Summary
Background
The prevalence, epidemiological and clinical heterogeneities, and impact profiles of individuals with preserved ratio impaired spirometry (PRISm), pre-COPD, young COPD, and mild COPD in general Chinese population were not known yet.
Methods
Data were obtained from the China Pulmonary Health study (2012–2015), a nationally representative cross-sectional survey that recruited 50,991 adults aged 20 years or older. Definitions of the four early disease status were consistent with the latest publications and the Global Initiative for Chronic Obstructive Lung Disease criteria.
Findings
The age-standardised prevalences of PRISm, pre-COPD, young COPD, and mild COPD were 5.5% (95% confidence interval, 4.3–6.9), 7.2% (5.9–8.8), 1.1% (0.7–1.8), and 3.1% (2.5–3.8), respectively. In summary, mild COPD was under more direct or established impact factor exposures, such as older age, male gender, lower education level, lower family income, biomass use, air pollution, and more accumulative cigarette exposures; young COPD and pre-COPD experienced more personal and parents’ events in earlier lives, such as history of bronchitis or pneumonia in childhood, frequent chronic cough in childhood, parental history of respiratory diseases, passive smoke exposure in childhood, and mother exposed to passive smoke while pregnant; pre-COPD coexisted with heavier symptoms and comorbidities burdens; young COPD exhibited worse airway obstruction; and most of the four early disease status harbored small airway dysfunction. Overall, older age, male gender, lower education level, living in the urban area, occupational exposure, frequent chronic cough in childhood, more accumulated cigarette exposure, comorbid with cardiovascular disease and gastroesophageal reflux disease were all associated with increased presence of the four early COPD status; different impact profiles were additionally observed with distinct entities. Over the four categories, less than 10% had ever taken pulmonary function test; less than 1% reported a previously diagnosed COPD; and no more than 13% had received pharmaceutical treatment.
Interpretation
Significant heterogeneities in prevalence, epidemiological and clinical features, and impact profiles were noted under varied defining criteria of early COPD; a unified and validated definition for an early disease stage is warranted. Closer attention, better management, and further research need to be administrated to these population.
Funding
Chinese Academy of Medical Sciences Institute of Respiratory Medicine Grant for Young Scholars (No. 2023-ZF-9); China International Medical Foundation (No. Z-2017-24-2301); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2021-I2M-1-049); National High Level Hospital Clinical Research Funding (No. 2022-NHLHCRF-LX-01); Major Program of National Natural Science Foundation of China (No. 82090011).
Keywords: Heterogeneity, Early COPD, PRISm, Pre-COPD, Young COPD, Mild COPD
Research in context.
Evidence before this study
We searched PubMed for articles published from inception to December 1, 2023, using the terms “early COPD” OR “early chronic obstructive pulmonary disease” OR “pre-COPD” OR “pre-chronic obstructive pulmonary disease” AND “heterogeneity”, and found no large clinical and epidemiological studies on the heterogeneity of early stage of COPD in China and even globally.
Added value of this study
To the best of our knowledge, this is the first and largest study in the world so far to estimate the prevalence and determine the heterogeneities of four early disease status of COPD, i.e. PRISm, pre-COPD, young COPD, and mild COPD, using a nationally representative population in China (i.e. the CPH study).
We reported the overall age-standardised prevalences of PRISm, pre-COPD, young COPD, and mild COPD were 5.5%, 7.2%, 1.1%, and 3.1%, respectively. The gender-specific and age-categorized prevalences were also provided. We comprehensively depicted and discussed the heterogeneities of epidemiological and clinical features across the four disease status, and found older age, male gender, lower education level, living in the urban area, occupational exposure, frequent chronic cough in childhood, more accumulated cigarette exposure, comorbid with CVD and GERD were common associated factors with the presence of the four early COPD entities. Different impact profiles were additionally observed. Over the four categories, less than 10% had ever taken pulmonary function test; less than 1% reported a previously diagnosed COPD; and no more than 13% had received pharmaceutical treatment.
Implications of all the available evidence
Significant heterogeneities were noted in prevalence, epidemiological and clinical characteristics, and impact profiles with different definitions of early COPD status; a unified and validated defining criteria is warranted. Closer attention, better management. and further research should be administrated to these population.
Introduction
According to the main report of China Pulmonary Health (CPH) study, the overall prevalence of chronic obstructive pulmonary disease (COPD) in general population aged 20 years or older was 8.6%, accounting for 99.9 million people in China.1 The Global Burden of Disease Study 2019 estimated COPD as the third leading cause of deaths ranked after ischemic heart disease and stroke both in China and globally.2 It is speculated that there may be a large amount of neglected or undiagnosed population who are at the edge of developing into COPD whereas without pulmonary function impairment, namely early stage of COPD.
In the fields of other major chronic diseases, such as diabetes, hypertension, cancer, and dementia, physicians and investigators have shed more lights on the early stage of the diseases, and have tried to deliver early intervention measures to prevent the condition deteriorating severely or rapidly.3, 4, 5, 6 These forward steps have gained encouraging achievements, notably have avoided heavier medical and economic burdens that were caused by late recognition of the diseases. It is expected that such progress could also be achieved in the fields of COPD research and management.
The recent Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2022–2024 reports reemphasized the early status of COPD, including preserved ratio impaired spirometry (PRISm), pre-COPD, young COPD, and mild COPD.7, 8, 9 However, currently there is still no a globally accepted consensus on the definition of early COPD. The similarities and differences in prevalence, epidemiological and clinical features, and developing trajectories, i.e. heterogeneities, underneath varied nomenclatures, have not yet been completely known.
To address above unanswered questions, current study analyzed the CPH survey data, firstly to report the prevalence and to clarify the epidemiological and clinical characteristics of the four early COPD status from multiple aspects (including sociodemographics, living environment, occupational exposure, personal and family histories, cigarette smoking, symptoms and comorbidities, physical examination, laboratory test, pulmonary function test, and diagnosis of COPD), and to determine the impact factors and corresponding association magnitudes that may contribute to early appearance of COPD, in the general Chinese population.
Methods
Study design and participants
The CPH study was a nationwide cross-sectional survey designed to investigate the pulmonary health of Chinese adults aged 20 years or older leveraging a multistage stratified sampling scheme between June 2012 and May 2015 in mainland China. Details of the design and sampling methods of the CPH study have been described previously.1,10 Briefly, a total number of 57,779 local permanent residents (i.e. who had been living in their current residence for one year or longer) recruited from ten representative provinces, autonomous regions, and municipalities were invited to participate in the study (Supplementary Figures S1 and S2). Individuals who were physically incapable of taking a spirometry test (i.e. because of thoracic, abdominal, or eye surgery, retinal detachment, or myocardial infarction in the past three months), who had been admitted to hospital for any cardiac condition in the past month, who had a heart rate greater than 120 beats per min, who had received antibacterial chemotherapy for tuberculosis, or who were pregnant or breastfeeding, were excluded. A detailed survey questionnaire, which incorporated multiple standardised questions and protocols (e.g. cigarette smoking, respiratory symptoms, general and COPD-specific health status, comorbidities, and pulmonary function test, etc.) were administrated to the participants. Finally, 50,991 adult residents who completed post-bronchodilator spirometry test and had reliable test results were included in further analyses (Supplementary Figure S3). A complete comparison of the included (n = 50,991) and excluded (n = 6788) participants is presented in Supplementary Table S1.
In current analyses, following criteria were used to define the four early COPD status and controls:
-
(1)
PRISm: In several studies, PRISm was defined as people with post-bronchodilator forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) ≥ 0.70 and FEV1 and/or FVC <80% predicted, or FEV1/FVC ≥ lower limit of normal (LLN) and FEV1 < LLN.11, 12, 13 In this study, PRISm was termed as people with post-bronchodilator FEV1/FVC ≥0.70 and FEV1 and/or FVC <80% predicted, and not diagnosed as asthma.
-
(2)
Pre-COPD: Han MK et al. proposed to define pre-COPD as people (importantly, of any age) who had respiratory symptoms (e.g. cough, sputum production, dyspnea, and/or exacerbation) with or without detectable structural (e.g. thoracic computed tomography (CT) emphysema, small and/or large airway impairments) and/or functional (e.g. low diffusion capacity for carbon monoxide (DLCO), hyperinflation, small airway obstruction, and/or accelerated FEV1 decline) abnormalities, in the absence of airflow limitation, and who might (or not) develop persistent airflow limitation (i.e. COPD) over time.14 Since lack of CT and DLCO assessments and cross-sectional setting in current study, pre-COPD was defined as people who presented with respiratory symptoms (including chronic cough, chronic sputum production, dyspnea, and/or exacerbation) and small airway dysfunction (SAD) but not diagnosed as COPD (i.e. post-bronchodilator FEV1/FVC ≥0.70) and asthma. The SAD judging criteria were consistent with a prior analysis of the CPH study.15
-
(3)
Young COPD: The GOLD 2022–2024 reports proposed to define young COPD as COPD patients with post-bronchodilator FEV1/FVC <0.70 diagnosed in the 20–50 year age range.7, 8, 9 In this study, similar criteria were applied, and the individuals comorbid with asthma were additionally excluded.
-
(4)
Mild COPD: The GOLD reports defined mild COPD as COPD patients with post-bronchodilator FEV1/FVC <0.70 and FEV1 ≥ 80% predicted.7, 8, 9 In this study, similar criteria were applied, and the individuals comorbid with asthma were additionally excluded.
Controls were defined as people who were not belonged to any of above four early COPD status, with pre- and post-bronchodilator FEV1/FVC ≥0.70, post-bronchodilator FEV1/FVC ≥ LLN, and not diagnosed as asthma.
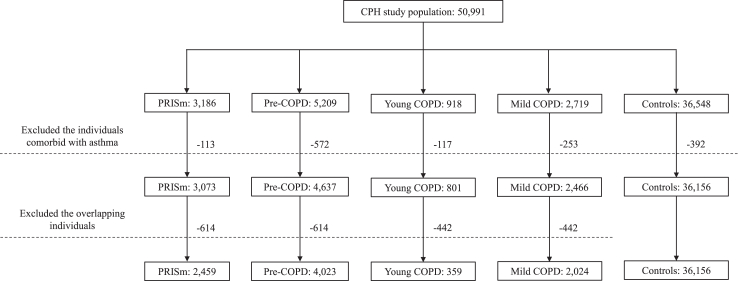
The definitions of respiratory symptoms were provided in Supplementary Text S1. To avoid potential confounding influence of asthma on the early COPD status, especially in a younger population, the individuals comorbid with asthma were both excluded from the cases and controls. Additionally, the overlapping individuals between the four disease categories were deleted from the analyses. Comparison of the theoretical and actual including criteria are presented in Table 1. The flow diagram and numbers of current study population are shown in Fig. 1.
Table 1.
Theoretical versus actual defining criteria for the four early COPD status in current study.
| Early COPD status | Theoretical criteria based on literatures and guidelines | Actual criteria used in this study |
|---|---|---|
| PRISm | People with post-bronchodilator FEV1/FVC ≥0.70 and FEV1 and/or FVC <80% predicted, or FEV1/FVC ≥ LLN and FEV1 < LLN.11, 12, 13 | People with post-bronchodilator FEV1/FVC ≥0.70 and FEV1 and/or FVC <80% predicted, and not diagnosed as asthma. |
| Pre-COPD | People (importantly, of any age) who had respiratory symptoms (e.g. cough, sputum production, dyspnea, and/or exacerbation) with or without detectable structural (e.g. thoracic CT emphysema, small and/or large airway impairments) and/or functional (e.g. low DLCO, hyperinflation, small airway obstruction, and/or accelerated FEV1 decline) abnormalities, in the absence of airflow limitation, and who might (or not) develop persistent airflow limitation (i.e. COPD) over time.14 | People who presented with respiratory symptoms (including chronic cough, chronic sputum production, dyspnea, and/or exacerbation) and SAD but not diagnosed as COPD (i.e. post-bronchodilator FEV1/FVC ≥0.70) and asthma. (The SAD judging criteria were consistent with a prior analysis in the CPH study.15) |
| Young COPD | COPD patients with post-bronchodilator FEV1/FVC <0.70 diagnosed in the 20–50 year age range.7, 8, 9 | COPD patients with post-bronchodilator FEV1/FVC <0.70 in the 20–50 year age range, and not diagnosed as asthma. |
| Mild COPD | COPD patients with post-bronchodilator FEV1/FVC <0.70 and FEV1 ≥ 80% predicted.7, 8, 9 | COPD patients with post-bronchodilator FEV1/FVC <0.70 and FEV1 ≥ 80% predicted, and not diagnosed as asthma. |
The four mutually exclusive early COPD status (i.e. PRISm, pre-COPD, young COPD, and mild COPD) were defined according to the latest Global Initiative for Chronic Obstructive Lung Disease reports and cutting-edge papers. COPD = chronic obstructive pulmonary disease. CPH = China Pulmonary Health. CT = computed tomography. DLco = diffusion capacity for carbon monoxide. FEV1 = forced expiratory volume in 1 s. FVC = forced vital capacity. LLN = lower limit of normal. PRISm = preserved ratio impaired spirometry. SAD = small airway dysfunction.
Fig. 1.
Flowchart of current study. The CPH study recruited a total of 50,991 Chinese adults aged 20 years or older. PRISm, pre-COPD, young COPD, and mild COPD were defined according to the latest Global Initiative for Chronic Obstructive Lung Disease reports and cutting-edge papers. The individuals comorbid with asthma and the overlapping individuals between the four categories were excluded and categorized into mutually exclusive four groups. COPD = chronic obstructive pulmonary disease. CPH = China Pulmonary Health. PRISm = preserved ratio impaired spirometry.
The study protocol was approved by the ethics review committees of the Capital Medical University (Beijing, China) and all other participating centers. Written informed consent were obtained from all participants.
Procedures
In the CPH study, implementation of questionnaire interview, physical examination and blood test were performed by trained medical staff at local community health centers. Details on operation of pulmonary function tests and quality controls have been reported previously.1,10,15 Pre- and post-bronchodilator spirometry tests were conducted with the use of a MasterScreen Pneumo PC spirometer (CareFusion, Yorba Linda, CA, USA) before and 20 min after 400 μg salbutamol inhalation, according to the American Thoracic Society and European Respiratory Society standard protocol.16 Up to eight forced expiratory manoeuvres and at least three acceptable and reproducible FEV1 and FVC variation within 150 mL, daily calibration with a three L syringe, and all data checked by an expert panel of senior physicians and technicians guaranteed the quality of pulmonary test results. Reference values for spirometry among Chinese people were used to determine LLN.17 The ratios of observed to predicted indicators were estimated based on the US general population references.18 Three parameters, i.e. maximal mid-expiratory flow, forced expiratory flow (FEF) at 50% of vital capacity, and FEF at 75% of vital capacity, were used to assess SAD, pre- and post-bronchodilator SAD, which was consistent with a prior analysis of the CPH study.15 Positive bronchodilator reversibility was defined as an increase of FEV1 with ≥12% and ≥200 mL from baseline, after 400 μg salbutamol administration.19
The survey questionnaire comprehensively collected the basic and sociodemographical information, epidemiological characteristics (e.g. impact factor exposure), family and personal histories, respiratory symptoms, management of the condition or disease, health-related quality of life, comorbidities, a range of physical measurements (e.g. anthropometry, blood pressure, oxygen saturation, pre- and post-bronchodilator spirometry tests, electrocardiogram, chest X-ray), and laboratory blood test results.
Most variables used in current analyses, e.g. cigarette smoking status, passive smoke exposure, biomass use, ambient particulate matter with a diameter less than 2.5 μm (PM2.5) exposure, history of bronchitis or pneumonia in childhood, chronic cough in childhood, health status (assessed by the 12-Item Short Form Health Survey),20 etc. have been described previously.1,10,15 Specifically, occupational exposure was determined as dust, allergen, and/or harmful gas exposure for more than 3 months at work; passive smoke exposure in childhood was defined as living with regular smokers at home before the age of 13; parental history of respiratory diseases referred to an asthma, chronic bronchitis, emphysema, COPD, pulmonary heart disease, congenital lung cyst, bronchiectasis, and/or rhinitis history of the participants' parents; exacerbation was defined as the participants’ daily activities and/or works were heavily affected by respiratory symptoms deteriorated; diabetes mellitus was defined as self-reported (i.e. ever diagnosed by a physician) and/or laboratory-determined (i.e. with fasting blood glucose ≥7 mmol/L) diabetes; gastroesophageal reflux disease (GERD) was defined as had a score of ≥8 with the use of the standardized GERD questionnaire21, 22, 23, 24; anxiety and depression were identified as had a score of ≥8 with the use of Hospital Anxiety and Depression Scale questionnaire respectively25, 26, 27; anemia was identified as self-reported (i.e. ever diagnosed by a physician) and/or laboratory-determined (i.e. hemoglobin <120 g/L for male and <110 g/L for female) disorder.
Statistical analyses
For data description, mean (standard deviation) and number and percentage were presented for continuous and categorical variables as appropriate.
Multinomial Logistic regression models were established to determine the associations of potential impact factors with the four early COPD status. The variables with a p value < 0.0001 (yielded from univariable analyses) and with a variance inflation factor <5 (to avoid multicollinearity) were entered into the multivariable-adjusted regression models. Complete cases (i.e. available with all variables of interest) were analyzed, with no imputation for missing data. The missingness of analyzed variables are listed in Supplementary Figure S4. To correct the potential interference on pulmonary function by other respiratory diseases, the individuals comorbid with chronic bronchitis and emphysema were further excluded from the final models.
Both the age-standardised prevalence estimation and multinomial Logistic regression analyses were adjusted for the study weights, which had accounted for 2010 China population census data, study sampling scheme, and several features of the survey, including oversampling for women, non-response, and other demographic differences between the sample and the total population.1,10
All statistical analyses were performed with the use of SAS (version 9.4; SAS Institute, Cary, NC, USA) and SUDAAN (version 11.0; Research Triangle Institute, Research Triangle Park, NC, USA).
Role of the funding source
The funders of the study had no roles in study design, data collection, data analysis, data interpretation, or writing of the manuscript. All authors were not precluded from accessing data in the study, and they accepted responsibility to submit for publication. We were not paid to write this manuscript by a pharmaceutical company or other agency.
Results
Age-standardised prevalence
The numbers and crude prevalences of PRISm, pre-COPD, young COPD, and mild COPD were listed in Supplementary Table S2. The crude prevalences of the four early disease entities in the general population were 4.8%, 7.9%, 0.7%, and 4.0%, respectively.
The age-standardised prevalences in entire population, separately for males and females, and stratified by age categories are shown in Table 2. The overall age-standardised prevalences were 5.5% (95% confidence interval, 4.3–6.9), 7.2% (5.9–8.8), 1.1% (0.7–1.8), and 3.1% (2.5–3.8), respectively. Among males, the age-standardised prevalences were 5.9% (4.3–8.0), 7.5% (6.2–9.2), 1.4% (0.9–2.2), and 4.4% (3.5–5.6), respectively. Among females, the age-standardised prevalences were 5.1% (4.1–6.3), 6.9% (5.4–8.8), 0.8% (0.5–1.4), and 1.7% (1.5–2.0), respectively.
Table 2.
Age-standardised prevalences of PRISm, pre-COPD, young COPD, and mild COPD in general Chinese population, separately for males and females, and stratified by age categories.
| Prevalence (%) | PRISm |
Pre-COPD |
Young COPD |
Mild COPD |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Males | Females | Total | Males | Females | Total | Males | Females | Total | Males | Females | |
| In entire population | 5.5 (4.3–6.9) | 5.9 (4.3–8.0) | 5.1 (4.1–6.3) | 7.2 (5.9–8.8) | 7.5 (6.2–9.2) | 6.9 (5.4–8.8) | 1.1 (0.7–1.8) | 1.4 (0.9–2.2) | 0.8 (0.5–1.4) | 3.1 (2.5–3.8) | 4.4 (3.5–5.6) | 1.7 (1.5–2.0) |
| By age categories (years) | ||||||||||||
| 20–29 | 7.6 (6.0–9.5) | 7.4 (5.4–10.0) | 7.8 (6.0–10.0) | 3.4 (2.5–4.6) | 3.5 (2.4–5.0) | 3.3 (2.3–4.8) | 0.9 (0.3–2.2) | 0.9 (0.3–2.7) | 0.8 (0.4–1.9) | 0 | 0 | 0 |
| 30–39 | 5.2 (3.9–6.8) | 5.7 (4.1–7.9) | 4.6 (3.3–6.4) | 5.3 (3.8–7.3) | 6.0 (3.8–9.4) | 4.6 (3.1–6.8) | 0.7 (0.4–1.2) | 0.7 (0.3–1.7) | 0.6 (0.3–1.1) | 0 | 0 | 0 |
| 40–49 | 5.5 (3.8–7.8) | 6.6 (3.9–11.0) | 4.3 (3.1–5.9) | 8.9 (6.6–11.7) | 10.2 (7.4–14.0) | 7.4 (5.4–10.2) | 1.7 (1.1–2.7) | 2.5 (1.7–3.6) | 1.0 (0.5–1.8) | 0 | 0 | 0 |
| 50–59 | 4.1 (3.2–5.3) | 3.9 (2.7–5.4) | 4.4 (3.5–5.6) | 9.9 (7.5–12.9) | 10.4 (7.8–13.7) | 9.4 (7.1–12.5) | NAa | NAa | NAa | 5.2 (4.2–6.3) | 7.4 (6.0–9.3) | 2.8 (2.2–3.5) |
| 60–69 | 3.6 (2.4–5.2) | 3.9 (2.7–5.6) | 3.2 (2.0–5.1) | 10.0 (7.6–12.9) | 9.7 (7.4–12.6) | 10.3 (7.1–14.7) | 0 | 0 | 0 | 10.8 (8.9–13.0) | 15.5 (12.4–19.1) | 5.9 (4.5–7.7) |
| ≥70 | 5.5 (3.6–8.4) | 6.6 (3.7–11.6) | 4.6 (3.2–6.4) | 10.0 (8.2–12.1) | 7.1 (6.0–8.4) | 12.5 (9.1–16.8) | 0 | 0 | 0 | 15.7 (10.8–22.3) | 22.8 (15.1–32.8) | 9.4 (6.9–12.6) |
| p value | 0.0003 | 0.0052 | 0.0027 | <0.0001 | 0.0001 | <0.0001 | 0.0038 | 0.0055 | 0.1794 | <0.0001 | <0.0001 | <0.0001 |
The age-standardised prevalences were calculated based on 2010 China population census data, and accounted for study sampling weights. For young COPD, the age-standardised prevalences were calculated for 20–29, 30–39, and 40–49 years categories, based on respective population weights for each age categories. Similar calculations were performed for PRISm, pre-COPD, and mild COPD, respectively.
The prevalence was not calculated for young COPD in the 50-59 years category because the maximum age of young COPD was 50 years, therefore the numbers in this category might not be representative for this age group. COPD = chronic obstructive pulmonary disease. NA = not applicable. PRISm = preserved ratio impaired spirometry.
Significant differences were noted across different age categories in almost all subgroups, except for young COPD in females. For PRISm, the highest prevalence was seen in the entire population with an age of 20–29 years, and the rate was a bit higher in females than in males. For pre-COPD, the highest prevalence was seen in the entire population and in females with an age of 60 years or older, whereas the highest rate was seen in males with an age of 40–59 years. For young COPD, the highest prevalence was seen in the entire population, males and females with an age of 40–49 years. For mild COPD, there were obvious trends as the age increasing, and the highest rate was seen in the 70 years or older category in the entire population, including males and females.
Heterogeneities in epidemiological and clinical characteristics
Sociodemographics, impact factor exposures, personal and family histories, respiratory symptoms, comorbidities, pulmonary function test results, and diagnoses of COPD for the study population and controls are presented in Table 3. As per its definition, young COPD was identified with a lowest age (mean ± standard deviation, 41.77 ± 7.64 years). PRISm also showed a relatively younger age (47.74 ± 15.67 years). Mild COPD had an oldest age (63.79 ± 7.53 years). The male percentages in PRISm, pre-COPD, young COPD, and mild COPD were 47.86%, 43.95%, 53.48%, and 61.66%, respectively; all higher than the controls. Mild COPD had the least people received middle and high school education or higher, and had the lowest family income.
Table 3.
Heterogeneities in sociodemographics, epidemiological and clinical characteristics of PRISm, pre-COPD, young COPD, and mild COPD, compared with controls.
| Variables | PRISm |
Pre-COPD |
Young COPD |
Mild COPD |
Controls |
|---|---|---|---|---|---|
| (n = 2459) | (n = 4023) | (n = 359) | (n = 2024) | (n = 36,156) | |
| Sociodemographics | |||||
| Age (years), mean ± SD | 47.74 ± 15.67 | 53.42 ± 12.30 | 41.77 ± 7.64 | 63.79 ± 7.53 | 47.30 ± 13.32 |
| Male, n (%) | 1177 (47.86) | 1768 (43.95) | 192 (53.48) | 1248 (61.66) | 13,837 (38.27) |
| Waist-to-hip ratio (%), mean ± SD | 87.87 ± 7.90 | 88.10 ± 6.58 | 87.88 ± 8.78 | 88.92 ± 6.53 | 86.76 ± 6.87 |
| Education level, n (%) | |||||
| Primary school or less | 551 (22.41) | 1196 (29.73) | 86 (23.96) | 916 (45.26) | 7932 (21.94) |
| Middle or high school | 1381 (56.16) | 2274 (56.52) | 218 (60.72) | 960 (47.43) | 21,218 (58.68) |
| College or higher | 527 (21.43) | 553 (13.75) | 55 (15.32) | 148 (7.31) | 7006 (19.38) |
| Urban residents, n (%) | 1698 (69.05) | 2774 (68.95) | 241 (67.13) | 1222 (60.38) | 23,075 (63.82) |
| Per capita annual household income (10 thousand CNY), mean ± SD | 1.49 ± 1.57 | 1.24 ± 1.52 | 1.16 ± 1.39 | 1.12 ± 1.17 | 1.38 ± 1.81 |
| Living environment | |||||
| Biomass use, n (%) | 485 (19.72) | 1205 (29.95) | 77 (21.45) | 786 (38.83) | 9282 (25.67) |
| Mold on the walls, n (%) | 592 (24.07) | 1163 (28.91) | 101 (28.13) | 563 (27.82) | 9007 (24.91) |
| Annual mean PM2.5 exposure (μg/m³), mean ± SD | 66.84 ± 14.70 | 69.15 ± 16.76 | 66.49 ± 15.23 | 70.28 ± 17.00 | 68.29 ± 15.47 |
| Occupational exposure | |||||
| Dust, allergen, or harmful gas exposure for more than three months, n (%) | 477 (19.40) | 1277 (31.74) | 104 (28.97) | 500 (24.70) | 8279 (22.90) |
| Personal and family histories | |||||
| Born with cesarean, n (%) | 30 (1.22) | 25 (0.62) | 1 (0.28) | 4 (0.20) | 350 (0.97) |
| Premature delivery, n (%) | 68 (2.77) | 105 (2.61) | 7 (1.95) | 48 (2.37) | 956 (2.64) |
| History of bronchitis or pneumonia in childhood, n (%) | 102 (4.15) | 292 (7.26) | 33 (9.19) | 98 (4.84) | 1440 (3.98) |
| Chronic cough in childhood, n (%) | |||||
| Rare | 2187 (88.94) | 3359 (83.49) | 306 (85.24) | 1796 (88.74) | 32,456 (89.77) |
| Sometimes | 179 (7.28) | 421 (10.46) | 29 (8.08) | 129 (6.37) | 2462 (6.81) |
| Frequent | 61 (2.48) | 196 (4.87) | 18 (5.01) | 69 (3.41) | 810 (2.24) |
| Parental history of respiratory diseases, n (%) | 207 (8.42) | 645 (16.03) | 39 (10.86) | 243 (12.01) | 3212 (8.88) |
| Cigarette smoke exposure | |||||
| Mother smoked while pregnant, n (%) | 117 (4.76) | 298 (7.41) | 21 (5.85) | 175 (8.65) | 1975 (5.46) |
| Mother exposed to passive smoke while pregnant, n (%) | 983 (39.98) | 1909 (47.45) | 177 (49.30) | 861 (42.54) | 16,937 (46.84) |
| Passive smoke exposure in childhood, n (%) | 1289 (52.42) | 2445 (60.78) | 207 (57.66) | 1124 (55.53) | 20,760 (57.42) |
| Smoking history, n (%) | |||||
| Never-smoker | 1764 (71.74) | 2552 (63.44) | 213 (59.33) | 1046 (51.68) | 27,323 (75.57) |
| Former smoker | 137 (5.57) | 250 (6.21) | 12 (3.34) | 238 (11.76) | 1562 (4.32) |
| Current smoker | 558 (22.69) | 1221 (30.35) | 134 (37.33) | 740 (36.56) | 7271 (20.11) |
| Smoke exposure (pack-years), n (%) | |||||
| 0 | 1764 (71.74) | 2552 (63.44) | 213 (59.33) | 1046 (51.68) | 27,323 (75.57) |
| 1–9 | 166 (6.75) | 250 (6.21) | 32 (8.91) | 90 (4.45) | 2454 (6.79) |
| 10–19 | 146 (5.94) | 264 (6.56) | 31 (8.64) | 154 (7.61) | 1855 (5.13) |
| ≥20 | 303 (12.32) | 806 (20.03) | 68 (18.94) | 658 (32.51) | 3554 (9.83) |
| Passive smoke exposure at home, n (%) | 1076 (43.76) | 1928 (47.92) | 164 (45.68) | 756 (37.35) | 16,741 (46.30) |
| Smokers living in the home, n (%) | |||||
| 0 | 1322 (53.76) | 2013 (50.04) | 186 (51.81) | 1220 (60.28) | 18,723 (51.78) |
| 1 | 909 (36.97) | 1564 (38.88) | 136 (37.88) | 623 (30.78) | 13,948 (38.58) |
| ≥2 | 167 (6.79) | 364 (9.05) | 28 (7.80) | 133 (6.57) | 2793 (7.72) |
| Symptoms and comorbidities | |||||
| Respiratory symptoms | |||||
| Cough, n (%) | 73 (2.97) | 1748 (43.45) | 95 (26.46) | 457 (22.58) | 1847 (5.11) |
| Sputum, n (%) | 110 (4.47) | 2238 (55.63) | 99 (27.58) | 528 (26.09) | 2656 (7.35) |
| Wheeze, n (%) | 90 (3.66) | 475 (11.81) | 35 (9.75) | 178 (8.79) | 925 (2.56) |
| Dyspnea, n (%) | 113 (4.60) | 1909 (47.45) | 95 (26.46) | 459 (22.68) | 1838 (5.08) |
| mMRC≥2, n (%) | 46 (1.87) | 623 (15.49) | 21 (5.85) | 131 (6.47) | 610 (1.69) |
| CAT≥10, n (%) | 29 (1.18) | 593 (14.74) | 67 (18.66) | 270 (13.34) | 310 (0.86) |
| Exacerbations in prior year, n (%) | 12 (0.49) | 202 (5.02) | 11 (3.06) | 35 (1.73) | 293 (0.81) |
| Self-reported diagnosed COPD, n (%) | 2 (0.08) | 20 (0.50) | 3 (0.84) | 11 (0.54) | 42 (0.12) |
| Pharmaceutical treatment, n (%) | 71 (2.89) | 498 (12.38) | 14 (3.90) | 134 (6.62) | 1103 (3.05) |
| Inhaled corticosteroid | 7 (0.28) | 49 (1.22) | 3 (0.84) | 11 (0.54) | 89 (0.25) |
| Inhaled bronchodilator | 8 (0.33) | 31 (0.77) | 2 (0.56) | 8 (0.40) | 58 (0.16) |
| Aminophylline | 6 (0.24) | 36 (0.89) | 6 (1.67) | 25 (1.24) | 57 (0.16) |
| Systemic corticosteroid | 6 (0.24) | 29 (0.72) | 3 (0.84) | 5 (0.25) | 58 (0.16) |
| Antibiotics | 58 (2.36) | 447 (11.11) | 11 (3.06) | 115 (5.68) | 993 (2.75) |
| Comorbidities, n (%) | |||||
| Rhinitis or allergic rhinitis | 135 (5.49) | 401 (9.97) | 15 (4.18) | 101 (4.99) | 2112 (5.84) |
| Pulmonary tuberculosis | 13 (0.53) | 45 (1.12) | 3 (0.84) | 19 (0.94) | 158 (0.44) |
| Chronic bronchitis | 49 (1.99) | 303 (7.53) | 18 (5.01) | 121 (5.98) | 525 (1.45) |
| Emphysema | 7 (0.28) | 20 (0.50) | 1 (0.28) | 18 (0.89) | 20 (0.06) |
| Bronchiectasis | 7 (0.28) | 26 (0.65) | 2 (0.56) | 11 (0.54) | 57 (0.16) |
| Pulmonary heart disease | 0 (0.00) | 8 (0.20) | 2 (0.56) | 1 (0.05) | 0 (0.00) |
| Pulmonary fibrosis | 1 (0.04) | 5 (0.12) | 1 (0.28) | 1 (0.05) | 14 (0.04) |
| Lung cancer | 1 (0.04) | 8 (0.20) | 0 (0.00) | 1 (0.05) | 12 (0.03) |
| Other malignant tumor | 3 (0.12) | 8 (0.20) | 0 (0.00) | 1 (0.05) | 16 (0.04) |
| Cardiovascular disease | 217 (8.82) | 658 (16.36) | 13 (3.62) | 285 (14.08) | 2566 (7.10) |
| Cerebrovascular disease | 14 (0.57) | 41 (1.02) | 2 (0.56) | 16 (0.79) | 156 (0.43) |
| Diabetes mellitus | 200 (8.13) | 306 (7.61) | 15 (4.18) | 193 (9.54) | 2091 (5.78) |
| Osteoporosis | 22 (0.89) | 101 (2.51) | 1 (0.28) | 36 (1.78) | 294 (0.81) |
| Gastroesophageal reflux disease | 113 (4.60) | 413 (10.27) | 24 (6.69) | 129 (6.37) | 1768 (4.89) |
| Depression | 266 (10.82) | 568 (14.12) | 42 (11.70) | 249 (12.30) | 3777 (10.45) |
| Anxiety | 208 (8.46) | 516 (12.83) | 39 (10.86) | 190 (9.39) | 3167 (8.76) |
| Anemia | 101 (4.11) | 201 (5.00) | 12 (3.34) | 63 (3.11) | 1347 (3.73) |
| Periodontitis | 289 (11.75) | 620 (15.41) | 27 (7.52) | 302 (14.92) | 4287 (11.86) |
| Pulmonary function test | |||||
| Ever took pulmonary function test, n (%) | 235 (9.56) | 392 (9.74) | 30 (8.36) | 149 (7.36) | 2878 (7.96) |
| Pre-bronchodilator FEV1/FVC (%), mean ± SD | 82.21 ± 8.37 | 74.41 ± 4.67 | 63.85 ± 11.99 | 65.30 ± 6.25 | 81.75 ± 5.59 |
| Post-bronchodilator FEV1/FVC (%), mean ± SD | 84.45 ± 7.67 | 77.68 ± 4.34 | 59.54 ± 9.62 | 65.29 ± 4.32 | 83.92 ± 5.33 |
| Post-bronchodilator FEV1%pred (%), mean ± SD | 76.73 ± 7.84 | 98.93 ± 12.94 | 65.34 ± 13.08 | 96.85 ± 14.76 | 105.07 ± 13.63 |
| Bronchodilator reversibility, n (%) | 111 (4.51) | 35 (0.87) | 24 (6.69) | 122 (6.03) | 1162 (3.21) |
| SAD, n (%) | |||||
| SAD | 1459 (59.33) | 4023 (100.00) | 320 (89.14) | 1939 (95.80) | 10,702 (29.60) |
| Pre-SAD | 191 (7.77) | 3177 (78.97) | 30 (8.36) | 290 (14.33) | 10,056 (27.81) |
| Post-SAD | 9 (0.37) | 1803 (44.82) | 0 (0.00) | 0 (0.00) | 4607 (12.74) |
| Diagnosis of COPD | |||||
| Diagnosis of COPD, n (%) | |||||
| Pre-bronchodilator FEV1/FVC <0.70 | 183 (7.44) | 667 (16.58) | 259 (72.14) | 1647 (81.37) | 0 (0.00) |
| Post-bronchodilator FEV1/FVC <0.70 | 0 (0.00) | 0 (0.00) | 359 (100.00) | 2024 (100.00) | 0 (0.00) |
| Post-bronchodilator FEV1/FVC < LLN | 113 (4.60) | 186 (4.62) | 359 (100.00) | 1667 (82.36) | 0 (0.00) |
| Severity of COPD, n (%) | |||||
| Mild | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2024 (100.00) | 0 (0.00) |
| Moderate | 0 (0.00) | 0 (0.00) | 317 (88.30) | 0 (0.00) | 0 (0.00) |
| Severe | 0 (0.00) | 0 (0.00) | 32 (8.91) | 0 (0.00) | 0 (0.00) |
| Very severe | 0 (0.00) | 0 (0.00) | 10 (2.79) | 0 (0.00) | 0 (0.00) |
CAT = chronic obstructive pulmonary disease assessment test. CNY = Chinese yuan. COPD = chronic obstructive pulmonary disease. FEV1 = forced expiratory volume in 1 s. FEV1%pred = percent of predicted value for forced expiratory volume in 1 s. FVC = forced vital capacity. LLN = lower limit of normal. mMRC = modified Medical Research Council. PM2.5 = particulate matter with a diameter less than 2.5 μm. PRISm = preserved ratio impaired spirometry. SAD = small airway dysfunction. SD = standard deviation.
Mild COPD had the most people exposed to biomass fuel (38.83%), and were living amongst the highest PM2.5 concentration (70.28 ± 17.00 μg/m³). Pre-COPD had the most people under occupational exposure (31.74%). Overall, mild COPD had the highest cigarette smoking rate (former and current smokers, 48.32%), with the most accumulated cigarette exposure (≥20 pack-years, 32.51%). Young COPD had the highest current smoking rate (37.33%). Pre-COPD had the most people with passive smoke exposure (47.92%), whereas mild COPD had the least proportion (37.35%).
Young COPD and pre-COPD had the most people with a history of bronchitis or pneumonia (9.19% and 7.26%, respectively), suffering frequent chronic cough (5.01% and 4.87%), and under passive smoke exposure (57.66% and 60.78%) in childhood; parents with a history of respiratory diseases (10.86% and 16.03%); and mothers exposed to passive smoke while pregnant (49.30% and 47.45%).
As per its definition, pre-COPD was presented with the most respiratory symptoms, including cough (43.45%), sputum (55.63%), wheeze (11.81%), dyspnea (47.45%), and exacerbations (5.02%). Young COPD also exhibited heavy symptom burdens and worse quality of life assessed by CAT. Pre-COPD coexisted with the most comorbidities, e.g. rhinitis or allergic rhinitis (9.97%), chronic bronchitis (7.53%), cardiovascular disease (CVD) (16.36%), GERD (10.27%), depression (14.12%), anxiety (12.83%), anemia (5.00%), and periodontitis (15.41%). Mild COPD had the second most comorbidities.
Moreover, less than 10% of the study population had ever taken pulmonary function test; less than 1% reported a previously diagnosed COPD; and no more than 13% had ever received pharmaceutical treatment (the pharmaceutical treatment rates were 2.89%, 12.38%, 3.90%, and 6.62% respectively in PRISm, pre-COPD, young COPD, and mild COPD). Most (88.30%) young COPD patients were identified as moderate severity with airflow obstruction; thus they exhibited the worst pulmonary function (even worse than mild COPD). Pre-COPD presented with the lowest reversibility rate (0.87%) after bronchodilator administration. Spirometry SAD was found in 59.33%, 100.00%, 89.14%, and 95.80% of respective population of PRISm, pre-COPD, young COPD, and mild COPD.
Heterogeneities in impact profiles
The potential and independent impact factors that were associated with discrete disease categories are presented in Supplementary Table S3 and Table 4, respectively. Overall, older age, male gender, lower education level, living in the urban area, occupational exposure, frequent chronic cough in childhood, more accumulated cigarette exposure, comorbid with CVD and GERD were all associated with increased prevalence of the four early COPD entities.
Table 4.
Multiple adjusted associations of the impact factors correlated with presence of PRISm, pre-COPD, young COPD, and mild COPD.
| Variables | PRISm vs controls |
Pre-COPD vs controls |
Young COPD vs controls |
Mild COPD vs controls |
Overall p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | ||
| Age (years) | 0.98 (0.97–1.00) | 0.0819 | 1.03 (1.02–1.04) | <0.0001 | NAa | NAa | 1.14 (1.12–1.16) | <0.0001 | <0.0001 |
| Male | 1.18 (0.83–1.66) | 0.3298 | 0.95 (0.78–1.15) | 0.5712 | 0.75 (0.39–1.44) | 0.3670 | 2.79 (2.00–3.90) | <0.0001 | <0.0001 |
| Waist-to-hip ratio (%) | 1.03 (1.00–1.06) | 0.0333 | 1.00 (0.98–1.02) | 0.9857 | 1.03 (1.01–1.05) | 0.0071 | 1.00 (0.98–1.03) | 0.9072 | 0.0599 |
| Education level | 0.0002 | ||||||||
| College or higher | ref. | ref. | ref. | ref. | |||||
| Middle or high school | 1.26 (0.80–1.98) | 0.2947 | 1.06 (0.73–1.54) | 0.7320 | 3.42 (1.39–8.42) | 0.0104 | 1.90 (1.14–3.18) | 0.0168 | |
| Primary school or less | 2.19 (1.20–4.00) | 0.0132 | 1.35 (0.90–2.01) | 0.1329 | 5.26 (2.47–11.22) | 0.0002 | 2.11 (1.10–4.05) | 0.0267 | |
| Urban residents | 1.18 (0.73–1.92) | 0.4839 | 1.45 (1.04–2.03) | 0.0319 | 1.74 (0.50–6.09) | 0.3659 | 0.75 (0.51–1.12) | 0.1517 | 0.0212 |
| Biomass use | 0.78 (0.49–1.26) | 0.2958 | 1.37 (1.08–1.74) | 0.0119 | 0.84 (0.22–3.22) | 0.7869 | 1.95 (1.23–3.12) | 0.0075 | 0.0646 |
| Dust, allergen, or harmful gas exposure for more than three months | 0.88 (0.62–1.23) | 0.4263 | 1.60 (1.09–2.34) | 0.0195 | 1.19 (0.43–3.30) | 0.7193 | 1.07 (0.67–1.73) | 0.7584 | <0.0001 |
| Chronic cough in childhood | <0.0001 | ||||||||
| Rare | ref. | ref. | ref. | ref. | |||||
| Sometimes | 1.20 (0.70–2.07) | 0.4808 | 1.53 (1.10–2.11) | 0.0138 | 2.04 (1.02–4.07) | 0.0438 | 0.85 (0.61–1.18) | 0.3069 | |
| Frequent | 1.45 (0.88–2.39) | 0.1399 | 3.33 (2.16–5.12) | <0.0001 | 6.98 (2.43–20.07) | 0.0011 | 2.77 (1.33–5.80) | 0.0095 | |
| Smoke exposure (pack-years) | <0.0001 | ||||||||
| 0 | ref. | ref. | ref. | ref. | |||||
| 1–9 | 1.19 (0.75–1.88) | 0.4434 | 1.30 (0.87–1.94) | 0.1932 | 1.34 (0.70–2.57) | 0.3525 | 0.79 (0.41–1.54) | 0.4708 | |
| 10–19 | 0.58 (0.38–0.88) | 0.0138 | 1.42 (0.93–2.18) | 0.0977 | 1.81 (0.89–3.66) | 0.0957 | 1.46 (0.99–2.16) | 0.0556 | |
| ≥20 | 1.95 (0.89–4.27) | 0.0914 | 3.05 (2.11–4.41) | <0.0001 | 5.49 (2.76–10.91) | 0.0001 | 2.57 (2.12–3.12) | <0.0001 | |
| Cardiovascular disease | 1.35 (0.90–2.04) | 0.1417 | 1.47 (1.17–1.84) | 0.0022 | 0.33 (0.13–0.85) | 0.0246 | 1.36 (0.89–2.09) | 0.1445 | 0.0009 |
| Gastroesophageal reflux disease | 1.23 (0.48–3.13) | 0.6519 | 1.72 (1.27–2.33) | 0.0015 | 2.99 (1.63–5.51) | 0.0014 | 1.11 (0.61–2.01) | 0.7224 | 0.0025 |
A fully adjusted multivariable multinomial logistic regression model was established between PRISm, pre-COPD, young COPD, and mild COPD versus controls to determine the associations with potential impact factors. The included covariates were age, male gender, waist-to-hip ratio, education level, urban residency, biomass use, occupational exposure, chronic cough in childhood, smoke exposure in pack-years, comorbid with cardiovascular disease and gastroesophageal reflux disease.
The association of age was not estimated for young COPD because age was included in the defining criteria of young COPD. CI = confidence interval. COPD = chronic obstructive pulmonary disease. NA = not applicable. OR = odds ratio. PRISm = preserved ratio impaired spirometry.
Specifically, older age, living in the urban area, biomass use, occupational exposure, frequent chronic cough in childhood, more accumulated cigarette exposure, comorbid with CVD and GERD were associated with increased prevalence of pre-COPD. Of these factors, frequent chronic cough in childhood (frequent cough: odds ratio [95% confidence interval] 3.33 [2.16–5.12], p < 0.0001; versus rare cough) and more accumulated cigarette exposure (≥20 pack-years: 3.05 [2.11–4.41], p < 0.0001; versus 0 pack-years) showed the strongest associations.
Higher waist-to-hip ratio, lower education level, frequent chronic cough in childhood, more accumulated cigarette exposure, comorbid with GERD were associated with increased prevalence of young COPD. Of these factors, frequent chronic cough in childhood (frequent cough: 6.98 [2.43–20.07], p = 0.0011; versus rare cough), more accumulated cigarette exposure (≥20 pack-years: 5.49 [2.76–10.91], p = 0.0001; versus 0 pack-years), lower education level (primary school or less: 5.26 [2.47–11.22], p = 0.0002; middle or high school: 3.42 [1.39–8.42], p = 0.0104; versus college or higher), and comorbid with GERD (with GERD: 2.99 (1.63–5.51), p = 0.0014; versus without GERD) showed the strongest associations.
In addition, higher waist-to-hip ratio and lower education level were correlated with increased prevalence of PRISm. Older age, male gender, lower education level, biomass use, frequent chronic cough in childhood, and more accumulated cigarette exposure were correlated with increased prevalence of mild COPD.
Discussion
This is the first and largest study in the world so far to estimate the prevalence, compare the epidemiological and clinical characteristics, and determine the heterogeneities of four early disease status of COPD, i.e. PRISm, pre-COPD, young COPD, and mild COPD, using a nationally representative population in China.
In this study, the overall age-standardised prevalences of PRISm, pre-COPD, young COPD, and mild COPD were 5.5%, 7.2%, 1.1%, and 3.1%, respectively. Compared to the overall prevalence of COPD (8.6%) and asthma (4.2%) in the CPH study, these data implicated pre-COPD that involving respiratory symptoms, pulmonary structure and function impairments affected a large proportion of people in the general population; the prevalence of PRISm was even higher than asthma; and the prevalence of young COPD was similar to the asthma which with airflow limitation (1.1%).1,10
Mild COPD was under more direct or established impact factor exposures, such as older age, male gender, lower education level, lower family income, biomass use, air pollution, and more accumulative cigarette exposures. Young COPD and pre-COPD experienced more personal and parents’ events in earlier lives, such as history of bronchitis or pneumonia in childhood, frequent chronic cough in childhood, parental history of respiratory diseases, passive smoke exposure in childhood, and mother exposed to passive smoke while pregnant. Pre-COPD coexisted with heavier symptoms and comorbidities burdens. Young COPD exhibited worse airway obstruction. Most of the four early disease entities harbored SAD. These findings indicated the disease burdens of early COPD cannot be solely assessed by pulmonary function, and symptoms are also important judged parameters; young COPD patients deserve more attention.
Additionally, older age, male gender, lower education level, living in the urban area, occupational exposure, frequent chronic cough in childhood, more accumulated cigarette exposure, comorbid with CVD and GERD were common associated factors with the presence of the four early COPD status. Of these impact factors, moving to live in a place with better environment, avoid occupational exposure, cigarette smoke cessation, and better controls of CVD and GERD could be considered to reduce the probability of suffering from early COPD. Moreover, different modifiable factors were noted for distinct status. Specifically, keeping fitness may prevent the occurrence of PRISm and young COPD; using clean energy for cooking and heating could reduce the presence of pre-COPD and mild COPD.
In this study, apart from intrinsic or physiopathological heterogeneities (which are not fully known yet) under different concepts, we thought varied definitions are also the origin of the heterogeneities. In addition, the latest GOLD reports suggested to use the term “early COPD” to discuss “biological early” only (not “clinical early”) when appropriate, for example in an experimental setting.7, 8, 9 Gogali A and Kostikas K. proposed a new term “latent COPD” for the individuals with structural and/or functional abnormalities and no/minimal symptoms to separate them from patients with overt COPD.28 However, we thought the “biological early COPD” and “latent COPD” still need more explanations. Moreover, the unstable state (i.e. probable transition to other categories) of the early disease status is another origin of the heterogeneities. For example, only a small proportion of PRISm and pre-COPD individuals would certainly progress to affirmed COPD in the coming years.11,13,14,29, 30, 31, 32, 33 To our knowledge, currently there was no a globally accepted consensus on the definition of early COPD, therefore a unified agreement is warranted. Moreover, in order to avoid the potential confounding interference of asthma in early and young COPD, we advocate to exclude the individuals comorbid with asthma from future studies of early COPD and studies attempting to describe lung function trajectories leading to COPD.
Early intervene this chronic airway disease evolution is one of the main aims in early COPD research. Several studies have tried to adopt cigarette smoke cessation,34,35 vaccination,36 pulmonary rehabilitation,37 or pharmacological therapy38 attempting to halt the disease progression in an early stage; however, the evidence on efficacy and safety of pharmacological intervention is scarce. In addition, the target population (e.g. PRISm, pre-COPD, young COPD, mild COPD, or latent COPD?) who could most benefit from early intervention is still unclear. Treatment trials for these population should be planned and well assessed.39
In this study, over the four disease groups, less than 10% of the participants had ever taken pulmonary function test; less than 1% reported a previously diagnosed COPD; and no more than 13% had received pharmaceutical treatment. These data highlighted a large amount of undiagnosed COPD that had not yet been identified, and underscored enormous unmet medical and public health needs for early COPD. The health administrative agencies should give more priorities to and allocate adequate resources to these population, such as improve the awareness of COPD, promote the application of pulmonary function test (especially in the primary and rural areas), improve self- and medical care of lung health, etc.
There were several limitations in this study. First, the CPH study was a cross-sectional survey, therefore we could not prospectively observe the evolution of these early disease status along time, and the impact profiles identified in current analysis should be cautiously interpretated. Second, not all literal indices should be used to define the four early disease entities were available in the CPH database. For example, thoracic CT scan, DLCO test, and annual decline of FEV1 were not accessible. These lacking information could be collected in future longitudinal studies.
Now we are implementing several prospective cohort studies to supplement the limits of current study. The Lung Health of Early COPD study (NCT05466396) has been initiated aiming to reveal the longitudinal change of COPD from an early stage. The Heterogeneity and Development of Early COPD study (NCT06096285) has been started to develop a novel multi-dimensional defining criteria for early COPD. The National COPD Screening Program (NCT05480176) has been established to detect the COPD high-risk population and undiagnosed COPD patients, aiming to address the lacking medical and public health needs in China.40
In conclusion, significant heterogeneities in prevalence, epidemiological and clinical features, and impact profiles were noted under varied defining criteria of early COPD; a unified and validated definition for an early disease stage is warranted. Closer attention, better management, and further research need to be administrated to these population.
Contributors
JL and KH conceived and designed the study. TY and CW supervised the work. JL did all the statistical analyses and was responsible for the data interpretation. All authors contributed to data collection, curation, and interpretation. KH and SW were responsible for this project and resource administration. TY and CW had full access and verification to all the data. JL drafted the manuscript. All authors critically revised the manuscript and approved the final version to be considered for publication.
Data sharing statement
The study data could be made available on reasonable request from the corresponding authors TY and CW.
Declaration of interests
We declare no competing interests.
Acknowledgements
This study was supported by Chinese Academy of Medical Sciences Institute of Respiratory Medicine Grant for Young Scholars (No. 2023-ZF-9, to JL); China International Medical Foundation (No. Z-2017-24-2301, to JL); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. 2021-I2M-1-049, to TY); National High Level Hospital Clinical Research Funding (No. 2022-NHLHCRF-LX-01, to TY); Major Program of National Natural Science Foundation of China (No. 82090011, to CW). We thank all the participants and participating centers involving in the China Pulmonary Health study. We also thank all the reviewers for their constructive comments; their insights have greatly improved the quality of our report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2024.101021.
Contributor Information
Ting Yang, Email: zryyyangting@163.com.
Chen Wang, Email: wangchen@pumc.edu.cn.
Appendix A. Supplementary data
References
- 1.Wang C., Xu J., Yang L., et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Collaborative Network . 2020. Global burden of disease study 2019 (GBD 2019) results.https://vizhub.healthdata.org/gbd-results/ [Google Scholar]
- 3.Echouffo-Tcheugui J.B., Perreault L., Ji L., Dagogo-Jack S. Diagnosis and management of prediabetes: a review. JAMA. 2023;329(14):1206–1216. doi: 10.1001/jama.2023.4063. [DOI] [PubMed] [Google Scholar]
- 4.Egan B.M., Stevens-Fabry S. Prehypertension--prevalence, health risks, and management strategies. Nat Rev Cardiol. 2015;12(5):289–300. doi: 10.1038/nrcardio.2015.17. [DOI] [PubMed] [Google Scholar]
- 5.Panegyres P.K., Berry R., Burchell J. Early dementia screening. Diagnostics. 2016;6(1) doi: 10.3390/diagnostics6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams S.J., Stone E., Baldwin D.R., Vliegenthart R., Lee P., Fintelmann F.J. Lung cancer screening. Lancet. 2023;401(10374):390–408. doi: 10.1016/S0140-6736(22)01694-4. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstructive Lung Disease . 2021. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2022 Report)https://goldcopd.org [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease . 2022. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 Report)https://goldcopd.org [Google Scholar]
- 9.Global Initiative for Chronic Obstructive Lung Disease . 2023. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2024 Report)https://goldcopd.org [Google Scholar]
- 10.Huang K., Yang T., Xu J., et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. 2019;394(10196):407–418. doi: 10.1016/S0140-6736(19)31147-X. [DOI] [PubMed] [Google Scholar]
- 11.Wan E.S., Hokanson J.E., Regan E.A., et al. Significant spirometric transitions and preserved ratio impaired spirometry among ever smokers. Chest. 2022;161(3):651–661. doi: 10.1016/j.chest.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan E.S., Balte P., Schwartz J.E., et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA. 2021;326(22):2287–2298. doi: 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higbee D.H., Granell R., Davey Smith G., Dodd J.W. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med. 2022;10(2):149–157. doi: 10.1016/S2213-2600(21)00369-6. [DOI] [PubMed] [Google Scholar]
- 14.Han M.K., Agusti A., Celli B.R., et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med. 2021;203(4):414–423. doi: 10.1164/rccm.202008-3328PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao D., Chen Z., Wu S., et al. Prevalence and risk factors of small airway dysfunction, and association with smoking, in China: findings from a national cross-sectional study. Lancet Respir Med. 2020;8(11):1081–1093. doi: 10.1016/S2213-2600(20)30155-7. [DOI] [PubMed] [Google Scholar]
- 16.Miller M.R., Hankinson J., Brusasco V., et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Jian W., Gao Y., Hao C., et al. Reference values for spirometry in Chinese aged 4-80 years. J Thorac Dis. 2017;9(11):4538–4549. doi: 10.21037/jtd.2017.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Asthma . 2023. Global strategy for asthma management and prevention (2023 report)www.ginasthma.org [Google Scholar]
- 20.Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Jones R., Junghard O., Dent J., et al. Development of the GerdQ, a tool for the diagnosis and management of gastro-oesophageal reflux disease in primary care. Aliment Pharmacol Ther. 2009;30(10):1030–1038. doi: 10.1111/j.1365-2036.2009.04142.x. [DOI] [PubMed] [Google Scholar]
- 22.Jonasson C., Wernersson B., Hoff D.A., Hatlebakk J.G. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–572. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 23.Wang B., Sun Q., Du Y., Mu K., Jiao J. Diagnosis and etiological analysis of gastroesophageal reflux disease by gastric filling ultrasound and GerdQ scale. J Healthc Eng. 2021;2021 doi: 10.1155/2021/5629067. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Liu Z., Gao X., Liang L., et al. Prevalence, general and periodontal risk factors of gastroesophageal reflux disease in China. J Inflamm Res. 2023;16:235–244. doi: 10.2147/JIR.S395777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 26.Lam C.L., Pan P.C., Chan A.W., Chan S.Y., Munro C. Can the Hospital Anxiety and Depression (HAD) Scale be used on Chinese elderly in general practice? Fam Pract. 1995;12(2):149–154. doi: 10.1093/fampra/12.2.149. [DOI] [PubMed] [Google Scholar]
- 27.Huang K., Huang K., Xu J., et al. Anxiety and depression in patients with chronic obstructive pulmonary disease in China: results from the China pulmonary health [CPH] study. Int J Chron Obstruct Pulmon Dis. 2021;16:3387–3396. doi: 10.2147/COPD.S328617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gogali A., Kostikas K. Latent COPD: a proposed new term in the disease nomenclature. Eur Respir J. 2023;61(5) doi: 10.1183/13993003.00535-2023. [DOI] [PubMed] [Google Scholar]
- 29.Wan E.S., Fortis S., Regan E.A., et al. Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene study. Am J Respir Crit Care Med. 2018;198(11):1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijnant S.R.A., De Roos E., Kavousi M., et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam Study. Eur Respir J. 2020;55(1) doi: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 31.He D., Sun Y., Gao M., et al. Different risks of mortality and longitudinal transition trajectories in new potential subtypes of the preserved ratio impaired spirometry: evidence from the English longitudinal study of aging. Front Med. 2021;8 doi: 10.3389/fmed.2021.755855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Padilla R., Montes de Oca M., Thirion-Romero I., et al. Trajectories of spirometric patterns, obstructive and PRISm, in a population-based cohort in Latin America. Int J Chron Obstruct Pulmon Dis. 2023;18:1277–1285. doi: 10.2147/COPD.S406208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divo M.J., Liu C., Polverino F., Castaldi P.J., Celli B.R., Tesfaigzi Y. From pre-COPD to COPD: a Simple, Low cost and easy to IMplement (SLIM) risk calculator. Eur Respir J. 2023;62(3) doi: 10.1183/13993003.00806-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee P.N., Fry J.S. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med. 2010;8:84. doi: 10.1186/1741-7015-8-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montes de Oca M., Laucho-Contreras M.E. Smoking cessation and vaccination. Eur Respir Rev. 2023;32(167) doi: 10.1183/16000617.0187-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon S., Joean O., Welte T., Rademacher J. The role of vaccination in COPD: influenza, SARS-CoV-2, pneumococcus, pertussis, RSV and varicella zoster virus. Eur Respir Rev. 2023;32(169) doi: 10.1183/16000617.0034-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troosters T., Janssens W., Demeyer H., Rabinovich R.A. Pulmonary rehabilitation and physical interventions. Eur Respir Rev. 2023;32(168) doi: 10.1183/16000617.0222-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Celli B.R., Anderson J.A., Cowans N.J., et al. Pharmacotherapy and lung function decline in patients with chronic obstructive pulmonary disease. A systematic review. Am J Respir Crit Care Med. 2021;203(6):689–698. doi: 10.1164/rccm.202005-1854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez F.J., Agusti A., Celli B.R., et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am J Respir Crit Care Med. 2022;205(3):275–287. doi: 10.1164/rccm.202107-1663SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lei J., Huang K., Pan J., et al. The national COPD screening programme in China: rationale and design. ERJ Open Res. 2023;9(2) doi: 10.1183/23120541.00597-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.