Abstract
Introduction
TPP1 variants have been identified as a causative agent of neuronal ceroid lipofuscinosis 2 disease, that ataxia is one of its clinical features. Therefore, here, molecular study of TPP1 variants is presented in an Iranian cohort and a novel pathogenic variant is described.
Methods
This investigation was conducted as a cross-sectional study in a tertiary referral hospital, Children’s Medical Center, Pediatrics Center of Excellence. Clinical presentations and pedigrees were documented. Patients with cerebellar ataxia were enrolled in this study. Next-generation sequencing was applied to confirm the diagnosis. Segregation and bioinformatics analyses were also done for the variants using Sanger sequencing.
Results
Forty-five patients were included in our study. The mean age of onset was 104 (+55.60) months (minimum = 31 months, maximum = 216 months). The majority of cases (73.3%) were born to consanguineous parents and only 1 patient (2.2%) had an affected sibling. Of the 45 patients, only 1 patient with a novel pathogenic variant (c.1425_1425+1delinsAT, p.A476Cfs*15) in the TPP1 gene was identified.
Discussion
The main strength of current study is the relatively large sample size. Besides, a novel pathogenic variant could be important toward the diagnosis and management of this condition. With significant advances in various therapies, early diagnosis could improve the treatments using personalized-based medicine.
Keywords: Autosomal recessive cerebellar ataxia, Neuronal ceroid lipofuscinosis 2 disease, Pathogenic variant, Pediatrics, TPP1
Introduction
Autosomal recessive ataxia is a large group of ataxias. On account of various pathogenic variants in different genes. Ataxia means lack of coordination and movement difficulty mainly due to cerebellum involvement [Fogel and Perlman, 2007]. The main Clinical presentations include gait disturbance, balance impairments, hypotonicity, dysarthria, and nystagmus [Perlman, 2004].
The TPP1 gene encodes a lysosomal enzyme called tripeptidyl-peptidase 1 which is a part of the serine family of carboxypeptidases or sedolysins. The enzyme is expressed in the central nervous system neurons 5 months after birth and gradually increases until it reaches its level at 3 years of age. The TPP1 deficiency leads to neuronal ceroid lipofuscinosis 2 disease (CLN2) [Warrier et al., 2013]. Up to now, 140 pathogenic variants were identified in the TPP1 gene, of which at least 115 pathogenic variants were seen in CLN2 (neuronal ceroid lipofuscinosis 2) [Gardner et al., 2019]. Clinical manifestations of the CLN2 consist of seizure usually presented at the age of 2–4 years, neurodevelopmental delay or regression, progressive dementia, visual or motor deterioration, and eventually death [Mole et al., 2018; Nickel et al., 2016, 2018]. Symptoms could be personated heterogeneously in the first 2 years, during infancy or in adolescence [Ju et al., 2002]. Late-onset manifestation in patients with CLN2 may also be due to the leaky gene expression [Kida et al., 2001; Kurachi et al., 2001]. However, unlike other sedolysins, endopeptidase activity of tripeptidyl-peptidase 1 is relatively weak and it removes the tripeptide sequence from the N-terminal terminus of proteins using an exopeptidase activity. To the best of our knowledge, no study on TPP1 variants has been published among Iranian population. Whole-exome sequencing of 45 patients showed a novel pathogenic variant in this population.
Materials and Methods
Study Population
This study was conducted as a cross-sectional study, in 2020–2021, a tertiary referral hospital Children’s Medical Center affiliated to Tehran University of Medical Sciences, Tehran, Iran. Diagnosis was made by expert pediatric neurologists, using the clinical presentation, neuroimaging, and serum biomarkers. Patients with a history of brain tumor, head trauma, myositis, or other anatomical or structural abnormality were excluded from the study. Age, age of presentation, family history, and consanguineous background if present were evaluated in all patients. All data were analyzed using SPSS version 22. Results are presented as the number (percent), mean (± standard deviation), and mean (interquartile range). The prevalence of new pathogenic variant was showed with number (percent).
Genomic DNA was extracted from peripheral blood [MWer et al., 1988]. Whole-exome sequencing was done using an Illumina NovaSeq™6000 Sequencing System platform with the mean depth of 100x for one case. The UCSC human reference genome GRCh38/hg38 version was used for alignment and variant calling. Annotation was performed using ProteinAnalysis Through Evolutionary Relationships, GeneMANIA (multiple association network integration algorithm), and Variant Effect Predictor (Ensembl). In the next step, the variants with a minimal allele frequency more than 0.01 were removed using the Exome Aggregation Database (http://exomad.broadinstitute.org/), the 1000 Genomes project (www.1000genomes.org), Exome Sequencing Project 6500 (http://evs.gs.washington.edu/EVS/), the Exome Aggregation Consortium database (http://exac.broadinstitute.org), the Greater Middle East Variome Project (http://igm.ucsd.edu/gme/), and the Iranome database (http://iranome.com/). The available bioinformatics tools including MutationTaster (http://www.mutationtaster.org/), CADD (https://cadd.gs.washington.edu/home), FATHMM (http://fathmm.biocompute.org.uk/), and GERP (http://mendel.stanford.edu/SidowLab/downloads/gerp/) were used to predict the pathogenicity of the novel variants.
Results
Patients’ Characteristics
Forty-five patients were enrolled in the present study. Twenty-six (57.8%) were females. The average age of the participants was 104 (±55.60) months. The average age of symptom onset was 26.66 (±30.02) months (minimum = 2 months, maximum = 144 months, mean interquartile range = 17 [6.25–34.5]). The most common signs and symptoms in patients are progressive gait disturbance (33.33%), cognitive deficits (26.67%), oculomotor apraxia (24.44%), and epilepsy (15.56%). The majority of cases (73.3%) were born to consanguineous parents but only one of them (2.2%) had an affected sibling.
Exome Sequencing Results and Segregation Analysis
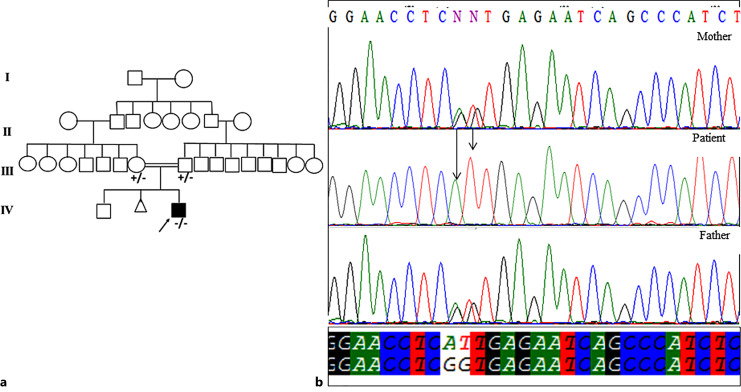
Only in 1 patient, a novel pathogenic homozygous variant, c.1425_1425+1delinsAT, was identified in TPP1 gene. The c.1425_1425+1delinsAT was not reported in the ESP, ExAC, 1000 Genome project, and Iranome databases. The variant was confirmed using Sanger sequencing and segregated with the disease within the family (Fig. 1a, b). c.1425_1425 + 1delinsAT leading to p.A476CfsX15, a pathogenic variant according to the American College of Medical Genetics and Genomics (ACMG) guideline. The patient was a 4-year-old boy referred to Ataxia Clinic because gait imbalance got worsen recently. He was the second child to consanguineous parents with a 8-year-old healthy brother. He was born by an elective and uneventful caesarian section following a full-term pregnancy. Early gross motor developmental milestones such as sitting and walking achieved on time. Delayed language development is noticed, as he could speak several words at the age of 4.
Fig. 1.
a Family pedigree of the studied patient. b Electropherograms of DNA sequence in the patient and his parents; the part marked with the arrow is the site of the novel variant.
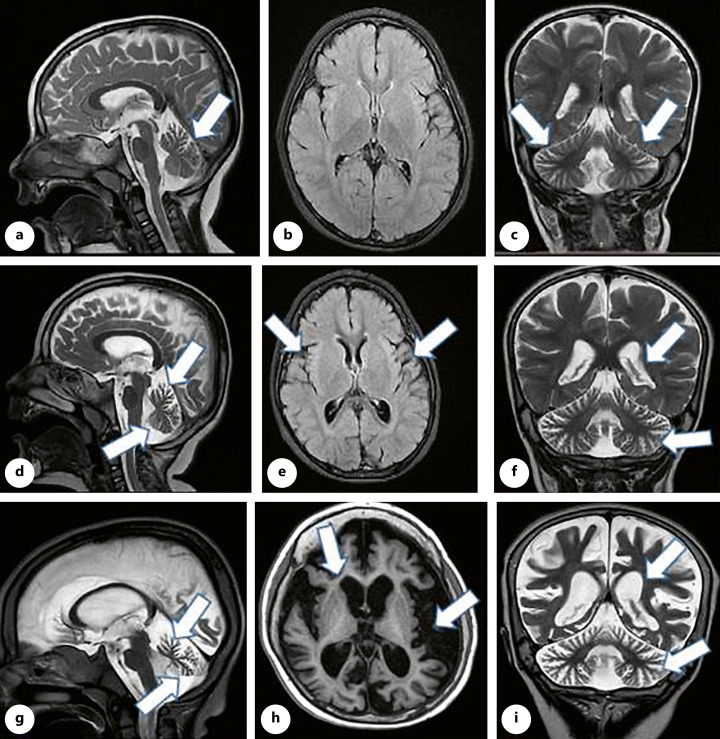
Parents got worried about esotropia when he was 4 months that leads to serial ophthalmologic evaluation. Over time, esotropia was disappeared while vision difficulty and diagnosis of Leber’s disease were noticeable. Gait problem and incoordination presented about 1 year ago and got worsen over recent previous months. Past medical history was remarkable for syndactyly/polydactyly correction surgery and laboratory data showed hypercalciuria. Family history revealed that his maternal first cousin suffers from nyctalopia. He was evaluated by brain magnetic resonance imaging. Neuroimaging findings contain progressive brain and cerebellum atrophy over 1 year (Fig. 2).
Fig. 2.
The first line pictures, a–c (2018, at age 1 year old), showed mild upper vermis atrophy (a), normal supra tentorial (b), and mild prominent cerebellar rugaes (c). The middle pictures, d–f (2019), presented progressive vermis atrophy (d), widening of sylvian fissure (e), and more prominent cerebellar rugaes as well as ventriculomegaly (f). The last line pictures, g–i (2022, at age 4 years old), revealed sever progressive cerebellar and cortical atrophy as well as ex vacuo ventriculomegaly.
Discussion
Forty-five patients were evaluated in a cohort study and a novel pathogenic variant (c.1425_1425 + 1delinsAT, p.A476Cfs * 15) in TPP1 gene in 1 patient was found. To the best of our knowledge, this variant is the third indel pathogenic variant affecting a splice site in TPP1 gene (Table 1). This pathogenic variant involves splice site of exon-intron 11. It is known that these types of pathogenic variants could lead to decrease or disrupt mRNA processing so that cause exon skipping or intron retention. We have previously reported pathogenic variants in some genetic disorders in our population. Specific pathogenic variants have been observed with high frequencies in some populations in many disorders, e.g., common pathogenic variants in CYP21A2 and GJB2 genes in different ethnicities living in Iran (Davoudi-Dehaghani et al., 2013; Mahdieh et al. [2016]; Rabbani et al. [2011]).
Table 1.
Previously reported indel pathogenic variants of TPP1
| Pathogenic variant (DNA) | Pathogenic variant (protein) | Position | Phenotype | Population | Ref |
|---|---|---|---|---|---|
| c.37dupC | p.Leu13ProfsX32 | E2 | LINCL | UK | [Kousi et al., 2012] |
| c.114delG | p.Trp38CysfsX12 | E3 | LINCL | UK | [Kousi et al., 2012] |
| c. 177-180delAAGA | p.E59DfsX20 | E3 | LINCL | Chinese | [Chang et al., 2012] |
| c.183_184delCT | p.S62Gfs*25 | E3 | LINCL | Chinese | [Lam et al., 2001] |
| c.274_274delT | p.Ser92Profs*3 | E4 | LSD | USA | [Sleat et al., 2009] |
| c.357_358insT | p.Leu120Serfs*18 | LINCL | USA | [Zhong et al., 2000] | |
| c.376_380delATCCG | p.Ile126Thrfs*10 | E4 | LINCL | USA | [Sleat et al., 1999] |
| c.381-17-4delTGTTCTCTGACCTC (IVS4) | Aberrant splicing | I4 | LINCL | Chinese | [Chang et al., 2012] |
| c.409-410insGCTG | p.E139GfsX1 | E5 | LINCL | Chinese | [Chang et al., 2012] |
| c.497dupA | p.His166GlnfsX22 | E5 | LINCL | Turkey | [Kousi et al., 2012] |
| c.538dupC | p.Leu180ProfsX9 | E6 | LINCL | Turkey | [Kousi et al., 2012] |
| c.822_837del | p.Leu275X | E7 | LINCL | UK | [Kousi et al., 2012] |
| c.688_688delT | p.Phe230Serfs*28 | E7 | LINCL | India | [Verma et al., 2015] |
| c.775_775delC | p.Arg259Valfs*17 | E7 | LINCL | Arab | [Goldberg-Stern et al., 2009] |
| c.992_993insCTC | p.331dupLeu | E8 | LINCL | NA | [Cooper et al., 2020] |
| c.969_976delGAGCTATG | p.Ser324Argfs*2 | E8 | LINCL | Italy | [Sleat et al., 1999] |
| c.1062delG | p.Leu355SerfsX72 | E8 | LINCL | Italy | [Kousi et al., 2012] |
| c.986_993delAGGACTCC | p.Glu329Alafs*12 | E8 | LINCL | Italy | [Santorelli et al., 2013] |
| c.1107-1108delTG | p.Gly370Lysfs*32 | E9 | LINCL | South America | [Kohan et al., 2013] |
| c.1424delC | p.Ser475TrpfsX13 | E11 | NCL | Canada | [Moore et al., 2008] |
| c.1425delinsAT | p.Ala476Cysfs*15/Aberrant splicing | I11 | LINCL | Iran | This study |
| c.1497delT | p.Gly501AlafsX18 | E12 | LINCL | Turkey | [Kousi et al., 2012] |
| c.1547_1548delTT | p.Phe516X | E12 | LINCL | Turkey | [Kousi et al., 2012] |
| c.1546-1547insTTCA | p.Asp517His fsX1 | E12 | LINCL | Chinese | [Chang et al., 2012] |
| c.1551+5_1551+6delGTinsTA | Splice defect | I12 | LINCL | Portugal | [Kousi et al., 2012] |
| c.1595_1596insA | p.Gln534Profs*74 | E13 | LINCL | USA | [Sleat et al., 1999] |
| c.1608_1618delCTGCTCTGGTC | p.Cys537Trpfs*67 | E13 | LINCL | USA | [Hofmann et al., 2002] |
| c.1677_1678delTC | p.Leu560Thrfs*47 | E13 | LINCL | USA | [Sleat et al., 1999] |
LINCL, late infantile neuronal ceroid lipofuscinosis; LSD, lysosomal storage disease.
The TPP1 protein starts with 19 amino acid signal peptides and has a 176 amino acid peptide deleted in its final form. The final form of the protein consists of the 368 amino acid chain tripeptidyl-peptidase 1. Most pathogenic variants in TPP1 are located in tripeptidyl-peptidase 1, while there are only three pathogenic variants in the propeptide section, and none of the pathogenic variants are located in the signal peptide section.
Other than autosomal recessive cerebellar ataxia, the TPP1 pathogenic variants have been studied under several neurologic disorders conditions; in 2019, Domowicz et al. investigated end-stage transcriptional changes associated with TPP1 inactivity in the brains of NCL mice. The NCL mouse model, in which TPP1 was disrupted by gene targeting, was referred to as the TPP1 - /- pathogenic variant and, using RNA-seq technology, changes in TPP1 expression in the forebrain/midbrain and cerebellum of 4-month-old homozygotes was compared with the control group. In 2018, Chen et al. studied TPP1 gene pathogenic variants in patients with seizures to find a link between genotype and phenotype for patients with different phenotypes. They used targeted sequencing of the new generation of the genome for 330 patients with seizures. They found a homozygous pathogenic variant for the TPP1 gene c.646 G > A /p.Val216Met), which existed in two siblings in the same family (an 8-year-old girl and a younger brother). Further studies of TPP1 gene pathogenic variants have shown that the prevalence of splicing pathogenic variants in patients with late-onset CLN2 is higher than in adolescents with CLN. Patients diagnosed with late-onset neonatal CLN have a higher rate of a splicing pathogenic variant in patients diagnosed with juvenile CLN, and there is no significant association between phenotype and a high number of destructive pathogenic variants. They concluded that based on the etiology, the relationship between the severity of the phenotype and gene pathogenic variant for TPP1 could explain different phenotypes, resulting from this gene pathogenic variant [Chen et al., 2019]. Compound heterozygous variants (c.1340GNA, p.R447H; c.790CNT, p.Q264X) in the TPP1 gene could be due to high carrier frequency of the variants. In 2015, Dy et al. reported a 10-year-old African-American /Caucasian girl with a normal neurodevelopmental status till 3 years of age, but neurodevelopmental regression occurred afterward due to a heterozygous TPP1 pathogenic variant in TPP1 gene. TPP1 activity was significantly decreased in blood and fibroblasts. Dy et al. concluded that TPP1 deficiency should be considered in differentiating cerebellar ataxia with early-onset age.
In 2013, Sun et al. identified the TPP1 gene as the gene responsible for autosomal recessive spinocerebellar ataxia (SCAR7). SCAR7 patients typically have ataxia and low serum TPP1 levels, although ocular disorders and seizures are not common in these patients. The clinical findings shown in our case associated with the TPP1 gene pathogenic variant to SCAR7. Despite the small sample size, they showed a correlation between genotype (pathogenic variant in the TPP1 gene) and phenotype. Finally, they hypothesized that lack of TPP1 gene leads to CLN2 disease and its decreased function leads to SCAR7 disease [Sun et al., 2013].
To the best of our knowledge, this study is the first study evaluating the TPP1 gene pathogenic variant in patients presented with autosomal recessive cerebellar ataxia; the main strength of the current study is a relatively large sample size. Besides, a novel pathogenic variant found could be important toward the diagnosis and management of this condition. With significant advances in various therapies, early identification of these patients leads to a change in treatment approach based on the etiology. By considering NCL as a devastating neurodegenerative disorder, CLN2 could be unique. Enzyme replacement therapy is considered as a promising therapeutic method [Mazurkiewicz-Bełdzińska et al., 2021]. Determining new pathogenic variants might play crucial role in precision medicine such as enzyme replacement therapy in these groups of neurodevelopmental disease.
Additional clinical and radiologic findings could lead to correct diagnosis. In the presented case, by considering vision difficulty as well as progressive brain (supratentorial) atrophy besides gait problems and cerebellar involvements, the underlying etiology might be more extensive than ataxia, as the main patient’s chief compliant. This clinical hypothesis was confirmed by genetic evaluation that revealed TPP1 pathogenic variant leads to CLN2.
Acknowledgments
We would like to thank the participant families and staffs of Growth and Development Research Center, Tehran University of Medical Sciences for technical assistance.
Statement of Ethics
This study has been approved by the Research Deputy and the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.CHMC.REC.1399.073). The written and verbal informed consent forms were obtained from all parents before enrollment of their offspring in the study. Author declares that the work described has been carried out in accordance with Declaration of Helsinki; the tests are based on molecular genetic testing guidelines.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was supported in part by the NIMAD proposal No 971846 received by Professor Mahmoud Reza Ashrafi and the Growth and Development Research Center.
Author Contributions
Nahid Vafaei: writing-original draft, conceptualization, and formal analysis. Ali Mohebbi: writing, data curation, and formal analysis. Zahra Rezaei and Morteza Heidari: formal analysis and resources. Sareh Hosseinpour, Ali Zare Dehnavi, Masoud Salehipour, and Ali Rabbani: Formal analysis. Azin Ghamari: review and editing. Nejat Mahdieh: writing – review and editing, conceptualization, formal analysis, and validation. Mahmoud Reza Ashrafi: validation, formal analysis, and project administrator.
Funding Statement
This study was supported in part by the NIMAD proposal No 971846 received by Professor Mahmoud Reza Ashrafi and the Growth and Development Research Center.
Data Availability Statement
There are no other data available.
References
- Chang X, Huang Y, Meng H, Jiang Y, Wu Y, Xiong H, et al. Clinical study in Chinese patients with late-infantile form neuronal ceroid lipofuscinoses. 2012;34, 739–45. [DOI] [PubMed] [Google Scholar]
- Chen Z-R, Liu D-T, Meng H, Liu L, Bian W-J, Liu X-R, et al. Homozygous missense TPP1 mutation associated with mild late infantile neuronal ceroid lipofuscinosis and the genotype-phenotype correlation. Seizure. 2019;69:180–5. 10.1016/j.seizure.2018.08.027. [DOI] [PubMed] [Google Scholar]
- Cooper DN, Ball EV, Stenson PD, Phillips AD, Evans K, Heywood S, et al. The human gene mutation database (HGMD®); 2020. [Google Scholar]
- Davoudi-Dehaghani E, Zeinali S, Mahdieh N, Shirkavand A, Bagherian H, Tabatabaiefar MA. A transversion mutation in non-coding exon 3 of the TMC1 gene in two ethnically related Iranian deaf families from different geographical regions; evidence for founder effect. Int J Pediatr Otorhinolaryngol. 2013;77(5):821–6. 10.1016/j.ijporl.2013.02.021. [DOI] [PubMed] [Google Scholar]
- Fogel BL, Perlman S. Clinical features and molecular genetics of autosomal recessive cerebellar ataxias. Lancet Neurol. 2007;6(3):245–57. 10.1016/S1474-4422(07)70054-6. [DOI] [PubMed] [Google Scholar]
- Gardner E, Bailey M, Schulz A, Aristorena M, Miller N, Mole SE. Mutation update: review of TPP1 gene variants associated with neuronal ceroid lipofuscinosis CLN2 disease. Hum Mutat. 2019;40(11):1924–38. 10.1002/humu.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg-Stern H, Halevi A, Marom D, Straussberg R, Mimouni-Bloch A. Late infantile neuronal ceroid lipofuscinosis: a new mutation in Arabs. Pediatr Neurol. 2009;41(4):297–300. 10.1016/j.pediatrneurol.2009.04.022. [DOI] [PubMed] [Google Scholar]
- Hofmann SL, Atashband A, Cho SK, Das AK, Gupta P, Lu J-Y. Neuronal ceroid lipofuscinoses caused by defects in soluble lysosomal enzymes (CLN1 and CLN2). Curr Mol Med. 2002;2(5):423–37. 10.2174/1566524023362294. [DOI] [PubMed] [Google Scholar]
- Ju W, Zhong R, Moore S, Moroziewicz D, Currie J, Parfrey P, et al. Identification of novel CLN2 mutations shows Canadian specific NCL2 alleles. J Med Genet. 2002;39(11):822–5. 10.1136/jmg.39.11.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida E, Golabek AA, Walus M, Wujek P, Kaczmarski W, Wisniewski KE. Distribution of tripeptidyl peptidase I in human tissues under normal and pathological conditions. J Neuropathol Exp Neurol. 2001;60(3):280–92. 10.1093/jnen/60.3.280. [DOI] [PubMed] [Google Scholar]
- Kohan R, Carabelos MN, Xin W, Sims K, Guelbert N, Cismondi IA, et al. Neuronal ceroid lipofuscinosis type CLN2: a new rationale for the construction of phenotypic subgroups based on a survey of 25 cases in South America. Gene X. 2013;516(1):114–21. 10.1016/j.gene.2012.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousi M, Lehesjoki AE, Mole SEJH. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2012;33(1):42–63. 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- Kurachi Y, Oka A, Itoh M, Mizuguchi M, Hayashi M, Takashima S. Distribution and development of CLN2 protein, the late-infantile neuronal ceroid lipofuscinosis gene product. Acta Neuropathol. 2001;102(1):20–6. 10.1007/s004010000321. [DOI] [PubMed] [Google Scholar]
- Lam C-W, Poon PM, Tong S-F, Ko C-HJA. Two novel CLN2 gene mutations in a Chinese patient with classical late-infantile neuronal ceroid lipofuscinosis. Am J Med Genet. 2001;99(2):161–3. . [DOI] [PubMed] [Google Scholar]
- Mahdieh N, Mahmoudi H, Ahmadzadeh S, Bakhtiyari S. GJB2 mutations in deaf population of Ilam (Western Iran): a different pattern of mutation distribution. Eur Arch Oto-Rhino-Laryngol. 2016;273(5):1161–5. 10.1007/s00405-015-3684-8. [DOI] [PubMed] [Google Scholar]
- Mazurkiewicz-Bełdzińska M, Del Toro M, Haliloğlu G, Huidekoper HH, Kravljanac R, Mühlhausen C, et al. Managing CLN2 disease: a treatable neurodegenerative condition among other treatable early childhood epilepsies. 2021, 1–8. [DOI] [PubMed] [Google Scholar]
- Mole SE, Gardner E, Schulz A, Xin WW. Molecular basis of CLN2 disease: a review and classification of TPP1 gene variants reported worldwide. Mol Genet Metabol. 2018;123(2):S97. 10.1016/j.ymgme.2017.12.255. [DOI] [Google Scholar]
- Moore S, Buckley D, MacMillan A, Marshall H, Steele L, Ray P, et al. The clinical and genetic epidemiology of neuronal ceroid lipofuscinosis in Newfoundland. Clin Genet. 2008;74(3):213–22. 10.1111/j.1399-0004.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes D, Polesky H. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids res. 1988;16(3):1215. 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel M, Jacoby D, Lezius S, Down M, Simonati A, Genter F, et al. Natural history of CLN2 disease: quantitative assessment of disease characteristics and rate of progression. Neuropediatrics. 2016;47(S 01):FV04–03. 10.1055/s-0036-1583730. [DOI] [Google Scholar]
- Nickel M, Simonati A, Jacoby D, Lezius S, Kilian D, Van de Graaf B, et al. Disease characteristics and progression in patients with late-infantile neuronal ceroid lipofuscinosis type 2 (CLN2) disease: an observational cohort study. Lancet Child Adolesc Health. 2018;2(8):582–90. 10.1016/S2352-4642(18)30179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman SL. Symptomatic and disease-modifying therapy for the progressive ataxias. Neurologist. 2004;10(5):275–89. 10.1097/01.nrl.0000141651.35193.67. [DOI] [PubMed] [Google Scholar]
- Rabbani B, Mahdieh N, Haghi Ashtiani MT, Akbari MT, Rabbani A. Molecular diagnosis of congenital adrenal hyperplasia in Iran: focusing on CYP21A2 gene. Iran J Pediatr. 2011;21(2):139–50. [PMC free article] [PubMed] [Google Scholar]
- Santorelli FM, Garavaglia B, Cardona F, Nardocci N, Dalla Bernardina B, Sartori S, et al. Molecular epidemiology of childhood neuronal ceroid-lipofuscinosis in Italy. 2013;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Ding L, Wang S, Zhao C, Wang Y, Xin W, et al. Mass spectrometry-based protein profiling to determine the cause of lysosomal storage diseases of unknown etiology. Mol Cell Proteomics. 2009;8(7):1708–18. 10.1074/mcp.M900122-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Gin RM, Sohar I, Wisniewski K, Sklower-Brooks S, Pullarkat RK, et al. Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder. Am J Hum Genet. 1999;64(6):1511–23. 10.1086/302427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Almomani R, Breedveld GJ, Santen GW, Aten E, Lefeber DJ, et al. Autosomal recessive spinocerebellar ataxia 7 (SCAR 7) is caused by variants in TPP1, the gene involved in classic late-infantile neuronal ceroid lipofuscinosis 2 disease (CLN 2 disease). Hum Mutat. 2013;34(5):706–13. 10.1002/humu.22292. [DOI] [PubMed] [Google Scholar]
- Verma J, Thomas DC, Sharma S, Jhingan G, Saxena R, Kohli S, et al. Inherited metabolic disorders: prenatal diagnosis of lysosomal storage disorders. Prenat Diagn. 2015;35(11):1137–47. 10.1002/pd.4663. [DOI] [PubMed] [Google Scholar]
- Warrier V, Vieira M, Mole SE. Genetic basis and phenotypic correlations of the neuronal ceroid lipofusinoses. Biochim Biophys Acta. 2013;1832(11):1827–30. 10.1016/j.bbadis.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Zhong N, Moroziewicz DN, Ju W, Jurkiewicz A, Johnston L, Wisniewski KE, et al. Heterogeneity of late-infantile neuronal ceroid lipofuscinosis. Genet Med. 2000;2(6):312–8. 10.1097/00125817-200011000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no other data available.