Abstract
Background
In anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV), histopathological assessment of affected tissue is often necessary for diagnosis and assessment of disease extent. There is a requirement for validated non-invasive biomarkers to avoid the need for serial tissue biopsies.
Methods
A systematic review of scientific databases from 2012 until present was performed to identify studies fulfilling the inclusion criteria. Studies were assessed for quality using the Strengthening the Reporting of Observational Studies in Epidemiology checklist for cohort, case–control and cross-sectional studies and the Risk of Bias Assessment tool for Non-randomised Studies, or the Cochrane Risk of Bias tool 2.0 for randomised controlled trials. A descriptive synthesis of the data for non-invasive (blood-based or urinary) biomarkers of AAV-related disease activity and organ damage was performed.
Results
Twenty-two high quality studies were included. These articles reported the value of blood-based and urinary biomarkers including anti-neutrophil cytoplasmic antibodies, immune cells, complement factors, gene expression profiles, cytokines, chemokines and other proteins in the assessment of disease activity and/or organ damage in patients with AAV. Many of these biomarkers involve the alternative complement pathway, neutrophil activation and macrophage activation.
Conclusion
This is the first contemporary systematic review synthesising the value of non-invasive biomarkers of AAV-related disease activity and organ damage. The incorporation of individual markers in combined biomarker profiles might enhance clinical decision-making. Many unmet needs were identified; few studies involve oeosinophilic granulomatosis with polyangiitis and patients with childhood-onset AAV. Further validation of the candidate biomarkers is warranted in large prospective studies to bridge the existing knowledge gaps and apply precision health to systemic vasculitis.
Keywords: Systemic vasculitis, Autoimmune Diseases, Cytokines, Granulomatosis with polyangiitis
WHAT IS ALREADY KNOWN ON THIS TOPIC
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a complex and heterogeneous disease leading to severe multisystem involvement.
Histopathological assessment of affected tissue, mainly the kidney, is often required for the diagnosis of ANCA-associated vasculitis and assessment of its activity, disease extent and prognosis.
Renal biopsy is invasive and implies associated risks such as haemorrhagic complications.
WHAT THIS STUDY ADDS
Various blood-based and urinary biomarkers including immune cell subsets, alternative complement pathway components and neutrophil-related gene expression scores can discriminate active AAV from remission.
Several non-invasive biomarkers assess AAV-related organ damage and correlate with specific histopathological features.
Nonetheless, this systematic review identifies multiple important knowledge gaps and unmet needs in AAV research.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The application of non-invasive biomarkers in validated multiplexed biomarker profiles may reduce the need for serial tissue biopsies in patients with AAV.
In-depth analysis of these biomarkers may further elucidate the pathophysiology of AAV and might lead to more targeted treatment options.
The reported knowledge gaps must be addressed before precision health can be applied to the field of AAV.
Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a complex and heterogeneous disease, characterised by autoantibody-mediated necrotising inflammation of small blood vessels often causing severe multisystem organ damage. Lungs and kidneys are especially at risk of permanent dysfunction. Pulmonary capillaritis may lead to diffuse alveolar haemorrhage, with an associated mortality of up to 25%.1 2 AAV-related kidney disease typically manifests as a pauci-immune rapidly progressive necrotising crescentic glomerulonephritis (GN), with a 5-year end-stage renal disease (ESRD)-free survival rate of 54% and a 5-year cumulative relapse rate of 35%.3 Prudent assessment of AAV-related organ involvement is therefore crucial.
Presently, a common, but invasive biomarker used to diagnose AAV and evaluate its activity, extent and prognosis is histopathological assessment of affected tissue, typically by kidney biopsy. Histopathological classification of ANCA-associated GN impacts patient outcomes with the focal class (≥50% normal glomeruli) involving the most favourable and the sclerotic class (≥50% globally sclerosed glomeruli) the most detrimental prognosis.4 However, biopsy-associated risks including haemorrhagic complications and psychological trauma in children may arise. Moreover, ANCA-associated GN often warrants repeated renal biopsies due to its relapsing-remitting course.
High-performing non-invasive biomarkers are therefore needed in the assessment of AAV-related disease activity and/or organ damage. Furthermore, biomarkers may guide personalised therapy as several targeted treatment options may be readily available in the future.5–7 Selecting the optimal treatment for individual patients will enhance drug efficacy while avoiding the toxicity of broad immunosuppression. Validated blood-based and/or urinary biomarker profiles may therefore aid in bringing the concept of precision health to the field of the systemic vasculitides.
The objectives of this systematic review were (1) to synthesise the available evidence of non-invasive biomarkers of disease activity and/or organ damage in AAV and (2) to delineate those biomarkers within the underlying pathophysiology of AAV by correlating them with histopathological features.
Methods
Search strategy
A systematic review of the literature was performed for the time frame 1 April 2012 to 1 April 2023. Searched databases included Ovid MEDLINE, Ovid Embase, PubMed, Cochrane Library, Web of Science and ClinicalTrials.gov. Searches were performed applying the Medical Subject Headings terms AAV, granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA), microscopic polyangiitis (MPA), Churg-Strauss syndrome, Wegener’s granulomatosis and biomarker. The full search strategy is outlined in the online supplemental materials.
rmdopen-2023-003579supp001.pdf (80.3KB, pdf)
Eligibility criteria
Studies were eligible if the following inclusion criteria were fulfilled: (1) AAV (or subtypes: GPA, EGPA, MPA) is the disease of interest; (2) the research concerns human data (children and/or adults); (3) the manuscript reports one or multiple non-invasive biomarkers assessing disease activity and/or organ damage; (4) sample size involves ≥3 patients; (5) the manuscript involves original research; (6) full-length article is available; and (7) the report is written in English. Reviews, case reports, (conference) abstracts, editorials and articles on imaging biomarkers were excluded. Studies resulting from the search strategy were screened for content by title and abstract. Relevant studies were selected for full-text review and assessed for the above inclusion criteria by two reviewers (TR and NG). A third reviewer (SB) made the final decision in case of disagreement.
Quality assessment
Selected studies were assessed for quality including risk of bias by applying the Strengthening the Reporting of Observational Studies in Epidemiology checklist for cohort, case–control and cross-sectional studies, the Risk of Bias Assessment tool for Non-randomised Studies and the Cochrane Risk Of Bias tool V.2.0 for randomised trials by two assessors (TR and SB).8–10 Studies were included in the systematic review on consensus of both assessors. Only studies with a low risk of bias were included.
Evidence synthesis
Data were clustered based on biomarkers assessing AAV-related disease activity versus organ damage. Moreover, data were grouped in blood-based versus urinary biomarkers. Data were further subdivided regarding the nature of the biomarker including antibodies, immune cells and subpopulations, complement factors, gene expression profiles and others such as cytokines and chemokines. Outcomes of the included studies were concisely summarised. Considering the nature of the included studies and their published data, a meta-analysis was not feasible.
Results
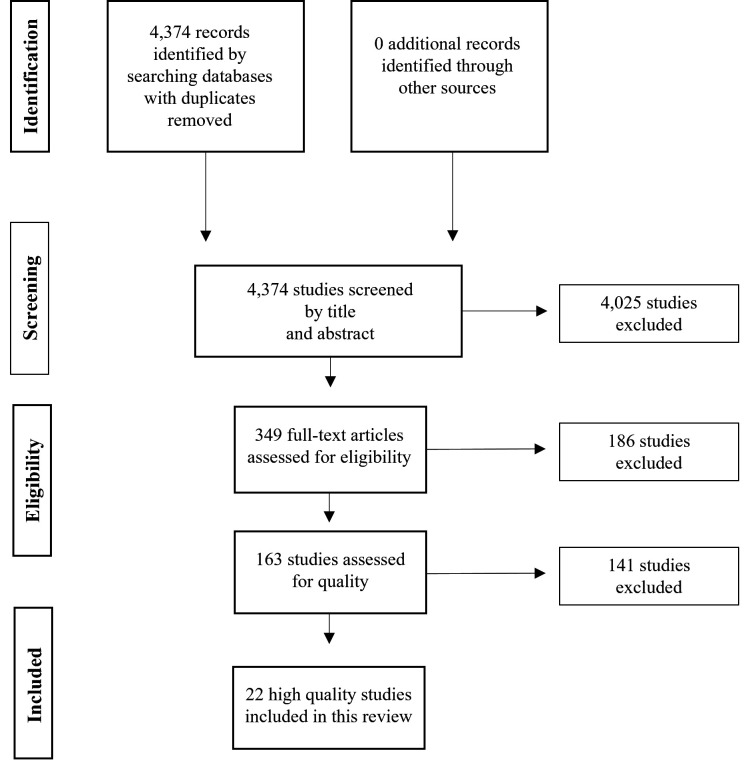
Our search strategy identified 4374 unique articles (figure 1). After screening of title and abstract, 349 relevant articles were selected for full-text assessment. Of those, 163 studies fulfilled the eligibility criteria and were assessed for quality. Twenty-two studies were scored as high quality and were included in this systematic review.
Figure 1.
Flowchart of the article selection process.
Study characteristics and assessed biomarkers
The selected studies included a total of 2362 patients with AAV, of which a majority were myeloperoxidase (MPO)-ANCA positive (≥52.5%) (table 1). Few patients with EGPA were included (n≥66). Few studies included juvenile patients (<18 years). Nineteen studies (86%) reported disease activity biomarkers, whereas three studies (14%) reported organ damage-related biomarkers. Reported non-invasive biomarkers include complement factors, immune cell subsets, cytokines and cytokine receptors, chemokines, gene expression profiles, ANCAs, scavenger receptors, immune checkpoint proteins, matrikines and matrixins, metabolites, glycoproteins, growth factors, mitochondrial DNA and lipocalins (table 2).
Table 1.
Patient characteristics of the included studies
| Study | Patients (n) | GPA n (%) | MPA n (%) | EGPA n (%) | PR3+ n (%) | MPO+ n (%) | Age (years*) | Sex (male/female) (n) |
| Watanabe et al11 | 271 | 34 (12.5) | 183 (67.5) | 15 (5.5) | 0 | 271 (100) | 73 (65–78) | 106/165 |
| Bunch et al12 | 54 | 18 (33.3)† | 18 (33.3)† | 0 | 23 (42.6) | 20 (37.0) | Active: 58 (48–68) Remission: 58 (38–66) |
30/24 |
| Matsumoto et al13 | 29 | 13 (44.8) | 16 (55.2) | 0 | 7 (24.1) | 21 (72.4) | 70 (57–79) | 13/16 |
| Chen et al14 | 82 | 14 (17.1) | 68 (82.9) | 0 | 0 | 82 (100) | 60±13 | 41/41 |
| Gou et al15 | 100‡ | NR | NR | NR | 0 | 100 (100) | Active: 55±36 Remission: 59±15 |
Active: 29/37 Remission: 21/33 |
| Grayson et al16 | 112 | 87 (77.7) | 25 (22.3) | 0 | 78 (69.6) | 34 (30.4) | Non-responder: 51±15 Responder: 52±18 | 53/59 |
| Yanaoka et al17 | 26 | 23 (88.5) | 3 (11.5) | 0 | 8 (30.8) | 18 (69.2) | 68 (58–75) | 7/19 |
| Monach et al18 | 186 | 139 (74.7) | 46 (24.7) | 0 | 124 (66.7) | 62 (33.3) | 52 (44–66) | 91/95 |
| Monach et al19 | 74 | 57 (77.0) | 16 (21.6) | 0 | 54 (73.0) | 20 (27.0) | 51 (40–63) | 33/41 |
| Ahn et al20 | 60 | 17 (28.3) | 32 (53.3) | 11 (18.3) | 7 (11.7)§ | 37 (61.7)§ | 59±14 | 20/40 |
| Yoon et al21 | 59 | 18 (30.5) | 30 (50.8) | 11 (18.6) | 7 (11.9) | 35 (59.3) | 60±14 | 21/38 |
| Sun et al22 | 32 | NR | NR | NR | 2 (6.3) | 30 (93.8) | 59±12 | 13/19 |
| Kambas et al23 | 17 | 11 (64.7) | 6 (35.3) | 0 | 9 (52.9) | 8 (47.1) | 58±12 | 5/12 |
| Aendekerk et al24 | 95 | 55 (57.9) | 37 (38.9) | 3 (3.2) | 45 (47.4)§ | 47 (49.5)§ | NR | 62/33 |
| O’Reilly et al, inception25 | 177¶ | 101 (57.1) | 54 (30.5) | 12 (6.8) | NR | NR | 64 (range 21–90) | 87/90 |
| O’Reilly et al, validation 125 | 155¶ | 94 (60.6) | 49 (31.6) | 6 (3.9) | NR | NR | 60 (range 17–95) | 97/58 |
| O’Reilly et al, validation 225 | 133** | 57 (42.9) | 59 (44.4) | 8 (6.0) | NR | NR | 60 (range 18–91) | 80/53 |
| O’Reilly et al, Ext. validation25 | 39 | 28 (71.8) | 11 (28.2) | 0 | NR | NR | 59 (range 31–82) | 16/23 |
| Dekkema et al26 | 154 | NR | NR | NR | 93 (60.4) | 61 (39.6) | Inception: 58 (47–67) Validation: 61 (48–74) |
87/67 |
| Wu et al27 | 256†† | |||||||
| Active | 173 | NR | NR | NR | 11 (6.4) | 162 (93.6) | 59±14 | 66/107 |
| Remission | 143 | NR | NR | NR | 4 (2.8) | 53 (37.1) | 60±12 | 61/82 |
| Gou et al28 | 29 | NR | NR | NR | 0 | 29 (100) | 58±15 | 12/17 |
| Sonnemann et al29 | 87 | 53 (60.9) | 34 (39.1) | 0 | 48 (55.2) | 39 (44.8) | 57 (48–64)‡‡ 44 (38–51)§§ 63 (55–71)¶¶ 67 (56–73)*** 50 (44–58)††† 64 (52–68)‡‡‡ |
54/33 |
| Sparding et al30 | 47 | NR | NR | NR | 21 (44.7) | 23 (48.9) | 62 (55–69) | 28/19 |
| Matsuda et al31 | 49 | 0 | 49 (100) | 0 | 3 (6.1)§§§ | 48 (98.0)§§§ | ILD: 71 (68–77) No ILD: 76 (71–82) |
25/24 |
| Wu et al32 | 39 | NR | NR | NR | 0 | 39 (100) | 58±100¶¶¶ 63±14**** 57±14†††† |
22/17 |
*Median (IQR) or mean±SD.
†Seven renal limited patients.
‡Twenty patients had both active and remission samples.
§One patient MPO-ANCA and PR3-ANCA positive.
¶Three anti-GBM disease patients were included.
**Five anti-GBM disease patients were included.
††Sixty patients had samples at both active disease stage and remission.
‡‡Active renal disease, inception cohort.
§§Active non-renal disease, inception cohort.
¶¶Remission, inception cohort.
***Active renal disease, validation cohort.
†††Active non-renal disease, validation cohort.
‡‡‡Remission, validation cohort.
§§§Two patients MPO-ANCA and PR3-ANCA positive.
¶¶¶Group of patients without kidney dysfunction .
****Group of patients who did not need dialysis.
††††Group of patients requiring dialysis.
ANCA, anti-neutrophil cytoplasmic antibody ; EGPA, eosinophilic granulomatosis with polyangiitis; Ext., external; GPA, granulomatosis with polyangiitis; ILD, interstitial lung disease; MPA, microscopic polyangiitis; MPO, myeloperoxidase; n, number; NR, not reported; PR3, roteinase 3.
Table 2.
Overview of the included non-invasive biomarkers of anti-neutrophil cytoplasmic antibody-associated vasculitis-related disease activity and/or organ damage
| Blood-based biomarkers of disease activity | Urinary biomarkers of disease activity |
ANCAs
Circulating immune cells
Complement factors
Gene expression profiles
Others
|
Complement factors
Others
|
| Blood-based biomarkers of organ damage | Urinary biomarkers of organ damage |
|
|
ANCA, anti-neutrophil cytoplasmic antibody .Bb, factor B (Bb); CFH, complement factor H; IL, interleukin; MPO, myeloperoxidase.sC5b-9, soluble (s)C5b-9.
Evidence synthesis: biomarkers of disease activity
Blood-based biomarkers of disease activity
ANCAs
The value of ANCAs in the assessment of AAV disease activity remains elusive. Watanabe et al found a significant association between MPO-ANCA reappearance and AAV relapse in a nested case–control study.11 Of the 181 patients with conversion to negative MPO-ANCA at remission, 25 (14%) experienced a relapse. Patients in the relapse group were more likely to convert to a positive MPO-ANCA compared with controls after adjusting for potential confounders including age, sex, AAV classification and baseline Birmingham Vasculitis Activity Score (BVAS) (76% vs 12%, p<0.0001; OR 26.2, 95% CI 8.2 to 101). Mean time between MPO-ANCA reappearance and relapse was 34 days.
Circulating immune cells
The role of specific immune cell subsets in the assessment of AAV-related disease activity is currently being explored. Flow cytometry-based immune cell phenotyping demonstrated a significant decrease in the percentage of CD5+ B cells (regulatory B cells) in patients with rituximab-naïve active AAV (median 17%, IQR 10–28) compared with those in remission (26%, IQR 21–36; p=0.02) and healthy controls (28%, IQR 21–35; p<0.001).12 The percentage of CD5+ B cells significantly increased during transition from active disease to remission following rituximab treatment (14%, IQR 10–17 vs 25%, IQR 17–45; p=0.008). Time to relapse was significantly shorter in those who repopulated B cells with ≤30% CD5+ cells compared with those with a normal amount of CD5+ B cells (median 7 months, IQR 3–11 vs 22 months, IQR 17–36; p=0.002). Matsumoto et al described higher levels of CD14++ CD16+ intermediate monocytes at AAV relapse compared with remission (median 58 /µL, IQR 34–79 vs 26 /µL, IQR 7.3–50, p=0.027 for relapse compared with first remission; 24 /µL, IQR 11–43, p=0.049 for relapse compared with second remission).13 CD14++ CD16+ intermediate monocyte levels correlated significantly with BVAS (β=0.82, 95% CI 0.25 to 1.4; p=0.005). Monocyte activation-related cytokines, including interleukin (IL)-1β (β=1.0, 95% CI 0.57 to 1.5, p<0.001), IL-6 (β=0.89, 95% CI 0.56 to 1.2, p<0.001), IL-8 (β=0.60, 95% CI 0.10 to 1.1, p=0.018) and tumour necrosis factor-α (β=0.91, 95% CI 0.43 to 1.4, p<0.001) showed significant associations with BVAS, which corroborates the main study results.
Complement factors
Increasing evidence suggests that the alternative complement pathway is involved in AAV pathophysiology. Lower levels of plasma complement factor H (CFH) were found in active MPO-ANCA positive patients with biopsy-confirmed renal involvement (417.87±119.74 µg/mL) compared with those in remission (551.33±114.12 µg/mL; p<0.001) and to age-matched and sex-matched healthy controls (559.72±87.92 µg/mL; p<0.001).14 CFH distinguished active disease from remission with an area under the curve (AUC) of 0.79 (95% CI 0.70 to 0.89; p<0.001) and an optimal cut-off value of 441.74 µg/mL (61% sensitivity, 89% specificity). CFH levels correlated significantly with BVAS (r=−0.34, p=0.002), renal function (r=−0.42, p<0.001 for serum creatinine; r=0.43, p<0.001 for estimated glomerular filtration rate (eGFR)) and the proportion of total crescents (r=−0.33, p=0.003) and cellular crescents (r=−0.37, p<0.001) on renal histopathology. Patients with CFH levels in the lowest tertile had the poorest renal outcome during follow-up. Plasma levels of soluble (s)C5b-9 (955.81±321.39 vs 345.99±106.84 ng/mL; p<0.01), C5a (51.65±34.75 vs 9.78±5.81 ng/mL; p<0.01), C3a (2178.67±668.91 vs 268.50±211.53 ng/mL; p<0.01) and activated factor B (Bb) (1.30±0.68 vs 0.62±0.34 ng/mL; p<0.01) were significantly higher in active MPO-ANCA positive patients with biopsy-confirmed renal involvement compared with remission.15 Properdin levels were significantly lower in active AAV compared with remission (10.35±5.64 vs 35.96±13.87 ng/mL; p<0.01). Bb levels correlated with the proportion of cellular crescents on renal histopathology (r=0.358, p<0.01).
Gene expression profiles
Patterns of gene expression at the cellular level may provide insights about disease activity. Grayson et al observed significant gene expression differences in 2346 transcripts between responders and non-responders in the Rituximab in ANCA-associated Vasculitis (RAVE) trial.16 An unsupervised hierarchical clustering model depicted a distinct cluster of 179 genes mainly including granulocyte-related genes. From this cluster, a granulocyte multigene composite score was calculated. The mean granulocyte composite score was higher in non-responders compared with responders (0.11, range −0.43 to 7.59 vs −0.27, range −0.46 to 3.70; p=0.02). In the responder group, granulocyte composite scores were higher at baseline compared with remission (−0.17, range −0.46 to 3.70 vs −0.39, range −0.47 to 0.53; p<0.01). Multivariate analyses (adjusted for potential confounders, including age, BVAS, neutrophil count, haemoglobin and use of glucocorticoids) demonstrated an association between higher composite scores and the inability to meet the primary outcome (ie, treatment response) in the RAVE trial (OR 2.13, 95% CI 1.16 to 3.90; p=0.01). An iterative weighted gene correlation network and random forest analysis by Yanaoka et al identified a neutrophil module, comprising pro-inflammatory genes such as S100A12 and S100A9 and neutrophil extracellular traps (NET)osis-related genes, as the most important gene module in the differentiation of patients with AAV with healthy controls.17 The neutrophil module correlated significantly with disease state as it was more abundant in patients with active AAV compared with patients in remission (p<0.05).
Other biomarkers
Several other blood-based biomarkers including cytokines and chemokines may help in differentiating active AAV from remission. Monach et al assessed multiple serum proteins in 186 patients with active, severe GPA or MPA participating in the RAVE trial.18 MMP-3 (AUC 0.89; optimal cut-off 38 ng/mL with sensitivity 82% and specificity 88%), CXCL13 (AUC 0.86; cut-off 70 pg/mL, sensitivity 77%, specificity 85%) and TIMP-1 (AUC 0.83; cut-off 270 ng/mL, sensitivity 78%, specificity 82%) comprised the best discrimination between active disease and remission. A follow-up study demonstrated that CXCL13, IL-6, IL-8, IL-15, IL-18BP and MMP-3 were significantly associated with AAV disease activity.19 Among these, IL-8, IL-15 and IL-18BP were the most promising serum proteins associated with AAV flare. Results were independent of age, sex, ANCA specificity, treatment and new-onset or relapsing disease. Serum chitinase-3-like 1 protein (YKL-40) levels were found to be significantly higher in patients with severe AAV (p=0.007) and high Five Factor Score (FFS) (p<0.001).20 YKL-40 was significantly associated with BVAS (β=0.301, 95% CI 0.005 to 0.035, p=0.012) and FFS (β=0.476, 95% CI 0.002 to 0.006, p<0.001) in multivariate analyses. Serum soluble programmed cell death protein 1 (sPD-1) correlated significantly with severe AAV in a cohort of 59 patients (380.7 pg/mL vs 180.3 pg/mL; p=0.015).21 sPD-1 correlated significantly with BVAS (β=0.367, p=0.004). Serum spinghosine-1-phosphate (S1P), important in C5a-mediated neutrophil activation, was significantly higher in active AAV compared with remission (1789.48±510.10 vs 1462.48±498.31 nmol/L, p<0.05).22 Eighteen out of 20 subjects (90%) with paired active disease versus remission samples had a decrease of their S1P level transitioning into remission. The staining of S1P receptors S1PR1-5 was assessed in renal biopsies of 24 patients with AAV. Mean optical density of S1PR2 correlated significantly with BVAS (r=0.477, p=0.019) and C-reactive protein (CRP) levels (r=0.560, p=0.046), S1PR3 with CRP (r=0.676, p=0.011) and cellular crescents (r=0.488, p=0.014), and S1PR4 with cellular crescents (r=0.451, p=0.027). Granulocytes of patients with active AAV release tissue factor (TF) expressing NETs, which was attenuated after treatment initiation.23 Higher serum levels of TF expressing microparticles were detected in patients with active AAV compared with remission (p<0.05). These levels correlated significantly with BVAS (r=0.496, p=0.006). Increased TF expression was observed in granulocytes isolated from bronchoalveolar lavage fluid from patients with AAV. TF expressing NETs were also seen in nasal biopsy samples from patients with AAV and glomerular AAV lesions.
Urinary biomarkers of disease activity
Multiplexed urinalysis may provide valuable information on glomerular inflammatory processes in AAV, reducing the need for renal biopsy. Aendekerk et al detected high urinary sCD163 levels (>30 ng/mmol; 94% sensitivity, 91% specificity) in 96 out of 110 patients (87%) with active ANCA-associated GN, compared with only 1 out of 15 patients (7%) without inflammation on renal biopsy (p<0.001).24 Patients with crescentic GN had higher urinary sCD163 levels compared with those in the focal, sclerotic or mixed class disease. sCD163 correlated with fibrinoid necrosis (r=0.48, p<0.001), capillary breaks (r=0.51, p<0.001) and crescents (r=0.70, p<0.001) on histopathology and inversely with the proportion of normal glomeruli (r=−0.49, p<0.001). O’Reilly et al found higher levels of normalised (ie, normalised ratio to urinary creatinine level to control for urinary flow rate variations) urinary sCD163 in patients with active renal vasculitis (0.56 ng/mmol) compared with renal vasculitis in remission (0.11 ng/mmol, p<0.001), active extrarenal vasculitis (0.12 ng/mmol, p<0.01) and extrarenal vasculitis in remission (0.1 ng/mmol, p<0.001) (AUC 0.94 with cut-off 0.3 ng/mmol).25 These results were validated in three independent cohorts. Dekkema et al demonstrated that serum and urinary sCD25, an IL-2 alpha receptor shed from T cells on activation, can complement urinary sCD163 in the assessment of ANCA-associated GN activity.26 Nonetheless, urinary sCD163 represented the single most reliable biomarker for active ANCA-associated GN. A decision tree comprising these three non-invasive biomarkers may be applied in clinical practice.
In a study by Wu et al normalised urinary epidermal growth factor (EGF) levels were significantly lower in patients with active renal AAV compared with those in remission (2.04±1.41 vs 2.63±1.31, p<0.001).27 EGF levels were significantly associated with eGFR(log2) (β=0.60, p<0.001 at active stage; β=0.74, p<0.001 at remission) and inversely with the grade of interstitial fibrosis and tubular atrophy (β=−0.37, p=0.005). Patients with renal treatment failure had lower normalised urinary EGF levels pretreatment compared with those with a good renal response (1.65±1.22 vs 2.16±1.26, p=0.04). EGF levels were a predictor of the composite endpoint, that is, ESRD or 30% reduction of eGFR, in multivariate analyses (HR 0.61, 95% CI 0.45 to 0.83, p=0.001).
Gou et al demonstrated that normalised urinary Bb (0.11 (0.04–0.21) vs 0.01 (0–0.03) µg/mg creatinine, p<0.001), C3a (3.25 (0.99–13.30) vs 0.02 (0.01–0.05) ng/mg creatinine, p<0.001), C5a (1.93 (0.67–9.01) vs 0.09 (0.01–0.05) ng/mg creatinine, p<0.001), sC5b-9 (52.73 (3.46–142.44) vs 0.20 (0–2.89) ng/mg creatinine, p<0.001), C1q (3.69 (0.91–4.76) vs 0.17 (0.04–0.39) ng/mg creatinine, p<0.001) and MBL levels (6.36 (2.15–43.57) vs 0.30 (0.16–0.87) ng/mg creatinine, p<0.001) were higher in patients with active ANCA-associated GN compared with remission.28 Bb levels correlated with both the serum creatinine (r=0.56, p=0.002) and the proportion of total crescents in the renal tissue (r=0.40, p=0.04) and inversely with the proportion of normal glomeruli (r=−0.49, p=0.009).
Urinary T cells may also inform us about ANCA-associated GN activity. Sonnemann et al performed flow cytometry to assess T-cell subset levels in the urine of 57 patients with AAV.29 CD3+, CD4+ and regulatory T (Treg) cell counts were significantly increased in patients with active ANCA-associated GN. CD3+ and Treg cell counts were the most promising biomarkers with an AUC of 0.95 and 0.92, respectively (cut-off 3149 CD3+ cells per 100 mL (sensitivity 90%, specificity 92%); 60 Treg cells per 100 mL (sensitivity 74%, specificity 96%)). The results were confirmed in an independent validation cohort. Interestingly, no differences in serum CD3+, CD4+ and Treg cell counts were found between the respective groups. Urinary T-cell subpopulations therefore differ from blood-based T-cell subpopulations in patients with AAV.
Evidence synthesis: biomarkers of disease-related organ damage
Blood-based biomarkers of disease-related organ damage
Currently, renal biopsy is the gold standard for the assessment of glomerular sclerosis and fibrosis. However, non-invasive methods such as blood-based and urinary biomarkers may complement tissue assessment. Serum endotrophin, a matrikine derived from collagen type IV, correlated with serum creatinine (r=0.81, p<0.0001) and inversely with eGFR (r=−0.82, p<0.0001) in 47 patients with AAV with renal involvement.30 Levels were higher in patients with chronic kidney disease (CKD) stage three (p<0.01), four (p<0.001) or five (p<0.0001), compared with those with CKD stage one. Serum levels correlated significantly with the extent of renal fibrosis ((log10 serum endotrophin) rpartial=0.32, p=0.003, when adjusted for serum creatinine levels) and to the percentage of sclerotic glomeruli (r=0.36, p<0.05). Endotrophin was able to identify patients with advanced interstitial fibrosis (AUC=0.799, p<0.001) and patients with advanced interstitial fibrosis and tubular atrophy (AUC=0.861, p<0.001). Higher levels were found in patients with crescentic (p<0.01) or mixed GN (p<0.001) versus focal GN. Baseline endotrophin levels could identify patients with worsening CKD over a 3-year follow-up period (AUC=0.807, 95% CI 0.615 to 0.931; p<0.001).
Biomarkers may also be of added value in the assessment of AAV-related lung involvement. High CCL2 serum levels in 49 patients with MPA were significantly associated with the presence of interstitial lung disease (ILD) in multivariate analyses (327.6 (IQR 296.8–461.6) pg/mL vs 249.1 (IQR 188.5–319.5) pg/mL; p=0.0018).31 The optimal CCL2 cut-off for ILD diagnosis was 276.2 pg/mL (AUC=0.77, sensitivity 88%, specificity 65%). Baseline CCL2 levels correlated with total lung fibrosis scores at 1-year follow-up (r=0.51, p=0.005). Immunohistochemistry analysis of lung biopsies from three patients with MPA-associated ILD revealed intense CCL2 staining in CD68+/CD163 macrophages and metaplastic epithelial cells.
Urinary biomarkers of disease-related organ damage
Normalised urinary endotrophin levels correlated with serum creatinine (r=0.69, p<0.0001), renal fibrosis (r=0.40, p<0.05), glomerular sclerosis (r=0.47, p<0.01) and inversely with eGFR (r=−0.72, p<0.0001) in patients with ANCA-associated GN.30 Levels were higher in patients with stage four (p<0.001) or five (p<0.0001) CKD, compared with those with stage one CKD. Urinary endotrophin discriminated patients with advanced interstitial fibrosis (AUC=0.759, p=0.002) and patients with advanced interstitial fibrosis and tubular atrophy (AUC=0.839, p<0.001). Urinary mitochondrial (mt)DNA levels correlated negatively with eGFR (r=−0.515, p<0.001) in 39 patients with MPO-ANCA-associated GN.32 Patients warranting dialysis at disease onset had the highest mtDNA levels. Levels were higher in patients with crescentic GN (4703.08±1744.31 copy/nmol creatinine) compared with those in the mixed (3258.14±1158.99, p=0.029) and focal class (2268.15±1897.63, p=0.001).
Discussion
This is the first contemporary comprehensive review synthesising the value of non-invasive biomarkers in the assessment of disease activity and organ damage in patients with AAV. Several interesting conclusions can be drawn from the evidence synthesis that may impact clinical decision-making as well as the overall understanding of AAV pathophysiology.
Summarised, various non-invasive biomarkers may complement our current assessment of AAV disease activity. The reappearance of MPO-ANCAs is a significant predictor of relapse. Measurement of immune cell subsets, neutrophil-related gene expression composite scores, alternative complement pathway factors and several other proteins including sCD25, MMP-3, CXCL13, TIMP-1, IL-8, IL-15, IL18-BP, YKL-40, sPD-1 and S1P may aid in discriminating active AAV from remission. Urinary levels of sCD163 and several alternative complement pathway components are increased in active ANCA-associated GN versus remission. Furthermore, some non-invasive biomarkers aid in the assessment of AAV-related organ damage. Serum and urinary endotrophin and urinary mtDNA levels reflect renal function. Interestingly, CCL2 levels reflect the presence of ILD. Several non-invasive biomarkers correlate with histopathological features, such as the proportion of renal crescents, glomerular sclerosis and lung fibrosis. Non-invasive biomarkers may therefore aid in predicting histopathology results and long-term prognosis. Collectively, application of validated blood-based and/or urinary biomarkers may reduce the requirement for invasive tissue biopsies in the future and may aid in further elucidating the pathophysiology of AAV.
Although the diagnostic role of ANCA is well-defined, the value of ANCA levels in the assessment of AAV disease activity remains less clear. ANCAs develop due to a loss of T and B-cell tolerance to the neutrophil proteins MPO and/or PR3.33 ANCAs will subsequently attach to these proteins and promote neutrophil activation. The only high quality study included in this systematic review evaluating the role of ANCAs in the assessment of disease activity demonstrated that the reappearance of MPO-ANCA following clinical remission is a significant predictor of relapse.11 This finding was recently confirmed by a meta-analysis of 20 studies, showing that a rise in ANCA level often precedes a relapse within 6 months (OR 3.65, 95% CI 1.66 to 8.03).34 An older meta-analysis of nine studies demonstrated that a rising ANCA titre at remission modestly predicted a relapse (summary estimates for positive likelihood 2.84, 95% CI 1.65 to 4.90).35 At a minimum, this data warrants caution in the follow-up and management of patients with AAV in clinical remission with a reappearing or rising ANCA level.
Mounting evidence indicates that the alternative complement pathway is involved in the pathophysiology of AAV. The first reports date back to 2007 and 2009, when it was demonstrated that C5-deficient and factor B-deficient mice are protected from developing GN in an MPO-AAV mouse model and C5a receptor deficient mouse develop less anti-MPO-induced necrotising crescentic GN.36 37 Renal involvement in AAV is historically described as a pauci-immune necrotising crescentic GN. Nonetheless, several studies reported the deposition of sC5b-9, C3c, C3d, C4d and factor B in glomeruli of patients with ANCA-associated GN.28 38 39 Glomerular Bb deposition correlated with the proportion of crescents and the extent of interstitial fibrosis and tubular atrophy, and inversely with the proportion of normal glomeruli.28 Concordantly, studies described in this review demonstrated the correlation between serum and urinary levels of several alternative complement pathway components and disease activity, renal function and the proportion of crescents on renal histopathology in patients with AAV.14 15 28 Those studies were largely corroborated by a meta-analysis by Moiseev et al comprising five studies, describing increased plasma levels of sC5b-9 and C5a in patients with active AAV compared with patients in remission.40 The important role of the alternative complement pathway in AAV pathophysiology has direct implications; complement cascade inhibition is a promising treatment strategy in patients with AAV. Avacopan, a C5a receptor antagonist, has shown to be a potential alternative to prednisone in combination with rituximab or cyclophosphamide in the remission induction of GPA and MPA.5 6 In addition, eculizumab, a C5 inhibitor, has shown efficacy in the glucocorticoid-free remission induction of a patient with severe MPO-ANCA associated GN.41 The above-described reports depict a translational and precision health success story, in which bench discoveries lead to significant benefit at the bedside of individual patients.
sCD163 emerged as a novel biomarker with potential relevance in several autoimmune diseases. CD163 is a scavenger receptor on M2 macrophages; when cleaved and released in the circulation, sCD163 functions as a marker of macrophage activation.42 43 In the current review we described the value of urinary sCD163 as a marker of active ANCA-associated GN.24 25 Urinary sCD163 levels have been demonstrated to distinguish active lupus nephritis from remission.43 44 Furthermore, urinary sCD163 levels correlate with IgA nephropathy severity.45 Urinary sCD163 is therefore a promising non-invasive biomarker in multiple autoimmune kidney diseases; future research should assess how this marker can be incorporated in our clinical decision-making.
Notwithstanding the potential value of the reported candidate biomarkers, several limitations and knowledge gaps remain. First, a meta-analysis of the reported study results was not feasible considering the reported data. However, this systematic review described 22 high quality articles published in the last decade and reported non-invasive biomarkers of AAV-related disease activity and/or organ damage. Second, few studies included patients with EGPA. Overall, EGPA is a neglected disease in translational and clinical research, possibly because of its rarity, heterogeneity and the challenges involving its diagnosis and classification. A deeper understanding of EGPA pathophysiology and associated candidate biomarkers is lacking. Third, this review involved few paediatric patients. Childhood-onset AAV is a rare but severe disease with treatment algorithms based on randomised controlled trials in adult patients or expert consensus guidelines, rather than paediatric studies. This highlights the critical need to increase childhood-onset AAV research. Fourth, many AAV studies were based in Chinese or Japanese centres, likely leading to an overwhelming majority of the included patients having MPO-ANCA positivity. Data from these studies may not be extrapolated to PR3-ANCA positive patients. Finally, the heterogeneity in the diagnosis, classification, clinical presentation and management of patients with AAV impedes an overarching assessment of AAV biomarkers. These unmet needs must be addressed before precision health can be applied to AAV.
In conclusion, several non-invasive biomarkers may be beneficial in the assessment of AAV-related disease activity and/or organ damage. Promising markers involve the alternative complement pathway, neutrophil activation and macrophage activation. Further validation of candidate biomarkers in large prospective cohorts and patients with childhood-onset AAV is warranted before routine clinical implementation. It seems unlikely that a single biomarker will be able to provide sufficient discriminative power to impact our clinical decision-making; multiplexed biomarker panels should therefore be developed and validated in patients with AAV. The ultimate goal is to enhance precision health by obtaining a more reliable assessment of disease extent, severity and prognosis at presentation precluding the need for serial biopsies.
Acknowledgments
We would like to thank Rachel Zhao, the librarian at Alberta Health Services, for her help with developing and executing the systematic search strategy. We would like to express our gratitude to the Jarock family for funding the Dawson Jarock Vasculitis Fellowship for Education in Pediatric Nephrology and Rheumatology.
Footnotes
Twitter: @rensontho
LH and SB contributed equally.
Contributors: All authors have contributed to the study design, data collection, data analysis and interpretation and preparation of the submitted manuscript. TR is responsible for the overall content as the guarantor.
Funding: TR was funded by the Dawson Jarock Vasculitis Fellowship for Education in Pediatric Nephrology and Rheumatology.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Jayne DR, Jones SJ, Severn A, et al. Severe pulmonary hemorrhage and systemic vasculitis in association with circulating anti-neutrophil cytoplasm antibodies of IgM class only. Clin Nephrol 1989;32:101–6. [PubMed] [Google Scholar]
- 2.Sacoto G, Boukhlal S, Specks U, et al. Lung involvement in ANCA-associated vasculitis. La Presse Médicale 2020;49:104039. 10.1016/j.lpm.2020.104039 [DOI] [PubMed] [Google Scholar]
- 3.Rhee RL, Hogan SL, Poulton CJ, et al. Trends in long-term outcomes among patients with antineutrophil cytoplasmic antibody-associated vasculitis with renal disease. Arthritis Rheumatol 2016;68:1711–20. 10.1002/art.39614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010;21:1628–36. 10.1681/ASN.2010050477 [DOI] [PubMed] [Google Scholar]
- 5.Jayne DRW, Bruchfeld AN, Harper L, et al. Randomized trial of C5A receptor inhibitor Avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017;28:2756–67. 10.1681/ASN.2016111179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jayne DRW, Merkel PA, Schall TJ, et al. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med 2021;384:599–609. 10.1056/NEJMoa2023386 [DOI] [PubMed] [Google Scholar]
- 7.Nozaki Y. New insights into novel therapeutic targets in ANCA-associated vasculitis. Front Immunol 2021;12:631055. 10.3389/fimmu.2021.631055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol 2013;66:408–14. 10.1016/j.jclinepi.2012.09.016 [DOI] [PubMed] [Google Scholar]
- 9.Sterne JAC, Savović J, Page MJ, et al. Rob2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 10.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe H, Sada K-E, Matsumoto Y, et al. Association between reappearance of myeloperoxidase-antineutrophil cytoplasmic antibody and relapse in antineutrophil cytoplasmic antibody-associated vasculitis subgroup analysis of nationwide prospective cohort studies. Arthritis Rheumatol 2018;70:1626–33. 10.1002/art.40538 [DOI] [PubMed] [Google Scholar]
- 12.Bunch DO, McGregor JG, Khandoobhai NB, et al. Decreased CD5+ B cells in active ANCA vasculitis and relapse after Rituximab. Clin J Am Soc Nephrol 2013;8:382–91. 10.2215/CJN.03950412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto K, Suzuki K, Yoshimoto K, et al. Longitudinal immune cell monitoring identified CD14++ CD16+ intermediate monocyte as a marker of relapse in patients with ANCA-associated vasculitis. Arthritis Res Ther 2020;22:145. 10.1186/s13075-020-02234-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S-F, Wang F-M, Li Z-Y, et al. Plasma complement factor H is associated with disease activity of patients with ANCA-associated vasculitis. Arthritis Res Ther 2015;17:129. 10.1186/s13075-015-0656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gou SJ, Yuan J, Chen M, et al. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 2013;83:129–37. 10.1038/ki.2012.313 [DOI] [PubMed] [Google Scholar]
- 16.Grayson PC, Carmona-Rivera C, Xu L, et al. Neutrophil-related gene expression and low-density Granulocytes associated with disease activity and response to treatment in antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol 2015;67:1922–32. 10.1002/art.39153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanaoka H, Nagafuchi Y, Hanata N, et al. Identifying the most influential gene expression profile in distinguishing ANCA-associated vasculitis from healthy controls. J Autoimmun 2021;119:102617. 10.1016/j.jaut.2021.102617 [DOI] [PubMed] [Google Scholar]
- 18.Monach PA, Warner RL, Tomasson G, et al. Serum proteins reflecting inflammation, injury and repair as biomarkers of disease activity in ANCA-associated vasculitis. Ann Rheum Dis 2013;72:1342–50. 10.1136/annrheumdis-2012-201981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monach PA, Warner RL, Lew R, et al. Serum biomarkers of disease activity in Longitudinal assessment of patients with ANCA‐Associated vasculitis. ACR Open Rheumatol 2022;4:168–76. 10.1002/acr2.11366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn SS, Yoon T, Park Y-B, et al. Serum Chitinase-3-like 1 protein is a useful biomarker to assess disease activity in ANCA-associated vasculitis: an observational study. Arthritis Res Ther 2021;23:77. 10.1186/s13075-021-02467-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoon T, Ahn SS, Jung SM, et al. Serum soluble programmed cell death protein 1 could predict the current activity and severity of antineutrophil cytoplasmic antibody-associated vasculitis: a Monocentric prospective study. Clin Exp Rheumatol 2019;37 Suppl 117:116–21. [PubMed] [Google Scholar]
- 22.Sun XJ, Wang C, Zhang LX, et al. Sphingosine-1-phosphate and its receptors in anti-neutrophil cytoplasmic antibody-associated vasculitis. Nephrol Dial Transplant 2017;32:1313–22. 10.1093/ndt/gfw427 [DOI] [PubMed] [Google Scholar]
- 23.Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived Microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote Thromboinflammation and the Thrombophilic state associated with the disease. Ann Rheum Dis 2014;73:1854–63. 10.1136/annrheumdis-2013-203430 [DOI] [PubMed] [Google Scholar]
- 24.Aendekerk JP, Timmermans SAMEG, Busch MH, et al. Urinary soluble CD163 and disease activity in biopsy-proven ANCA-associated glomerulonephritis. Clin J Am Soc Nephrol 2020;15:1740–8. 10.2215/CJN.07210520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Reilly VP, Wong L, Kennedy C, et al. Urinary soluble CD163 in active renal vasculitis. J Am Soc Nephrol 2016;27:2906–16. 10.1681/ASN.2015050511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dekkema GJ, Abdulahad WH, Bijma T, et al. Urinary and serum soluble CD25 complements urinary soluble CD163 to detect active renal anti-neutrophil cytoplasmic autoantibody-associated vasculitis: a cohort study. Nephrol Dial Transplant 2019;34:234–42. 10.1093/ndt/gfy018 [DOI] [PubMed] [Google Scholar]
- 27.Wu L, Li X-Q, Goyal T, et al. Urinary epidermal growth factor predicts renal prognosis in antineutrophil cytoplasmic antibody-associated vasculitis. Ann Rheum Dis 2018;77:1339–44. 10.1136/annrheumdis-2017-212578 [DOI] [PubMed] [Google Scholar]
- 28.Gou SJ, Yuan J, Wang C, et al. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol 2013;8:1884–91. 10.2215/CJN.02790313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnemann J, Klocke J, Bieringer M, et al. Urinary T cells identify renal antineutrophil cytoplasmic antibody-associated vasculitis and predict prognosis: a proof of concept study. Kidney Int Rep 2023;8:871–83. 10.1016/j.ekir.2023.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparding N, Genovese F, Rasmussen DGK, et al. Endotrophin, a collagen type VI-derived Matrikine, reflects the degree of renal fibrosis in patients with IgA nephropathy and in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 2022;37:1099–108. 10.1093/ndt/gfab163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsuda S, Kotani T, Kuwabara H, et al. Ccl2 produced by CD68+/CD163+ Macrophages as a promising clinical biomarker of microscopic polyangiitis-interstitial lung disease. Rheumatology (Oxford) 2021;60:4643–53. 10.1093/rheumatology/keab064 [DOI] [PubMed] [Google Scholar]
- 32.Wu S-J, Yang X, Xu P-C, et al. Urinary mitochondrial DNA is a useful biomarker for assessing kidney injury of antineutrophil cytoplasmic antibody -associated vasculitis. Clin Chim Acta 2020;502:263–8. 10.1016/j.cca.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 33.Kitching AR, Anders H-J, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers 2020;6:71. 10.1038/s41572-020-0204-y [DOI] [PubMed] [Google Scholar]
- 34.Al-Soudi A, Vegting Y, Klarenbeek PL, et al. A systematic review and meta-analysis on the value of serial ANCA level evaluation. Front Med (Lausanne) 2022;9:844112. 10.3389/fmed.2022.844112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomasson G, Grayson PC, Mahr AD, et al. Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis-a meta-analysis. Rheumatology (Oxford) 2012;51:100–9. 10.1093/rheumatology/ker280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao H, Schreiber A, Heeringa P, et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 2007;170:52–64. 10.2353/ajpath.2007.060573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiber A, Xiao H, Jennette JC, et al. C5A receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 2009;20:289–98. 10.1681/ASN.2008050497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing G, Chen M, Liu G, et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated Pauci-immune vasculitis. J Clin Immunol 2009;29:282–91. 10.1007/s10875-008-9268-2 [DOI] [PubMed] [Google Scholar]
- 39.Hilhorst M, van Paassen P, van Rie H, et al. Complement in ANCA-associated glomerulonephritis. Nephrol Dial Transplant 2017;32:1302–13. 10.1093/ndt/gfv288 [DOI] [PubMed] [Google Scholar]
- 40.Moiseev S, Lee JM, Zykova A, et al. The alternative complement pathway in ANCA-associated vasculitis: further evidence and a meta-analysis. Clin Exp Immunol 2020;202:394–402. 10.1111/cei.13498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribes D, Belliere J, Piedrafita A, et al. Glucocorticoid-free induction regimen in severe ANCA-associated vasculitis using a combination of Rituximab and Eculizumab. Rheumatology (Oxford) 2019;58:2335–7. 10.1093/rheumatology/kez190 [DOI] [PubMed] [Google Scholar]
- 42.Yang G, Guo N, Yin J, et al. Elevated soluble Cd163 predicts renal function deterioration in lupus nephritis: a cohort study in Eastern China. J Int Med Res 2021;49:03000605211049963. 10.1177/03000605211049963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta R, Yadav A, Aggarwal A. Urinary soluble CD163 is a good biomarker for renal disease activity in lupus nephritis. Clin Rheumatol 2021;40:941–8. 10.1007/s10067-020-05343-6 [DOI] [PubMed] [Google Scholar]
- 44.Endo N, Tsuboi N, Furuhashi K, et al. Urinary soluble CD163 level reflects glomerular inflammation in human lupus nephritis. Nephrol Dial Transplant 2016;31:2023–33. 10.1093/ndt/gfw214 [DOI] [PubMed] [Google Scholar]
- 45.Gong S, Jin S, Li Y, et al. Urinary soluble CD163 levels predict IgA nephropathy remission status. Front Immunol 2021;12:769802. 10.3389/fimmu.2021.769802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003579supp001.pdf (80.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request.