Abstract
Radiation therapy (RT) plays an essential role in the management of esophageal cancer. Because the esophagus is a centrally located thoracic structure there is a need to balance the delivery of appropriately high dose to the target while minimizing dose to nearby critical structures. Radiation dose received by these critical structures, especially the heart and lungs, may lead to clinically significant toxicities, including pneumonitis, pericarditis, and myocardial infarction. Although technological advancements in photon RT delivery like intensity modulated RT have decreased the risk of such toxicities, a growing body of evidence indicates that further risk reductions are achieved with proton beam therapy (PBT). Herein we review the published dosimetric and clinical PBT literature for esophageal cancer, including motion management considerations, the potential for reirradiation, radiation dose escalation, and ongoing esophageal PBT clinical trials. We also consider the potential cost-effectiveness of PBT relative to photon RT.
Introduction
Esophageal cancer remains one of the deadliest cancers despite progress in the treatment of this disease over the past several decades. Worldwide there are an estimated 456,000 new esophageal cancer cases and 400,000 deaths annually (1). Although the incidence of esophageal cancer in the United States is lower than in many Asian and African countries, the annual number of expected American deaths from esophageal cancer (approximately 16,000) still rivals the predicted number of new diagnoses (approximately 18,000) (2).
For patients with locally advanced esophageal cancer deemed suitable for surgery, the standard treatment recommendation is neoadjuvant chemoradiation (CRT) followed by esophagectomy. This is based on prospective data showing superior local control (LC) and overall survival (OS) compared with esophagectomy alone (3). For those who are not surgical candidates definitive CRT is recommended, which results in a significantly greater likelihood of survival at 5 years compared with radiation therapy (RT) alone (4). Thus, RT plays a central role in the treatment of locally advanced esophageal cancer regardless of surgical appropriateness.
Radiation therapy for esophageal cancer is challenging because of the central location of the esophagus within the thorax, resulting in a need to delicately balance minimizing radiation dose to nearby critical structures (ie, heart, lung, and spinal cord) while maintaining an effectively high dose to the target. Lung and heart doses in particular have been shown to increase the likelihood of pneumonitis, post-operative pulmonary complications (5, 6), heart wall motion abnormalities, coronary artery disease, pericarditis, and myocardial infarction (7–9).
Technological advances in RT delivery have led to an evolution in the treatment of esophageal cancer over the last half century by achieving increasingly better normal tissue sparing while maintaining accurate dose delivery to the target. In the era of 2-dimensional (2D) planning, generous treatment ports were used to ensure adequate target coverage, but 2D RT also exposed significant volumes of normal tissues to high doses and led to serious complications. Increasing conformity around the target resulting in superior normal tissue sparing was later achieved using 3-dimensional conformal RT (3D-CRT), and this was subsequently improved upon even further with the advent of intensity modulated radiation therapy (IMRT) (10–12). For example, Chandra et al (10) found that 7-field IMRT compared with 3D-CRT significantly reduced the percentage of lung receiving at least 10 Gy (V10) from 40.4% to 29.2% (P=.01), V20 from 19.3% to 13.5% (P=.01), and mean lung dose (MLD) from 14.8 Gy to 11.8 Gy (P=.01). This decrease in normal tissue dose achieved with IMRT has been suggested to result in clinically meaningful outcomes (13, 14). A study from MD Anderson Cancer Center reported a propensity score–adjusted comparison of long-term clinical outcomes between 3D-CRT and IMRT. Intensity modulated RT was associated with significantly higher OS, but there was no difference in cancer-related deaths or pulmonary-related deaths. The key difference was seen in the patients who received 3D-CRT, who had a significantly higher risk of cardiac-related deaths (P=.049) and other-cause deaths (72.6% vs 52.9%, P<.0001) (13). These data highlight that treatment outcomes are not only dependent on the ability to deliver adequate radiation dose to esophageal cancers, but that the overall health of the patient is directly affected by treatment-related toxicity. These toxicities could be further reduced using advanced treatment delivery technologies, such as IMRT and proton beam therapy (PBT). In fact, the physics of PBT is ideally suited for tumors of the esophagus as compared with photon RT because of the significantly reduced exit dose through the heart and lungs.
Proton beam therapy has historically been used to treat select cancers; however, a growing collection of studies suggest that PBT is not only safe and effective in treating esophageal cancer, but that the side-effect profile may also be improved over traditional photon-based techniques. Herein we review published dosimetric and clinical PBT literature for esophageal cancer, including some of the exciting potential applications of PBT that capitalizes on the toxicity sparing effects of PBT. We also consider the potential cost-effectiveness of PBT relative to photon-based techniques.
Dosimetric Comparison of Proton Versus Photon Therapy
Proton beam therapy has traditionally been delivered using a passive scattering technique, in which placing scattering material in the path of the proton beam spreads out a proton beam while compensators and collimators are used to conform dose to the target. The potential benefits of passive scattering proton therapy (PSPT) over 3D-CRT for esophageal cancer were suggested by a comparative treatment planning study published in 1998 (15). Both radiation modalities provided excellent target coverage, although sparing of the heart, lungs, spinal cord, and kidneys favored PSPT for all patients. The mean tumor control probability increased by a mean 20%-units (2%- to 23%-units) using the best proton plan, assuming a 5% normal tissue complication probability. On the basis of these data the authors predicted that radiation dose escalation above 40–50 Gy(RBE [relative biological effectiveness]) would be more feasible using protons. Passive scattering proton therapy was shown in a recently published dosimetric comparison with 3D-CRT to also be advantageous when prescribing a total dose of 60 Gy(RBE) in 30 fractions (16). Passive scattering proton therapy was delivered using an anterior–posterior/posterior–anterior (AP/PA) beam arrangement for most patients, whereas 3D-CRT was given using AP/PA and oblique beams to limit the spinal cord dose. Significant normal tissue sparing was achieved using PSPT, including lung V5-V20, mean lung dose, and heart V30-V50 (P<.001), and this translated into reduced cardiac and pulmonary morbidity based on normal tissue complication probability calculations. Dose escalation using PBT is more thoroughly discussed below.
Passive scattering proton therapy has been shown to reduce normal tissue dose compared with IMRT. Zhang et al performed a comparative planning study of 2-beam PSPT (AP/PA), 3-beam PSPT (AP/posterior obliques), and IMRT for distal esophageal or gastroesophageal junction (GEJ) cancer (17). The prescription dose was 50.4 Gy(RBE) in 28 fractions. Although target volume coverage was similar between all plans, PSPT delivered significantly less dose to the lung than IMRT. Not surprisingly, the ability to spare the lungs from doses less than 20 Gy(RBE) was better using 2 versus 3 proton beams, whereas heart sparing was superior using 3 beams because of the ability to deliver more dose posteriorly. The risk of postoperative pulmonary complications was estimated to be 18.5% using IMRT, compared with 5% using 2-beam PSPT and 11% using 3-beam PSPT. The identification of several feasible beam arrangements can allow for personalization of PBT by accounting for patients’ baseline cardiac and/or pulmonary health and anatomy, and then choosing which beam arrangement is preferred to maximize sparing the organ most at risk from radiation toxicity. Another important aspect of this study was evaluation of dosimetric effects due to motion using 4-dimensional (4D) CT scans compared with 3D average CT scans. Target coverage was suggested to differ by at least 2% according to the presence of diaphragmatic motion and stomach filling, highlighting the need to account for and minimize differences in anatomy on a day to day basis to the greatest extent possible when using proton therapy for esophageal cancer.
Investigators from Loma Linda University performed a dosimetric comparison of 3D-CRT, IMRT, and PSPT for 10 patients with either distal esophageal or GEJ cancers (17). The prescription dose was 50.4 Gy(RBE) in 28 fractions. The 3D-CRT plans consisted of a 4-field box, IMRT plans used a step-and-shoot approach with 6–9 beams, and PSPT plans were generated using 2 beams at lateral and oblique angles. Compared with IMRT and 3D-CRT, PSPT provided similar target coverage but delivered significantly reduced dose to various volumes of the liver, spinal cord, lung (including V5, V20, and mean dose), and heart (including V25, V40, and mean dose). Not only was the whole heart dose evaluated, but dose to individual substructures of the heart, including the left anterior descending artery, left ventricle, and pericardium, was also determined. Passive scattering proton therapy was the best at limiting dose to these substructures. For example, the mean left anterior descending artery dose between PSPT, IMRT, and 3D-CRT plans was 0.4 Gy(RBE), 17.6 Gy(RBE), and 15.1 Gy(RBE), respectively, which may reduce the risk of late clinically significant cardiac morbidity (18).
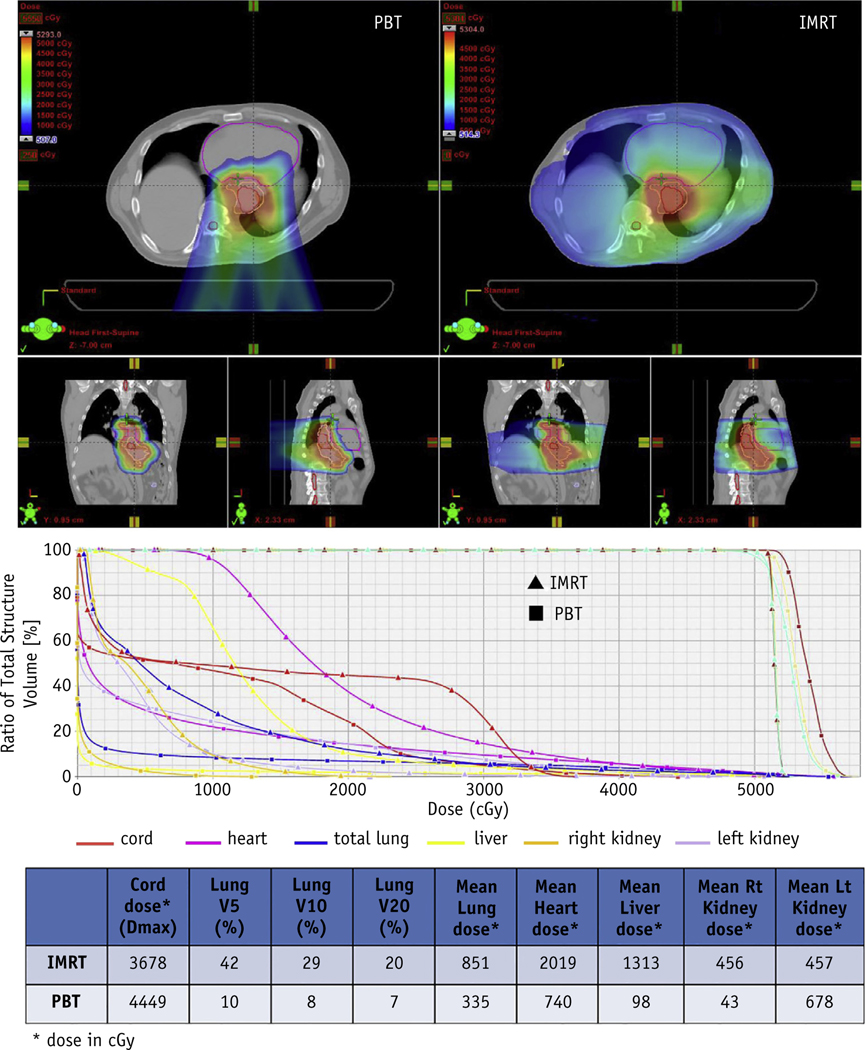
Although PSPT essentially eliminates exit dose to normal tissues compared with photon therapy, the deposition of high doses proximal to the target is not as highly conformal. Pencil beam scanning, which is also commonly referred to as intensity modulated proton therapy (IMPT), is a more recent technological advancement in which magnets steer the proton beam to cover, or “paint,” the target volume layer by layer. Pencil beam scanning utilization is increasing because of its ability to deliver a dose distribution that conforms to both the distal and proximal edges of the target volume. A study from Mayo Clinic showed significantly improved sparing of the lungs, heart, kidneys, liver, and small bowel, although spinal cord and skin dose were higher using either 1-field (single PA) or 2-field (posterior obliques) pencil beam scanning compared with IMRT in patients with distal esophageal cancer (19). Welsh et al. evaluated IMPT and IMRT plans for patients with distal esophageal cancer who were prescribed 65.8 Gy(RBE) to the GTV and 50.4 Gy(RBE) to the PTV in 28 fractions (20). Intensity modulated RT was delivered using a simultaneous integrated boost, whereas IMPT was delivered using 3 unique beam arrangements: AP/PA, left posterior oblique/right posterior oblique (LPO/RPO), and AP/LPO/RPO. All 3 IMPT beam arrangements provided greater sparing of the heart, lungs, liver, and spinal cord compared with IMRT. Similar to the data presented above from Zhang et al, cardiac sparing was increased when LPO/RPO beams were used. Figure 1 illustrates the dosimetric advantages of IMPT compared with IMRT for treatment of distal esophageal cancer.
Fig. 1.
Significant normal tissue sparing is achieved with Proton Beam Therapy (PBT), which in this case is done with Intensity Modulated Proton Therapy, compared with intensity modulated radiation therapy (IMRT) for distal esophageal cancer.
A study from MD Anderson Cancer Center compared motion robust IMPT, PSPT, and volumetric modulated arc therapy (VMAT) plans for 11 patients with distal esophageal or GEJ cancer (21). All plans were prescribed a total dose of 50.4 Gy(RBE) in 28 fractions, and 100% of the internal clinical target volume was required to receive the prescription dose. The mean dose conformity index for the internal clinical target volume was 0.55, 0.55, and 0.45 for motion robust IMPT, VMAT, and PSPT, respectively (21). Intensity modulated proton therapy was associated with significant reductions in mean dose to the heart and liver compared with PSPT (Table 1).
Table 1.
Motion robust IMPT achieves improved heart, lung, and liver sparing compared with PSPT and VMAT for esophageal cancer
| Parameter | Motion robust IMPT | PSPT | VMAT | P |
|---|---|---|---|---|
|
| ||||
| Heart mean [Gy(RBE)] | 9.9 ± 3.1 | 12.3 ± 3.2 | .001 | |
| 17.1 ± 4.2 | .001 | |||
| Heart V40 (%) | 9.2 ± 4.4 | 13.9 ± 4.8 | .001 | |
| 8.5 ± 5.0 | .3 | |||
| Lung mean [Gy(RBE)] | 3.0 ± 0.9 | 4.4 ± 1.3 | .001 | |
| 6.3 ± 1.3 | .001 | |||
| Lung V5 (%) | 11.4 ± 3.4 | 18.7 ± 5.6 | .001 | |
| 36.0 ± 8.8 | .001 | |||
| Lung V20 (%) | 5.7 ± 1.7 | 7.9 ± 2.3 | .002 | |
| 8.1 ± 1.9 | .001 | |||
| Liver mean [Gy(RBE)] | 4.0 ± 2.5 | 3.5 ± 1.6 | .13 | |
| 9.8 ± 3.1 | .002 | |||
| Liver V30 (%) | 5.4 ± 3.0 | 6.0 ± 2.8 | .04 | |
| 7.0 ± 2.6 | .01 | |||
Abbreviations: IMPT = intensity modulated proton therapy; PSPT = passive scattering proton therapy; RBE = relative biological effectiveness; VMAT = volumetric modulated arc therapy.
Values are mean ± standard deviation.
Motion Management Considerations for Proton Beam Therapy
The dose distribution using PBT is affected to a much greater extent by changes in tissue density than photon RT. As a result there is concern about using PBT in the presence of significant target motion. This especially pertains to targets in the thorax and upper abdomen, including the distal esophagus, that move as a result of diaphragmatic excursion. Furthermore, motion generally affects the dose distribution for IMPT to a greater degree than PSPT. Although IMPT has better dose conformity than PSPT and delivers less integral dose than IMRT, the clinical implementation of IMPT for distal esophageal cancer is challenging, with the concern of dosimetric uncertainty caused by respiratory motion. In general, changes of radiologic path length, as measured by water equivalent thickness (WET), of the proton beam could result in substantial change in dose distribution (22, 23). Although the range of motion for the GTV in distal esophagus is typically a few millimeters, diaphragmatic motion is larger and on the order of centimeters (24, 25). Because the diaphragm moves during respiration, this results in changes to the WET in the beam path, which can cause significant interplay effects.
Various motion management strategies have been proposed, and some have been implemented (26–36). For PSPT, motion robust treatment planning strategies were evaluated by Pan et al (37) on the basis of the average, inspiration, and expiration CT scans. This analysis concluded that a 1- to 3.5-cm smearing margin on the inspiration CT scan was the most robust (37). For IMPT, a study from Mayo Clinic reported on the benefit of using maximum monitor unit threshold-based repainting, which was especially useful when using a 1-field (PA) approach (38). Yu et al (39) reported a strategy to select IMPT beam angles that are robust to diaphragmatic motion. Motion robust beam angles were determined by examining the change of WET along the beam path during a full cycle of free-breathing motion at various angles. The beam angles that yielded the smallest value of the maximum temporal change of WET were considered to be the most robust. They concluded that the most motion robust beam angles are generally posterior with median gantry angle of 200° (range, 180°−220°), because these beam angles pass through a relatively less mobile portion of the diaphragm. The study also determined that motion robust optimization based on the average image sets of 4D CT and the 2 extreme phases of 4D CT (the end of inhale and exhale phases) was able to significantly reduce the motion-induced dose uncertainty for target volume coverage. The motion-induced dose uncertainty was evaluated by both the overall dose uncertainty after multiple fractions, and the dose uncertainty induced by interplay effect in any single fraction. For the IMPT plans calculated with a conventional optimization algorithm, the average dose uncertainty over the whole course was more than 1 Gy(RBE) for the dose–volume parameter D95 (the minimum dose received by 95% of the treatment volume), and it was reduced 50% with the motion robustness optimization. The average dose error due to interplay effects in any one fraction was also reduced with the motion robustness optimization [from 0.6 to 0.4 Gy(RBE)]. These results suggest that IMPT plans for distal esophageal cancers that are relatively insensitive to respiratory and diaphragmatic motion can be developed.
Clinical Experience of PBT for Esophageal Cancer
Reported clinical experiences for PBT have been limited to single-institution studies. One of the initial case reports came from the University of Tsukuba (40), with the recent update from the same group in 51 patients with esophageal squamous cell carcinoma (ESCC) treated between 1985 and 2005 (41). Most patients had locally advanced disease; 27 patients (53%) were clinically node positive, whereas 34 (67%) had clinical stage T3 or T4 disease. The median primary tumor length was 6 cm (range, 2–15 cm). Thirty-three were treated to a median dose of 46 Gy(RBE) with photon with a PBT boost to 80 Gy(RBE) [range, 70–90 Gy(RBE)], and 18 patients were treated with PBT alone [median dose of 79 Gy(RBE), range 62–98 Gy(RBE)]. No patients were treated with concurrent chemotherapy. No treatment interruptions were attributed to RT, such as esophagitis or hematologic toxicities. Only 6 patients (11.7%) developed grade 3 esophagitis, and the majority of patients experienced only low-grade toxicities. One patient developed an aspiration pneumonia and had to discontinue therapy, and 1 patient died of esophageal hemorrhage at the site of the primary tumor. The 5-year OS was 21.1%, and median survival was approximately 21 months. The 5-year LC rate was 38%. Because concurrent CRT is the standard of care for most patients, the University of Tsukuba group also recently reported their initial experience in 40 patients treated with definitive PBT and concurrent chemotherapy with cisplatin and 5-fluorouracil (42). Twenty-one patients (53%) were clinically node positive, whereas T3/T4 disease was diagnosed in 15 patients (28%). The patients were treated to 60 Gy(RBE) after an initial 40–50 Gy(RBE) to a larger AP/PA field arrangement. There were no grade 3 or higher cardiopulmonary toxicities reported. The 3-year OS was 70%, and 2-year disease-free survival (DFS) and locoregional control rates were 77% and 66%, respectively.
In contrast to ESCC, esophageal adenocarcinomas (EACs) demonstrate different clinical outcomes, toxicities, and operability after concurrent chemotherapy and PBT. The treatment using PBT and concurrent chemotherapy for EAC was reported in 2 single-institution experiences from MD Anderson Cancer Center. The first was in 62 consecutive patients reported in 2012 (43). Most patients had EAC (76%), stage II-III disease (84%), and were prescribed a median dose of 50.4 Gy(RBE) in 28 fractions. Nearly 50% underwent preoperative therapy. The majority of the acute side effects were esophagitis, nausea, anorexia, and radiation dermatitis. Grade 3 toxicities were seen in <10%, with 2 cases of grade 2 and 3 radiation pneumonitis. The pathologic complete response (pCR) rate was 28%. Post-operative complications were mostly pulmonary (6.5%), cardiac (atrial fibrillation in 8%), and wound complications (3.2%). The 3-year overall, relapse-free, distant metastasis-free, and locoregional-free survival rates were 51.7%, 40.5%, 66.7%, and 56.5%, respectively.
Neoadjuvant PBT compared with photon RT may reduce the incidence and severity of postoperative complications, including pulmonary, cardiac, gastrointestinal (GI), and wound complications related to reduction in radiation dose to the lungs, heart, stomach, and anterior/lateral skin and soft tissue. A second study from MD Anderson reported the outcomes of preoperative CRT in 444 patients treated between 1998 and 2011 (5). During this period, 208, 164, and 72 patients were treated with 3D, IMRT, or PBT, respectively. The investigators evaluated the incidence and the clinical predictors of the most common postoperative complications (pulmonary, cardiac, wound, and GI) within 30 days of surgery. A number of factors predicted for pulmonary, GI, and cardiac complications, but the type of radiation modality used was the only factor associated with pulmonary or GI complications. On multivariate analysis, only radiation modality and preradiation diffuse capacity of the lung for carbon monoxide were independently associated with pulmonary complications. For GI complications, radiation modality only trended toward significance. There was a significant increase in pulmonary complications for 3D-CRT compared with IMRT (odds ratio [OR] 4.10; 95% confidence interval [CI] 1.37–12.29) or 3D-CRT compared with PBT (OR 9.13; 95% CI 1.83–45.42), but a trend for IMRT being worse compared with PBT (OR 2.23; 95% CI 0.86–5.76). When dosimetric factors such as the MLD and mean heart dose were added to the multivariable regression model, MLD replaced radiation modality as the strongest predictor of pulmonary complications. The results suggest that although radiation modality was predictive of post-operative pulmonary complications, it was the ability of the individual radiation technologies to deliver a lower MLD that was most predictive of lower pulmonary complications. The median hospital length of stay was also significantly different after PBT, IMRT, and 3D-CRT (8 vs 10 vs 12 days, respectively, P<.0001) (44). Combining photon and proton data from 3 institutions, investigators at MD Anderson, Mayo Clinic, and University of Maryland examined the impact of preoperative RT modality (proton, n=111, vs photon, n=471) on postoperative outcomes in a retrospective study of 582 patients with esophageal cancer treated with trimodality therapy between 2007 and 2013 (45). Proton beam therapy was associated with lower rates of postoperative complications (41% vs 56%, P=.005), including pulmonary (14% vs 28%, P=.003), cardiac (12% vs 19%, P=.10), GI (19% vs 22%, P=.5), and wound (5% vs 15%, P=.002) complications. Mean hospital length of stay was 9 days for PBT and 12 days for photon RT (P<.0001). Mortality rates at 90 days after surgery were 4.2% for photon RT and 0.9% for PBT (P=.15).
Proton beam therapy may also reduce certain treatment-related toxicities during or shortly after CRT. In the aforementioned multi-institution study of 582 patients with esophageal cancer treated with photon (n=471) or proton (n=111) CRT, proton CRT was associated with lower incidences of acute grade ≥2 nausea (29% vs 50%, P<.001), fatigue (27% vs 33%, P<.001), and hematologic toxicity (2% vs 26%, P<.001) (46). This was despite greater treatment intensity (higher rate of induction chemotherapy and higher RT dose) in patients who received proton (vs photon) CRT. The improved tolerance of proton-based CRT for esophageal cancer observed in these studies suggests that intensification of treatment (such as RT dose escalation and/or additional systemic therapy agents) should be explored.
The literature suggesting that clinically meaningful improvements for esophageal cancer patients may be achieved with PBT have exclusively used PSPT. Although the evidence supporting the expanded use of IMPT is currently limited to dosimetric comparisons, the potential to further reduce normal tissue dose beyond what is achievable with PSPT has garnered increasing interest. Clinical outcomes of IMPT for esophageal cancer are needed, and prospective evaluation is ongoing, as will be discussed in greater detail below. We should remain cognizant of the larger effect of motion on dose delivery in IMPT compared with PSPT plans; motion robust IMPT planning techniques are promising, as mentioned above. Furthermore, although image guidance for PBT is currently limited to 2D imaging, the increasing availability of cone-beam CT for PBT is expected to allow for better assessment of tumor motion, improve treatment accuracy, and potentially permit safe use of more selective treatment uncertainty margins.
Incorporating PBT into Dose Escalation
The value of radiation dose escalation for esophageal cancer has been debated for many years. The Radiation Therapy Oncology Group 94–04/INT 0123 study conducted by Minsky et al (47) compared a group of unresectable and primarily ESCC patients (85%) who were randomized to either 50.4 Gy and 64.8 Gy with concurrent 5-fluorouracil and cisplatin and demonstrated a lack of benefit to the high-dose irradiation arm for both local recurrence and OS rates. Notably, of the 11 deaths in the high-dose arm, 7 occurred before receiving at least 50.4 Gy. Moreover, an outdated radiation technique was used (2D treatment planning and larger margins than are now considered to be standard). The potential benefit of radiation dose intensification using modern radiation technique has yet to be clearly shown, although emerging data suggest that there may be a rationale to consider this treatment approach.
Minsky et al (47) demonstrated no OS benefit to dose escalation in the definitive setting for ESCC, although multiple retrospective studies suggest a benefit to escalating radiation doses for both ESCC and EAC. In a study by Zhang et al (48) that evaluated 69 patients with unresectable ESCC and EAC who underwent CRT, those who received >51 Gy had significantly higher 3-year LC, DFS, and OS. The clinical complete response rate was doubled in the higher dose group, to 46% from 23% in the lower dose group (P=.048), but there was a greater proportion of ESCC in the higher dose arm (81% vs 61%), demonstrating both a likelihood of the responsiveness of ESCC to higher radiation doses and the difficulty in escalating doses to the more distal EACs without the use of more advanced radiation technologies (ie, no IMRT was used in this study to spare cardiac tissue) (49). Similarly, He et al (49) asked a similar question about dose escalation in the definitive management of ESCC and found that doses of >50.4 Gy decreased local failures (17.9% vs 34.3%, P=.024) but did not improve the complete response rate when delivered with modern techniques and concurrent chemotherapy. Additionally, the improvement in LC came at the cost of increased toxicity without impacting OS (49).
Early outcomes from 2 prospective chemoradiation dose escalation trials in unresectable esophageal cancer patients have been recently presented (50, 51). A phase 1/2 trial at MD Anderson Cancer Center used a simultaneous integrated boost to prescribe 50.4 Gy to regions of subclinical disease and 63 Gy to gross disease, all in 28 fractions (50). This study included 38 patients (T3 84%, N1 34%, N2 24%) with 50% ESCC. With median follow-up of 16.8 months, grade 3 toxicity included esophagitis (8%), dysphagia (24%), nausea (5%), and anorexia (8%). No grade 4 or higher toxicity was reported. Locoregional failure occurred in 37%, which is favorable compared with historical control. Outcomes of a phase 2 trial from Shantou University were also recently reported, including 59 ESCC patients (T3/4 78%, N1 72%) (51). Fifty-four grays in 1.8-Gy fractions was prescribed to a larger region for microscopic disease coverage, whereas the primary tumor was simultaneously prescribed 66 Gy in 2.2-Gy fractions, all in 30 fractions. Grade 3 nonhematologic toxicity was 27%, although 2 patients experienced grade 5 esophageal ulcer/fistula. With median follow-up of 19 months, 1- and 2-year local and regional control were 94.3% and 90%, and 90.7% and 88.3%, respectively. These 2 trials provide support for continued evaluation of dose escalation in unresectable esophageal cancer patients. Two prospective randomized dose escalation trials are currently underway, one in France (CONCORDE, NCT01348217) and one in the Netherlands (ART DECO, NTR3532).
For neoadjuvant therapy, there also seems to be evidence of a dose–response relationship (52). A meta-analysis of 26 trials demonstrated a higher likelihood of pCR with increasing doses of RT (53). According to the available literature, escalating doses of RT can improve pCR rates; however, with studies of limited sizes it is challenging to demonstrate an OS benefit (54). The ability to achieve a pCR using CRT may obviate the need for surgery, because surgery in combination with CRT for ESCC provides primarily a LC benefit without improvement in OS over CRT alone (55, 56). Multiple studies have demonstrated that the pCR rates of ESCC are superior when identical doses of neoadjuvant CRT are administered to both EAC and ESCC histologies, with some improvement in OS for ESCC when surgery and CRT are combined (57). Rates of locoregional recurrence after neoadjuvant CRT and surgery are 14% compared with 34% without CRT (58); therefore, dose escalation may reduce the need for surgery by achieving a durable pCR. Using this rationale, there may be room for improvement in intensification of radiation dose using PBT.
Using PBT is a logical next step for assessing the potential merits of dose escalation for esophageal cancer, and several studies suggest that this is feasible. Koyama et al (59) used PBT alone or as a boost after photon RT without chemotherapy for the definitive management of ESCC. Fiducial markers were implanted at the proximal and distal edges of the primary tumor. Fraction sizes ranged from 2.5 to 3.7 Gy(RBE) given once daily (owing to limited proton machine availability), and photon RT was delivered in 1.8- to 2.0-Gy fractions daily. Overall mean total doses were 77.7 Gy(RBE) for T1 tumors and 80.7 Gy(RBE) for T3-T4 tumors. Results were very promising for early stage T1 lesions, with 5-year DFS of 100% and 49% for T2-T3 tumors. Local recurrences in the more advanced groups occurred in the middle of the tumor site and at the margin of the field. Another series from Sugahara et al (60) included 46 ESCC patients. Proton beam therapy was delivered up to 89.5 Gy(RBE) [median 82 Gy(RBE)] and combined photon/proton plans delivered up to 87.4 Gy(RBE) [median 76 Gy(RBE)] also without concurrent chemotherapy. The complete response rate for T1-T2 tumors was 100%, for T3 was 85%, and for T4 was 20%. Overall, 48% of patients developed esophageal ulcers, which were observed to occur 2–14 months after the start of treatment. Mizumoto et al (61) evaluated 51 ESCC patients treated without chemotherapy and with median doses of PBT to 79 Gy(RBE) [range, 62–98 Gy(RBE)] and mixed photon/proton plans with a median of 80 Gy(RBE) [range, 70–90 Gy(RBE)]. Nearly 80% of these patients had a complete response within 4 months of finishing RT. Finally, another regimen incorporated a hyperfractionated concomitant proton boost to a median dose of 78 Gy(RBE); median survival was 31.5 months (61).
The Potential of PBT for Reirradiation
Because of its capability to better spare normal tissues, PBT is likely better suited than photon RT for reirradiation of locally recurrent or persistent esophageal cancer. However, the clinical outcomes of reirradiation using PBT are limited to a single report of 11 patients (62). Five of 11 patients were locally controlled at a median follow-up of 11.3 months. Eight patients died with a median OS of 13 months. One case of grade 4 esophagopleural fistula occurred, and 3 patients developed neutropenic fever. One patient had late esophageal stenosis that required a permanent feeding tube. These results suggest that durable LC may be achieved after PBT reirradiation with acceptable toxicity and indicate the need for continued study of reirradiation for esophageal cancer.
Cost-Effectiveness of PBT
Any consideration of proton beam therapy must carefully balance the increased costs associated with delivery of PBT against any incremental improvement in outcomes. The 2015 Medicare reimbursement rate for daily delivery of complex PBT is $1071.95 (Current Procedural Terminology (CPT) code 77,525) versus $421 (Healthcare Common Procedure Coding System (HCPS) code G6015) for delivery of complex hospital-based IMRT. Over a course of 23–28 fractions that are typically used for esophageal cancer, this will result in a substantial increase in initial treatment costs. To determine whether this increase in treatment costs is warranted, formal cost-effectiveness analyses are necessary.
Studying cost-effectiveness of PBT for esophageal carcinoma has been difficult, in part owing to the paucity of published clinical outcomes and toxicity data (42). Cost-effectiveness studies most commonly consist of modeling studies (eg, Markov or Monte Carlo simulations) that are based on known probabilities for experiencing specific outcomes (survival, toxicity) (63). The probability of experiencing an outcome is extracted from published literature, and costs are typically estimated according to Medicare reimbursement figures. A main output measure of such analyses is quality-adjusted life-years (QALYs), which accounts for both the quality and quantity of life after a specific treatment (eg, PBT vs photons). In a cost-effectiveness analysis, the costs associated with each incremental QALY gained from each treatment, termed the incremental cost-effectiveness ratio, is additionally evaluated. The incremental cost-effectiveness ratio is a surrogate for whether a modality is termed “cost-effective” by payers and policy makers, and has been informally proposed as the $50,000/QALY “willingness to pay” threshold, although this value has been debated extensively (64).
No study to date has formally evaluated the cost-effectiveness of PBT for esophageal cancer, but recently published data from a multi-institutional comparative effectiveness of PBT versus photon therapy raises the possibility to perform such an analysis (45). Although OS was not significantly different between the cohorts, use of PBT was associated with decreased acute GI and hematologic toxicities after CRT (45), significantly reduced post-operative GI, pulmonary, and wound complications (5, 44), and a 25% reduction in postoperative length of hospital stay (44). Because there are significant medical costs associated with each of these, future cost-effectiveness studies are required to analyze the incremental lifetime costs of PBT that account for both treatment and posttreatment costs.
Medicare reimbursement for PBT decreased by nearly 10% in 2015, and will likely decrease further in the next few years with the development of a single-room proton facility. Formal economic evaluation of PBT for esophageal cancer will be performed as a secondary endpoint of the ongoing randomized study of PBT and IMRT, and will provide further insight into this important issue.
Clinical Trials
Despite promising clinical results from the previously mentioned single-institution experiences of esophageal PBT, prospective trials comparing PBT with standard photon technologies like 3D-CRT or IMRT will be necessary to provide high-quality evidence demonstrating the value of PBT. There are currently 4 active clinical trials in the United States evaluating the role of PBT for esophageal cancer. These include a phase 2 trial from Loma Linda University (NCT01684904) (“A Phase II Trial of Proton Chemotherapy for Resectable Esophageal or Esophagogastric Junction Cancer”), a phase 1 dose escalation trial from University of Pennsylvania (NCT02213497) (“Dose Escalation of Neoadjuvant Proton Beam Radiotherapy With Concurrent Chemotherapy in Locally Advanced Esophageal Cancer”), and a prospective observational study from the Mayo Clinic (NCT02452021) (Pencil Beam Scanning Proton Radiotherapy for Esophageal Cancer). Last, there is an accruing randomized trial from MD Anderson Cancer Center that began in the spring of 2012 (NCT01512589) (“Phase IIB Randomized Trial of PBT versus IMRT for the Treatment of Esophageal Cancer”). The central hypothesis is that PBT can reduce severe side effects of treatment with or without improving the progression-free survival time. The primary endpoint is “total toxicity burden” and progression-free survival. The total toxicity burden is the patient’s overall severe adverse events using a composite scoring system that is a collective score of multiple types of rare but severe adverse events that the patients may experience over time during and after the treatment course. Secondary objectives include (1) patient-reported outcomes of symptoms during and after treatment using the M. D. Anderson Symptom Index and the EQ5D quality of life questionnaires; (2) clinician-reported toxicities using Common Terminology Criteria for Adverse Events v4.0; (3) QALY comparison; and (4) cost–benefit economic analysis. Any patient with nonmetastatic esophageal cancer eligible for CRT could enroll. The randomization to PBT or IMRT is stratified according to whether patients (1) are deemed resectable or unresectable before starting therapy; (2) received induction chemotherapy; (3) have stage I-II versus stage III disease; (4) have adenocarcinoma or squamous carcinoma; and (5) are younger or older or equal to 65 years of age. Patients randomized to PBT can receive either PSPT or IMPT, and for IMRT, various techniques including VMAT could be used. Four-dimensional CT simulation is required for all patients, with standard target delineation and daily kilovoltage imaging setup. The prescription dose is 50.4 Gy(RBE) in 28 daily fractions. Patients are followed until progression of disease or death, whichever comes first. Several interim analyses are planned to compare the total toxicity burden and PFS of PBT versus IMRT, with early closure if there are significant difference in any of these co-primary endpoints. The target accrual is 180 patients. As of August 2015, 79 patients were registered and randomized, but only 62 patients were treated on the trial. The most common reasons for not being treated on study were denial of insurance coverage (13 of 17, 76%, 11 were from the proton group) and patient preference (either wanting PBT after being randomized to IMRT [n=2] or wanting IMRT after being randomized to PBT [n=2]). This study will hopefully clarify the role of PBT for the treatment of esophageal cancer.
Conclusion
There is a growing body of evidence to suggest that the dosimetric benefits of PBT may result in clinically significant reduction in acute and long-term treatment-related toxicities compared with photon RT. Radiation and/or chemotherapy intensification, as well as reirradiation, are promising future applications of PBT for esophageal cancer. Strong consideration should be given to enrolling eligible patients to available esophageal PBT clinical trials.
Footnotes
Conflict of interest: none.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090–1098. [DOI] [PubMed] [Google Scholar]
- 4.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593–1598. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;64:692–699. [DOI] [PubMed] [Google Scholar]
- 7.Hatakenaka M, Yonezawa M, Nonoshita T, et al. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: Magnetic resonance evaluation. Int J Radiat Oncol Biol Phys 2012;83:e67–e73. [DOI] [PubMed] [Google Scholar]
- 8.Gayed I, Gohar S, Liao Z, et al. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging 2009;25:487–495. [DOI] [PubMed] [Google Scholar]
- 9.Beukema JC, van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015;114:85–90. [DOI] [PubMed] [Google Scholar]
- 10.Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005; 77:247–253. [DOI] [PubMed] [Google Scholar]
- 11.Kole TP, Aghayere O, Kwah J, et al. Comparison of heart and coronary artery doses associated with intensity-modulated radiotherapy versus three-dimensional conformal radiotherapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:1580–1586. [DOI] [PubMed] [Google Scholar]
- 12.Nicolini G, Ghosh-Laskar S, Shrivastava SK, et al. Volumetric modulation arc radiotherapy with flattening filter-free beams compared with static gantry IMRT and 3D conformal radiotherapy for advanced esophageal cancer: A feasibility study. Int J Radiat Oncol Biol Phys 2012;84:553–560. [DOI] [PubMed] [Google Scholar]
- 13.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freilich J, Hoffe SE, Almhanna K, et al. Comparative outcomes for three-dimensional conformal versus intensity-modulated radiation therapy for esophageal cancer. Dis Esophagus 2015;28:352–357. [DOI] [PubMed] [Google Scholar]
- 15.Isacsson U, Lennernas B, Grusell E, et al. Comparative treatment planning between proton and x-ray therapy in esophageal cancer. Int J Radiat Oncol Biol Phys 1998;41:441–450. [DOI] [PubMed] [Google Scholar]
- 16.Makishima H, Ishikawa H, Terunuma T, et al. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res 2015;56:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Zhao Kl, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2008;72:278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor CW, Bronnum D, Darby SC, et al. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol 2011;100:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funk RK, Tryggestad EJ, Kazemba BD, et al. Dosimetric comparison of imrt vs pencil-beam scanning proton therapy for distal esophageal cancer. Int J Particle Ther 2015;2:360–361. [Google Scholar]
- 20.Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: A dosimetric study. Int J Radiat Oncol Biol Phys 2011;81:1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Zhang X, Liao L, et al. Motion-robust intensity-modulated proton therapy for distal esophageal cancer. Med Phys 2016;In Press [DOI] [PubMed] [Google Scholar]
- 22.Mori S, Chen GT. Quantification and visualization of charged particle range variations. Int J Radiat Oncol Biol Phys 2008;72:268–277. [DOI] [PubMed] [Google Scholar]
- 23.Mori S, Wolfgang J, Lu HM, et al. Quantitative assessment of range fluctuations in charged particle lung irradiation. Int J Radiat Oncol Biol Phys 2008;70:253–261. [DOI] [PubMed] [Google Scholar]
- 24.Yaremko BP, Guerrero TM, McAleer MF, et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2008;70:145–153. [DOI] [PubMed] [Google Scholar]
- 25.Wang JZ, Li JB, Wang W, et al. Changes in tumour volume and motion during radiotherapy for thoracic oesophageal cancer. Radiother Oncol 2015;114:201–205. [DOI] [PubMed] [Google Scholar]
- 26.Bert C, Saito N, Schmidt A, et al. Target motion tracking with a scanned particle beam. Med Phys 2007;34:4768. [DOI] [PubMed] [Google Scholar]
- 27.Chang JY, Li H, Zhu XR, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys 2014;90:809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furukawa T, Inaniwa T, Sato S, et al. Moving target irradiation with fast rescanning and gating in particle therapy. Med Phys 2010;37: 4874. [DOI] [PubMed] [Google Scholar]
- 29.Knopf AC, Hong TS, Lomax A. Scanned proton radiotherapy for mobile targets—the effectiveness of re-scanning in the context of different treatment planning approaches and for different motion characteristics. Phys Med Biol 2011;56:7257–7271. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Kardar L, Li X, et al. On the interplay effects with proton scanning beams in stage III lung cancer. Med Phys 2014;41:021721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori S, Inaniwa T, Furukawa T, et al. Amplitude-based gated phase-controlled rescanning in carbon-ion scanning beam treatment planning under irregular breathing conditions using lung and liver 4DCTs. J Radiat Res 2014;55:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori S, Zenklusen S, Inaniwa T, et al. Conformity and robustness of gated rescanned carbon ion pencil beam scanning of liver tumors at NIRS. Radiother Oncol 2014;111:431–436. [DOI] [PubMed] [Google Scholar]
- 33.Riboldi M, Orecchia R, Baroni G. Real-time tumour tracking in particle therapy: Technological developments and future perspectives. Lancet Oncol 2012;13:e383–e391. [DOI] [PubMed] [Google Scholar]
- 34.Schätti A, Zakova M, Meer D, et al. Experimental verification of motion mitigation of discrete proton spot scanning by re-scanning. Phys Med Biol 2013;58:8555–8572. [DOI] [PubMed] [Google Scholar]
- 35.Seco J, Robertson D, Trofimov A, et al. Breathing interplay effects during proton beam scanning: Simulation and statistical analysis. Phys Med Biol 2009;54:N283–N294. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi W, Mori S, Nakajima M, et al. Carbon-ion scanning lung treatment planning with respiratory-gated phase-controlled rescanning: Simulation study using 4-dimensional CT data. Radiat Oncol 2014;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan X, Zhang X, Li Y, et al. Impact of using different four-dimensional computed tomography data sets to design proton treatment plans for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2009;73:601–609. [DOI] [PubMed] [Google Scholar]
- 38.Tryggestad EJ, Beltran CJ, Funk RK, et al. 4D robustness of proton pencil beam scanning for esophageal cancer using a GPU-based Monte Carlo dose engine. Int J Particle Ther 2015;2:364–365. [Google Scholar]
- 39.Yu J, Zhang X, Liao L, et al. Motion-robust intensity-modulated proton therapy for distal esophageal cancer. Med Phys 2015;In press [DOI] [PubMed] [Google Scholar]
- 40.Shibuya S, Takase Y, Watanabe M, et al. Usefulness of proton irradiation therapy as preoperative measure for esophageal cancer. Dis Esoph 1989;2:99–104. [Google Scholar]
- 41.Mizumoto M, Sugahara S, Nakayama H, et al. Clinical results of proton-beam therapy for locoregionally advanced esophageal cancer. Strahlenther Onkol 2010;186:482–488. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa H, Hashimoto T, Moriwaki T, et al. Proton beam therapy combined with concurrent chemotherapy for esophageal cancer. Anticancer Res 2015;35:1757–1762. [PubMed] [Google Scholar]
- 43.Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:e345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallemeier CL, Chuong M, Merrell KW, et al. Impact of Neoadjuvant Proton vs. Photon Chemoradiotherapy on Post-Operative Outcomes in Patients with Esophageal Cancer Treated with Trimodality Therapy: A Multi-Institutional Analysis. Int J Particle Ther 2015;2:79–80. [Google Scholar]
- 46.Chuong M, Bhooshan N, Allen PK, et al. A Multi-Institutional Analysis of Acute Toxicity after Neoadjuvant Chemoradiation Using Photons or Protons in Trimodality Esophageal Cancer Patients. Int J Particle Ther 2015;2:88–89. [Google Scholar]
- 47.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167–1174. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z, Liao Z, Jin J, et al. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys 2005;61:656–664. [DOI] [PubMed] [Google Scholar]
- 49.He L, Allen PK, Potter A, et al. Re-evaluating the optimal radiation dose for definitive chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Oncol 2014;9:1398–1405. [DOI] [PubMed] [Google Scholar]
- 50.Welsh JW, Seyedin SN, Hofstetter W, et al. Prospective Phase 1/2 Clinical Trial: Evaluating Dose-Escalation for Esophageal Cancer. Int J Radiat Oncol Biol Phys 2015;93:S12–S13. [Google Scholar]
- 51.Chen J, Li D, Zhai T, et al. Phase 2 Study of Simultaneous Modulated Accelerated Radiation Therapy Combined With Chemotherapy for Esophageal Squamous Cell Carcinoma: Early Outcomes. Int J Radiat Oncol Biol Phys 2015;93:S13. [Google Scholar]
- 52.Shridhar R, Chuong M, Weber J, et al. Preoperative dose painting IMRT chemoradiation to 56 Gy in esophageal cancer doubles pathologic complete response rate without increasing toxicity. Int J Radiat Oncol Biol Phys 2012;84:S42. [Google Scholar]
- 53.Geh JI, Bond SJ, Bentzen SM, et al. Systematic overview of preoperative (neoadjuvant) chemoradiotherapy trials in oesophageal cancer: evidence of a radiation and chemotherapy dose response. Radiother Oncol 2006;78:236–244. [DOI] [PubMed] [Google Scholar]
- 54.Chargari C, Soria JC, Deutsch E. Controversies and challenges regarding the impact of radiation therapy on survival. Ann Oncol 2013;24:38–46. [DOI] [PubMed] [Google Scholar]
- 55.Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160–1168. [DOI] [PubMed] [Google Scholar]
- 56.Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310–2317. [DOI] [PubMed] [Google Scholar]
- 57.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 58.Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32: 385–391. [DOI] [PubMed] [Google Scholar]
- 59.Koyama S, Tsujii H, Yokota H, et al. Proton beam therapy for patients with esophageal carcinoma. Jpn J Clin Oncol 1994;24: 144–153. [PubMed] [Google Scholar]
- 60.Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys 2005;61:76–84. [DOI] [PubMed] [Google Scholar]
- 61.Mizumoto M, Sugahara S, Okumura T, et al. Hyperfractionated concomitant boost proton beam therapy for esophageal carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e601–606. [DOI] [PubMed] [Google Scholar]
- 62.Plastaras JP, Berman AT, Freedman GM. Special cases for proton beam radiotherapy: re-irradiation, lymphoma, and breast cancer. Semin Oncol 2014;41:807–819. [DOI] [PubMed] [Google Scholar]
- 63.Decision analytic modelling in the economic evaluation of health technologies. A consensus statement. Consensus Conference on Guidelines on Economic Modelling in Health Technology Assessment. Pharmacoeconomics 2000;17:443–444. [DOI] [PubMed] [Google Scholar]
- 64.Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med 2003; 163:1637–1641. [DOI] [PubMed] [Google Scholar]