Abstract
Background
Stavudine remains a component of combination antiretroviral therapy (ART) in resource‐constrained countries due to its relatively low cost despite the WHO recommendation for its phasing out as a strategy to reduce stavudine associated toxicities. Where stavudine is still in use, it is recommended at a dose lower than the standard dose in order to reduce stavudine related toxicity.
Objectives
To compare the safety and virologic efficacy of low dose versus high dose stavudine for treating HIV‐1 infection.
Search methods
The comprehensive search strategy developed by the Cochrane HIV/AIDS Review Group was used to identify randomised controlled trials that compared the use of low dose versus high dose stavudine. The last search was conducted in February 2014 and the searches covered the period 1996 to 2014.
Selection criteria
Randomised controlled trials comparing the use of low dose and high dose stavudine as part of ART combination therapy for treating adults.
Data collection and analysis
Two reviewers independently selected eligible trials, assessed methodological quality of the included studies and completed data extraction and analysis.
Main results
The search identified 3952 abstracts which were scanned for relevance. Three trials met the inclusion criteria (Milinkovic 2007; McComsey 2008; Sanchez‐Conde 2005). All three trials were conducted in developed countries, participants were ART experienced and all had sustained virologic suppression at baseline. A total of 157 participants were recruited to the trials. Sample sizes ranged from 24 to 92 and more than 79% of participants were male.The studies were at a high risk of selection, performance/detection and selective outcome reporting biases. Some baseline characteristics differed among the groups, including triglyceride levels in two studies and body mass index in one study. In light of variation in the design and follow‐up of the study results, no meta‐analysis was performed and the results of single studies are presented. There was no significant difference in virologic suppression in the included studies (Milinkovic 2007; McComsey 2008; Sanchez‐Conde 2005); Risk Ratio (RR) 1.09 (95% CI: 0.93 to 1.28), 0.94 (95% CI:0.59 to 1.50) and 1.03 (95% CI: 0.90 to 1.18) respectively. Symptomatic hyperlactatemia was seen in the high dose arm of the Milinkovic 2007 study; RR 0.21 (95% CI: 0.01 to 4.66), in no participants in the McComsey 2008 trial and not reported on in the Sanchez‐Conde 2005 trial. McComsey 2008 and Milinkovic 2007 demonstrated a reduction in bone mineral density (BMD), reduction in limb fat and an increase in triglycerides in the high dose arms. The studies did not indicate that any participants discontinued treatment due to adverse events.
Authors' conclusions
This systematic review identified only three small trials that evaluated virologic efficacy and safety of high dose versus low dose stavudine. All three trials were conducted in developed countries and none reported from developing countries yet stavudine remains a component of ART combination therapy in many developing countries. It was not possible to perform a meta‐analysis on these trails. Individual results from the trials were imprecise and have not identified a clear advantage in virologic efficacy or safety between low and high dose stavudine. Furthermore, enrolled participants were treatment experienced with sustained virologic suppression and so existing data cannot be generalized to settings where stavudine is currently used in ART naive patients with high viral loads. Stavudine dose reduction trials in ART naive patients, in developing countries where stavudine is still being used are warranted as the phasing out of stavudine that is recommended by WHO may not be immediately universally feasible.
Keywords: Female, Humans, Male, Anti‐HIV Agents, Anti‐HIV Agents/administration & dosage, Anti‐HIV Agents/adverse effects, Developing Countries, HIV Infections, HIV Infections/drug therapy, HIV Infections/virology, HIV‐1, Randomized Controlled Trials as Topic, Stavudine, Stavudine/administration & dosage, Stavudine/adverse effects, Viral Load, Viral Load/drug effects
Plain language summary
Low dose versus high dose stavudine for treating people with HIV infection
Stavudine has been the most widely used antiretroviral agent as part of the combination therapy for treating HIV‐1 infection in low‐income countries. The use of stavudine has been associated with complications of redistribution of fat in the body, abnormalities in insulin and lipids, lactic acidosis and nerve problems. Some of these complications may be life‐threatening. Strategies to avoid or reduce the risk of these complications have included using alternative drugs where available or using lower doses of the stavudine.
The comprehensive search strategy developed by the Cochrane HIV/AIDS Review Group was used to identify trials that compared the safety and efficacy in suppressing the viral load of low dose versus high dose stavudine in the context of treating HIV‐1 with combination antiretroviral therapy. The searches covered the period 1996 to 2014.The search identified 3952 trials and only three met the inclusion criteria, all the included trials were conducted in developed countries, the number of participants ranged from 24 to 92 and the majority were male. The efficacy of suppressing the viral load was found to be the same in all the trials whether high dose or low dose of stavudine was used. McComsey 2008 and Milinkovic 2007 demonstrated a reduction in bone mineral density (BMD), reduction in limb fat and an increase in triglycerides in the high dose arms. While there was no demonstration of a difference in efficacy of viral load suppression between high dose and low dose stavudine in the included trials, participants included in these trials were already treated with antiretroviral therapy and had suppressed viral load. The fact that participants already had suppression of the viral load and the studies were small, meant it would be difficult to demonstrate the differences in viral load suppression between the two groups. The studies did not indicate that any participants discontinued treatment due to adverse events.
This review identified only trials that tested the safety and efficacy in suppressing the viral load of low dose compared to high dose stavudine. These trials were small, conducted in developed countries and included participants with suppressed viral loads that had been on antiretroviral treatment for a long time. Individual results from the trials have not identified a clear advantage in viral load suppression or safety between low and high dose stavudine.Studies that evaluate the safety and efficacy in viral load suppression need to be conducted particularly in developing countries where stavudine is still being used and probably needed to either sustain treatment programs or where alternatives are limited.
Background
The HIV/AIDS epidemic is one of the worst catastrophes of our time. At the end of 2013 an estimated 35 million (33.2 ‐ 37.2 million) people were estimated to be living with HIV‐1 globally (UNAIDS 2014). Sub‐Saharan Africa is home to 10% of the world's population, yet more than 60% of all adults and children infected with HIV‐1 globally, live in this region. The introduction of antiretroviral therapy (ART) in 1996 revolutionalised the care of patients infected with HIV‐1 and drastically reduced the morbidity and mortality associated with HIV‐1 infection. When taken correctly, ART successfully decreases the HIV‐1 viral load, improves immunological function, delaying clinical events and death. WHO guidelines recommend initiation of combination ART in resource‐constrained countries with one non‐nucleoside reverse‐transcriptase inhibitor (NNRTI) and two nucleoside reverse transcriptase inhibitors (NRTI) as first line therapy while protease inhibitors are an option for inclusion in first‐line regimens in high‐income countries (Gilks 2006).
Access to ART is currently being scaled up in low to middle income countries with NRTIs forming the cornerstone of ART. NRTIs inhibit HIV‐1 reverse transcriptase, thus preventing HIV‐1 DNA formation. NRTIs interact in the same way with human mitochondrial DNA polymerase gamma, the enzyme responsible for mitochondrial DNA replication. It has been proposed that NRTI toxicities are caused by cellular mitochondrial DNA depletion and subsequent mitochondrial dysfunction (Brinkman 1998; Lewis 1995; Kakuda 2000; Brinkman 1999). The severity of NRTI‐associated toxicity depends on the degree of mitochondrial DNA depletion. The rank order of NRTI according to their ability to cause mitochondrial dysfunction in vitro is greatest with zalcitabine, followed in declining order by didanosine, stavudine and zidovudine. The least toxic agents are abacavir, lamivudine and tenofovir, which are all considered equal (Birkus 2002).
Toxicities associated with NRTIs include hepatic steatosis, lactic acidosis, metabolic complications and fat redistribution or HIV‐associated lipodystrophy syndrome (HALS), which might lead to increased risk in morbidity especially cardiovascular disease. In particular, stavudine has been consistently the NRTI most associated with lactic acidosis, peripheral neuropathy and lipoatrophy (Birkus 2002). In a study that investigated the incidence of diabetes among HIV‐infected patients in the Adverse Events of Anti‐HIV Drugs (D:A:D) cohort, stavudine was significantly associated with new‐onset diabetes (De Wit 2008)
At the beginning of combination ART scale up in resource‐limited countries, the WHO recommended stavudine as one of the NRTI agents used for first‐line treatment, owing to its relatively low cost. Stavudine related toxicities necessitated consideration of alternative strategies. A meta‐analysis conducted in 2007 showed that there was no significant difference in virologic efficacy associated with the use of a low dose stavudine compared with the standard dose of 40 mg BD for patients weighing 60 kg or more and 30 mg BD for patients weighing less than 60 kg (Hill 2007). Furthermore, the safety profile was reportedly better at the lower dosage than the standard dosage. Findings of this meta‐analysis led to the modification of the WHO treatment guidelines for adults and stavudine use was recommended at lower than standard dosages (WHO 2007). The non‐thymidine analogue NRTI tenofovir (TDF) or zidovudine (AZT) was subsequently recommended as first‐line therapy replacing stavudine where resources permit as an additional strategy to reduce stavudine associated complications (WHO 2010).
Stavudine remains an option where TDF or AZT are contraindicated or unavailable. Furthermore, the AZT or TDF options are more expensive, require more laboratory monitoring and have higher initial discontinuation rates (Gallant 2004; Gallant 2006). It remains questionable whether stavudine should be completely phased out of the armamentarium for first‐line management of HIV‐1 infection, particularly in resource‐constrained settings where the cost of drugs and laboratory monitoring dictate sustainability of treatment programs. The WHO currently recommends stavudine at a dose of 30 mg BD regardless of weight as opposed to the previous standard dose of 40 mg BD or greater for patients with a body weight of 60 kg or more and 30 mg BD dosage for those with a body weight of less than 60 kg.
The aim of this systematic review was to apply the Cochrane Collaboration Methodology to evaluate the evidence supporting the effects of stavudine at dosages that are lower than standard dosage and the applicability of available data in resource‐limited settings where stavudine use plays a critical role. The protocol was published in the Cochrane Database of Systematic Reviews (Magula 2008).
Objectives
The objective was to compare the safety and virologic efficacy of low dose stavudine (30 mg BD or less for patients with a body weight of 60 kg or more and less than 30 mg BD for patients with a body weight of less than 60 kg) versus high dose stavudine (40 mg BD or greater for patients with a body weight of 60 kg or more and 30 mg BD dosage for patients with a body weight of less than 60 kg) for treating HIV‐1 infection in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised, controlled blinded and non‐blinded clinical trials
Types of participants
HIV infected adults treated with combination ART
Types of interventions
Low dose stavudine versus high dose stavudine
Low dose stavudine is described as the stavudine dosage of 30 mg BD or less for patients with a body weight of 60 kg or more and less than 30 mg BD for patients with a body weight of less than 60 kg
High dose stavudine is described as the standard dosage of 40 mg BD or greater for patients with a body weight of 60 kg or more and 30 mg BD dosage for those with a body weight of less than 60 kg
Types of outcome measures
Primary Outcomes:
Viral load < 200 copies/ml
Major side‐effects leading to drug discontinuation such as lactic acidosis, pancreatitis or severe peripheral neuropathy
Secondary Outcomes:
Less severe side‐effects e.g. mild peripheral neuropathy, lipodystrophy, rash, etc.
Search methods for identification of studies
The HIV/AIDS Cochrane Review Group assisted with searching for studies that compared low dose stavudine versus high dose stavudine in the treatment of HIV‐1 infection. The search strategy was based on that of the HIV/AIDS Cochrane Review Group.
A search was undertaken using Medical Subject Headings (MeSH) and free text terms. All languages were included. The strategy was combined with the search strategy for RCTs as recommended by The Cochrane Collaboration. Studies conducted before and after Food and Drug Administration approval of stavudine were included. Each study was analysed in terms of the efficacy and safety outcomes.
Electronic searches
The following electronic databases were searched for randomised controlled trials: MEDLINE (Appendix 1); AIDSearch (Appendix 2); EMBASE and CINAHL (Appendix 3). The Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effectiveness, Gateway and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library were also searched (Appendix 4). Searches covering the time period 1996 to 2008 were conducted on 10 September 2008. The searches were repeated on 5 June 2009 for the period 2008 to 2009, on 23 November 2012 for the period 2009 to 2012 and on 5 February 2014 for the period 2012‐2014.
Searching other resources
The abstracts of relevant conferences, including the International AIDS Conference and the Conference on Retroviruses and Opportunistic Infections were also reviewed. The reference lists of all review articles and primary articles identified were also searched. The search strategy was iterative, in that references of included studies were searched for additional references. Contact was also made with investigators in the field for additional studies.
Data collection and analysis
Our methodology for data collection and analysis was based on the guidance of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008). Two authors independently selected potentially relevant studies by scanning the titles, abstracts, and descriptor terms of all downloaded material from the electronic searches. Irrelevant reports were discarded, and the full articles were obtained for all potentially relevant or uncertain reports.
Selection of studies
Two authors independently applied the inclusion criteria, using an eligibility form specific to this review. There were no disagreements between the two authors that required a third arbiter. Studies were reviewed for relevance, based on study design, types of participants, exposures and outcome measures. Authors were contacted to provide further clarification of data where necessary and possible.
Data extraction and management
Two authors independently extracted data using a standardized data extraction form. The following characteristics were extracted from each included study:
Administrative details: identification; author(s); published or unpublished; year of publication; number of studies included in paper; year in which study was conducted; details of other relevant papers cited.
Details of study: study design; type, duration and completeness of follow‐up; country and location of the study (developed versus developing country).
Characteristics of participants: prior exposure to antiretroviral therapy (type and duration); disease stage; baseline CD4 count; baseline HIV‐1 RNA level.
Details of intervention: types and doses of drugs used; duration of therapy; adverse events (method of surveillance for adverse events and type: minor or severe) and adherence measures (if reported).
Outcome data: death or occurrence of new event (death or AIDS defining event, lipodystrophy, lactic acidosis, peripheral neuropathy); subsequent and final CD4 counts and HIV‐1 RNA levels dichotomised as having reached, or as not having reached, a non‐detectable level, i.e. less than 200 copies/ml or in its continuous form.
Assessment of risk of bias in included studies
We used the Cochrane Collaboration tool (Higgins 2008) for assessing the risk of bias for each individual study, and present results in summary tables. For randomised trials, the Cochrane tool assesses risk of bias in individual studies across six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential biases.
Assessment of reporting biases
We minimised the potential for reporting bias by using comprehensive search strategies, which included searching scientific literature from a wide range of databases, published or unpublished, written in any language.
Data synthesis
For dichotomous data the overall measure of effect was calculated as a relative risk, with 95% confidence intervals, using the random effects model. We used an intention‐to‐treat analysis (ITT). Our definition of ITT includes analysing participants in their originally randomised groups, and also uses the initial number of randomised participants per group as the denominator. Continuous data was reported in medians and the duration of follow‐up and outcomes measured were different for each of the studies, the data is therefore reported separately for each of the studies.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
The search conducted for period 1996 to 2012 identified 3952 abstracts which were then scanned for relevance Figure 1. Seven studies were selected for application of eligibility criteria. Three studies met the inclusion criteria (Milinkovic 2007; McComsey 2008; Sanchez‐Conde 2005). The Sanchez‐Conde 2005 trial was conducted in Madrid, Spain and it enrolled 92 participants. The Milinkovic 2007 trial was conducted in Barcelona, Spain. It had three arms that compared high dose versus low dose stavudine versus switching to tenofovir. Thirty‐one participants were enrolled in the stavudine dose comparison arms. The McComsey 2008 trial was also a small trial (N=24) conducted in the United States of America. All three trials included participants who were receiving antiretroviral therapy with suppressed HIV‐1 viral loads. The median baseline CD4 count in the Milinkovic 2007 trial was 569 cells/mm3 (IQR 417 to 811) and in the McComsey 2008 trial the median CD4 cell count was 558 cells/mm3 (IQR 207 to 1698). The mean CD4 count in the Sanchez‐Conde 2005 trial was more than 670 cells/mm3. Participants were predominantly male in all three trials; over 90% in the Milinkovic 2007, over 80% in the Sanchez‐Conde 2005 and 79% in the McComsey 2008 trials. The median duration on stavudine therapy was 4.5 years. The baseline characteristics differed between the two arms of the McComsey 2008 trial with a higher median triglyceride level in the low dose arm of 175mg/dL (IQR: 52 to 777) and a median triglyceride level of 113mg/dL (IQR: 63 to 181) in the high dose arm, p=0.02. The body mass index was also higher in the low dose arm at median 26.6(IQR: 22 to 35) and 23 (IQR: 20 to 27) in the high dose arm, p=0.003. Lean body mass was higher in the low dose arm at median 62,051g (IQR: 39,754 to 74,637) and 51,703 (IQR: 41,469 to 64,277) in the high dose arm, p=0.04. There was no difference in the baseline bone mineral density (BMD) or lipoatrophy score with a total median 1.18g/cm2 (0.99 to 1.36) and 5 (0 to 12) respectively.
1.
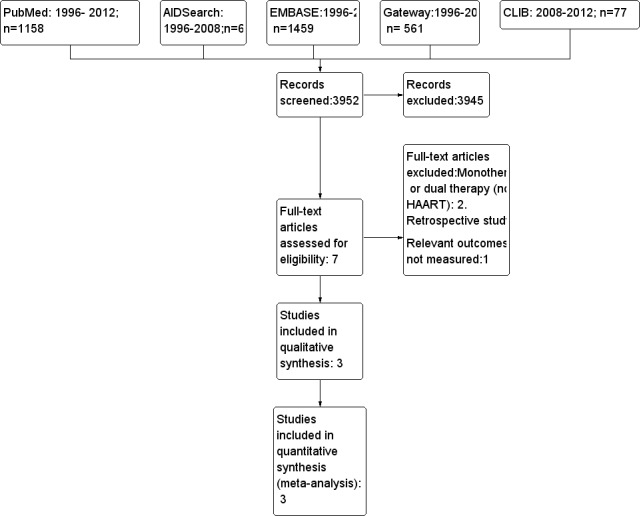
Study flow prisma diagram.
Participants of the Milinkovic 2007 trial were on ART for a median duration of more than 6 years. The low dose and the high dose arms differed in their baseline triglyceride level with a median 207 mg/dL (IQR: 124 to 229) in the low dose arm and 141 mg/dL (IQR: 122 to 313) in the high dose arm. There was no difference in the baseline BMD between the two arms. The mean duration on stavudine in the Sanchez‐Conde 2005 trial was 12 months. The mean baseline triglyceride level in this trial was 158 mg/mL in the low dose arm and 161 mg/mL in the high‐dose arm.
In two of the trials that were excluded, Pollar 1997; Lange 1998, the participants were not treated with combination ART but with monotherapy or dual therapy (Lange 1998) or dual therapy only (Pollar 1997). One study, Makinson 2008, was a systematic review, Wolf 2004; Mashitisho 2013 were retrospective studies. Menezes 2012 compared the early effects of low dose and standard dose stavudine with tenofovir on adipocyte mitochondrial DNA number, gene expression and metabolic parameters in black South African HIV‐1 infected patients. This trial was excluded from the current review due to the short follow‐up of four weeks that would not have allowed for evaluation of the primary and secondary outcomes of this review. Pujades‐Rodriquez 2011 was a large study that compared the incidence and timing of toxicity associated with the use of a low dose stavudine (30 mg) compared with high dose stavudine (40 mg). The study was excluded from the review because it was an observational study. The study Fabian 2008 was identified through communication with authors for additional unidentified studies but was excluded because it was a retrospective study.
Risk of bias in included studies
2.
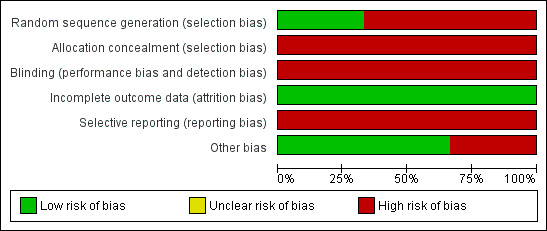
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
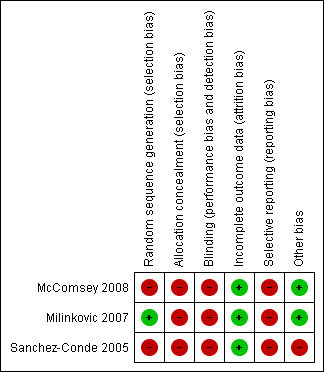
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
GENERATION OF ALLOCATION SEQUENCE
The Milinkovic 2007 trial used a randomisation list that was computer generated. Participants were stratified according to current treatment (PI or non‐NRTI). The method used for generation of allocation sequence is thought to be adequate. In the McComsey 2008 trial the randomisation schedule was prepared by the data manager who was not linked to patient care. The randomisation is described as 3:2 but the method used to generate allocation sequence is not described in detail. The method used to generate allocation sequence in the Sanchez‐Conde 2005 is not described.
ALLOCATION CONCEALMENT
The Sanchez‐Conde 2005; Milinkovic 2007 and McComsey 2008 trials did not describe details of allocation concealment and since the trials were open‐labeled, there may not have been sufficient concealment of allocation.
BLINDING
There was no blinding in the Milinkovic 2007 and Sanchez‐Conde 2005 for both participants and providers as this was an open‐labeled trial. It is unclear if the outcome assessors were blinded. The McComsey 2008 was also open‐labeled with no blinding for participants and providers, however, the outcome assessors were blinded. The DEXA scans were read centrally by a reader who was blinded to the timing of the DEXA scan and participants' characteristics. The laboratory personnel was also blinded to sample characteristics.
INCOMPLETE OUTCOME DATA
The Sanchez‐Conde 2005 randomised 45 participants to continue with 40 mg twice a day stavudine and 47 participants to reduce to 30 mg twice a day. Forty‐one (45%) did not complete the 12‐month follow‐up period. Six participants were lost to follow‐up, eight withdrew voluntarily, twelve changed antiretroviral therapy for reasons other than virologic failure and six were discontinued because they were started on treatment for hepatitis C infection. Nine participants were discontinued because they developed virologic failure. The specific time points for the development of virologic failure is not reported.
The Milinkovic 2007 randomised 22 participants to continue high dose stavudine and 19 to reduce to low dose. One participant in the high dose arm was excluded due to symptomatic hyperlactataemia at baseline. Altogether three participants were lost to follow‐up, two in the low dose arm and one in the high dose arm. Analysis was by intention to treat using the policy of last‐date‐carried‐forward analysis adopted for patients with missing data for the remaining follow‐up period.
Of the 24 participants enrolled in the McComsey 2008 trial, 21 (88%) completed the trial. Two participants in the low dose arm and one in the high dose arm did not complete the trial. One participant in the low dose arm withdrew at week four because of scheduling conflict and the second participant in the low dose arm died of an overdose of illegal drugs and respiratory failure that was unrelated to study participation at week 37. The withdrawal of the one participant in the high dose arm was because of incarceration. It is unclear if intention‐to‐treat analysis was used.
Effects of interventions
The three trials that were eligible for inclusion differed in the primary outcomes they measured and duration of follow‐up. The cut‐off for viral load suppression is reported at different levels in each of the studies with the McComsey 2008 trial using a cut‐off of 50 copies/mL, the Sanchez‐Conde 2005 also at 50 copies/mL and Milinkovic 2007 using a cut‐off of 200 copies/mL. The proportion of participants achieving viral load suppression below 200 copies/mL is shown in Analysis 1.1Figure 4. The proportion of participants developing symptomatic hyperlactatemia are shown in Analysis 2.1Figure 5. The rest of the outcomes reported in the two trials McComsey 2008; Milinkovic 2007 on lipids, limb fat mass, fat mitochondrial DNA, glucose, lactate and bone mineral density are all reported in median (IQR) in both studies and are shown in Table 1.
1.1. Analysis.

Comparison 1 Low dose versus high dose stavudine, Outcome 1 Proportion of participants with HIV RNA < 200 copies/ml.
4.
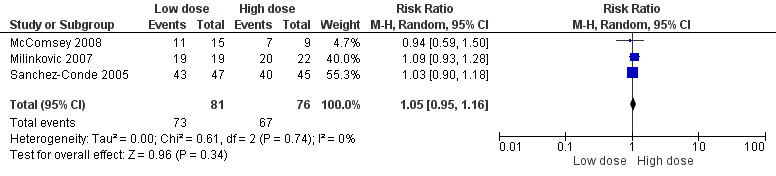
Forest plot of comparison: 2 Low dose vs high dose stavudine, outcome: 2.1 Proportion of participants with HIV‐1 RNA < 200 copies/ml.
2.1. Analysis.

Comparison 2 Low dose versus high dose stavudine, Outcome 1 Proportion of participants developing symptomatic hyperlactatemia.
5.

Forest plot of comparison: 3 Low dose versus high dose stavudine, outcome: 3.1 Proportion of participants developing symptomatic hyperlactatemia.
1. Secondary Outcomes.
| McComsey 2008 | Milinkovic 2007 | Sanchez‐Conde 2005 | |||||||
| Outcome Measure |
Low dose | High‐dose | P value | Low‐dose | High dose | p‐value | Low dose | High dose | P value |
| Limb fat, g |
189 (‐2256 to 3173) | ‐194(‐1160 to 5098) | 0.45 | 527 (‐343 to 694) | ‐182 (‐469 to‐50) | 0.12 | ND | ND | |
| Limb fat % | 6.9 (‐23.8 to 34.4) | ‐5.0 (‐39.8 to 92.4) | 0.23 | ND | ND | ND | ND | ||
| Trunk fat, g |
804 (‐4356 to 2898) | ‐50 (‐1560 to 7233) | 0.80 | 937.5 (‐336 to 1316) | ‐145 (‐461 to 161) | ND | ND | ||
| Total lean body mass,g | ‐155(‐4917 to 6261) | ‐187(‐3176 to 3911) | 0.45 | 394 (‐577 to 1032) | 816 (‐683 to 1220) | ND | ND | ||
| Triglyceride, mg/dL |
‐38 (‐606 to 82) | 5 (‐74 to 64) | 0.16 | ‐16.5 (‐62 to 18.5) | 11 (‐21 to 51) | ND | ND | ||
| Total cholesterol,mg/dL |
‐1 (‐59 to 24) | ‐11.5 (‐44 to 11) | 0.56 | 1.5 (‐7 to 22) | ‐3 (‐7 to 22) | ND | ND | ||
| HDL Cholesterol,mg/dL |
6 (‐11 to 15) | 0 (‐35 to 16) | 0.15 | 1 (‐5 to 6) | ‐2 (‐7 to 3) | ND | ND | ||
| Fat MtDNA Copes/Cell |
40 (‐49 to 261) | 39 (‐97 to 115) | 0.36 | ND | ND | ND | ND | ||
| PMBC mtDNA Copies/Cell | 1.5 (9 to 121) | ‐2.5 (‐46 to 8) | 0.22 | ND | ND | ND | ND | ||
| Total Lean Mass Change,g | 394 (‐577 to 1.032) | 816 (‐683 to 1220) | 0.99 | ND | ND | ||||
| Glucose | ‐4 (‐2.3 to 51 ) | 3 (‐51 to 18) | 0.91 | ‐1.5 (‐11 to 4) | 2.1 ( ‐7 to 9) | ND | ND | ||
| Lactate | 0 (‐3 to 4) | 3 (‐1 to 6) | ND | ND | |||||
| BMD g/cm2 |
0.0 (‐1.2 to 4) | ‐1.7 (‐6.3 to 0.8) | 0.003 | 0.007(0.003 to 0.012) | ‐0.001(‐0.007 to 0.0100) | ND | ND | ||
ND= Not Done
This trial compared stavudine at the standard dose of 40 mg twice a day with a reduced dose of 30 mg twice a day. Participants that were enrolled were receiving a stable ART regimen that included stavudine at 40 mg twice a day. Participants were randomised to either continue the standard dose or change to the reduced dose of 30 mg twice a day. At entry, all participants had a viral load of <200copies/ml for at least six months. Participants were followed up for 24 weeks.
PRIMARY OUTCOMES
Viral load suppression:
Two of 22 participants in the high dose arm and none of the 19 in the low dose arm experienced viral load rebound after a 24 week follow‐up. The Risk Ratio (RR) for suppressing viral load was 1.09 (95% CI: 0.93 to 1.28) (Table 1). The lower limit cut‐off for the HIV‐1 RNA was 200 copies/ml.
There were no cases of lactic acidosis and other major side‐effects leading to drug discontinuation such as pancreatitis or severe peripheral neuropathy were not reported.
SECONDARY OUTCOMES (Table 1)
Symptomatic hyperlactatemia
The RR for developing symptomatic hyperlactatemia was 0.23 (95% CI 0.01 to 4.51) with two of the 22 participants in the high dose arm developing symptomatic hyperlactatemia at lactate levels >22 mg/dL.
Body composition
There was an increase in the median limb fat from baseline to 24 weeks of follow‐up in the low dose arm of 527g (95% CI: ‐343 to 694) and a reduction in the high dose arm of ‐182g (95% CI: ‐469 to ‐50). The difference in limb fat change from baseline to 24 weeks follow‐up between the low dose and the high dose arm was not statistically significant, p=0.12. There was an increase in the median trunk fat from baseline to 24 weeks follow‐up in the low dose arm of 937.5g (95% CI: ‐336 to 1316) and a reduction in the high dose arm of ‐145g (95% CI: ‐461 to 161). There was an increase in the median total lean body mass in both the low dose arm 394g (95% CI: ‐577 to 1032) from baseline to 24 weeks follow‐up and in the high dose arm 816g (95% CI: ‐683 to 1220).
Metabolic changes
There was a reduction in triglycerides in the low dose arm at 24 weeks with a median change of ‐16.5mg/dL (IQR: ‐62 to 18.5) and an increase of 11 mg/dL (IQR: ‐21 to 51) in the high dose arm. The change in total cholesterol was median 1.5 mg/dL (IQR: ‐7 to 22) in the low dose arm and ‐3 mg/dL (IQR: ‐7 to 22) in the high dose arm. There was a modest increase in the median HDL cholesterol of 1 mg/dL (IQR: ‐5 to 6) in the low dose arm and a reduction of ‐2 mg/dL (IQR: ‐7 to 3) in the high dose arm. The median change in BMD in the low dose arm was 0.007g/cm2 (IQR: 0.003 to 0.012) and ‐0.001g/cm2 (‐0.007 to 0.010) in the high dose arm.
This trial randomised participants in a ratio of 3:2 to either continue the standard dose of stavudine at 40 mg twice a day for weight ≥ 60 kg and 30 mg twice a day for weight < 60 kg or reduced stavudine to 20 mg twice a day for weight ≥ 60 kg or 15 mg for weight < 60 kg. Participants enrolled in the study were on stable ART containing stavudine for 24 weeks or more. All had viral load suppression of <50 copies/ml or branched DNA < 75 copies/ml. Participants were followed up for 48 weeks.
PRIMARY OUTCOME
Viral load suppression
Eleven of 15 participants in the low dose arm and seven of nine participants in the high dose arm had viral load suppression. The RR for developing viral load suppression was 0.94 (95% CI: 0.59 to 1.50) (Analysis 1.1). The lower limit cut‐off for HIV‐1 RNA was 50 copies/ml.
There were no major side‐effects that led to discontinuation of therapy.
SECONDARY OUTCOMES (Table 1)
Symptomatic hyperlactataemia
No participants had symptomatic hyperlactatemia in both the low dose and high dose arms.
Limb fat changes:
There was an increase in limb fat in the low dose arm with median change at 48 weeks of 189g( IQR: ‐2256 to 3173) and reduction in limb fat in the high dose arm was ‐194 (IQR: ‐160 to 5098); p= 0.45. The limb fat percentage gain in the low dose arm was 6.0% (IQR: ‐23.8 to 34.4) with a loss in the high dose arm of ‐5.0% (IQR: ‐39.8 to 92.4). Consistent with the limb fat changes, there was a trunk fat gain at 48 weeks in the low dose arm of 804g (IQR: ‐4356 to 2898) and a reduction in the high dose arm of ‐50g(IQR: ‐1560 to 7233); p=0.80. A loss in total lean body mass was seen in both arms with a median change of ‐155g (IQR: ‐4917 to 6261) in the low dose arm and ‐187g (‐3176 to 3911) in the high dose arm, p=0.45.
Metabolic changes:
There was a reduction in triglycerides in the low dose arm at 48 weeks with a median change of ‐38 mg/dL (IQR: ‐606 to 82) and an increase in the high dose arm of 5 mg/dL (IQR: ‐74 to 64); p=0.16. There was a reduction in total cholesterol in both arms but more in the high dose arm with median change in the high dose arm of ‐11.5 mg/dL (IQR: ‐44 to 11) and the low dose arm of ‐1 mg/dL (IQR: ‐59 to 24); p=0.56. There was no change in BMD from baseline in the low dose arm but a median reduction in BMD in the high dose arm of ‐1.7g/cm2 (‐6.3 to 0.8), p=0.003.
This trial randomised half of the participants to standard dose stavudine, 40 mg twice a day and the other half to reduce dosage from 40 mg twice a day to 30 mg twice a day. All participants were on a stavudine containing regimen and had a suppressed viral load with plasma HIV‐1 RNA < 50 copies/mL over at least the previous three months. The mean time on stavudine prior to enrolment was 12 months. Participants were followed up for 12 months.
PRIMARY OUTCOME
Viral load suppression
Forty‐three of 47 participants in the low dose arm and 40 of 45 participants in the high dose arm had viral load suppression. The RR for developing viral load suppression was 1.03 (95% CI: 0.90‐1.18) Analysis 1.1. Fourty‐one participants did not complete the 12 month follow‐up.
Since the drop out rate in this trial was very high, a number of case scenarios were considered as possible outcomes. The first scenario considered was a best case scenario for the low dose arm where in all the participants that dropped out had virologic suppression. The RR for this scenario was 1.96 (95% CI: 1.42‐2.71) Analysis 1.2Figure 6. In the low dose worst case scenario, where all those that dropped out did not have virologic suppression, the RR was 0.72 (95% CI: 0.57‐0.91) Analysis 1.3.
1.2. Analysis.
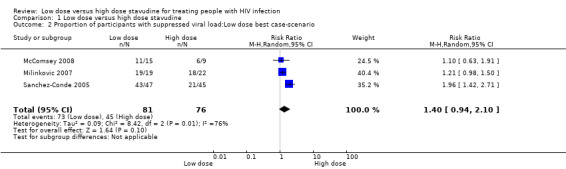
Comparison 1 Low dose versus high dose stavudine, Outcome 2 Proportion of participants with suppressed viral load:Low dose best case‐scenario.
6.
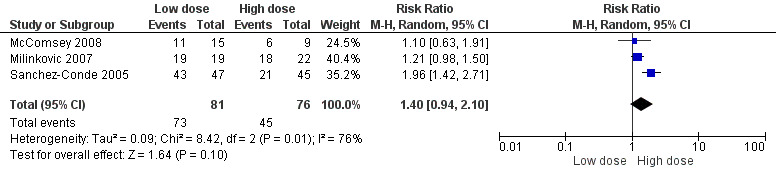
Forest plot of comparison: 1 Low dose versus high dose stavudine, outcome: 1.2 Low dose best case‐scenario.
1.3. Analysis.
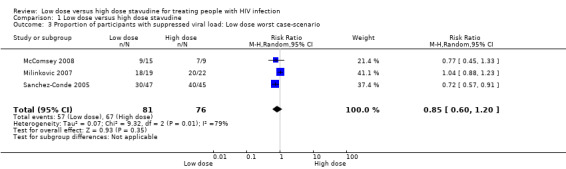
Comparison 1 Low dose versus high dose stavudine, Outcome 3 Proportion of participants with suppressed viral load: Low dose worst case‐scenario.
SECONDARY OUTCOMES
No data is reported on symptomatic hyperlactatemia, limb fat and metabolic changes.
Discussion
Summary of main results
This review evaluated the safety and virologic efficacy of the use of low dose compared with high dose stavudine as part of ART. The review identified only three randomised controlled trials that tested the safety and virologic efficacy of low dose compared with high dose stavudine. The most significant finding of this review is that in all three trials that met criteria for inclusion (Sanchez‐Conde 2005; Milinkovic 2007; McComsey 2008), participants were all ART experienced and had sustained virologic suppression at the time of enrolment. Since these studies were small and participants already virologically suppressed, this meant finding an outcome of virologic efficacy was highly unlikely, as it would be for any primary outcome. The duration of follow‐up was different in each of the trials, however, there was no significant difference in virologic suppression between the low dose and high dose arms at the end of follow‐up for each of the trials. None of the three trials reported the CD4 cell count outcomes at the end of follow‐up.
There were no differences in lactic acidosis between the low dose and high dose stavudine arms and no reported major side‐effects that led to discontinuation of treatment in all three trials. There was no associated significant changes in body fat composition. Both McComsey 2008 and Milinkovic 2007 trials showed a reduction in BMD in the high dose arms. Total lean body mass increased in both arms of Milinkovic 2007 and decreased in both arms of McComsey 2008. In the Milinkovic 2007 and McComsey 2008 there was an increase in limb fat in both the low dose arms and a reduction in both high dose arms, however the changes were not statistically significant. There was also an increase in triglycerides for the high dose arms of both trials. In theses trials, high dose stavudine was associated with a reduction in BMD, reduction in limb fat and an increase in triglycerides.
There was a high drop out rate in the Sanchez‐Conde 2005 trial but no significant difference in viral load suppression and outcomes of metabolic changes were not reported on.
Overall completeness and applicability of evidence
Metabolic toxicities associated with the use of stavudine have led to investigations to reduce these toxicities including reducing the dose of stavudine or switching to less toxic non‐thymidine analogues such as tenofovir as in the trials included in this review (Sanchez‐Conde 2005; Milinkovic 2007; McComsey 2008). The use of stavudine remains a cornerstone of ART in many resource‐constrained countries. Stavudine is an alternative agent in the first line therapy in many patients with contra‐indications for use of tenofovir or AZT, where monitoring for tenofovir or AZT is not available or where stocks of these preferred agents are in short supply. Although the WHO now recommends the phasing out of stavudine as the main strategy of reducing stavudine related toxicities, this strategy may not be feasible for the majority of countries with limited resources due to high costs. Phasing out completely of stavudine, an agent that is relatively inexpensive, may impact negatively on sustainability of antiretroviral programs.
This review found that the clinical trials that have been conducted on stavudine dose reduction were performed in developed countries, the majority of participants were male and all were ART experienced with suppressed viral load. Evidence is most needed in resource‐constrained countries as stavudine is no longer used at large scale in resource‐rich countries. However, the fact that these studies were conducted in patients who were ART experienced with suppressed viral load, makes the data not to be applicable to countries where stavudine is used in ART naive patients who do not have virologic suppression. Furthermore, sub‐Saharan Africa now accounts for 67% of all people living with HIV‐1 infection, with 60% of those living with the infection being women UNAIDS 2010, the majority of the included participants are men and do not reflect background demographics in resource constrained countries.
A multi‐site randomized controlled trial that is aimed at demonstrating the non‐inferiority of low dose stavudine compared with tenofovir when each is combined with lamivudine and efavirenz Venter 2013 has drawn much debate between researchers on the one hand who believe this investigation is warranted and researchers, HIV activists and people living with HIV on the other hand who believe that stavudine should not be investigated.Andrieux‐Meyer 2012; Venter 2012 At the heart of the debate is the stavudine related toxicity that has led the WHO to recommend phasing out of stavudine use altogether. The investigators of the study argue for dose optimization to reduce stavudine related toxicity so that stavudine can be kept as an alternative agent for cost‐effective purposes or where options are limited. The opposition argues that such an investigation should not be carried out as stavudine is more toxic than the currently recommended standard of care with tenofovir. The study is being conducted in India, South Africa and Uganda and recruitment is anticipated to be concluded at the end of 2013 with results at the beginning of 2016. The stavudine dose of 20 mg BD chosen for this investigation is lower than the dose recommended in the WHO and South African National antiretroviral treatment guidelines.WHO 2010; DOH 2013 The study is funded by the Bill and Melinda Gates Foundation.
Quality of evidence and potential biases in the review
The trials that have been conducted comparing the effectiveness and safety of low dose and high dose stavudine have been small trials, approximately 80% of participants were male. The methods of sequence generation were well described for Milinkovic 2007 trial, not described in detail for the McComsey 2008 trial and not described at all for the Sanchez‐Conde 2005 trial. Since the included participants were already on ART, the randomisation was based on reducing or maintaining the standard dose. Providers and participants were not blinded and it is likely that there was no allocation concealment. Using the GRADE criteria it is felt the overall quality of evidence in the three studies included in this review is low, due to limitations in the trials as described above and in the characteristics of studies table.
Agreements and disagreements with other reviews and studies
Findings of this review are in agreement with those of a previous one (Hill 2007) where virologic suppression was found not to be significantly different whether low dose or high dose stavudine was used, however the two reviews differ significantly on included studies. The current review has included three randomised controlled trials whereas Hill 2007 included fifteen studies. Hill 2007 included nine clinical trials (Browne 1993; Petersen 1995; Pollard 1999; Anderson 1995; Ruxrungtham 2000; Slangphoe 2004; Ribera 2005; Milinkovic 2007; Sanchez‐Conde 2005), and six observational cohort studies (Koegl 2003; Urbina A 2005; Hanvanich 2003; Delpierre 2005; Armitage 1994; Cross 2002). Observational cohort studies and clinical trials not conducted in the context of ART were excluded from this review.
A significant reduction in BMD was found in the high dose arm of the McComsey 2008 but this was not shown in the Milinkovic 2007. There were no differences in lactic acidosis and other major side‐effects such as pancreatitis and peripheral neuropathy were not reported on in the Milinkovic 2007; Sanchez‐Conde 2005 trials. McComsey 2008 reported that there were no major side‐effects that led to discontinuation of therapy. While complications such as neuropathy and lactic acidosis were not seen, there was reduction in BMD, reduction in limb fat and an increase in triglycerides in the high dose arms. These findings are different from reports in Africa where low rates of lipid abnormalities (Buchacz 2008), high rates of lactic acidosis (Geddes 2006), and neuropathy (Maritz 2010) have been reported. It may be that Africans metabolize drugs differently as shown by Wang 2006, where they found significantly higher steady state levels of efavirenz in association with CYP2B6*16 among Africans compared to Swedes and Turks.
The WHO Guidelines Development Group made an addendum (WHO 2007) to the 2006 WHO guidelines on antiretroviral therapy for HIV‐1 infection in adults and adolescents following evaluation of evidence from a systematic review of nine randomised trials, six observational cohort studies (Hill 2007) and other complementary studies (Wolf 2004; Sanchez‐Conde 2005). The new recommendation suggests initiation of stavudine at a lower dose of 30 mg twice a day regardless of body weight in ART naive patients. The WHO has further recommended the phasing out of stavudine as a strategy to reduce stavudine related toxicitiesWHO 2010, however this recommendation is not universally feasible due to the higher cost of alternative agents and the pressing need to expand ART programs.
Authors' conclusions
Implications for practice.
The use of stavudine has been linked to severe metabolic complications and fat redistribution or HIV‐associated lipodystrophy syndrome (HALS), hence the strategy to reduce the dose of stavudine in an attempt to lower adverse effects while maintaining virologic efficacy. While individual trial results have not identified clear advantages in virologic efficacy and lactic acidosis of either low dose or high dose arms, these trials were conducted in ART experienced patients with sustained viral load suppression. It is not known whether the use of low dose stavudine could contribute to treatment failure in patients who are treated with low dose stavudine as first line treatment in resource constrained countries where alternatives to stavudine may be unavailable or in short supply.
Implications for research.
Only three trials that evaluated the efficacy and safety of high dose versus low dose stavudine in the setting of combination ART were identified. All three trials were conducted in developed countries, in predominantly males. All enrolled patients were ART experienced for many years and had sustained virologic suppression. The WHO Guidelines Development Group revised the initiation dose of stavudine in ART naive patients to the lower dose of 30 mg BD, regardless of body weight, based on available evidence (Hill 2007; Sanchez‐Conde 2005; Wolf 2004), and subsequently recommended the phasing out of stavudine. This review shows that evidence for virologic efficacy at low dose stavudine in ART naive patients in low‐income countries, where stavudine is still being used, and required to sustain treatment programs is lacking. Therefore dose reduction studies in ART naive patients may be warranted if stavudine continues to be used, as the phasing out of stavudine may not be universally feasible. The impact of stavudine dose reduction on treatment failure in ART naive patients is unknown. Randomized controlled trials investigating the efficacy and safety of low dose stavudine in ART naive patients are needed if stavudine continues to be used as it is presently. While the activism against investigation of stavudine is acknowledged, the reality is that stavudine continues to be used, including in ART naive patients, where there are no alternatives, at doses for which evidence is sparse. Given the criticism that has been attracted by the clinical trial that is currently ongoing, investigating low dose stavudine versus tenofovir, Venter 2013 it is unlikely that a clinical trial that compares low versus high dose stavudine in the future would be acceptable to some researchers and people affected by HIV. Therefore trials investigating the safety and efficacy of low dose stavudine will require much innovation including education of people affected by HIV.
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 11 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We wish to thank the SACC HIV/AIDS Review Group: Nandi Siegfried, Joy Oliver, Elizabeth Pienaar, Babalwa Zani, Tamara Kredo for mentorship and their constant support including conduct of the searches; Mr Fundile Habana and Sr Yolandie Kriel for administrative support; Andrew Hill for his contribution to protocol development; Prof AA Motala and Prof UG Lalloo for mentorship.
Appendices
Appendix 1. MEDLINE search strategy
Database: PubMed 1996 ‐ 2008
Date: 29 August 2008
| Search | Most Recent Queries | Time | Result |
| #7 | Search #3 AND #4 AND #5 Limits: Publication Date from 1996/01/01 to 2008/08/29 | 06:20:20 | 852 |
| #6 | Search #3 AND #4 AND #5 Limits: Publication Date from 1996/01/01 to 2008 | 06:15:24 | 852 |
| #5 | Search STAVUDINE OR D4T OR ZERIT OR STAVIR | 06:14:29 | 2144 |
| #4 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | 06:14:03 | 2934596 |
| #3 | Search #1 OR #2 | 06:13:56 | 271703 |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) | 06:13:38 | 92062 |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | 06:13:23 | 240864 |
Database: PubMed 2008 ‐ 2009
Date: 5 June 2009
| Most Recent Queries | Time | Result | |
| #7 | Search #3 AND #4 AND #5 Limits: Publication Date from 2008/08/29 to 2009/06/05 | 08:59:49 | 44 |
| #6 | Search #3 AND #4 AND #5 | 08:55:13 | 942 |
| #5 | Search STAVUDINE OR D4T OR ZERIT OR STAVIR | 08:55:00 | 2281 |
| #4 | Search randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR comparative study [mh] OR evaluation studies [mh] OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | 08:52:45 | 3094485 |
| #3 | Search #1 OR #2 | 08:52:23 | 284387 |
| #2 | Search Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])) OR ((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])) | 08:52:09 | 98311 |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | 08:51:55 | 251542 |
Database: PubMed (2009 – 2012)
Date: 23 November 2012
| Search | Query | Items found |
| #7 | Search ((#3 AND #4 AND #5)) AND ("2009/06/01"[Date ‐ Publication] : "2012/11"[Date ‐ Publication]) | 262 |
| #6 | Search (#3 AND #4 AND #5) | 1797 |
| #5 | Search (stavudine OR d4T OR zerit OR stavir) | 2750 |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) | 2631802 |
| #3 | Search (#1 AND #2) | 78433 |
| #2 | Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab]))) | 124461 |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) | 289617 |
Appendix 2. AIDSearch strategy
Database: AIDSearch1996 ‐ 2008
Date: 10 September 2008
| Set# | Matches | Search Strategy |
| #1 | 339,088 | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) |
| #2 | 197,754 | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) |
| #3 | 100,549 | (HIGHLY ACTIVE ANTIRETROVIRAL THERAPY) OR (ANTI‐RETROVIRAL AGENTS) OR (ANTIVIRAL AGENTS) OR ((ANTI) AND (HIV)) OR ANTIRETROVIRAL* OR ((ANTI) AND (RETROVIRAL*)) OR HAART OR ((ANTI) AND (ACQUIRED IMMUNODEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNEDEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNO‐DEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUNE‐DEFICIENCY)) OR ((ANTI) AND (ACQUIRED IMMUN*) AND (DEFICIENCY)) |
| #4 | 349,168 | #1 OR #3 |
| #5 | 3,598 | STAVUDINE OR D4T OR ZERIT OR STAVIR |
| #6 | 1,831 | #2 AND #4 AND #5 |
| #7 | 361 | #6 AND PY=1996‐1998 |
| #8 | 225 | #6 AND PY=1999‐2000 |
| #9 | 297 | #6 AND PY=2001‐2003 |
| #10 | 474 | #6 AND PY=2004‐2006 |
| #11 | 363 | #6 AND PY=2007‐2008 |
Appendix 3. EMBASE strategy
Database: EMBASE 1996 ‐ 2008
Date: 10 September 2008
| No. | Query | Results | Date |
| #2 | ((('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection'))) OR (((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (('human immunodeficiency virus'/de OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')))) OR (((('b cell lymphoma'/de OR 'b cell lymphoma') OR ('b cell lymphoma'/de OR 'b cell lymphoma')) OR (('b cell lymphoma'/de OR 'b cell lymphoma') OR ('b cell lymphoma'/de OR 'b cell lymphoma')))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) | 296,987 | 10 Sep 2008 |
| #3 | (('human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine') OR ('human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine')) OR ('anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab) OR ('anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab) OR ('anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab) OR ('anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab) OR ('anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab) OR ('anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab) OR ('anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab) OR ('anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab) OR ('anti hiv':ti OR 'anti hiv':ab) OR (antiretrovir*:ti OR antiretrovir*:ab) OR ('anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab) OR (haart:ti OR haart:ab) OR ('aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab) OR ((('anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent') OR ('anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent'))) OR ((('antiretrovirus agent'/de OR 'antiretrovirus agent') OR ('antiretrovirus agent'/de OR 'antiretrovirus agent'))) OR ((('antivirus agent'/de OR 'antivirus agent') OR ('antivirus agent'/de OR 'antivirus agent'))) OR ((('highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy') OR ('highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy'))) | 87,156 | 10 Sep 2008 |
| #4 | #2 OR #3 | 324,367 | 10 Sep 2008 |
| #5 | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR ((('crossover procedure'/de OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure'))) OR ((('double‐blind procedure'/de OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure'))) OR ((('single‐blind procedure'/de OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure'))) OR ((('randomized controlled trial'/de OR 'randomized controlled trial') OR ('randomized controlled trial'/de OR 'randomized controlled trial'))) OR (allocat*:ti OR allocat*:ab) | 818,276 | 10 Sep 2008 |
| #6 | ('stavudine'/de OR 'stavudine') OR ('d4t'/de OR 'd4t') OR ('zerit'/de OR 'zerit') OR stavir | 9,558 | 10 Sep 2008 |
| #7 | #4 AND #5 AND #6 AND [1996‐2008]/py | 818 | 10 Sep 2008 |
Database: EMBASE 2008 ‐ 2009
Date: 5 June 2009
| No. | Query | Results | Date |
| #1 | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection')) OR (('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/de OR 'human immunodeficiency virus')) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab) OR ('acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab) OR ('acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab) OR ('acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab) OR ('acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab) AND [embase]/lim | 203,546 | 05 Jun 2009 |
| #2 | ('human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine') OR ('anti human immunedeficiency':ti OR 'anti human immunedeficiency':ab) OR ('anti human immunodeficiency':ti OR 'anti human immunodeficiency':ab) OR ('anti human immuno‐deficiency':ti OR 'anti human immuno‐deficiency':ab) OR ('anti human immune‐deficiency':ti OR 'anti human immune‐deficiency':ab) OR ('anti acquired immune‐deficiency':ti OR 'anti acquired immune‐deficiency':ab) OR ('anti acquired immunedeficiency':ti OR 'anti acquired immunedeficiency':ab) OR ('anti acquired immunodeficiency':ti OR 'anti acquired immunodeficiency':ab) OR ('anti acquired immuno‐deficiency':ti OR 'anti acquired immuno‐deficiency':ab) OR ('anti hiv':ti OR 'anti hiv':ab) OR (antiretrovir*:ti OR antiretrovir*:ab) OR ('anti retroviral':ti OR 'anti retroviral':ab OR 'anti retrovirals':ti OR 'anti retrovirals':ab OR 'anti retrovirus':ti OR 'anti retrovirus':ab) OR (haart:ti OR haart:ab) OR ('aids vaccine':ti OR 'aids vaccine':ab OR 'aids vaccines':ti OR 'aids vaccines':ab) OR ('anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent') OR (('antiretrovirus agent'/de OR 'antiretrovirus agent')) OR (('antivirus agent'/de OR 'antivirus agent')) OR (('highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy')) AND [embase]/lim | 67,830 | 05 Jun 2009 |
| #3 | #1 OR #2 | 223,483 | 05 Jun 2009 |
| #4 | ((random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR ((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab)) OR ((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab)) OR (assign*:ti OR assign*:ab) OR (allocat*:ti OR allocat*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/de OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/de OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/de OR 'single‐blind procedure')))) OR (((('randomized controlled trial'/exp OR 'randomized controlled trial') OR ('randomized controlled trial'/de OR 'randomized controlled trial')) OR (('randomized controlled trial'/exp OR 'randomized controlled trial') OR ('randomized controlled trial'/de OR 'randomized controlled trial'))))) AND [embase]/lim | 651,916 | 05 Jun 2009 |
| #5 | ('stavudine'/de OR 'stavudine') OR ('d4t'/de OR 'd4t') OR ('zerit'/de OR 'zerit') OR stavir AND [embase]/lim | 9,645 | 05 Jun 2009 |
| #6 | #3 AND #4 AND #5 AND [2008‐2009]/py | 100 | 05 Jun 2009 |
Database: EMBASE (2009 – 2012)
Date: 23 November 2012
| No. | Query | Results |
| #12 | #3 AND #9 AND #10 AND [embase]/lim AND [1‐6‐2009]/sd NOT [23‐11‐2012]/sd | 541 |
| #11 | #3 AND #9 AND #10 | 2021 |
| #10 | 'stavudine'/de OR stavudine:ab,ti OR 'd4t'/de OR d4t:ab,ti OR 'zerit'/de OR zerit:ab,ti OR 'stavir'/de OR stavir:ab,ti | 12739 |
| #9 | #4 NOT #8 | 1643300 |
| #8 | #5 NOT #7 | 2131733 |
| #7 | #5 AND #6 | 17226685 |
| #6 | 'human'/de OR 'human' | 17608466 |
| #5 | 'animal'/de OR 'animal' OR 'nonhuman'/de OR 'nonhuman' OR 'animal experiment'/de OR 'animal experiment' | 19358418 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1724036 |
| #3 | #1 AND #2 | 131034 |
| #2 | 'human immunodeficiency virus vaccine'/de OR 'human immunodeficiency virus vaccine' OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/de OR 'anti human immunodeficiency virus agent' OR 'antiretrovirus agent'/de OR 'antiretrovirus agent' OR 'highly active antiretroviral therapy'/de OR 'highly active antiretroviral therapy' OR 'highly active antiretroviral therapy':ab,ti | 152508 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | 391197 |
Appendix 4. Gateway search
Database: GATEWAY 1996 ‐ 2008
Date: 11 September 2008
Meeting abstracts: 517 records
| Search Number | Search | Items Found |
| #6 | Search: (((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))))) AND ((((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*))) AND (STAVUDINE OR D4T OR ZERIT OR STAVIR) Limit: 1996:2008 | 560 |
| #5 | Search: STAVUDINE OR D4T OR ZERIT OR STAVIR | 4276 |
| #4 | Search: (((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) | 180676 |
| #3 | Search: ((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw]))))) | 378475 |
| #2 | Search: (("Antiretroviral Therapy, Highly Active"[MeSH] OR "Anti‐Retroviral Agents"[MeSH] OR "Antiviral Agents"[MeSH:noexp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw])))) OR ((((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))) | 116403 |
| #1 | Search: (((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL))) | 108093 |
Database: PubMed 2008 ‐ 2009
Date: 5 June 2009
No meeting abstracts were found
| Search Number | Search | Items Found |
| #6 | Search: (((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw]))) OR (((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw]))))) AND ((((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*))) AND (STAVUDINE OR D4T OR ZERIT OR STAVIR) Limit: 2008/08/29:2009/06/05 | 44 |
| #5 | Search: STAVUDINE OR D4T OR ZERIT OR STAVIR | 4409 |
| #4 | Search: (((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR (CLINICAL TRIAL) OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL*) AND (MASK* OR BLIND* )))) OR ((PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) | 5091143 |
| #3 | Search: ((((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL)))) OR ((Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw]))) OR (((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw])))) | 391233 |
| #2 | Search: (Antiretroviral Therapy, Highly Active[MeSH] OR Anti‐Retroviral Agents[MeSH] OR Antiviral Agents[MeSH:NoExp] OR ((anti) AND (hiv[tw])) OR antiretroviral*[tw] OR ((anti) AND (retroviral*[tw])) OR HAART[tw] OR ((anti) AND (acquired immunodeficiency[tw]))) OR (((anti) AND (acquired immunedeficiency[tw])) OR ((anti) AND (acquired immuno‐deficiency[tw])) OR ((anti) AND (acquired immune‐deficiency[tw])) OR ((anti) AND (acquired immun*) AND (deficiency[tw]))) | 122328 |
| #1 | Search: (((HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME))) OR (((ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL))) | 367717 |
Appendix 5. CLIB
Database: CLIB 2008 ‐ 2009
Date: 5 June 2009
| ID | Search | Hits |
| #1 | "HIV Infections" OR HIV OR hiv OR "hiv‐1*" OR "hiv‐2*" OR hiv1 OR hiv2 OR "hiv infect*" OR "human immunodeficiency virus" OR "human immunedeficiency virus" OR "human immuno‐deficiency virus" OR "human immune‐deficiency virus" OR ("human immun*" AND "deficiency virus") OR "acquired immunodeficiency syndrome" OR "acquired immunedeficiency syndrome" OR "acquired immuno‐deficiency syndrome" OR "acquired immune‐deficiency syndrome" OR ("acquired immun*" AND "deficiency syndrome") OR "viral sexually transmitted diseases" | 8116 |
| #2 | ANTIRETROVIRAL THERAPY HIGHLY ACTIVE single term (MeSH) OR ANTI‐HIV AGENTS explode all trees (MeSH) OR ANTIVIRAL AGENTS single term (MeSH) OR AIDS VACCINES single term (MeSH) OR ANTI HIV OR ANTIRETROVIRAL* OR ANTI RETROVIRAL* OR AIDS VACCIN* | 3577 |
| #3 | (#1 OR #2) | 8338 |
| #4 | STAVUDINE OR D4T OR ZERIT OR STAVIR | 347 |
| #5 | (#3 AND #4), from 2008 to 2009 | 24 |
Database: CLIB 2009 – 2012
Date: 23 November 2012
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 6786 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2265 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or HIV INFECT* or HUMAN IMMUNODEFICIENCY VIRUS or HUMAN IMMUNEDEFICIENCY VIRUS or HUMAN IMMUNE‐DEFICIENCY VIRUS or HUMAN IMMUNO‐DEFICIENCY VIRUS or HUMAN IMMUN* DEFICIENCY VIRUS or ACQUIRED IMMUNODEFICIENCY SYNDROME or ACQUIRED IMMUNEDEFICIENCY SYNDROME or ACQUIRED IMMUNO‐DEFICIENCY SYNDROME or ACQUIRED IMMUNE‐DEFICIENCY SYNDROME or ACQUIRED IMMUN* DEFICIENCY SYNDROME | 10995 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 21 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 22 |
| #6 | #1 or #2 or #3 or #4 or #5 | 11072 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 844 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 2271 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 2919 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 286 |
| #11 | ANTI HIV or ANTIRETROVIRAL* or ANTI RETROVIRAL* or AIDS VACCIN* | 5000 |
| #12 | #7 or #8 or #9 or #10 or #11 | 7904 |
| #13 | #6 and #12 | 5375 |
| #14 | STAVUDINE or D4T or ZERIT or STAVIR | 423 |
| #15 | #13 and #14 from 2009 to 2012, in Trials | 53 |
Appendix 6. WHO International Clinical Trials Registry Platform
Date: 23 November 2012
Search strategy: HIV and STAVUDINE
Number of records retrieved: 207 records for 202 trials
| Recruitment status | Main ID | Public Title | Date of Registration | |
| Not recruiting | NCT01694017 | Economic, Clinical and Quality of Life Assessment in Patients on Antiretroviral Therapy | 18/09/2012 | |
| Not Recruiting | CTRI/2012/08/002931 | A clinical trial to study the effect of RECEPTOL® , a pure natural nano peptide ‐ antiviral and immunomodulator drug, in patients with HIV / AIDS | 29‐08‐2012 | |
| Not Recruiting | PACTR201208000402280 | STALEO | 13/08/2012 | |
| Recruiting | ACTRN12612000696897 | A trial of BIT225 in patients with HIV‐1 infection, to study the safety, concentration and distribution in the body, and anti‐viral activity of the drug. | 29/06/2012 | |
| Not Recruiting | CTRI/2012/06/002716 | Study of effectiveness of Stavudine 20mg taken two times daily in patients suffering from HIV‐1 infection | 06‐06‐2012 | |
| Not recruiting | NCT01601899 | Differences Between Stavudine and Tenofovir Each Combined With Lamivudine and Efavirenz in SA HIV‐infected Patients | 14/05/2012 | |
| Not Recruiting | EUCTR2010‐021651‐79‐IT | 13/04/2012 | ||
| Authorised | EUCTR2012‐000198‐21‐ES | A study to assess the efficacy and safety of the withdrawal of nucleos/tide analogues with resistance in multitreated HIV‐1‐infected subjects with virological suppression | 29/03/2012 | |
| Not Recruiting | EUCTR2010‐023749‐30‐IT | 13/03/2012 | ||
| Not Recruiting | CTRI/2012/03/002471 | study with HIV‐HBV coinfected patients. | 02‐03‐2012 | |
| Recruiting | NCT01359800 | Study to Assess the Seroprevalence of Anti‐Tat Antibodies in HIV‐infected Patients | 17/05/2011 | |
| Not recruiting | NCT01255371 | A Multicentre Trial of Second‐line Antiretroviral Treatment Strategies in African Adults Using Atazanavir or Lopinavir/Ritonavir | 29/11/2010 | |
| Not recruiting | NCT01215149 | A Trial to Evaluate the Safety and Immunogenicity of Ad26‐ENVA and Ad35‐ENV HIV Vaccines in Healthy HIV‐uninfected Adult Volunteers | 04/10/2010 | |
| Recruiting | NCT01178684 | Epidermal Nerve Fiber Density, Fat and Mitochondrial Parameters in Thai HIV+ Patients on d4T and HIV‐ Patients | 11/06/2010 | |
| Recruiting | NCT01146873 | Treatment Options for Protease Inhibitor‐exposed Children | 08/06/2010 | |
| Not recruiting | ISRCTN55748789 | Effectiveness of generic split adult tablets and paediatric fixed dose combination (FDC) of d4T/3TC/NVP in the treatment of HIV infected Malawian children | 20/05/2010 | |
| Recruiting | ISRCTN69078957 | Children with human immunodeficiency virus (HIV) in Africa ‐ pharmacokinetics and acceptability/adherence of simple antiretroviral regimens (CHAPAS‐3 trial) Recruitment status Main ID Public title Date of registration Recruiting PACTR201006000222401 Children with human immunodeficiency virus (HIV) in Africa, pharmacokinetics adn acceptability/adherence of simple antiretroviral regimen (CHAPAS‐3) 15/06/2010 |
15/04/2010 | |
| Recruiting | NCT01069809 | Safety and Efficacy Study of AGS‐004 During Analytical Treatment Interruption | 16/02/2010 | |
| Authorised | EUCTR2008‐007765‐23‐IT | MoLO study ‐ Evaluation of cost/efficacy ratio of monotherapy with lopinavir/ritonavir versus standard in patients treated with protesi inhibotors in virologic suppressison. ‐ ND | 11/12/2009 | |
| Not recruiting | NCT00998582 | Artery Elasticity After Switch From Epzicom to Truvada | 19/10/2009 | |
| Recruiting | NCT00986063 | Genotype Based Personalized Prescription of Nevirapine | 27/09/2009 | |
| Recruiting | ISRCTN37737787 | Europe ‐ Africa Research Network for Evaluation of Second‐line Therapy Recruitment status Main ID Public title Date of registration Not recruiting NCT00988039 Europe‐Africa Research Network for Evaluation of Second‐line Therapy 30/09/2009 |
22/09/2009 | |
| Not recruiting | NCT00944879 | Preparing for Adolescent HIV Vaccine Trials in South Africa: | 20/07/2009 | |
| Recruiting | NCT00940771 | Equivalence of Boosted Atazanavir Based Regimens and Currently Effective HAART Regimens | 15/07/2009 | |
| Not Recruiting | CTRI/2008/091/000021 | Siddha medicine for HIV | 10‐06‐2009 | |
| Not recruiting | NCT01025830 | Triomune Bioequivalence With Innovators | 21/04/2009 | |
| Not recruiting | NCT00872417 | Study on the Antiviral Therapy and Immune Reconstitution of Chinese HIV/AIDS Patients | 30/03/2009 | |
| Not Recruiting | EUCTR2009‐010189‐48‐IT | 09/02/2009 | ||
| Not recruiting | NCT00830856 | Early Versus Delayed Antiretroviral Therapy (ART) in the Treatment of Cryptococcal Meningitis in Africa | 27/01/2009 | |
| Not recruiting | NCT00703898 | Durability of Nevirapine‐Based Antiretroviral Regimen | 20/06/2008 | |
| Not recruiting | NCT00672191 | Phase II Study of AGS‐004 as an Immunotherapeutic in Antiretroviral Therapy (ART)‐Treated Subjects Infected With HIV | 05/05/2008 | |
| Not recruiting | NCT00669487 | A 72‐week Randomized Clinical Trial Comparing the Safety and Efficacy of Three Initial Antiretroviral Regimens ‐GPO‐VIR S (d4T/3TC/NVP) for 24 Weeks Followed by GPO‐VIR Z (AZT/3TC/NVP) vs GPO‐VIR Z vs TDF/FTC/NVP | 24/04/2008 | |
| Not recruiting | NCT00647946 | Study to Evaluate Changes in Limb Fat When Switching From a Thymidine Analogue | 27/03/2008 | |
| Not recruiting | NCT00617643 | Nevirapine Drug Levels in HIV Positive Patients Also Receiving Rifampicin for Tuberculosis | 05/02/2008 | |
| Not recruiting | NCT00618176 | Three Generic Nevirapine‐Based Antiretroviral Treatments in Chinese Patients:Multicentric Observation Cohort | 05/02/2008 | |
| Not Recruiting | EUCTR2007‐003418‐32‐GB | A Phase III, Randomised, Open‐ Label Study Comparing the Safety and Efficacy of Switching Stavudine or Zidovudine to Tenofovir Disoproxil Fumarate versus Continuing Stavudine or Zidovudine in Virologically Suppressed HIV‐Infected Children Taking Highly Active Antiretroviral Therapy. | 11/10/2007 | |
| Not Recruiting | EUCTR2005‐004025‐26‐IT | THERAPEUTIC SIMPLIFICATION WITH THYMIDINE ANALOGUR SPARING REGIMENS IN PATIENTS ON EFFECTIVE HAART: A CONTROLLED, RANDOMIZED STUDY | 12/09/2007 | |
| Not recruiting | NCT00490152 | Microbicides Acceptability Among Sexually Active Young Women | 21/06/2007 | |
| Authorised | EUCTR2006‐002805‐30‐IT | Strategic long term, immunologically driven treatment interruptions in previously naive patients starting HAART: a controlled, randomized, multicenter study ‐ (Therapeutic Structured Interruptions Study) | 19/06/2007 | |
| Recruiting | NCT00476606 | A Prospective Cohort of Children With HIV Infection | 20/05/2007 | |
| Not recruiting | NCT00471614 | Effects of Uridine Supplementation on Metabolic Side Effects of Stavudine and Zidovudine | 08/05/2007 | |
| Authorised | EUCTR2006‐006076‐38‐GB | The Liverpool HIV TDM Registry: Studying influences upon plasma HIV drug exposure ‐ The Liverpool HIV TDM Registry | 17/04/2007 | |
| Not recruiting | NCT00457665 | Mechanisms of Lipodystrophy in HIV‐Infected Pateints | 04/04/2007 | |
| Not recruiting | NCT00455585 | Comparison of Plasma Drug Levels of Triomune 40 With Those of the Originator Products | 02/04/2007 | |
| Not recruiting | NCT00441298 | Safety and Effectiveness Study of a Candidate Vaginal Microbicide for Prevention of HIV | 27/02/2007 | |
| Not recruiting | NCT00427297 | Optimizing Pediatric HIV‐1 Treatment in Infants With Prophylactic Exposure to Nevirapine, Nairobi, Kenya | 22/01/2007 | |
| Recruiting | NCT00428116 | Optimizing Pediatric HIV‐1 Treatment, Nairobi, Kenya | 22/01/2007 | |
| Not recruiting | NCT00528957 | Safety and Efficacy of Switching From Stavudine or Zidovudine to Tenofovir DF in HIV‐1 Infected Children | 03/01/2007 | |
| Authorised | EUCTR2006‐003425‐81‐GB | Host genetic factors influencing drug disposition and response to HIV treatment ‐ Pharmacogenetics of HIV Therapy | 23/11/2006 | |
| Not recruiting | NCT00381212 | A Pilot Study to Investigate the Safety and Immunologic Activity AGS‐004 an Autologous HIV Immunotherapeutic Agent. | 25/09/2006 | |
| Not recruiting | NCT00380770 | HIV/AIDS Kaposis Sarcoma: Comparison of Response to HAART vs HAART Plus CXT | 25/09/2006 | |
| Not recruiting | NCT00342355 | Antiretroviral Therapy for Advanced HIV Disease in South Africa | 19/06/2006 | |
| Not recruiting | NCT00312832 | Study Comparing Reducing the Dose of Stavudine Versus Switching to Tenofovir in HIV‐Infected Patients Receiving Antiretroviral Therapy | 07/04/2006 | |
| Not recruiting | NCT00312091 | Drug Levels of Tablet and Liquid Forms of Lamivudine, Nevirapine, and Stavudine in HIV Infected Thai Children | 05/04/2006 | |
| Not recruiting | ISRCTN31084535 | Children with human immunodeficiency virus (HIV) in Africa ‐ Pharmacokinetics and Adherence of Simple Antiretroviral Regimens | 23/02/2006 | |
| Not recruiting | NCT00282581 | Safety Study of MVA Smallpox Vaccine in HIV‐Positive Subjects Who Are Vaccinia Naive | 26/01/2006 | |
| Not recruiting | NCT00270556 | Phase II Comparator Study of Substitution of Tenofovir or Abacavir Receiving Thymidine Analogue as Part of HAART. | 23/12/2005 | |
| Not recruiting | NCT00255840 | Evaluation of Two Anti‐HIV Treatment Strategies in Resource‐Limited South African Communities | 16/11/2005 | |
| Not recruiting | ISRCTN04750658 | Randomised open multicentre trial comparing stavudine versus abacavir, both combined with lamivudine/efavirenz, in Human Immunodeficiency Virus (HIV) infected antiretroviral naïve patients | 08/11/2005 | |
| Not recruiting | NCT00235222 | Evaluation of Viral Efficacy and Safety of a Reduced Dose of Stavudine (d4T): THE PHOENIX STUDY | 06/10/2005 | |
| Not recruiting | NCT00215839 | HRN 004‐ Peginterferon a‐2a Plus Ribavirin for Chronic Hepatitis C Infection in HIV Infected Persons Who Have Failed to Achieve a Sustained Virologic Response Following Previous Interferon Therapy | 20/09/2005 | |
| Not recruiting | NCT00189930 | An Evaluation of Immunogenicity and Safety of Two Doses of MVA‐nef vs. MVA‐BN in HIV‐1 Infected Patients | 12/09/2005 | |
| Not recruiting | NCT00192660 | HIV Infection And Metabolic Abnormalities Protocol 1 (HAMA001) | 12/09/2005 | |
| Not recruiting | NCT00197613 | The Adult Antiretroviral Treatment and Resistance Study (Tshepo) | 12/09/2005 | |
| Not recruiting | NCT00158821 | Study of Treatment of Antiretroviral‐naive, HIV‐1‐Infected Patients Comparing Tenofovir Disoproxil Fumarate Administered in Combination With Lamivudine and Efavirenz vs. Stavudine, Lamivudine and Efavirenz. | 07/09/2005 | |
| Not recruiting | NCT00143702 | D4T or Abacavir Plus Vitamin Enhancement in HIV‐Infected Patients (DAVE) | 31/08/2005 | |
| Not recruiting | NCT00135369 | Switching HIV‐1 Infected Subjects From a Highly Active Anti‐Retroviral Treatment (HAART) Regimen Dosed Twice Daily or More Frequently to a Once‐Daily Regimen | 25/08/2005 | |
| Not recruiting | NCT00127972 | 2NN & CHARM Long‐Term Follow‐up Study | 08/08/2005 | |
| Not Recruiting | EUCTR2004‐000441‐38‐IT | A Large, Simple Trial Comparing Two Strategies for Management of Anti‐Retroviral Therapy Recruitment status Main ID Public title Date of registration Authorised EUCTR2004‐000441‐38‐IE Large, Simple Trial Comparing Two Strategies for Management of Anti‐Retroviral Therapy (The SMART Study) ‐ SMART 24/11/2006 |
01/08/2005 | |
| Not recruiting | NCT00116298 | Rollover Study for Zerit (Stavudine) ER Studies (‐096, ‐099) | 28/06/2005 | |
| Not recruiting | NCT00116116 | DART II ‐ A Phase IV Study of 3 Antiretroviral Medicines in Combination, in HIV Patients Who Have Not Been Previously Treated With Antiretroviral Therapy | 27/06/2005 | |
| Not Recruiting | EUCTR2004‐001827‐39‐IT | NATIONAL, MULTICENTER, RANDOMISED, OPEN STUDY TO VALUATE THE EFFICACY OF DIFFERENT THERAPEUTIC STRATEGIES TO AVOID THE IMMUNOLOGIC FAILURE IN MULTIRESISTENT HIV‐1 INFECTED PATIENTS. | 27/06/2005 | |
| Not Recruiting | EUCTR2004‐004055‐19‐DE | A Phase IIb randomized, partially blinded, dose‐finding trial of TMC278 in antiretroviral naive HIV‐1 infected subjects. ‐ N/A Recruitment status Main ID Public title Date of registration Authorised EUCTR2004‐004055‐19‐GB A Phase IIb randomized, partially blinded, dose‐finding trial of TMC278 in antiretroviral naive HIV‐1 infected subjects. ‐ N/A 22/03/2005 |
17/03/2005 | |
| Authorised | EUCTR2004‐000623‐16‐GB | A retrospective study to compare the 3‐year antiviral efficacy of nevirapine and efavirenz in combination with D4T and 3TC in 2NN patients ‐ 2NN Follow‐up study | 17/02/2005 | |
| Not recruiting | NCT00103532 | Healthy Choices to Promote Health and Reduce Risk in HIV‐Infected Youth | 09/02/2005 | |
| Not recruiting | NCT00102089 | HIV‐1 Vaccine Booster in Previously Immunized Uninfected Adult Volunteers | 20/01/2005 | |
| Not recruiting | NCT00100646 | Anti‐HIV Treatment Interruptions in HIV Infected Adults in South Africa | 04/01/2005 | |
| Not recruiting | NCT00100594 | Efavirenz and Lamivudine/Zidovudine for Treatment‐Naive HIV Infected People in Wenxi County, Shanxi Province, China | 03/01/2005 | |
| Not recruiting | NCT00100048 | A Study to Evaluate the Safety and Efficacy of an Investigational Drug in HIV Infected Patients (0518‐004)(COMPLETED) | 22/12/2004 | |
| Not recruiting | NCT00080522 | Strategies for Delivering Anti‐HIV Therapy in South Africa | 06/04/2004 | |
| Not recruiting | NCT00074581 | Preventing Sexual Transmission of HIV With Anti‐HIV Drugs | 16/12/2003 | |
| Not recruiting | NCT00055120 | When to Start Anti‐HIV Drugs in Patients With Opportunistic Infections | 19/02/2003 | |
| Not recruiting | NCT00050895 | Comparing the Safety, Effectiveness, and Tolerability of Three Anti‐HIV Drug Regimens for Treatment‐Naive Patients | 30/12/2002 | |
| Not recruiting | NCT00040300 | A 14‐Day Study of Racivir When Used in Combination in HIV‐Infected Males | 24/06/2002 | |
| Not recruiting | NCT00039741 | Anti‐HIV Drug Regimens and Treatment‐Switching Guidelines in HIV Infected Children Recruitment status Main ID Public title Date of registration Not recruiting ISRCTN73318385 PENPACT 1: A phase II/III randomised, open‐label study of combination antiretroviral regimens and treatment‐switching strategies in antiretroviral naive children >30 days and <18 years of age 19/07/2002 |
07/06/2002 | |
| Not recruiting | NCT00036452 | A Study to Compare Anti‐HIV Drugs Given Twice a Day or Once a Day, With or Without Direct Observation | 10/05/2002 | |
| Not recruiting | NCT00034086 | Study of Anti‐HIV Therapy Intensification | 22/04/2002 | |
| Not recruiting | NCT00021632 | Effects of Ribavirin on Zidovudine or Stavudine | 26/07/2001 | |
| Recruiting | NCT00017992 | Emtricitabine Given Once A Day With Other Anti‐HIV Drugs in Children With HIV | 23/06/2001 | |
| Not recruiting | NCT00014937 | Simplified Drug Regimens for HIV Patients in ACTG 388 or Patients Who Responded to A First Potent Combination Regimen | 14/04/2001 | |
| Not recruiting | NCT00013520 | Comparison of Three Different Initial Treatments Without Protease Inhibitors for HIV Infection | 16/03/2001 | |
| Not recruiting | NCT00007202 | Safety and Effectiveness of a Three‐Drug Combination Treatment for Recently Infected or Converted HIV Patients | 15/12/2000 | |
| Not recruiting | NCT00006443 | Effectiveness of Anti‐HIV Drugs in Patients Who Have Not Received Previous Anti‐HIV Drugs During Different Stages of HIV Infection | 03/11/2000 | |
| Not recruiting | NCT00006415 | A Study of Patients Who Recently Have Been Infected With HIV | 19/10/2000 | |
| Not recruiting | NCT00006397 | Differences Between Women and Men Taking a Combination of Indinavir, Ritonavir, Enteric‐Coated Didanosine, and Stavudine Who Previously Took Anti‐HIV Drugs | 10/10/2000 | |
| Not recruiting | NCT00006339 | Safety and Effectiveness of an Anti‐HIV Drug Combination With and Without Hydroxyurea in Patients With Early HIV Infection | 04/10/2000 | |
| Not recruiting | ISRCTN58987964 | An open randomised trial to evaluate the activity and tolerability of combinations of reverse transcriptase and protease inhibitors, including induction therapy, in individuals with Human Immunodeficiency Virus‐1 (HIV‐1) infection and CD4 cell counts greater than 25 x 10 to the power of 6 per litre | 03/10/2000 | |
| Not recruiting | NCT00006208 | A Comparison of Emtricitabine and Stavudine Used With Didanosine Plus Efavirenz in HIV‐Infected Patients Who Have Not Taken Anti‐HIV Drugs | 11/09/2000 | |
| Not recruiting | NCT00006190 | A Study to Determine How and Why HIV‐Infected Subjects on Anti‐viral Treatment Develop Lipodystrophy | 25/08/2000 | |
| Not recruiting | NCT00006144 | A Study of HIV‐Disease Development in Aging | 07/08/2000 |
Data and analyses
Comparison 1. Low dose versus high dose stavudine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants with HIV RNA < 200 copies/ml | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.95, 1.16] |
| 2 Proportion of participants with suppressed viral load:Low dose best case‐scenario | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.94, 2.10] |
| 3 Proportion of participants with suppressed viral load: Low dose worst case‐scenario | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.60, 1.20] |
Comparison 2. Low dose versus high dose stavudine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants developing symptomatic hyperlactatemia | 2 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
McComsey 2008.
| Methods | Participants were randomised at a ratio of 3:2 to reduce the dose of stavudine or continue the standard dose for 48 weeks | |
| Participants | Participants were recruited in the Special Immunology Unit of University Hospitals of Cleveland (Cleveland,OH) and in the MacGregor Infectious Diesease Clinic of the Hospital of University of Pennsylvania (Philadelphia, PA) between November 2004 and December 2005 Age≥ 18, on stable ARV therapy containing stavudine for ≥ 24 weeks, Viral load < 50 copies/ml or branched DNA < 75 copies/ml. Participants had to have at least one of the following markers or risk factors for mitochondrial toxicity: 1. self‐reported lipoatrophy, defined as fat loss in the face, extremities, and/or buttocks, and confirmed by the investigator; 2. 2 consecutive elevated lactate measurements; 3. regular excessive use of alcohol within the past year, defined as alcohol intake of >40 g per day for men (or >30 g per day for women) on >3 days per week; 4. hepatitis C infection; 5. female sex; 6. overweight (body mass index [BMI] of > 25 kg/m2; 7. use of didanosine at time of screening. |
|
| Interventions | Low dose (20 mg stavudine for weight ≥ 60 kg or 15 mg stavudine for weight < 60 kg); High dose (stavudine 40 mg for weight ≥ 60 kg or stavudine 30 mg for weight < 60kg). All other antiretroviral drugs continued, the drugs are not specified | |
| Outcomes | Primary endpoints: Changes in fat and PBMC mtDNA levels Secondary end‐points: Changes in triglyceride levels; total, non‐high‐density lipoprotein (HDL) cholesterol levels; lactate levels; lactate/pyruvate ratio; DEXA‐measured changes in limb fat, and BMD; CD4 cell count; and HIV‐1 RNA level |
|
| Notes | Bristol‐Myers Squibb and the Clinical Core of the Penn Center for AIDS Research (AI45008). The mitochondrial assays were supported by National Institute of Allergy and Infectious Diseases (AI‐60484 to G.M.). G.M. has received research grant support and serves as consultant and speaker for Bristol‐Myers Squibb, Glaxo‐SmithKline, Gilead, and Abbott. I.F. is a speaker and consultant for Bristol‐Myers Squibb. All other authors: no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization described as ratio of 3:2. The method used to generate allocation sequence is not described in detail. |
| Allocation concealment (selection bias) | High risk | Method used to conceal allocation not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open labelled trial. Participants and providers not blinded. Dexa scans were read centrally by a reader who was blinded to timing of DEXA and participants' characteristics. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 24 participants enrolled, 21 completed the study. Two participants in the low dose and one in the high dose stavudine arm. Reasons for attrition are reported clearly. |
| Selective reporting (reporting bias) | High risk | Secondary endpoint of CD4 cell counts not reported |
| Other bias | Low risk | |
Milinkovic 2007.
| Methods | Single centre, randomised, open‐label | |
| Participants | Documented HIV‐1 infection, viral load < 200 copies/ml for at least 6/12 prior to inclusion, total body weight > 60kg, moderate to severe clinical lipoatrophy in at least 1 region, stable triple antiretroviral therapy including stavudine 40mg twice daily for at least six preceding months. Other drugs included Non‐nucleoside reverse transcription inhibitor, Protease inhibitors, triple NRTI regimen. None of the patients were receiving lipid‐lowering and/or anti‐diabetic therapy at baseline | |
| Interventions | Participants were randomly assigned to either continue stavudine 40mg twice daily or to reduce the dose of stavudine from 40mg to 30mg twice daily | |
| Outcomes | Primary endpoint: Median change in limb fat mass measured by DEXA at week 24 Secondary endpoints: median change in the following‐ total and central fat mass; lipid and mitochondrial parameters; viral load; and CD4 Tcell count |
|
| Notes | Local ethics committee approval, all patients signed informed consent Funding Source:The study was supported in part by grants: PI02590 from Fondo de Investigaciones Sanitarias; RIS G03/173 from Red Tematica Cooperativa de Investigacion en SIDA, Ministerio de Sanidad y Consumo; Fundacio La Marato de TV3 (020210), Redes de Investigacion en Mitocondrias (V2003‐REDC06E‐0); and by Grups de Recerca de la Generalitat de Catalunya (SGR 0300/2005). The authors state that the funding sources played no role in the design of the study, data collection, data analysis, data interpretation or writing of the report |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomisation list was computer generated in blocks. Participants were stratified according to current treatment, PIs and NRTIs |
| Allocation concealment (selection bias) | High risk | Allocation concealment not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. Participants and providers not blinded. Unclear if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was by intention to treat using the policy of last‐date‐carried‐forward analysis adopted for participants with missing data for the remaining follow‐up period. Follow‐up for all participants adequately described. |
| Selective reporting (reporting bias) | High risk | Secondary endpoint of CD4 cell counts not reported |
| Other bias | Low risk | |
Sanchez‐Conde 2005.
| Methods | Participants receiving HAART including stavudine were allocated to either reduce the dose of stavudine or continue with the standard dose | |
| Participants | Participants receiving a stavudine containing regimen in the first semester of 2003 at the Department of Infectous Diseases and Service Pharmacy, Hospital Carlos III, Madrid, Spain HIV‐1 RNA < 50 copies/mL during at least previous 3 months Those receiving hepatitis C therapy with interferon and ribavirin or those receiving interleukin‐2 were excluded |
|
| Interventions | Half of the participants were allocated to reduce stavudine dose from 40mg to 30mg BD and the other half continued with 40mg BD Participants continued all other antiretroviral drugs |
|
| Outcomes | Plasma HIV‐1 RNA and CD4 T‐cell measurements Virologic failure defined as repeated values of plasma HIV‐1 RNA above 50 copies/mL |
|
| Notes | Baseline age not reported Funding source: In part by grants from Fundacion Investigacion y Educacion en SIDA (IES) and Red de Investigacion en SIDA (RIS project 173) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were allocated to either reduce or continue the stavudine dosage. The method used to generate allocation sequence is not described. |
| Allocation concealment (selection bias) | High risk | The method used to conceal allocation is not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were allocated to either reduce their stavudine dose or continue their dose. It is assumed that both participants and providers were not blinded, however, it is unclear if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 92 Participants were included in the trial, 47 in the low dose and 45 in the high dose arm. 41 participants did not complete the 12 month follow‐up period. Loss to follow‐up=6; Initiation of Hepatitic C therapy=6; Drug switch for reasons other than virologic failure=12; Voluntary withdrawal=8. The lack of completion was relatively evenly distributed. Intention‐to‐treat analysis was used with missing=failure |
| Selective reporting (reporting bias) | High risk | Nine participants are reported to have experienced virologic failure (4 in the low dose group and 5 in the high dose group), however, the HIV‐1 RNA measurements are not reported. The mean CD4 count is reported as not significantly different between the two groups but the mean values are not reported. |
| Other bias | High risk | Age at study entry not reported |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fabian 2008 | Retrospective |
| Lange 1998 | Participants treated with monotherapy or dual therapy |
| Makinson 2008 | Review |
| Mashitisho 2013 | Retrospective |
| Menezes 2012 | Too short follow up (four weeks) for documentation of outcomes |
| Pollar 1997 | Participants treated with dual therapy |
| Pujades‐Rodriquez 2011 | Observational |
| Wolf 2004 | Retrospective study |
Characteristics of studies awaiting assessment [ordered by study ID]
Ribera 2005.
| Methods | Randomized controlled trial |
| Participants | 54 HAART naive patients |
| Interventions | Stavudine 40mg or 30mg twice a day as part of HAART for 48 weeks |
| Outcomes | Rates of virologic suppression and CD4 cell count increase |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Venter 2013.
| Trial name or title | A randomized, double‐blind, multi‐centre, parallel‐group Phase 3b study to demonstrate non‐inferiority of stavudine (20 mg twice daily) compared with tenofovir disoproxil fumarate (300 mg once daily) when administered in combination with lamivudine and efavirenz in antiretroviral‐naive patients infected with HIV‐1 |
| Methods | Randomized clinical trial |
| Participants | HIV‐1 infected adults |
| Interventions | Stavudine 20 mg twice daily compared with tenofovir disoproxil fumarate 300mg daily |
| Outcomes | Undetectable plasma HIV‐1 RNA levels (<50 copies/ml) at week 48 |
| Starting date | 2013 |
| Contact information | fventer@wrhi.ac.za |
| Notes |
Differences between protocol and review
Viral load outcome has been changed from 400 copies/ml to 200 copies/ml as more sensitive tests have become available. However, each of the studies had the outcome measured at the level of the cut‐off for each individual study. The 200 copies/ml cut‐off is a convenient cut‐off as it is the highest cut‐off among the included studies There has been a change in authorship between the protocol and review stages.
Contributions of authors
NM and MD conducted eligibility of the search results, data extraction and quality assessment. NM entered data, conducted the analyses and wrote the first draft of the review. MD provided feedback into results and manuscript.
Sources of support
Internal sources
-
Discovery Foundation Fellowship, Other.
Scholarship
-
University of KwaZulu‐Natal Competitive Grant, Other.
Scholarship
-
University of KwaZulu‐Natal Doctoral Grant, Other.
Scholarship
External sources
-
Medical Research Council Self Initiated Grant, Other.
Scholarship
Declarations of interest
NM has received funding from the Discovery Foundation, University of KwaZulu‐Natal and the Medical Research Council. There are no potential conflict of interest to declare by both authors.
New
References
References to studies included in this review
McComsey 2008 {published data only (unpublished sought but not used)}
- McComsey GA, Lo Re V 3rd, O'Riordan M, Walker UA, Lebrecht D, Baron E, et al. Effect of reducing the dose of stavudine on body composition, bone density, and markers of mitochondrial toxicity in HIV‐infected subjects: a randomized, controlled study. Clinical Infectious Disease 2008;46:1290‐1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milinkovic 2007 {published and unpublished data}
- Milinkovic A, Martinez E, López S, Lazzari E, Miró O, Vidal S, et al. The impact of reducing stavudine dose versus switching to tenofovir on plasma lipids, body composition and mitochondrial function in HIV‐infected patients. Antiviral Therapy 2007;12:407‐415. [PubMed] [Google Scholar]
Sanchez‐Conde 2005 {published data only}
- Sanchez‐Conde M, Mendoza C, Jimenez‐Nacher I, Barreiro P, Gonzalez‐Lahoz J, Soriano V. Reductions in stavudine dose might ameliorate mitochondrial‐associated complications without compromising antiviral activity. HIV Clin Trials 2005 Jul‐Aug;6(4):197‐202. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fabian 2008 {published data only}
- Fabian J, Venter WDF, Mkhabela L, et.al. Symptomatic hyperlactateamia in adults on antiretroviral therapy: A single‐center experience. S Afr Med J 2008;98:795‐800. [PubMed] [Google Scholar]
Lange 1998 {unpublished data only}
- Lange J, Cooper D, Kunanusont C, Teeratakulpisarn S, Ruxrungtham K, Ungsedhapand C, Phanuphak P. Study HIV‐NAT 002: the safety and efficacy of didanosine (DDI) +/‐ stavudine (d4T) in high/low dose combinations in an antiretroviral therapy naive Thai adult population with CD4 counts 150‐350/mm3 and predominantly infected with HIV‐1 clade E. International Conference on AIDS. December 1998; Vol. 12, issue 12241:ICA 12/98387016.
Makinson 2008 {published data only}
- Makinson A, Moing VL, Kouanfack C, Laurent C, Delaporte E. Safety of stavudine in the treatment of HIV infection with a special focus on resource‐limited settings. Expert Opinion 2008;7(3):283‐293. [DOI] [PubMed] [Google Scholar]
Mashitisho 2013 {unpublished data only}
- Mashitisho B. The efficacy of 20 mg Stavudine‐containing regimen in viral suppression in human immunodeficiency virus infected patients at Dr George Mukhari Hospital. FIDSSA Conference 2013.
Menezes 2012 {published data only}
- Menezes C, Duarte R, Dickens C, Dix‐Peek T, Amsterdam D, John MA, et al. The early effects of stavudine compared with tenofovir on adipocyte gene expression, mitochondrial DNA copy number and metabolic parameters in South African HIV‐infected patients: a randomized trial. HIV Medicine 2012;11:244. [DOI] [PubMed] [Google Scholar]
Pollar 1997 {published data only}
- Pollar RB, Peterson D, Hardy D, Pedneault L, Rutkiewics V, Pottage J, et al. Randomized double‐blind study of combination therapy with didanosine and stavudine in HIV‐infected individuals. Antiviral therapy 1997;2(Suppl 3):89‐93. [Google Scholar]
Pujades‐Rodriquez 2011 {published data only}
- Pujades‐Rodrigues M, Dantony E, Pinoges L, et.al. Toxicity Associated with Stavudine Dose Reduction from 40 to 30 mg in First‐line Antiretroviral Therapy. PLoS ONE 2011;6(11):e28112.doi:10.1371/journal.pone.0028112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2004 {unpublished data only}
- Wolf E, Koegl C, Hoffmann C, Ruemmelein N, Postel N, Jaegel‐Guedes E, Jaeger H. Low dose stavudine: less side effects but as effective as standard dose. International Conference on AIDS. 2004; Vol. 15:abstract no. WePeB5861.
References to studies awaiting assessment
Ribera 2005 {published and unpublished data}
- Ribera E, Paradinero J, Domingo P. A randomized study comparing the efficacy and tolerability of low‐dose versus standard‐dose stavudine in antiretroviral‐naive patients(ETOX study). 3rd International Conference on AIDS Pathogenesis, Rio de Jenero, Brazil. July 2005.
References to ongoing studies
Venter 2013 {published data only}
- Venter WDF. A Randomized , Double Blind, Multi Center, Parallel Group Phase 3b Study to Demonstrate Non‐inferiority of Stavudine (20 mg Twice Daily) Compared With Tenofovir Disoproxil Fumarate (300 mg Once Daily) When Administered in Combination With Lamivudine and Efavirenz in Antiretroviral‐Naive Patients Infected With HIV‐1. ongoing 2016.
Additional references
Anderson 1995
- Anderson RE, Dunkle LM, Smaldone L, Adler M, Wirtz C, et al. Design and implementation of the stavudine parallel track programme. Comparison of safety and efficacy of two doses of stavudine in a simple trial in the US parallel track programme. J Infect Dis 1995;171:118‐122. [DOI] [PubMed] [Google Scholar]
Andrieux‐Meyer 2012
- Isabelle Andrieux‐Meyer, Polly Clayden, Simon Collins, et.al. WHY IT'S TIME TO SAY GOODBYE TO STAVUDINE...EVERYWHERE. SAJHIV March 2012:17‐19. [Google Scholar]
Armitage 1994
- Armitage P, Berry G. Statistical Methods in Medical research, 3rd edn.. Blackwell scientific publications, Oxford, UK 1994.
Birkus 2002
- Birkus G, Hitchcock JMH, Cihlar T. Assessment of mitochondrial toxicity in human cells treated with tenofovir: comparison with other nucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother 2002;46:716‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brinkman 1998
- Brinkman K, ter Hofstede J, Burger D. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 1998;12:1735‐1744. [DOI] [PubMed] [Google Scholar]
Brinkman 1999
- Brinkman K, Smeitink J, Romijin J. Mitochondrial toxicity induced by nucleoside‐analogue reverse‐transcriptase inhibitors is key factor in the pathogenesis of antiretroviral‐therapy‐related lipodystrophy. Lancet 1999;354:1112‐1115. [DOI] [PubMed] [Google Scholar]
Browne 1993
- Browne MJ, Mayer KH, Chafee SB, Dudley MN, Posner MR, Steinberg SM, et al. 2'3'‐ didehydro‐3' ‐ deoxythymidine (d4T) in patients with AIDS or AIDS‐related complex: a phase I trial. J Infect Dis 1993;167:21‐29. [DOI] [PubMed] [Google Scholar]
Buchacz 2008
- Buchacz K, Weidle PJ, Moore D, Were W, Mermin J, Downing R, et al. Changes in lipid profile over 24 months among adults on first‐line highly active antiretroviral therapy in the home‐based AIDS care program in rural Uganda. J Acquir Immune Defic Syndr Mar 2008;47(3):304‐11. [DOI] [PubMed] [Google Scholar]
Cross 2002
- Cross J, Lee H, Westelinck A, Nelson J, Grudzinskas C, Peck C. Postmarketing drug dosage changes of 499 FDA approved new molecular entities. Pharmaco Drug Safety 2002;11:439‐446. [DOI] [PubMed] [Google Scholar]
De Wit 2008
- Stephanie De Wit, Caroline A Sabin, Rainer Weber, et al. Incidence and Risk Factors for New‐Onset Diabetes in HIV‐Infected Patients. Diabetes Care 2008;31:1224‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delpierre 2005
- Delpirre C, Cuzin L, Alvarez M. Lowering stavudine dosages does not compromise anti‐viral efficacy in HIV‐1 infected patients. IAS conference on pathogenesis. Rio de janiero, Brazil. July 2005.
DOH 2013
- DOH. The South African Antiretroviral Treatment Guidelines. http://www.doh.gov.za/docs/policy/2013/ART_Treatment_Guidelines_Final_25March2013.pdf 2013.
Gallant 2004
- Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral‐naive patients: a 3‐year randomized trial. JAMA 2004;292(2):191‐201. [DOI] [PubMed] [Google Scholar]
Gallant 2006
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 2006;354(3):251‐260. [DOI] [PubMed] [Google Scholar]
Geddes 2006
- Geddes R, Knight S, Moosa MY, Reddi A, Uebel K, Sunpath H. A high incidence of nucleoside reverse transcriptase Inhibitor (NRTI)‐induced lactic acidosis in HIV‐infected in a South African context. S Afr Med J August 2006;96(8):722‐4. [PubMed] [Google Scholar]
Gilks 2006
- Gilks CF, Crowley S, Ekpini R, Gove S, Perriens J, Souteyrand Y, et al. The WHO public‐health approach to antiretroviral treatment against HIV in resource‐limited settings. Lancet 2006;368:505‐510. [DOI] [PubMed] [Google Scholar]
Hanvanich 2003
- Hanvanich M, Prasanthai V, Riengchan P, et al. Reductiion of d4t dosage improves lipoatrophy without virologic failure. Antiviral Therapy 2003;8(suppl. 1):S392 abstr749. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley‐Blackwell, 2008. [Google Scholar]
Hill 2007
- Hill A, Ruxrungtham K, Mattana H, et al. Systematic review of clinical trials evaluating low doses of stavudine as part of antiretroviral treatment. Expert Opin. Pharmacother 2007;8(5):679‐688. [DOI] [PubMed] [Google Scholar]
Kakuda 2000
- Kakuda T. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitors‐induced mitrochondrial toxicity. Clin Ther 2000;22:685‐708. [DOI] [PubMed] [Google Scholar]
Koegl 2003
- Koelgl C, Wolf E, Postel N, et al. Low dose stavudine as effective as standard dose but less side effects. 9th European AIDS conference (EACS) Warsaw 25 October 2003;9:8/5. [Google Scholar]
Lewis 1995
- Lewis W, Dalakas M. Mitochondrial toxicity of antiviral drugs. Nat Med 1995;1:417‐422. [DOI] [PubMed] [Google Scholar]
Magula 2008
- Magula N, Dedicoat M. Low dose versus high dose stavudine for treating people with HIV infection (Protocol). Cochrane Database of Systematic Reviews 2008, (4):Art. No. DOI:10.1002/14651858.CD007497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maritz 2010
- Maritz J, Benatar M, Dave JA, Harrison TB, Badri M, Levitt NS, Heckman JM. HIV neuropathy in South Africans: frequency, characteristics, and risk factors. Muscle Nerve May 2010;41(5):599‐606. [DOI] [PubMed] [Google Scholar]
Petersen 1995
- Petersen E, Ramirez‐Ronda C, Hardy D, et al. Dose‐relatted activity of stavudine in patients infected with human immunodeficiency virus. J Inf. Dis 1995;171(Suppl1):S131‐S139. [DOI] [PubMed] [Google Scholar]
Pollard 1999
- Pollard R, Peterson D, Hardy D, et al. Safety and antiretroviral effects of combined didanosine and stavudine therapy in HIV infected individuals with CD4 of 200 to 500 cells/mm3. J Acq Immuno Sydr 1999;22:39‐45. [DOI] [PubMed] [Google Scholar]
Ruxrungtham 2000
- Ruxrungtham K, Kroon E, Ungsedhapand C, et al. A randomised, dose‐finding study with didanosine plus stavudine versus didanosine alone in antiviral‐naive, HIV‐infected Thai patients. AIDS 2000;14:1375‐1382. [DOI] [PubMed] [Google Scholar]
Slangphoe 2004
- Slangphoe U, Srikaew S, Walwavaravuth C, et, al. Efficacy and safety of half dose compared to full dose stavudine (d4t) and zidovudine (AZT) in combination with didanosine (ddl) in Thai HIV‐infected patients: 96 week results of ACTT002/ARV065 study. World AIDS Conference. Bangkok, Thailand. July 2004:Abstr.WePeB5952.
UNAIDS 2010
- UNAIDS takes action to empower women and girls to protect themselves from HIV. Unaids.orghttp://data.unaids.org/pub/PressRelease/2010/20100302_pr_womenhiv_en.pdf. Accessed 25 August 2010.
UNAIDS 2014
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global AIDS Response Progress Reporting 2014.. http://www.unaids.org/sites/default/files/media_asset/GARPR_2014_guidelines_en_0.pdf [accessed 27 January 2015].
Urbina A 2005
- Urbina AJibilian A, Nibbe Y. Evaluatioon of viral efficacy and safety of a reduced dose of stavudine (d4T); the PHOENIX study. European AIDS treatment conference Dublin, Ireland. November 2005.
Venter 2012
- WD Francois Venter, Steve Innes, Mark Cotton. LOW‐DOSE STAVUDINE TRIALS: A PUBLIC HEALTH PRIORITY FOR DEVELOPING COUNTRIES. SAJHIV March 2012:20‐21. [Google Scholar]
Wang 2006
- Wang J, Sonnerborg A, Rane A, Josephson F, Lundgren S, Stahle L, Ingelman‐Sundberg M. Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics 2006 Mar;16(3):191‐8. [DOI] [PubMed] [Google Scholar]
WHO 2007
- Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a public health approach. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf accessed 05 January 2013. [PubMed]
WHO 2010
- Antiretroviral therapy for HIV infection in adults and adolescents. http://www.who.int/hiv/pub/arv/adult2010/en/index.html accessed 5 January 2013.


