Abstract
Pigs infected with hemolytic F4+ strains of enterotoxigenic Escherichia coli often develop septicemia secondary to intestinal infection. We tested the hypothesis that inactivation of hemolysin would reduce the ability of F4+ enterotoxigenic E. coli to cause septicemia in swine following oral inoculation. Inactivation of the hemolysin structural gene (hlyA) did not decrease the incidence of septicemia in the gnotobiotic piglet model.
Escherichia coli is an important cause of diarrheal disease and extraintestinal infections in animals and humans (26). Strains that produce enterotoxins (enterotoxigenic E. coli [ETEC]) are among the most important causes of diarrhea in neonatal swine and cattle (20, 45) and are a common cause of septicemia in preweaned swine (8). E. coli bacteria that cause septicemia express virulence determinants that mediate their spread from the intestine to extraintestinal sites and that mediate bacterial persistence and proliferation in these locations (8). Putative virulence determinants include fimbriae, polysaccharide capsules, O-antigen capsules, lipopolysaccharide, aerobactin, hemolysin, and other cytotoxins (8). P fimbriae and hemolysin are the most important virulence factors that distinguish bacteremia-causing isolates from fecal control strains in human diabetic patients (3). The introduction of hemolysin genes into E. coli bacteria increases the virulence of these strains when inoculated into the peritoneal cavity or bloodstream in laboratory rodents (7, 33, 42). Hypothetically, hemolysin might increase virulence by increasing the availability of iron in the absence of aerobactin (27), mediating toxic effects on leukocytes and other nucleated cells (43), potentiating the effects of endotoxin (43), and mediating serum resistance (31). However, despite evidence suggesting that hemolysin increases the virulence of E. coli, there is little information regarding its role in vivo (43).
It has been shown that hemolytic ETEC strains naturally cause extraintestinal disease in preweaned and weaned swine, manifested clinically by sudden death and pathologically by lesions caused by endotoxic shock (9, 23). These strains most often belong to serogroups O8, O149, and O157 and are gene probe positive for hemolysin, K88 (F4) fimbriae, heat-labile enterotoxin I (LT-I), and heat-stable enterotoxin b (STb) (9, 23, 24). Experimental inoculation of gnotobiotic piglets with hemolytic strains of E. coli causes bacteremia and lesions identical to those seen in naturally occurring cases of hemolytic-ETEC-induced endotoxic shock (9, 14, 16, 23, 24).
The results of these and other experiments using animal models and the association of hemolytic E. coli with extraintestinal disease in swine led us to hypothesize that inactivation of hemolysin would decrease the ability of F4+ ETEC to cause secondary bacterial septicemia in this species. We used the neonatal gnotobiotic piglet as a model because it is naturally susceptible to infection by F4+ ETEC and is suitable for the study of E. coli invasion (15, 16, 19). The objectives of this study were (i) to generate a stable nonhemolytic mutant from a hemolytic F4+ ETEC strain by gene replacement and (ii) to compare the virulence of the hemolytic and nonhemolytic isogenic strains in gnotobiotic piglets.
Bacterial strains.
The bacterial strains and plasmids used in this study are shown in Table 1. ETEC strain 2534-86 serotype O8:K87:NM:F4ac was isolated from the small intestine of a 2-week-old pig with enteric colibacillosis and secondary bacterial septicemia (23). Strain 2534-86 was Kms at a concentration of 50 μg/ml and was shown to be positive for F4 fimbrial antigen by indirect immunofluorescence assay (11). Strain 2534-86 was shown to be LT-I+, STb+, hemolysin-positive (Hly+), intimin negative (EaeA−), negative for Shiga toxins 1 and 2, and negative for cytotoxic necrotizing factors 1 and 2 by Southern hybridization and PCR analyses (4, 5, 13, 22, 36, 44).
TABLE 1.
E. coli strains and plasmids used
| Strain or plasmid | Relevant characteristic(s) | Vector | Reference |
|---|---|---|---|
| E. coli strains | |||
| 2534-86 | O8:K87:NM:F4ac, HlyA+ LT-I+ STb+ | 23 | |
| S17-1λpir | RP4-2-Tc::Mu Km::Tn7 pro res Mod+ λPir+ | 17, 32 | |
| WAM1808 | K12 | This study | |
| WAM2317 | Nalr mutant of 2534-86 | This study | |
| WAM2335 | ΔhlyA mutant of WAM2317 | This study | |
| Plasmids | |||
| pCVD442 | MobRP4+ R6K+ SacB+ Apr | pGP704 | 6 |
| pWAM04 | HlyCABD+ Apr | pUC19 | 44 |
| pUC4K | Kmr Apr | pUC7 | 38 |
| pWAM1097 | HlyCA+ Apr | pUC19 | 39 |
| pWAM1661 | HlyC+ ΔhlyA Apr Kmr | pUC19 | 39 |
| pWAM1746 | HlyC+ ΔhlyA Apr Kmr | pCVD442 | This study |
| pDAS102 | LT-I+ Apr | pUC8 | 4 |
| pDAS103 | STb+ Apr | pUC8 | 13 |
Genetic and recombinant DNA procedures.
Recombinant DNA procedures were done as described by Sambrook et al. (29). Total genomic DNA was extracted by a guanidium isothiocyanate-EDTA-Sarkosyl lysis procedure, digested with PvuII, and separated by 0.75% agarose gel electrophoresis in Tris-acetate-EDTA buffer (29). Gene probes for hlyCABD and the Kmr marker were prepared from the 7.0-kb AvaI-A fragment of pWAM04 (44) and from the 1.2-kb BamHI-B fragment of pUC4K, respectively. Gene probes for LT-I and STb were prepared from the 850-bp HindIII fragment of plasmid pDAS102 and from the 460-bp HinfI fragment of plasmid pDAS103, respectively (4, 13, 22, 36). The probes were directly labeled with horseradish peroxidase, and the hybridization products were detected with a chemiluminescence detection system (ECL direct nucleic acid labeling and detection system; Amersham International, Little Chalfont, Buckinghamshire, England).
Construction of hlyA deletion mutant.
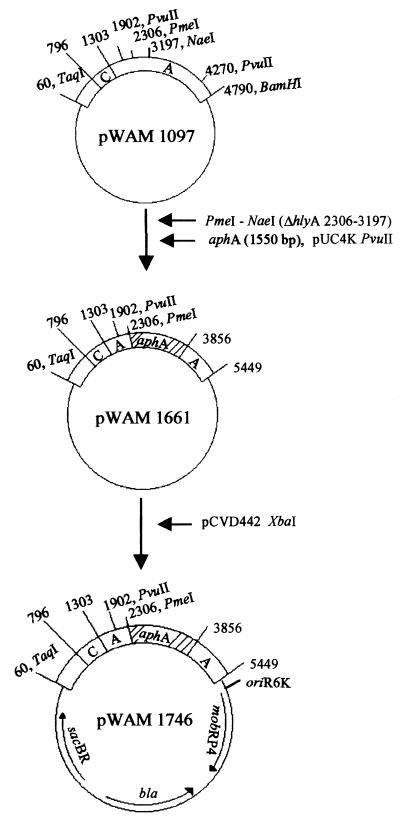
A nonhemolytic mutant of strain 2534-86 was constructed as follows (Fig. 1). Plasmid pWAM1097 contains hlyCA from E. coli urinary tract isolate J96, cloned in pUC19 (39). Plasmid pWAM1661 was constructed by removing PmeI-NaeI (hlyA2306–3197) in pWAM1097 and replacing it with a Kmr cassette (aphA) from pUC4K (38, 39). This hlyCA region containing the Kmr cassette was cloned into the XbaI site of pCVD442 and transformed into S17-1λpir to yield donor strain WAM1746. WAM1746 was mated to a spontaneous nalidixic acid-resistant mutant of strain 2534-86, designated WAM2317. Bacterial matings were done at 37°C for 9 to 12 h on 0.45-μm-pore-size filters on Luria-Bertani (LB) agar containing 100 mM sodium citrate. Mating mixtures were subcultured overnight at 37°C on blood agar plates containing nalidixic acid (50 μg/ml) and kanamycin (50 μg/ml) (BA-NAL-KM plates). Five transconjugant colonies with decreased hemolytic zones on BA-NAL-KM plates were selected from the mating and pooled into one culture. This culture was incubated with aeration at 37°C for 5 h, pelleted, and plated for counterselection on LB agar plates containing 5% sucrose, nalidixic acid, kanamycin, and no NaCl (LB-NAL-KM-sucrose). After a 16-h incubation at 28°C, isolated colonies on the LB-NAL-KM-sucrose plates were patched onto LB plates containing 100 μg of ampicillin per ml and onto BA-NAL-KM plates. One nonhemolytic Aps colony, designated WAM2335, was selected for further examination. A liquid hemolysis assay (2) with dilutions made in 20 mM CaCl2 detected no hemolytic activity in culture supernatant fluids of WAM2335, whereas the peak activity of WAM2317 was approximately 200 50% lysis hemolytic units/ml.
FIG. 1.
Construction of plasmid pWAM1746 for an Hly− donor strain. aphA encodes aminoglycoside phosphotransferase, bla encodes β-lactamase, and sacBR encodes levansucrase. oriR6K, origin of replication of R6K; mobRP4, mobilization region of RP4.
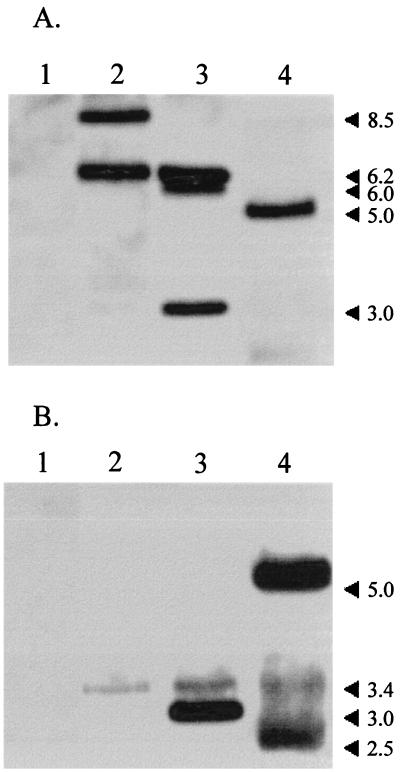
Southern blot analyses of PvuII digests of WAM2317 genomic DNA probed for hlyCABD contained hybridizable fragments of 8.5 and 6.2 kb (Fig. 2A). In contrast, digests of WAM2335 probed for hlyCABD contained hybridizable fragments of 6.2, 6.0, and 3.0 kb (Fig. 2A). The 3.0-kb fragment also hybridized to the aphA gene probe (Fig. 2B). Southern blot analyses of HindIII digests of WAM2335 genomic DNA contained hybridizable bands for LT-I and STb. Expression of F4 fimbrial antigen in WAM2335 was demonstrated by an indirect immunofluorescence assay (data not shown).
FIG. 2.
Southern hybridization analysis of PvuII digests of genomic DNA of negative control WAM1808 (lanes 1), recipient strain WAM2317 (lanes 2), and nonhemolytic mutant strain WAM2335 (lanes 3) and XbaI digest of plasmid DNA of donor strain WAM1746 (lanes 4). Horseradish peroxidase-labeled probes for hlyCABD (7.0-kb AvaI fragment of pWAM04) (A) and the kanamycin resistance gene aphA (1.2-kb BamHI fragment of pUC4K) (B) were used. Numbers at right are molecular size markers.
Gnotobiotic piglet experiments.
Seven gnotobiotic piglets from one litter were derived by closed hysterotomy and reared in sterile isolator units according to standard procedures (18). When the piglets were 8 days old, swab samples from the nasal cavity and rectum and blood samples from the orbital sinus were obtained from each piglet under anesthesia (20 mg of tiletamine hydrochloride-zolazepam hydrochloride [Telazol; Fort Dodge Labs, Fort Dodge, Iowa] per kg of body weight [12, 40]). When the piglets were 9 days old, four were inoculated orally with WAM2335 and three were inoculated orally with WAM2317. Piglets in both groups were inoculated by instilling 1.0 × 109 CFU into the milk replacer. After inoculation, the piglets were examined every 2 to 4 h for decreased rectal temperature, anorexia, vomiting, diarrhea, depression, and a moribund condition. Pre- and postinoculation blood samples, the latter when piglets were moribund or at 65 h postinoculation, were collected in sterile blood collection tubes for bacterial culture.
Rectal and nasal swab specimens and ileal, liver, and lung specimens were cultured aerobically and anaerobically by standard procedures on sheep blood agar (5% sheep blood in Trypticase soy agar; Remel Labs, Lenexa, Kans.). Blood samples were cultured aerobically and anaerobically in commercial brain heart infusion broth culture vials (Septi-Check; BBL Labs, Cockeysville, Md.). Bacterial isolates were identified by standard procedures. Clinical parameters and mean colony counts per gram of ileal mucosa were compared by using Student’s t test (34).
Specimens of duodenum, jejunum, ileum, spiral colon, thymus, lung, liver, spleen, kidney, and brain were collected at necropsy, fixed in 10% neutral buffered formalin, processed for histopathology by standard procedures, and examined by light microscopy. Additional sections were stained by an immunohistochemical procedure (28) with rabbit polyclonal anti-O8 antiserum (E. coli Reference Center, The Pennsylvania State University). One block of paraffin-embedded ileum from a piglet inoculated with WAM2335 was further processed and examined with a transmission electron microscope (model 201; Philips, Eindhoven, The Netherlands). The F4 adherence phenotype of each piglet was determined by a brush border adherence assay (1).
All piglets became depressed, anorectic, and diarrheic after inoculation. All piglets developed diarrhea by 22 h postinoculation, and it persisted until euthanasia or death (38 to 65 h postinoculation). Three of four piglets inoculated with WAM2335 and one of three piglets inoculated with WAM2317 became hypothermic and moribund at 36 to 42 h postinoculation (P > 0.10).
Cultures of preinoculation rectal and nasal specimens from three piglets inoculated with WAM2335 and one piglet inoculated with WAM2317 yielded Staphylococcus spp. The preinoculation blood samples from all piglets were sterile. Cultures of the ileal specimens collected at necropsy yielded the respective E. coli inoculum strain from each of the piglets. Mean CFUs of WAM2335 and WAM2317 per gram of ileal mucosa were not significantly different (P > 0.05). WAM2335 was isolated from the liver, lung, and blood of three of four inoculated piglets. WAM2317 was isolated from the lungs of the three inoculated piglets but from the liver and blood of only one of these piglets (P > 0.10).
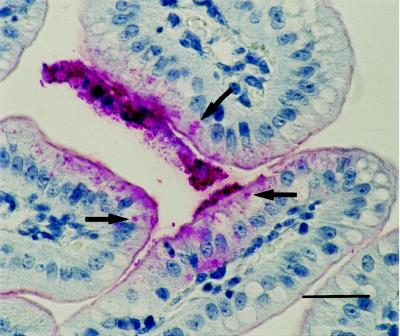
All seven piglets were determined to be of F4 adherence phenotype A (i.e., susceptible to F4ab, F4ac, and F4ad organisms) in the brush border adherence assay. Histologically, coliform bacteria were adherent to the small intestinal villous epithelium in all piglets inoculated with WAM2317 and WAM2335. In addition, they were within cytoplasmic vacuoles in two of four piglets inoculated with WAM2335 and in two of three piglets inoculated with WAM2317. Immunostained sections of small intestine from the same blocks of tissue had O8-positive bacteria in these locations (Fig. 3). Stomach, small intestine, spiral colon, liver, lung, kidney, and brain from three of four piglets inoculated with WAM2335 and from one of three piglets inoculated with WAM2317 were hyperemic and contained microthrombi (P > 0.10). The thymic cortices from all piglets had fragmented nuclei and cellular debris.
FIG. 3.
Light micrograph of jejunum of a gnotobiotic piglet inoculated with hemolytic E. coli strain WAM2317. O8-positive bacteria in villous enterocytes (arrows) are stained red; staining was done by the immunohistochemical staining technique. Bar = 10 μm.
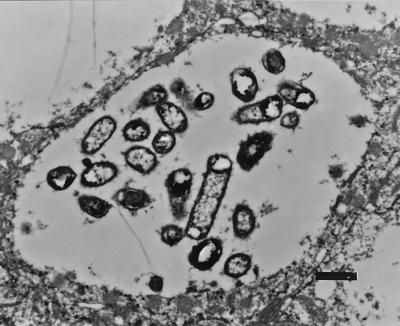
Ultrastructurally, WAM2335 organisms were adhered to the apical surfaces of villous enterocytes and within cytoplasmic vacuoles in these cells. Bacteria on the surfaces of enterocytes were either loosely or intimately adhered to microvilli. Occasionally, intimately adhered bacteria were seen in invaginations in the apical surfaces of enterocytes with disrupted microvilli. Individual bacteria were present in small cytoplasmic vacuoles (data not shown), whereas multiple bacteria were often present in large cytoplasmic vacuoles (Fig. 4). Bacteria also were seen within cytoplasmic vacuoles of M cells (data not shown).
FIG. 4.
Transmission electron micrograph of formalin-fixed ileum of a gnotobiotic piglet inoculated with nonhemolytic mutant E. coli strain WAM2335. Numerous bacteria are shown within a large cytoplasmic vacuole in an enterocyte. Bar = 1 μm.
In this study we provide the first report of the construction of an Hly− isogenic mutant of a porcine ETEC strain. The hlyA gene in this strain was inactivated by double homologous recombination following introduction of an HlyC+ΔhlyA fragment on a conjugatable suicide plasmid. The occurrence of a deletion event in hlyCA in conjunction with Kmr cassette insertion into this region was confirmed by Southern blot analysis.
We hypothesized that inactivation of hlyA would decrease the ability of ETEC to cause secondary septicemia in swine. However, inactivation of hlyA did not reduce the incidence of diarrhea or septicemia in neonatal gnotobiotic piglets inoculated with isogenic Hly+ and Hly− strains. Our results confirm and extend those of Smith and Linggood, who, by using an ETEC strain cured of its Hly plasmid, found that hemolysin played no role in diarrhea (33). Either hemolysin plays no role in septicemia in this model or other virulence factors obscured its effects. Animal-to-animal variation also may have influenced the outcome (21) and may explain why only one of three piglets inoculated with the Hly+ parent strain developed septicemia despite the fact that all three had bacterial colonization of the lungs.
The finding of ETEC within cytoplasmic vacuoles of columnar enterocytes may reflect the predisposition of neonatal gnotobiotic piglet intestine to phagocytose bacteria and/or an invasive capacity of the serotype used for inoculation (16, 19, 25, 30, 35). ETEC is not considered to be invasive in the classical sense (26). However, the results of studies of natural and experimental infections in piglets less than 1 week old sugggested that ETEC serotype O8:K87 (previously termed G7), in particular, is invasive (16, 19, 30, 35). Ultrastructural examination of the intestine was not done in these previous studies with E. coli O8:K87, and to our knowledge, E. coli bacteria within cytoplasmic vacuoles of enterocytes had not been seen in piglets inoculated with other ETEC serotypes. However, they have been seen in gnotobiotic piglets inoculated with enteropathogenic E. coli, enterohemorrhagic E. coli, and septicemia-inducing E. coli (21, 25, 37, 41). Interestingly, more recent studies have shown that a classical human ETEC strain (H10407) invades human intestinal cell lines and that the capacity for invasion is located on two separate, chromosomal loci, designated tia and tib (toxigenic invasion loci A and B [10]). Based on our results and those from other studies, we hypothesize that intestinal invasion plays a role in the pathogenesis of some ETEC infections.
A criterion important in order for an extraintestinal infection to occur is the ability of the strain to survive and to persist in the bloodstream and extraintestinal tissues (8). In the present study, both the nonhemolytic mutant and hemolytic parent strain survived and caused bacterial septicemia in the process. Hypothetically, hemolysin might enhance the ability of E. coli to survive in the bloodstream by mediating resistance to complement and phagocytes and by serving as an alternative mechanism for the acquisition of iron in the absence of aerobactin (31, 43). Further studies with the enterically infected gnotobiotic piglet model could address the effects of hemolysin on extraintestinal infection. These include competition experiments with Hly+ and Hly− organisms, studies of the effects of lower inoculum levels, studies addressing the time of onset and level of bacteremia, and studies of the effects of indigenous enteric bacteria (e.g., Lactobacillus spp. and Streptococcus faecium).
Acknowledgments
We thank Shahaireen Pellett, Doreen Bailey, and Rick Roscetti for technical assistance.
This study was supported by a grant from the Nebraska Pork Producers Association, Inc., and by NC-62 Regional Research Funds.
Footnotes
Published as journal series no. 12184 of the Agricultural Research Division, University of Nebraska.
REFERENCES
- 1.Baker D R, Billey L O, Francis D H. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet Microbiol. 1997;54:123–132. doi: 10.1016/s0378-1135(96)01277-1. [DOI] [PubMed] [Google Scholar]
- 2.Bohach G A, Cavalieri S J, Snyder I S. Purification of Escherichia coli α-hemolysin. Methods Enzymol. 1988;165:137–146. doi: 10.1016/s0076-6879(88)65023-3. [DOI] [PubMed] [Google Scholar]
- 3.Brauner A, Katouli M, Östenson C G. P-fimbriation and haemolysin production are the most important virulence factors in diabetic patients with Escherichia coli bacteremia: a multivariate statistical analysis of seven bacterial virulence factors. J Infect. 1995;31:27–31. doi: 10.1016/s0163-4453(95)91271-1. [DOI] [PubMed] [Google Scholar]
- 4.Dallas W S, Gill D M, Falkow S. Cistrons encoding Escherichia coli heat-labile toxin. J Bacteriol. 1979;139:850–858. doi: 10.1128/jb.139.3.850-858.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Rycke J, Guillot J F, Boivin R. Cytotoxins in non-enterotoxigenic strains of Escherichia coli isolated from calves. Vet Microbiol. 1987;15:137–157. doi: 10.1016/0378-1135(87)90139-8. [DOI] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emödy T, P, Safonova N V, Kuch B, Golutva N K. Alpha-haemolysin: an additive virulence factor in Escherichia coli. Acta Microbiol Acad Sci Hung. 1980;27:333–342. [PubMed] [Google Scholar]
- 8.Fairbrother J M, Ngeleka M. Extraintestinal Escherichia coli infections in pigs. In: Gyles C L, editor. Escherichia coli in domestic animals and humans. Wallingford, United Kingdom: CAB International; 1994. pp. 221–236. [Google Scholar]
- 9.Faubert C, Drolet R. Hemorrhagic gastroenteritis caused by Escherichia coli in piglets: clinical, pathological and microbiological findings. Can Vet J. 1992;33:251–256. [PMC free article] [PubMed] [Google Scholar]
- 10.Fleckenstein J M, Kopecko D J, Warren R L, Elsinghorst E A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–2265. doi: 10.1128/iai.64.6.2256-2265.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis D H. Use of immunofluorescence, Gram’s staining, histologic examination, and seroagglutination in the diagnosis of porcine colibacillosis. Am J Vet Res. 1983;44:1884–1888. [PubMed] [Google Scholar]
- 12.Huhn R G, Osweiler G D, Switzer W P. Application of the orbital sinus bleeding technique to swine. Lab Anim Care. 1969;19:403–405. [PubMed] [Google Scholar]
- 13.Lee C H, Moseley S L, Moon H W, Whipp S C, Gyles C L, So M. Characterization of the gene encoding heat-stable toxin II and preliminary molecular epidemiological studies of enterotoxigenic Escherichia coli heat-stable toxin II producers. Infect Immun. 1983;42:264–268. doi: 10.1128/iai.42.1.264-268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer R C, Rhoades H E, Saxena S P, Simon J. Escherichia coli isolated from domestic animals pathogenic for gnotobiotic piglets. Infect Immun. 1971;3:735–738. doi: 10.1128/iai.3.6.735-738.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer R C, Rhoades H E, Simon J. Susceptibility of gnotobiotic swine to Escherichia coli isolated from nonenteric human infections. Appl Microbiol. 1972;23:1167–1169. doi: 10.1128/am.23.6.1167-1169.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer R C, Simon J. Experimental peracute colibacillosis: gastrointestinal lesions in gnotobiotic piglets. Vet Pathol. 1972;9:360–367. doi: 10.1177/030098587200900506. [DOI] [PubMed] [Google Scholar]
- 17.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miniats O P, Jol D. Gnotobiotic pigs—derivation and rearing. Can J Comp Med. 1978;42:428–437. [PMC free article] [PubMed] [Google Scholar]
- 19.Miniats O P, Mitchell L, Barnum D A. Response of gnotobiotic pigs to Escherichia coli. Can J Comp Med. 1970;34:269–276. [PMC free article] [PubMed] [Google Scholar]
- 20.Moon H W, Bunn T O. Vaccines for preventing enterotoxigenic Escherichia coli infections in farm animals. Vaccine. 1993;11:213–220. doi: 10.1016/0264-410X(93)90020-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moseley S L, Huq I, Alim A R M A, So M, Samadpour-Motalebi M, Falkow S. Detection of enterotoxigenic Escherichia coli by DNA colony hybridization. J Infect Dis. 1980;142:892–898. doi: 10.1093/infdis/142.6.892. [DOI] [PubMed] [Google Scholar]
- 23.Moxley R A, Erickson E D, Breisch S. Proceedings of the George A. Young Swine Conference and Annual Nebraska SPF Swine Conference. 1988. Shock associated with enteric colibacillosis in suckling and weaned swine; pp. 33–38. [Google Scholar]
- 24.Moxley, R. A. Unpublished data.
- 25.Murata H, Yaguchi H, Namioka S. Relationship between the intestinal permeability to macromolecules and invasion of septicemia-inducing Escherichia coli in neonatal piglets. Infect Immun. 1979;26:339–347. doi: 10.1128/iai.26.1.339-347.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro J P, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Opal S M, Cross A S, Gemski P, Lyhte L W. Aerobactin and α-hemolysin as virulence determinants in Escherichia coli isolated from human blood, urine, and stool. J Infect Dis. 1990;161:794–796. doi: 10.1093/infdis/161.4.794. [DOI] [PubMed] [Google Scholar]
- 28.Rogers D G, Andersen A A, Hunsaker B D. Lung and nasal lesions caused by a swine chlamydial isolate in gnotobiotic pigs. J Vet Diagn Investig. 1996;8:45–55. doi: 10.1177/104063879600800108. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Saunders C N, Stevens A J, Spence J B. Escherichia coli infection in piglets. Res Vet Sci. 1960;1:28–35. [Google Scholar]
- 31.Siegfried L, Kmeťová M, Janigová V, Šašinka M, Takáčová V. Serum response of Escherichia coli strains causing dyspepsia and urinary tract infection: relation to alpha-hemolysin production and O type. Infect Immun. 1995;63:4543–4545. doi: 10.1128/iai.63.11.4543-4545.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–791. [Google Scholar]
- 33.Smith H W, Linggood M A. Observations on the pathogenic properties of the K88, hly and ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971;4:467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- 34.Snedecor G W, Cochran W G. Statistical methods. 7th ed. Ames: Iowa State University Press; 1980. pp. 54–59. [Google Scholar]
- 35.Sojka W J, Lloyd M K, Sweeney E J. Escherichia coli serotypes associated with certain pig diseases. Res Vet Sci. 1960;1:17–27. [Google Scholar]
- 36.Sommerfelt H, Svennerholm A-M, Kalland K H, Haukanes B-I, Bjorvatn B. Comparative study of colony hybridization with synthetic oligonucleotide probes and enzyme-linked immunosorbent assay for identification of enterotoxigenic Escherichia coli. J Clin Microbiol. 1988;26:530–534. doi: 10.1128/jcm.26.3.530-534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Staley T E, Jones E W, Corley L D. Attachment and penetration of Escherichia coli into intestinal epithelium of the ileum in newborn pigs. Am J Pathol. 1969;56:371–392. [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor L A, Rose R E. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 1988;16:358. doi: 10.1093/nar/16.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas W D, Jr, Wagner S P, Welch R A. A heterologous membrane protein domain fused to the C-terminal ATP-binding domain of HlyB can export Escherichia coli hemolysin. J Bacteriol. 1992;174:6771–6779. doi: 10.1128/jb.174.21.6771-6779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thurmon J C, Benson G J, Tranquilli W J, Olson W A, Tracy C H. The anesthetic and analgesic effects of Telazol and xylazine in pigs: evaluating clinical trials. Vet Med. 1988;83:841–845. [Google Scholar]
- 41.Tzipori S, Gibson R, Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infect Immun. 1989;57:1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welch R A, Dellinger E P, Minshew B, Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981;294:665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- 43.Welch R A, Bauer M E, Kent A D, Leeds J A, Moayeri M, Regassa L B, Swenson D L. Battling against host phagocytes: the wherefore of the RTX family of toxins? Infect Agents Dis. 1995;4:254–272. [PubMed] [Google Scholar]
- 44.Welch R A, Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988;170:1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson R A, Francis D H. Fimbriae and enterotoxins associated with Escherichia coli serogroups isolated from pigs with colibacillosis. Am J Vet Res. 1986;47:213–217. [PubMed] [Google Scholar]