Summary
Patients with inflammatory diseases such as rheumatoid arthritis treated with IL-6 pathway antagonists are particularly prone to potentially serious infections. However, diagnosis of such events can be hindered by the normality of usual inflammatory biomarkers such as C reactive protein (CRP). In this retrospective monocentric study, we included all patients treated with tocilizumab for an inflammatory disease (mostly rheumatoid arthritis) and hospitalised in an unscheduled manner between 2009 and 2020 for any symptoms that raised suspicion of infection to explore the discrimating capacity of easily-accessible biomarkers to diagnose infections in patients treated with anti-IL6 receptor. We showed here for the first time that eosinopenia (eosinophil count (EC) <0.05 G/L) and a low ratio EC/neutrophil count (NC) (EC/NC×1000<11.8) could be relevant biomarkers for the diagnosis of infection in tocilizumab-treated patients. Moreover, despite what was expected, CRP level above 5 mg/L can be an additional biomarker of infection.
Results
Patients treated with IL-6 pathway antagonists like tocilizumab are more prone to infections,1 but diagnosis of such complications remains challenging and is often delayed as NC and CRP increase is usually abrogated by treatment.2 Consequently, reliable IL-6-independant biomarkers of infection are needed. Eosinopenia and low ratio between EC and NC (EC/NC×1000) in blood have already been reported to be associated with bacterial infections in other settings.3 The aim of this original study was to assess if such biomarkers were associated with the diagnosis of infection in tocilizumab-treated patients.
This single-centre, retrospective and observational study included all patients treated with tocilizumab for an inflammatory disease and hospitalised in an unscheduled manner between 2009 and 2020 for any symptoms that raised suspicion of infection. Final diagnoses were made according to current guidelines, depending on clinical symptoms, laboratory and imaging tests results. Areas under the receiver operating characteristic curve (AUC) were performed to assess the discriminating capacity of EC, CRP, NC, lymphocyte count (LC) and EC/NC×1000 at admission to predict the diagnosis of infection.
Forty-one patients out of 163 treated with tocilizumab for an inflammatory disease were hospitalised in an unscheduled manner during the study period. Median age was 59 years (IQR (52–66)). Thirty-four (82.9%) patients were female. An infectious disease was diagnosed in 20 patients (48.8%). The most common infections were pneumonia (30.0%), joint or bone infection (25.0%) and gastrointestinal tract infection (15.0%). Four (20.0%) patients had a bacteraemia, including three associated with another infected organ. No relevant differences were found between infected and non-infected patients regarding age, sex, inflammatory disease and steroid treatment. Median absolute value of EC at admission was lower in infected patients (0.06 (IQR (0.01–0.16)) G/L) than in non-infected patients (0.20 (IQR (0.11–0.29)) G/L; p=0.010) as were LC (1.40 (IQR (0.97–1.64)) G/L vs 1.84 (IQR(1.35–2.30)) G/L, p=0.014) and EC/NC×1000 ratio (6.54 (IQR (1.33–30.60)) vs 48.50 (IQR (27.50–76.70)), p<0010), whereas CRP level (3.00 (IQR (1.10–120.30)) mg/L vs 0.65 (IQR (0.30–1.58)) mg/L, p=0.008) and NC (7.43 (IQR (5.46–9.48)) G/L vs 3.90 (IQR (2.27–6.90)) G/L, p=0.020) were significantly increased in infected patients (online supplemental table 1). To better help physicians in the diagnosis, thresholds for these biomarkers can be helpful: An EC≤0.04 G/L yielded a sensitivity (Se) of 0.50 (95% CI (29.9 to 70.1)) and a specificity (Sp) of 0.91 (95% CI (0.711 to 0.983)). A CRP concentration increase above 5 mg/L was observed for more than half of the infected patients (11/19) in contrast to only 3 patients (3/20) in the non-infected group (SE 0.58 (95% CI (0.36 to 0.77)), Sp 0.85 (95% CI (0.64 to 0.95))).
rmdopen-2023-003998supp001.pdf (93.7KB, pdf)
AUC for EC, CRP, NC, LC and EC/NC×1000 all showed good discriminating performances between infected and non-infected patients in the whole studied population (figure 1).
Figure 1.
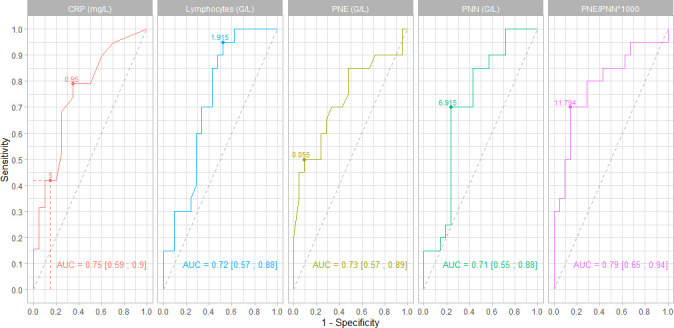
Areas under the receiver operating characteristic and their 95% CIs were performed to assess the discrimination capacity of C reactive protein (CRP), lymphocytes count (LC), eosinophil count (EC), neutrophil count (NC) and EC/NC×1000. For each parameter, thresholds suggested by Youden Index are represented. Se and 1−Sp for CRP<5 mg/L is represented on the first curve. CRP, C reactive protein; Se, sensitivity; Sp, specificity.
Some publications have previously reported the interest of EC and of combining EC and NC to help predicting the probability of infection at hospital admission in immunocompetent populations.4 Vigouroux et al have reported that the combination of eosinopenia and an NC>10 500 cells/mm3 allowed to differentiate septic from crystal-induced arthritis with a good Sp and positive predictive value.5 We showed here for the first time that an EC<0.05 G/L and EC/NC×1000<11.8 could be relevant biomarkers for the diagnosis of infection in tocilizumab-treated patients. Moreover, despite what was expected, CRP level above 5 mg/L can be an additional biomarker of infection. In conclusion, this original study suggests that all those easily available parameters should be used to maximise Se in the screening of infection in patients undergoing treatment with IL-6 pathway antagonists.
Footnotes
Contributors: AG made substantial contribution to acquisition of all clinical data, analyses and interpretation of data and in drafting the article. MP made substantial contribution to statistical analyses and in revising the manuscript critically. MH made substantial contribution to statistical analyses and in revising the manuscript critically. JHS made substantial contribution to acquisition of all clinical data and in revising the manuscript critically. AS made substantial contribution to conception of the study and in revising the manuscript critically. AR made substantial contributions to conception and design, interpretation of data and in revising the manuscript critically for important intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
The data that support the findings of this study are mostly available within the article and supplementary data. More deidentified data (particularly comparison between eosinopenic and non-eosinopenic patients) could be shared on reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Database was constituted in accordance with the reference methodology MR004 of the Commission Nationale de l’Informatique et des Libertés (no 2206749, 13/09/2018). As such, non-opposition was obtained from each patient included in the study for the use of their deidentified medical record data and data management was in compliance with current French legislation governing nominative personal data, the General Data Protection Regulation of the European Union, and the French legislation pertaining to informatics and liberties of 6 January 1978 and its modification in 2018.
References
- 1.Rutherford AI, Subesinghe S, Hyrich KL, et al. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis 2018;77:905–10. 10.1136/annrheumdis-2017-212825 [DOI] [PubMed] [Google Scholar]
- 2.Nagai Y, Yokogawa N, Shimada K, et al. Comparison of the clinical characteristics and severity of community-acquired pneumonia between patients with rheumatoid arthritis treated with tocilizumab and those treated with TNF inhibitor. Mod Rheumatol 2019;29:782–7. 10.1080/14397595.2018.1515059 [DOI] [PubMed] [Google Scholar]
- 3.Abidi K, Khoudri I, Belayachi J, et al. Eosinopenia is a reliable marker of sepsis on admission to medical intensive care units. Crit Care 2008;12:R59. 10.1186/cc6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouldoires B, Gil H, Soumagne T, et al. A predictive bacterial infection score according to eosinophil level: An observational study. Rev Med Interne 2018;39:10–6. 10.1016/j.revmed.2017.10.425 [DOI] [PubMed] [Google Scholar]
- 5.Vigouroux A, Ostertag A, Crémieux A-C, et al. Eosinopenia to differentiate crystal-induced and septic arthritis. Ann Rheum Dis 2022;81:1201–2. 10.1136/annrheumdis-2022-222322 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003998supp001.pdf (93.7KB, pdf)
Data Availability Statement
The data that support the findings of this study are mostly available within the article and supplementary data. More deidentified data (particularly comparison between eosinopenic and non-eosinopenic patients) could be shared on reasonable request to the corresponding author.


