Abstract
Streptococcal pyrogenic exotoxin C (SPEC), when injected intradermally, induces erythema in unsensitized rabbits. In the present study, we examined whether this erythema induction is due to the T-cell stimulatory activity of SPEC as a superantigen. Analysis by using single-residue mutant SPECs indicated that mutant SPECs Y15I, A16E, and Y17I, in which tyrosine 15, alanine 16, and tyrosine 17 were replaced with isoleucine, glutamic acid, and isoleucine, respectively, exhibited significantly reduced mitogenic activity for Vβ2+ human T cells in vitro, and Y15I showed as much as a 1,000-fold reduction. Y15I mutant SPEC, however, retained the ability to bind to major histocompatibility complex class II antigen and to form a homodimer, implying that residue 15 is critically important for the interaction of SPEC with T-cell antigen receptor β chains. When injected intradermally into normal rabbits, wild-type SPEC induced a characteristic erythema after 3 h in a dose-dependent fashion, which was associated with polymorphonuclear and mononuclear cell infiltration. This erythema formation was found to be severely suppressed by systemic pretreatment with cyclosporin A, suggesting the involvement of host T cells. Y15I mutant SPEC exhibited nearly 1,000-fold less erythema induction in vivo than wild-type SPEC. Altogether, the present results strongly suggest that erythema induction in rabbits by SPEC is attributable mostly to its T-cell stimulatory activity as a superantigen.
Characteristic symptoms caused by pathogenic bacterial infection in hosts are often ascribed to the production of unique exotoxins. Some of them induce direct injuries of selected host tissues, while others affect the host immune system, thereby inducing a variety of inflammatory responses (5, 22, 48). Among the latter are a series of bacterial exotoxins called superantigens, including staphylococcal enterotoxins (SEs), toxic shock syndrome toxin 1 (TSST-1), and streptococcal pyrogenic exotoxins (SPEs). These exotoxins are similar in that they can, in their intact forms, directly bind to both β chains of T-cell antigen receptors (TCR) with selected Vβ regions and major histocompatibility complex (MHC) class II antigens, irrespective of antigenic peptides in the grooves (31). They can thus polyclonally stimulate a set of T cells to produce a variety of cytokines, such as interleukin-1 (IL-1), IL-2, IL-6, IL-8, tumor necrosis factor (TNF), and gamma interferon, either directly or indirectly via other types of cells (11, 13, 16, 17, 24, 35, 37). They have been considered to be potential causes or aggravating factors of toxic shock syndrome, rheumatic fever, guttate psoriasis, Kawasaki disease, and atopic dermatitis (32, 46, 51). Some of the pathogenic effects seen in these diseases are believed to be due to the T-cell-stimulating activities of these toxins as superantigens. Fatal shock in mice by systemic injection of SEB, for instance, is considered to be mediated by TNF secreted by rapidly and massively activated T cells (34). It was also reported that the lethal shock induced by TSST-1 in rabbits correlated with the T-cell-stimulating activity (9). On the other hand, the emetic activity of SEA and SEB was shown to be independent of their T-cell stimulatory activity, since the mutant exotoxins devoid of superantigenicity still retained the emetic activity (3, 18). It thus remains to be carefully elucidated whether individual pathogenic effects of the superantigens in vivo are indeed due to their superantigenicity or ascribed to other biological activities of the exotoxins.
SPEA and SPEC, known as scarlet fever toxins, have various biological properties, including pyrogenicity, skin rash formation, and enhancement of endotoxin shock (7). Additionally, the SPEs can induce streptococcal toxic shock syndrome (STSS), which is characterized by acute-onset erythema, fever, hypotension, and multiorgan failure (46). Although SPEs are known to be typical superantigens, it remains to be seen whether such variable characteristic pathogenic activities are due to their superantigenicity in vivo. In the present study, we examined, by mutational analysis, whether or not erythema, a representative skin lesion, induced in rabbits by SPEC is dependent on its T-cell-stimulating activity.
MATERIALS AND METHODS
Cloning of the speC gene.
The speC gene was amplified from the DNA of Streptococcus pyogenes T18P, provided by P. M. Schlievert, Minneapolis, Minn., with a PCR using a sense primer (5′-CCGGGATCCGACTCTAAGAAAGACATT-3′) and an antisense primer (5′-GGCGGATCCTTATTTTTCAAGATAAATATCGA-3′) both carrying BamHI restriction sites. After digestion with BamHI, the fragment encoding mature SPEC with no leader sequence was cloned into the BamHI site of plasmid pUC118 and then recloned into the EcoRI and PstI sites of an M13mp19 phage vector (M13mp19W).
Site-directed mutagenesis.
Replacement of amino acids at the N-terminal region of SPEC (see Fig. 1) was carried out by the method of Kunkel et al. (29). Briefly, single-stranded DNA of M13mp19W was prepared from Escherichia coli CJ236 harboring M13mp19W, and each mutagenic oligonucleotide encoding a designated amino acid mutation at each position was annealed. Polymerase and ligase reactions were performed in vitro, and the resulting double-stranded DNA was transformed into E. coli BMH71-18mutS. E. coli MV1184 was infected with phage particles obtained from the resulting clones. The entire genes were sequenced by dideoxy-chain termination, and it was confirmed that only the intended mutations had occurred, with no secondary mutations. BamHI fragments containing the wild-type or mutated speC gene were isolated and cloned into the BamHI site of expression vector pGEX-2T (Pharmacia, Tokyo, Japan). These recombinant plasmids were transformed into E. coli MC1061.
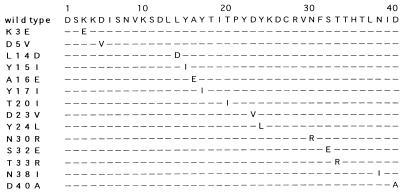
FIG. 1.
Amino acid substitutions in mutant SPECs. Mutagenesis was introduced on the residues located in the N terminus of SPEC. In each amino acid sequence, dashes indicate identical amino acid residues and the substituted amino acid is shown in the one-letter code.
Purification of recombinant SPECs.
Expression and purification of fusion proteins in the GST Gene Fusion System (Pharmacia) were carried out as suggested by the manufacturer. Briefly, the culture of E. coli MC1061 transformed with recombinant pGEX-2T plasmids was grown, and fusion proteins were induced with isopropyl-β-d-thiogalactopyranoside (IPTG) for 5 h, harvested, disrupted by sonication in ice-cold phosphate-buffered saline (PBS) containing 1% Triton X-100, and centrifuged to obtain a soluble lysate. The lysate was applied to glutathione-Sepharose 4B (Pharmacia). After unbound materials were washed off thoroughly with PBS, the SPECs were cleaved off from the glutathione S-transferase (GST) fusion proteins bound to the beads by thrombin (Sigma, St. Louis, Mo.) as previously described (50). The homogeneity of the purified SPECs was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Endotoxin contamination of purified SPECs was checked by the Limulus amebocyte lysate test (PYROTELL, Associates of Cape Cod, Inc.) and found to be less than 0.3 endotoxin units/ml in all recombinant SPEC samples.
Anti-SPEC serum.
Antiserum against recombinant wild-type SPEC was produced in rabbits by injecting 50 μg of purified recombinant wild-type SPEC once in Freund’s complete adjuvant and twice more in Freund’s incomplete adjuvant. It was confirmed that the antiserum immunoreacted similarly to all of the mutant recombinant SPECs by Western blotting analysis.
Quantification of recombinant SPECs.
The protein concentration of the recombinant SPECs was determined by enzyme-linked immunosorbent assay (ELISA). A SPEC-specific sandwich ELISA was developed as described before (38) by using anti-SPEC immunoglobulin G-coated polystyrene beads and horseradish peroxidase-conjugated Fab′ of anti-SPEC immunoglobulin G. The Bradford dye-binding assay was done by using a protein assay kit (Bio-Rad, Tokyo, Japan) with bovine serum albumin as the standard.
Assay for mitogenic activity.
Human peripheral blood lymphocytes (PBL) were isolated from normal heparinized blood by density centrifugation over Ficoll-Paque (Pharmacia, Uppsala, Sweden), washed, and resuspended in RPMI 1640 medium supplemented with 2 mM glutamine, antibiotics, 5 × 10−5 M 2-mercaptoethanol, and 10% fetal calf serum. PBL were cultured in round-bottom microtiter wells (105 cells/well) in the absence or presence of various concentrations of wild-type or mutant SPECs for 3 days. The cultures were pulsed with 0.5 μCi of [3H]thymidine (specific activity, 83.0 Ci/mmol; Amersham International plc.) per well for the last 16 h and harvested, and [3H]thymidine uptake was determined with a scintillation counter (Beckman LS6500).
Flow cytometric analysis.
For flow cytometric analysis, human PBL were cultured in a 24-well plate at 106/ml in the absence or presence of wild-type SPEC or mutant SPEC Y15I (final concentration, 100 pg/ml) for 6 days. Cultured PBL were double stained with a biotin-conjugated anti-OKT3 monoclonal antibody (MAb; PharMingen, San Diego, Calif.) and a fluorescein isothiocyanate-conjugated anti-Vβ2 MAb (Immunotech, Marseille, France), followed by phycoerythrin-avidin, and analyzed by a FACScan (Becton Dickinson, Mountain View, Calif.) as described before (36).
Assay for binding to MHC class II.
Binding of toxins to MHC class II antigens was examined by using a competition assay as described before (1, 15), with slight modifications. Briefly, Raji cells (106/ml) were preincubated with or without various concentrations of wild-type or mutant SPECs at 37°C for 1 h. FluorX reactive dye-conjugated wild-type SPEC (2 μg/ml) was then added, and the cells were again incubated at 37°C for 2 h. After a washing with PBS, cells were analyzed by a FACScan.
Induction of skin erythema.
An erythematous reaction was induced in rabbits by intradermal injection of SPEC as described by Kamezawa et al. (25, 26). The hair was clipped from the backs of Japanese white rabbits, and 2 days later, toxin samples (25 μl) at various concentrations were injected intradermally into the clipped areas. Four hours later, diameters of erythema formed around the injection sites were measured. Biopsy specimens were taken from the injection sites, fixed with 10% (vol/vol) formalin, sectioned in paraffin, and stained with hematoxylin and eosin. To evaluate the effect of cyclosporin A, 4 ml of PBS containing 25% rabbit serum with or without cyclosporin A (16 mg/kg), supplied by Novartis Pharma (Tokyo, Japan), was administered intravenously 5 min before and 2 h after toxin injection.
RESULTS
Generation of single-residue mutant SPECs with compromised superantigenicity and examination of the critical role of residues at positions 15 to 17 of the N-terminal region.
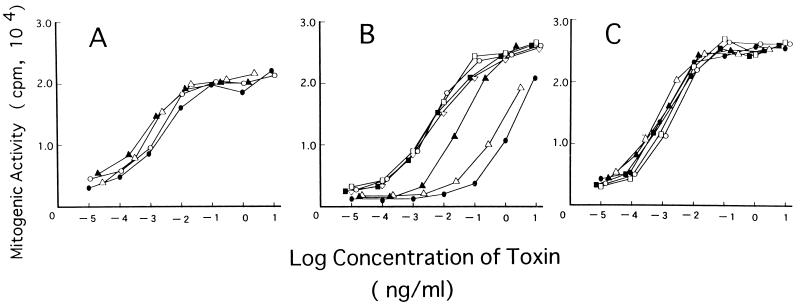
We intended first to identify the residues of SPEC that are critical for its superantigenicity by mutational analysis. SPEC has only weak amino acid sequence identity (around 20%) with other well-characterized bacterial superantigens, the N-terminal region being particularly unique (4, 14, 31). However, since it was reported previously that the superantigenic activity of SEB critically depends on the N-terminal region (27), we focused on the N-terminal region of SPEC. As listed in Fig. 1, 14 single-amino-acid mutant forms of SPEC were generated by site-directed mutagenesis as GST fusion proteins and purified by GST-Sepharose 4B, and GST was cleaved off. All of the purified mutant SPECs were detected as single 24-kDa proteins with a probable dimer band (see below) identical to that of wild-type SPEC by SDS-PAGE analysis and similarly immunoreacted to the rabbit polyclonal anti-SPEC antibody (data not shown). Protein concentrations of the purified mutant and wild-type SPEC were determined by sandwich ELISA with the anti-SPEC antibody. Normal human PBL were cultured in the absence or presence of various concentrations of either wild-type or mutant SPECs for 3 days, and [3H]thymidine uptake was assayed. As shown in Fig. 2, wild-type SPEC induced potent mitogenic activity on normal PBL in a dose-dependent fashion, producing a significant proliferative response at as little as 1 pg/ml and a maximum response at 0.1 ng/ml and higher concentrations. Among the 14 mutant SPECs, mutant Y15I showed a nearly 1,000-fold reduction of mitogenic activity, a 1-ng/ml protein concentration inducing a proliferative response comparable to that induced by wild-type SPEC at 1 pg/ml. Also, the A16E and Y17I mutant SPECs exhibited 300- and 10-fold reductions of mitogenic activity compared with wild-type SPEC, respectively. The other 11 mutant SPECs retained mitogenic activity comparable to that of wild-type SPEC. These results indicate that three consecutive residues (15 to 17) in the N-terminal region play critical roles in determining the mitogenic activity of SPEC against normal lymphocytes.
FIG. 2.
Mitogenic activity of wild-type and mutant SPECs. Normal human PBL were incubated with each toxin for 56 h and pulsed with 0.5 μCi of [3H]thymidine for 16 h, and [3H]thymidine incorporation was determined. Values are means of triplicate determinations. (A) ○, wild-type SPEC; •, K3E: ▵, D5V; ▴, L14D. (B) ○, wild-type SPEC; •, Y15I; ▵, A16E; ▴, Y17I; □, T20I; ■, D23V; ▿, Y24L. (C) ○, wild-type SPEC; •, N30R; ▵, S32E; ▴, T33R; □, N38I; ■, D40A.
Y15I mutant SPEC exhibits severely compromised Vβ2-specific superantigenic activity with fully retained class II MHC-binding activity.
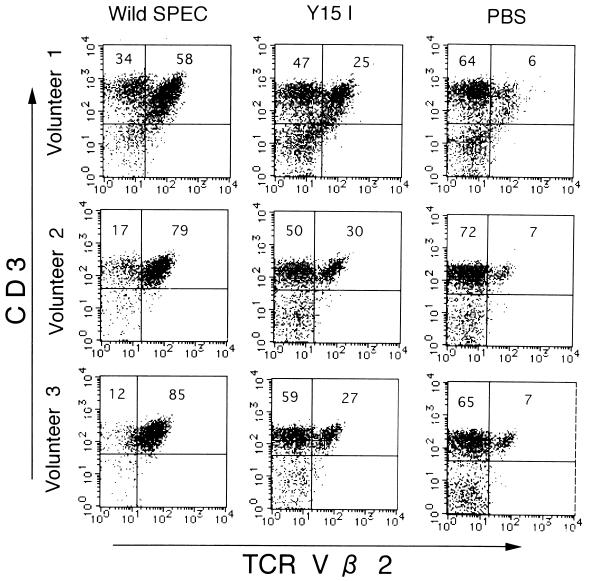
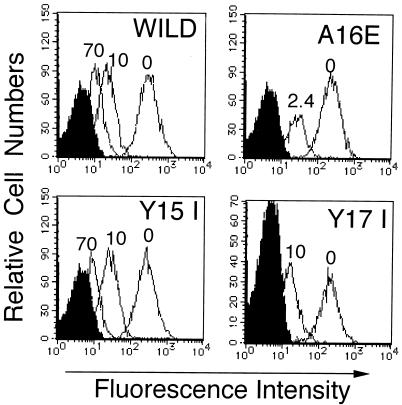
To confirm that the mitogenic activity of SPEC was indeed due to superantigenicity, the phenotypes of PBL stimulated with SPEC were examined by two-color flow cytometry. As shown for PBL from volunteer 1 in Fig. 3, wild-type SPEC induced a pronounced expansion of Vβ2+ T cells (58% of all T cells) compared with unstimulated PBL (6%) on day 6 of culture, conforming to a previous report (10). On the other hand, the Y15I SPEC mutant induced not only reduced mitogenic activity but also much less Vβ2+ T-cell expansion (25%) than did wild-type SPEC. Essentially identical results were obtained by using the PBL from three unrelated healthy donors. These results thus indicate that N-terminal Y15 plays an important role in the Vβ2-specific superantigenic activity of SPEC. The possibility that the reduced superantigenicity of Y15I SPEC is due to impaired binding to MHC class II was then directly assessed. MHC class II-bearing Raji cells were preincubated with various concentrations of either wild-type or mutant SPECs, extensively washed, and then incubated with wild-type SPEC conjugated with FluorX reactive dye (2 μg/ml), followed by flow cytometric analysis. As shown in Fig. 4, pretreatment with excess concentrations of Y15I (10 and 70 μg/ml) competitively inhibited the binding of fluorescence-labeled SPEC to a degree comparable to that achieved by wild-type SPEC. The A16E and Y17I mutants similarly inhibited the binding of labeled wild-type SPEC quite effectively at 2.4 and 10 μg/ml, respectively. These results strongly suggest that the binding activity of the three mutant SPECs to MHC class II molecules was not affected significantly.
FIG. 3.
Vβ2-specific expansion of T cells. Expansion of Vβ2+ T cells on SPEC-stimulated human PBL was analyzed by two-color flow cytometry. PBL (106/ml) from three healthy volunteers were incubated with PBS, wild-type SPEC, or mutant SPEC Y15I (final toxin concentration, 100 pg/ml) for 6 days, double stained with a biotin-conjugated anti-OKT3 MAb and a fluorescein isothiocyanate-conjugated anti-Vβ2 MAb, followed by phycoerythrin-avidin, and analyzed by a FACScan. The flow cytometric profiles of three individual samples of PBL are shown.
FIG. 4.
Binding of wild-type and mutant SPECs to MHC class II. The ability of each toxin to bind to MHC class II was examined in a competition assay. Raji cells were preincubated with various concentrations of toxins (wild-type SPEC, 10 and 70 μg/ml; Y15I, 10 and 70 μg/ml; A16E, 2.4 μg/ml; Y17I, 10 μg/ml), washed, incubated with wild-type SPEC (2 μg/ml) conjugated with FluorX reactive dye, and then analyzed by flow cytometry.
Mutation Y15I does not affect the dimer formation of SPEC in vitro.
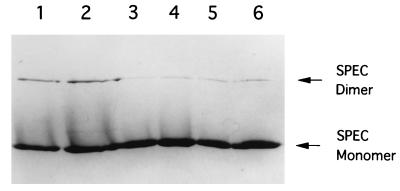
Recent crystallographic and biochemical studies revealed that SPEC molecules form dimers at neutral or alkaline pH and implied that such dimerization plays a unique role in the superantigenicity of SPEC (30, 43). We therefore addressed the question of whether the Y15I mutation somehow affects dimer formation. Recombinant wild-type and Y15I mutant SPECs were purified as before, incubated in various-pH buffers, and analyzed by SDS-PAGE. As shown in Fig. 5, a band corresponding to the dimer was detected in addition to the monomer band of wild-type SPEC, the former being the most prominent under alkaline conditions (pH 8.8), in agreement with a previous report (30). For the Y15I SPEC, an essentially identical dimer formation pattern was observed (Fig. 5), indicating that the Y15I mutation does not significantly affect the dimerization of SPEC.
FIG. 5.
Dimer formation of wild-type SPEC and Y15I mutant SPEC. Wild-type SPEC and Y15I mutant SPEC were incubated in 0.5 M Tris-HCl buffer, boiled in sample buffer, and analyzed by SDS-PAGE. Lanes: 1, 3, and 5, 2 μg of wild-type SPEC; 2, 4, and 6, 2 μg of Y15I mutant SPEC; 1 and 2, 0.5 M Tris-HCl buffer and sample buffer adjusted to pH 8.8; 3 and 4, buffers adjusted to pH 6.8; 5 and 6, buffers adjusted to pH 5.0.
SPEC locally injected into the skin induces a characteristic erythematous lesion in vivo which is inhibited by cyclosporin A.
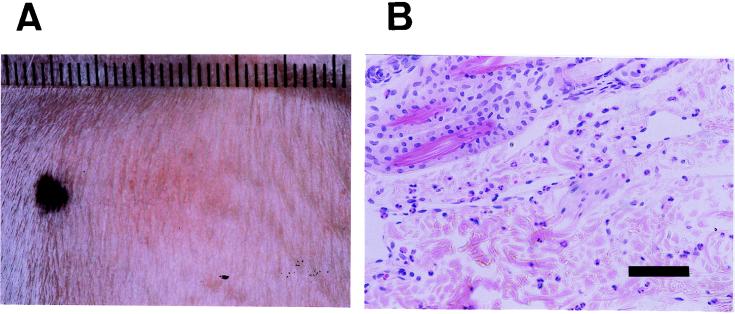
To examine its effects in vivo, SPEC (200 ng) was injected intradermally into a normal unsensitized rabbit. As shown in Fig. 6A, it induced a characteristic erythematous lesion at the injection site, which became evident at around 3 h, peaked at 4 h, and lasted at least 24 h. The erythematous reaction was augmented by a repeat injection at a 1-week interval, irrespective of the injection site. Histological examination of the lesion revealed infiltration of neutrophils and mononuclear cells, which was also enhanced by the repeated SPEC injection (Fig. 6B). To elucidate the possible involvement of T cells in erythema induction, we examined the effect of pretreatment with cyclosporin A, which is known to selectively suppress the activation of T cells in vivo (12, 49). Cyclosporin A (16 mg/kg) dissolved in 4 ml of PBS containing 25% rabbit serum was administered intravenously 5 min before and 2 h after an intradermal injection of wild-type SPEC (200 ng). Erythematous lesion diameters were measured 4 h after the injection of SPEC. The means and standard deviations of five independent measurements of diameters in three animals per group were as follows: vehicle, 12.4 ± 2.3 mm; cyclosporin A, 3.7 ± 1.7 mm. These results show that intravenous injection of cyclosporin A before and after the intradermal injection of SPEC nearly completely suppressed erythema formation, strongly suggesting that host T cells are required for erythema formation by SPEC.
FIG. 6.
Erythematous reaction induced in a rabbit by intradermal injection of SPEC. (A) Erythema that developed 4 h after wild-type SPEC (200 ng) was injected intradermally into an unsensitized rabbit. (B) Histology of an erythematous lesion that developed 4 h after wild-type SPEC (200 ng) was injected intradermally into a rabbit that had received an injection of the same dose of wild-type SPEC 1 week previously. Hematoxylin-and-eosin staining. Bar, 50 μm.
Y15I mutant SPEC with compromised superantigenic activity exhibits proportionally reduced ability to induce erythema in vivo.
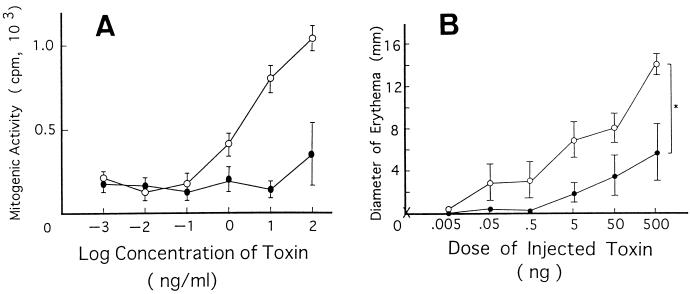
Unlike other superantigens, SPEC is reported to show no mitogenicity against mouse T cells (30, our unpublished data), and thus, we first wished to know whether it could function as a superantigen in rabbits as well. As shown in Fig. 7A, wild-type SPEC indeed exhibited mitogenic activity against rabbit PBL in vitro, while Y15I mutant SPEC did so only marginally, conforming to the results obtained with human PBL. We then addressed the question of whether the characteristic erythema induction in vivo by SPEC is indeed dependent on its superantigenic activity. To compare the activity quantitatively, we injected rabbits intradermally with various concentrations of either wild-type or Y15I mutant SPEC and 4 h later inspected the injected sites of the skin for the development of typical erythema. As shown in Fig. 7B, the injection of wild-type SPEC induced erythematous lesions in a dose-dependent fashion. On the other hand, the ability of Y15I SPEC to induce erythema in vivo was roughly 100- to 1,000-fold lower than that of wild-type SPEC, as judged from the dose-response curve. Thus, even as much as 500 ng of Y15I could induce erythema of a size comparable to that induced by 0.5 to 5 ng of wild-type SPEC. This was roughly equivalent to the degree of reduction of T-cell mitogenic activity of the mutant SPEC in vitro. These results thus strongly suggest that the ability of SPEC to induce erythematous skin lesions in vivo is directly dependent on its T-cell-stimulating activity as a superantigen.
FIG. 7.
(A) Mitogenic activities of wild-type SPEC and Y15I mutant SPEC. Unsensitized rabbit PBL were incubated with various concentrations of either wild-type SPEC or Y15I mutant SPEC for 95 h, pulsed with 0.5 μCi of [3H]thymidine for 20 h, and harvested. [3H]thymidine incorporation was then determined. Values are means ± standard deviations of triplicate measurements. ○, wild-type SPEC; •, Y15I mutant SPEC. (B) Erythema induced by wild-type SPEC and Y15I mutant SPEC. Various concentrations of either wild-type SPEC or Y15I mutant SPEC were injected intradermally into unsensitized rabbits and 4 h later, diameters of erythematous lesions formed around injection sites were measured. Values are means ± standard deviations for groups of four animals. ○, wild-type SPEC; •, mutant SPEC Y15I; ×, PBS. ∗, P < 0.002 (Student’s t test).
DISCUSSION
SPEs have recently been recognized as one of the most potent causative factors of STSS and guttate psoriasis (46). Although erythema seen in these diseases, as well as aggravation of atopic dermatitis by focal streptococcal infections, has been speculated to be associated with the T-cell-stimulating superantigen activity of these toxins, the exact causal relationship remains unknown. In the present study, we intended to examine the etiologic relationship between the T-cell-stimulating superantigen activity of SPECs and SPEC-induced erythema by producing single-residue mutant SPECs with compromised superantigen activity and examining their in vivo effects.
A number of structural analyses of superantigenic activity have been carried out with SEA (19, 21, 41), SEB (23, 27, 33, 52), TSST-1 (6, 9), and SPEA (20). Extensive mutational analysis of staphylococcal enterotoxins (SEB) by Kappler et al. indicated that the N-terminal region, in particular regions 1 (residues 9 to 23) and 2 (residues 41 to 53), is critically important for T-cell mitogenic activity (27). Among the mutants with severely reduced mitogenic activity, all of those with region 2 mutations exhibited little class II MHC-binding activity, while the activity of region 1 mutants was retained fully or only partially affected (27). More recently, crystallographic analyses of SEB revealed that the binding sites for TCR and MHC class II molecules are indeed located in the N-terminal region (23, 52). Crystallographic analyses of SEA, SEB, SEC2, and TSST-1 revealed the marked similarity of the three-dimensional structures of these toxins (2, 8, 28, 39, 40, 42, 44, 47, 52). Among these bacterial superantigens, however, SPEC shares much less overall amino acid sequence homology, i.e., only around 20% identity.
In the present study, we focused on the importance of the N-terminal region of SPEC for mutagenesis. Among 14 mutants with single-residue mutations in the N-terminal region (residues 3 to 40), a Y15I mutant exhibited a roughly 1,000-fold reduction of in vitro T-cell mitogenesis, while the two adjacent mutant SPECs, A16E and Y17I showed 300- and 10-fold reductions, respectively, and the rest showed activity comparable to that of wild-type SPEC. Impaired mitogenicity of Y15I SPEC was associated with the reduction of Vβ2+ T-cell predominance after the stimulation of normal PBL in vitro, indicating that superantigenicity specific for T cells was indeed compromised in the mutant SPEC. The ability to bind to class II MHC antigens, on the other hand, was hardly affected in all three mutant SPECs, as judged by a competition assay, resembling in nature the region 1 mutant forms of SEB. Most recently, the crystallographic structure of SPEC has been revealed (43). According to the model, SPEC has the usual superantigen fold, comprising domains I and II. However, the region corresponding to the low-affinity MHC binding site at domain I in other superantigens is used to create a dimer in SPEC, although the C-terminal zinc-dependent high-affinity MHC-binding site analogous to SEA is retained in domain II. Y15, which is in the first α helix outside domain I, is not located in either the MHC-binding or the dimerization region presented in this model. This is compatible with our present findings that the Y15I mutation did not affect MHC binding or dimer formation. The exact site of SPEC binding to the TCR β chain remains to be verified. Our present results, however, imply that three consecutive residues (Y15, A16, and Y17) in the N-terminal α helix play an important role in the interaction of SPEC with the TCR β chain, either directly as a binding region or indirectly by keeping proper folding between domains I and II.
When injected intradermally into a normal rabbit, wild-type SPEC was found to induce a characteristic erythematous lesion in a dose-dependent fashion, with as little as 5 ng being effective. The lesion became visible at around 3 h after the injection and lasted for at least 24 h, which is different from the kinetics of classical allergic skin reactions (types I, II, and IV). Although the reaction could be reproducibly induced by a first injection of SPEC in normal rabbits, unlike a conventional hypersensitivity reaction, it was also noted that repeated injection resulted in a progressively augmented erythematous response irrespective of the injection sites, conforming to the early report of Schlievert et al. (45). It thus appears that injection with the exotoxin at low doses has a priming effect as well. Histological examination revealed prominent cellular infiltrate consisting of polymorphonuclear and mononuclear cells. Our unpublished immunohistochemistry results, obtained by using a polyclonal anti-rabbit T-cell antibody, suggested the presence of significant numbers of T cells in the lesion. However, since reliable monospecific anti-T-cell reagents for rabbits are unavailable, we examined the effect of cyclosporin A, which is known to selectively suppress T-cell functions (12, 49). Results clearly indicated that systemic pretreatment of a normal rabbit with cyclosporin A (16 mg/kg) nearly completely suppressed erythema induction by SPEC, strongly suggesting that host T cells play a crucial role in erythema formation.
Compared with the wild-type, Y15I mutant SPEC exhibited a significantly reduced ability to induce erythema in vivo. A dose-response analysis indicated that the activity of Y15I was at least 100- to 1,000-fold less than that of wild-type SPEC. This was quite compatible with the finding that Y15I showed roughly 1,000-fold less T-cell mitogenic activity in vitro than did wild-type SPEC for both human and rabbit PBL. Given this result together with the effect of cyclosporin A, it is thus quite conceivable that the characteristic erythema formation by SPEC is mostly attributable to its activity in stimulating a set of T cells polyclonally as a superantigen. Such stimulation may well lead to rapid production of various cytokines, such as TNF, affecting vascular cells, as well as chemokines, as shown in vitro (16, 17, 35, 37), thereby causing local inflammation even in unsensitized hosts. Cytokines produced by MHC class II-bearing monocyte lineage cells secondarily stimulated by such activated T cells might also contribute to the manifestation of inflammation.
A number of findings indicate that the fatal shock syndrome induced by systemic exposure to SEs is due mostly to their superantigenicity (9, 34). On the other hand, it is not clear how much the superantigenicity is responsible for the local, often unique symptoms caused by the exotoxins. For instance, it was reported that the emetic activity and T-cell stimulatory activity are apparently dissociated in certain mutant SEs (3, 18). Our present results strongly argue that the characteristic skin erythema induced by SPEC is largely dependent on its T-cell stimulatory activity. Single-residue mutant SPECs with severely compromised superantigenicity should help to further elucidate the possible involvement of the T-cell stimulatory activity in other forms of pathogenesis caused by streptococcal infection, such as fever, STSS, and aggravation of atopic dermatitis.
REFERENCES
- 1.Abrahmsén L, Dohlsren M, Segrén S, Björk P, Jonsson E, Kalland T. Characterization of two distinct MHC class II binding sites in superantigen staphylococcal enterotoxin A. EMBO J. 1995;14:2978–2986. doi: 10.1002/j.1460-2075.1995.tb07300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya K R, Passalacqua E F, Jones E Y, Harlos K, Stuart D I, Brehm R D, Tranter H S. Structural basis of superantigen action inferred from crystal structure of toxic-shock syndrome toxin-1. Nature. 1994;367:94–97. doi: 10.1038/367094a0. [DOI] [PubMed] [Google Scholar]
- 3.Alber G, Hammer D K, Fleischer B. Relationship between enterotoxic- and lymphocyte-stimulating activity of staphylococcal enterotoxin B. J Immunol. 1990;144:4501–4504. [PubMed] [Google Scholar]
- 4.Betley M J, Borst D W, Legassa L B. Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem Immunol. 1992;55:1–35. [PubMed] [Google Scholar]
- 5.Betley M J, Miller V L, Mekalanos J J. Genetics of bacterial enterotoxins. Annu Rev Microbiol. 1986;40:577–605. doi: 10.1146/annurev.mi.40.100186.003045. [DOI] [PubMed] [Google Scholar]
- 6.Blanco L, Choi E M. Mutants of staphylococcal toxic shock syndrome toxin 1: mitogenicity and recognition by a neutralizing monoclonal antibody. Infect Immun. 1990;58:3020–3028. doi: 10.1128/iai.58.9.3020-3028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illness. Crit Rev Microbiol. 1990;17(4):251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 8.Bohach G A, Chi Y, Stauffacher C V. Crystallization and preliminary X-ray diffraction analysis of staphylococcal enterotoxin C2. Protein. 1992;13:152–157. doi: 10.1002/prot.340130208. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre P F, Heeg H, Cullen C, Lian C-J. Toxicity of recombinant toxic shock syndrome toxin 1 and mutant toxins produced by Staphylococcus aureus in a rabbit infection model of toxic shock syndrome. Infect Immun. 1993;61:793–799. doi: 10.1128/iai.61.3.793-799.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun M A, Gerlach D, Hartwig U F, Ozegouski J-H, Romagé F, Carrel S, Köhler W, Fleischer B. Stimulation of human T cells by streptococcal “superantigen” erythrogenic toxins (scarlet fever toxin) J Immunol. 1993;150:2457–2466. [PubMed] [Google Scholar]
- 11.Carlsson R, Sjogren H O. Kinetics of IL-2 and interferon-gamma production, expression of IL-2 receptors, and cell proliferation in human mononuclear cells exposed to staphylococcal enterotoxin A. Cell Immunol. 1985;96:175–183. doi: 10.1016/0008-8749(85)90349-1. [DOI] [PubMed] [Google Scholar]
- 12.Emmel E A, Verweij C L, Durand D B, Higgins K M, Lacy E, Crabtree G R. Cyclosporin A specifically inhibits functions of nuclear proteins involved in T cell activation. Science. 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 13.Fast D J, Schlievert P M, Nelson R D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989;57:291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goshorn S C, Schlievert P M. Nucleotide sequence of streptococcal pyrogenic exotoxin type C. Infect Immun. 1988;56:2518–2520. doi: 10.1128/iai.56.9.2518-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grossman D, Van M, Mollick J A, Highlander S K, Rich R R. Mutation of the disulfide loop in staphylococcal enterotoxin A. Consequences for T cell recognition. J Immunol. 1991;147:3274–3281. [PubMed] [Google Scholar]
- 16.Hackett S P, Stevens D L. Streptococcal toxic shock syndrome synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992;165:879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- 17.Hackett S P, Stevens D L. Superantigens associated with staphylococcal and streptococcal toxic shock syndrome are potent inducers of tumor necrosis factor β synthesis. J Infect Dis. 1993;168:232–235. doi: 10.1093/infdis/168.1.232. [DOI] [PubMed] [Google Scholar]
- 18.Harris T O, Grossman D, Kappler J W, Marrack P, Rich R R, Betley M J. Lack of complete correlation between emetic and T-cell-stimulatory activities of staphylococcal enterotoxins. Infect Immun. 1993;61:3175–3183. doi: 10.1128/iai.61.8.3175-3183.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris T O, Betley M J. Biological activities of staphylococcal enterotoxin type A mutants with N-terminal substitutions. Infect Immun. 1995;63:2133–2140. doi: 10.1128/iai.63.6.2133-2140.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartwig U F, Fleischer B. Mutations affecting MHC class II binding of the superantigen streptococcal erythrogenic toxin A. Int Immunol. 1993;5:869–875. doi: 10.1093/intimm/5.8.869. [DOI] [PubMed] [Google Scholar]
- 21.Hedlund G, Dohlstein M, Hermann T, Buell G, Lando P A, Segren S, Schrimsher J, Macdonald H R, Sjogren H D, Kalland T. A recombinant C-terminal fragment of staphylococcal enterotoxin A binds to human MHC class II products but does not activate T cells. J Immunol. 1991;147:4082–4085. [PubMed] [Google Scholar]
- 22.Iandolo J J. Genetic analysis of extracellular toxins of Staphylococcus aureus. Annu Rev Microbiol. 1989;43:375–402. doi: 10.1146/annurev.mi.43.100189.002111. [DOI] [PubMed] [Google Scholar]
- 23.Jardetzky T S, Brown J H, Gorga J C, Stern L J, Urban R G, Chi Y, Stauffacher C, Strominger J L, Willey D C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 24.Jupin C, Anderson S, Damais C, Alouf J E, Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988;167:752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamezawa Y, Nakahara T. Purification and characterization of streptococcal erythrogenic toxin type A produced by Streptococcus pyogenes strain NY-5 cultured in the synthetic medium NCTC-135. Comparison with the dialyzed medium (TP-medium)-derived toxin. Microbiol Immunol. 1989;33:183–194. doi: 10.1111/j.1348-0421.1989.tb01512.x. [DOI] [PubMed] [Google Scholar]
- 26.Kamezawa Y, Nakahara T, Abe Y, Kato I. Increased vascular permeability, erythema, and leukocyte emigration induced in rabbit skin by streptococcal erythrogenic toxin type A. FEMS Lett. 1990;68:159–162. doi: 10.1111/j.1574-6968.1990.tb04141.x. [DOI] [PubMed] [Google Scholar]
- 27.Kappler J W, Herman A, Clements J, Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992;175:387–396. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Urban R G, Strominger L L, Willey D C. Toxic shock syndrome toxin-1 complexed with a class II major histocompatibility molecule HLA-DR1. Science. 1994;266:1870–1874. doi: 10.1126/science.7997880. [DOI] [PubMed] [Google Scholar]
- 29.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–384. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 30.Li L-P, Tiedemann R E, Moffat S L, Fraser J D. The superantigen streptococcal pyrogenic exotoxin C (SPE-C) exhibits a novel mode of action. J Exp Med. 1997;186:375–383. doi: 10.1084/jem.186.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 32.Mcfadden J M, Noble W C, Camp R D R. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic dermatitis. Br J Dermatol. 1993;128:631–632. doi: 10.1111/j.1365-2133.1993.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 33.Metzroth B, Fleischer B. Concomitant loss of conformation and superantigenic activity of staphylococcal enterotoxin B deletion mutant proteins. Infect Immun. 1993;61:2445–2452. doi: 10.1128/iai.61.6.2445-2452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miethke T, Gaus H, Wahl C, Heeg K, Wagner H. T cell-dependent shock induced by a bacterial superantigen. Chem Immunol. 1992;55:172–184. [PubMed] [Google Scholar]
- 35.Müller-Alouf H, Alouf J E, Gerlach D, Ozegowski J-H, Fitting C, Cavaillon J-M. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect Immun. 1994;62:4915–4921. doi: 10.1128/iai.62.11.4915-4921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura E, Kubota H, Sato M, Sugie T, Yoshida O, Minato N. Involvement of NK1+CD4−CD8− α β T cells and endogenous IL-4 in non-MHC-restricted rejection of embryonal carcinoma in genetically resistant mice. J Immunol. 1997;158:5338–5348. [PubMed] [Google Scholar]
- 37.Norrby-Teglund A, Norgren M, Holm S E, Andersson U, Andersson J. Similar cytokine induction profiles of a novel streptococcal exotoxin, MF, and pyrogenic exotoxins A and B. Infect Immun. 1994;62:3731–3738. doi: 10.1128/iai.62.9.3731-3738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oku Y, Uesaka Y, Hirayama T, Takeda Y. Development of a highly sensitive bead-ELISA to detect bacterial protein toxins. Microbiol Immunol. 1988;32:807–816. doi: 10.1111/j.1348-0421.1988.tb01442.x. [DOI] [PubMed] [Google Scholar]
- 39.Papageorgiou A C, Acharya K R. Crystal structure of the superantigenic enterotoxin C2 from Staphylococcus aureus reveals a zinc-binding site. Structure. 1995;3:769–779. doi: 10.1016/s0969-2126(01)00212-x. [DOI] [PubMed] [Google Scholar]
- 40.Passalacqua E F, Brehm R D, Acharya K R, Tranter H S. Crystallization and preliminary X-ray analysis of a microbial superantigen staphylococcal enterotoxin C2. J Mol Biol. 1993;233:170–172. doi: 10.1006/jmbi.1993.1493. [DOI] [PubMed] [Google Scholar]
- 41.Pontzer C, Russel J K, Johnson H M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989;143:280–284. [PubMed] [Google Scholar]
- 42.Prasad G S, Earhart C A, Murray D L, Novick R P, Schlievert P M, Ohlendorf D H. Structure of toxic shock syndrome-1. Biochemistry. 1993;32:13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- 43.Roussel A, Anderson B F, Baker H M, Fraser J D, Baker E N. Crystal structure of the streptococcal superantigen SPE-C: dimerization and zinc binding suggest a novel mode of interaction with MHC class II molecules. Nat Struct Biol. 1997;4:635–642. doi: 10.1038/nsb0897-635. [DOI] [PubMed] [Google Scholar]
- 44.Schad E M, Zaitseva I, Zaitseva V N, Dohlsten M, Kalland T, Schlievert P M, Ohlendorf D H, Svensson L A. Crystal structure of the superantigen staphylococcal enterotoxin type A. EMBO J. 1995;14:3292–3301. doi: 10.1002/j.1460-2075.1995.tb07336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlievert P M, Bettin K M, Watson D W. Reinterpretation of the Dick test: role of group A streptococcal pyrogenic exotoxin. Infect Immun. 1979;26:467–472. doi: 10.1128/iai.26.2.467-472.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlievert P M. Role of superantigen in human disease. J Infect Dis. 1993;167:997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- 47.Schlievert, P. M., G. A. Bohach, D. H. Ohlendorf, C. V. Stauffacher, D. Y. M. Leung, D. A. Murray, C. A. Earhart, L. M. Jablonski, M. L. Hoffmann, and Y. Chi. 1995. Molecular structure of staphylococcal and streptococcal superantigens. J. Clin. Immunol. 15(Suppl. 6):4s–10s. [DOI] [PubMed]
- 48.Seas C L, Kaper J B. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigal N H, Dumont F J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- 50.Smith D B, Johnson K S. Single-step purification of polypeptides expressed in Escherichia coli as fusion with glutathione s-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 51.Strange P, Skov L, Lisby S, Nielsen P L, Baadsgaad O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996;132:27–33. [PubMed] [Google Scholar]
- 52.Swaminathan S, Furey W, Pletcher J, Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992;359:801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]