- 148.Yim AP, Chan AT, Lee TW, et al. . Thoracoscopic talc insufflation versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg 1996; 62: 1655–1658. doi: 10.1016/S0003-4975(96)00808-9 [DOI] [PubMed] [Google Scholar]
- 149.Arapis K, Caliandro R, Stern JB, et al. . Thoracoscopic palliative treatment of malignant pleural effusions: results in 273 patients. Surg Endosc 2006; 20: 919–923. doi: 10.1007/s00464-005-0534-6 [DOI] [PubMed] [Google Scholar]
- 150.Cardillo G, Facciolo F, Carbone L, et al. . Long-term follow-up of video-assisted talc pleurodesis in malignant recurrent pleural effusions. Eur J Cardiothorac Surg 2002; 21: 302–305. doi: 10.1016/S1010-7940(01)01130-7 [DOI] [PubMed] [Google Scholar]
- 151.Yoon DW, Cho JH, Choi YS, et al. . Predictors of survival in patients who underwent video-assisted thoracic surgery talc pleurodesis for malignant pleural effusion. Thorac Cancer 2016; 7: 393–398. doi: 10.1111/1759-7714.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bayman EO, Parekh KR, Keech J, et al. . A prospective study of chronic pain after thoracic surgery. Anesthesiology 2017; 126: 938–951. doi: 10.1097/ALN.0000000000001576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fitzgerald DB, Sidhu C, Budgeon C, et al. . Australasian Malignant PLeural Effusion (AMPLE)-3 trial: study protocol for a multi-centre randomised study comparing indwelling pleural catheter (±talc pleurodesis) versus video-assisted thoracoscopic surgery for management of malignant pleural effusion. Trials 2022; 23: 530. doi: 10.1186/s13063-022-06405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huggins JT, Maldonado F, Chopra A, et al. . Unexpandable lung from pleural disease. Respirology 2018; 23: 160–167. doi: 10.1111/resp.13199 [DOI] [PubMed] [Google Scholar]
- 155.Hassan M, Gadallah M, Mercer RM, et al. . Predictors of outcome of pleurodesis in patients with malignant pleural effusion: a systematic review and meta-analysis. Expert Rev Respir Med 2020; 14: 645–654. doi: 10.1080/17476348.2020.1746647 [DOI] [PubMed] [Google Scholar]
- 156.Light RW, Jenkinson SG, Minh VD, et al. . Observations on pleural fluid pressures as fluid is withdrawn during thoracentesis. Am Rev Respir Dis 1980; 121: 799–804. doi: 10.1164/arrd.1980.121.5.799 [DOI] [PubMed] [Google Scholar]
- 157.Boshuizen RC, Sinaasappel M, Vincent AD, et al. . Pleural pressure swing and lung expansion after malignant pleural effusion drainage: the benefits of high-temporal resolution pleural manometry. J Bronchology Interv Pulmonol 2013; 20: 200–205. doi: 10.1097/LBR.0b013e31829af168 [DOI] [PubMed] [Google Scholar]
- 158.Feller-Kopman D, Berkowitz D, Boiselle P, et al. . Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg 2007; 84: 1656–1661. doi: 10.1016/j.athoracsur.2007.06.038 [DOI] [PubMed] [Google Scholar]
- 159.Lentz RJ, Lerner AD, Pannu JK, et al. . Routine monitoring with pleural manometry during therapeutic large-volume thoracentesis to prevent pleural-pressure-related complications: a multicentre, single-blind randomised controlled trial. Lancet Respir Med 2019; 7: 447–455. doi: 10.1016/S2213-2600(18)30421-1 [DOI] [PubMed] [Google Scholar]
- 160.Wong A, Patail H, Ahmad S. The absent sinusoid sign. Ann Am Thorac Soc 2019; 16: 506–508. doi: 10.1513/AnnalsATS.201810-654CC [DOI] [PubMed] [Google Scholar]
- 161.Herman DD, Cooper AZ, Esguerra V. Is the finding of an absent “sinusoid sign” on lung ultrasound meaningful? Ann Am Thorac Soc 2019; 16: 1075. doi: 10.1513/AnnalsATS.201904-309LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Salamonsen MR, Lo AKC, Ng ACT, et al. . Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest 2014; 146: 1286–1293. doi: 10.1378/chest.13-2876 [DOI] [PubMed] [Google Scholar]
- 163.Rathinam S, Waller DA. Pleurectomy decortication in the treatment of the “trapped lung” in benign and malignant pleural effusions. Thorac Surg Clin 2013; 23: 51–61. doi: 10.1016/j.thorsurg.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 164.Mishra EK, Clive AO, Wills GH, et al. . Randomized controlled trial of urokinase versus placebo for nondraining malignant pleural effusion. Am J Respir Crit Care Med 2018; 197: 502–508. doi: 10.1164/rccm.201704-0809OC [DOI] [PubMed] [Google Scholar]
- 165.Saydam O, Karapinar K, Gokce M, et al. . The palliative treatment with intrapleural streptokinase in patients with multiloculated malignant pleural effusion: a double-blind, placebo-controlled, randomized study. Med Oncol 2015; 32: 612. doi: 10.1007/s12032-015-0612-0 [DOI] [PubMed] [Google Scholar]
- 166.Okur E, Baysungur V, Tezel C, et al. . Streptokinase for malignant pleural effusions: a randomized controlled study. Asian Cardiovasc Thorac Ann 2011; 19: 238–243. doi: 10.1177/0218492311410874 [DOI] [PubMed] [Google Scholar]
- 167.Roberts ME, Rahman NM, Maskell NA, et al. . British Thoracic Society Guideline for pleural disease. Thorax 2023; 78: Suppl. 3, s1–s42. doi: 10.1136/thorax-2022-219784 [DOI] [PubMed] [Google Scholar]
- 168.Baird A-M, Gray S. Chapter 22 – Epigenetics of cisplatin resistance. In: Gray SG, ed. Epigenetic Cancer Therapy. 2nd Edn. London, Elsevier Inc., 2023; pp. 577–611. [Google Scholar]
- 169.Zhao WY, Chen DY, Chen JH, et al. . Effects of intracavitary administration of Endostar combined with cisplatin in malignant pleural effusion and ascites. Cell Biochem Biophys 2014; 70: 623–628. doi: 10.1007/s12013-014-9965-9 [DOI] [PubMed] [Google Scholar]
- 170.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020; 20: 651–668. doi: 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gandhi L, Garassino MC. Pembrolizumab plus chemotherapy in lung cancer. N Engl J Med 2018; 379: e18. doi: 10.1056/NEJMc1808567 [DOI] [PubMed] [Google Scholar]
- 172.Garon EB, Rizvi NA, Hui R, et al. . Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372: 2018–2028. doi: 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 173.Grosu HB, Arriola A, Stewart J, et al. . PD-L1 detection in histology specimens and matched pleural fluid cell blocks of patients with NSCLC. Respirology 2019; 24: 1198–1203. doi: 10.1111/resp.13614 [DOI] [PubMed] [Google Scholar]
- 174.Kawachi H, Tamiya M, Tamiya A, et al. . Association between metastatic sites and first-line pembrolizumab treatment outcome for advanced non-small cell lung cancer with high PD-L1 expression: a retrospective multicenter cohort study. Invest New Drugs 2020; 38: 211–218. doi: 10.1007/s10637-019-00882-5 [DOI] [PubMed] [Google Scholar]
- 175.Morita M, Tamiya M, Fujimoto D, et al. . Prediction of patients with a tumor proportion score >50% who do not respond to first-line monotherapy with pembrolizumab. BMC Cancer 2020; 20: 93. doi: 10.1186/s12885-020-6582-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Usui K, Sugawara S, Nishitsuji M, et al. . A phase II study of bevacizumab with carboplatin-pemetrexed in non-squamous non-small cell lung carcinoma patients with malignant pleural effusions: North East Japan Study Group Trial NEJ013A. Lung Cancer 2016; 99: 131–136. doi: 10.1016/j.lungcan.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 177.Chellappan DK, Leng KH, Jia LJ, et al. . The role of bevacizumab on tumour angiogenesis and in the management of gynaecological cancers: a review. Biomed Pharmacother 2018; 102: 1127–1144. doi: 10.1016/j.biopha.2018.03.061 [DOI] [PubMed] [Google Scholar]
- 178.Takemoto S, Fukuda M, Yamaguchi H, et al. . Phase II study of ramucirumab and docetaxel for previously treated non-small cell lung cancer patients with malignant pleural effusion: protocol of PLEURAM study. Thorac Cancer 2020; 11: 389–393. doi: 10.1111/1759-7714.13279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Prager GW, Lackner EM, Krauth MT, et al. . Targeting of VEGF-dependent transendothelial migration of cancer cells by bevacizumab. Mol Oncol 2010; 4: 150–160. doi: 10.1016/j.molonc.2010.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ishii H, Yazawa T, Sato H, et al. . Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs). Lung Cancer 2004; 45: 325–337. doi: 10.1016/j.lungcan.2004.02.021 [DOI] [PubMed] [Google Scholar]
- 181.Huang R, Zhan Q, Zhou X, et al. . Continuous administration of recombinant human endostatin (Endostar): a pre-clinical safety study. Exp Ther Med 2012; 3: 1018–1022. doi: 10.3892/etm.2012.534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Greene J, Segaran A, Lord S. Targeting OXPHOS and the electron transport chain in cancer; molecular and therapeutic implications. Semin Cancer Biol 2022; 86: 851–859. doi: 10.1016/j.semcancer.2022.02.002 [DOI] [PubMed] [Google Scholar]
- 183.Peters S, Camidge DR, Shaw AT, et al. . Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017; 377: 829–838. doi: 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 184.Shaw AT, Solomon BJ, Chiari R, et al. . Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: a multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol 2019; 20: 1691–1701. doi: 10.1016/S1470-2045(19)30655-2 [DOI] [PubMed] [Google Scholar]
- 185.Lee KWC, Li MSC, Gai W, et al. . Testing for EGFR variants in pleural and pericardial effusion cell-free DNA in patients with non-small cell lung cancer. JAMA Oncol 2023; 9: 261–265. doi: 10.1001/jamaoncol.2022.6109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Skoulidis F, Li BT, Dy GK, et al. . Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med 2021; 384: 2371–2381. doi: 10.1056/NEJMoa2103695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jänne PA, Riely GJ, Gadgeel SM, et al. . Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N Engl J Med 2022; 387: 120–131. doi: 10.1056/NEJMoa2204619 [DOI] [PubMed] [Google Scholar]
- 188.Shen B, Tan M, Wang Z, et al. . The meta-analysis of bevacizumab combined with platinum-based treatment of malignant pleural effusions by thoracic perfusion. J Oncol 2022; 2022: 1476038. doi: 10.1155/2022/1476038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nie K, Zhang Z, You Y, et al. . A randomized clinical study to compare intrapleural infusion with intravenous infusion of bevacizumab in the management of malignant pleural effusion in patients with non-small-cell lung cancer. Thorac Cancer 2020; 11: 8–14. doi: 10.1111/1759-7714.13238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Biaoxue R, Xiguang C, Hua L, et al. . Thoracic perfusion of recombinant human endostatin (Endostar) combined with chemotherapeutic agents versus chemotherapeutic agents alone for treating malignant pleural effusions: a systematic evaluation and meta-analysis. BMC Cancer 2016; 16: 888. doi: 10.1186/s12885-016-2935-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang W, Jiang X, Zhang Y, et al. . Intracavitary chemotherapy with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) is not superior to TKI monotherapy in controlling malignant pleural effusion recurrence in EGFR-mutated lung cancer patients. J Thorac Dis 2019; 11: 3712–3720. doi: 10.21037/jtd.2019.09.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Seto T, Ushijima S, Yamamoto H, et al. . Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer: a multi-institutional phase II trial. Br J Cancer 2006; 95: 717–721. doi: 10.1038/sj.bjc.6603319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kleontas A, Sioga A, Pandria N, et al. . Clinical factors affecting the survival of patients diagnosed with non-small cell lung cancer and metastatic malignant pleural effusion, treated with hyperthermic intrathoracic chemotherapy or chemical talc pleurodesis: a monocentric, prospective, randomized trial. J Thorac Dis 2019; 11: 1788–1798. doi: 10.21037/jtd.2019.05.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jones DR, Taylor MD, Petroni GR, et al. . Phase I trial of intrapleural docetaxel administered through an implantable catheter in subjects with a malignant pleural effusion. J Thorac Oncol 2010; 5: 75–81. doi: 10.1097/JTO.0b013e3181c07ddc [DOI] [PubMed] [Google Scholar]
- 195.Işık AF, Şanlı M, Yılmaz M, et al. . Intrapleural hyperthermic perfusion chemotherapy in subjects with metastatic pleural malignancies. Respir Med 2013; 107: 762–767. doi: 10.1016/j.rmed.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 196.Choi MG, Park S, Oh DK, et al. . Effect of medical thoracoscopy-guided intrapleural docetaxel therapy to manage malignant pleural effusion in patients with non-small cell lung cancer: a pilot study. Thorac Cancer 2019; 10: 1885–1892. doi: 10.1111/1759-7714.13158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sheffield BS, Hwang HC, Lee AF, et al. . BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015; 39: 977–982. doi: 10.1097/PAS.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 198.Scherpereel A, Opitz I, Berghmans T, et al. . ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020; 55: 1900953. doi: 10.1183/13993003.00953-2019 [DOI] [PubMed] [Google Scholar]
- 199.Louw A, Badiei A, Creaney J, et al. . Advances in pathological diagnosis of mesothelioma: what pulmonologists should know. Curr Opin Pulm Med 2019; 25: 354–361. doi: 10.1097/MCP.0000000000000578 [DOI] [PubMed] [Google Scholar]
- 200.Illei PB, Rusch VW, Zakowski MF, et al. . Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res 2003; 9: 2108–2113. [PubMed] [Google Scholar]
- 201.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. . Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–2644. doi: 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 202.Baas P, Scherpereel A, Nowak AK, et al. . First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021; 397: 375–386. doi: 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 203.Zhou N, Rice DC, Tsao AS, et al. . Extrapleural pneumonectomy versus pleurectomy/decortication for malignant pleural mesothelioma. Ann Thorac Surg 2022; 113: 200–208. doi: 10.1016/j.athoracsur.2021.04.078 [DOI] [PubMed] [Google Scholar]
- 204.Butchart EG, Ashcroft T, Barnsley WC, et al. . Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976; 31: 15–24. doi: 10.1136/thx.31.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Paajanen J, Jaklitsch MT, Bueno R. Contemporary issues in the surgical management of pleural mesothelioma. J Surg Oncol 2023; 127: 343–354. doi: 10.1002/jso.27152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Muruganandan S, Jeffery E, McIntyre C, et al. . Nutrition, exercise, and complementary medicine: potential role in mesothelioma? Current Pulm Rep 2016; 5: 20–27. doi: 10.1007/s13665-016-0133-0 [DOI] [Google Scholar]
- 207.Jeffery E, Lee YCG, Newton RU, et al. . Body composition and nutritional status in malignant pleural mesothelioma: implications for activity levels and quality of life. Eur J Clin Nutr 2019; 73: 1412–1421. doi: 10.1038/s41430-019-0418-9 [DOI] [PubMed] [Google Scholar]
- 208.Jeffery E, Lee YCG, Newton RU, et al. . Changes in body composition in patients with malignant pleural mesothelioma and the relationship with activity levels and dietary intake. Eur J Clin Nutr 2022; 76: 979–986. doi: 10.1038/s41430-021-01062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Peddle-McIntyre CJ, Baker MK, Lee YCG, et al. . The feasibility of a pragmatic distance-based intervention to increase physical activity in lung cancer survivors. Eur J Cancer Care 2018; 27: e12722. doi: 10.1111/ecc.12722 [DOI] [PubMed] [Google Scholar]
- 210.Peddle-McIntyre CJ, Singh F, Thomas R, et al. . Exercise training for advanced lung cancer. Cochrane Database Syst Rev 2019; 2: CD012685. doi: 10.1002/14651858.CD012685.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peddle-McIntyre CJ, Muruganandan S, McVeigh J, et al. . Device assessed activity behaviours in patients with indwelling pleural catheter: a sub-study of the Australasian Malignant PLeural Effusion (AMPLE)-2 randomized trial. Respirology 2023; 28: 561–570. doi: 10.1111/resp.14451 [DOI] [PubMed] [Google Scholar]
- 212.Peddle-McIntyre CJ, Cavalheri V, Boyle T, et al. . A review of accelerometer-based activity monitoring in cancer survivorship research. Med Sci Sports Exerc 2018; 50: 1790–1801. doi: 10.1249/MSS.0000000000001644 [DOI] [PubMed] [Google Scholar]




