Abstract
Lifestyle and environmental factors are key determinants in disease causality and progression in neurological conditions, including multiple sclerosis (MS). Lack of exercise, poor diet, tobacco smoking, excessive alcohol intake, social determinants of health, concomitant medications, poor sleep and comorbidities can exacerbate MS pathological processes by impacting brain health and depleting neurological reserves, resulting in more rapid disease worsening. In addition to using disease-modifying therapies to alter the disease course, therapeutic strategies in MS should aim to preserve as much neurological reserve as possible by promoting the adoption of a “brain-healthy” and “metabolically-healthy” lifestyle. Here, we recommend self-regulated lifestyle modifications that have the potential to improve brain health, directly impact on disease progression and improve outcomes in people with MS. We emphasise the importance of self-management and adopting a multidisciplinary, collaborative and person-centred approach to care that encompasses the healthcare team, family members and community support groups.
Keywords: multiple sclerosis, brain health, lifestyle, disease progression, outcomes, holistic care, recommendations
1. Introduction
Multiple sclerosis (MS) is an inflammatory demyelinating and degenerative disease of the central nervous system (CNS) affecting the brain and spinal cord (1–3). MS can cause a range of symptoms, including sensory, motor, or visual disturbance, fatigue and cognitive impairment (4). MS can occur at any age, including during childhood and adolescence, with the global average age of MS diagnosis being 32 years (1). MS is a heterogenous disease, with some people having a variable rate of recovery characterised by periods of relapse and remission while others have progressive disease (5–7). Due to the heterogeneity of MS, life for people with the disease and their families and friends can be unpredictable, with negative impacts on their quality of life (QoL) (1). Furthermore, MS is the leading cause of nontraumatic neurological disability in young people, which has major implications for societal costs due to loss of productivity and healthcare costs (1).
Although repair mechanisms and remodelling of the CNS can partially abate the underlying pathological insults in MS, eventually, the compensatory capacity is not temporally aligned with the ongoing inflammatory disease activity (2, 4). Neurological reserve capacity is defined as the ability of the brain to effectively buffer changes associated with normal aging and cope with pathological damage. The baseline reserve is depleted by factors such as aging, neurologic disease progression and health comorbidities, but can be preserved by protective factors such as effective treatments and healthy lifestyles (8). Individual people have varying degrees of reserve at disease onset (9); however, as this reserve is gradually exhausted, the consequences of MS become clinically apparent, with steady increases in physical, mental and cognitive disability. Treatment with disease-modifying therapies (DMTs) can help preserve the neurological reserve and lead to a lower risk of disability (8).
Metabolic health relates to levels of blood glucose, triglycerides, high-density lipoprotein cholesterol, blood pressure, and waist circumference. Lifestyle and environmental factors are key determinants in disease causality and progression in neurological conditions, including MS (10, 11). Lack of exercise, poor diet, smoking, excessive alcohol intake, social determinants of health (SDOH), concomitant medications, poor sleep and comorbidities can exacerbate MS pathological processes by impacting brain health and depleting neurological reserves, resulting in more rapid disease worsening (2, 4). Therefore, in addition to using DMTs to slow down the disease course, therapeutic strategies in MS should aim to preserve as much neurological reserve as possible by promoting the adoption of a “brain-healthy” and “metabolically-healthy” lifestyle (2, 4). Here, we recommend self-regulated lifestyle modifications that have the potential to significantly improve brain health and metabolic health in people with MS and directly or indirectly slow down disease progression and improve outcomes.
2. What is brain health?
A recent position paper published by the World Health Organization (WHO) defined brain health as “the state of brain functioning across cognitive, sensory, social–emotional, behavioural and motor domains, allowing a person to realise their full potential over the life course, irrespective of the presence or absence of disorders” (12). Accordingly, brain health is measured as the brain’s ability to grow and build strong neuronal connections, repair neuronal damage and compensate over time in ways that allow people to think, feel, move and interact with the world around them. The WHO identified five major determinants of human brain health optimisation across the life course, namely: physical health, environment, safety and security, lifelong learning and social connections, and access to quality services (12). Brain health can be optimised throughout people’s lifetimes by minimising exposure to risk factors, where possible, and enhancing factors that promote neuroplasticity and recovery from brain injury (12). To optimise brain health in people with MS, it is important to understand how the underlying pathological processes impact brain function, and the resulting sequelae. However, it is also important to identify and optimise modifiable factors that may improve or maintain brain health throughout the disease course. Recommendations for healthcare professionals to maintain brain health in people with MS are shown in Table 1. As the evidence discussed herein shows, it is never too late for people with and without MS to reduce lifestyle risk factors, even for those already experiencing disability.
Table 1.
Recommendations for healthcare professionals to maintain brain health in people with MS.
| Promote prompt diagnosis and early treatment with DMT |
|
| Discuss infection prevention and treatment |
|
| Discuss strategies to improve emotional well-being |
|
| |
| |
| |
| Encourage people with MS to adopt lifestyle changes that preserve cognitive reserve and develop resilience to stress |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Ensure regular monitoring of people with MS in MS clinic and via partners (e.g., GP) |
|
| |
| |
| |
| |
| |
|
*See later section for more specific guidance.
CBT, cognitive behavioural therapy; DMT, disease modifying therapy; GP, general practitioner; NHS, National health System; MS, multiple sclerosis.
2.1. Enhance cognitive reserve
Despite MS being considered a disease of young adults, the mean age of the population with MS is increasing, resulting in an increased incidence of MS among the elderly (13). Ageing in people with MS is associated with cognitive impairment, particularly regarding information processing speed and multitasking, as seen in people with dementia due to primary neurodegenerative conditions such as Alzheimer’s disease (14). A similar cognitive profile between young and old people with MS suggests that the cognitive impairment in MS is probably a continuous process starting from the earliest stages of the disease and increasing over its course (14). To slow down brain ageing and maintain neuroplasticity, it is vital that people with MS stay as cognitively active as possible (15). Therefore, people with MS should be encouraged to learn and to connect with others regularly. A short daytime nap has been shown to help with cognitive performance in the workplace (16). High resilience (the ability to achieve, retain or regain a level of physical or emotional health after illness or loss), perseverance and stamina increase healthy ageing and well-being, particularly the ability to maintain healthy lifestyle habits, independence and participation (17, 18).
3. Optimising brain health in people with MS
3.1. Adopt a multidisciplinary, collaborative and person-centred approach to care that encompasses the healthcare team, family members and community support groups
People with MS have different needs for information, advice and support, and these needs change as MS progresses. Within healthcare services, patients should have a single point of contact who can coordinate access to care from a multidisciplinary team with expertise in MS (19). Care should be tailored to the individual patient and be responsive to their changing needs. To enable and maintain healthy lifestyle behaviours, people with MS may require support from family members and the broader community. Within routine clinical practice, it is crucial to incorporate the ability for healthcare providers to signpost people with MS and their families to local support groups who can assist them with lifestyle behaviours (e.g., smoking cessation support, physical activity programmes, healthy eating advice).
3.2. Identify and treat symptoms promptly
3.2.1. Infections
Infections trigger proinflammatory reactions in the brain; preventing infections can positively impact disease progression by minimising inflammatory mechanisms. Mounting evidence from different fields of research supports the pivotal role of the Epstein–Barr virus (EBV) in the development of MS (20). Ongoing research aims to clarify the causative role of EBV in MS; potential mechanisms include the promotion of neuroinflammation via autoimmunity or direct viral infection.
There is increasing evidence that people with MS have a higher risk of overall infections and infections leading to hospitalisation compared with the general population (21, 22). All types of infection are increased, including viral, fungal, pneumonia and influenza, and opportunistic infections (22). Urinary tract infections (UTIs) are the most common infection treated by MS specialists, followed by aspiration pneumonia (22). Poor oral hygiene is reported in some people with MS, and there is evidence that people with MS may be at higher risk of periodontal disease than the general population (23). Proactive infection monitoring, management and self-care education for people with MS, such as being provided with the recently developed home UTI testing kit, can help identify infections early and improve patient well-being, particularly in those with more severe disabilities, who typically have poor outcomes from infection (24, 25). Comprehensive MS bladder management pathways have been developed to increase awareness of the importance of bladder problems in people with MS and how to best manage them.
3.2.2. Bladder and bowel symptoms
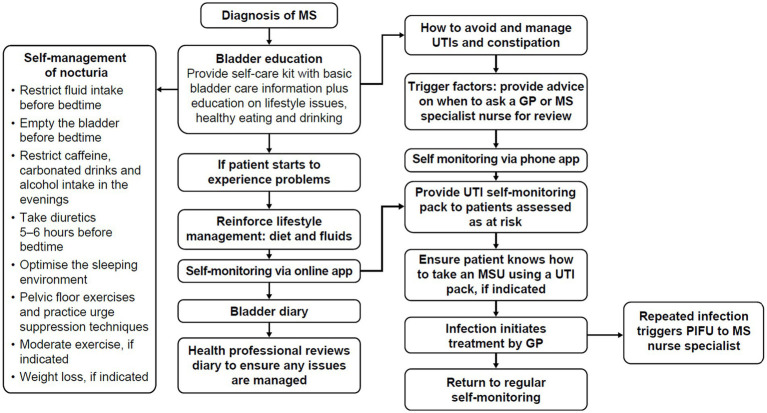
Bladder and bowel symptoms have a high impact on QoL (25). Bladder problems contribute to debilitating sleep disturbance (e.g., via nocturia) and are significantly correlated with fatigue, daytime sleepiness and depression. Despite these detrimental impacts, people with MS can be reluctant to seek help, due to a lack of awareness around potential treatments, or the social stigma associated with bladder dysfunction; they may choose to self-manage symptoms alone. Although self-management is recommended to identify UTIs early, other lifestyle modifications such as reduction of bladder irritants like caffeine and alcohol can help to decrease and manage bladder and bowel symptoms, which in turn help to alleviate sleep disruption for some people with MS (Figure 1).
Figure 1.
Self-management pathway for bladder dysfunction in people with MS. Adapted from Thomas et al. (25, 46). GP, general practitioner; MS, multiple sclerosis; MSU, mid-stream urine culture; PIFU, patient-initiated follow-up; UTI, urinary tract infection.
3.2.3. Depression and anxiety
Up to 50% of people with MS are reported to experience depression at some point in their life (26), and the incidence of suicide is higher in people with MS compared with the general population (26, 27). Depressive symptoms in people with MS may be experienced differently by females and males, with females experiencing greater overall symptoms and greater somatic symptoms compared with males (28). Social isolation may increase the risk of depression and anxiety in people with MS; loneliness is reported by 60% of people living with MS (29, 30).
Many factors play a role in developing depression in people with MS, including a link to increased inflammatory activation of the immune system affecting both the periphery and the CNS. Chronic inflammation caused by stress has a causative association with chronic diseases, including depression (31). In addition, a role for immune activation in depression is supported by response to treatments targeting immune pathways, which are effective in treating depression and fatigue in some cases (17).
Depression can be very disabling; it affects QoL, productivity and brain health (32). However, low mood is not necessarily related to depression. Depression and physical symptoms of MS can be quite similar, e.g., brain fog, lower motivation for certain activities, reduced energy levels or withdrawal. Moreover, people with depression do not always realise that they have depression – in fact, other family members may notice signs first (33). It is important to identify early signs of depression and obtain a clinical diagnosis as soon as possible, as depression can lead to increased symptoms and decreased QoL in people with MS (32). There is a vicious cycle between (1) depression worsening MS symptoms (through inactivity and negative automatic thoughts), and (2) the physical impact of MS symptoms worsening depression. This can be treated with psychological therapies such as cognitive behavioural therapy (CBT), acceptance commitment therapy (ACT) and counselling.
Anxiety is reported to occur in up to 45% of people with MS (34). Females and people with a relapsing–remitting MS (RRMS) disease course and a worse degree of functional disability show higher rates of anxiety symptoms than their counterparts (34). As with depression, psychological therapies such as CBT, ACT and counselling are treatment options.
3.2.4. Cardiovascular symptoms
Vascular damage in the brain may contribute to neurodegeneration and is associated with lower cognitive function in both people with MS and in the general population (35). A recent UK study reported that at the time of MS diagnosis, people with MS have an increased prevalence of vascular risk factors, including hypertension and diabetes, compared with matched controls (36). It is therefore important to prevent, or identify and control, conditions that increase the risk of cardiovascular disease, including diabetes mellitus, hypothyroidism, hypercholesterolaemia, dyslipidaemia, hypertension, sedentary lifestyle and obesity. The impact of comorbid conditions in people with MS is discussed in more detail later in this review.
3.2.5. Sleep disorders
Sleep disorders are more prevalent in people with MS than in the general population and include sleep-related breathing disorders, restless legs syndrome, periodic limb movement disorders, secondary narcolepsy, rapid eye movement sleep behaviour disorder and propriospinal myoclonus (37). Magnetic resonance imaging (MRI) has shown a link between sleep disorders and lesions in specific CNS regions (37). Management of sleep disorders can be complex in people with MS, and multidisciplinary care, with referral to sleep medicine specialists and other specialities (e.g., urologists), may be required.
Changes in sleep quality over time impact how people with MS experience a whole host of symptoms, and there is evidence that poor sleep quality may be a trigger for MS relapse and can negatively influence MS outcomes (38). There is a complex relationship between MS symptoms and sleep, with some symptoms exacerbating others, leading to a vicious cycle of sleep disruption, whilst some known MS symptoms may themselves be caused by a lack of sleep (39) Because pain sensitivity may be heightened by sleep loss (40), researchers recommend the management of sleep and anxiety in people with MS to decrease their pain levels (41).
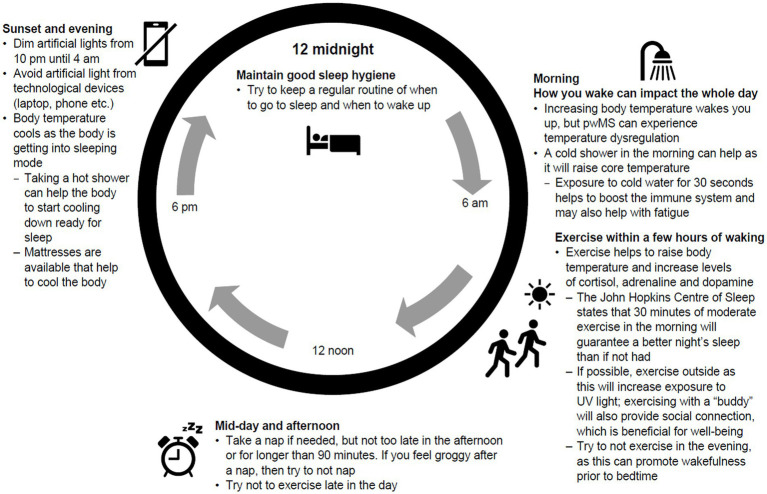
Education around good sleep hygiene and the impact of lifestyle choices on sleep quality need to be available to people with MS, with CBT offered for those with chronic insomnia (42). Self-management of sleep disruption should be encouraged by helping people with MS understand the circadian rhythm and how body temperature, light exposure and nutrition can impact sleep architecture (Figure 2). Tailoring sleep hygiene advice to the individual is important for those with MS, considering symptoms, medication and side effects, and ensuring information on physical activity, natural light and nutrition are personalised (43–46).
Figure 2.
Guidance on good sleep hygiene and sleep architecture. UV, ultraviolet.
Medications, including DMTs and symptomatic medications (e.g., baclofen, gabapentin, oxybutynin), can affect sleep in people with MS either by causing insomnia or hypersomnia; the underlying mechanisms vary [Table 2; (47–49)].
Table 2.
Medications commonly used in the treatment of MS with the potential to disrupt sleep and proposed adaptations to avoid or decrease sleep disruption.
| Medication | Indication in MS | Effects on sleep | PSG data | Proposed MoA | Adaptation |
|---|---|---|---|---|---|
| DMTs | |||||
| Interferon-beta | First-line DMT | Fatigue, hypersomnolence, insomnia | Reduction of sleep efficiency | Reduces antigen presentation and T-cell proliferation; alters cytokine expression | Switch from evening to morning injection |
| Glatiramer acetate | First-line DMT | More frequent awakenings and daytime somnolence; increased anxiety and irritability | Not available | Partly due to increase in anti-inflammatory cytokines (IL-4 and IL-10) through activation of the Th-2 cell pathway of immunity | |
| Natalizumab | Second-line DMT | Improvement in fatigue, daytime sleepiness and depression | Not available | Monoclonal antibody against the α-4 subunit of α-4 β-1 integrin that inhibits the ability of activated T cells to migrate across the blood–brain barrier into the CNS | |
| Symptomatic medications | |||||
| Methylprednisolone | Acute relapse | Insomnia | Decreased REM sleep | Decreased cytokine cascade, activation of B and T cells and ability of immune cells to penetrate the CNS | Lifestyle changes, CBT, pharmacological intervention (if needed) |
| Modafinil | Fatigue | Insomnia | Reduced sleep latency | Unknown | |
| Methylphenidate | Fatigue | Insomnia | REM sleep suppression | Increased catecholamine release and reuptake inhibition | |
| Amantadine | Fatigue | Insomnia | Not available | Presynaptic dopamine release | |
| Pemoline | Fatigue | Not available | CNS stimulant | ||
| 4-Aminopyridine | Fatigue | Insomnia | Not available | Block of K channels in neurons | |
| Baclofen | Spasticity | Sedation | Total sleep time increased and reduced wake after sleep onset | GABA-B receptor agonist | |
| Clonazepam | Spasticity, anxiety | Somnolence | Increased total sleep time, reduced sleep latency and wake after sleep onset, increased spindle activity and reduced REM sleep | GABA-A receptor agonist | |
| Tizanidine | Spasticity | Daytime drowsiness | Improvement in sleep induction and maintenance | Central α-2 adrenoreceptor agonist | |
| SSRI | Depression, anxiety | Insomnia or sedation | Decreased total sleep time, increased stage 1 sleep, decreased REM sleep, increased sleep latency, “Prozac eyes” and periodic limb movements | Inhibition of serotonin reuptake | |
| Gabapentin | Pain seizures | Sleepiness | Decreased sleep stage 1, increased sleep stage 3, reduced periodic limb movements, increased REM sleep | May promote formation of GABA in the CNS | |
| Oxybutynin | Urinary frequency | Sedation | Decreased REM sleep and increased REM sleep latency | Anticholinergic agent | |
| Cannabis-based medicinal extracts | Spasticity, bladder dysfunction, central neuropathic pain | Improved sleeping difficulty and sleep quality, diminished awakenings and sleepiness | Not available | Inhibition of smooth muscle contraction, interaction with the cholinergic receptor system and/or synergism with anticholinergic medication, analgesic properties | |
Adapted from Brass et al. (47) and Lanza et al. (48). CBT, cognitive behavioural therapy; CNS, central nervous system; DMT, disease-modifying therapy; GABA, gamma-amino-butyric acid; IL, interleukin; K, potassium; MoA, mechanism of action; MS, multiple sclerosis; PSG, polysomnography; REM, rapid eye movement; SSRI, selective serotonin reuptake inhibitor.
3.2.6. Comorbidities
Certain comorbidities are more prevalent in people with MS than in the general population, including cerebrovascular, cardiovascular, neurological and musculoskeletal diseases, psychiatric disorders and thyroid disease (50–52). Comorbidities influence the course of MS, have medical and socioeconomic consequences (50) and also have impact on the treatment response and on switching between treatments, as they are associated with lower persistence on DMTs (51). To mitigate the challenges of comorbidities, it is important to monitor their occurrence in the MS population and to consider the medical and social consequences of these different conditions.
The most common comorbidities in people with MS admitted as inpatients in England during the financial year 2020/2021 (n = 31,275) were hypertension (24.8% of admissions), abnormality of gait and mobility (likely related to musculoskeletal conditions; 17.1%), disorders of the urinary system (other than MS-related; 15.3%), depressive episode (13.9%), type 2 diabetes (11.7%), smoking (10.9%), asthma (10.9%), other anxiety disorders (9.0%), obesity (8.9%) and chronic ischaemic heart disease (7.5%) (53).
The relationship between comorbidities and MS is complex: they may be due to a direct causal relationship, common risk factors for disease or a secondary association (50). For example, thyroid disorders appear to affect people with MS more than the general population. However, thyroid dysfunction is a known side effect of some DMTs, particularly alemtuzumab and, to a lesser extent, interferon-beta. It is, therefore, important to assess the true causal association (50).
Issues related to comorbidities should be part of patient counselling, especially regarding lifestyle factors, as preventing or effectively treating comorbidities can alleviate the burden of MS (Table 3) (50). Furthermore, differential diagnosis between MS symptoms and symptoms caused by other, undiagnosed conditions is essential to ensure effective management and avoid increased disease burden. Without specific screening programmes in general practice, monitoring of weight, blood pressure and relevant blood tests should take part in secondary care.
Table 3.
Recommendations to identify and manage comorbid conditions in people with MS.
| Regularly assess comorbid conditions in people with MS and take proactive steps to improve conditions where possible |
|
|
|
| Provide practical guidance on how to live well with MS and comorbid conditions |
|
|
|
MS, multiple sclerosis.
3.2.7. Life stage—Menopause and multiple sclerosis
Since MS is typically diagnosed in patients aged 20 to 40 years old, most women living with MS will undergo menopause after MS diagnosis (54, 55). It has been reported that in women with MS, menopause is associated with a reduced ARR, but an increase in disease-related disability (56, 57). However, the effect of menopause on MS management and progression is not fully understood, as most randomised controlled trials are not powered to evaluate DMTs in menopausal populations (58, 59).
Menopause, which typically occurs in women between the ages of 45 and 55 years, is a normal physiological transition caused by the loss of ovarian follicular function and a decline in circulating oestrogen levels (60, 61). Perimenopause, which often lasts for several years, refers to the period from when these symptoms are first observed and ends 1 year after the final menstrual period. Some women experience premature menopause before they reach 40 years, which may be due to certain chromosomal abnormalities, autoimmune disorders, or other unknown causes (60). Symptoms of the perimenopause often include hot flushes, night sweats, worsening mood, depression and/or anxiety, worsening fatigue, worsening bladder control, and difficulty in sleeping/insomnia (60).
MS is more than twice as common in females than in males (62). Following diagnosis, MS will impact women through all stages of their life, including puberty, family planning, pregnancy and childbearing, breast feeding, infertility treatment, menopause and cancer prevention (e.g., vaccination against papillomavirus, smear tests to detect cervical cancer) (63, 64). Several immunologic changes have been reported in post-menopausal women, including decreased CD4 T lymphocytes, B lymphocytes and natural killer cells cytotoxic activity, and increased proinflammatory responses, which all have effects on infection and autoimmunity. These immunologic changes overlap with age-related changes and are driven by oestrogen deprivation.
Some studies have shown a shift in MS onset to an older age, with a higher frequency of late-onset forms in women; the possible effect of menopause on the susceptibility to MS should be considered in these patients (59). This effect may be attributable to the postmenopausal proinflammatory state and deprivation of the neuroprotective effects of oestrogen and progestins, which can reduce the resilience of the CNS and thereby facilitate the onset of MS when other predisposing factors are present (58, 59).
The hormonal changes during perimenopause can affect physical, emotional, mental and social well-being. The symptoms of MS and perimenopause can frequently overlap, including fatigue and disturbances in cognition, mood, sleep, bladder and sexual function (55). This can create challenges in ascertaining the likely cause of symptoms to be treated. Once perimenopause or menopause is diagnosed, an explanation of the stages of menopause should be provided, along with the benefits and risks of treatments for menopausal symptoms. Some strategies have been suggested for managing menopause and MS-related symptoms simultaneously (Table 4) (65). It is important to use a holistic approach to healthcare that addresses both conditions.
Table 4.
Recommendations to identify and manage the menopause in women with MS.
| Provide guidance for women with MS who are menopausal |
|
| |
| Suggestions for managing symptoms of the menopause and MS-related symptoms simultaneously |
|
| |
| |
|
MS, multiple sclerosis.
Hormone Replacement Therapy (HRT) can be used to supplement hormones that are lost during the menopausal transition, and conventionally contains both oestrogen and progesterone components (66). Hormone replacement therapy can be offered to women with MS experiencing menopausal symptoms (67). A survey focusing on the changes in severity of MS symptoms with the menopause and use of HRT showed that although MS symptoms worsened with the menopause, 75% of respondents who had tried HRT reported an improvement in symptoms (68). HRT has also been associated with better physical quality of life in postmenopausal women with MS (69). In addition, a phase 2 study (n = 164) found that women who took oestriol with glatiramer acetate reduced the number of relapses compared with women who took only glatiramer acetate, although this reduction did not meet statistical significance (70). Despite these results, HRT has not shown consistent benefits in patients with MS, and patients should consult their healthcare practitioners for personalized advice on HRT use (71).
It is important to acknowledge menopause as a significant aspect of MS care and to manage related symptoms. Optimizing sleep quality, mood and hot flush quantity/interference could substantially improve mental health in menopausal women with MS (61). As such, healthcare providers should be encouraged to consider menopause when tailoring treatment plans for women with MS.
3.3. Early treatment with DMT and lifestyle interventions
Accumulation of disability in MS may occur as relapse-associated worsening or steady progression independent of relapse activity (72). Studies have consistently shown a correlation between the number of relapses in the first years of MS and disability accumulation in the long term (73). Furthermore, a shorter time interval between the first two relapses predicts faster disease progression (4, 73). In line with these epidemiological observations, large numbers of MRI inflammatory lesions at disease onset and accumulation over the first 5 years were shown to be correlated with poor prognosis (74). Overall, these data support the notion that early focal inflammatory activity contributes to faster brain degeneration in the long term (75). Indeed, evidence shows that implementation of early aggressive treatment with DMTs is correlated with significant improvements in clinical metrics of disease, including relapse rate, disability scores and MRI activity; however, its impact on a patients’ overall QoL is limited, suggesting MS may still subtly progress over time despite effective control of inflammatory activity (2, 76). Consequently, effective DMT and a “brain-healthy” lifestyle should be initiated as soon as the disease is diagnosed to protect the neurological reserve and to maximise lifelong brain health (4, 77). This has recently been further underpinned by the adoption, for the first time, of MS DMT to the World Health Organization’s Essential Medicines List (78).
3.3.1. Movement and physical activity
Movement involving a range of physical activities, including exercise, is recommended as part of the symptomatic management of MS. The beneficial effects of movement and physical activities are well documented and include improved muscle strength (79), increased mobility (80), improved QoL (81), reduced fatigue (82) and reduced depression (83). Therapeutic modalities vary from aerobic exercises, aquatic exercise, balance and resistance training, yoga, tai chi and Pilates to virtual reality and video games (83, 84). Aquatic exercise may lead to increased blood flow to the brain and increased levels of the anti-inflammatory brain-derived neurotrophic factor (84). Furthermore, aquatic exercise may help to improve mobility and maintain body temperature, which may help to counter any temporary exacerbation of MS symptoms due to Uhthoff’s phenomenon (a worsening of neurological function for <24 h in response to increases in body temperature) (85). To optimise the benefits associated with movement, activities should be regular, include the FITT principles (Frequency, Intensity, Time and Type) and be meaningful to the person with MS to facilitate compliance (Table 5) (86).
Table 5.
Recommendations to increase or maintain exercise and physical activity in people with MS.
| Provide guidance on how to maintain an active lifestyle and engage in physical activity at all stages of MS – work with partners (e.g., physiotherapy, healthcare providers, community and charity-based organisations) where needed |
|
| |
| |
| |
|
MS, multiple sclerosis.
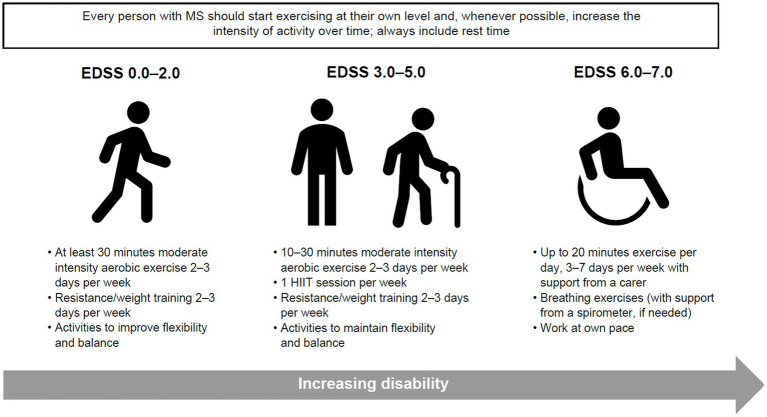
Clinical studies investigating the impact of movement and physical activity on people with MS tend to focus on more mobile individuals, with fewer studies on those with limited mobility; however, physical activity is important, and if possible, should be promoted for all people with MS, irrespective of their disability levels. In previously inactive people, it is important to start exercise as early as possible after MS diagnosis to reduce long-term disability. Therefore, people with MS should be encouraged and supported to participate in physical activity throughout their MS journey, and to incorporate suitable exercises or stretches into their daily life (Figure 3). Similarly, healthcare providers and stakeholders can create opportunities to promote and embed physical activity within MS care to prevent the negative consequences of inactivity and optimise brain health.
Figure 3.
Guidance on minimal amount of physical activity for people with MS to improve or maintain function, reduce disease activity and prevent disability. Adapted from Kalb et al. (87). EDSS, Expanded Disability Status Scale; HIIT, high intensity interval training; MS, multiple sclerosis.
3.3.2. Diet and nutrition
The composition, structure and function of the brain are dependent on the availability of appropriate nutrients, in particular lipids, amino acids, vitamins and minerals. It is, therefore, logical that food intake and quality have an impact on brain function. In addition, endogenous gut hormones, neuropeptides, neurotransmitters and the gut microbiota are affected directly by the composition of the diet (88). The emergence of the new research field of nutritional psychiatry offers promise in identifying which dietary components are most important for mental health and for whom, under which circumstances and at which specific dosages these nutritional interventions have preventative and therapeutic efficacy.
Three aspects of MS pathophysiology can be impacted by diet and have the potential to affect disease outcomes, which are: modification of the inflammatory state, protection against neurodegeneration and promotion of injury repair (89).
A wide range of nutrient and dietary approaches have been studied in people with MS, but definitive recommendations are limited owing to the limited quality, duration and scope of many trials, and the inherent challenges of conducting and interpreting nutrition research (89–94). Conclusive evidence supporting the notion that dietary changes can impact disability levels are lacking, but meta-analyses have shown improvements in QoL and fatigue (89, 91, 95). Limited evidence suggests that calorie restriction may help to achieve weight loss in people with MS and may improve emotional health (96). In addition, as people with MS are at greater risk of cardiovascular disease (97, 98), it would be prudent for doctors to stress the use of a diet which controls sources of saturated fat and fried food.
Nutritional psychiatry studies have reported that high fruit and vegetable intake increases happiness, mental health and well-being (88). Diets high in fruit, vegetables, fish and whole grains and the Mediterranean diet have also been linked to reduced risk of depression and reduced risk of dementia, and intake of antioxidant polyphenols in the elderly has been associated with improved cognitive abilities (99, 100). High-fibre diets and a Mediterranean diet promote a diverse gut microbiota and are associated with a reduced likelihood of depression (88). Meanwhile, a processed and ultra-processed Western-style diet is associated with an increased risk of mood disorders, cognitive impairments (particularly memory impairments) and increased anxiety-like behaviour (101). Fibre (<30 g/day), red meat (>70 g/day) and saturated fat (>20 g/day) consumption in women with MS was associated with worse QoL outcomes compared with a control population who were not diagnosed with MS (102). The recommendations to improve diet and nutrition are shown in (Table 6). Although the above-mentioned diets have shown some benefits for people with MS, the results are not yet conclusive and further study is required. Specific, high-quality diets created for people with MS may be a valuable resource for patients, providing a framework for patients who want more information on healthy eating as well as a support network (103, 104).
Table 6.
Recommendations to improve diet and nutrition in people with MS.
| Discuss different diets that have shown potential benefits on health outcomes |
|
| |
| |
| |
| |
| |
| |
| Provide details of foods to avoid |
|
| Provide practical guidance to help adopt a healthy diet |
|
| |
| |
| |
| |
| |
| |
| |
|
MS, multiple sclerosis.
3.3.3. Tobacco smoking and alcohol
Tobacco smoking is a risk factor both for developing MS and for more rapid disease progression and higher levels of disability (105–108). The relative risk for MS development is approximately 1.5 for tobacco smokers compared with non-smokers (105, 108). Several studies have reported associations between current smoking and MS progression, worsening of clinical symptoms and neurological disability, higher rates of brain atrophy and decreased time to conversion from RRMS to secondary progressive MS (106, 107). In addition to the direct impact of smoking on MS pathogenesis, there is an association between tobacco smoking and depression and anxiety in people with MS (107). Although vaping is less harmful than smoking cigarettes and is a useful tool for smoking cessation, the long-term risks are unknown (109).
The impact of tobacco smoking on long-term grey matter atrophy and clinical disability was studied in people with RRMS (106). After 10 years, tobacco smoking was associated with lower total white matter volume and higher logT2 lesion volume, lower deep grey matter volume and more severe walking impairment. Data from the UK MS Registry showed that over 8 years, the rate of motor deterioration was accelerated in tobacco smokers vs. non-smokers (110). In addition, over the same period, the rate of motor deterioration reduced to the rate observed in non-smokers in those who gave up tobacco smoking. This study shows that it is never too late for people with MS to benefit from giving up tobacco smoking. A good example of a designated programme to help people quit smoking or reduce/stop alcohol intake is the Better Health programme run by the NHS in England and Wales, which includes access to apps designed to assist with smoking cessation and reducing alcohol intake (111).
The relationship between alcohol and MS risk is heterogeneous between studies, but there is a lack of evidence to support a protective effect of alcohol on MS risk in the UK population (112). However, in general, alcohol in excess has detrimental effects on health, and this also applies to people with MS (4). It is, therefore, prudent to use a screening tool to assess whether drinking behaviour in people with MS is pathological (113, 114).
There is a need for large, prospective, longitudinal trials to investigate how alcohol intake impacts MS disease incidence and progression and how alcohol drinking behaviour changes with disease activity over time. Recommendations to reduce smoking and alcohol intake in people with MS are shown in Table 7.
Table 7.
Recommendations to decrease smoking and alcohol intake in people with MS.
| Ascertain smoking habits and alcohol intake in people with MS |
|
| |
| Discuss poor outcomes associated with smoking and alcohol |
|
| |
| |
| Provide practical guidance to help people with MS reduce or stop smoking/alcohol |
|
| |
| |
|
MS, multiple sclerosis; NHS, National health service.
3.3.4. Emotional well-being
There are multiple evidence-based methods used to alleviate depression and anxiety in people with MS, including CBT, ACT, exposure therapy, mindfulness, virtual reality and music therapy (115–118) (Table 8). Enhanced physical activity and self-efficacy are also important means by which healthcare professionals can help people with MS to reduce their levels of anxiety (119). However, there is no definitive solution, and different approaches will likely suit different people. A person-centred approach to care is key.
Table 8.
Recommendations to improve emotional well-being in people with MS.
| Discuss the prevalence of depression and anxiety in people with MS and their consequences on outcomes |
|
| |
| |
| Provide practical guidance to help people with MS reduce the likelihood of depression and anxiety |
|
| |
| |
| |
|
ACT, acceptance commitment therapy; CBT, cognitive behaviour therapy; MS, multiple sclerosis.
3.4. Assess social determinants of health
Although many of the lifestyle or behaviour changes mentioned above in relation to brain health are important considerations, many social determinants of health (SDOH) also affect people with MS and these often cannot be changed. SDOH are the conditions in which people are born, grow, live, work and age. These circumstances are the non-medical factors that influence health outcomes. It has been suggested that SDOH account for 30 to 55% of health outcomes (120). According to the World Bank, as of 2022, over one-third of the global population (3.1 in 8.0 billion) resides in low-to-middle-income countries (121, 122) where healthcare inequities and lack of access to quality care are key challenges. Access to healthcare is not only a feature of low-to-middle income countries, with access in high income countries being impacted by different insurance models or the structure of the healthcare systems (123–125).
SDOH may be specific to the individual: sex, gender, race, ethnicity, education, employment, socioeconomic status, safety (e.g., presence of domestic violence and abuse or local crime rate) or to the local social and natural environment. SDOH also include structural factors associated with the societies we live in, which vary according to international location and include access to healthcare, access to and quality of food, presence of air pollution and availability of social support (120, 126). These are often aspects of life that we do not choose or have very little control over.
Many SDOH interact and intersect with each other, leading to poorer outcomes, in either an additive or even a multiplicative manner (120). High health expenditure per capita is more strongly associated with higher national MS prevalence than low health care expenditure per capita (127). This suggests that theories attributing variations in MS prevalence primarily to latitude effects on vitamin D are incomplete. However, as national wealth increases with latitude, there is likely to be an underestimation of MS prevalence in countries with low health expenditure (127). Assessing and addressing SDOH has already successfully improved outcomes in other chronic diseases and could also be beneficial in people with MS (53, 120). Unemployment has a large impact on people with MS, with worse outcomes for those who are unemployed (120). A study in France found a socioeconomic gradient in people with relapsing-onset MS, with individuals considered to live in conditions of deprivation exposed to a greater excess death rate (128).
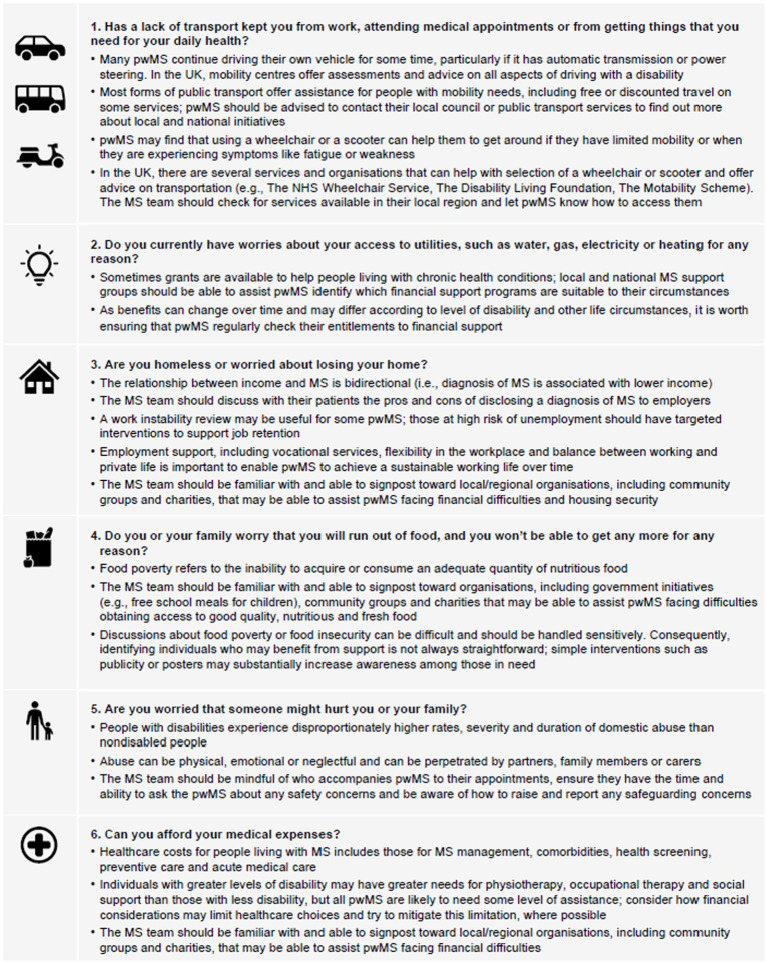
Screening for SDOH is usually only performed in employment, so neurologists may not have access to this information. A validated screening tool (Core-5) exists for other health conditions (129) and an adapted version has been developed that may be useful in clinical practice for patients with MS (120). Although not all of the questions in the Core-5 tool are applicable across all healthcare and welfare systems, they provide a framework for systematic inquiry to identify individuals with the greatest need of support (120). Figure 4 presents screening questions that could be used to identify SDOH in people with MS, along with guidance on how the MS team may be able to offer support. Facilitating opportunities for people with MS to talk to each other can positively change perception of MS (131) and is a positive feature of targeted education programmes early in the disease course (Table 9).
Figure 4.
Suggested screening questions for social determinants of health in people with MS and considerations for how to offer practical support. Adapted from Bradywood et al. (130) and Dobson et al. (120). MS, multiple sclerosis; NHS, National Health Service; pwMS, people with MS; UK, United Kingdom.
Table 9.
Recommendations to address social determinants of health (SDOH) in people with MS.
| Regularly assess SDOH in people with MS |
|
|
|
|
| Provide people with MS with support on how to stay in employment as long as they are able or want to |
|
|
|
|
MS, multiple sclerosis; SDOH, social determinants of health.
4. Conclusion
For people with MS, early diagnosis and prompt treatment are essential components of a therapeutic strategy that offers the best chance of preserving CNS tissue and minimising, or delaying, further decline. Consequently, both an effective DMT and a “brain-healthy” lifestyle should be adopted as soon as MS is diagnosed to protect the neurological reserve and maximise lifelong brain health.
Care for people with MS should be tailored to the individual and responsive to their changing needs. To enable and maintain healthy lifestyle behaviours, people with MS may require support from family members and the broader community. It is therefore important to incorporate within routine clinical practice the ability for healthcare providers to signpost people with MS and their families to local support groups that can assist them with lifestyle behaviours (e.g., smoking cessation support, physical activity programmes, healthy eating advice). Early educational input and ongoing support for people with MS are also critical to ensure that they proactively engage in the management of their MS and have the knowledge and resources to manage comorbid conditions and adopt healthy lifestyle practices. This can help to alleviate the burden of MS and improve overall QoL.
Our recommendations, therefore, aim to preserve as much neurological reserve as possible by using an appropriate DMT to minimise disease activity early in the disease process and by adopting a “brain-healthy” lifestyle to delay disease progression. It is increasingly clear that healthy lifestyle practices should be seen as an essential form of treatment for MS, alongside pharmacologic and therapeutic medicine. A multidisciplinary, collaborative and person-centred approach to care that encompasses the healthcare team, family members and community support groups will enable people with MS to access appropriate support. In addition, there is a need for ongoing research and innovation in the field of MS to further progress and define diagnostic and therapeutic strategies, and ultimately improve outcomes.
Author contributions
GG: Resources, Writing - review & editing. HF: Resources, Writing - review & editing. KS: Resources, Writing - review & editing. RM: Resources, Writing - review & editing. AMS: Resources, Writing - review & editing. IP: Resources, Writing - review & editing. LS: Resources, Writing - review & editing. ASc: Resources, Writing - review & editing. CB: Resources, Writing - review & editing. RS: Resources, Writing - review & editing. SH: Resources, Writing - review & editing. AW: Resources, Writing - review & editing. SJ: Resources, Writing - review & editing. CP: Resources, Writing - review & editing. ASt: Conceptualization, Resources, Writing - review & editing.
Acknowledgments
We are profoundly grateful to Samantha Coates, whose initial draft was deeply influenced by the pivotal content from the “BeewellwithMS” podcast. The invaluable interviews led by the host and founder, Dr. Agne Straukiene, were essential in providing content that significantly shaped the manuscript. Editorial support for this document was adeptly provided by Excel Scientific Solutions (Fairfield, CT, USA) with funding from Biogen.
Funding Statement
The preparation of this manuscript was funded by Biogen without any content input.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.MS International Federation . Atlas of MS 3rd edition (2020) Available at:https://www.msif.org/wp-content/uploads/2020/12/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf (Accessed August 22, 2023).
- 2.Giovannoni G, Popescu V, Wuerfel J, Hellwig K, Iacobaeus E, Jensen MB, et al. Smouldering multiple sclerosis: the 'real MS'. Ther Adv Neurol Disord. (2022) 15:17562864211066751. doi: 10.1177/17562864211066751, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reich DS, Lucchinetti CF, Calabresi PA. Multiple sclerosis. N Engl J Med. (2018) 378:169–80. doi: 10.1056/NEJMra1401483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. (2016) 9:S5–S48. doi: 10.1016/j.msard.2016.07.003, PMID: [DOI] [PubMed] [Google Scholar]
- 5.Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry. (2014) 85:67–75. doi: 10.1136/jnnp-2012-304333, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. (2018) 4:43. doi: 10.1038/s41572-018-0041-4 [DOI] [PubMed] [Google Scholar]
- 7.Kuhlmann T, Moccia M, Coetzee T, Cohen JA, Correale J, Graves J, et al. Multiple sclerosis progression: time for a new mechanism-driven framework. Lancet Neurol. (2023) 22:78–88. doi: 10.1016/s1474-4422(22)00289-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandstadter R, Katz Sand I, Sumowski JF. Beyond rehabilitation: a prevention model of reserve and brain maintenance in multiple sclerosis. Mult Scler. (2019) 25:1372–8. doi: 10.1177/1352458519856847, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Rooij SR. Are brain and cognitive reserve shaped by early life circumstances? Front Neurosci. (2022) 16:825811. doi: 10.3389/fnins.2022.825811, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peel C. Evidence for non-medical management in MS: optimised management and patient participation. Br J Neurosci Nurs. (2021) 17:S31–3. doi: 10.12968/bjnn.2021.17.Sup1.S31 [DOI] [Google Scholar]
- 11.Alfredsson L, Olsson T. Lifestyle and environmental factors in multiple sclerosis. Cold Spring Harb Perspect Med. (2019) 9:9. doi: 10.1101/cshperspect.a028944, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Optimizing brain health across the life course: WHO position paper (2022) Available at:https://www.who.int/publications/i/item/9789240054561 (Accessed August 22, 2023).
- 13.Wandall-Holm MF, Andersen MA, Buron MD, Magyari M. Aging with multiple sclerosis: age-related factors and socioeconomic risks. Front Neurol. (2022) 13:818652. doi: 10.3389/fneur.2022.818652, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branco M, Ruano L, Portaccio E, Goretti B, Niccolai C, Patti F, et al. Aging with multiple sclerosis: prevalence and profile of cognitive impairment. Neurol Sci. (2019) 40:1651–7. doi: 10.1007/s10072-019-03875-7, PMID: [DOI] [PubMed] [Google Scholar]
- 15.The MS Trust . Cognitive reserve (2018) Available at:https://mstrust.org.uk/a-z/cognitive-reserve (Accessed February 10, 2023).
- 16.Dutheil F, Danini B, Bagheri R, Fantini ML, Pereira B, Moustafa F, et al. Effects of a short daytime nap on the cognitive performance: a systematic review and meta-analysis. Int J Environ Res Public Health. (2021) 18:10212. doi: 10.3390/ijerph181910212, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CH, Giuliani F. The role of inflammation in depression and fatigue. Front Immunol. (2019) 10:1696. doi: 10.3389/fimmu.2019.01696, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploughman M, Downer MB, Pretty RW, Wallack EM, Amirkhanian S, Kirkland MC, et al. The impact of resilience on healthy aging with multiple sclerosis. Qual Life Res. (2020) 29:2769–79. doi: 10.1007/s11136-020-02521-6, PMID: [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Care Excellence . Multiple sclerosis. Quality standard [QS108] (2016) Available at:https://www.nice.org.uk/guidance/qs108 (Accessed August 22, 2023).
- 20.Aloisi F, Giovannoni G, Salvetti M. Epstein-Barr virus as a cause of multiple sclerosis: opportunities for prevention and therapy. Lancet Neurol. (2023) 22:338–49. doi: 10.1016/S1474-4422(22)00471-9, PMID: [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Hu K, Song H, Pawitan Y, Piehl F, Fang F. Infections among individuals with multiple sclerosis, Alzheimer's disease and Parkinson's disease. Brain Commun. (2023) 5:fcad065. doi: 10.1093/braincomms/fcad065, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lechner-Scott J, Waubant E, Levy M, Hawkes C, Giovannoni G. Is multiple sclerosis a risk factor for infections? Mult Scler Relat Disord. (2020) 41:102184. doi: 10.1016/j.msard.2020.102184, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manchery N, Henry JD, Nangle MR. A systematic review of oral health in people with multiple sclerosis. Community Dent Oral Epidemiol. (2020) 48:89–100. doi: 10.1111/cdoe.12512, PMID: [DOI] [PubMed] [Google Scholar]
- 24.The MS Trust . Managing relapses (2022) Available at:https://mstrust.org.uk/information-support/ms-symptoms-diagnosis/mnaging-ms-relapses (Accessed February 10, 2023).
- 25.Multiple Sclerosis Academy . MS awareness week: the importance of bladder management (2022) Available at:https://neurologyacademy.org/articles/ms-awareness-week-the-importance-of-bladder-management (Accessed August 22, 2023).
- 26.Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry. (2005) 76:469–75. doi: 10.1136/jnnp.2004.054635, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boeschoten RE, Braamse AMJ, Beekman ATF, Cuijpers P, van Oppen P, Dekker J, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci. (2017) 372:331–41. doi: 10.1016/j.jns.2016.11.067, PMID: [DOI] [PubMed] [Google Scholar]
- 28.Mayo CD, Lacey C, Gawryluk JR. Differences in symptoms of depression between females and males with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. (2021) 51:102884. doi: 10.1016/j.msard.2021.102884, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Ling S, Moss B, Wang Z, Sullivan AB. Exploring the impact of the COVID-19 pandemic on social isolation and mental health in people with MS. Mult Scler Relat Disord. (2022) 68:104186. doi: 10.1016/j.msard.2022.104186, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hepworth J, Nathan H. Evaluation of MS Society local groups’ role in reducing loneliness for people affected by MS (2018) Available at:https://www.mssociety.org.uk/sites/default/files/2020-08/Local-groups---Reducing-Loneliness-and-Isolation.pdf (Accessed August 22, 2023).
- 31.Kim I-B, Lee J-H, Park S-C. The relationship between stress, inflammation, and depression. Biomedicine. (2022) 10:1929. doi: 10.3390/biomedicines10081929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JL, Reimer MA, Metz LM, Patten SB. Major depression and quality of life in individuals with multiple sclerosis. Int J Psychiatry Med. (2000) 30:309–17. doi: 10.2190/PGWT-UXJ0-7UEH-LGRY [DOI] [PubMed] [Google Scholar]
- 33.Bains N, Abdijadid S. Major depressive disorder In: StatPearls. Treasure Island, FL: StatPearls Publishing; (2023) [PubMed] [Google Scholar]
- 34.Filser M, Buchner A, Fink GR, Gold SM, Penner IK. The manifestation of affective symptoms in multiple sclerosis and discussion of the currently available diagnostic assessment tools. J Neurol. (2023) 270:171–207. doi: 10.1007/s00415-022-11359-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marrie RA, Patel R, Figley CR, Kornelsen J, Bolton JM, Graff LA, et al. Effects of vascular comorbidity on cognition in multiple sclerosis are partially mediated by changes in brain structure. Front Neurol. (2022) 13:910014. doi: 10.3389/fneur.2022.910014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palladino R, Marrie RA, Majeed A, Chataway J. Management of vascular risk in people with multiple sclerosis at the time of diagnosis in England: a population-based study. Mult Scler. (2023) 29:671–9. doi: 10.1177/13524585231164296, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foschi M, Rizzo G, Liguori R, Avoni P, Mancinelli L, Lugaresi A, et al. Sleep-related disorders and their relationship with MRI findings in multiple sclerosis. Sleep Med. (2019) 56:90–7. doi: 10.1016/j.sleep.2019.01.010, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Buratti L, Iacobucci DE, Viticchi G, Falsetti L, Lattanzi S, Pulcini A, et al. Sleep quality can influence the outcome of patients with multiple sclerosis. Sleep Med. (2019) 58:56–60. doi: 10.1016/j.sleep.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 39.Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. (2017) 9:151–61. doi: 10.2147/nss.S134864, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krause AJ, Prather AA, Wager TD, Lindquist MA, Walker MP. The pain of sleep loss: a brain characterization in humans. J Neurosci. (2019) 39:2291–300. doi: 10.1523/jneurosci.2408-18.2018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amtmann D, Askew RL, Kim J, Chung H, Ehde DM, Bombardier CH, et al. Pain affects depression through anxiety, fatigue, and sleep in multiple sclerosis. Rehabil Psychol. (2015) 60:81–90. doi: 10.1037/rep0000027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medline Plus . Sleep and your health (2022) Available at:https://medlineplus.gov/ency/patientinstructions/000871.htm (Accessed August 22, 2023).
- 43.Pattnaik H, Mir M, Boike S, Kashyap R, Khan SA, Surani S. Nutritional elements in sleep. Peer E med. (2022) 14:e32803. doi: 10.7759/cureus.32803, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kline CE. The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am J Lifestyle Med. (2014) 8:375–9. doi: 10.1177/1559827614544437, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park I, Díaz J, Matsumoto S, Iwayama K, Nabekura Y, Ogata H, et al. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci Rep. (2021) 11:4410. doi: 10.1038/s41598-021-83817-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas S, Bradley J, Cole G, Girvan M, Metcalfe G, Naik P, et al. The neurogenic bladder: developing a consensus bladder and bowel management pathway for people with MS. Br J Nurs. (2022) 31:1088–95. doi: 10.12968/bjon.2022.31.21.1088, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Brass SD, Duquette P, Proulx-Therrien J, Auerbach S. Sleep disorders in patients with multiple sclerosis. Sleep Med Rev. (2010) 14:121–9. doi: 10.1016/j.smrv.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 48.Lanza G, Ferri R, Bella R, Ferini-Strambi L. The impact of drugs for multiple sclerosis on sleep. Mult Scler. (2017) 23:5–13. doi: 10.1177/1352458516664034 [DOI] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence . Multiple sclerosis in adults: Management London, UK: National Institute for health and care excellence (2022) Available at:https://www.nice.org.uk/guidance/ng220 (Accessed July 6, 2023).
- 50.Magyari M, Sorensen PS. Comorbidity in multiple sclerosis. Front Neurol. (2020) 11:851. doi: 10.3389/fneur.2020.00851, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laroni A, Signori A, Maniscalco GT, Lanzillo R, Russo CV, Binello E, et al. Assessing association of comorbidities with treatment choice and persistence in MS: a real-life multicenter study. Neurology. (2017) 89:2222–9. doi: 10.1212/WNL.0000000000004686, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Mahmoudi E, Sadaghiyani S, Lin P, Kamdar N, Norcott A, Peterson MD, et al. Diagnosis of Alzheimer's disease and related dementia among people with multiple sclerosis: large cohort study, USA. Mult Scler Relat Disord. (2022) 57:103351. doi: 10.1016/j.msard.2021.103351, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Straukiene A, Stross R, Pomeroy I, Fisniku L, Berry G, Peel C, et al. Poster presented at 37th congress of the European committee for treatment & research in multiple sclerosis In: Well-being gap: One-size MS service does not fit all (2021)
- 54.Bove R, Okai A, Houtchens M, Elias-Hamp B, Lugaresi A, Hellwig K, et al. Effects of menopause in women with multiple sclerosis: an evidence-based review. Front Neurol. (2021) 12:554375. doi: 10.3389/fneur.2021.554375, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gklinos P, Dobson R. Menopause in women with multiple sclerosis: clinical symptoms, therapeutic considerations and possible impact on the course of the disease. Br J Neurosci Nurs. (2023) 19:S34–8. doi: 10.12968/bjnn.2023.19.Sup1.S34 [DOI] [Google Scholar]
- 56.Bove R, Healy BC, Musallam A, Glanz BI, De Jager PL, Chitnis T. Exploration of changes in disability after menopause in a longitudinal multiple sclerosis cohort. Mult Scler. (2016) 22:935–43. doi: 10.1177/1352458515606211, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shahraki Z, Rastkar M, Rastkar E, Mohammadifar M, Mohamadi A, Ghajarzadeh M. Impact of menopause on relapse rate and disability level in patients with multiple sclerosis (MS): a systematic review and meta-analysis. BMC Neurol. (2023) 23:316. doi: 10.1186/s12883-023-03332-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Houtchens MK, Bove R. A case for gender-based approach to multiple sclerosis therapeutics. Front Neuroendocrinol. (2018) 50:123–34. doi: 10.1016/j.yfrne.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 59.Lorefice L, Fenu G, Fronza M, Murgia F, Frau J, Coghe G, et al. Menopausal transition in multiple sclerosis: relationship with disease activity and brain volume measurements. Front Neurol. (2023) 14:1251667. doi: 10.3389/fneur.2023.1251667, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World Health Organization . Menopause factsheet. Available at:https://www.who.int/news-room/fact-sheets/detail/menopause (Accessed October 20, 2023).
- 61.Morales-Rodriguez D, Anderson A, Nylander A, Hsu S, Singh J, Rowles W, et al. Well-being at midlife: correlates of mental health in ambulatory menopausal women with multiple sclerosis. Mult Scler. (2023) 29:1493–502. doi: 10.1177/13524585231197056, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Public Health England . Multiple sclerosis: Prevalence, incidence and smoking status - data briefing (2020) Available at:https://www.gov.uk/government/publications/multiple-sclerosis-prevalence-incidence-and-smoking-status/multiple-sclerosis-prevalence-incidence-and-smoking-status-data-briefing (Accessed August 22, 2023).
- 63.Ciampi E, Ceccarelli A, Zuluaga MI. Editorial: women in multiple sclerosis and other demyelinating disorders: a global perspective. Front Neurol. (2023) 14:1148659. doi: 10.3389/fneur.2023.1148659, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zeydan B, Atkinson EJ, Weis DM, Smith CY, Gazzuola Rocca L, Rocca WA, et al. Reproductive history and progressive multiple sclerosis risk in women. Brain Commun. (2020) 2:fcaa185. doi: 10.1093/braincomms/fcaa185, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The multiple sclerosis trust . MS and the menopause. Available at:https://mstrust.org.uk/news/ms-and-menopause (Accessed October 20, 2023).
- 66.Harper-Harrison G, Shanahan MM. Hormone replacement therapy. Treasure Island (FL): StatPearls; (2023). [PubMed] [Google Scholar]
- 67.National Institute for Health and Care Excellence . Menopause: diagnosis and management (2015) Available at:www.nice.org.uk/guidance/ng23 (Accessed August 28, 2023). [PubMed]
- 68.Smith R, Studd JW. A pilot study of the effect upon multiple sclerosis of the menopause, hormone replacement therapy and the menstrual cycle. J R Soc Med. (1992) 85:612–3. doi: 10.1177/014107689208501008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bove R, White CC, Fitzgerald KC, Chitnis T, Chibnik L, Ascherio A, et al. Hormone therapy use and physical quality of life in postmenopausal women with multiple sclerosis. Neurology. (2016) 87:1457–63. doi: 10.1212/WNL.0000000000003176, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. (2016) 15:35–46. doi: 10.1016/S1474-4422(15)00322-1, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Midaglia L, Otero S, Baro F, Montalban X, Tintore M. Menopause and multiple sclerosis: influence on prognosis and role of disease-modifying drugs and hormonal replacement therapy. Mult Scler. (2022) 28:173–82. doi: 10.1177/1352458520952022, PMID: [DOI] [PubMed] [Google Scholar]
- 72.Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. (2020) 77:1132–40. doi: 10.1001/jamaneurol.2020.1568, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. (2010) 133:1914–29. doi: 10.1093/brain/awq118, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. (2008) 131:808–17. doi: 10.1093/brain/awm329, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. (2006) 253:98–108. doi: 10.1007/s00415-005-0934-5 [DOI] [PubMed] [Google Scholar]
- 76.He A, Spelman T, Manouchehrinia A, Ciccarelli O, Hillert J, McKay K. Association between early treatment of multiple sclerosis and patient-reported outcomes: a nationwide observational cohort study. J Neurol Neurosurg Psychiatry. (2022) 94:284–9. doi: 10.1136/jnnp-2022-330169, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hobart J, Bowen A, Pepper G, Crofts H, Eberhard L, Berger T, et al. International consensus on quality standards for brain health-focused care in multiple sclerosis. Mult Scler. (2019) 25:1809–18. doi: 10.1177/1352458518809326, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Health Organization . WHO endorses landmark public health decisions on essential medicines for multiple sclerosis. Geneva: World Health Organization; (2023). [Google Scholar]
- 79.Sá MJ. Exercise therapy and multiple sclerosis: a systematic review. J Neurol. (2014) 261:1651–61. doi: 10.1007/s00415-013-7183-9 [DOI] [PubMed] [Google Scholar]
- 80.Snook EM, Motl RW. Effect of exercise training on walking mobility in multiple sclerosis: a meta-analysis. Neurorehabil Neural Repair. (2009) 23:108–16. doi: 10.1177/1545968308320641, PMID: [DOI] [PubMed] [Google Scholar]
- 81.Anthony W, Gidugu V. Systematic review of the effects of exercise and physical activity on psychological and quality of life outcomes for individuals with multiple sclerosis, 1996–2011. Boston: Boston University, Sargent College, Center for Psychiatric Rehabilitation; (2012). [Google Scholar]
- 82.Heine M, van de Port I, Rietberg MB, van Wegen EE, Kwakkel G. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev. (2015) 2015:CD009956. doi: 10.1002/14651858.CD009956.pub2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kyriakatis GM, Besios T, Lykou PM. The effect of therapeutic exercise on depressive symptoms in people with multiple sclerosis - a systematic review. Mult Scler Relat Disord. (2022) 68:104407. doi: 10.1016/j.msard.2022.104407 [DOI] [PubMed] [Google Scholar]
- 84.Hao Z, Zhang X, Chen P. Effects of different exercise therapies on balance function and functional walking ability in multiple sclerosis disease patients-a network meta-analysis of randomized controlled trials. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph19127175, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Panginikkod S, Rayi A, Rocha Cabrero F, Rukmangadachar LA. Uhthoff phenomenon In: StatPearls. Treasure Island (FL): StatPearls Publishing LLC; [PubMed] [Google Scholar]
- 86.Stennett A, De Souza L, Norris M. The meaning of exercise and physical activity in community dwelling people with multiple sclerosis. Disabil Rehabil. (2020) 42:317–23. doi: 10.1080/09638288.2018.1497715, PMID: [DOI] [PubMed] [Google Scholar]
- 87.Kalb R, Brown TR, Coote S, Costello K, Dalgas U, Garmon E, et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler. (2020) 26:1459–69. doi: 10.1177/1352458520915629, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adan RAH, van der Beek EM, Buitelaar JK, Cryan JF, Hebebrand J, Higgs S, et al. Nutritional psychiatry: towards improving mental health by what you eat. Eur Neuropsychopharmacol. (2019) 29:1321–32. doi: 10.1016/j.euroneuro.2019.10.011, PMID: [DOI] [PubMed] [Google Scholar]
- 89.Guerrero Aznar MD, Villanueva Guerrero MD, Cordero Ramos J, Eichau Madueno S, Morales Bravo M, Lopez Ruiz R, et al. Efficacy of diet on fatigue, quality of life and disability status in multiple sclerosis patients: rapid review and meta-analysis of randomized controlled trials. BMC Neurol. (2022) 22:388. doi: 10.1186/s12883-022-02913-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Katz SI. The role of diet in multiple sclerosis: mechanistic connections and current evidence. Curr Nutr Rep. (2018) 7:150–60. doi: 10.1007/s13668-018-0236-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Snetselaar LG, Cheek JJ, Fox SS, Healy HS, Schweizer ML, Bao W, et al. Efficacy of diet on fatigue and quality of life in multiple sclerosis: a systematic review and network meta-analysis of randomized trials. Neurology. (2023) 100:e357–66. doi: 10.1212/WNL.0000000000201371, PMID: [DOI] [PubMed] [Google Scholar]
- 92.Stoiloudis P, Kesidou E, Bakirtzis C, Sintila SA, Konstantinidou N, Boziki M, et al. The role of diet and interventions on multiple sclerosis: a review. Nutrients. (2022) 14:1150. doi: 10.3390/nu14061150, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vitolins MZ, Case TL. What makes nutrition research so difficult to conduct and interpret? Diabetes Spectr. (2020) 33:113–7. doi: 10.2337/ds19-0077, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Staudacher HM, Yao CK, Chey WD, Whelan K. Optimal Design of Clinical Trials of dietary interventions in disorders of gut-brain interaction. Am J Gastroenterol. (2022) 117:973–84. doi: 10.14309/ajg.0000000000001732, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fitzgerald KC, Tyry T, Salter A, Cofield SS, Cutter G, Fox R, et al. Diet quality is associated with disability and symptom severity in multiple sclerosis. Neurology. (2018) 90:e1–e11. doi: 10.1212/wnl.0000000000004768, PMID: [DOI] [PubMed] [Google Scholar]
- 96.Fitzgerald KC, Vizthum D, Henry-Barron B, Schweitzer A, Cassard SD, Kossoff E, et al. Effect of intermittent vs. daily calorie restriction on changes in weight and patient-reported outcomes in people with multiple sclerosis. Mult Scler Relat Disord. (2018) 23:33–9. doi: 10.1016/j.msard.2018.05.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Albuquerque LDS, Damasceno NRT, Maia FN, Carvalho BM, Maia CSC, D'Almeida JAC, et al. Cardiovascular risk estimated in individuals with multiple sclerosis: a case-control study. Mult Scler Relat Disord. (2021) 54:103133. doi: 10.1016/j.msard.2021.103133, PMID: [DOI] [PubMed] [Google Scholar]
- 98.Kaplan TB, Berkowitz AL, Samuels MA. Cardiovascular dysfunction in multiple sclerosis. Neurologist. (2015) 20:108–14. doi: 10.1097/NRL.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 99.Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi M, et al. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Med. (2017) 15:23. doi: 10.1186/s12916-017-0791-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shannon OM, Ranson JM, Gregory S, Macpherson H, Milte C, Lentjes M, et al. Mediterranean diet adherence is associated with lower dementia risk, independent of genetic predisposition: findings from the UK biobank prospective cohort study. BMC Med. (2023) 21:81. doi: 10.1186/s12916-023-02772-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hecht EM, Rabil A, Martinez Steele E, Abrams GA, Ware D, Landy DC, et al. Cross-sectional examination of ultra-processed food consumption and adverse mental health symptoms. Public Health Nutr. (2022) 25:3225–34. doi: 10.1017/s1368980022001586, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coe S, Tektonidis TG, Coverdale C, Penny S, Collett J, Chu BTY, et al. A cross sectional assessment of nutrient intake and the association of the inflammatory properties of nutrients and foods with symptom severity in a large cohort from the UK multiple sclerosis registry. Nutr Res. (2021) 85:31–9. doi: 10.1016/j.nutres.2020.11.006, PMID: [DOI] [PubMed] [Google Scholar]
- 103.Overcoming MS. The multiple sclerosis diet. Available at:https://overcomingms.org/recovery-program/diet (Accessed October 20, 2023).
- 104.Society MS. Special diets and MS. Available at:https://www.mssociety.org.uk/care-and-support/everyday-living/eating-and-drinking/special-diets-and-ms (Accessed October 20, 2023).
- 105.Degelman ML, Herman KM. Smoking and multiple sclerosis: a systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord. (2017) 17:207–16. doi: 10.1016/j.msard.2017.07.020, PMID: [DOI] [PubMed] [Google Scholar]
- 106.Lie IA, Wesnes K, Kvistad SS, Brouwer I, Wergeland S, Holmoy T, et al. The effect of smoking on long-term gray matter atrophy and clinical disability in patients with relapsing-remitting multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. (2022) 9:e200008. doi: 10.1212/NXI.0000000000200008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vong V, Simpson-Yap S, Phaiju S, Davenport RA, Neate SL, Pisano MI, et al. The association between tobacco smoking and depression and anxiety in people with multiple sclerosis: a systematic review. Mult Scler Relat Disord. (2023) 70:104501. doi: 10.1016/j.msard.2023.104501 [DOI] [PubMed] [Google Scholar]
- 108.Wingerchuk DM. Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord. (2012) 5:13–22. doi: 10.1177/1756285611425694, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Limb M. Vaping is "unlikely to be risk free," finds evidence review. BMJ. (2022) 378:o2361. doi: 10.1136/bmj.o2361, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Rodgers J, Friede T, Vonberg FW, Constantinescu CS, Coles A, Chataway J, et al. The impact of smoking cessation on multiple sclerosis disease progression. Brain. (2022) 145:1368–78. doi: 10.1093/brain/awab385, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Health Service . Kickstart your health (2023) Available at:www.nhs.uk/better-health/ (Accessed August 28, 2023).
- 112.Dreyer-Alster S, Achiron A, Giovannoni G, Jacobs BM, Dobson R. No evidence for an association between alcohol consumption and multiple sclerosis risk: a UK Biobank study. Sci Rep. (2022) 12:22158. doi: 10.1038/s41598-022-26409-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Office for Health Improvement & Disparities . Guidance on the 5 alcohol use screening tests (2020) Available at:www.gov.uk/government/publications/alcohol-use-screening-tests/guidance-on-the-5-alcohol-use-screening-tests#alcohol-use-disorders-identification-test-audit (Accessed August 28, 2023).
- 114.Public Health England . How to use the ASSIST-lite screening tool to identify alcohol and drug use and tobacco smoking (2021) Available at:https://www.gov.uk/government/publications/assist-lite-screening-tool-how-to-use/how-to-use-the-assist-lite-screening-tool-to-identify-alcohol-and-drug-use-and-tobacco-smoking#introduction (Accessed August 22, 2023).
- 115.Hind D, Cotter J, Thake A, Bradburn M, Cooper C, Isaac C, et al. Cognitive behavioural therapy for the treatment of depression in people with multiple sclerosis: a systematic review and meta-analysis. BMC Psychiatry. (2014) 14:5. doi: 10.1186/1471-244X-14-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sihvonen AJ, Sarkamo T, Leo V, Tervaniemi M, Altenmuller E, Soinila S. Music-based interventions in neurological rehabilitation. Lancet Neurol. (2017) 16:648–60. doi: 10.1016/S1474-4422(17)30168-0 [DOI] [PubMed] [Google Scholar]
- 117.Simpson R, Posa S, Langer L, Bruno T, Simpson S, Lawrence M, et al. A systematic review and meta-analysis exploring the efficacy of mindfulness-based interventions on quality of life in people with multiple sclerosis. J Neurol. (2023) 270:726–45. doi: 10.1007/s00415-022-11451-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thompson B, Moghaddam N, Evangelou N, Baufeldt A, Das Nair R. Effectiveness of acceptance and commitment therapy for improving quality of life and mood in individuals with multiple sclerosis: a systematic review and meta-analysis. Mult Scler Relat Disord. (2022) 63:103862. doi: 10.1016/j.msard.2022.103862, PMID: [DOI] [PubMed] [Google Scholar]
- 119.Fahy A, Maguire R. Potentially modifiable associates of anxiety in people with multiple sclerosis: a systematic review. Disabil Rehabil. (2022) 44:8201–12. doi: 10.1080/09638288.2021.2022776, PMID: [DOI] [PubMed] [Google Scholar]
- 120.Dobson R, Rice DR, D'Hooghe M, Horne R, Learmonth Y, Mateen FJ, et al. Social determinants of health in multiple sclerosis. Nat Rev Neurol. (2022) 18:723–34. doi: 10.1038/s41582-022-00735-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.World Bank . Population, Total for lower middle income countries, retrieved from FRED, Federal Reserve Bank of St. Louis(2022) Available at:https://fred.stlouisfed.org/series/SPPOPTOTLLMC (Accessed October 15, 2023).
- 122.United Nations Department of Economic and Social Affairs PD. World population prospects 2022: Summary of results New York(2022) Available at:https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf (Accessed October 15, 2023).
- 123.Chiu C, Park M, Hoffman T, Campbell M, Bishop M. Descriptive analysis of free-text comments on healthcare priorities and experiences in a national sample of people with multiple sclerosis. Mult Scler Relat Disord. (2019) 34:141–9. doi: 10.1016/j.msard.2019.06.023 [DOI] [PubMed] [Google Scholar]
- 124.Hartung DM. Economics of multiple sclerosis disease-modifying therapies in the USA. Curr Neurol Neurosci Rep. (2021) 21:28. doi: 10.1007/s11910-021-01118-x [DOI] [PubMed] [Google Scholar]
- 125.Langer-Gould A, Klocke S, Beaber B, Brara SM, Debacker J, Ayeni O, et al. Improving quality, affordability, and equity of multiple sclerosis care. Ann Clin Transl Neurol. (2021) 8:980–91. doi: 10.1002/acn3.51326, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Simpson-Yap S, Nag N, Jakaria M, Jelinek GA, Neate S. Sociodemographic and clinical characteristics of diet adherence and relationship with diet quality in an international cohort of people with multiple sclerosis. Mult Scler Relat Disord. (2021) 56:103307. doi: 10.1016/j.msard.2021.103307, PMID: [DOI] [PubMed] [Google Scholar]
- 127.Hwang S, Garcia-Dominguez MA, Fitzgerald KC, Saylor DR. Association of multiple sclerosis prevalence with sociodemographic, health systems, and lifestyle factors on a national and regional level. Neurology. (2022) 99:e1813–23. doi: 10.1212/wnl.0000000000200962 [DOI] [PubMed] [Google Scholar]
- 128.Wilson S, Calocer F, Rollot F, Fauvernier M, Remontet L, Tron L, et al. Effects of socioeconomic status on excess mortality in patients with multiple sclerosis in France: a retrospective observational cohort study. Lancet Reg Health Eur. (2023) 24:100542. doi: 10.1016/j.lanepe.2022.100542, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bechtel N, Jones A, Kue J, Ford JL. Evaluation of the core 5 social determinants of health screening tool. Public Health Nurs. (2022) 39:438–45. doi: 10.1111/phn.12983 [DOI] [PubMed] [Google Scholar]
- 130.Bradywood A, Leming-Lee TS, Watters R, Blackmore C. Implementing screening for social determinants of health using the Core 5 screening tool. BMJ Open Qual. (2021) 10:10. doi: 10.1136/bmjoq-2021-001362, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garabedian M, Perrone E, Pileggi C, Zimmerman V. Support group participation: effect on perceptions of patients with newly diagnosed multiple sclerosis. Int J MS Care. (2020) 22:115–21. doi: 10.7224/1537-2073.2018-099, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]