Summary
Pinpointing functional, structural, and redox-sensitive cysteines is a central challenge of chemoproteomics. Here, we present a protocol comprising two dual-enrichment cysteine chemoproteomic techniques that enable capture of cysteines (Cys-LoC) and quantification of cysteine oxidation state (Cys-LOx) in a localization-specific manner. We describe steps for utilizing TurboID-mediated protein biotinylation for enrichment of compartment-specific proteins, followed by click-mediated biotinylation and enrichment of cysteine-containing peptides. Thus, changes to compartment-specific cysteine identification and redox state can be assessed in a variety of contexts.
For complete details on the use and execution of this protocol, please refer to Yan et al. (2023).1
Subject areas: Molecular/Chemical Probes, Proteomics, Systems biology
Graphical abstract
Highlights
-
•
Combining proximity labeling and click chemistry enables dual-enrichment proteomics
-
•
Dual-enrichment proteomics enables profiling of compartment-specific cysteines
-
•
Cys-LoC identifies compartment-specific cysteines
-
•
Cys-LOx quantifies cysteine oxidation state in a compartment-specific manner
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Pinpointing functional, structural, and redox-sensitive cysteines is a central challenge of chemoproteomics. Here, we present a protocol comprising two dual-enrichment cysteine chemoproteomic techniques that enable capture of cysteines (Cys-LoC) and quantification of cysteine oxidation state (Cys-LOx) in a localization-specific manner. We describe steps for utilizing TurboID-mediated protein biotinylation for enrichment of compartment-specific proteins, followed by click-mediated biotinylation and enrichment of cysteine-containing peptides. Thus, changes to compartment-specific cysteine identification and redox state can be assessed in a variety of contexts.
Before you begin
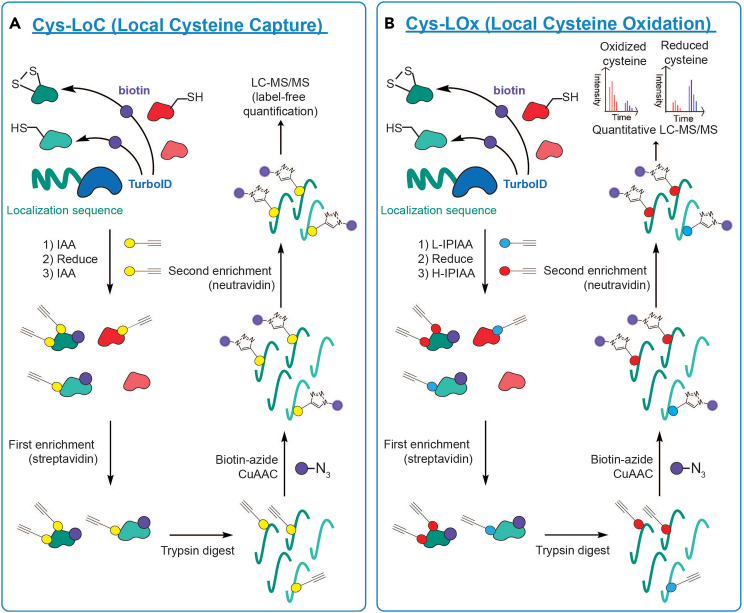
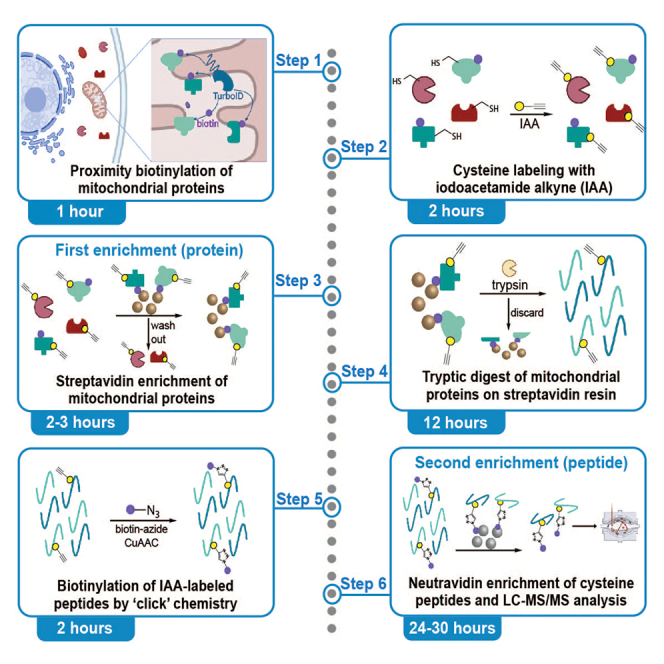
We recently reported two parallel chemoproteomics methods that enable compartment-specific cysteine identification (Local Cysteine Capture; Cys-LoC) (Figure 1A) and localization-specific quantification of cysteine oxidation state (Local Cysteine Oxidation; Cys-LOx)1 (Figure 1B). The protocol overcomes limitations of established methods for profiling cysteine redox state,2,3,4,5,6 which do so on a global scale, thus masking subcellular-specific information. Our protocol utilizes two enrichment steps, one on the protein level and one on the peptide level, for isolation of compartment specific cysteines. The first step uses a localized TurboID7 construct for proximity biotinylation of proteins in a specific subcellular compartment (e.g., mitochondria), enabling enrichment of proteins from this compartment. For Cys-LoC, cysteines within these proteins are capped with an iodoacetamide alkyne (IAA) probe, to enable subsequent capture of compartment-specific cysteine peptides via peptide-level biotinylation and enrichment (Figure 1A). For Cys-LOx, natively reduced cysteines are capped by an isotopically “light” isopropyl iodoacetamide alkyne probe (L-IPIAA), followed by reduction of natively oxidized cysteines, to enable capping of the oxidized subset with an isotopically “heavy” IPIAA probe (H-IPIAA), such that reduced and oxidized cysteines are differentially labeled (Figure 1B). For both Cys-LoC and Cys-LOx, peptides containing (IP)IAA-labeled cysteines are biotinylated through copper-catalyzed azide-alkyne cycloaddition (‘click’ chemistry), enabling enrichment of cysteine-containing peptides. Subsequently, enriched cysteine peptides are analyzed and quantified by liquid chromatography–tandem mass spectrometry (LC-MS/MS).
Figure 1.
Workflows for (A) Cys-LoC (Local Cysteine Capture) and (B) Cys-LOx (Local Cysteine Oxidation)
(A) A localized TurboID construct (e.g., mitochondria-localized) will biotinylate proteins within a single subcellular compartment, which are represented here as green-colored proteins. Cells are then lysed, and all natively reduced cysteines are labeled with iodoacetamide alkyne (IAA) prior to addition of DTT to reduce natively oxidized cysteines, as DTT can interfere with iodoacetamide labeling of cysteines. In order to ensure comprehensive profiling of localized cysteines, natively oxidized cysteines are then reduced and capped by a sequential round of IAA labeling (using an excess of IAA relative to DTT). Streptavidin enrichment then enriches biotinylated proteins from a single cellular compartment (e.g., mitochondria), and an on-resin trypsin digest releases peptides from the enriched proteins. IAA-labeled peptides are then conjugated to biotin-azide via CuAAC ‘click’ chemistry, and biotinylated peptides are enriched by neutravidin. Enriched peptides are then analyzed by label-free LC-MS/MS to identify cysteines from the specified subcellular location.
(B) A localized TurboID construct (e.g., mitochondria-localized) will biotinylate proteins within a single subcellular compartment. After cell lysis, natively reduced cysteines are labeled with “light” isopropyl iodoacetamide alkyne (L-IPIAA). Natively oxidized cysteines are then reduced and capped by “heavy” isopropyl iodoacetamide alkyne (H-IPIAA), such that reduced and oxidized cysteines are differentially labeled. Streptavidin enrichment then enriches biotinylated proteins from a single cellular compartment (e.g., mitochondria), and an on-resin trypsin digest releases peptides from the enriched proteins. IPIAA-labeled peptides are then conjugated to biotin-azide via CuAAC ‘click’ chemistry, and biotinylated peptides enriched by neutravidin. Enriched peptides are then analyzed by quantitative LC-MS/MS to quantify redox states of the cysteines from the specified subcellular location.
The protocol below describes the steps for both local cysteine capture (Cys-LoC) and local cysteine oxidation state quantitation (Cys-LOx). For Cys-LOx, we describe the protocol for interrogation of mitochondrial cysteine oxidation state changes in response to lipopolysaccharide (LPS) and interferon-gamma (IFNγ) in immortalized bone marrow-derived macrophages (iBMDM). These stimuli were chosen for iBMDM cells as this cell line is known to adopt a pro-inflammatory program in response to LPS and IFNγ treatment, which results in robust generation of reactive oxygen species (ROS) and reactive nitrogen species (RNS) via induction of nitric oxide synthase (iNOS).8,9,10,11 This induction correlates with complete ablation of mitochondrial oxidative phosphorylation.12 While the increase in ROS and RNS in response to LPS and IFNγ is well established, the mitochondria-specific cysteines with stimuli-sensitive oxidation states were not elucidated prior to our recent report using this protocol.1 Demonstrating the broad utility of our protocol, we have also performed the protocol in HEK293T cells and in additional subcellular compartments (Golgi, endoplasmic reticulum, cytosol, nucleus). Given the relevance of oxidative modifications to many cellular processes, the generalizability of Cys-LOx is useful for interrogation of additional cellular stimulants and systems, and could be applied, for example, to interrogate the oxidation-sensitive cysteines that contribute to phase separation of membraneless organelles such as stress granules,13 cysteines that are sensitive to oxidative modifications in response to viral infection,14,15 and identify redox-sensitive cysteines within cellular models of aging.5,16 The protocol described herein utilizes stably expressed localized TurboID constructs. Notably, the protocol has been found to generalize to transient expression systems.
Acquire plasmid for stable expression of mitochondrial-localized TurboID
Timing: 1 week
-
1.All plasmids used in this protocol and in the original study1 for stable expression of localized TurboID have been deposited on Addgene for public use:
-
a.FUGW-mito-TurboID: Addgene #200963.
-
b.FUGW-cytosol-TurboID: Addgene #200961.
-
c.FUGW-ER-TurboID: Addgene #200965.
-
d.FUGW-Golgi-TurboID: Addgene #200964.
-
e.pCMV-VSV-G lentiviral packaging: Addgene #8454.
-
f.pCMV-dR8.2 dvpr lentiviral packaging: Addgene #8455.
-
a.
Note: This protocol will detail the use of the mito-TurboID construct. If an alternative cellular compartment is desired, the corresponding plasmid can be purchased from Addgene and used in the same manner. Alternatively, a desired localization sequence can be fused to TurboID and subcloned into a vector compatible with stable expression (e.g. lentiviral transduction).
Employ lentiviral transduction to stably express mitochondrial-localized TurboID in immortalized bone-marrow derived macrophages (iBMDMs)
Timing: 1–2 weeks
-
2.
Culture HEK293T cells in a 10 cm dish with DMEM media at 37°C under 5% CO2. Supplement DMEM media with 10% fetal bovine serum (FBS) without antibiotics.
CRITICAL: It is critical to culture HEK293T cells for lentiviral production in antibiotic-free media.
-
3.Generate mitochondrial-localized TurboID lentivirus.
-
a.Transfect one 10-cm dish of HEK293T cells at 70%–80% confluency by combining the following in a microcentrifuge tube in the following order:
-
i.10 μg of the FUGW-mito-TurboID plasmid (Addgene #200963).
-
ii.4 μg of the pCMV-VSV-G lentiviral packaging plasmid (Addgene #8454).
-
iii.8 μg of pCMV-dR8.2 dvpr lentiviral packaging plasmid (Addgene #8455).
-
iv.1 mL reduced-serum Opti-MEM media.
-
v.66 μL of Lipofexin (Lamda Biotech Inc.).
-
i.
-
b.Gently mix the transfection cocktail and let incubate in the hood for 15 min.
-
c.Add transfection cocktail dropwise to plate of HEK293T cells in 10 mL antibiotic-free media and let incubate for 6 h.
-
d.After 6 h, replace media with fresh antibiotic-free media (10 mL) and allow cells to incubate for 48 h for lentiviral generation.
-
e.After 48 h, collect media (containing lentivirus) and store at 4°C.
-
f.Give cells fresh antibiotic-free media (10 mL ) and allow cells to incubate for an additional 24 h.
-
g.After 24 h, collect the lentivirus-containing media and add to the previously harvested media (total collected media is 20 mL).
-
h.To the 20 mL of lentivirus-containing media, add 6.66 mL of Lenti-X concentrator (Takara Bio, #631232) (1/3 total volume of media) and incubate for 16 h at 4°C.
-
i.Pellet the lentivirus by centrifuging at 1500 g for 45 min at 4°C, aspirate the supernatant, and resuspend the pellet (live virus) in 500 μL serum-free DMEM media.
-
j.Store the lentivirus in 100 μL aliquots at −80°C or use directly in step 4.
-
a.
-
4.Transduce immortalized bone marrow-derived macrophages (iBMDMs) with mitochondria- localized TurboID lentivirus.
-
a.Culture iBMDMs in 10-cm dish in DMEM at 37°C under 5% CO2. Supplement DMEM media with 10% fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 5% (v/v) macrophage colony stimulating factor (M-CSF). Grow cells to ∼75% confluency.
-
b.Add 250 μL of reconstituted virus to the dish of cells and let incubate for 24–48 h.
-
a.
-
5.
Passage cells ∼5 times.
-
6.
Because the mito-TurboID construct within the FUGW-mito-TurboID plasmid is tagged with GFP, successfully transduced cells will be fluorescent. Therefore, employ fluorescence activated cell sorting (FACS) to sort and collect the GFP-positive cells. Expand these cells for use in the following steps.
Note: For long term storage of generated cell lines, resuspend cells in fetal bovine serum (FBS) containing 10% DMSO and aliquot 3–5 × 106 cells in this suspension per cryogenic tube. Place cryogenic tubes at -80°C for 24–48 h, then transfer to a liquid nitrogen cryogenic freezer for long term storage.
Culture and seed iBMDMs that are stably expressing mitochondrial-localized TurboID
Timing: 3 days
-
7.
Culture iBMDM cells that are stably expressing mitochondria-localized TurboID in a 10 cm dish in DMEM media at 37°C under 5% CO2. Supplement DMEM medium with 10% fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 5% (v/v) macrophage colony stimulating factor (M-CSF).
Note: Any cell line that is stably expressing mitochondria-localized TurboID can be used. Here, we selected immortalized bone marrow-derived macrophages (iBMDMs) to interrogate cysteines with redox states sensitive to an inflammatory response initiated by lipopolysaccharide and interferon gamma.
-
8.
When iBMDM cells reach 90%–100% confluent, remove the culture medium and add 5 mL DPBS to wash cells. Aspirate DPBS.
-
9.
Add 1 mL 0.25% (1×) Trypsin solution to the dish and place the dish in a CO2 cell incubator for ∼3 min at 37°C.
-
10.
Add 5 mL supplemented DMEM media to inhibit the trypsin. Pipet the cell suspension several times to remove all cells from the plate and collect the cell suspension in a 15-mL falcon tube.
-
11.
Spin the cells down at 500 g for 3 min at 4°C, aspirate media, and resuspend cells in 3 mL fresh media.
-
12.
Count cells and seed ∼3 × 106 iBMDM cells per 10-cm dish in 10 mL DMEM media supplemented with 10% dialyzed fetal bovine serum, 100 U/mL penicillin, 100 mg/mL streptomycin, and 5% (v/v) macrophage colony stimulating factor (M-CSF).
CRITICAL: To limit background signal observed during the protocol, it is critical to seed cells that will be used for the experiment in media that is supplemented with dialyzed fetal bovine serum and without biotin. This limits endogenous biotinylation of nonspecific proteins.
Note: Seed 3 10-cm dishes of cells per experimental conditions used (for 3 biological replicates per condition). For example, we performed the Cys-LOx protocol with an inflammation-stimulated condition (3 dishes) and a control condition (3 dishes), requiring a total of 6 10-cm dishes of iBMDM cells for the experiment.
Synthesize required chemical probes
Timing: 3–5 days
- 13.
- 14.
- 15.
- 16.
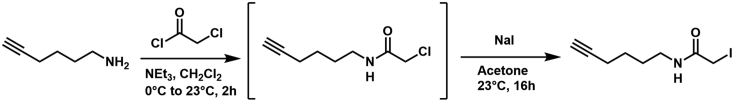
Scheme 1.
N-(hex-5-yn-1-yl)-2-iodoacetamide: To an oven dried 25-mL round-bottom flask, purged with argon, was added hex-5-yn-1-amine (100 mg, 118 μL, 1 Eq, 1.03 mmol) and methylene chloride (4.0 mL)
The solution was cooled to 0°C and triethylamine (208 mg, 287 μL, 2 Eq, 2.06 mmol) was added followed by dropwise addition of chloroacetyl chloride (174 mg, 124 μL, 1.5 Eq, 1.54 mmol). Solution was allowed to warm to ambient temperature and stir for 2 h. After completion, as determined by TLC, the solution was diluted with saturated Aq. sodium bicarbonate (5 mL) and extracted with methylene chloride (3 x 5 mL). The combined organic layers were washed with brine (1 x 5 mL) and dried over anhydrous sodium sulfate. The crude solution of chloroacetamide alkyne (CAA) was concentrated down and redissolved in anhydrous acetone (5 mL) followed by addition of sodium iodide (309 mg, 2 Eq, 2.06 mmol). The mixture was then allowed to stir for 14–16 h in the absence of light. After 16 h the acetone was removed under reduced pressure and the resulting residue was redissolved in water and extracted with methylene chloride (3 x 5 mL). The combined organic layers were washed with saturated sodium thiosulfate (1 x 2 mL) followed by brine (1 x 5 mL) and dried over anhydrous sodium sulfate. The resulting material was purified by flash column chromatography using a gradient elution of 10%–50% Ethyl acetate in hexanes to yield the desired product as a beige solid (175 mg, 64%).
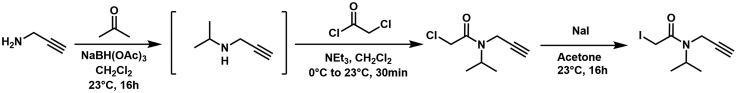
Scheme 2.
Propargylamine and acetone were condensed with sodium triacetoxyborohydride, followed by chloroacetylation, and Finkelstein to yield the desired light isopropyl iodoacetamide alkyne
For full synthetic detail please see Desai et al., 20226 (compound 4).
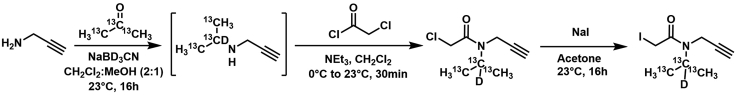
Scheme 3.
Propargylamine and 13C3-acetone were condensed with sodium cyanoborodeuturide, followed by chloroacetylation, and Finkelstein to yield the desired heavy isopropyl iodoacetamide alkyne
For full synthetic detail please see Desai et al., 20226 (compound 5).
Scheme 4.
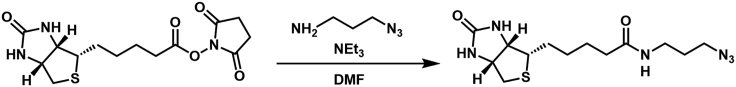
(+)-Biotin-NHS was condensed with 3-azido-propan-1-amine to yield the desired biotin azide
For full synthetic detail please see Cao et al., 202118 (compound 6).
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco’s modified Eagle’s medium (DMEM) | Thermo Fisher Scientific | Cat#: 11995073 |
| Dulbecco’s phosphate-buffered saline (DPBS) | Thermo Fisher Scientific | Cat#: 14190250 |
| Gibco Trypsin-EDTA (0.25%), phenol red | Fisher Scientific | Cat#: 25-200-114 |
| Fetal bovine serum | Avantor Seradigm | Cat#: 1500-500 |
| Fetal bovine serum (FBS), dialyzed, US origin | Thermo Fisher Scientific | Cat#: 26400044 |
| Penicillin-Streptomycin (10,000 U/mL) | Thermo Fisher Scientific | Cat#: 15140122 |
| Recombinant M-CSF generated from CMG14-12 culture supernatant | Lam et al., 200019 | N/A |
| Opti-MEM reduced serum medium | Thermo Fisher Scientific | Cat#: 31985070 |
| LipoFexin | Lambda Biotech | Cat#: TS310 |
| Lenti-X concentrator | Takara Bio | Cat#: 631232 |
| Lipopolysaccharide (LPS) | InvivoGen | Cat#: tlrl-smlps |
| Recombinant murine IFN-γ | PeproTech | Cat#: 315-05 |
| Cycloheximide | Fisher Scientific | Cat#: AC357420010 |
| D-biotin | Combi-Blocks | Cat#: SS-7910 |
| RIPA buffer with Triton|r X-100 (5×) | Fisher Scientific | Cat#: AAJ62885AE |
| Light isopropyl iodoacetamide alkyne (L-IPIAA) | Desai et al.6 | N/A |
| Heavy isopropyl iodoacetamide alkyne (H-IPIAA) | Desai et al.6 | N/A |
| Iodoacetamide alkyne (IAA) | This paper (See “before you begin”) | N/A |
| 10× PBS | Bioland | Cat#: PBS01-02 |
| Streptavidin agarose | Fisher Scientific | Cat#: PI20353 |
| DL-dithiothreitol 98.0+% (DTT) | Fisher Scientific | Cat#: D107125G |
| Trypsin, TPCK treated | Worthington Biochem | Cat#: LS003740 |
| CuSO4 | Fisher Scientific | Cat#: BP346-500 |
| TBTA | Click Chemistry Tools | Cat#: 1061-1G |
| Tert-butanol | Fisher Chemical | Cat#: A401-1 |
| Biotin-azide | Cao et al.18 | N/A |
| Sodium ascorbate | Spectrum Chemical | S1349 |
| Sera-Mag SpeedBead carboxylate-modified (E3) magnetic particles | Cytiva | Cat#: 65152105050250 |
| Sera-Mag SpeedBead carboxylate-modified (E7) magnetic particles | Cytiva | Cat#: 45152105050250 |
| Acetonitrile | Fisher Scientific | Cat#: A998-4 |
| MOPS | Fisher Scientific | Cat#: AAA1291422 |
| Sodium phosphate | Fisher Scientific | Cat#: S397-500 |
| NaCl | Fisher Scientific | Cat#: S271-500 |
| NeutrAvidin agarose | Fisher Scientific | Cat#: PI29200 |
| Formic acid, 99.0+%, Optima LC/MS grade | Fisher Scientific | Cat#: A117-50 |
| UltraPure distilled water | Invitrogen | Cat#: 10977015 |
| Dimethyl sulfoxide (DMSO) | Fisher BioReagents | Cat#: BP231-100 |
| Urea | Fisher Chemical | Cat#: U15-3 |
| Hex-5-yn-1-amine | Combi-Blocks | Cat#: QH-9488 |
| Chloroacetyl chloride | Sigma-Aldrich | Cat#: 22880-100ML |
| Triethylamine | Fisher Scientific | Cat#: T0424500ML |
| Sodium iodide | Fisher Scientific | Cat#: S324-100 |
| Acetone | Fisher Chemical | Cat#: A18-20 |
| Anhydrous DCM | Fisher Scientific | Cat#: MDX08364 |
| Propargylamine | Combi-Blocks | Cat#: OS-7456 |
| Sodium triacetoxyborohydride | Fisher Scientific | Cat#: AC291821000 |
| Acetone (13C3, 99%) | Cambridge Isotope Laboratories | Cat#: CLM-1334-1 |
| Sodium cyanoborodeuteride | Sigma-Aldrich | Cat#: 190020 |
| Methanol, Optima LC/MS grade | Fisher Chemical | Cat#: A456-4 |
| Biotin-NHS | Combi-Blocks | Cat#: QA-1959 |
| 3-Azido-1-propanamine | Fisher Scientific | Cat#: 50-1742-832 |
| Anhydrous DMF | VWR | Cat#: 200004-428 |
| Critical commercial assays | ||
| BCA Protein Assay | Thermo Fisher Scientific | Cat#: PI23227 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | Cat#: CRL-3216 RRID: CVCL_0045 |
| Cre-J2 viral supernatant immortalized bone-marrow-derived macrophages | Laboratory of Stephen Smale | N/A |
| Recombinant DNA | ||
| FUGW-Mito-TurboID | Yan et al.1 | Plasmid#: 200963 |
| pCMV-VSV-G lentiviral packaging plasmid | Addgene | Plasmid#: 8454 |
| pCMV-dR8.2 dvpr lentiviral packaging plasmid | Addgene | Plasmid#: 8455 |
| Software and algorithms | ||
| FragPipe | Kong et al.,20 da Veiga Leprevost et al.,21 Yu et al.22 | https://fragpipe.nesvilab.org/ |
| Perseus | Tyanova et al.23 | https://maxquant.net/perseus/ |
| R-4.2.3 | The Comprehensive R Archive Network (CRAN) | https://cran.rstudio.com/ |
| Thermo XCalibur | Thermo Fisher Scientific | RRID: SCR_014593 |
| 230119_mouse_process.py | Yan et al.1 | https://github.com/BackusLab/cysloc |
| Volcano_AJ-CysLOx.Rmd | This paper | https://github.com/BackusLab/cyslox_volcano |
| Other | ||
| CO2 cell incubator | Thermo Fisher Scientific | Cat#: 370 |
| SpeedVac concentrator | Savant Instruments, Inc. | Serial No.: SC100-OK47429-1A |
| Orbitrap Eclipse Tribrid mass spectrometer | Thermo Fisher Scientific | Cat#: FSN04-10000 |
| EASY-nLC 1200 System | Thermo Fisher Scientific | Cat#: LC140 |
| Prelubricated 1.7 mL microcentrifuge tube | Costar | Cat#: 3207 |
| General long-term storage cryogenic tubes | Fisher | Cat#: 03-337-7D |
| LN2 freezer | Thermo Scientific | Cat#: 8209 |
| Magnetic rack | Amazon | Cat#: B0812XLPVK |
| Fluorescence-activated cell sorter (FACS) | BD Biosciences | BD Aria |
| Bulk C18 reverse-phase resin | https://dr-maisch.com/ | Particle size, 1.9 m; pore size, 100 Å |
| 100 μm inner diameter (ID) fused silica capillary | Molex | NA |
| Thermo Scientific SureSTART 0.3 mL glass snap top microvials for <2 mL samples, level 3 high-performance applications | Fisher Scientific | Cat#: 03-452-365 |
| Thermo Scientific SureSTART 11 mm snap caps, level 3 high-performance applications | Fisher Scientific | Cat#: 03-452-372 |
Materials and equipment
Click master mix
| Reagent | Stock concentration | Solvent used | Amount of stock (for 1 click reaction) | Final concentration in click reaction |
|---|---|---|---|---|
| TBTA | 1.7 mM | See TBTA recipe below | 12 μL | 0.102 mM |
| Copper sulfate | 50 mM | Molecular biology grade H2O | 4 μL | 1 mM |
| Biotin-azide | 200 mM | DMSO | 4 μL | 4 mM |
| Sodium ascorbate | 250 mM | Molecular biology grade H2O | 4 μL | 5 mM |
Note: click master mix should be made fresh each time the click reaction is performed.
1× IAP Buffer
| Reagent | Final concentration |
|---|---|
| MOPS | 50 mM |
| Na2HPO4 | 10 mM |
| NaCl | 50 mM |
| pH | 7.5 |
Note: A 10× stock of IAP buffer can be made and stored at 4°C for up to 1 year. 1× stock can also be stored at 4°C for up to 1 year.
Neutravidin elution buffer
| Reagent | Percentage (v/v) |
|---|---|
| Acetonitrile | 80% |
| UltraPure distilled Water | 19.9% |
| Formic acid | 0.1% |
Note: Neutravidin elution buffer should be made fresh each time the neutravidin elutions are performed. Formic acid used to make buffer should be less than 6 months old and stored at 4°C.
LC-MS/MS reconstitution buffer
| Reagent | Percentage (v/v) |
|---|---|
| Acetonitrile | 5% |
| UltraPure distilled Water | 94% |
| Formic acid | 1% |
Note: LC-MS/MS reconstitution buffer should be made fresh for each experiment on the day the peptides are reconstituted. Formic acid used to make buffer should be less than 6 months old and stored at 4°C.
TBTA (50×)
-
•
8.85 mg TBTA dissolved in 200 μL DMSO.
TBTA (1×)
-
•
Dilute 50× TBTA 1:10 by adding 20 μL 50× TBTA to 180 μL DMSO (this is now 5× TBTA).
-
•
Dilute 5× TBTA 1:5 by adding 200 μL of 5× TBTA to 800 μL of tert-Butanol (this is now 1× TBTA).
Note: 50× TBTA can be stored at −80°C for 6 months. 1× TBTA can be stored at ambient temperature for 1 year.
RIPA buffer (1×)
-
•
Dilute 5× RIPA buffer 1:5 in UltraPure distilled water (e.g., add 10 mL 5× RIPA buffer to 40 mL UltraPure distilled water).
Note: 1× RIPA buffer can be stored at 4°C for 1 month.
PBS buffer (1×)
-
•
Dilute 10× PBS buffer 1:10 in UltraPure distilled water (e.g., add 100 mL 10× PBS buffer to 900 mL UltraPure distilled water).
Note: 1× PBS buffer can be stored at ambient temperature for 6 months.
Step-by-step method details
Stimulate cells with lipopolysaccharide and interferon gamma and initiate biotinylation of mitochondrial proteins
Timing: 24 h
Having seeded 6 10-cm dishes of iBMDM cells (3 for the control condition and 3 for the stimulated condition) as detailed in the “before you begin” section above, an inflammatory response will now be initiated in 3 dishes by stimulating cells with lipopolysaccharide (LPS) and interferon gamma (IFNγ), followed by biotinylation of mitochondrial proteins in all conditions for enrichment and downstream analysis. Ultimately, the oxidation state of mitochondrial cysteines will be quantified in both the unstimulated and stimulated conditions, in order to identify changes in response to LPS and IFNγ.
CRITICAL: As detailed in the “before you begin” section above, cells should be seeded in media containing dialyzed fetal bovine serum to limit nonspecific biotinylation.
-
1.Initiate an inflammatory response by treating cells with lipopolysaccharide (LPS) and interferon gamma (IFNγ) (Figure 2).
-
a.Culture 6 10-cm dishes of iBMDM cells to ∼70% confluency.
-
b.Treat 3 plates to a final concentration of 20 ng/mL IFNγ and a final concentration of 100 ng/mL LPS for 24 h.
-
i.The IFNγ stock is made at 10 μg/mL in UltraPure distilled water and kept at -80°C. The LPS stock is made at 1 mg/mL in UltraPure distilled water and kept at -80°C. For treatment, a working stock is made at 10 μg/mL by diluting the stock in media.
-
i.
-
c.Treat the 3 control plates with the same volume of UltraPure distilled water for 24 h.
-
a.
-
2.Inhibit translation of newly synthesized mitochondrial-localized TurboID by treating cells with cycloheximide.
-
a.18 h after the LPS + IFNγ treatment (e.g., 6 h before biotin treatment), treat all 6 dishes to a final concentration of 100 μg/mL cycloheximide using a freshly made stock solution of cycloheximide prepared at 100 mg/mL in DMSO. This step is meant to inhibit translation of mitochondrial-localized TurboID to limit nonspecific biotinylation by TurboID that is newly translated and being trafficked to the mitochondria.
-
a.
-
3.Initiate biotinylation of mitochondrial-localized proteins (Figure 2).
-
a.24 h after the LPS + IFNγ treatment, treat all 6 dishes to a final concentration of 500 μM D-biotin for 1 h. This step will initiate mitochondrial-localized TurboID to biotinylate proteins within the mitochondria.
-
i.The D-biotin stock is made at 100 mM in cell-culture grade DMSO and is made fresh for each experiment.
-
i.
-
a.
-
4.Harvest all cells.
-
a.Inspect cells under a microscope to ensure that the majority are still adherent to the plate.
-
b.Aspirate media and wash each plate gently with 5 mL ice cold DPBS. Aspirate DPBS.
-
c.Add 5 mL of ice cold DPBS to each dish. Gently lift cells with cell lifter and transfer cell suspension to 15-mL falcon tube.
-
d.Pellet cells by spinning at 1800 g for 3 min.
-
e.Aspirate DPBS and resuspend cell pellet in 5 mL DPBS to wash. Pellet cells by spinning at 1800 g for 3 min.
-
f.Aspirate DPBS, resuspend cell pellet in 1 mL DPBS, and transfer cell suspension to a 1.5 mL microcentrifuge tube. Pellet cells by spinning at 1800 g for 3 min.
-
g.Aspirate DPBS and continue to next step to lyse cells or flash freeze cell pellet in liquid nitrogen and store at -80°C until ready to continue the protocol.
-
a.
CRITICAL: It is critical to perform all the DPBS washes of the cell pellet in order to remove excess biotin from the cell suspension. If excess biotin from the cell treatment is carried over to the cell lysate, the protein-level enrichment will not be efficient.
Note: If many cells are not adherent after the cell treatment, the media can be collected in a 15-mL falcon tube and spun to collect floating cells. The cells that are harvested in the following steps can then be added to this fraction to collect all cells.
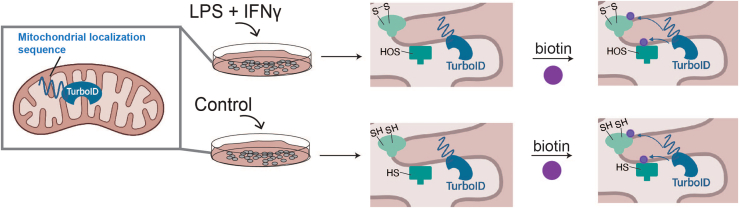
Figure 2.
Workflow for cell stimulation and mitochondrial protein biotinylation
Cells stably expressing mitochondria-localized TurboID are stimulated with lipopolysaccharide (LPS) and interferon gamma (IFNγ) to activate an inflammatory response. As a result of stimulation, specific mitochondrial cysteines undergo oxidation. All cells are then treated with biotin to initiate biotinylation of mitochondrial proteins via the biotin ligase TurboID.
Cysteine labeling with iodoacetamide alkyne and streptavidin enrichment of mitochondrial proteins
Timing: 16–24 h
In this step natively reduced cysteines are capped with iodoacetamide alkyne (IAA) (Cys-LoC) or isotopically “light” isopropyl iodoacetamide alkyne (IPIAA) (Cys-LOx) followed by streptavidin enrichment to enrich mitochondrial proteins that were previously biotinylated by mitochondrial- localized TurboID. On streptavidin, natively oxidized cysteines will be reduced by DTT such that they are then amenable to an additional round of (IP)IAA labeling. For the Cys-LoC protocol (local cysteine capture), the second round of labeling will be done with the same IAA as the first round, to ensure comprehensive profiling of localized cysteines and to ensure the highest efficiency of cysteine capping is achieved (capping prior to DTT addition). For the Cys-LOx protocol (local cysteine oxidation), this second round of labeling will be done with isotopically “heavy” IPIAA, such that reduced and oxidized cysteines are differentially labeled. An on-streptavidin trypsin digest will then release mitochondrial peptides from the beads.
-
5.Lyse all cell pellets.
-
a.Resuspend cell pellets in 500 μL of 1× RIPA buffer and mix by pipetting.
-
b.Incubate on ice for 30 min.
-
c.Centrifuge cell lysate at 21,100 g for 10 min to clear insoluble debris from soluble lysate and transfer soluble lysate to new microcentrifuge tube.
-
a.
-
6.Measure the soluble lysate concentration by BCA assay according to manufacturer’s microplate procedure.
-
a.Link to manufacturer’s protocol.
-
a.
-
7.
Normalize all lysates to ∼2 mg/mL (or the lowest lysate concentration) with 1× RIPA buffer.
-
8.Cap reduced cysteines with iodoacetamide alkyne (IAA) (Cys-LoC) or isotopically “light” isopropyl iodoacetamide alkyne (L-IPIAA) (Cys-LOx) (Figure 3).
-
a.Transfer 500 μL of each normalized lysate to a new microcentrifuge tube.
-
b.Add 2 μL of 500 mM stock IAA or L-IPIAA to each lysate such that the final concentration of (IP)IAA in each sample is 2 mM. Vortex for 2–3 s or until homogenous(for Cys-LoC, IAA is added prior to DTT reduction here to maximize efficiency of cysteine labeling, as DTT can hinder iodoacetamide capping of cysteines).
-
i.IAA and L-IPIAA stocks are prepared at 500 mM in DMSO.
-
i.
-
c.Incubate treated samples in the dark for 1 h at 23°C (ambient temperature).
-
a.
-
9.Prepare streptavidin bead solution.
-
a.Add 300 μL of the streptavidin resin (50 μL/sample) to 10 mL 1× PBS.
-
b.Spin down beads at 1500 g for 3 min.
-
c.Aspirate 1× PBS and resuspend streptavidin beads in 3 mL of 2 M urea in 1× RIPA (500 μL/sample).
-
a.
-
10.Enrich biotinylated proteins on streptavidin.
-
a.Add 500 μL of the streptavidin solution to each of the cell lysate samples so the final volume of each sample is 1 mL.
-
b.Rotate the samples at 23°C (ambient temperature) for 1.5 h.
-
a.
-
11.Wash streptavidin beads.
-
a.Pellet streptavidin resin in each microcentrifuge tube by spinning at 1500 g for 2 min.
-
b.Aspirate supernatant.
-
c.Wash resin by resuspending streptavidin beads in 1 mL of 2 M urea in 1× RIPA.
-
d.Spin down beads and aspirate supernatant.
-
e.Wash resin by resuspending streptavidin beads in 1 mL 1× RIPA and spin down beads. Aspirate supernatant and repeat this wash.
-
f.Wash resin by resuspending streptavidin beads in 1 mL 1× PBS and spin down beads. Aspirate supernatant and repeat this wash.
-
g.Wash resin by resuspending streptavidin beads in 1 mL UltraPure distilled water and spin down beads. Aspirate supernatant and repeat this wash.
-
h.Spin down beads and aspirate supernatant.
-
a.
-
12.Reduce natively oxidized cysteines and cap with second round of (IP)IAA (for Cys-LoC, a second round of IAA labeling is performed to comprehensively profile cysteines, including natively oxidized cysteines).
-
a.Resuspend streptavidin beads in 200 μL of 6 M urea in 1× PBS.
-
b.Add 1 μL of freshly prepared 200 mM DTT stock (prepared in UltraPure distilled water) to each of the samples and incubate at 65°C for 15 min.
-
c.For Cys-LoC protocol (subcellular localized cysteine capture): treat each sample with 2 μL of 200 mM IAA stock (stock prepared in DMSO) (Figures 3A and 3C) such that the final concentration of IAA will be 2 mM.
-
d.For Cys-LOx protocol (subcellular localized oxidation state quantitation): treat each sample with 2 μL of 200 mM H-IPIAA (stock prepared in DMSO) (Figures 3B and 3C) such that the final concentration of H-IPIAA will be 2 mM.
-
e.Rotate samples in the dark at 23°C (ambient temperature) for 1 h.
-
a.
CRITICAL: Be sure to use the correct isotope of (IP)IAA in the second round of labeling depending on the desired protocol (Cys-LoC or Cys-LOx). Cys-LoC uses the same IAA for both capping steps as this protocol is meant to identify cysteines within the mitochondrial proteome for comprehensive profiling of the mitochondrial cysteinome, while Cys-LOx is meant to quantify the redox state of mitochondrial cysteines, and thus uses sequential labeling with “light” IPIAA and “heavy” IPIAA, such that reduced and oxidized cysteines are differentially labeled.
-
13.Initiate trypsin digest of proteins on streptavidin resin (Figure 4, step 1).
-
a.Add 400 μL of 1× PBS to each sample to reduce urea concentration to 2 M.
-
b.Pellet streptavidin beads by spinning at 1500 g for 2 min and aspirate supernatant.
-
c.Resuspend streptavidin beads in 150 μL of 2 M urea in 1× PBS.
-
d.Add 3 μL of 1 mg/mL trypsin to each sample, vortex lightly, and incubate in a shaking incubator for 14–16 h at 37°C and 200 rpm.
-
i.Trypsin stock made by reconstituting 10 mg trypsin (Worthington Biochem, LS003740) in 10 mL 50 mM acetic acid (1 mg/mL). 50 μL trypsin aliquots can be stored at −80°C for future use.
-
i.
-
a.
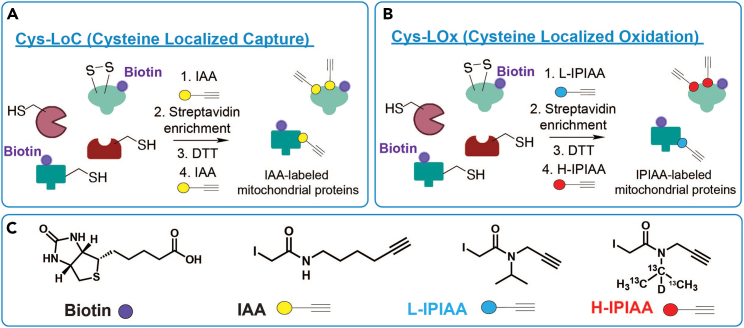
Figure 3.
Labeling of cysteines with (IP)IAA and streptavidin enrichment of mitochondrial proteins for (A) Cys-LoC and (B) Cys-LOx
(A) For Cys-LoC, cell lysates are treated with iodoacetamide alkyne (IAA) to alkylate all reduced cysteines. Mitochondrial proteins that were previously biotinylated by TurboID are then enriched on streptavidin resin. Oxidized cysteines are then reduced with DTT and capped with IAA on resin.
(B) For Cys-LOx, cell lysates are treated with light isopropyl iodoacetamide alkyne (L-IPIAA) to alkylate all reduced cysteines. Mitochondrial proteins that were previously biotinylated by TurboID are then enriched on streptavidin resin. Oxidized cysteines are then reduced with DTT and capped with heavy isopropyl iodoacetamide alkyne (H-IPIAA) on resin.
(C) Chemical structures of biotin, IAA, L-IPIAA, and H-IPIAA.
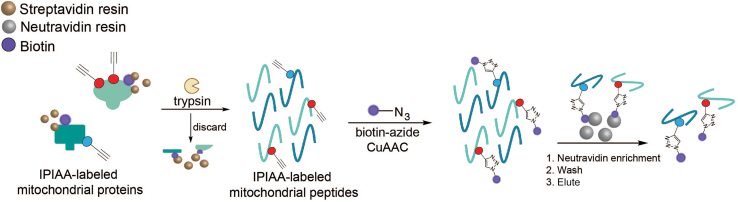
Figure 4.
(IP)IAA-labeled mitochondrial peptides are enriched via sequential avidin enrichments
IPIAA-labeled mitochondrial proteins that were previously enriched on streptavidin resin are then trypsin digested into peptides off the resin (step 1). Digested IPIAA-labeled peptides are then conjugated to biotin-azide via CuAAC- mediated ‘click’ chemistry (step 2). Biotinylated cysteine peptides are then enriched on neutravidin resin (step 3), and eluted cysteine peptides are used for LC-MS/MS analysis in the following steps.
Conjugation of biotin-azide to (IP)IAA-labeled cysteine peptides
Timing: 3 h
In this step, copper catalyzed azide-alkyne cycloaddition (CuAAC) ‘click’ chemistry is used to conjugate biotin-azide to (IP)IAA-labeled cysteine peptides to enable enrichment on neutravidin resin in the following step.
-
14.Separate digested peptides from streptavidin resin.
-
a.Following the trypsin digest, pellet streptavidin resin by spinning at 1500 g for 2 min.
-
b.Transfer supernatant of each sample (which contains peptides) to prelubricated (low-bind) microcentrifuge tubes.
-
c.Wash streptavidin resin with 50 μL of UltraPure distilled water, vortex for 2–3 s until resuspended, and spin at 1500 g for 2 min.
-
d.Combine the 50 μL peptide fraction with the previous fraction from the same sample, such that the total sample volume is ∼200 μL.
-
a.
-
15.Click biotin-azide onto (IP)IAA-labeled peptides (Figure 4, step 2).
-
a.Prepare enough click master mix (Table 1) for 7 click reactions (e.g., multiply the amounts in Table 1 by 7) to provide excess than the 6 reactions that will be done.
 CRITICAL: It is critical to prepare the click master mix by combining the ingredients in the order listed in Table 1. Click master mix should also be prepared fresh each time the click reaction is performed.
CRITICAL: It is critical to prepare the click master mix by combining the ingredients in the order listed in Table 1. Click master mix should also be prepared fresh each time the click reaction is performed. -
b.Add 24 μL of click master mix to each 200 μL sample and vortex for 2–3 s or until homogenous.
-
c.Let reaction incubate at 23°C (ambient temperature) for 1 h.
-
a.
-
16.Remove excess click reagents from peptides.
-
a.Prepare 240 μL solution of Sera-Mag SpeedBead Carboxylate-Modified Magnetic Particles (40 μL/sample) by combining each type of bead 1:1 (120 μL each type, E3 and E7) and washing 3× with 1 mL UltraPure distilled water using magnetic rack. Resuspend beads in 240 μL UltraPure distilled water.
-
b.Add 40 μL of SpeedBead Carboxylate-Modified Magnetic Particles solution to each peptide sample and shake at 1000 rpm for 5 min at 23°C (ambient temperature).
-
c.Transfer each sample to a 15 mL falcon tube and add 3.5 mL 100% acetonitrile (LC-MS grade) and shake at 1000 rpm for 10 min at 23°C (ambient temperature) to bind peptides to the SpeedBead Carboxylate-Modified Magnetic beads.
-
d.Wash the SpeedBead Carboxylate-Modified Magnetic Particles (which now are bound by peptides) with 1 mL 100% acetonitrile by resuspending the beads and then using the magnetic rack to aspirate the wash. Repeat this wash 2 additional times (3 washes total).
-
e.Elute peptides from beads by resuspending beads in 100 μL 2% DMSO in water and shaking at 1000 rpm for 30 min at 37°C.
-
f.Use magnetic rack to transfer eluant (containing peptides) to a pre-lubricated microcentrifuge tube.
-
g.Repeat elution and add second eluant fraction to the previous fraction from the same sample so that total sample volume is ∼200 μL.
-
a.
Table 1.
Recipe for click master mix
| Reagent | Stock concentration | Solvent used | Amount of stock (for 1 click reaction) | Final concentration in click reaction (after addition to 200 μL peptide sample) |
|---|---|---|---|---|
| TBTA | 1.7 mM | See “materials and equipment” | 12 μL | 0.102 mM |
| Copper sulfate | 50 mM | Molecular biology grade H2O | 4 μL | 1 mM |
| Biotin-azide | 200 mM | DMSO | 4 μL | 4 mM |
| Sodium ascorbate | 250 mM | Molecular biology grade H2O | 4 μL | 5 mM |
Neutravidin enrichment of biotinylated cysteine peptides
Timing: 3–4 h
In this step, biotinylated peptides are enriched on neutravidin resin to isolate (IP)IAA-labeled cysteine peptides. This enrichment will enable analysis of these peptides by liquid chromatography tandem mass spectrometry (LC-MS/MS) to identify labeled mitochondrial cysteines (Cys-LoC) or quantify the oxidation state of mitochondrial cysteines (Cys-LOx).
-
17.Prepare neutravidin resin.
-
a.Add 300 μL of neutravidin resin (50 μL/sample) to 10 mL 1× IAP buffer (Table 2) and spin down at 2000 g for 8 min.
-
b.Aspirate IAP buffer and repeat this wash 2 additional times (3 washes total).
-
c.Resuspend neutravidin resin in 4.8 mL of IAP buffer (800 μL/sample).
-
a.
-
18.Enrich biotinylated peptides on neutravidin (Figure 4, step 3).
-
a.Add 800 μL of neutravidin solution to each sample of eluted peptides.
-
b.Rotate at 23°C (ambient temperature) for 2 h.
-
a.
-
19.Wash neutravidin beads.
-
a.Pellet neutravidin resin in each microcentrifuge tube by spinning at 1500 g for 2 min and aspirate supernatant.
-
b.Wash resin by resuspending neutravidin beads in 1 mL of 1× PBS buffer. Then spin down beads and aspirate supernatant. Repeat the 1× PBS wash 2 additional times (3 washes total).
-
c.Wash resin by resuspending neutravidin beads in 1 mL UltraPure distilled water. Then spin down beads and aspirate supernatant. Repeat the water wash 2 additional times (3 washes total).
-
a.
-
20.Elute biotinylated peptides from neutravidin resin.
-
a.Resuspend neutravidin resin (which is now bound to biotinylated peptides) in 60 μL of neutravidin elution buffer (Table 3) and rotate at 23°C (ambient temperature) for 10 min.Note: A stock of freshly prepared neutravidin elution buffer should be prepared directly before the 3 rounds of elution and the stock used for each elution.
-
b.Pellet neutravidin resin by spinning at 1500 g for 2 min and transfer the supernatant (containing eluted peptides) to a new pre-lubricated microcentrifuge tube.
-
c.Resuspend the neutravidin resin in 60 μL of neutravidin elution buffer and incubate at 72°C for 10 min.
-
d.Pellet neutravidin resin by spinning at 1500 g for 2 min and transfer the supernatant (containing eluted peptides) to the previous eluant fraction of the same sample (total sample volume now ∼120 μL).
-
e.Resuspend the neutravidin resin in 40 μL of neutravidin elution buffer and vortex for 2–3 s or until resuspended.
-
f.Pellet neutravidin resin by spinning at 1500 g for 2 min and transfer the supernatant (containing eluted peptides) to the previous eluant fractions of the same sample (total sample volume now ∼160 μL).
-
a.
-
21.
Freeze eluted samples in liquid nitrogen and store at -80°C.
Pause Point: Samples can either be stored at -80°C until ready to continue the protocol, or frozen and directly carried on to step 22.
Table 2.
1× IAP buffer
| Reagent | Final concentration |
|---|---|
| MOPS | 50 mM |
| Na2HPO4 | 10 mM |
| NaCl | 50 mM |
Table 3.
Neutravidin elution buffer
| Reagent | Percentage (v/v) |
|---|---|
| Acetonitrile | 80% |
| UltraPure distilled Water | 19.9% |
| Formic acid | 0.1% |
Prepare peptide samples for liquid chromatography tandem mass spectrometry analysis (LC-MS/MS)
Timing: 2–3 h
In this step peptide samples are speedvacced to remove the neutravidin elution buffer from the peptides to be analyzed. Samples are then reconstituted in LC-MS/MS-compatible buffer and run on an Orbitrap Eclipse mass spectrometer.
-
22.Speedvac frozen peptide samples.
-
a.Load frozen peptide samples (in neutravidin elution buffer) into a speedvac with the caps opened.
-
b.Speedvac samples by turning on centrifugation and vacuum for 1–2 h such that the neutravidin elution buffer is removed from the peptides.
-
a.
CRITICAL: It is critical to open the Eppendorf tube caps when speedvaccing such that the solvent can evaporate.
CRITICAL: When removing samples from the speedvac, close the valve to the vacuum and let pressure equalize before turning off the centrifugation. If centrifugation is turned off before pressure stabilizes, the samples may be sucked out.
-
23.
Remove samples from speedvac and store solid peptides at −80°C until ready to run on the mass spectrometer or continue to step 24.
Note: It is likely that the solid peptide pellet will not be visible following speedvaccing.
Pause Point: Solid peptide samples can be stored at -80°C until ready to run on the mass spectrometer.
-
24.Resuspend peptides in LC-MS/MS reconstitution buffer (Table 4).
-
a.Prepare LC-MS/MS reconstitution buffer as detailed in Table 4.
-
b.Resuspend solid peptides in 15 μL of LC-MS/MS reconstitution buffer and transfer solution to a SureSTART 0.3 mL Glass Snap Top Microvial secured with at SureSTART 11 mm Snap Cap.
-
c.Place each vial in the autosampler of the Thermo EASY-nLC 1200 and inject 5 μL of each sample into mass spectrometer for analysis. For acquisition details continue to the following section.
-
a.
Note: LC-MS/MS reconstitution buffer should made fresh each time samples are prepared.
Note: Each sample should be run twice, such that there are 2 technical replicates for each biological replicate.
Table 4.
LC-MS/MS reconstitution buffer
| Reagent | Percentage (v/v) |
|---|---|
| Acetonitrile | 5% |
| UltraPure distilled Water | 94% |
| Formic acid | 1% |
Liquid chromatography tandem mass spectrometry (LC-MS/MS) parameters and acquisition
Timing: 24–30 h (∼2 h/sample)
This section details the parameters used on the Orbitrap Eclipse mass spectrometer for acquisition of raw data.
-
25.
For separation of peptides, pack a fused silica capillary column with bulk C18 reverse-phase resin up to ∼18 cm in length.24
-
26.Separate the peptides with the Thermo EASY-nLC 1200 integrated nano-HPLC system directly interfaced with an Eclipse Tribrid Mass Spectrometer using a 70-min gradient elution as detailed in Table 5.
-
a.Mobile phase A consists of 0.1% formic acid and 3% DMSO in water, and mobile phase B consists of 80% acetonitrile, 3% DMSO, and 0.1% formic acid in water.
-
a.
-
27.Peptides are eluted and subjected to electrospray ionization for analysis by the Orbitrap Eclipse mass spectrometer.
-
a.Voltage: 2200 V.
-
b.Sheath gas flow: 0.
-
c.Ion transfer tube temperature: 300°C.
-
d.Acquisition software: Xcalibur using data-dependent acquisition (DDA).
-
e.MS1 parameters: single full mass spectrum (375–1,600 m/z), 120,000 resolution, 30% RF lens, normalized AGC target (percentage): 250.
-
f.MS2 parameters: 1.6 m/z isolation window, HCD collision with 30% normalized collision energy (NCE), 15,000 resolution.
-
g.Cycle time: 3 s (max scans collected per cycle time).
-
h.Max injection time: auto.
-
i.Filters: 5 × 104 intensity threshold, includes charge states 2–7, monoisotopic precursor selection (MIPS): peptide, dynamic exclusion: excludes after 1 time, exclusion duration 30 s, mass tolerance 10 ppm.
-
a.
-
28.
Once all data is acquired, continue to the “quantification and statistical analysis” section for a protocol on data processing.
Table 5.
Liquid chromatography gradient for separation of peptides
| Time | Duration | Flow (nL/min) | Mobile phase B (percentage of solvent) |
|---|---|---|---|
| 00:00 | 00:00 | 300 | 3 |
| 05:00 | 05:00 | 220 | 10 |
| 15:00 | 10:00 | 220 | 20 |
| 64:00 | 49:00 | 220 | 47 |
| 66:00 | 02:00 | 250 | 95 |
| 70:00 | 04:00 | 250 | 95 |
Expected outcomes
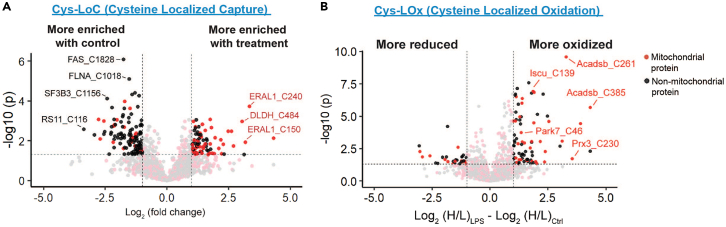
A successful Cys-LoC and/or Cys-LOx experiment should ultimately yield approximately 500–1500 enriched cysteine peptides, although this number is variable depending upon the unique system applied (Figure 5). For the Cys-LoC protocol, a number of cysteines should be enriched with treatment (more capture with treatment) and a number will likely be enriched with control (less capture with treatment) (Figure 5A), indicating either a change in abundance of the cysteine-containing protein, or a change relative localization of TurboID (e.g., increased/decreased localization specificity). For the Cys-LOx protocol, out of the total enriched cysteine peptides, a handful should show statistically significant changes in the redox state between the control and stimulated conditions (if the stimulation is expected to affect redox state) (Figure 5B). For the control condition, the majority of cysteines should have a negative H/L ratio, as most cysteines exist in a predominantly reduced state.
Figure 5.
Volcano plots after processing Cys-LoC and Cys-LOx data
(A) Volcano plot depiction of cysteines enriched by Cys-LoC in both a control and experimental group (note here that the treatment was cycloheximide and dialyzed FBS, in comparison to the control which was no cycloheximide and non-dialyzed FBS). P values were calculated from a Student's t test. Cysteines that are enriched with treatment represent those that had a higher intensity in the treatment group, whereas cysteines that are enriched in the control group represent those that had a lower intensity in the treatment group (adapted from Yan et al.).1
(B) Volcano plot depiction of Cys-LOx data from both a control and experimental group (here the treatment is LPS + IFNγ). P values were calculated from a Student's t test. Instead of comparisons of intensity between the two groups as in the Cys-LoC analysis, this plot represents comparison of H/L IPIAA ratios (indicative of redox state). Because a higher H/L ratio indicates a more oxidized cysteine, cysteines that appear on the right side of the volcano plot represent those that are more oxidized with treatment (LPS + IFNγ), and cysteines that appear on the left side of the volcano plot represent those that are more reduced with treatment (adapted from Yan et al.).1
To achieve successful enrichment of cysteine peptides, several conditions must be met. First, the mitochondrial localized TurboID should be successfully expressed in all cellular conditions, and this construct should initiate substantial biotinylation in the presence of exogenous biotin (with little background in the absence of biotin). Second, the streptavidin enrichment should efficiently enrich all biotinylated proteins (mitochondrial proteins) in a compartment-specific manner. A successful protein- level streptavidin enrichment should yield ∼500-1500 proteins with a high specificity for the mitochondria (>30% enriched proteins should be mitochondrial proteins, see Yan et al.).1 The number of enriched proteins after the protein-level enrichment will likely be similar to that of the cysteines identified after both enrichments due to the inevitable loss of peptides during the sample preparation and the I(PI)AA labeling not being 100% efficient. Lastly, the enrichment of cysteine-containing peptides on neutravidin resin should be efficient based on successful click chemistry and sufficient washing away of excess click materials.
Quantification and statistical analysis
-
1.Process .raw mass spectrometry data using MSFragger through FragPipe v19.0 (for Cys-LoC).
-
a.Instructions for configuration of FragPipe.
-
b.Database: uniprot_mouse_swissprot_1042022.fasta.fas with 50% decoys.
-
c.MSFragger: Variable modifications: 15.9949 on methionine (methionine oxidation), 42.0106 on N-terminus (acetylation), 463.2366 on cysteine (IAA modification). Precursor and fragment mass tolerance was set as 20 ppm. Missed cleavages were allowed up to 1. Peptide length was set 7 - 50 and peptide mass range was set 500 - 5000.
-
d.Quant (MS1): Ionquant (v1.8.9), LFQ (Add MaxLFQ), MaxLFQ min ions: 2, Match Between Runs (MBR), Normalize intensity across runs.
-
a.
-
2.Process .raw mass spectrometry data using MSFragger through FragPipe v19.0 (for Cys-LOx).
-
a.Instructions for configuration of FragPipe.
-
b.Database: uniprot_mouse_swissprot_1042022.fasta.fas with 50% decoys.
-
c.MSFragger: Variable modifications: 15.9949 on methionine (methionine oxidation), 42.0106 on N-terminus, 463.2366 on cysteine (light IPIAA modification), and 467.2529 on cysteine (heavy IPIAA modification). Precursor and fragment mass tolerance was set as 20 ppm. Missed cleavages were allowed up to 1. Peptide length was set 7 - 50 and peptide mass range was set 500 - 5000.
-
d.Quant (MS1): MS1 labeling quant was enabled with Light set as C+463.2366 and Heavy set as C+467.2529. MS1 intensity ratio of heavy and light labeled cysteine peptides were reported with Ionquant (v1.8.9).
-
a.
-
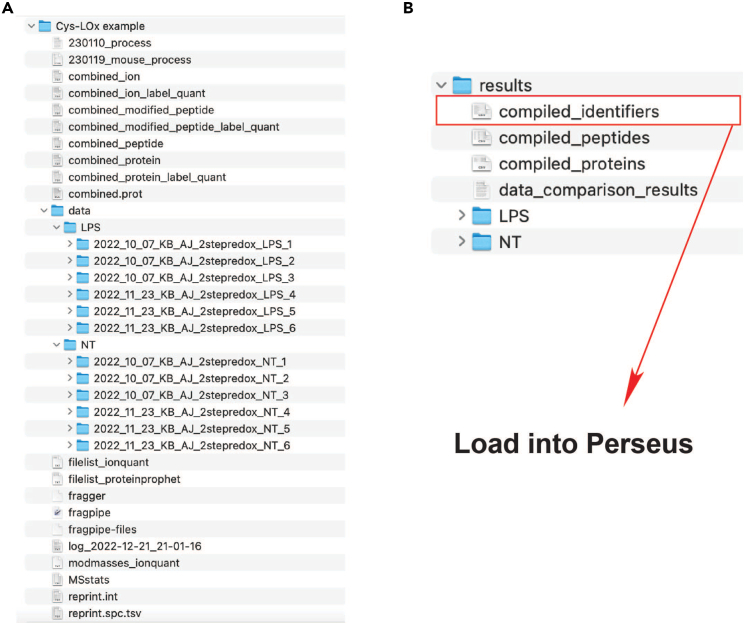
3.Use custom script 230119_mouse_process.py (https://github.com/BackusLab/cysloc) to aggregate cysteine identifiers across all replicates.
-
a.Generate a parent folder within which to organize all FragPipe output files. Within the parent folder, create a folder called ‘data’ that contains subfolders for each experimental condition (Figure 6A). Transfer the FragPipe output folder for each replicate (6 per condition) to their respective condition subfolder (Figure 6A). The parent folder should also contain all the additional FragPipe output files, in addition to the Python script 230119_mouse_process.py.
-
b.Open a terminal window to run the pipeline through Python.
-
c.Navigate to the parent folder in the terminal window.
-
d.Enter this line of code to run the Python script for Cys-LOx:>python3 230119_process.py -lpm '463.2366' -hpm '467.2529' -proref '2022-10-04-decoys-contam-uniprot_mouse_swissprot_1042022.fasta.fas'
-
e.Enter this line of code to run the Python script for Cys-LoC:>python3 230119_process.py -exp 'lfq' -proref '2022-10-04-decoys-contam-uniprot_mouse_swissprot_1042022.fasta.fas'
-
f.Execution of this command will generate a folder called ‘results’ within the parent folder, containing outputs of compiled data from the FragPipe search (Figure 6B).
-
a.
-
4.Filter processed data with Perseus.
-
a.Load the ‘compiled_identifiers.tsv’ output from the ‘results’ folder (Figure 6B) into Perseus and enter the 12 columns (for 12 replicates ) containing the ‘median’ ratio values (for Cys-LOx) or raw intensity values (for Cys-LoC) into the ‘Main’ category in Perseus. All other columns can be entered into the ‘Text’ box.
-
b.Grouping of Samples: under Annotate Rows > Categorical Annot., give all of the replicates from a single condition the same name (e.g., all 6 control replicates can be named ‘control’ and all experimental replicates can be named ‘experiment’). This will group all replicates into 2 distinct groups for statistical analysis.
-
c.Filtering According to Groups: under Filter Rows > Filter based on valid values, the minimum number of values should be at least 2 ‘in each group.’ This will retain all identifiers that have at least 2 values (out of 6 replicates) in both conditions.
-
d.Perform a 2-sample t-test with default FDR to obtain fold change values for each cysteine.
-
e.Export the filtered data for use in step 5.
-
a.
-
5.
Use any desired program (e.g., https://github.com/ashleyjulio/Cys-LOx_Volcano) to plot each identifier (each quantified cysteine ratio) on a volcano plot that depicts the fold change of the intensity (Cys-LoC) or difference value of the ratio (Cys-LOx) for each cysteine against the p-value. Any cysteine with a fold change > 1 and p-value <0.05 can be considered significantly enriched- either more or less abundant (Cys-LoC) or more or less oxidized (Cys-LOx) in response to the cellular treatment/stimulation (Figure 5). For Cys-LoC, if the intensity of the cysteine becomes more positive with treatment, that cysteine became more susceptible to identification with treatment as a result of increased biotinylation of the cysteine-containing protein in the organelle of interest relative to the untreated condition (e.g., increase in relative protein abundance or increase in TurboID localization specificity), whereas if the intensity is reduced with treatment, that cysteine became less susceptible to identification with treatment (Figure 5A). For Cys-LOx, if the ratio becomes more positive in response to treatment, the treatment induced oxidation of the cysteine. If the ratio becomes more negative in response to treatment, the treatment induced reduction of the cysteine (Figure 5B).
Figure 6.
Layout of folders for running script for compilation of data
(A) A parent folder (here called ‘Cys-LOx example’) contains a folder called ‘data,’ which contains subfolders of the two conditions (NT = no treatment, LPS = LPS + IFNγ). Each experimental condition subfolder contains the FragPipe output folders for each corresponding replicate. The parent folder also contains the Python script file (here called ‘230119_mouse_process’) and all the additional FragPipe output files.
(B) Contents of the ‘results’ file that is generated upon execution of the command in step 3d. The ‘compiled_identifiers.tsv’ output is loaded directly into Perseus for further processing.
Limitations
Although both Cys-LoC and Cys-LOx are versatile platforms for the identification of compartment-specific cysteines and quantification of compartment-specific cysteine oxidation state, respectively, the protocol is subject to limitations. First, the overexpression of the TurboID construct may lead to nonspecific biotinylation events (e.g., biotinylation of non-mitochondrial proteins) as a result of a small fraction of the construct being mislocalized and/or being active during trafficking. The protocol attempts to mitigate this through the use of cycloheximide, however, this introduces an additional limitation in that cycloheximide is known to cause cell stress as a result of translation inhibition, which may lead to misrepresentation of the oxidation state of specific cysteines. Furthermore, the protocol relies on detection of the same cysteines across multiple experiments, which can decrease overall coverage, due in part to the stochastic nature of data dependent acquisition.
Troubleshooting
Problem 1
Very few cysteine peptides are identified/quantified after completing the protocol.
Potential solution
-
•
Increase protein lysate input to at least 1000 μg per sample (increase volume or lysate concentration in step 8a).
-
•
Increase biotin exposure time and/or concentration (step 3a).
-
•
Adjust the LC gradient in step 26 to optimize peptide elution within the gradient window by increasing the time with 95% mobile phase B (e.g., 95% B for 10 min at the end of the method) or increasing the percentage of mobile phase B earlier on in the liquid chromatography method (e.g., use 20% B starting at 5 min).
-
•
Stringently wash cells with 1× PBS (3+ times) after harvesting in step 4 to get rid of the excess biotin and enhance the efficiency of the streptavidin enrichment.
-
•
Stringently wash peptides with acetonitrile on SpeedBead Carboxylate-Modified Magnetic Particles after click reaction (>3 times) in step 16 to get rid of the excess biotin-azide and enhance the efficiency of the neutravidin enrichment.
Problem 2
Many nonspecific peptides (e.g., peptides from an undesired cellular compartment) were enriched after completing the protocol.
Potential solution
-
•
Ensure that the cell culture medium is absent of biotin (e.g., if cells are cultured in RPMI, verify that it is biotin-free RPMI).
-
•
Optimize cycloheximide (CHX) treatment time in step 2. Longer treatment time will provide ample time for inhibition of translation of the TurboID construct and will thus allow all trafficked TurboID to reach the organelle of interest prior to biotinylation, thus limiting nonspecific biotinylation. However, cells should not be treated with CHX for too long to avoid excessive cell stress and turnover of the currently existing pool of TurboID itself, and thus it is optimal to test multiple time points for CHX treatment.
-
•
Run a similar experiment with no biotin added to acquire a ‘streptavidin/neutravidin’ background dataset, such that the identified peptides can be ruled out as non-specific binders to the resins used.
Problem 3
For Cys-LOx, none or very few cysteines show differential oxidation states in response to cell treatment/stimulation.
Potential solution
-
•
Optimize the cell stimulation conditions (concentrations/times) in step 1.
-
•
Run the protocol with a treatment that is known to induce oxidation changes (e.g., lipopolysaccharide as described in step 1) to ensure this problem is treatment-specific.
-
•
Include the L-IPIAA in the RIPA lysis buffer (final concentration 2 mM) such that reduced cysteines are being capped while the cells lyse (step 5), in order to mitigate changes to cysteine oxidation state that may occur as a result of cell lysis.
Problem 4
For Cys-LoC, none or very few cysteines show differential enrichment in response to cell treatment/stimulation.
Potential solution
-
•
While this may not be unexpected depending on the specific treatment performed (since differential enrichment for Cys-LoC indicates a change in cysteine abundance or a change in TurboID localization specificity), if differential enrichment is expected, the following potential solutions can explored:
-
•
Optimize the cell stimulation conditions (concentrations/times) in step 1.
-
•
Run a control experiment using cycloheximide as the ‘treatment’ (step 1) and excluding cycloheximide from the control condition to determine whether differential enrichment is achieved upon translation inhibition as expected.
Problem 5
For Cys-LOx, the majority of cysteines in the control condition have H/L ratios with positive, or near positive, values. This indicates that most cysteines are existing at a predominantly oxidized state, which is not typically the case.
Potential solution
-
•
Start cell lysis (step 5) less than 16 h after cell harvesting (step 4) (e.g., do not let cell pellets sit at -80°C for more than 16 h).
-
•
Re-synthesize fresh L-IPIAA and H-IPIAA probes, limit freeze thaw cycles of the aliquots, and keep aliquots in amber microcentrifuge tubes at −80°C.
-
•
Take extra care that all treatments performed with L-IPIAA or H-IPIAA (step 8 and 12) are done in the dark.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Keriann Backus (kbackus@mednet.ucla.edu).
Technical contact
Questions regarding execution of the described protocol can be directed to the technical contact, Ashley Julio (ajulio@g.ucla.edu).
Materials availability
All plasmids generated or used for this protocol are deposited on Addgene. The synthesis of all required probes can be found in the “before you begin” section of this paper. Synthesis of IAA was adapted from Backus et al.17 The synthesis of IPIAA reagents was reported in Desai et al.6 Synthesis of biotin-azide was reported in Cao et al.18
Data and code availability
For data and code associated with this protocol, please refer to Yan et al.1 All code is deposited and publicly available on GitHub (https://github.com/BackusLab/cysloc and https://github.com/ashleyjulio/Cys-LOx_Volcano). An archived version of the Cys-LoC and Cys-LOx data processing pipeline is available at Zenodo (https://doi.org/10.5281/zenodo.10482958) and an archived version of the Cys-LOx volcano plot script is available at Zenodo (https://doi.org/10.5281/zenodo.10480961).
Acknowledgments
This study was supported by a Packard Fellowship 2020-71388 (K.M.B.), National Institutes of Health DP2 OD030950-01 (K.M.B.), TRDRP T31DT1800 (T.Y.), and NIGMS UCLA Chemistry Biology Interface T32GM136614 (A.R.J.). iBMDM cells were graciously provided by the laboratory of Dr. Stephen T. Smale. The graphical abstract, Figure 2, Figure 3, and Figure 4 were created using BioRender.com. We acknowledge Nikolas R. Burton and Lisa Boatner for their guidance on the synthesis and computational pipelines, respectively.
Author contributions
Conceptualization, K.M.B. and T.Y.; experiment, A.R.J. and T.Y.; methodology, A.R.J. and T.Y.; writing – original draft, A.R.J. and K.M.B.; writing – review and editing, A.R.J., T.Y., and K.M.B.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Ashley R. Julio, Email: ajulio@g.ucla.edu.
Keriann M. Backus, Email: kbackus@mednet.ucla.edu.
References
- 1.Yan T., Julio A.R., Villanueva M., Jones A.E., Ball A.B., Boatner L.M., Turmon A.C., Nguyễn K.B., Yen S.L., Desai H.S., et al. Proximity-labeling chemoproteomics defines the subcellular cysteinome and inflammation-responsive mitochondrial redoxome. Cell Chem. Biol. 2023;30:811–827.e7. doi: 10.1016/j.chembiol.2023.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffrey S.R., Snyder S.H. The biotin switch method for the detection of S-nitrosylated proteins. Sci. STKE. 2001;2001:pl1. doi: 10.1126/stke.2001.86.pl1. [DOI] [PubMed] [Google Scholar]
- 3.Leichert L.I., Gehrke F., Gudiseva H.V., Blackwell T., Ilbert M., Walker A.K., Strahler J.R., Andrews P.C., Jakob U. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu L., Li Z., Liu K., Tian C., He J., He J., He F., Xu P., Yang J. A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes. Nat. Protoc. 2020;15:2891–2919. doi: 10.1038/s41596-020-0352-2. [DOI] [PubMed] [Google Scholar]
- 5.Xiao H., Jedrychowski M.P., Schweppe D.K., Huttlin E.L., Yu Q., Heppner D.E., Li J., Long J., Mills E.L., Szpyt J., et al. A Quantitative Tissue-Specific Landscape of Protein Redox Regulation during Aging. Cell. 2020;180:968–983.e24. doi: 10.1016/j.cell.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desai H.S., Yan T., Yu F., Sun A.W., Villanueva M., Nesvizhskii A.I., Backus K.M. SP3-Enabled Rapid and High Coverage Chemoproteomic Identification of Cell-State-Dependent Redox-Sensitive Cysteines. Mol. Cell. Proteomics. 2022;21 doi: 10.1016/j.mcpro.2022.100218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Branon T.C., Bosch J.A., Sanchez A.D., Udeshi N.D., Svinkina T., Carr S.A., Feldman J.L., Perrimon N., Ting A.Y. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 2018;36:880–887. doi: 10.1038/nbt.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey J.D., Diotallevi M., Nicol T., McNeill E., Shaw A., Chuaiphichai S., Hale A., Starr A., Nandi M., Stylianou E., et al. Nitric Oxide Modulates Metabolic Remodeling in Inflammatory Macrophages through TCA Cycle Regulation and Itaconate Accumulation. Cell Rep. 2019;28:218–230.e7. doi: 10.1016/j.celrep.2019.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorsbach R.B., Murphy W.J., Lowenstein C.J., Snyder S.H., Russell S.W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J. Biol. Chem. 1993;268:1908–1913. doi: 10.1016/S0021-9258(18)53940-5. [DOI] [PubMed] [Google Scholar]
- 10.Lowenstein C.J., Alley E.W., Raval P., Snowman A.M., Snyder S.H., Russell S.W., Murphy W.J. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc. Natl. Acad. Sci. USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Held T.K., Weihua X., Yuan L., Kalvakolanu D.V., Cross A.S. Gamma interferon augments macrophage activation by lipopolysaccharide by two distinct mechanisms, at the signal transduction level and via an autocrine mechanism involving tumor necrosis factor alpha and interleukin-1. Infect. Immun. 1999;67:206–212. doi: 10.1128/IAI.67.1.206-212.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van den Bossche J., Baardman J., Otto N.A., van der Velden S., Neele A.E., van den Berg S.M., Luque-Martin R., Chen H.-J., Boshuizen M.C.S., Ahmed M., et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. doi: 10.1016/j.celrep.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Emara M.M., Fujimura K., Sciaranghella D., Ivanova V., Ivanov P., Anderson P. Hydrogen peroxide induces stress granule formation independent of eIF2α phosphorylation. Biochem. Biophys. Res. Commun. 2012;423:763–769. doi: 10.1016/j.bbrc.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciriolo M.R., Palamara A.T., Incerpi S., Lafavia E., Buè M.C., De Vito P., Garaci E., Rotilio G. Loss of GSH, oxidative stress, and decrease of intracellular pH as sequential steps in viral infection. J. Biol. Chem. 1997;272:2700–2708. doi: 10.1074/jbc.272.5.2700. [DOI] [PubMed] [Google Scholar]
- 15.Khomich O.A., Kochetkov S.N., Bartosch B., Ivanov A.V. Redox Biology of Respiratory Viral Infections. Viruses. 2018;10:392. doi: 10.3390/v10080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go Y.-M., Jones D.P. Redox theory of aging: implications for health and disease. Clin. Sci. 2017;131:1669–1688. doi: 10.1042/CS20160897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backus K.M., Correia B.E., Lum K.M., Forli S., Horning B.D., González-Páez G.E., Chatterjee S., Lanning B.R., Teijaro J.R., Olson A.J., et al. Proteome-wide covalent ligand discovery in native biological systems. Nature. 2016;534:570–574. doi: 10.1038/nature18002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao J., Boatner L.M., Desai H.S., Burton N.R., Armenta E., Chan N.J., Castellón J.O., Backus K.M. Multiplexed CuAAC Suzuki-Miyaura Labeling for Tandem Activity-Based Chemoproteomic Profiling. Anal. Chem. 2021;93:2610–2618. doi: 10.1021/acs.analchem.0c04726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lam J., Takeshita S., Barker J.E., Kanagawa O., Ross F.P., Teitelbaum S.L. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong A.T., Leprevost F.V., Avtonomov D.M., Mellacheruvu D., Nesvizhskii A.I. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry-based proteomics. Nat. Methods. 2017;14:513–520. doi: 10.1038/nmeth.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Veiga Leprevost F., Haynes S.E., Avtonomov D.M., Chang H.-Y., Shanmugam A.K., Mellacheruvu D., Kong A.T., Nesvizhskii A.I. Philosopher: a versatile toolkit for shotgun proteomics data analysis. Nat. Methods. 2020;17:869–870. doi: 10.1038/s41592-020-0912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu F., Haynes S.E., Nesvizhskii A.I. IonQuant Enables Accurate and Sensitive Label-Free Quantification With FDR-Controlled Match-Between-Runs. Mol. Cell. Proteomics. 2021;20 doi: 10.1016/j.mcpro.2021.100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyanova S., Temu T., Sinitcyn P., Carlson A., Hein M.Y., Geiger T., Mann M., Cox J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13:731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 24.Jami-Alahmadi Y., Pandey V., Mayank A.K., Wohlschlegel J.A. A Robust Method for Packing High Resolution C18 RP-nano-HPLC Columns. J. Vis. Exp. 2021;171:e62380. doi: 10.3791/62380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For data and code associated with this protocol, please refer to Yan et al.1 All code is deposited and publicly available on GitHub (https://github.com/BackusLab/cysloc and https://github.com/ashleyjulio/Cys-LOx_Volcano). An archived version of the Cys-LoC and Cys-LOx data processing pipeline is available at Zenodo (https://doi.org/10.5281/zenodo.10482958) and an archived version of the Cys-LOx volcano plot script is available at Zenodo (https://doi.org/10.5281/zenodo.10480961).