Abstract
Background:
Transcranial alternating current stimulation (tACS)—a noninvasive brain stimulation technique that modulates cortical oscillations through entrainment—has been demonstrated to alter oscillatory activity and enhance cognition in healthy adults. TACS is being explored as a tool to improve cognition and memory in patient populations with mild cognitive impairment (MCI) and Alzheimer’s disease (AD).
Objective:
To review the growing body of literature and current findings obtained from the application of tACS in patients with MCI or AD, highlighting the effects of gamma tACS on brain function, memory, and cognition. Evidence on the use of brain stimulation in animal models of AD is also discussed. Important parameters of stimulation are underscored for consideration in protocols that aim to apply tACS as a therapeutic tool in patients with MCI/AD.
Findings:
The application of gamma tACS has shown promising results in the improvement of cognitive and memory processes that are impacted in patients with MCI/AD. These data demonstrate the potential for tACS as an interventional stand-alone tool or alongside pharmacological and/or other behavioral interventions in MCI/AD.
Conclusions:
While the use of tACS in MCI/AD has evidenced encouraging results, the effects of this stimulation technique on brain function and pathophysiology in MCI/AD remains to be fully determined. This review explores the literature and highlights the need for continued research on tACS as a tool to alter the course of the disease by reinstating oscillatory activity, improving cognitive and memory processing, delaying disease progression, and remediating cognitive abilities in patients with MCI/AD.
1. Introduction
Mild cognitive impairment (MCI) is a neurological syndrome characterized by deficits in memory, language, thinking, or judgment. While cognitive deficits associated with MCI are not severe enough to limit independence or activities of daily living, 10–20% of MCI patients will go on to develop dementia-level impairments over a one-year period, and MCI is often a precursor to Alzheimer’s disease (AD) [1–4]. Currently, there are no existing FDA-approved pharmacological or behavioral intervention designed to treat MCI or to slow or prevent progression from MCI to AD or other forms of dementia. AD—the most common neurodegenerative dementia—is a complex, multifactorial disorder commonly characterized by progressive impairments in memory, thinking, judgement and reasoning skills, language and communication, and changes in personality and behavior [5,6]. Multiple factors contribute to the pathogenesis of AD, including amyloid-β deposition, tau accumulation, aberrant microglia activity and astrocyte-mediated inflammation, loss of neurons and synapses, and altered cortical oscillations within and between brain networks [7]. There is no known cure for AD. Commonly employed pharmacologic treatment options (e. g. acetylcholinesterase inhibitors) are designed to palliate disease symptoms and are only mildly effective [8]. Emerging AD treatments (i. e., aducanumab, lecanemab [9]) aimed at decreasing amyloid plaque burden in the brain have thus far shown only modest clinical benefits and can have adverse side effects (e.g., brain swelling and/or bleeding [10–12]). Thus, while research and development of treatments and diagnostics for AD has made recent progress, there remains a critically urgent need for novel and efficacious intervention strategies aimed at cognitive remediation to delay or avert further decline.
Transcranial alternating current stimulation (tACS) stands out as a potentially promising intervention for persons suffering from neurodegenerative disorders of cognition. It has shown potential in enhancing cognition and memory processes in older adults with normal age-related cognitive decline [13], and early evidence suggests that it may be effective in patients with MCI and AD [14–20]. This noninvasive brain stimulation (NIBS) technique offers a safe and painless approach to alter cortical excitability and impact neuroplasticity to ultimately improve cognitive and behavioral processes. By applying a weak sinusoidal alternating current set to a specific frequency (Hz) to a target brain region(s) using electrodes placed over the scalp, tACS modulates endogenous cortical oscillations in the brain through mechanisms of entrainment to regulate and improve brain network communication [20–25]. A small but growing number of studies have shown that tACS is capable of altering oscillatory aberrations which are known to occur in MCI/AD patients and reinstate patterns of cortical oscillations that are associated with successful cognitive and memory performance [16,17, 26–28]. Evidence of tACS-induced cognitive improvements in persons with MCI/AD is notably associated with gamma stimulation, a frequency range with prominent involvement in hippocampal-mediated memory processes that is usually impaired in early disease stages [29–32].
This mini-review provides an overview on the use of gamma tACS as a tool for cognitive remediation in persons with MCI or AD and summarizes the literature in this area. We provide a synopsis of oscillatory activity in the human brain, evidence from studies that assessed gamma stimulation and its impact on underlying disease pathology in animal models of AD, and preliminary evidence of gamma tACS on disease pathology in AD patients. Lastly, we discuss parameters of stimulation that are important to consider for optimizing treatment response, and provide an outlook on potential therapeutic applications in patients with MCI/AD.
2. The importance of gamma oscillatory activity in the human brain
Neural or cortical oscillations refer to the regular fluctuations in local field potentials that reflect the input of tens of thousands of neurons [33–35]. Oscillatory activity is fundamental to information transfer and temporal organization of neural activity patterns in large-scale brain networks related to behavior and memory [7,36]. Excitatory and inhibitory neuronal activity that occurs within brain circuits operates at several distinct time scales, and their dynamic interactions contribute to different frequencies of oscillations. Oscillatory frequencies, from slowest to fastest, include delta (~0.5–4 Hz), theta (~4–8 Hz), alpha (~8–12 Hz), beta (~12–30 Hz), and gamma (~30–100 Hz) [7,37]. Mounting evidence from studies measuring oscillatory activity via intracranial electroencephalography (EEG), surface EEG, and magnetoencephalography (MEG) has shown that specific frequency bands and distinct synchronization patterns across frequency bands are vital for numerous cognitive and memory processes [37,38]. In particular, the gamma frequency has been shown to play a critical role in object representation [39], visual feature binding [40], and many higher order cognitive functions [13,41–43].
Synchronization of oscillations supports the coordination of information-sharing and communication across brain regions within networks that are essential for creating and maintaining memories [44]. Cross-frequency phase-amplitude coupling (PAC), in which the phase of slower oscillations modulates the amplitude of faster oscillations, has been proposed as a general mechanism that underlies memory processing, such as encoding, storage, and retrieval [45]. PAC is known to occur between gamma and theta frequency bands where the slower frequency (theta) drives the faster frequency band (gamma) [13,46,47]. Changes in theta-gamma activity highly correlate with the occurrence of long-term potentiation (LTP), and theta-gamma PAC are associated with alterations of synaptic plasticity [45,48]. Converging evidence indicates that theta-gamma PAC and gamma synchronization are important to successful memory maintenance [49–51] and coordination of memory reactivation in healthy individuals [13]. The application of gamma tACS in healthy individuals has associated with improved cognitive performance [52,53].
3. Aberrant gamma oscillations in neurodegenerative disease
Neurodegenerative syndromes that impact cognition and memory have been linked to impaired gamma oscillatory activity in persons with MCI or AD as well as mouse models of AD [54–56]. Abnormal gamma oscillatory activity has been exhibited as significantly reduced spectral power and desynchronized theta-gamma coupling compared to healthy age-matched controls [13]. These aberrant alterations are linked to lower short-term memory performance, such as working memory-related tasks [13] and long-term episodic memory processing [57] – one of the hallmark features of AD and a defining feature in amnestic MCI [58]. At the neurophysiological level, theta-gamma coupling is thought to represent a neural code for temporal ordering, given that different neuronal assemblies fire at consecutive time points [50,59–62]. In addition, desynchronization between the phase and amplitude of theta and gamma is associated with decreased connectivity between brain regions that are involved in memory networks [60,63]. Applying tACS in patients with MCI or AD to restore gamma oscillatory activity as a stand-alone therapeutic intervention or as an adjunctive therapy alongside existing pharmacological and/or behavioral interventions may offer a powerful treatment option to improve cognition and memory processes in these patients [16,26,64–66]. See Fig. 1 for a visual summary of this review and Table 1 for a summary of data.
Fig. 1.
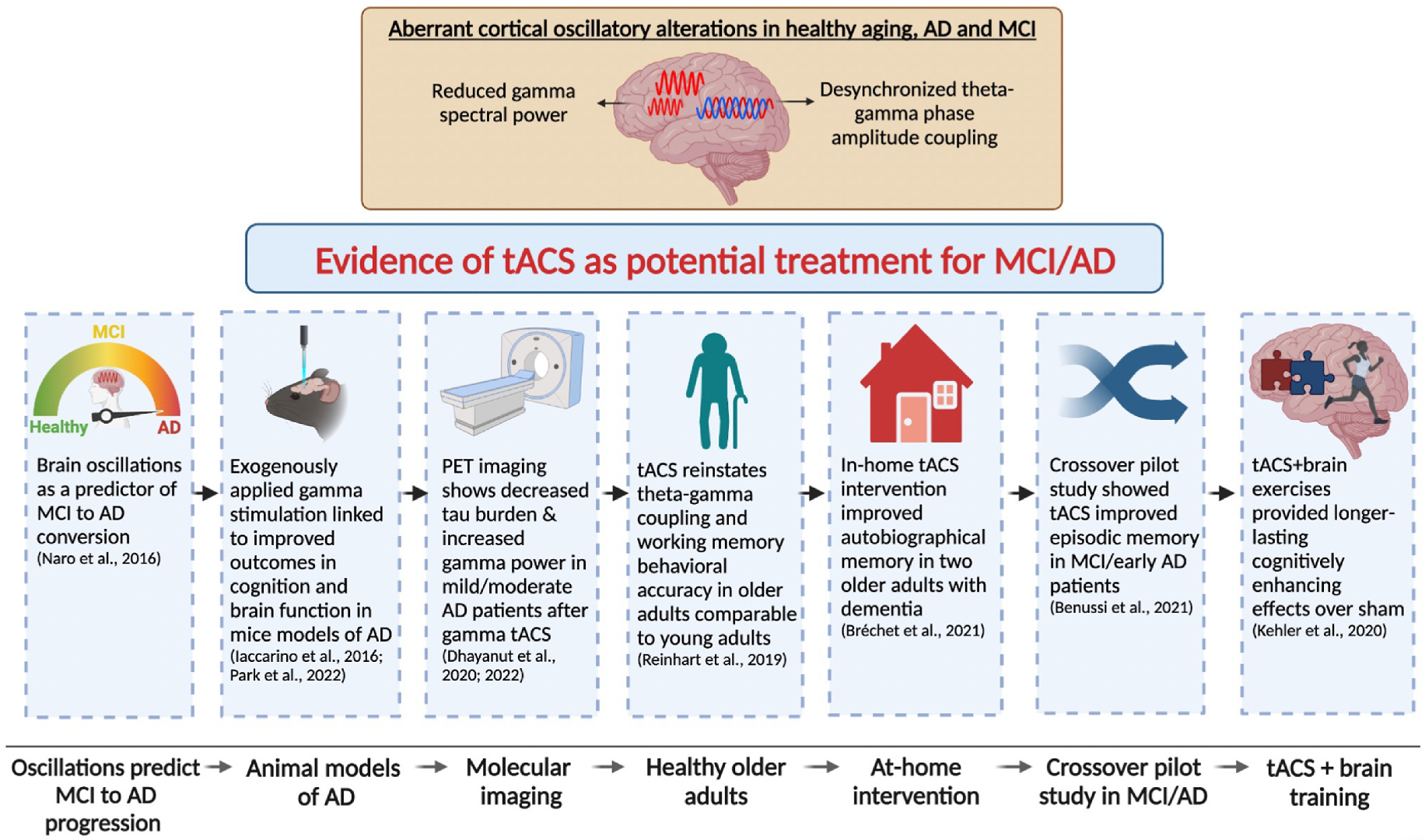
Summary of articles exploring gamma stimulation in animal models of AD and tACS in aging and MCI/AD patients.
Table 1.
Summary table of articles exploring gamma stimulation in patients with MCI/AD and animal models of AD.
| Author, year | Population | Study Design | Session Number | Session Duration (mins) | Stimulation Parameters | Stimulation Location | Concurrent Task | Task | Cognitive Domain | Outcome Measures | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Benussi et al., 2021 | MCI/AD patients | Within- subject crossover | 1 | 60 | Conventional tACS; 40 Hz gamma; 3 mA peak-to-peak intensity | Anode: medial parietal cortex/ precuneus (Pz); Cathode: right deltoid muscle | Online, offline | Rey auditory verbal learning (RAVL) test, Face-name associations task (FNAT) | Episodic memory | Accuracy on RAVL and FNAT | Active tACS showed significant improvement on RAVL and long delayed recall scores compared to sham stimulation. |
| Benussi et al., 2022 | MCI/AD patients | Within-subject crossover | 1 | 60 | Conventional tACS; 40 Hz gamma; 3 mA peak-to-peak intensity | Anode: medial parietal cortex/ precuneus (Pz); Cathode: right deltoid muscle | Online, offline | RAVL and FNAT | Episodic memory | Accuracy on RAVL and FNAT; Gamma EEG activity (pre-post change); Cholinergic levels (pre-post change) | Active tACS showed significant improvement on RAVL immediate and delayed recall scores compared to sham. FNAT scores improved after active but not sham. Cholinergic levels increased after active tACS but not sham. Increased gamma in parietal lobes correlated with task improvements. |
| Bréchet et al., 2021 | AD patients | Open label pilot study | 70 | 20 | HD-tACS; 40 Hz gamma; 4 mA peak-to-peak intensity | Anodes: left angular gyrus (BA39/40); Cathodes: C3, P2, P7, T7 | Offline | Autobiographical memory task | Episodic memory | MoCA (non-visual version); Memory Index Score (NACC UDS) | Improvement in cognitive testing every 2 weeks over 14 week intervention compared with baseline performance; study demonstrated feasibility and safety of home-based tACS. |
| Dhaynaut et al., 2020 | Mild to moderate AD patients | Open label pilot study | 20 | 60 | tACS; 40 Hz gamma; intensity not reported | Anode/Cathode: bilateral temporal lobes | N/A | N/A | N/A | PET imaging: amyloid-β, p-Tau, and microglia | Trend of decreased intracerebral Tau burden in 3/5 patients; intracerebral amyloid-p and microglial activation was not influenced by tACS. |
| Dhaynaut et al., 2022 | Mild to moderate AD patients | Proof of principle case series | 20 | 60 | tACS; 40 Hz gamma; 2 mA intensity | Anode/Cathode: bilateral temporal lobes (T7-T8; P7-P8) | N/A | N/A | N/A | PET imaging: amyloid-β, p-Tau, and microglia EEG: Gamma spectral power | Significant decrease of 2% p-Tau burden in 3/4 patients; decrease in microglia activation in 1/4 patients; intracerebral amyloid-β was not influenced by tACS; gamma spectral power increased from baseline to post-treatment. |
| Iaccarino et al., 2016 | Multiple mouse models of AD | Between-subject | 1 | 60 | Light flicker (20, 40, or 80 Hz) versus random flicker or dark exposure | Targeting primary visual cortex | N/A | N/A | N/A | Levels of amyloid-β and microglia in visual cortex | Reduction of amyloid-p peptides and concomitant microglial response in the visual cortex after 40 Hz gamma entrainment, over 20 Hz, 80 Hz, and random flicker or darkness conditions. |
| Kehler et al., 2020 | MCI/mild to moderate dementia patients | Between-subject | 40 | 60 | tACS; 40 Hz gamma; 1.5 mA peak-to-peak intensity | Anode: left dorsolateral prefrontal cortex (F3); Cathode: contralateral supraorbital area | Online, offline | MindTriggers: brain exercises; Wechsler Memory Scale (WMS-IV) | Memory function: verbal, auditory, visual, shortterm, and working memory | WMS-IV score (pre-post change score) | Active and sham groups significantly improved on WMS immediately after intervention. Active tACS maintained improvements significantly better than sham at one month followup. |
| Park et al., 2022 | Mouse model of AD | Between-subject | 72 | 46.67 | Light flicker (40 Hz) + treadmill exercise | Targeting primary visual cortex | N/A | N/A | N/A | Levels of amyloid-β, tau, apoptosis and inflammatory markers | Light flicker (40 Hz) + exercise over dark condition significantly reduced amyloid-β, tau protein levels, and apoptosis. |
| Sprugnoli et al., 2021 | Mild to moderate AD patients | Open label pilot study | 10 to 20 sessions | 60 | HD-tACS; 40 Hz gamma; 4 mA peak-to-peak intensity | Targeting right temporal lobe (T8) | Online | Viewing documentaries | Attention | Arterial Spin Label (ASL) MRI (pre-post change) | Blood perfusion significantly increased in bilateral temporal lobes after tACS treatment. Perfusion change positively correlated with changes in episodic memory and spectral power changes in gamma EEG activity. |
4. Gamma stimulation in animal models of AD
Altered gamma oscillations, including reduced synchronization and decreased spectral power, have been demonstrated in multiple mouse models of AD [55,56,67,68]. A small but growing number of studies have used light flicker gamma stimulation in well-established models of AD and suggest that exogenous gamma stimulation may be beneficial to cognition and the alteration of underlying disease pathology [68–70].
Using light flicker treatment to manipulate gamma activity in multiple mouse models with AD pathology, Iaccarino et al. (2016) examined the relationship between gamma oscillations and change in amyloid-β levels. Three-month-old mice with elevated amyloid-β levels, but no onset of plaque formation or cognitive decline, underwent 1-h light flicker treatment (20, 40, 80 Hz in constant light, random interval flicker or darkness) over the course of seven days. After 1 h of stimulation, researchers observed a marked reduction of amyloid-β peptides in the visual cortex and a concomitant decreased microglial response after 40 Hz gamma entrainment in AD mice models [69]. These effects were not observed for 20 Hz, 80 Hz, random flicker or darkness conditions.
More recently, Park et al. (2022) evaluated a combination of exercise and 40 Hz gamma light flickering over 12 weeks in a 5-month-old 3xTg AD mouse model to assess whether favorable effects occur in earlier stages of cognitive dysfunction. Spatial learning and memory, hippocampal amyloid-β, tau, neuroinflammation, proinflammatory expression, mitochondrial function, and neuroplasticity were examined. Results showed a significant reduction in amyloid-β and tau protein levels that associated with protection against cognitive decline by reducing neuroinflammation and proinflammatory cytokines [70]. Additionally, mitochondrial function improved, apoptosis was lowered, and markers of synapse-related protein expression significantly increased. Response to treatment was most effective in the exercise paired with 40 Hz light flickering condition compared to exercise alone or 40 Hz light flickering alone. Improvements were comparable to levels observed in a wild-type age-matched control group.
Collectively, preliminary evidence from mouse models of AD indicates that exogenously applied gamma stimulation is linked to improved outcomes in cognition and neural function. These studies provide key evidence that driving gamma oscillations may induce an overall neuroprotective response and modulate underlying AD neuropathy. While the mechanisms that underlie these changes are not yet fully understood, these data shed light on the potential for modulating gamma oscillatory activity in humans as a novel treatment approach to alleviate disease pathology and improve cognitive function in patients with limited effective treatment options.
5. Gamma tACS on Tau PET imaging in mild to moderate AD
PET imaging research has indicated the effect of gamma tACS in patients with AD. Dhaynaut et al. (2020) examined the impact of 40 Hz tACS targeting bilateral temporal lobes (over 4 weeks) on modulation of microglial levels, tau and amyloid- β in a small sample of five amyloid-positive patients with AD. Results revealed a decreased trend in intracerebral tau burden following tACS in 3 of 5 patients in the temporal lobes [71]. While tau burden showed a reduction after tACS intervention, the amount of intracerebral amyloid-β and microglial activation was not significantly influenced by stimulation. Another PET study by Dhaynaut et al. (2022) revealed that repeated sessions of 40 Hz tACS increased gamma spectral power and decreased over 2% of p-tau burden in 3 of 4 patients with mild to moderate AD [19]. Amyloid-β levels were not impacted by stimulation, but microglia activation decreased in one patient. Despite the underpowered nature of these preliminary studies, the data provide early evidence regarding the potential beneficial impact of gamma tACS protocols on AD pathology.
In a pilot study by Bréchet et al. (2021), 10 or 20 sessions of gamma tACS was demonstrated to induce significant gamma EEG activity in AD patients. In line with results in mice models, PET also confirmed tACS-induced modulation of amyloid-β and tau deposition, implying that tACS could influence underlying aspects of AD pathology. However, 10 or 20 sessions of stimulation were not sufficient to translate into apparent behavioral effects. Prolonged and multi-week laboratory-based interventions can prove challenging for patients and their caregivers (Clinical Trials NCT03412604 data, as cited by Bréchet et al., 2021). Due to the nature of neurodegenerative syndromes, it is likely that persons with AD may benefit from greater numbers of tACS sessions, as repeated sessions have been shown to correlate with greater behavioral enhancement and LTP effects in healthy individuals and post-stroke motor recovery [72–74]. Moreover, patient populations and caregivers may benefit from at-home stimulation approaches to reduce the burden and challenges of protocols that require patients to arrive on-site for repeated treatments [75].
Another study by Bréchet et al. (2021) provides preliminary evidence on the feasibility of a remotely monitored, caregiver-administered, home-based tACS intervention to improve autobiographical memory in two older adults with dementia. Daily 20-min sessions of 40 Hz gamma tACS to the left angular gyrus were administered over 14 weeks for a total of 70 sessions per participant. Preliminary results showed the participants substantially improved on episodic memory testing and memory index scores (NACC UDS delayed recall; scores were not statistically analyzed but demonstrated improvements – patient 1: baseline recall 0 words increased to 5 post-intervention; patient 2: baseline recall 5 words increased to 11 post-intervention) [76]. Although underpowered, this study is one of the first to demonstrate safety and feasibility of employing gamma tACS treatment in dementia patients with caregivers delivering at-home treatment.
6. tACS effects on episodic memory processing in MCI/AD
Episodic memory—an important process that enables the encoding and recollection of personal life events—is characteristically impaired in patients with MCI and AD [58,77]. Episodic memory processing is supported by a widespread and complex network of brain regions which includes neocortical association areas and subcortical structures (i.e., medial temporal lobe – hippocampus, parahippocampus, entorhinal cortex, perirhinal cortex) and the prefrontal cortex [78]. Another important structure, the precuneus, which is part of the medial posterior parietal cortex, is involved in episodic memory processes and plays a role in memory integration, visuo-spatial imagery, retrieval and self-processing operations (e.g., first-person perspective and experience of agency) [79–81]. The precuneus has robust connectivity to prefrontal and subcortical structures involved in episodic memory, and is a brain region that can be targeted with NIBS [26]. Reductions in gamma oscillatory activity typically precedes episodic memory impairment in MCI/AD [26,54,82,83]. Gamma oscillations are crucial in binding perceptual features and contextual information from diverse brain regions into episodic representations [57].
In two open-label pilot studies, Sprugnoli et al. (2021) examined the impact of multiple sessions of 40 Hz tACS targeting the temporal lobe (1 h sessions over 2–4 weeks) on cerebral perfusion measured via arterial spin label (ASL) MRI, neurophysiology measured by EEG, and episodic memory performance in 15 patients with mild to moderate AD. ASL MRI revealed a significant increase in blood perfusion in the temporal lobes bilaterally from baseline to post-intervention. Perfusion changes were positively correlated with changes in episodic memory performance and gamma spectral power [17].
Recently, Benussi et al. (2021) explored the impact of 40 Hz gamma tACS (3.0 mA peak-to-peak intensity) in 20 patients with MCI/AD in a randomized, double-blind, sham-controlled crossover pilot study. Active gamma tACS involved a single 1-h session targeting the Pz (i.e., medial parietal cortex and precuneus), a key node involved in the episodic memory network. Results from twenty patients with MCI/AD showed that active tACS significantly improved auditory verbal learning and long delayed recall scores compared to sham. Moreover, an indirect measure of cholinergic transmission, evaluated via short latency afferent inhibition using transcranial magnetic stimulation (TMS), was increased only in active gamma versus sham condition [26].
In a follow-up study, Benussi et al. (2022) examined 40 Hz gamma tACS (3.0 mA peak-to-peak intensity; 1 h duration) targeting the precuneus in 60 patients with AD. The impact of active versus sham tACS was evaluated on episodic memory and cholinergic transmission. A significant correlation between enhancement of episodic memory and indirect measures of cholinergic neurotransmission was observed after active gamma tACS versus sham. Results were corroborated by pre- and post-EEG changes which demonstrated gamma frequency entrainment and suggested site-specific stimulation effects as increased gamma activity was observed over the posterior parietal cortices and precuneus [27]. These promising results in MCI/AD patients suggest that the use of tACS to enhance gamma oscillatory activity has the potential for therapeutic benefit in the context of memory processing with site-specific effects to brain regions impacted by MCI/AD.
7. Maintenance of gamma tACS treatment effects on cognition in mild-moderate dementia
A pilot study by Kehler et al. (2020) investigated the effects of 40 Hz gamma tACS (1.5 mA intensity peak-to-peak) paired with computerized brain exercises to assess whether working memory and cognitive function could be improved in a cohort of older adults with a diagnosis of mild to moderate dementia. The protocol involved brain exercises in the lab (two 30-min sessions per day, 5 days/week over the course of 4 consecutive weeks). Seventeen patients completed the study (11 randomized to active versus 6 sham). Patients were evaluated at baseline, immediately post-intervention, and 1-month post-intervention to assess whether treatment effects were maintained. Both active and sham groups exhibited a significant improvement from baseline to immediate post-intervention on cognitive assessments. However, at the 1-month follow up, the active group demonstrated significantly higher performance on cognitive testing, suggesting that active tACS paired with brain exercises provided longer-lasting cognitively enhancing effects over sham stimulation [16]. To our knowledge, this is currently the only study that has identified a positive result on maintenance of enhanced cognition after gamma tACS at a long-term time point in patients with dementia. The results suggest that gamma tACS, in combination with rigorous brain exercises, could promote better working memory and cognitive function in clinical populations than brain exercises alone with a more sustainable impact. To disentangle potential effects of brain exercises versus tACS-induces effects, future studies that employ these methods should ensure that stimulation effects are isolated from the brain exercise effect (e.g., randomized within-group designs with control tasks) to better demonstrate the strength of stimulation effects.
8. Brain oscillations as a predictor of MCI to AD conversion
Alterations in oscillatory features and rate of change may be useful biomarkers to predict conversion from MCI to AD. Naro et al. (2016) demonstrated that gamma tACS could identify MCI patients at a high risk of developing dementia by applying 10 min of gamma stimulation to M1, PMA, SMA, DLPFC and DMPFC in the left hemisphere in MCI and AD patients. The study utilized a neuropsychological battery to assess the global cognitive status, frontal functions, verbal and non-verbal memory, language, attention, and executive functions. Neuropsychological improvement in healthy elderly controls and individuals with MCI who responded to the tACS protocol (MCI-R) was linked to gamma band oscillation (GBO) power increase. On the other hand, AD and MCI patients who did not respond to the tACS protocol (MCI-NR) did not show any tACS-induced GBO increase. Compared to controls, MCI-R demonstrated abnormal DMPFC-tACS induced GBO increase. The MCI-NR patients that did not show significant clinical or electrophysiological DMPFC after-effects progressed to AD over a two-year period even though these MCI-NR patients had baseline clinical scores and GBO power magnitudes similar to other MCI participants [84]. Naro and colleagues suggest that measuring sensitivity of GBO to external perturbations may offer insights into cognitive reserve, its impact on cognitive domains and predict the developmental trajectories of AD. In particular, they found individuals that exhibit abnormal GBO modulation within frontoparietal networks may be considered at potential risk of developing dementia. Thus, tACS may serve as a useful tool for early identification of at-risk MCI patients for better prevention and disease management strategies to avert and mitigate potential progression to AD.
9. Importance of tACS parameters
Parameters of tACS govern its impact on neural structures and ultimately its effect on brain function and behavior. Thus, they must be considered carefully in the development of tACS protocols. This includes numerous modifiable settings, such as frequency (Hz), duration, intensity (mA), electrode size/location (conventional versus HD-tACS), number and frequency of sessions, online versus offline application (e. g., during or before task), and target brain region(s).
Frequency (Hz) of stimulation is essential to consider, as tACS has been shown to entrain oscillations at the level of applied stimulation. With respect to stimulation frequency, one important consideration is whether to employ a standardized frequency (Hz) across participants, versus a personalized frequency that can be obtained during task performance or resting state. A wide range of cognitive capabilities are mediated by the dynamic modulation of oscillatory activity between and within different regions of the brain (e.g., beta and gamma oscillations mediating interactions between prefrontal and sensory cortices to direct attention) [23,85]. Studies in healthy individuals have shown evidence of tACS altering oscillatory power at frequency bands outside of the applied stimulation (e.g., 10 Hz alpha increasing theta power during phonological word decisions) [86].
Evidence of gamma tACS altering lower frequency bands in MCI patients was shown by Kim et al. (2021). In that crossover study, MCI patients received gamma tACS (targeting dorsolateral prefrontal cortex), tDCS, and sham stimulation in separate sessions in order to compare effects of stimulation techniques on cognition and neurophysiology. Gamma tACS associated with improved performance on executive function tasks compared to tDCS and sham. However, tACS increased beta power and activity, while tDCS and sham did not associate with improved performance, and tDCS decreased slow frequency activity and beta power. Kim and colleagues suggest that gamma tACS may have facilitated cognitive function due to plasticity alterations within the stimulated area or surrounding network regions [28]. Open questions remain as to the impact of frequency (Hz) of stimulation on frequency bands outside of the applied range and warrant further exploration. Differences exist at the biophysical level of brain tissue in healthy individuals versus MCI/AD [87]. It is especially critical to understand how tACS may differentially impact other frequency bands or brain regions within and between networks outside of the target stimulation region to optimize the application in patients with MCI or AD. Relatedly, in the case of neurodegenerative disorders, determining the right brain target entails consideration of whether to target degenerated tissue or relatively spared regions that may aid in compensatory processes, as well as the intensity of current, and the number and duration of sessions to achieve the intended stimulation effects.
Given the diversity of parameter choices, it is unsurprising that variability exists in response to stimulation between- and within-subjects and across studies in healthy individuals and patient populations [88–92]. Moreover, the influence of inherent differences such as brain and skull anatomy are known to alter the pattern and intensity of current flow that reaches the brain. It may be especially important in the context of MCI/AD to use personalized approaches such as cortical activity mapping, topographic scalp distribution, and computational modeling as a guide to identify target region(s) and dose of stimulation due to the neurodegenerative nature of the syndrome [93].
The application of tACS in the context of neurodegenerative disease states is still in its infancy, and studies typically use varied parameter settings. Of note, it is the gamma frequency range that shows abnormal cortical oscillations in MCI/AD; typically, 40 Hz gamma stimulation is applied in patient populations across studies. Whether 40 Hz frequency versus higher or lower gamma frequencies versus personalized frequency is the most appropriate choice remains to be de termined. As the field continues to grow, the assessment of tACS efficacy in systematic meta-analytic approaches will be invaluable in identifying how differing parameters, including personalized versus standardized frequency, might modulate response to stimulation and aid in more optimized behavioral outcomes.
10. Conclusion
The high prevalence of MCI and AD and the current limitations of treatment for these disorders warrant novel therapeutic approaches. TACS, particularly in the gamma frequency range, offers the potential of an effective treatment protocol. Gamma stimulation has been linked to improvements in cognition in human and animal models of AD. Impaired episodic memory, a notable characteristic of MCI/AD, is associated with decreased gamma band oscillations which may be reinstated with tACS. Early evidence from PET imaging suggests that gamma tACS may also have the ability to reduce tau burden, an important factor underlying AD pathology. Given the ability of tACS in modulating brain oscillations and consequently influencing cognitive processes, different studies have corroborated evidence on the effective use of tACS in improving episodic memory in MCI/AD populations. Gamma tACS also shows a positive impact on other memory processes, such as associative, declarative, autobiographical and working memory through the administration of gamma tACS to different target brain areas, such as Pz, left prefrontal cortex, DLPFC, and left angular gyrus. In addition, tACS and gamma band oscillations can also serve as a useful tool to identify MCI patients at risk of progressing into AD and thus help inform appropriate preventive measures.
In light of these encouraging results, further research is needed to strengthen direct evidence on the impact of gamma tACS in the treatment of MCI/AD. As memory processes are multifaceted and complex, questions remain as to which memory functions and respective brain areas should be targeted in MCI/AD. Moreover, future research is needed to gain greater insight into whether personalized tACS yields a higher benefit over standardized frequency, the appropriate dose, intensity and duration required for optimal treatment effects in MCI/AD.
The future of a novel and more effective therapeutic interventions for MCI/AD calls for RCTs using within-group designs, and larger sample sizes to provide more a robust understanding of the use of tACS for cognitive remediation. As studies emerge in this important area, meta-analyses on aggregate statistics [20], and the incorporation of different neuroscience tools and techniques to elucidate and leverage the potential of tACS stimulation will be greatly beneficial to understand variables and parameters of stimulation that may modulate or optimize response to this form of brain stimulation.
Acknowledgements
This work was supported by a Moss Rehabilitation Research Institute/University of Pennsylvania post-doctoral training fellowship (NIH 5T32HD071844–05) and the Laboratory for Cognition and Neural Stimulation at the University of Pennsylvania.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Farias ST, Mungas D, Reed BR, Harvey D, Decarli C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch Neurol 2009;66:1151–7. 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Varatharajah Y, Ramanan VK, Iyer R, Vemuri P, Weiner MW, Aisen P, et al. Predicting short-term MCI-to-AD progression using imaging, CSF, genetic factors, cognitive resilience, and demographics. Sci Rep 2019;9. 10.1038/s41598-019-38793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment. Arch Neurol 1999;56:303. 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- [4].Kelley B, Petersen R. Alzheimer’s disease and mild cognitive impairment. Neurol Clin 2007;25:1–34. 10.1016/j.ncl.2007.03.008.Alzheimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ahmed S, Haigh A-MF, de Jager CA, Garrard P. Connected speech as a marker of disease progression in autopsy-proven Alzheimer’s disease. Brain 2013;136:3727–37. 10.1093/brain/awt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996;46:130–5. 10.1212/WNL.46.1.130. [DOI] [PubMed] [Google Scholar]
- [7].Adaikkan C, Tsai LH. Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci 2020;43:24–41. 10.1016/j.tins.2019.11.001. [DOI] [PubMed] [Google Scholar]
- [8].Marucci G, Buccioni M, Ben DD, Lambertucci C, Volpini R, Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology 2021;190. 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- [9].van Dyck CH, Swanson CJ, Aisen P, Bateman RJ, Chen C, Gee M, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med 2022;388:9–21. 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- [10].Lythgoe MP, Jenei K, Prasad V. Regulatory decisions diverge over aducanumab for Alzheimer’s disease, 376. BMJ Publishing Group; 2022. 10.1136/bmj-2021-069780. [DOI] [PubMed] [Google Scholar]
- [11].Mahase E FDA approves controversial Alzheimer’s drug despite uncertainty over effectiveness. BMJ 2021;373. 10.1136/bmj.n1462. [DOI] [PubMed] [Google Scholar]
- [12].Dave AS, Beizer JL. The controversial use of aducanumab (aduhelm) for Alzheimer’s disorder. 2022. [Google Scholar]
- [13].Reinhart RMG, Nguyen JA. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci 2019. 10.1038/s41593-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Birba A, Ibanez A, Sedeño L, Ferrari J, García AM, Zimerman M. Non-invasive brain stimulation: a new strategy in mild cognitive impairment? Front Aging Neurosci 2017;9:1–13. 10.3389/fnagi.2017.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Meinzer M, Lindenberg R, Phan MT, Ulm L, Volk C, Flöel A. Transcranial direct current stimulation in mild cognitive impairment: behavioral effects and neural mechanisms. Alzheimer’s Dementia 2015;11:1032–40. 10.1016/j.jalz.2014.07.159. [DOI] [PubMed] [Google Scholar]
- [16].Kehler L, Francisco CO, Uehara MA, Moussavi Z. The effect of transcranial alternating current stimulation (tACS) on cognitive function in older adults with dementia. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 2020;2020-July:3649–53. 10.1109/EMBC44109.2020.9175903. [DOI] [PubMed] [Google Scholar]
- [17].Sprugnoli G, Munsch F, Cappon D, Paciorek R, Macone J, Connor A, et al. Impact of multisession 40Hz tACS on hippocampal perfusion in patients with Alzheimer’s disease. Alzheimer’s Res Ther 2021;13. 10.1186/s13195-021-00922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Koch G, Bonnì S, Pellicciari MC, Casula EP, Mancini M, Esposito R, et al. Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer’s disease. Neuroimage 2018;169:302–11. 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- [19].Dhaynaut M, Sprugnoli G, Cappon D, Macone J, Sanchez JS, Normandin MD, et al. Impact of 40 Hz transcranial alternating current stimulation on cerebral tau burden in patients with Alzheimer’s disease: a case series. J Alzheim Dis 2022;85:1667–76. 10.3233/JAD-215072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nissim NR, McAfee DC, Edwards S, Prato A, Lin JX, Lu Z, et al. Efficacy of transcranial alternating current stimulation in the enhancement of working memory performance in healthy adults: a systematic meta-analysis. Neuromodulation: Technology at the Neural Interface 2023. 10.1016/j.neurom.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Neuling T, Ruhnau P, Weisz N, Herrmann CS, Demarchi G. Faith and oscillations recovered: on analyzing EEG/MEG signals during tACS. Neuroimage 2017;147:960–3. 10.1016/j.neuroimage.2016.11.022. [DOI] [PubMed] [Google Scholar]
- [22].Herrmann CS. Modeling-informed tACS allows shaping oscillatory activity in specific brain networks. Brain Stimul 2017;10:387. 10.1016/j.brs.2017.01.144. [DOI] [Google Scholar]
- [23].Fröhlich F, Sellers KK, Cordle AL. Targeting the neurophysiology of cognitive systems with transcranial alternating current stimulation. Expert Rev Neurother 2014;15:145–67. 10.1586/14737175.2015.992782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Herrmann CS, Rach S, Neuling T, Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci 2013;7:279. 10.3389/fnhum.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Antal A, Luber B, Brem AK, Bikson M, Brunoni AR, Cohen Kadosh R, et al. Non-invasive brain stimulation and neuroenhancement. Clin Neurophysiol Pract 2022;7:146–65. 10.1016/j.cnp.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Benussi A, Cantoni V, Cotelli MS, Cotelli M, Brattini C, Datta A, et al. Exposure to gamma tACS in Alzheimer’s disease: a randomized, double-blind, sham-controlled, crossover, pilot study. Brain Stimul 2021;14:531–40. 10.1016/j.brs.2021.03.007. [DOI] [PubMed] [Google Scholar]
- [27].Benussi A, Cantoni V, Grassi M, Brechet L, Michel CM, Datta A, et al. Increasing brain gamma activity improves episodic memory and restores cholinergic dysfunction in Alzheimer’s disease. Ann Neurol 2022;92:322–34. 10.1002/ana.26411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim J, Kim H, Jeong H, Roh D, Kim DH. tACS as a promising therapeutic option for improving cognitive function in mild cognitive impairment: a direct comparison between tACS and tDCS. J Psychiatr Res 2021;141:248–56. 10.1016/j.jpsychires.2021.07.012. [DOI] [PubMed] [Google Scholar]
- [29].Güntekin B, Erdal F, Bölükbas B, Hanoglu L, Yener G, Duyğun R. Alterations of resting-state Gamma frequency characteristics in aging and Alzheimer’s disease. Cogn Neurodyn 2022. 10.1007/s11571-022-09873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hampel H, Bürger K, Teipel SJ, Bokde ALW, Zetterberg H, Blennow K. Core candidate neurochemical and imaging biomarkers of Alzheimer’s disease. Alzheimer’s Dementia 2008;4:38–48. 10.1016/j.jalz.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [31].Griffiths BJ, Parish G, Roux F, Michelmann S, van der Plas M, Kolibius LD, et al. Directional coupling of slow and fast hippocampal gamma with neocortical alpha/beta oscillations in human episodic memory. Proc Natl Acad Sci U S A 2019;116:21834–42. 10.1073/pnas.1914180116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Manippa V, Palmisano A, Nitsche MA, Filardi M, Vilella D, Logroscino G, et al. Cognitive and neuropathophysiological outcomes of gamma-tACS in dementia: a systematic review. Neuropsychol Rev 2023. 10.1007/s11065-023-09589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Telenczuk B, Dehghani N, le Van Quyen M, Cash SS, Halgren E, Hatsopoulos NG, et al. Local field potentials primarily reflect inhibitory neuron activity in human and monkey cortex. Sci Rep 2017;7. 10.1038/srep40211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol 2005;116:2719–33. 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- [35].Zoefel B, ten Oever S, Sack AT. The involvement of endogenous neural oscillations in the processing of rhythmic input: more than a regular repetition of evoked neural responses. Front Neurosci 2018;12:1–13. 10.3389/fnins.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hanslmayr S, Axmacher N, Inman CS. Modulating human memory via entrainment of brain oscillations. Trends Neurosci 2019;42:485–99. 10.1016/j.tins.2019.04.004. [DOI] [PubMed] [Google Scholar]
- [37].Herrmann CS, Strüber D, Helfrich RF, Engel AK. EEG oscillations: from correlation to causality. Int J Psychophysiol 2016;103:12–21. 10.1016/j.ijpsycho.2015.02.003. [DOI] [PubMed] [Google Scholar]
- [38].Gross J Magnetoencephalography in cognitive neuroscience: a primer. Neuron 2019;104:189–204. 10.1016/j.neuron.2019.07.001. [DOI] [PubMed] [Google Scholar]
- [39].Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: a possible role for object representation. Int J Psychophysiol 2000;38:211–23. 10.1016/S0167-8760(00)00166-5. [DOI] [PubMed] [Google Scholar]
- [40].Honkanen R, Rouhinen S, Wang SH, Palva JM, Palva S. Gamma oscillations underlie the maintenance of feature-specific information and the contents of visual working memory. Cerebr Cortex 2015;25:3788–801. 10.1093/cercor/bhu263. [DOI] [PubMed] [Google Scholar]
- [41].Traikapi A, Konstantinou N. Gamma oscillations in Alzheimer’s disease and their potential therapeutic role. Front Syst Neurosci 2021;15. 10.3389/fnsys.2021.782399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, et al. Brain oscillatory substrates of visual short-term memory capacity. Curr Biol 2009;19:1846–52. 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- [43].Noguchi Y, Kakigi R. Temporal codes of visual working memory in the human cerebral cortex: brain rhythms associated with high memory capacity. Neuroimage 2020;222. 10.1016/j.neuroimage.2020.117294. [DOI] [PubMed] [Google Scholar]
- [44].Bikbaev A, Manahan-Vaughan D. Relationship of hippocampal theta and gamma oscillations to potentiation of synaptic transmission. Front Neurosci 2008;2:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bergmann TO. Brain state-dependent brain stimulation. Front Psychol 2018;9:1–4. 10.3389/fpsyg.2018.02108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Axmacher N, Henseler MM, Jensen O, Weinreich I, Elger CE, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci U S A 2010;107:3228–33. 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Osipova D, Takashima A, Oostenveld R, Fernández G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 2006;26:7523–31. 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci 2011;12:105–18. 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- [49].Lisman J Working memory: the importance of theta and gamma oscillations. Curr Biol 2010;20:R490–2. 10.1016/j.cub.2010.04.011. [DOI] [PubMed] [Google Scholar]
- [50].Lisman J, Buzsáki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull 2008;34:974–80. 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lisman JE, Jensen O. The theta-gamma neural code. Neuron 2013;77:1002–16. 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lee TL, Lee H, Kang N. A meta-analysis showing improved cognitive performance in healthy young adults with transcranial alternating current stimulation. NPJ Sci Learn 2023;8. 10.1038/s41539-022-00152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hoy KE, Bailey N, Arnold S, Windsor K, John J, Daskalakis ZJ, et al. The effect of γ-tACS on working memory performance in healthy controls. Brain Cognit 2015;101:51–6. 10.1016/j.bandc.2015.11.002. [DOI] [PubMed] [Google Scholar]
- [54].Stam CJ, van Cappellen van Walsum AM, Pijnenburg YAL, Berendse HW, de Munck JC, Scheltens P, et al. Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol 2002;19. [DOI] [PubMed] [Google Scholar]
- [55].Gillespie AK, Jones EA, Lin YH, Karlsson MP, Kay K, Yoon SY, et al. Apolipoprotein E4 causes age-dependent disruption of slow gamma oscillations during hippocampal sharp-wave ripples. Neuron 2016;90:740–51. 10.1016/j.neuron.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007;55:697–711. 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev 2010;34:1023–35. 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Irish M, Lawlor BA, Coen RF, O’Mara SM. Everyday episodic memory in amnestic mild cognitive impairment: a preliminary investigation. BMC Neurosci 2011;12. 10.1186/1471-2202-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Goodman MS, Kumar S, Zomorrodi R, Ghazala Z, Cheam ASM, Barr MS, et al. Theta-Gamma coupling and working memory in Alzheimer’s dementia and mild cognitive impairment. Front Aging Neurosci 2018;10:1–10. 10.3389/fnagi.2018.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lisman JE, Jensen O. The theta-gamma neural code. Neuron 2013;77:1002–16. 10.1016/j.neuron.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cognit Sci 2014;18:16–25. 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- [62].Lisman JE, Idiart MAP. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science 1979;267:1512–5. 10.1126/science.7878473.1995. [DOI] [PubMed] [Google Scholar]
- [63].Reinhart RMG, Nguyen JA. Synchronizing rhythmic brain circuits. Nat Neurosci 2019;22. 10.1038/s41593-019-0371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].van der Plas M, Hanslmayr S. Entraining neurons via noninvasive electric stimulation improves cognition. PLoS Biol 2020;18. 10.1371/journal.pbio.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bréchet L, Yu W, Biagi MC, Ruffini G, Gagnon M, Manor B, et al. Patient-tailored, home-based non-invasive brain stimulation for memory deficits in dementia due to Alzheimer’s disease. Front Neurol 2021;12. 10.3389/fneur.2021.598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dhaynaut M, Sprugnoli G, Cappon Balos D, Macone J, Sanchez J, Normandin M, et al. Impact of 40 Hz transcranial alternating current stimulation on cerebral tau burden in patients with Alzheimer’s disease: a case series. J Alzheim Dis 2021;85:1–10. 10.3233/JAD-215072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016;540:230–5. 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Martorell AJ, Paulson AL, Suk HJ, Abdurrob F, Drummond GT, Guan W, et al. Multi-sensory gamma stimulation ameliorates alzheimer’s-associated pathology and improves cognition. Cell 2019;177:256–271.e22. 10.1016/j.cell.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016;540:230–5. 10.1038/nature20587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Park SS, Park HS, Kim CJ, Baek SS, Park SY, Anderson C, et al. Combined effects of aerobic exercise and 40-Hz light flicker exposure on early cognitive impairments in Alzheimer’s disease of 3*Tg mice. J Appl Physiol 2022;132:1054–68. 10.1152/japplphysiol.00751.2021. [DOI] [PubMed] [Google Scholar]
- [71].Dhaynaut M, Cappon D, Paciorek R, Macone J, Connor A, Guehl N, et al. Effects of modulating gamma oscillations via 40Hz transcranial alternating current stimulation (tACS) on Tau PET imaging in mild to moderate Alzheimer’s Disease. 2020. [Google Scholar]
- [72].Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 2013;6:424–32. 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- [73].Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 2007;25:123–9. [PubMed] [Google Scholar]
- [74].Esposito S, Trojsi F, Cirillo G, de Stefano M, di Nardo F, Siciliano M, et al. Repetitive transcranial magnetic stimulation (rTMS) of dorsolateral prefrontal cortex may influence semantic fluency and functional connectivity in frontoparietal network in mild cognitive impairment (MCI). Biomedicines 2022;10. 10.3390/biomedicines10050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bystad M, Grønli O, Rasmussen ID, Gundersen N, Nordvang L, Wang-Iversen H, et al. Transcranial direct current stimulation as a memory enhancer in patients with Alzheimer’s disease: a randomized, placebo-controlled trial. Alzheimer’s Res Ther 2016;8:13. 10.1186/s13195-016-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Bréchet L, Yu W, Biagi MC, Ruffini G, Gagnon M, Manor B, et al. Patient-tailored, home-based non-invasive brain stimulation for memory deficits in dementia due to Alzheimer’s disease. Front Neurol 2021;12:1–12. 10.3389/fneur.2021.598135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pause BM, Zlomuzica A, Kinugawa K, Mariani J, Pietrowsky R, Dere E. Perspectives on episodic-like and episodic memory. Front Behav Neurosci 2013. 10.3389/fnbeh.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 2010;35:86–104. 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 2006;129:564–83. 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- [80].Trimble MR, Cavanna AE. Chapter 3.7 the role of the precuneus in episodic memory. Handb Behav Neurobiol 2008;18:363–77. 10.1016/S1569-7339(08)00220-8. [DOI] [Google Scholar]
- [81].Stillesjö S, Nyberg L, Wirebring LK. Building memory representations for exemplar-based judgment: a role for ventral precuneus. Front Hum Neurosci 2019;13:1–16. 10.3389/fnhum.2019.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, et al. Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 2005;26:165–71. 10.1016/j.neurobiolaging.2004.03.008. [DOI] [PubMed] [Google Scholar]
- [83].Rizzolo L, Narbutas J, van Egroo M, Chylinski D, Besson G, Baillet M, et al. Relationship between brain AD biomarkers and episodic memory performance in healthy aging. Brain Cognit 2021;148:105680. 10.1016/j.bandc.2020.105680. [DOI] [PubMed] [Google Scholar]
- [84].Naro A, Corallo F, de Salvo S, Marra A, di Lorenzo G, Muscara N, et al. Promising ` role of neuromodulation in predicting the progression of mild cognitive impairment to dementia. J Alzheimers Dis 2016;53:1375–88. 10.3233/jad-160305. [DOI] [PubMed] [Google Scholar]
- [85].Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Curr Opin Neurobiol 2011;21:475–85. 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- [86].Moliadze V, Sierau L, Lyzhko E, Stenner T, Werchowski M, Siniatchkin M, et al. After-effects of 10 Hz tACS over the prefrontal cortex on phonological word decisions. Brain Stimul 2019;12:1464–74. 10.1016/j.brs.2019.06.021. [DOI] [PubMed] [Google Scholar]
- [87].Yin CH, Li SO, Zhao WN, Feng JC. Brain imaging of mild cognitive impairment and Alzheimer’s disease. Neural Regen Res 2013;8:435–44. 10.3969/j.issn.1673-5374.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Guerra A, Asci F, Zampogna A, D’Onofrio V, Petrucci S, Ginevrino M, et al. Gamma-transcranial alternating current stimulation and theta-burst stimulation: inter-subject variability and the role of BDNF. Clin Neurophysiol 2020;131:2691–9. 10.1016/j.clinph.2020.08.017. [DOI] [PubMed] [Google Scholar]
- [89].Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul 2014;7:468–75. 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- [90].Albizu A, Fang R, Indahlastari A, O’Shea A, Stolte SE, See KB, et al. Machine learning and individual variability in electric field characteristics predict tDCS treatment response. Brain Stimul 2020;13. 10.1016/j.brs.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-Del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul 2014;7:372–80. 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- [92].Bland NS, Sale M v. Current challenges: the ups and downs of tACS. Exp Brain Res 2019;237:3071–88. 10.1007/s00221-019-05666-0. [DOI] [PubMed] [Google Scholar]
- [93].del Felice A, Castiglia L, Formaggio E, Cattelan M, Scarpa B, Manganotti P, et al. Personalized transcranial alternating current stimulation (tACS) and physical therapy to treat motor and cognitive symptoms in Parkinson’s disease: a randomized cross-over trial. Neuroimage Clin 2019;22:101768. 10.1016/j.nicl.2019.101768. [DOI] [PMC free article] [PubMed] [Google Scholar]


