Highlights
-
•
Functional Motor Disorders are common and disabling and diagnosis can be challenging.
-
•
There are a range of clinical neurophysiological techniques that can assist in providing a positive diagnosis.
-
•
Here we provide a state of the art practical review of these techniques, including established and emerging tests.
Keywords: Functional Neurological Disorder, Functional Movement Disorders, Electrophysiology, Clinical neurophysiology
Abstract
Functional Motor Disorders are common and disabling. Clinical diagnosis has moved from one of exclusion of other causes for symptoms to one where positive clinical features on history and examination are used to make a “rule in” diagnosis wherever possible. Clinical neurophysiological assessments have developed increasing importance in assisting with this positive diagnosis, not being used simply to demonstrate normal sensory-motor pathways, but instead to demonstrate specific abnormalities that help to positively diagnose these disorders. Here we provide a practical review of these techniques, their application, interpretation and pitfalls. We also highlight particular areas where such tests are currently lacking in sensitivity and specificity, for example in people with functional dystonia and functional tic-like movements.
1. Introduction
Functional Motor Disorders are a subtype of Functional Neurological Disorder (FND), a common and disabling condition which spans motor, seizure-like, sensory and cognitive symptoms (Espay et al., 2018). In general terms, FND is a disorder where the primary problem appears to be one of accessing or controlling the body normally, despite normal motivation and normal basic function of the nervous system. The term dissociative disorder is used to describe this phenomenon within some classification systems, which speaks to this type of “disconnection” from the body. FND has occupied a complex position between neurology and psychiatry with explanatory models tending to focus on one aspect of the other; however more integrated approaches are now developing traction.
Functional Motor Disorders span a wide range of phenomena including weakness, tremor, dystonia, jerks (myoclonus, tic-like movements) and gait disturbance. As well as occurring in isolation, they can be seen as a prodrome to or overlay on other disorders such as Parkinson’s disease and Multiple Sclerosis (e.g. Onofrj et al., 2022, Piliavska et al., 2023).
The twin pillars of clinical diagnosis rely on inconsistency and incongruence (Espay et al., 2018). Inconsistency refers to variability of the observed phenomena over time, for example tremor which varies in anatomical distribution, frequency or changes with distraction. Incongruity refers to the way in which a functional disorder breaks basic rules of anatomy and physiology or is different from the pattern seen in disorders known to be due to structural damage/degeneration of the nervous system. Examples here would include a tubular visual field defect (which breaks the basic laws of optics). One can see that diagnosis based on incongruity from what is seen in typical neurological disease (rather than basic anatomy or physics) is a rather problematic criteria as it makes an assumption that the patterns of symptoms and signs seen in typical neurological disease are all known, which is untrue.
Neurophysiology has been used for many years as a diagnostic aid in functional movement disorders, especially for functional tremor and myoclonus. Gupta and Lang proposed a specific category of diagnostic certainty for functional movement disorders of laboratory supported diagnosis (Gupta and Lang, 2009). This allows in a formal way the incorporation of laboratory (typically neurophysiological) assessments that allow a positive diagnosis. This, therefore, is not using investigations such as neurophysiology to demonstrate normal function of the nervous system. Instead, the concept is that there are specific techniques that can positively demonstrate inconsistency and incongruence to complement clinical examination.
Here we provide a clinically focused review of the neurophysiological techniques that can be useful in positive diagnosis of functional motor disorders.
2. Functional tremor
2.1. Electrophysiological methods in functional tremor
Functional tremor is the most common functional movement disorder (Carson et al., 2016, Tinazzi et al., 2020). Clinical neurophysiology can support the diagnosis and strengthen the level of diagnostic certainty in clinically challenging cases (Deuschl et al., 2022, Hallett, 2016, Schwingenschuh et al., 2016, Vial et al., 2019). A recent survey-based study found that electrophysiology was used by 60 % of movement disorder specialists on a regular basis for a laboratory-supported diagnosis of functional myoclonus or tremor. About one third of respondents had no access to such laboratory studies. There were considerable differences between countries in practice patterns and access to testing (LaFaver et al., 2020).
The recommended standard equipment includes two accelerometers, a four-channel electromyography (EMG), a metronome, which is available as free online tool or mobile app, and a 500-gram weight that can be attached to the limb. Tremor recordings are most often obtained from both upper limbs and are performed at rest, at posture (with and without weight loading), and during movement. Recording may be performed over additional sites based on the presence of tremor in other body parts such as the lower limbs or the head. Testing usually includes tapping studies, performance of ballistic movements or other distraction manoeuvres (Deuschl et al., 2022).
Recorded signals are analyzed in the time and frequency domains (Schwingenschuh et al., 2016, Vial et al., 2019). In patients with presumed functional tremor, tremor recordings mainly aim to identify electrophysiological correlates of the clinical hallmarks of functional tremor, namely variability, distractibility and entrainment, co-contraction, and synchronicity (Deuschl et al., 2022).
2.2. Electrophysiological features of functional tremor
Clinical neurophysiology is useful to demonstrate the large variation of tremor amplitude and frequency commonly seen in functional tremor (O'Suilleabhain and Matsumoto, 1998). The frequency usually ranges between 6 and 11 Hz for functional hand tremors (Brown and Thompson, 2001).
The normal allocation of attention during aimed movement has been shown to be altered in functional tremor, where the attention is disproportionately directed towards the ongoing visual feedback from the moving hand (Huys et al., 2021). The strongest positive clinical and also electrophysiological features of functional tremor are therefore distractibility and entrainment. To investigate if these are present, sEMG electrodes and accelerometers are mounted on the tremulous limb and on the corresponding contralateral limb. Tremor is recorded while voluntary rhythmic repetitive movements at various frequencies given by a metronome and ballistic movements directed by the examiner are performed with the contralateral limb. The tremor is considered as entrained if it takes up exactly the frequency of the voluntary tapping, which is defined by significant coherence between the EMG spectra of the tremulous and the tapping extremity at the tapping frequency (McAuley and Rothwell, 2004, Schwingenschuh et al., 2016). The patient needs to be instructed to tap at a low amplitude. This helps to minimize the chance of mechanical transmission between the limbs, which could be misinterpreted as coherence (Vial et al., 2019). If the original tremor frequency peak persists and an additional tremor peak emerges at the frequency of the tapping, this finding may correspond to a mirror movement and should not be confused with entrainment (Merchant et al., 2018).
While pure entrainment is seen in only about one third of patients with functional tremor, significant changes in tremor frequency and marked intraindividual variability during tapping are by far more common (Schwingenschuh et al., 2011b, Schwingenschuh et al., 2016, Zeuner et al., 2003). Indeed, entrainment may be relatively infrequent precisely because so many patients experience complete or near complete suppression of tremor with distraction. Frequency analyses may reveal an incorrect tapping performance, which is another positive sign for a functional tremor (Schwingenschuh et al., 2011b, Zeuner et al., 2003).
In the ballistic movement test, the patient is asked to perform a quick movement with one hand while observing for a pause in the functional tremor of the opposite hand during the quick movement (Kumru et al., 2004, Schwingenschuh et al., 2011a, Schwingenschuh et al., 2011b).
The electrophysiological equivalent of the clinical ‘‘coactivation sign’’ is a short, approximately 300 ms, tonic coactivation phase on EMG before the onset of tremor bursts (Deuschl et al., 1998). This arises because coactivation of agonist–antagonist muscles leads to an isometric-type tremor. There are no tonic-coactivation signs before tremor onset in other tremor disorders (Chen and Chen, 2020).
Approximately half of the patients with functional tremor show significant coherence between two tremulous limbs. This is regarded as another positive sign for functional tremor, as - with the exception of orthostatic tremor - most patients with bilateral tremors have independent tremor rhythms in different affected body parts (Raethjen et al., 2004, Schwingenschuh et al., 2011b). Standard coherence analyses use a single coherence estimate for the total time interval under investigation. In contrast, wavelet coherence analysis enables to detect variations in coherence and phase difference between two signals over time. Based on the results of a recent study, the authors concluded that wavelet coherence analysis could be a useful additional tool to discriminate functional tremor from other tremors and that wavelet coherence analysis might be superior to standard coherence analysis (Kramer et al., 2018).
Functional tremors may show an increase of the tremor amplitude during loading of the limb. The increase of tremor amplitudes in functional tremor may arise from increased coactivation to maintain oscillation (Schwingenschuh et al., 2011b). Other characteristic features are absence of finger tremor (Deuschl et al., 1998), and involvement of fewer limb segments (O'Suilleabhain and Matsumoto, 1998).
2.3. Performance of electrophysiological test batteries in functional tremor
The clinical presentation of functional tremor varies widely and it is therefore not surprising that only some, but not all, of the neurophysiological features described above are observed in individuals with functional tremor and therefore a battery of tests is usually needed for making the diagnosis (Chen and Chen, 2020, Schwingenschuh et al., 2011b). Such a test battery including assessment of tonic coactivation at tremor onset, tapping performance at three frequencies, frequency shift / suppression/ entrainment with tapping, response to ballistic movements and loading the limb, and coherence analysis (score of ≥ 3 out of 10 positive tests suggests functional tremor) has been validated in a large prospective study including 40 patients with functional upper limb tremor and 72 patients with tremors of other aetiologies, and showed good sensitivity (89.5 %), specificity (95.9 %), and inter-rater reliability (Schwingenschuh et al., 2016). Other neurophysiological criteria for the diagnosis of functional tremor that have been proposed are frequency change during entrainment tests, frequency change during distractibility tests, and frequency variability > 1.75 Hz (score of ≥ 2 out of 3 positive tests suggests functional tremor) (Kramer et al., 2018).
Most neurophysiological studies have been performed in patients with functional limb tremors. It has also been reported as an adjunct to the clinical diagnosis of functional palatal tremor with electromyographic recordings with and without motor distraction with time-locked video recordings (Vial et al., 2019), although change with distraction is often clinically very clear.
The demonstration of functional tremors does not exclude another neurological disorder because functional and other neurological disorders can coexist in the same patient. Most electrophysiological studies have only included patients with pure functional tremor, thus its value for identifying patients with functional overlay and for differentiating pure functional tremor from functional overlay is unknown (Schwingenschuh and Deuschl, 2016). In clinical practice, this is of particular interest in tremor patients, who are referred for deep brain stimulation (DBS) or ablation procedures (i.e., radiofrequency thalamotomy, magnetic resonance guided focused ultrasound) because of medication refractory tremor. Functional movement disorders are regarded a contraindication to performing these procedures, but failure to recognise this diagnosis has led to unnecessary DBS and other interventions with no or no lasting benefit (Pauls et al., 2017). In a recent retrospective study of 87 medication-refractory essential tremor patients referred for presurgical workup, nine patients were clinically suspected of functional tremor by the DBS neurologist. Electrophysiological criteria used for a diagnosis of functional tremor were distractibility, entrainment, increased variability of tremor features spontaneously or with tasks, variability in tremor vector, variability of tremor frequency >2 Hz, or high coherence. Electrophysiology confirmed functional tremor features in 7/9. Electrophysiology newly identified 5 additional cases of functional tremor. There were 12 total confirmed cases of functional tremor, which was present isolated in 1, and mixed with ET in 11 (Chou et al., 2022). Presence of a functional movement disorder may also be considered among the possible reasons for failure of surgical interventions in patients with tremor and recognizing this entity is essential to avoid further unnecessary invasive therapies (Alshimemeri et al., 2022).
Future prospective studies need to evaluate the usefulness of above-mentioned test batteries in cases with clinically undetermined tremors and in patients with a tremor disorder and functional overlay (Thomsen et al., 2020).
3. Functional weakness (and associated sensory loss)
Functional weakness/paralysis is a common motor presentation. Classic clinical signs include Hoover’s sign where weakness of hip extension returns to normal when the movement is triggered by contralateral hip flexion. Most patients with functional weakness also complain of sensory disturbance – either complete sensory loss, or more commonly a range of more subtle alterations in sensory experience from numbness to dysesthesia. Neurophysiological assessments are quite commonly performed to investigate such symptoms, typically with the expectation that these tests will be normal, ruling out (in theory) a typical neurological disease process, rather than ruling in a functional one. However, more complex tasks have been employed, largely in experimental rather than clinical settings, to attempt to provide a positive diagnosis of functional motor/sensory impairment.
In patients with functional weakness (and indeed other movement disorders) the examination of central motor tracts and peripheral nerves should produce normal results (Cantello et al., 2001, Jang and Seo, 2019, Janssen et al., 1976, Liepert et al., 2008, Liepert et al., 2009, Valls-Sole, 2016). For example, single pulse Transcranial Magnetic Stimulation (TMS) can be used to assess motor evoked potential (MEP) latencies and amplitudes. If these parameters are outside the normal range, a structural lesion can be assumed which would not be compatible with a pure Functional Motor Disorder (FMD).
Typically, FMD patients with weakness, and some other functional movement disorders such as fixed dystonia, report the inability to perform voluntary movements without being able to explain what prevents these movements. This suggests the presence of a form of motor inhibition. To further explore this hypothesis, motor excitability has been tested during a motor imagery task. In healthy subjects, the imagination of a movement results in a considerable, task specific increase in corticospinal excitability for muscles involved in the imagined movement (Facchini et al., 2002, Suzuki et al., 2021). In two studies, FMD patients with a unilateral functional paresis were asked to imagine index finger adductions (Liepert et al., 2008, Liepert et al., 2009). Recordings were taken from the first dorsal interosseous muscle. Compared to the control condition (MEPs recorded at rest), patients exhibited a decrease of MEP amplitudes while imagining an index finger movement with the affected side. This suggests an “active“ inhibitory process and was present in patients with flaccid paresis as well as patients with fixed dystonia. This finding indicates that the clinical presentation is less relevant for the observed phenomenon. Both, flaccid as well as dystonic movement disorders, seem to share the same type of inhibition. Moreover, it was demonstrated that, in some patients, the clinically non-affected side also showed significantly less increase of motor excitability during motor imagery than the healthy age-matched control group. This supports the idea of an underlying generalized inhibition. Of course, TMS results cannot discriminate from which brain areas this motor inhibition originates. However, neuroimaging data support the idea of an involvement of dorsal anterior cingulate, dorsolateral prefrontal, inferior frontal cortices on cognitive control and motor inhibition and of the supplementary motor area on motor planning (Perez et al., 2015).
Liepert et al. also studied motor excitability during motor imagery and action observation in FMD patients with a functional weakness of one or both lower extremities (Liepert et al., 2011). Similar to results obtained in the upper extremities, motor imagery of a foot dorsiflexion was associated with a MEP amplitude reduction as compared to the TMS recording at rest. In contrast, action observation induced an increase of motor excitability comparable to healthy subjects. This finding suggests that the perspective (first-person or third-person) could be relevant for the occurrence of motor inhibition in FMD patients.
Some other MEP abnormalities have been found in FMD patients: Changes of MEP amplitudes in response of an auditory cue signal were examined in FMD patients and healthy controls. Following the cue, subjects were asked to perform ramp-and-hold contractions during which TMS was applied. MEP sizes showed a significantly higher variability in FMD patients. The authors presented this finding as coefficients of variance and suggested that this variance is a supportive parameter for the diagnosis of FMD (Morita et al., 2008). During voluntary contraction, MEP amplitude increases, MEP latency decreases and MEP duration is prolonged. An inverse relationship between short-interval intracortical inhibition and MEP duration has been described, suggesting that motor cortical mechanisms contribute to MEP duration (van den Bos et al., 2017). In a retrospective data analysis, MEP duration increase during voluntary contraction was studied in 5 FMD patients and was found to be absent (Brum et al., 2015). This interesting finding deserves further exploration.
Typically, sensory evoked potentials (SEPs) obtained by repetitive electrical stimulation of the median nerve or the tibial nerve are unremarkable in FMD patients and serve as an indicator of intact somatosensory pathways, thus supporting the assumption of a non-structural but rather functional impairment in these patients (Hallett, 2016, Harvey et al., 2006, Kaplan et al., 1985). However, several publications report transient abnormalities in the shape or amplitude of cortical SEPs. Levy & Mushin published SEP recordings in 9 FMD patients and found smaller cortical SEP amplitudes when stimulating the nerve with near-threshold intensity (Levy and Mushin, 1973). An increase of the stimulus intensity was associated with a normalization of the SEP amplitude. SEPs also normalized after recovery. Yazici et al. reported 2 FMD patients with severe gait disturbances and an almost complete loss of the cortical SEP. After recovery, cortical SEPs became normal (Yazici et al., 2004). As a similar case, a 12-year-old FMD patient was reported to have a transient loss of cortical SEPs which became normal after remission of symptoms (Yu et al., 2021). Powell et al. (2019) presented the case of a 22-year-old man with the diagnosis of FMD and a transient latency delay and amplitude reduction of the tibial SEP. However, other researchers questioned the pure functional origin of this finding and argued that FMD is often associated with other neurologic disorders which, for example, could have caused a transient demyelination (Hallett et al., 2020). Gurses et al. published the case of a FMD patient with normal N20 potential (median nerve SEP) but enlarged amplitudes of the P25 and N33 components of the cortical potential (Gurses et al., 2008).
Therefore, although in most FMD patients SEP recordings are within normal range, several reports suggest subtle abnormalities of the cortical SEP. In almost all cases, a decrease of the potential amplitude was found, suggesting an inhibition. The fact that these abnormalities were reversed within days (following clinical recovery) supports the idea of a functional, not a structural deficit. A possible explanation for these findings was presented by Vuilleumier et al. who described a contralateral hypoactivation of basal ganglia and thalamus in FMD patients with a unilateral sensorimotor loss (Vuilleumier et al., 2001). However, the fact that SEPs amplitude abnormalities can only be found in a minority of FMD patients might be explained by pathophysiological heterogeneity in people with FMD.
4. Functional jerks, myoclonus and tics
Clinical diagnosis of functional jerks, myoclonus and tics can be complex as there is significant clinical overlap between functional and other causes of jerky movements both in the movement phenomenology and in co-morbidities. For example, patients with functional jerks may feel an urge preceding their jerks and may be able to suppress their movement briefly too, but these are also characteristic features of tics in Tourette’s syndrome (Ganos et al., 2018). Psychiatric comorbidity such as anxiety disorders, obsessive–compulsive disorders and depression are common phenomena in both patients with functional jerks and organic myoclonus or tics. Clinical neurophysiological testing is therefore often particularly useful in the positive diagnosis of a functional jerky movement disorder (Zutt et al., 2017).
4.1. Clinical neurophysiology of jerky movement disorders: Jerk-locked back-averaging and the Bereitschaftspotential
Jerky movement disorders such as myoclonus, tics and functional jerks are best studied using combined surface EMG and EEG recordings (van der Veen et al., 2021). Although polymyography is more easily obtained, and some characteristics such as muscle recruitment pattern and muscle burst duration can be assessed using EMG only, the co-registration of EEG allows investigation of cortical transients preceding the jerks, which adds valuable diagnostic information (Hallett et al., 2021).
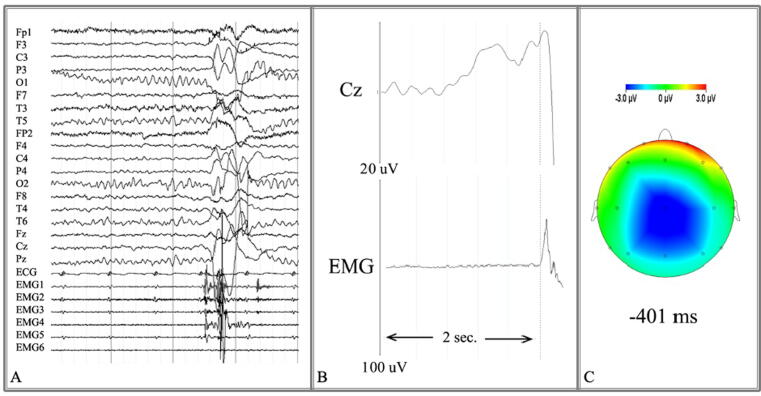
Analysis of EMG-EEG is performed offline, and the hallmark is jerk-locked back-averaging. This method increases the signal to noise ratio spectacularly and time-locks cortical activity to jerky movements, enabling the detection of cortical transients directly preceding a jerky movement (van der Veen et al., 2022). In short, EMG bursts corresponding to jerky movements are identified visually, and markers are placed at burst onset of the first muscle involved. A clear onset point needs to be identifiable, which means the procedure may not be possible in patients with high frequency jerks. For each burst, an EMG-EEG segment lasting a few seconds before and after the jerk is selected (e.g., jerk ± 2 s). Next, the signal is averaged across all segments, decreasing background noise. For averaging to be effective, at least 100 segments are ideal. After jerk-locked back-averaging, the neurophysiologist is left with a visual representation of the association in time between cortical activity and the jerky movement itself (Fig. 1).
Fig. 1.
A) Segment of co-registered EEG (top) and EMG (bottom) traces. A jerk is visible as muscle bursts in the EMG traces; the corresponding EEG traces are affected by movement artifact. B) Jerk-locked back-averaging of many segments results in a visual representation of the association in time between cortical activity at the vertex (Cz) and the jerky movement (EMG). In this case, a Bereitschaftspotential is visible, characterised by a slowly rising negative deflection over the vertex within 2 s before movement onset. C) Visual representation of the negative deflection (in blue) over the vertex 400 ms before movement onset. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The presence of a Bereitschaftspotential (BP), or readiness potential, preceding the jerky movement can be verified in this visual representation. First described in 1965 (Kornhuber and Deecke, 1965), the BP is generally thought to represent a ramping up of EEG activation prior to voluntary actions (Shibasaki and Hallett, 2006). It is characterised by a slowly rising negative deflection over the vertex, occurring within two seconds before movement onset. The potential consists of an early component, comprising a slow increase of EEG negativity about 1.5 s before movement onset, and a late component, comprising a steeper increase of negativity around 400–500 ms prior to movement onset. Investigation of the BP can aid the differentiation of jerky movement disorders, as will be discussed below.
4.2. Differentiating functional jerks from myoclonus
When differentiating between functional jerks and myoclonus, investigation of the BP is of great diagnostic value (van der Veen et al., 2021). In two studies, a BP upon visual inspection was reported in 47–86 % of patients with functional jerks and none with myoclonus (Beudel et al., 2018, van der Salm et al., 2012).This makes the presence of a BP a specific, positive sign for functional myoclonus when compared against organic myoclonus, but less so when compared with organic tics. Note however, that the test does not have a high sensitivity and that the absence of a BP therefore does not rule out functional jerks.
A second diagnostic sign, also based on jerk-locked back-averaging, consists of event related desynchronisation in the broad beta range (13–45 Hz) before onset of functional jerks. Significant event-related desynchronisation has been identified in 62–65 % of patients with functional jerks, but not in patients with organic myoclonus (Beudel et al., 2018, Meppelink et al., 2016). Adding investigation of event-related desynchronisation to BP assessment improves diagnostic sensitivity, without loss of specificity, and thus improves the ability to discriminate between functional jerks and myoclonus.
Of course, the presence of electrophysiology findings befitting cortical myoclonus should be taken as evidence against functional jerks. For instance, brief EMG discharges with bursts lasting < 50–100 ms, EEG seizure discharges, and a cortical wave over the contralateral sensorimotor cortex preceding the movement by 10–40 ms all suggest a non-functional myoclonus. See van der Veen et al., 2022 for a practical review on the clinical neurophysiology of myoclonus.
Propriospinal myoclonus deserves special attention in this section because it is a specific presentation of jerky movements, which upon evaluation is considered to be functional in most patients (van der Salm et al., 2010). Non-functional propriospinal myoclonus resulting from a spinal lesion is rare (van der Salm et al., 2014). Propriospinal myoclonus was originally hypothesised to arise from a spiral generator that transmits activity up and down the spinal cord via propriospinal pathways (Brown et al., 1991), resulting in axial jerks typically involving flexion of the trunk, hips and knees. On EMG, the activation pattern of propriospinal myoclonus consists of bilateral rostral and caudal recruitment originating from the spinal cord. A consistent recruitment pattern fits a diagnosis of propriospinal myoclonus, whereas recruitment variability is indicative of functional jerks. A BP could be identified in 63–86 % of patients with functional jerks, and none of the patients with organic myoclonus (Erro et al., 2013, van der Salm et al., 2014). Therefore, assessment of recruitment variability and BP is recommended for patients presenting with axial jerks. Clinical neurophysiological testing can be impactful, because clinical diagnosis of propriospinal myoclonus is known to be unreliable (Erro et al., 2013) and a delay in diagnosis predicts a worse outcome in patients with functional axial jerks (Erro et al., 2014).
4.3. Differentiating functional jerks from tics
To differentiate functional jerks from motor tics, investigation of the BP is also the most important test. One study compared the presence of a BP in functional jerks and tics in Tourette patients: a BP was found in 86 % of patients with functional jerks and 43 % of Tourette patients (van der Salm et al., 2012). The onset of BP was significantly earlier for functional jerks, with a median onset time of 1.2 s prior to functional jerks versus a median onset time of 0.9 s prior to motor tics. Note, however, that the range in BP onset time in functional jerks and motor tics did overlap (Table 1). Two earlier studies investigating only motor tics reported a BP in 0 out of 6 (Obeso et al., 1981) and 2 out of 5 (Karp et al., 1996) Tourette patients. Other clinical neurophysiological features that have been investigated in patients with motor tics, such as reduced motor cortex excitability upon TMS or reduced pre-pulse inhibition (van der Veen et al., 2022), have not been investigated in functional jerks and therefore unfortunately do not carry discriminatory value. Overall, the presence and onset time of the BP may aid in the differentiation of functional jerks versus motor tics, however, a clinical neurophysiological test distinguishing these two movement disorders with high sensitivity and specificity is currently lacking. It is possible that other pre-movement electrophysiological potentials such as beta event-related desynchronisation might provide more discriminatory power between tics and functional tic-like movements (Hallett M, personal communication).
Table 1.
Presence and onset time of the Bereitschaftspotential in functional jerks, myoclonus and tics (data derived from van der Salm et al., 2012).
| Presence of BP preceding jerky movements |
Onset time BP (ms) | |
|---|---|---|
| Functional jerks | 47–86 % | 1195 (700–2410) |
| Myoclonus | 0 % | – |
| Motor tics | 43 % | 915 (510–1700) |
Onset time is reported as median (range).
5. Functional dystonia
Functional dystonia may manifest with paroxysmal or continuous rapid involuntary movements or with painful fixed posturing of one body part (Ganos et al., 2014). This is the third most common FMD, it usually affects the lower limbs, but it may also manifest as tonic spasms of one side of the face or the neck muscles. Functional dystonia poses great diagnostic challenge (Morgante et al., 2012), as no study has defined the phenomenological features which might help to differentiate it from idiopathic, genetic or other secondary dystonias. Indeed, a minor peripheral injury might predate both functional dystonia and adult onset idiopathic dystonia (Macerollo et al., 2019), albeit people with functional dystonia tend to have sudden onset, evidence of fixed dystonia, and acute peripheral trauma (Ercoli et al., 2021). Distractibility is extremely difficult to achieve in people with functional dystonia, especially when they have fixed postures and, when functional dystonia is mobile, it can be challenging to differentiate it clinically from genetic dystonia whose phenotype can be complex due to combination with other movement disorders. Finally, functional dystonia might occur in the context of idiopathic dystonia syndromes (Tinazzi et al., 2021a, Tinazzi et al., 2021b).
Despite different electrophysiological techniques have been employed to understand the pathophysiology of functional dystonia, there are no validated electrophysiological measures. Some studies showed similarities between functional dystonia and idiopathic dystonia with decreased cortical and spinal inhibition tested respectively with TMS (Avanzino et al., 2008, Espay et al., 2006) and forearm reciprocal inhibition (Espay et al., 2006) (Table 2). Specifically, functional dystonia and idiopathic dystonia share decreased short and long intracortical inhibition in both affected and unaffected body districts decreased cortical silent period and reduced first phase of reciprocal inhibition (Espay et al., 2006). Moreover, tactile temporal discrimination threshold, a marker of somatosensory processing once considered an endophenotype of genetic and idiopathic dystonic syndromes, is equally increased in functional dystonia and idiopathic dystonia (Morgante et al., 2011).
Table 2.
Electrophysiological tests performed in Functional and Idiopathic Dystonia.
| Test | Idiopathic Dystonia | Functional Dystonia |
|---|---|---|
| Electrophysiological differences | ||
| BR recovery cycle | ↓ | ⇔ |
| S-M plasticity | ↑ | ⇔ |
| Pain Tolerance | ⇔ | ↑ |
| Electrophysiological similarities | ||
| SICI | ↓ | ↓ |
| LlCI | ↓ | ↓ |
| CSP | ↓ | ↓ |
| SAI | ⇔ ↓ | ⇔ |
| LAI | ⇔ ↓ | ⇔ |
| Pain Thresholds | ⇔ | ⇔ |
| TDT | ↑ | ↑ |
BR = blink reflex; BP = Bereitschaftspotential; LAI = long afferent inhibition; LICI = long intracortical inhibition; SAI = short afferent inhibition; S-M = sensorimotor; SICI = short intracortical inhibition; TDT = tactile temporal discrimination threshold. Data summarised from: (Avanzino et al., 2008, Espay et al., 2006, Schwingenschuh et al., 2011a, Quartarone et al., 2003, Morgante et al., 2018, Morgante et al., 2011).
Other studies have found difference a group level between functional dystonia and idiopathic dystonia, but specificity and sensitivity of such tests is unknown. Brainstem excitability assessed by the blink reflex recovery cycle has been found normal in 9 out of 10 subjects with functional blepharospasm compared to those with idiopathic blepharospasm, who exhibited disinhibition of brainstem interneurons (Schwingenschuh et al., 2011a). Sensorimotor cortex plasticity probed with the paired associative stimulation protocol is also normal in functional dystonia, opposite from idiopathic dystonia in which lack of topographical specificity of the effects of paired TMS + peripheral nerve stimulation is demonstrated (Quartarone et al., 2003). Interestingly, subjects with complex regional pain syndrome type 1 (CRPS-I) and fixed hand posture, a nosographic entity that has many clinical similarities to functional dystonia (Popkirov et al., 2019), also disclose normal sensorimotor cortex plasticity (Morgante et al., 2017).
Pain is a frequent manifestation of functional dystonia, reported in 47.4 % of subjects (Tinazzi et al., 2021a, Tinazzi et al., 2021b). The sensory-discriminative and cognitive-emotional component of pain in patients can be assessed by testing pain thresholds and pain tolerance, by delivering electrical pulses of increasing intensity. Pain tolerance is intensity at which painful sensation is reported as intolerable and it is a measure of the cognitive-emotional component of pain. People with functional dystonia with persistent symptoms have increased pain tolerance compared to those with paroxysmal functional dystonia and subjects with Cervical Dystonia (Morgante et al., 2018).
All the electrophysiological and psychophysiological measures above described have been tested in small cohorts and found differences at group level. Assessment of sensitivity and specificity in large cohorts and correlation with specific sub-types of functional dystonia is needed in order to use these measures for diagnostic purposes.
6. Functional gait disorders
Gait abnormalities are seen in approximately 40 % of people with a functional movement disorder. Most commonly, these are seen in combination with other signs, although 6 %-9% of patients are reported to have a pure functional gait disorder (Baik and Lang, 2007, Baizabal-Carvallo et al., 2020). Diagnosis of functional gait is challenging as no single walking pattern is pathognomonic (Nonnekes et al., 2020). Historically, functional gaits were often been labelled as ‘bizarre’, but other neurological disorders can also produce bizarre gait features (i.e. ‘hobby horse gait’ in patients with dystonia type 4 (DYT4) or choreatic gait in people with Huntington’s disease) (Wilcox et al., 2011).
In line with other functional movement disorders, diagnosis of functional gait is based on the presence of inconsistencies and incongruity (Espay et al., 2018, Nonnekes et al., 2020). Importantly, the presence of inconsistencies alone is not enough, as these can also be seen in other neurological gait disorders. For example, as dystonia is typically task-specific, it may be present during forward walking, but not when running or walking backwards. This highlights the dangers of using incongruity with other neurological disorder as a diagnostic criterion rather than incongruity with anatomy and physiology, as outlined in the introduction.
In the diagnostic process of gait disorders, the presenting signs should the starting point for a tailored search into the origin (Nonnekes et al., 2018). Specific gait and balance tests, such as tandem gait, the pull-test or walking backwards, can help to reveal inconsistencies or incongruencies, supporting a diagnosis of functional gait. For example, persons with a functional gait often have claims of poor balance, and may walk-broad based and seek support of walls and doorposts. However, when asked to perform tandem gait, they may be able to do his without sidesteps, or may display exaggerated performance with prolonged single-leg stance. Another example of incongruity is an exaggerated postural response after a light touch or tap on the shoulders (Coebergh et al., 2021, Geroin et al., 2022). The neurophysiological complimentary tests build out from these clinical observations. For example, improvement in gait and posture with distraction can be seen clinically, but can also be objectively documented (perhaps more sensitively than clinical examination in some situations) using posturography (Gandolfi et al., 2021, Wolfsegger et al., 2013). Gandolfi et al. (2023) have recently extended this work, demonstrating that certain spatio-temporal parameters of gait tend to normalise under dual-task conditions.
To understand the possible neurophysiological correlates of functional gait, knowledge of normal gait control is required. This has largely been derived from animal work and in minor part from imaged gait (Jahn et al., 2008, Takakusaki et al., 2023), as until recently, measuring brain activity in humans during actual gait was not possible. Recent technological advances now allow for the study of cortical activity during actual waking, for example using ambulatory electroencephalography (Tosserams et al., 2022).
Gait depends on a complex locomotor network, involving spinal central pattern generators, brainstem mesencephalic and cerebellar locomotor regions, and corticostriatal output projecting from the motor cortices (Takakusaki et al., 2023). In addition, distributed cortical areas contribute to adjustment and adaptation of walking. This involves the integration of multisensory feedback and comparison with efference copies of the motor command. The parietotemporal cortex is thought to play an important role in multisensory integration and the comparison between feedforward models (i.e. efference copy) and feedback models (based on multisensory feedback) (Barthel et al., 2018, de Haan and Dijkerman, 2020). The output of the comparison between the feedback feedforward model is being used to update future motor programs, which is thought to take place at the prefrontal cortices and supplementary and premotor areas. There is evidence, however, that comparison between the efference copy and sensory feedback not only occurs at cortical level, but at all levels of the locomotor network, including the spinal cord and brainstem (Takakusaki et al., 2023).
Functional gait disorder is hypothesized to result from dysfunction in the cortical sensorimotor control circuitry comparing feedforward and feedback models (Hallett et al., 2022), in which the feedforward model is overweighed under the influence of previous expectations, attention and emotion (Edwards et al., 2012). A classic paradigm to study sensorimotor control is stepping onto a broken or stationary escalator that had previously been experienced as moving (Bronstein et al., 2009), and this has also been applied to people with functional gait (Lin et al., 2020). Stepping onto a stationary escalator results in subjective and objective instability, known as the locomotor after-effect, and these disappear with repeated stepping onto the stationary escalator (Reynolds and Bronstein, 2003). Translated to gait control; with repeated stepping onto the stationary escalator, the feedforward program is being updated using multisensory information. In the study of Lin et al., 14 people with functional gait and 17 healthy control subjects walked five times onto a stationary sled, then five times onto a moving sled and then again five times onto a stationary sled (Lin et al., 2020). Participants were aware of the change in conditions. Both people with functional gait and controls were able to accommodate their gait in response to the moving sled with similar learning curves, indicating normal motor learning in the context of a postural challenge requiring an automatic reaction. However, when the sled was returned to a stationary position, there was a persistence of the locomotor after-effects (i.e. increased trunk displacement and gait velocity) in people with functional gait compared to the healthy controls. These findings most likely indicate an abnormal scaling between the predicted and actual movement, and inadequate updating of future motor programs.
7. Conclusions
Clinical neurophysiological assessment has a direct and important contribution to make in the positive diagnosis of functional movement disorders. As outlined above, this is well beyond the traditional role of demonstrating normality of specific neural pathways in people with presumed functional disorders. Instead, and building on clinical and pathophysiological advances, it has developed into a useful tool to provide an additional level of diagnostic certainty.
There remain specific areas of need, for example in the diagnosis of certain forms of functional dystonia, and in the differentiation of functional tics from other causes of tics. There is also an issue of availability, with many movement disorders specialists lacking access to specialised neurophysiological testing. This is an important issue to resolve given the paramount importance of making a clear positive diagnosis of a functional movement disorder. It is only through this that the patient can access the appropriate treatment and not be exposed to unnecessary medical and surgical interventions.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alshimemeri S., Vargas-Mendez D., Chen R., Lipsman N., Schwartz M.L., Lozano A.M., et al. Functional tremor developing after successful MRI-guided focused ultrasound thalamotomy for essential tremor. J. Neurol. Neurosurg. Psychiatry. 2022 doi: 10.1136/jnnp-2021-327524. [DOI] [PubMed] [Google Scholar]
- Avanzino L., Martino D., van de Warrenburg B.P., Schneider S.A., Abbruzzese G., Defazio G., et al. Cortical excitability is abnormal in patients with the “fixed dystonia” syndrome. Mov. Disord. 2008;23(5):646–652. doi: 10.1002/mds.21801. [DOI] [PubMed] [Google Scholar]
- Baik J.S., Lang A.E. Gait abnormalities in psychogenic movement disorders. Mov. Disord. 2007;22(3):395–399. doi: 10.1002/mds.21283. [DOI] [PubMed] [Google Scholar]
- Baizabal-Carvallo J.F., Alonso-Juarez M., Jankovic J. Functional gait disorders, clinical phenomenology, and classification. Neurol. Sci. 2020;41(4):911–915. doi: 10.1007/s10072-019-04185-8. [DOI] [PubMed] [Google Scholar]
- Barthel C., Nonnekes J., van Helvert M., Haan R., Janssen A., Delval A., et al. The laser shoes: a new ambulatory device to alleviate freezing of gait in Parkinson disease. Neurology. 2018;90(2):e164–e171. doi: 10.1212/WNL.0000000000004795. [DOI] [PubMed] [Google Scholar]
- Beudel M., Zutt R., Meppelink A.M., Little S., Elting J.W., Stelten B.M.L., et al. Improving neurophysiological biomarkers for functional myoclonic movements. Parkinsonism Relat. Disord. 2018;51:3–8. doi: 10.1016/j.parkreldis.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronstein A.M., Bunday K.L., Reynolds R. What the “Broken Escalator” phenomenon teaches us about balance. Ann. N.Y. Acad. Sci. 2009;1164:82–88. doi: 10.1111/j.1749-6632.2009.03870.x. [DOI] [PubMed] [Google Scholar]
- Brown P., Thompson P.D. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov. Disord. 2001;16(4):595–599. doi: 10.1002/mds.1145. [DOI] [PubMed] [Google Scholar]
- Brown P., Thompson P.D., Rothwell J.C., Day B.L., Marsden C.D. Axial myoclonus of propriospinal origin. Brain. 1991;114:197–214. [PubMed] [Google Scholar]
- Brum M., Cabib C., Valls-Sole J. Clinical value of the assessment of changes in MEP duration with voluntary contraction. Front. Neurosci. 2015;9:505. doi: 10.3389/fnins.2015.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantello R., Boccagni C., Comi C., Civardi C., Monaco F. Diagnosis of psychogenic paralysis: the role of motor evoked potentials. J. Neurol. 2001;248(10):889–897. doi: 10.1007/s004150170075. [DOI] [PubMed] [Google Scholar]
- Carson A., Hallett M., Stone J. Assessment of patients with functional neurologic disorders. Handb. Clin. Neurol. 2016;139:169–188. doi: 10.1016/B978-0-12-801772-2.00015-1. [DOI] [PubMed] [Google Scholar]
- Chen K.S., Chen R. Principles of electrophysiological assessments for movement disorders. J. Mov. Disord. 2020;13(1):27–38. doi: 10.14802/jmd.19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C.Z., Ahlskog J.E., Klassen B.T., Coon E.A., Ali F., Bower J.H., et al. Utility of routine surface electrophysiology to screen for functional tremor prior to surgical treatment of essential tremor. Clin. Park. Relat. Disord. 2022;7 doi: 10.1016/j.prdoa.2022.100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coebergh J., Zimianiti I., Kaski D. Shoulder-tap test for functional gait disorders a sign of abnormal anticipatory behavior. Neurology. 2021;97(23):1070–1071. doi: 10.1212/WNL.0000000000012886. [DOI] [PubMed] [Google Scholar]
- de Haan E.H.F., Dijkerman H.C. Somatosensation in the brain: a theoretical re-evaluation and a new model. Trends Cogn. Sci. 2020;24(7):529–541. doi: 10.1016/j.tics.2020.04.003. [DOI] [PubMed] [Google Scholar]
- Deuschl G., Koster B., Lucking C.H., Scheidt C. Diagnostic and pathophysiological aspects of psychogenic tremors. Mov. Disord. 1998;13(2):294–302. doi: 10.1002/mds.870130216. [DOI] [PubMed] [Google Scholar]
- Deuschl G., Becktepe J.S., Dirkx M., Haubenberger D., Hassan A., Helmich R.C., et al. The clinical and electrophysiological investigation of tremor. Clin. Neurophysiol. 2022;136:93–129. doi: 10.1016/j.clinph.2022.01.004. [DOI] [PubMed] [Google Scholar]
- Edwards M.J., Adams R.A., Brown H., Parees I., Friston K.J. A Bayesian account of 'hysteria'. Brain. 2012;135:3495–3512. doi: 10.1093/brain/aws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercoli T., Defazio G., Geroin C., Marcuzzo E., Fabbrini G., Bono F., et al. Sudden onset, fixed dystonia and acute peripheral trauma as diagnostic clues for functional dystonia. Mov. Disord. Clin. Pract. 2021;8(7):1107–1111. doi: 10.1002/mdc3.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erro R., Bhatia K.P., Edwards M.J., Farmer S.F., Cordivari C. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov. Disord. 2013;28(13):1868–1873. doi: 10.1002/mds.25627. [DOI] [PubMed] [Google Scholar]
- Erro R., Edwards M.J., Bhatia K.P., Esposito M., Farmer S.F., Cordivari C. Psychogenic axial myoclonus: clinical features and long-term outcome. Parkinsonism Relat. Disord. 2014;20(6):596–599. doi: 10.1016/j.parkreldis.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Espay A.J., Morgante F., Purzner J., Gunraj C.A., Lang A.E., Chen R. Cortical and spinal abnormalities in psychogenic dystonia. Ann. Neurol. 2006;59(5):825–834. doi: 10.1002/ana.20837. [DOI] [PubMed] [Google Scholar]
- Espay A.J., Aybek S., Carson A., Edwards M.J., Goldstein L.H., Hallett M., et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol. 2018;75(9):1132–1141. doi: 10.1001/jamaneurol.2018.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini S., Muellbacher W., Battaglia F., Boroojerdi B., Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol. Scand. 2002;105(3):146–151. doi: 10.1034/j.1600-0404.2002.1o004.x. [DOI] [PubMed] [Google Scholar]
- Gandolfi M., Fiorio M., Geroin C., Prior M., De Marchi S., Amboni M., et al. Motor dual task with eyes closed improves postural control in patients with functional motor disorders: a posturographic study. Gait Posture. 2021;88:286–291. doi: 10.1016/j.gaitpost.2021.06.011. [DOI] [PubMed] [Google Scholar]
- Gandolfi M., Fiorio M., Geroin C., Torneri P., Menaspà Z., Smania N., Giladi N., Tinazzi M. Dual tasking affects gait performance but not automaticity in functional gait disorders: a new diagnostic biomarker. Parkinsonism Relat. Disord. 2023;108 doi: 10.1016/j.parkreldis.2023.105291. [DOI] [PubMed] [Google Scholar]
- Ganos C., Edwards M.J., Bhatia K.P. The phenomenology of functional (psychogenic) dystonia. Mov. Disord. Clin. Pract. 2014;1(1):36–44. doi: 10.1002/mdc3.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganos C., Rothwell J., Haggard P. Voluntary inhibitory motor control over involuntary tic movements. Mov. Disord. 2018;33(6):937–946. doi: 10.1002/mds.27346. [DOI] [PubMed] [Google Scholar]
- Geroin C., Nonnekes J., Erro R., Camozzi S., Bloem B.R., Tinazzi M. Shoulder-Touch test to reveal incongruencies in persons with functional motor disorders. Eur. J. Neurol. 2022 doi: 10.1111/ene.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Lang A.E. Psychogenic movement disorders. Curr. Opin. Neurol. 2009;22(4):430–436. doi: 10.1097/WCO.0b013e32832dc169. [DOI] [PubMed] [Google Scholar]
- Gurses N., Temucin C.M., Lay Ergun E., Ertugrul A., Ozer S., Demir B. Evoked potentials and regional cerebral blood flow changes in conversion disorder: a case report and review. Turk Psikiyatri Derg. 2008;19(1):101–107. [PubMed] [Google Scholar]
- Hallett M. Neurophysiologic studies of functional neurologic disorders. Handb. Clin. Neurol. 2016;139:61–71. doi: 10.1016/B978-0-12-801772-2.00006-0. [DOI] [PubMed] [Google Scholar]
- Hallett M., LaFaver K., Maurer C.W., Merchant S.H.I., Vial F. Reader response: therapeutic benefits of early electrophysiological testing in a functional neurology case. Neurol. Clin. Pract. 2020;10(6):e52. doi: 10.1212/CPJ.0000000000000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., DelRosso L.M., Elble R., Ferri R., Horak F.B., Lehericy S., Mancini M., Matsuhashi M., Matsumoto R., Muthuraman M., Raethjen J., Shibasaki H. Evaluation of movement and brain activity. Clin. Neurophysiol. 2021;132(10):2608–2638. doi: 10.1016/j.clinph.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., Aybek S., Dworetzky B.A., McWhirter L., Staab J.P., Stone J. Functional neurological disorder: new subtypes and shared mechanisms. Lancet Neurol. 2022;21(6):537–550. doi: 10.1016/S1474-4422(21)00422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey S.B., Stanton B.R., David A.S. Conversion disorder: towards a neurobiological understanding. Neuropsychiatr. Dis. Treat. 2006;2(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- Huys A.M.L., Haggard P., Bhatia K.P., Edwards M.J. Misdirected attentional focus in functional tremor. Brain. 2021;144(11):3436–3450. doi: 10.1093/brain/awab230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K., Deutschlander A., Stephan T., Kalla R., Hufner K., Wagner J., et al. Supraspinal locomotor control in quadrupeds and humans. Prog. Brain Res. 2008;171:353–362. doi: 10.1016/S0079-6123(08)00652-3. [DOI] [PubMed] [Google Scholar]
- Jang S.H., Seo Y.S. Diagnosis of conversion disorder using diffusion tensor tractography and transcranial magnetic stimulation in a patient with mild traumatic brain injury. Diagnostics (Basel) 2019;9(4) doi: 10.3390/diagnostics9040155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen B.A., Theiler R., Grob D., Dvorak J. The role of motor evoked potentials in psychogenic paralysis. Spine (Phila Pa 1976) 1995;20(5):608–611. doi: 10.1097/00007632-199503010-00019. [DOI] [PubMed] [Google Scholar]
- Kaplan B.J., Friedman W.A., Gravenstein D. Somatosensory evoked potentials in hysterical paraplegia. Surg. Neurol. 1985;23(5):502–506. doi: 10.1016/0090-3019(85)90246-0. [DOI] [PubMed] [Google Scholar]
- Karp B.I., Porter S., Toro C., Hallett M. Simple motor tics may be preceded by a premotor potential. J. Neurol. Neurosurg. Psychiatry. 1996;61(1):103–106. doi: 10.1136/jnnp.61.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhuber H.H., Deecke L. Changes in the brain potential in voluntary movements and passive movements in man: readiness potential and reafferent potentials. Pflugers Arch. Gesamte Physiol. Menschen Tiere. 1965;284:1–17. [PubMed] [Google Scholar]
- Kramer G., Van der Stouwe A.M.M., Maurits N.M., Tijssen M.A.J., Elting J.W.J. Wavelet coherence analysis: A new approach to distinguish organic and functional tremor types. Clin. Neurophysiol. 2018;129(1):13–20. doi: 10.1016/j.clinph.2017.10.002. [DOI] [PubMed] [Google Scholar]
- LaFaver K., Lang A.E., Stone J., Morgante F., Edwards M., Lidstone S., et al. Opinions and clinical practices related to diagnosing and managing functional (psychogenic) movement disorders: changes in the last decade. Eur. J. Neurol. 2020;27(6):975–984. doi: 10.1111/ene.14200. [DOI] [PubMed] [Google Scholar]
- Levy R., Mushin J. The somatosensory evoked response in patients with hysterical anaesthesia. J. Psychosom. Res. 1973;17(2):81–84. doi: 10.1016/0022-3999(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Liepert J., Hassa T., Tuscher O., Schmidt R. Electrophysiological correlates of motor conversion disorder. Mov. Disord. 2008;23(15):2171–2176. doi: 10.1002/mds.21994. [DOI] [PubMed] [Google Scholar]
- Liepert J., Hassa T., Tuscher O., Schmidt R. Abnormal motor excitability in patients with psychogenic paresis. A TMS Study. J Neurol. 2009;256(1):121–126. doi: 10.1007/s00415-009-0090-4. [DOI] [PubMed] [Google Scholar]
- Liepert J., Hassa T., Tuscher O., Schmidt R. Motor excitability during movement imagination and movement observation in psychogenic lower limb paresis. J. Psychosom. Res. 2011;70(1):59–65. doi: 10.1016/j.jpsychores.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Lin D., Castro P., Edwards A., Sekar A., Edwards M.J., Coebergh J., et al. Dissociated motor learning and de-adaptation in patients with functional gait disorders. Brain. 2020;143(8):2594–2606. doi: 10.1093/brain/awaa190. [DOI] [PubMed] [Google Scholar]
- Macerollo A., Edwards M.J., Huang H.C., Lu M.K., Chen H.J., Tsai C.H., et al. Peripheral trauma and risk of dystonia: What are the evidences and potential co-risk factors from a population insurance database? PLoS One. 2019;14(5):e0216772. doi: 10.1371/journal.pone.0216772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley J., Rothwell J. Identification of psychogenic, dystonic, and other organic tremors by a coherence entrainment test. Mov. Disord. 2004;19(3):253–267. doi: 10.1002/mds.10707. [DOI] [PubMed] [Google Scholar]
- Meppelink A.M., Little S., Oswal A., Erro R., Kilner J., Tijssen M.A.J., et al. Event related desynchronisation predicts functional propriospinal myoclonus. Parkinsonism Relat. Disord. 2016;31:116–118. doi: 10.1016/j.parkreldis.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S.H., Haubenberger D., Hallett M. Mirror movements or functional tremor masking organic tremor. Clin. Neurophysiol. Pract. 2018;3:107–113. doi: 10.1016/j.cnp.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante F., Tinazzi M., Squintani G., Martino D., Defazio G., Romito L., et al. Abnormal tactile temporal discrimination in psychogenic dystonia. Neurology. 2011;77(12):1191–1197. doi: 10.1212/WNL.0b013e31822f0449. [DOI] [PubMed] [Google Scholar]
- Morgante F., Edwards M.J., Espay A.J., Fasano A., Mir P., Martino D. Diagnostic agreement in patients with psychogenic movement disorders. Mov. Disord. 2012;27(4):548–552. doi: 10.1002/mds.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgante F., Naro A., Terranova C., Russo M., Rizzo V., Risitano G., et al. Normal sensorimotor plasticity in complex regional pain syndrome with fixed posture of the hand. Mov. Disord. 2017;32(1):149–157. doi: 10.1002/mds.26836. [DOI] [PubMed] [Google Scholar]
- Morgante F., Matinella A., Andrenelli E., Ricciardi L., Allegra C., Terranova C., et al. Pain processing in functional and idiopathic dystonia: an exploratory study. Mov. Disord. 2018;33(8):1340–1348. doi: 10.1002/mds.27402. [DOI] [PubMed] [Google Scholar]
- Morita H., Shimojima Y., Nishikawa N., Hagiwara N., Amano N., Ikeda S. Size variance of motor evoked potential at initiation of voluntary contraction in palsy of conversion disorder. Psychiatry Clin. Neurosci. 2008;62(3):286–292. doi: 10.1111/j.1440-1819.2008.01795.x. [DOI] [PubMed] [Google Scholar]
- Nonnekes J., Goselink R.J.M., Ruzicka E., Fasano A., Nutt J.G., Bloem B.R. Neurological disorders of gait, balance and posture: a sign-based approach. Nat. Rev. Neurol. 2018;14(3):183–189. doi: 10.1038/nrneurol.2017.178. [DOI] [PubMed] [Google Scholar]
- Nonnekes J., Ruzicka E., Serranova T., Reich S.G., Bloem B.R., Hallett M. Functional gait disorders: a sign-based approach. Neurology. 2020;94(24):1093–1099. doi: 10.1212/WNL.0000000000009649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeso J.A., Rothwell J.C., Marsden C.D. Simple tics in Gilles de la Tourette's syndrome are not prefaced by a normal premovement EEG potential. J. Neurol. Neurosurg. Psychiatry. 1981;44(8):735–738. doi: 10.1136/jnnp.44.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onofrj M., Russo M., Carrarini C., Delli Pizzi S., Thomas A., Bonanni L., Espay A.J., Sensi S.L. Functional neurological disorder and somatic symptom disorder in Parkinson's disease. J. Neurol. Sci. 2022;15:433. doi: 10.1016/j.jns.2021.120017. [DOI] [PubMed] [Google Scholar]
- O'Suilleabhain P.E., Matsumoto J.Y. Time-frequency analysis of tremors. Brain. 1998;121:2127–2134. doi: 10.1093/brain/121.11.2127. [DOI] [PubMed] [Google Scholar]
- Pauls K.A.M., Krauss J.K., Kampfer C.E., Kuhn A.A., Schrader C., Sudmeyer M., et al. Causes of failure of pallidal deep brain stimulation in cases with pre-operative diagnosis of isolated dystonia. Parkinsonism Relat. Disord. 2017;43:38–48. doi: 10.1016/j.parkreldis.2017.06.023. [DOI] [PubMed] [Google Scholar]
- Perez D.L., Dworetzky B.A., Dickerson B.C., Leung L., Cohn R., Baslet G., et al. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin. EEG Neurosci. 2015;46(1):4–15. doi: 10.1177/1550059414555905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piliavska K., Dantlgraber M., Dettmers C., Jöbges M., Liepert J., Schmidt R. Functional neurological symptoms are a frequent and relevant comorbidity in patients with multiple sclerosis. Front. Neurol. 2023;14:1077838. doi: 10.3389/fneur.2023.1077838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkirov S., Hoeritzauer I., Colvin L., Carson A.J., Stone J. Complex regional pain syndrome and functional neurological disorders - time for reconciliation. J. Neurol. Neurosurg. Psychiatry. 2019;90(5):608–614. doi: 10.1136/jnnp-2018-318298. [DOI] [PubMed] [Google Scholar]
- Quartarone A., Bagnato S., Rizzo V., Siebner H.R., Dattola V., Scalfari A., et al. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Raethjen J., Kopper F., Govindan R.B., Volkmann J., Deuschl G. Two different pathogenetic mechanisms in psychogenic tremor. Neurology. 2004;63(5):812–815. doi: 10.1212/01.wnl.0000137012.35029.6b. [DOI] [PubMed] [Google Scholar]
- Reynolds R.F., Bronstein A.M. The broken escalator phenomenon. Aftereffect of walking onto a moving platform. Exp. Brain Res. 2003;151(3):301–308. doi: 10.1007/s00221-003-1444-2. [DOI] [PubMed] [Google Scholar]
- Schwingenschuh P., Deuschl G. Functional tremor. Handb. Clin. Neurol. 2016;139:229–233. doi: 10.1016/B978-0-12-801772-2.00019-9. [DOI] [PubMed] [Google Scholar]
- Schwingenschuh P., Katschnig P., Edwards M.J., Teo J.T., Korlipara L.V., Rothwell J.C., et al. The blink reflex recovery cycle differs between essential and presumed psychogenic blepharospasm. Neurology. 2011;76(7):610–614. doi: 10.1212/WNL.0b013e31820c3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingenschuh P., Katschnig P., Seiler S., Saifee T.A., Aguirregomozcorta M., Cordivari C., et al. Moving toward “laboratory-supported” criteria for psychogenic tremor. Mov. Disord. 2011;26(14):2509–2515. doi: 10.1002/mds.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingenschuh P., Saifee T.A., Katschnig-Winter P., Macerollo A., Koegl-Wallner M., Culea V., et al. Validation of “laboratory-supported” criteria for functional (psychogenic) tremor. Mov. Disord. 2016;31(4):555–562. doi: 10.1002/mds.26525. [DOI] [PubMed] [Google Scholar]
- Shibasaki H., Hallett M. What is the Bereitschaftspotential? Clin. Neurophysiol. 2006;117(11):2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Kaneko N., Sasaki A., Tanaka F., Nakazawa K., Nomura T., et al. Muscle-specific movement-phase-dependent modulation of corticospinal excitability during upper-limb motor execution and motor imagery combined with virtual action observation. Neurosci. Lett. 2021;755 doi: 10.1016/j.neulet.2021.135907. [DOI] [PubMed] [Google Scholar]
- Takakusaki K., Takahashi M., Noguchi T., Chiba R. Neurophysiological mechanisms of gait disturbance in advanced Parkinson's disease patients. Neurol. Clin. Neurosci. 2023;11:201–217. [Google Scholar]
- Thomsen B.L.C., Teodoro T., Edwards M.J. Biomarkers in functional movement disorders: a systematic review. J. Neurol. Neurosurg. Psychiatry. 2020;91(12):1261–1269. doi: 10.1136/jnnp-2020-323141. [DOI] [PubMed] [Google Scholar]
- Tinazzi M., Morgante F., Marcuzzo E., Erro R., Barone P., Ceravolo R., et al. Clinical correlates of functional motor disorders: an Italian multicenter study. Mov. Disord. Clin. Pract. 2020;7(8):920–929. doi: 10.1002/mdc3.13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinazzi M., Geroin C., Erro R., Marcuzzo E., Cuoco S., Ceravolo R., et al. Functional motor disorders associated with other neurological diseases: beyond the boundaries of “organic” neurology. Eur. J. Neurol. 2021;28(5):1752–1758. doi: 10.1111/ene.14674. [DOI] [PubMed] [Google Scholar]
- Tinazzi M., Geroin C., Marcuzzo E., Cuoco S., Ceravolo R., Mazzucchi S., et al. Functional motor phenotypes: to lump or to split? J. Neurol. 2021;268:4737–4743. doi: 10.1007/s00415-021-10583-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosserams A., Weerdesteyn V., Bal T., Bloem B.R., Solis-Escalante T., Nonnekes J. Cortical correlates of gait compensation strategies in Parkinson disease. Ann. Neurol. 2022;91(3):329–341. doi: 10.1002/ana.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J. The utility of electrodiagnostic tests for the assessment of medically unexplained weakness and sensory deficit. Clin. Neurophysiol. Pract. 2016;1:2–8. doi: 10.1016/j.cnp.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos M.A., Geevasinga N., Menon P., Burke D., Kiernan M.C., Vucic S. Physiological processes influencing motor-evoked potential duration with voluntary contraction. J. Neurophysiol. 2017;117(3):1156–1162. doi: 10.1152/jn.00832.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Salm S.M., Koelman J.H., Henneke S., van Rootselaar A.F., Tijssen M.A. Axial jerks: a clinical spectrum ranging from propriospinal to psychogenic myoclonus. J. Neurol. 2010;257(8):1349–1355. doi: 10.1007/s00415-010-5531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Salm S.M., Tijssen M.A., Koelman J.H., van Rootselaar A.F. The bereitschaftspotential in jerky movement disorders. J. Neurol. Neurosurg. Psychiatry. 2012;83(12):1162–1167. doi: 10.1136/jnnp-2012-303081. [DOI] [PubMed] [Google Scholar]
- van der Salm S.M., Erro R., Cordivari C., Edwards M.J., Koelman J.H., van den Ende T., et al. Propriospinal myoclonus: clinical reappraisal and review of literature. Neurology. 2014;83(20):1862–1870. doi: 10.1212/WNL.0000000000000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen S., Klamer M.R., Elting J.W.J., Koelman J., van der Stouwe A.M.M., Tijssen M.A.J. The diagnostic value of clinical neurophysiology in hyperkinetic movement disorders: a systematic review. Parkinsonism Relat. Disord. 2021;89:176–185. doi: 10.1016/j.parkreldis.2021.07.033. [DOI] [PubMed] [Google Scholar]
- van der Veen S., Caviness J.N., Dreissen Y.E.M., Ganos C., Ibrahim A., Koelman J., et al. Myoclonus and other jerky movement disorders. Clin. Neurophysiol. Pract. 2022;7:285–316. doi: 10.1016/j.cnp.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial F., Kassavetis P., Merchant S., Haubenberger D., Hallett M. How to do an electrophysiological study of tremor. Clin. Neurophysiol. Pract. 2019;4:134–142. doi: 10.1016/j.cnp.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Chicherio C., Assal F., Schwartz S., Slosman D., Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Wilcox R.A., Winkler S., Lohmann K., Klein C. Whispering dysphonia in an Australian family (DYT4): a clinical and genetic reappraisal. Mov. Disord. 2011;26(13):2404–2408. doi: 10.1002/mds.23866. [DOI] [PubMed] [Google Scholar]
- Wolfsegger T., Pischinger B., Topakian R. Objectification of psychogenic postural instability by trunk sway analysis. J. Neurol. Sci. 2013;334(1–2):14–17. doi: 10.1016/j.jns.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Yazici K.M., Demirci M., Demir B., Ertugrul A. Abnormal somatosensory evoked potentials in two patients with conversion disorder. Psychiatry Clin. Neurosci. 2004;58(2):222–225. doi: 10.1111/j.1440-1819.2003.01221.x. [DOI] [PubMed] [Google Scholar]
- Yu T., Ye J., Deng Y., Luo R., Wan C. Abnormal somatosensory evoked potentials in a child with motor conversion disorder: a case report. Psychiatry Clin. Neurosci. 2021;75(10):319–320. doi: 10.1111/pcn.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuner K.E., Shoge R.O., Goldstein S.R., Dambrosia J.M., Hallett M. Accelerometry to distinguish psychogenic from essential or parkinsonian tremor. Neurology. 2003;61(4):548–550. doi: 10.1212/01.wnl.0000076183.34915.cd. [DOI] [PubMed] [Google Scholar]
- Zutt R., Elting J.W., van der Hoeven J.H., Lange F., Tijssen M.A.J. Myoclonus subtypes in tertiary referral center. Cortical myoclonus and functional jerks are common. Clin. Neurophysiol. 2017;128(1):253–259. doi: 10.1016/j.clinph.2016.10.093. [DOI] [PubMed] [Google Scholar]