Abstract
This study aimed to investigate the potential use of okra and psyllium mucilage as co-carrier wall materials with whey protein and gum Arabic polymers for encapsulation of fenugreek oil to mask its undesirable flavor and promote their health benefits. Particle size, zeta potential, encapsulation efficiency, morphological properties and fatty acid profiles of crude and encapsulated oils were examined using zeta-sizer, SEM and GC-MS techniques. Crude and encapsulated fenugreek oils were added as functional ingredients during production of pan bread and biscuits. The quality characteristics (baking quality, color and organoleptic properties) of bread and biscuits as well as microbiological properties of bred samples were evaluated. Results showed that the forming microcapsules had sphere particles with the size of 5.05 and 31.64 μm for okra and pysillium mucilage, respectively and had smooth continuous surfaces with no holes or fractures. Fatty acids analysis showed that fenugreek oil is superior functional edible oil, rich in unsaturated fatty acids. The organoleptic properties of products were improved when fat replaced with encapsulated fenugreek oil with okra or psyllium mucilage. Likewise, encapsulated fenugreek oil showed antimicrobial activity in bread samples during storage period. On contrary, Bread and biscuits incorporated with crude fenugreek oil gained the lowest scores for all organoleptic parameters. Regarding these results, encapsulated fenugreek oil presents good fat alternatives in dough formulations with acceptable technological, sensory and antimicrobial properties. However, further investigations still needed regarding the biological activity of encapsulated fenugreek oil and its utilization as a food supplement in other food products.
Keywords: Fenugreek oil, Okra mucilage, Psyllium mucilage, Fat replacers, Bread, Biscuit
Graphical abstract
1. Introduction
Excess saturated and trans fat intake is the main factor for increasing of triglycerides and LDL-cholesterol which are associated with the obesity and development of cardiovascular disease [[1], [2], [3]]. Therefore, decreasing such fat consumption has become an immediate demand for the national health authorities [4,5]. Moreover, saturated fats replacement with unsaturated ones is another healthy demand in the recent years [6,7]. However, fat reduction in food systems faces several challenges, as beside the nutritional role, fat plays an important role in food processing and affects the overall palatability and consumer acceptance [8,9]. Also, unsaturated fats are very sensitive to moisture, temperature, light and oxygen [[10], [11], [12]]. Furthermore, the undesirable odor and taste of many unsaturated fats remains one of the challenges facing their incorporation in food products [6,13].
To meet these challenges and to maintain the consumer acceptance, fat mimics and microencapsulation technology could be employed. Fat replacers that emerged in food production could be subdivided according to their chemical structure and functions to fat substitutes (lipid-based) and fat mimics (carbohydrate- and protein-based) [[14], [15], [16]]. Most of fat mimics cannot fully replace fat in food products [17,18], but they have good properties as carrier materials in encapsulation technology [19,20]. Recently, plant biopolymers have gained high attention as sustainable and cost-effective fat replacers and carrier materials in encapsulation technology [21,22]. Biopolymers, generally recognized as safe (GRAS), are diversely used in food production as coatings, emulsifiers, binders, and gelling agents [23,24]. Among biopolymers, mucilage exhibits distinctive functional properties not only in food but also in pharmaceutical formulations [25].
Psyllium (Plantago ovata) and okra (Abelmoschus esculentus L. Moench) are important sources of mucilage. Psyllium mucilage is a natural anionic polysaccharide, mainly branched arabinoxylan, composed of d–xylose, L-arabinose and D-galactoronic acid [26,27]. While, okra mucilage is a pectic polysaccharide, that composed of L-rhamnose, D-galactose and L-galacturonic acid [28,29]. So far, okra and psyllium mucilage has been studied as natural emulsifier agents, fat replacers and carrier wall materials in the encapsulation technology [[30], [31], [32], [33], [34], [35]].
Fenugreek oil is a good source of phytonutrients as omega fatty acids (ω3, ω6 and ω9), sterols, alkaloids and saponins [[36], [37], [38]]. Several studies investigated the fatty acids composition of fenugreek oil and showed that it was dominated by oleic (C18:1), linoleic (C18:2) and linolenic (C18:2) acids [[39], [40], [41], [42]]. However, the fatty acids of fenugreek oil differ according to the geographic and cultivation conditions [[43], [44], [45]]. Due to its unique composition, fenugreek oil has various health promoting benefits. For instance, it reduces LDL cholesterol and triglycerides of high blood levels [36,46,47], which subsequently minimizes the risk of cardiovascular disease [48,49]. Also, it acts as a phytoestrogen that involved in lactation and ovulation, regulations [[50], [51], [52]]. Furthermore, it considered as safe and cheap herbal treatment for men infertility [39,53].
Fenugreek oil showed a potent antimicrobial activity against several bacterial and fungal strains. Regarding the above mentioned observations, fenugreek oil can be implemented in food and pharmaceutical industries [[54], [55], [56]]. Despite of these advantages of fenugreek oil, there are many challenges for applying fenugreek oil in food products, such as the bitter taste and strong nasty smelling of oil [43,57]. Therefore, the present study aimed to study the application of okra or psyllium mucilage in combination with gum Arabic and whey protein as carrier wall materials for fenugreek oil encapsulation to mask the bitter taste of fenugreek oil and to protect the unsaturated fatty acids. Also, the produced capsules were used as fat substitute in pan bread and fat mimics in biscuit preparation. Particle size, encapsulation efficiency, morphology of microcapsules and fatty acid compositions of crude and encapsulated oil, as well as the physical and sensory properties of bread and biscuits were investigated.
2. Materials and methods
2.1. Materials
Fenugreek, psyllium and okra seeds were sown on November at National Research Centre farm and harvested on March, May and April respectively. Ethanol, methanol, petroleum ether and hexane were of analytical grade and purchased from Fisher Scientific Chemical (Loughborough, UK). Plate count agar was obtained from Bio-World, USA and Malt extract agar plate was obtained from HiMedia Limited, India. Tryptic soya agar and sodium methoxide were purchased from Merck, Germany. Other materials used in pan bread and biscuit production such as wheat flour, dry yeast, sugar, salt, and fats were purchased from the local market, Dokki, Giza, Egypt.
2.2. Extraction of psyllium and okra mucilage
Mucilage extraction and purification was carried out according to Woolfe et al. [58] with some modifications. Psyllium seeds were powdered and okra fruits were sliced, boiled in ethanol for 20 min to remove pigments and to deactivate the enzymes. The treated psyllium seeds and okra fruits were homogenized in distilled water at the ratio of 1:5 (W/V). The mixtures were centrifuged at 5000 rpm for 15 min and the clear supernatants were collected. The mucilage was precipitated with three volumes of ethanol and stored overnight in a refrigerator. The precipitated mucilage was separated, washed with more ethanol and freeze-dried.
2.3. Extraction fenugreek seeds oil
Fenugreek oil was extracted from milled seeds using petroleum ether (40–60C) with an ultrasonic assessed method (Ultrasonic UP650, Acculab Inc., the USA) without raising the heat over 35 °C. The collected extract was evaporated by rotary evaporator (Heidolph, Germany) at 40 °C. The resultant oil was filtered, packed in dark brown bottle and stored at −20 °C until use.
2.4. Preparation of fenugreek seeds oil microcapsules
Fenugreek oil microcapsules were prepared using a mixture of whey protein (WP), gum Arabic (GA) and okra (OM) or psyllium mucilage (PM) with the ratio of 2:2:1 as wall materials. For emulsions preparation, the wall materials were dispersed in de-ionized water at 25 °C ± 2 under magnetic stirring overnight to complete hydration of the polymers and then were mixed with fenugreek oil using a homogenizer Ultra-Turrax T25 Basic (IKA, Wilmington, NC) at 18,000 rpm for 20 min in ice bath. The emulsions were prepared containing 42 g dry wall material/liter of total emulsion, with emulsifying agents (3g of span 80) and 5 g of fenugreek oil. The emulsions were spray dried using Mini spray drier (B-290, Buchi, Germany) with inlet air temperature of 160 ± 5 °C, and outlet air temperature of 90 ± 5 °C with a feed flow rate of 300 ml/h.
2.5. Particle size and zeta potential properties of encapsulated fenugreek oil
Droplet size (particle size and zeta potential) and polydispersity index (PDI) measurements were performed using Malvern Zeta-sizer Nano ZS Instrument, Worcestershire, UK with manufacturer's software.
2.6. Encapsulation efficiency measurement
Encapsulation efficiency (EE) was determined as the ratio of fenugreek oil retained inside the capsules after removal of surface oil as described by Munshi and Kumar [59]. Briefly, 2 g of microcapsules were washed with 20 ml hexane under magnetic stirrer at 200 rpm for 60 s. After 10 min, the mixture was filtered through Whatman filter paper (No. 41). The powder residue was again washed twice with 5 ml of hexane. The collected hexane was combined and evaporated and dried at 105 °C to constant weight and the surface oil was calculated. Encapsulation efficiency was calculated as follow: EE (%) = (Total oil - Surface oil)/(Total oil) × 100.
2.7. Morphological properties of encapsulated fenugreek oil
The surface morphology and elemental analysis were performed with Scanning Electron Microscopy-Energy Dispersive X-ray (SEM-EDAX) (JEOL JEM–2100, Tokyo, USA) at 5 kV. Twenty microliters of dispersed capsules in ethanol was placed on a film-coated 200-mesh copper specimen grid for 10 min and the fluid excess was eliminated using filter paper. The grid was then stained with one drop of 3 % phosphotungstic acid and allowed to dry for 3 min. The coated grid was dried and examined under the SEM microscope.
2.8. Determination of fatty acids profile of fenugreek seeds oil
2.8.1. Preparation of fatty acid methyl esters
Aliquot (20 mg) of fenugreek oil into screw-capped tube and 1 ml of 1 % sodium methoxide in methanol was added. Tubes were homogenized in a vortex for 20 s. Then, 1 mL of hexane was added, which led to separation two layer; the organic layer was collected, dried over anhydrous Na2SO4, and injected in GC-MS.
2.8.2. Gas chromatography–mass spectrometry analysis (GC-MS) for FAME
The GC-MS system (Agilent Technologies) equipped with gas chromatograph (7890B), mass spectrometer detector (5977A) and HP-5MS column (30 m × 0.25 mm internal diameter and 0.25 μm film thickness) was used. Analyses were carried out using helium as the carrier gas at a flow rate of 1.0 ml/min, injection volume of 1 μl and the following temperature program: 50 °C for 1 min; rising at 20 °C/min to 200 °C and held for 5 min; rising at 3 °C/min to 230 °C and held for 23 min. The injector and detector were held at 250 °C. Mass spectra were obtained by electron ionization (EI) at 70 eV and using a spectral range of m/z 20–550 and solvent delay 1.8 min. Identification of different constituents was determined by comparing the spectrum fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
2.9. Preparation of bread samples
The straight dough method for pan bread production was carried out according to the method described in AACC [60]. The ingredients consisted of wheat flour (100 g), water (60 g), dry yeast (2 g), salt (1 g), sugar (4 g) and fat (2 g). Sunflower oil was used as the fat source in the control bread, and it was replaced with fenugreek oil (2 g) or encapsulated oil (20 g). The ingredients were mixed for 4 min at slow speed (30 rpm) and for additional 6 min at fast speed (60 rpm). The resulted dough was left to rest for 20 min at 30 °C (first proofing) then divided, rolled and molded automatically in a molding machine. Each piece was placed in aluminium pan and left to ferment for 60 min at 37 °C (final proofing) then baked in an electrical oven at 250 °C for 20 min implementing a steaming step for the first 7 min of baking. Pan bread samples were separated from the aluminium pan and allowed to cool at room temperature.
2.10. Preparation of biscuit samples
Control biscuit sample was made according to the standard procedure [60] using wheat flour (100 g), sugar powder (15 g), shortening (20 g), salt (1 g), sodium bicarbonate (1.11 g), 14.66 mL of dextrose solution (5.93 %) and required water. Fat reduced biscuit samples were prepared by replacing the shortening in the control formula with fenugreek oil (2 g) or encapsulated oil (20 g). The corresponding biscuit dough therefore contained 2 %, fat in their respective formulations. All dry ingredients were mixed together in a dough mixer for 3 min, then all liquid ingredients were added to the dry mixture and mixed at low speed for 3 min then water added as require to obtained suitable smooth dough. The dough was left to rest for 5 min then sheeted to a thickness of 3 mm and cut into circle pieces using a 50 mm diameter dough cutter template. The biscuits were baked at 180 °C for 12 min, and allowed to cool at room temperature for 1 h before sensory evaluation.
2.11. Baking qualities of pan bread and biscuit samples
Volume (cm3) and weight (g) of pan bread samples were estimated according to the method described in AACC [60]. Specific volume (g/cm3) was calculated by dividing of the volume to weight. Biscuits were evaluated for diameter, thickness and spread ratio.
2.12. Color attributes of pan bread and biscuit samples
The color parameters of pan bread and biscuit samples were evaluated using Hunter, Lab Scan XE, Reston VA., calibrated with a white standard tile of Hunter Lab color standard (LX No. 16379) x = 77.26, y = 81.94 and z = 88.14 (L* = 92.43, a* = −0.88, b* = 0.21). The results were expressed in accordance with the CIELAB system where: L (L = 0 [black], L = 100 [white]), a (-a = greenness, +a = redness), b* (-b = blueness, +b = yellowness). Total color differences (ΔE) were calculated using the following equation:
| ΔE = [(ΔL)2 + (Δa) 2 + (Δb) 2] 0.5 |
2.13. Sensory evaluation pan bread and biscuit samples
The sensory evaluation of produced pan bread was done as described by AACC [60] using ten panelists from the Food Technology Department staff in National Research Center. The quality score of pan bread included color (20), texture (20), taste (20), flavor (20), general appearance (20) and overall acceptability was calculated as the sum-scores of the previous parameters for each sample (100).
The biscuit samples were evaluated for color, appearance, texture, taste, flavor and overall acceptability on a 9-point hedonic scale by ten panelists from the Food Technology Department staff in National Research Center according to the method of Sudha et al. [61].
2.14. Microbiological analysis
Representative sample (10 g) were taken and homogenized in 90 ml of 0.9 % NaCl. Serial 10 fold dilutions were prepared in saline tubes, and 1 ml of solution was used for microbial counting. Microbial enumeration was performed using pour plate method according to APHA [62] and FDA [63].
Total viable counting was performed using the plate count agar medium as recommended by Refs. [62,63]. Dilutions were made which were transferred to sterilized plates (1 ml per plate approximately). At the end of 2 days of aerobic incubation at 35 °C, the plates were analyzed for total growing population (CFU/g).
Molds and yeasts counts were examined using malt extract agar according to Galloway and Burgess [64]. Dilutions were made which were transferred to sterilized plates (1 ml per plate approximately). At the end of 3 days incubation period at 30 °C, the plates were analyzed for yeast and fungal population (CFU/g). Growing colonies morphology on specific agar media, and microscopic examination for target colonies were performed [62,63].
Typical spore count tests involve the heating of a reconstituted powder sample to 80 °C for 12 min before cooling, culturing on tryptic soya agar and incubation at 35 °C for 48hr and enumerating colonies [65,66].
2.15. Statistical analysis
Data were analyzed by standard procedures for analysis of variance and least significant difference test (LSD) to compare the means and determine the effect of treatments using the Statistical Analysis Software (SAS). The probability value of p˂0.05 was used as the criteria for significant differences. All experiments were performed in triplicate, and data are expressed as means ± SE.
3. Results and discussion
3.1. Particle size and encapsulation efficiency measurement
The mean particle size, polydispersity index (PDI), zeta potential and encapsulation efficiency of fenugreek oil microcapsules prepared with okra and psyllium mucilage are presented in Table 1. Mucilage source significantly influenced (p < 0.05) the particle size and zeta potentials of the produced capsules. The particle size of microcapsules containing okra and psyllium mucilage was 5.05 and 31.64 μm, respectively and its zeta potentials was −3.12 and −0.613 mV, respectively. These effects could be due to the difference in the molecular structure, monosaccharide compositions and sequences as well as the configuration of glycoside linkages and their position in the backbone [67]. Psyllium mucilage is a xylan polymer in which the pyranose ring of ß-d-xylose forms a linear backbone through β 1–3 and β 1–4 glucosidic linkage [68]. Psyllium mucilage is composed of xylose (74.65 %), arabinose (22.6 %) and traces of other sugars with about 35 % non-reducing terminal residues [69]. Okra mucilage is pectic polysaccharide composed of repeating unit of (1–4)-galacturonic acid and (1–2)-rhamnose residues with disaccharide side chains, which exhibit a specific degree of acetylation [70].
Table 1.
Particle size and encapsulation efficiency of encapsulated fenugreek oil.
| Parameter |
Encapsulated oil |
LSD | |
|---|---|---|---|
| WP:GA:OM | WP:GA:PM | ||
| Z-average (μm) | 5.05b ± 0.59 | 31.64a±0.79 | 4.242 |
| Polydispersity index | 0.23 ± 0.06 | 0.59 ± 0.12 | NS |
| Zeta potential (mV) | −3.12 b ± 0.05 | −0.613a ±0.13 | 0.599 |
| Encapsulation efficiency (%) | 90.90a±2.15 | 82.76b ± 1.51 | 6.585 |
- WP = whey protein, GA = gum Arabic, OM = okra mucilage, PM = psyllium mucilage, NS = not significant.
- Data in the same raw followed by different superscript letters are significantly different at p < 0.05.
The structure of okra mucilage provides a large number of surface hydroxyl groups (−OH) [71], which explain the high zeta potential value of microcapsules containing okra mucilage compared to those containing psyllium mucilage. Zeta potential is one of the factors that affect the emulsions stability as it reveals the existence of electrostatic repulsion between the emulsion droplets preventing its flocculation and aggregation [72]. So, the bigger particles of psyllium mucilage containing capsules may be due to back to the rapid flocculation of these particles after homogenization process, which lead to higher atomization and larger particles of spry-dried powders.
This theory was further supported by PDI result, which was 0.23 and 0.59 for okra and psyllium microcapsules, respectively. Polydispersity index is an indicator for the distribution of particle size; PDI < 0.3 indicates stable and homogeneous particles with uniform size distribution, while PDI > 0.3 indicates unstable and inhomogeneous particles with wide size distribution [73,74]. Also, zeta potential is an indicator for the stability of microencapsulated products [75]. The particles with high zeta potential have high repulsion strength, and consequently high stability [76]. Thus, microcapsules containing okra mucilage were more stable compared to those containing psyllium mucilage (Table 1).
Encapsulation efficiency of wall materials formulated with okra mucilage were significantly higher (p < 0.05) compared to psyllium mucilage (Table 1). The small particles are usually consistent with low surface oil, consequently high encapsulation efficiency and reduced lipid oxidation rate [77,78]. Although the obtained results showed okra mucilage as a promising co-wall material compared to psyllium mucilage, further investigation regarding the concentration of mucilage and the use of other encapsulation wall materials.
3.2. Morphological properties of encapsulated fenugreek oil
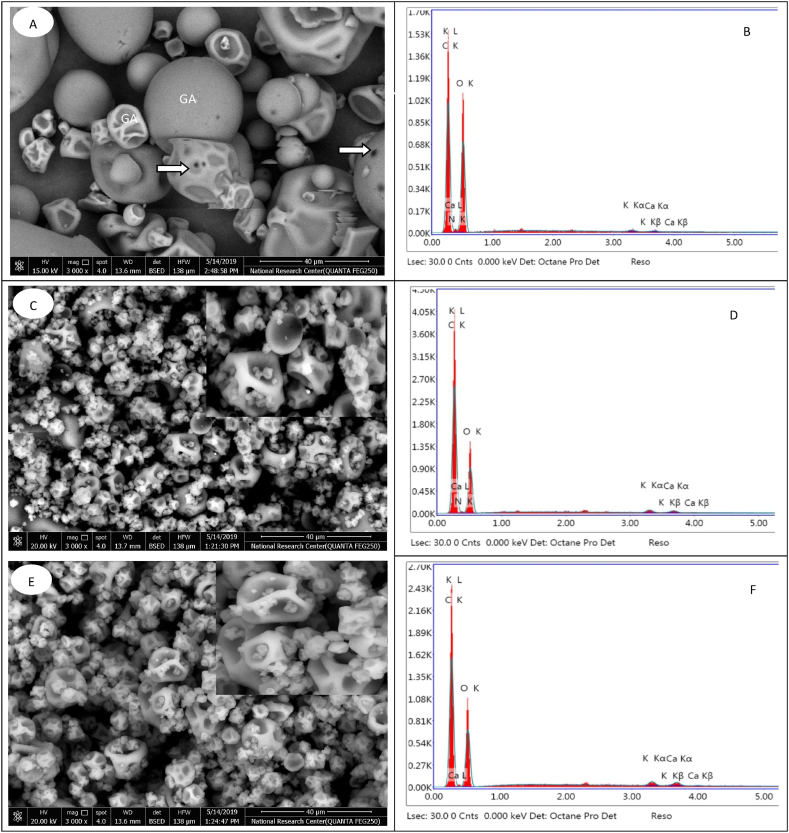
Morphological characteristics of neat whey protein and gum Arabic and spray-dried fenugreek oil microcapsules produced with the mixture of WP: GA: OM and WP: GA: PM matrices and their EDAX are presented in Fig. 1. SEM image of the neat Whey protein and gum Arabic powders showed that whey protein has big microspheres with a smooth surface, while gum Arabic has smaller irregular sphere particles. Also, SEM image showed many holes, cracks and dents on the surface of some of them (Fig. 1A). Similar results were reported by Sulaiman [79] and Prieto and Lagaron [80].
Fig. 1.
SEM-EDAX images of whey protein + gum arabic (A, B) and encapsulated fenugreek oil via whey protein + gum arabic + okra mucilage (C,D) and whey protein + gum arabic + psyllium mucilage (E,F).
Capsules produced by different matrices showed different morphological properties. Generally, most of the produced microcapsules had spherical structure with concavities on the surface. The occurrence of these concavities is common in spray-drying technique due to the rapid evaporation of liquid droplets at the beginning of the drying process [81]. Similar results were previously reported by Su et al. [82] and Rocha et al. [83]. In general, microspheres produced by combination of whey proteins, gum Arabic and both okra and psyllium mucilage matrices showed smaller spherical particles, free of holes, cracks, dents, fissures, or collapsing, which indicate b better protection for the unsaturated fatty acids in the encapsulated oils (Fig. 1C–E). The absence of fissures and holes is an interesting feature to ensure better protection and complete coverage of the core material.
In EDAX data, whey protein and gum Arabic consisted of carbon (46.86 %), oxygen (48.23 %), nitrogen (4.53 %), potassium (0.22 %) and calcium (0.16 %) (Fig. 1B). Fenugreek oil microcapsules containing okra or psyllium mucilage showed somewhat different composition. The carbon, potassium and calcium present in higher quantities (56.57 and 56.11 %; 0.53 and 0.81 % and 0.49 and 0.83 %, respectively), while the oxygen and nitrogen present in lower quantities (38.39 and 38.26 % and 4.02 and 4.0 %, respectively) (Fig. 1D–F). The variation of these constituents could play a role in the development of particles during emulsion preparation and spray-drying steps of encapsulation process, but this point still need further investigation. The obtained results are close to those previously reported by Singh et al. [47] for okra and psyllium mucilage.
3.3. Fatty acid profiles of crude and encapsulated fenugreek oil
Fatty acid profiles of crude fenugreek oil and those encapsulated using psyllium and okra mucilage are presented in Table 2. Fenugreek oil was rich in polyunsaturated fatty acids (PUFAs), including linoleic acid (C18:2) and linolenic acid (C18:3), representing 36.17 % and 25.17 % of total fatty acids, respectively. Oleic acid (C18:1) was the dominant monounsaturated fatty acid (MUFAs) making up 16.94 % of total fatty acids. Among the saturated fatty acids (SFAs), palmitic acid (C16:0) was the predominant followed by stearic acid (C18:0), representing 11.69 and 4.33 % of total fatty acids, respectively. Capric (C10:0), lauric (C12:0), myristic (C14:0), pentadecylic (C15:0), margaric (C17:0), arachidic (C20:0) and behenic (C22:0) contribute lower amounts (0.22–1.37 %) to the fenugreek fatty acids profile. Similar results were reported by Sulieman et al. [43] and Al Jasass and Al Jasser [36] for fenugreek seeds oil cultivated under Sudan and Saudi Arabia conditions. Furthermore, Gu et al. [40] found that fatty acid profiles of fenugreek oil extracted by subcritical butane and accelerated solvent extraction showed higher linoleic content (42.71–42.80 %) and lower linolenic and oleic contents (26.03–26.15 % and 14.24–14.40 %, respectively).
Table 2.
Fatty acid profiles of crude and encapsulated fenugreek oil.
| Fatty acids | Crude oil |
Encapsulated oil |
LSD | |
|---|---|---|---|---|
| WP:GA:OM | WP:GA:PM | |||
| Saturated fatty acids – SFAs (%) | ||||
| Capric acid (C10:0) | 0.22 ± 0.012 | 0.25 ± 0.014 | 0.23 ± 0.015 | NS |
| Lauric acid (C12:0) | 0.42 ± 0.023 | 0.51 ± 0.028 | 0.48 ± 0.031 | NS |
| Myristic acid (C14:0) | 0.39b ± 0.022 | 0.55a±0.031 | 0.44ab ± 0.029 | 0.124 |
| Methyl myristic acid | 0.42a±0.023 | 0.14b ± 0.008 | 0.13b ± 0.009 | 0.068 |
| Pentadecylic acid (C15:0) | 0.70a±0.039 | 0.41b ± 0.023 | 0.42b ± 0.028 | 0.138 |
| Palmitic acid (C16:0) | 11.69 ± 0.415 | 12.16 ± 0.553 | 11.33 ± 0.402 | NS |
| Margaric acid (C17:0) | 0.56 ± 0.031 | 0.54 ± 0.030 | 0.62 ± 0.041 | NS |
| Stearic acid (C18:0) | 4.33 ± 0.240 | 4.37 ± 0.243 | 4.89 ± 0.320 | NS |
| Arachidic acid (C20:0) | 1.37b ± 0.076 | 2.08a±0.115 | 2.35a±0.154 | 0.5371 |
| Behenic acid (22:0) | 0.51a±0.028 | 0.46ab ± 0.026 | 0.38b ± 0.025 | 0.119 |
| Monounsaturated fatty acids – MUFAs (%) | ||||
| Palmitoleic acid (C16:1) | 0.42b ± 0.023 | 0.78a±0.043 | 0.81a±0.053 | 0.187 |
| Oleic acid (C18:1) | 16.94 ± 0.432 | 17.05 ± 0.435 | 17.72 ± 0.629 | NS |
| Gondoic acid (C20:1) | 0.65 ± 0.036 | 0.50 ± 0.028 | 0.57 ± 0.037 | NS |
| Polyunsaturated fatty acids – PUFAs (%) | ||||
| Linoleic acid (C18:2) | 36.17 ± 0.922 | 37.06 ± 0.945 | 36.49 ± 0.930 | NS |
| Linolenic acid (C18:3) | 25.17 ± 0.894 | 23.11 ± 0.820 | 23.12 ± 0.821 | NS |
- WP = whey protein, GA = gum Arabic, OM = okra mucilage, PM = psyllium mucilage, NS = not significant.
- Data in the same raw followed by different superscript letters are significantly different at p < 0.05.
From the nutritional point of view, the obtained data showed that fenugreek oil has a unique fatty acids profile, rich in USFAs, especially PUSFAs. Linoleic and linolenic acids have many biological functions and play an important role in the growth reproduction of body systems [84,85]. Also, the role of oleic acid in cardiovascular disease prevention and treatment was stated [86]. Furthermore, it is worth to mention that fatty acids from plants possess antimicrobial activity as reported by Ref. [87]. Linoleic and oleic acids inhibited the growth of Gram-positive B. subtilis, S. aureus and Micrococcus kristinae and linoleic acid also showed activity against B. cereus and B. pumilis [88]. Also, Lee et al. [89] reported the MIC of lineolenic acid on B. cereus and S. aureus was 20 and 50 ppm respectively.
However, PUFAs are sensitive to oxygen and temperature, and thus limits their industrial applications [90]. In this concern, the baking process at 180–240 °C can initiate the thermal decomposition and formation of trans polyunsaturated fatty acid [91]. So, the encapsulation technique could be used to protect poly-unsaturated rich oil and increase its stability during thermal treatment [92,93]. Except pentadecylic, behenic and Methyl myristic acids, which significantly (p < 0.05) decreased in encapsulated oil, the fatty acid profiles of encapsulated oil, were closed to the fatty acid profile of crude oil. Furthermore, palmitoleic and arachidic acids significantly increased in fenugreek oil encapsulated via psyllium and okra mucilage.
3.4. Baking quality of pan bread and biscuits
Baking quality was measured in terms of weight, volume and specific volume for pan bread, and thickness, diameter and spread ratio for biscuits as presented in Table 3. Utilization of both crude and encapsulated fenugreek oil as fat substitutes had no significant effect (p < 0.05) on the weight pan bread loaves. While, both oils significantly affected the volume and specific volume parameters of bread samples. This could be due to the fact that the addition of encapsulated oil to bread formulations dilutes their gluten content and affects the structure of gluten matrix. These effects lead to reduced gas retention ability of the dough during fermentation and reduced oven spring during baking process [[94], [95], [96]]. Furthermore, Beikzadeh et al. [97] studied the effect of psyllium husk on the quality characteristics of sponge cakes and found a decrease in the volume and an increase in cake density. They attributed this result to the high water absorption of psyllium husk through hydrogen bonding interaction between the hydroxyl groups of water and those of polysaccharide molecules.
Table 3.
Baking quality of pan bread and biscuit samples.
| Parameters | Control | Crude oil | Encapsulated oil |
LSD | ||||
|---|---|---|---|---|---|---|---|---|
| WP:GA:OM | WP:GA:PM | |||||||
| Pan bread rowhead | ||||||||
| Weight (g) | 143.00 ± 3.00 | 135.75 ± 0.25 | 144.50 ± 0.50 | 143.25 ± 0.25 | NS | |||
| Volume (cm3) | 476.00a±1.00 | 452.50b ± 2.50 | 324.00c±1.00 | 347.50d ± 2.50 | 7.476 | |||
| Specific volume (cm3/g) | 3.33a±0.06 | 3.33a±0.03 | 2.24c±0.02 | 2.43b ± 0.23 | 0.140 | |||
| Biscuit rowhead | ||||||||
| Thickness (cm) | 0.71b ± 0.01 | 0.81a±0.01 | 0.66c±0.05 | 0.67bc±0.01 | 0.049 | |||
| Diameter (cm) | 4.75a±0.05 | 4.20b ± 0.05 | 4.95a±0.05 | 4.85a±0.05 | 0.259 | |||
| Spread ratio | 6.69b ± 0.02 | 5.19c±0.25 | 7.56a±0.14 | 7.24a±0.03 | 0.417 | |||
- WP = whey protein, GA = gum Arabic, OM = okra mucilage, PM = psyllium mucilage, NS = not significant.
- Data in the same raw followed by different superscript letters are significantly different at p < 0.05.
On the other hand, baking quality parameters of the fat reduced biscuit samples (Table 3) showed different trends. The control biscuit sample had a diameter of 4.75 cm, which significantly reduced to 4.20 cm for biscuit sample containing 2 % crude oil. On contrary, a significant increase in the thickness of this biscuit sample from 0.71 to 0.81 cm was observed. Replacement of fat with encapsulated fenugreek oil via whey protein and mucilage as fat mimics showed significant improvements on these parameters. Also, spread ratio increased with the incorporation of encapsulated oil via okra and psyllium to 7.56 and 7.24 compared to 6.69 and 5.18 for the control biscuit and those containing 2 % crude fenugreek oil, respectively. Similar results were reported by Sudha et al. [61] for fat reduced biscuits with maltodextrin and polydextrose. Since fats are utilized in biscuit production as lubricants to coat the starch and gluten granules for softening the dough and decrease its elastic nature, which is the main responsible for the low thickness and spread ratio of biscuits [98]. The emulsifying activity of encapsulated oil was able to restore the baking quality parameters of fat-reduced biscuits.
3.5. Color attributes of pan bread and biscuits
Color parameters (Lightness (L*), redness (a*), yellowness (b*) and total color differences (ΔE)) of pan bread and biscuit samples are shown in Table 4. Addition of crude fenugreek oil to bread formula significantly increased a*, b* and ΔE parameters, while L* parameter showed insignificant effect compared to the control sample. Yellowness value increased to 31.72 compared to 29.71 for the control sample. This could be due to the presence of yellow pigment in fenugreek oil. Bread samples incorporated with encapsulated oil via okra mucilage showed the highest L* value (67.92) and the lowest a* value (11.20) compared to 48.97 and 17.36 for the control sample, respectively. While, the highest b* (39.25) and a* (20.06) values were recorded for bread sample incorporated with encapsulated oil via psyllium mucilage. On the other hand, fat-reduced biscuits showed significant (p˂ 0.05) lower L* value and higher a* and b* values, in comparison to the control sample.
Table 4.
Color attributes of pan bread and biscuit samples.
| Parameters | Control | Crude oil |
Encapsulated oil |
LSD | ||||
|---|---|---|---|---|---|---|---|---|
| WP:GA:OM | WP:GA:PM | |||||||
| Pan bread rowhead | ||||||||
| Lightness (L*) | 48.97c±0.29 | 48.52c±0.39 | 67.92a±0.29 | 55.58b ± 0.38 | 1.332 | |||
| Redness (a*) | 17.36c±0.11 | 18.43b ± 0.11 | 11.20d ± 0.37 | 20.06a±0.23 | 0.898 | |||
| Yellowness (b*) | 29.71d ± 0.18 | 31.72c±0.12 | 33.75b ± 0.39 | 39.25a±0.16 | 0.930 | |||
| Total color differences (ΔE) | 0.00d | 2.11c±0.13 | 20.60a±0.32 | 11.92b ± 0.30 | 1.130 | |||
| Biscuits rowhead | ||||||||
| Lightness (L*) | 78.26a±0.39 | 68.93b ± 1.47 | 64.49c±0.61 | 69.09b ± 0.83 | 3.596 | |||
| Redness (a*) | 4.49b ± 0.25 | 10.16a±0.61 | 12.39a±0.84 | 10.78a±0.40 | 2.229 | |||
| Yellowness (b*) | 29.25c±0.24 | 33.41b ± 0.30 | 36.27a±0.28 | 35.69a±0.32 | 1.115 | |||
| Total color differences (ΔE) | 0.00c | 11.51b ± 1.38 | 17.16a±0.97 | 12.61b ± 0.95 | 3.78 | |||
- WP = whey protein, GA = gum Arabic, OM = okra mucilage, PM = psyllium mucilage.
- Data in the same raw followed by different superscript letters are significantly different at p < 0.05.
It is well known that the favorable golden brown color of the baked product crusts is mainly due to Maillard and caramelization reactions that take place during baking process [99,100]. The previous results mean that the addition of okra mucilage decreased the favorable color of pan bread. These trends were further indicated through the ΔE values. The color parameters of bread sample containing crude oil (ΔE = 2.11) were close to those of the control sample, followed by psyllium mucilage bread (ΔE = 11.92). While, bread samples incorporated with encapsulated oil via okra mucilage showed the highest ΔE value (20.60). Similar trends were observed when the color parameters of biscuit sample were considered.
3.6. Sensory evaluation of pan bread and biscuits
Overall sensory scores (color, texture, taste, flavor, appearance, and overall acceptability) of pan bread samples are illustrated in Table 5. Pan bread characters, except texture and appearance, were significantly affected by crude and encapsulated fenugreek oil incorporation. Among the tested samples, pan bread containing crude oil gained the lowest scores for taste and flavor parameters. This could be due the effect of bitter taste and flavor of fenugreek oil. The obtained results showed that encapsulated fenugreek oil via both okra and psyllium mucilage could be an alternative for reducing these problems. Taste and flavor parameters significantly improved for bread samples incorporated with encapsulated oil. This finding was previously stated by Fávaro-Trindade et al. [81] and Sihombing and Ananingsih [101] during encapsulation of casein hydrolysate and Curcuma aeruginosavia to attenuate its bitter taste. Also, Munshi and kumar [59] stated that the bitter taste of the fenugreek seed oil would be masked through its encapsulation via maltodextrin and fenugreek mucilage.
Table 5.
Organoleptic properties of pan bread and biscuit samples.
| Parameters | Control | Crude oil |
Encapsulated oil |
LSD |
|||
|---|---|---|---|---|---|---|---|
| WP:GA:OM | WP:GA:PM | ||||||
| Pan bread rowhead | |||||||
| Color (20) | 18.25ab ± 0.63 | 18.50a±0.65 | 18.00ab ± 0.41 | 16.50b ± 0.65 | 1.820 | ||
| Texture (20) | 17.75 ± 1.03 | 18.25 ± 0.85 | 17.00 ± 0.82 | 16.75 ± 0.85 | NS | ||
| Taste (20) | 19.00a±0.41 | 15.75c±0.48 | 17.75b ± 0.25 | 18.00b ± 0.11 | 1.043 | ||
| Flavor (20) | 18.75a±0.49 | 15.25c±0.49 | 17.25b ± 0.48 | 18.00ab ± 0.41 | 1.424 | ||
| Appearance (20) | 18.50 ± 0.65 | 18.25 ± 0.85 | 17.25 ± 1.03 | 17.25 ± 0.63 | NS | ||
| OAC (100) | 92.25a±1.93 | 87.00ab ± 1.58 | 85.75b ± 1.93 | 88.00ab ± 2.48 | 6.187 | ||
| Biscuits rowhead | |||||||
| Color (9) | 9.00a±0.40 | 6.50c±0.50 | 7.50bc±0.50 | 8.00ab ± 0.41 | 1.406 | ||
| Texture (9) | 8.25a±0.25 | 6.50b ± 0.29 | 7.50ab ± 0.65 | 7.50ab ± 0.50 | 1.338 | ||
| Taste (9) | 8.50a±0.29 | 6.25b ± 0.25 | 7.75a±0.63 | 8.50a±0.29 | 1.218 | ||
| Flavor (9) | 8.25a±0.48 | 6.50b ± 0.500 | 7.75ab ± 0.63 | 8.25a±0.500 | 1352 | ||
| Appearance (9) | 8.25a±0.25 | 6.00b ± 0.421 | 7.25a±0.48 | 7.50a±0.28 | 1.134 | ||
| OAC (9) | 8.50a±0.29 | 6.25b ± 0 | 7.00b ± 0.58 | 8.25a±0.22 | 1.133 | ||
- WP = whey protein, GA = gum Arabic, OM = okra mucilage, PM = psyllium mucilage, OAC = overall acceptability, NS = not significant.
- Data in the same raw followed by different superscript letters are significantly different at p < 0.05.
Also, Sensory evaluation of fat-reduced biscuit samples containing crude and encapsulated fenugreek oil is presented in Table 5. Results showed that fat-reduced biscuits containing 2 % crude fenugreek oil gained significant (p˂ 0.05) lower scores for all organoleptic parameters compared to the other samples. This is mainly due to the lack of fat that holds the incorporated air during the creaming process and contributes the crisp or crunchy structure and aroma development of the biscuits [102,103]. On the other hand, there were no significant differences between the organoleptic parameters biscuit samples containing encapsulated fenugreek oil and those of the control biscuits. Regarding these results, encapsulated fenugreek oil presents good fat mimic in the soft dough formulations for biscuits production.
3.7. Microbiological analysis of pan bread during storage period
Table 6 shows the result of microbiological analysis of bread samples during storage period (7d). The results indicated that the control bread samples had the highest significant bacterial and fungal counts during storage period. Crude fenugreek oil showed antibacterial and antifungal activity and significantly (p < 0.05) inhibits the growth of bacteria and fungi. Bread samples incorporated crude fenugreek oil had lower microbial count than the control samples by about one log cycle during storage period. The antimicrobial activity of fenugreek oil may be attributed to their chemical constituents especially their content of fatty acids [104].
Table 6.
Microbiological analysis of pan bread during storage (log CFU/g).
| Storage time (d) | Control | Crude oil |
Encapsulated oil |
LSD | |||
|---|---|---|---|---|---|---|---|
| WP:GA:OM | WP:GA:PM | ||||||
| Total bacterial count rowhead | |||||||
| 0 | 3.13 ± 0.03 | 3.32 ± 0.06 | 2.93 ± 0.23 | 2.95 ± 0.17 | NS | ||
| 3 | 5.17a±0.01 | 4.38b ± 0.04 | 4.11c±0.07 | 3.88d ± 0.03 | 0.162 | ||
| 7 | 6.39a±0.01 | 5.54b ± 0.02 | 4.89c±0.01 | 4.84c±0.02 | 0.062 | ||
| Total fungal count rowhead | |||||||
| 0 | ND | ND | ND | ND | – | ||
| 3 | 5.11a±0.02 | 4.26b ± 0.01 | 4.15c±0.02 | 4.11c±0.15 | 0.066 | ||
| 7 | 6.48a±0.15 | 5.59b ± 0.09 | 5.44c±0.05 | 5.37d ± 0.51 | 0.044 | ||
- WP = whey protein, GA = gum Arabic, OM = okra mucilage, P M = psyllium mucilage, NS = not significant, ND = not detected.
- Data in the same raw followed by different superscript letters are significantly different at p < 0.05.
Encapsulated fenugreek oil also showed antibacterial and antifungal activity more than the activity of crude oil as the bread samples incorporated with encapsulated fenugreek oil had the lowest bacterial and fungal counts with more than one log cycle less than the control samples. It was noteworthy that psyllium microcapsule showed higher antimicrobial activity compared to okra microcapsule. The higher antimicrobial activity of encapsulated oil may be related to the small particle size, high stability and sustained release [26].
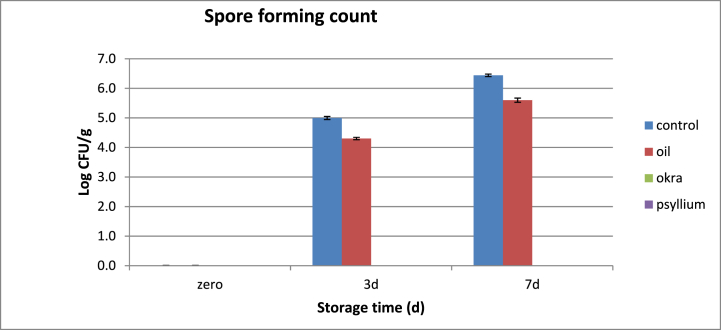
Regarding the spore forming count, the encapsulated fenugreek oil either via okra or psyllium mucilage showed strong activity against spore forming bacteria as it prevent the growth of spore forming bacteria along the storage period (Fig. 2). Mansuri et al. [56] stated the antibacterial activity of fenugreek oil nanoemulsion against Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. The members of Bacillus genus (B. subtilis, B. cereus, and B. licheniformis) are the causative organisms for the ropeness phenomenon, which is a bacterial decomposition of the bread making it unsafe and improper for consumption [105]. Also, Nampuak and Tongkhao [106] showed that okra mucilage exerted strong inhibitory effects against Bacillus cereus, Staphylococcus aureus and Listeria monocytogenes. The antibacterial activity of okra mucilage was attributed to its ability to destroy the cell membranes and cell walls, denature cytoplasmic proteins and cell lysis. However, literatures related to the activity of psyllium mucilage against spore forming bacteria are very scarce.
Fig. 2.
Spore forming bacterial analysis of pan bread during storage.
4. Conclusion
Fenugreek seed oil is functional edible oil rich in polyunsaturated fatty acids and associated with several health benefits, however its bitter taste and low stability are the main drawbacks of it industrial application. Masking the bitter taste through encapsulation via okra or psyllium mucilage as copolymers is an effective way to include these health promoters not only in human nutrition, but also in pharmaceutics. Combination of whey proteins, gum Arabic and okra or psyllium mucilage matrices offer the possibility for high encapsulation efficiency (90.90 and 82.76 %, respectively). Morphological analysis showed integrated microspheres free of holes, cracks, dents, fissures, or collapsing, which indicate better protection for the unsaturated fatty acids in the encapsulated oils. Fenugreek oil encapsulated with mucilage as a copolymer presented novel functional fat replacers. It represents good fat mimics suitable for production of low fat biscuit with acceptable quality. Also, it can be used as natural preservative to improve their nutritional value and increase the shelf-life of bread. Although okra mucilage showed superior properties as co-wall material, bread and biscuit containing psyllium mucilage had more acceptable organoleptic properties and lower microbial count.
Data availability statement
Data generated and utilized for analyses of results presented in this manuscript are available from the corresponding author on reasonable requests.
Additional information
No additional information is available for this paper.
CRediT authorship contribution statement
Ayman A. Mohammad: Writing – review & editing, Writing – original draft, Validation, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation. Fathy M. Mehaya: Writing – original draft, Methodology, Investigation, Data curation. Salah H. Salem: Writing – original draft, Methodology, Formal analysis, Data curation. Heba M. Amer: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors like to thank the National Research Centre (NRC) for support this work through the project no. AR110213.
Contributor Information
Ayman A. Mohammad, Email: aymnmohamed79@yahoo.com.
Heba M. Amer, Email: dr.heba_nrc@yahoo.com.
References
- 1.Aune D., Sen A., Prasad M., Norat T., Janszky I., Tonstad S., Romundstad P., Vatten L.J. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:2156. doi: 10.1136/bmj.i2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs M. A., Petersen K.S., Kris-Etherton P.M. Saturated fatty acids and cardiovascular disease: replacements for saturated fat to reduce cardiovascular risk. Healthcare. 2017;5(2):29. doi: 10.3390/healthcare5020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Froyen E. Risk Factors for Cardiovascular Disease. Intech Open.; 2021. The effects of linoleic acid consumption on lipid risk Markers for cardiovascular disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyseni L., Atkinson M., Bromley H., Orton L., Lloyd-Williams F., McGill R., Capewell S. The effects of policy actions to improve population dietary patterns and prevent diet-related non-communicable diseases: scoping review. Eur. J. Clin. Nutr. 2017;71(6):694–711. doi: 10.1038/ejcn.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization. Regional Office for Europe; 2022. WHO European Regional Obesity Report 2022. [Google Scholar]
- 6.Keenan D.F., Resconi V.C., Smyth T.J., Botinestean C., Lefranc C., Kerry J.P., Hamill R.M. The effect of partial-fat substitutions with encapsulated and unencapsulated fish oils on the technological and eating quality of beef burgers over storage. Meat Sci. 2015;107:75–85. doi: 10.1016/j.meatsci.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Hooper L., Martin N., Jimoh O.F., Kirk C., Foster E., Abdelhamid A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020;(8) doi: 10.1002/14651858.CD011737.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colla K., Costanzo A., Gamlath S. Fat replacers in baked food products. Foods. 2018;7:192. doi: 10.3390/foods7120192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcorta A., Porta A., Tárrega A., Alvarez M.D., Vaquero M.P. Foods for plant-based diets: challenges and innovations. Foods. 2021;10(2):293. doi: 10.3390/foods10020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrow C.J., Nolan C., Holub B.J. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J. Funct.Foods. 2009;1:38–43. [Google Scholar]
- 11.Liu K., Liu Y., Chen F. Effect of storage temperature on lipid oxidation and changes in nutrient contents in peanuts. Food Sci. Nutr. 2019;7(7):2280–2290. doi: 10.1002/fsn3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musakhanian J., Rodier J.D., Dave M. Oxidative stability in lipid formulations: a review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech. 2022;23(5):151. doi: 10.1208/s12249-022-02282-0. [DOI] [PubMed] [Google Scholar]
- 13.Raghavendra S.N., Patricia A., Hampana N.N., Mahalakshmi D. Effect of fats and oils on different properties of flours used in bakery products. J. Nutr. Food Sci. 2022;12(1):1–7. [Google Scholar]
- 14.Peng X., Yao Y. Carbohydrates as fat replacers. Annu. Rev. Food Sci. Technol. 2017;8:331–351. doi: 10.1146/annurev-food-030216-030034. [DOI] [PubMed] [Google Scholar]
- 15.Nourmohammadi N., Austin L., Chen D. Protein-based fat replacers: a focus on fabrication methods and fat-mimic mechanisms. Foods. 2023;12(5):957. doi: 10.3390/foods12050957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva R.C.D., Ferdaus M.J., Foguel A., da Silva T.L.T. Oleogels as a fat substitute in food: a current review. Gels. 2023;9(3):180. doi: 10.3390/gels9030180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Temkov T.M., Mureșan V. Tailoring the structure of lipids, oleogels and fat replacers by different approaches for solving the trans-fat issue—a review. Foods. 2021;10(6):1376. doi: 10.3390/foods10061376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srikanth D., Gopi D., Sunil C.K., Michael K., Rawson A. Proteins as fat replacers in the food industry. Fat Mimetics for Food Applications. 2023;1:33–154. [Google Scholar]
- 19.Ray S., Raychaudhuri U., Chakraborty R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016;13:76–83. [Google Scholar]
- 20.Subramani T., Ganapathyswamy H. An overview of liposomal nano-encapsulation techniques and its applications in food and nutraceutical. J. Food Sci. Technol. 2020;57(10):3545–3555. doi: 10.1007/s13197-020-04360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freitas T.K.F.S., Oliveira V.M., De Souza M.T.F., Geraldino H.C.L., Almeida V.C., L Fávaro S., Garcia J.C. Optimization of coagulation-flocculation process for treatment of industrial textile wastewater using okra (A. esculentus) mucilage as natural coagulant. Ind. Crops Prod. 2015;76:538–544. [Google Scholar]
- 22.Das A., Ringu T., Ghosh S., Pramanik N. A comprehensive review on recent advances in preparation, physicochemical characterization, and bioengineering applications of biopolymers. Polym. Bull. 2022:1–66. doi: 10.1007/s00289-022-04443-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma F., Wang R., Li X., Kang W., Bell A.E., Zhao D., Liu X., Chen W. Physical properties of mucilage polysaccharides from Dioscorea opposita Thunb. Food Chem. 2020;311 doi: 10.1016/j.foodchem.2019.126039. [DOI] [PubMed] [Google Scholar]
- 24.Singh R., Barreca D. Analysis of gums and mucilages. Recent Adv. Nat. Prod. Anal. 2020:663–676. [Google Scholar]
- 25.Tosif M.M., Najda A., Bains A., Kaushik R., Dhull S.B., Chawla P., Walasek-Janusz M. A comprehensive review on plant-derived mucilage: characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers. 2021;13:1066. doi: 10.3390/polym13071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar D., Pandey J., Kumar P., Raj V. Psyllium mucilage and its use in pharmaceutical field: an overview. Curr. Synth. Syst. Biol. 2017;5:2332. [Google Scholar]
- 27.Dybka-Stępień K., Otlewska A., Góźdź P., Piotrowska M. The renaissance of plant mucilage in health promotion and industrial applications: a review. Nutrients. 2021;13(10):3354. doi: 10.3390/nu13103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha P., Ubaidulla U., Nayak A.K. Okra (Hibiscus esculentus) gum-alginate blend mucoadhesive beads for controlled glibenclamide release. Int. J. Biol. Macromol. 2015;72:1069–1075. doi: 10.1016/j.ijbiomac.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Nie X.R., Fu Y., Wu D.T., T Huang T., Jiang Q., Zhao L., Zhang Q., Lin D.R., Chen H., Qin W. Ultrasonic-assisted extraction, structural characterization, chain conformation, and biological activities of a pectic-polysaccharide from okran (Abelmoschus esculentus) Mol. 2020;25:1155. doi: 10.3390/molecules25051155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gemede H.F., Haki G.D., Beyene F., Rakshit S.K., Woldegiorgis A.Z. Indigenous Ethiopian okra (Abelmoschus esculentus) mucilage: a novel ingredient with functional and antioxidant properties. Food Sci. Nutr. 2018;6:563–571. doi: 10.1002/fsn3.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zbikowska A., Kowalska M., Pieniowska J. Assessment of shortcrust biscuits with reduced fat content of microcrystalline cellulose and psyllium as fat replacements. J. Food Process. Preserv. 2018;42 [Google Scholar]
- 32.Kang S., Wang H., Xia L., Chen M., Li L., Cheng J., Li X., Jiang S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020;229 doi: 10.1016/j.carbpol.2019.115402. [DOI] [PubMed] [Google Scholar]
- 33.Zhu X.M., Xu R., Wang H., Chen J.Y., Tu Z.C. Structural properties, bioactivities, and applications of polysaccharides from Okra [Abelmoschus esculentus (L.) Moench]: a review. J. Agric. Food Chem. 2020;68:14091–14103. doi: 10.1021/acs.jafc.0c04475. [DOI] [PubMed] [Google Scholar]
- 34.Dantas T.L., Alonso Buriti F.C., Florentino E.R. Okra (Abelmoschus esculentus L.) as a potential functional food source of mucilage and bioactive compounds with technological applications and health benefits. Plants. 2021;10(8):1683. doi: 10.3390/plants10081683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cakmak H., Ilyasoglu-Buyukkestelli H., Sogut E., Ozyurt V.H., Gumus-Bonacina C.E., Simsek S. A review on recent advances of plant mucilages and their applications in food industry: extraction, functional properties and health benefits. Food. Hydrocoll. Health. 2023 [Google Scholar]
- 36.Al-Jasass F.M., Al-Jasser M.S. The scientific world journal; 2012. Chemical Composition and Fatty Acid Content of Some Spices and Herbs under Saudi Arabia Conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syed Q.A., Rashid Z., Ahmad M.H., Shukat R., Ishaq A., Muhammad N., U Rahman H.U. Nutritional and therapeutic properties of fenugreek (Trigonella foenum-graecum): a review. Int. J. Food Prop. 2020;23(1):1777–1791. [Google Scholar]
- 38.Mariod A.A., editor. Multiple Biological Activities of Unconventional Seed Oils. Academic Press; 2022. [Google Scholar]
- 39.Al-khalisy M.H.H. Treatment of men infertility using low doses of fenugreek oil extract. Adv. Life Sci. Technol. 2015;29 [Google Scholar]
- 40.Gu L.B., Liu X.N., Liu H.M., Pang H.L., Qin G.Y. Extraction of fenugreek (Trigonella foenum-graceum L.) seed oil using subcritical butane: characterization and process optimization. Mol. 2017;22:228. doi: 10.3390/molecules22020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munshi M., Arya P., Kumar P. Physico-chemical analysis and fatty acid profiling of fenugreek (Trigonella Foenum-Graecum) seed oil using different solvents. J. Oleo Sci. 2020;69(11):1349–1358. doi: 10.5650/jos.ess20137. [DOI] [PubMed] [Google Scholar]
- 42.Symoniuk E., Ksibi N., Wroniak M., Lefek, Ratusz K. Oxidative stability analysis of selected oils from unconventional raw materials using rancimat apparatus. Appl. Sci. 2022;12(20) [Google Scholar]
- 43.Sulieman A.M.E., O Ali A., Hemavathy J. Lipid content and fatty acid composition of fenugreek (Trigonella foenum-graecum L.) seeds grown in Sudan. Int. J. Food Sci. Technol. 2008;43(2):380–382. [Google Scholar]
- 44.Yasothai R. Fatty acid composition of fenugreek (Trigonella foenum-graecum L.) seed and Galactomannan depleted fenugreek residue. Pharm. Innov. J. The Pharma. 2021;10(7):1509–1511. [Google Scholar]
- 45.Rajabihashjin M., Zeinalabedini M., Asghari A., Ghaffari M.R., Salekdeh G.H. Impact of environmental variables on yield related traits and bioactive compounds of the Persian fenugreek (Trigonella foenum-graecum L.) populations. Sci. Rep. 2022;12(1):7359. doi: 10.1038/s41598-022-10940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sfar M., Jaouani A., Ben-Mahrez K., Skhiri H.A., Rayana C.B., Chemli R., Slama F.B. The effect of fenugreek on blood glucose, lipid parameters, insulin and resistin in obese women with type 2 diabetes. Hum. J. 2018;11(3):108–123. [Google Scholar]
- 47.Singh U., Chamoli M., Singh K.P., Ram L., Jangir S., Maheshwari R.K. Amazing health benefit of fenugreek (Trigonella foenum-graecum LINN.) Int. J. Environ. Health Sci. 2022;19 [Google Scholar]
- 48.Heshmat‐Ghahdarijani K., Mashayekhiasl N., Amerizadeh A., Teimouri Jervekani Z., Sadeghi M. Effect of fenugreek consumption on serum lipid profile: a systematic review and meta‐analysis. Phytother Res. 2020;34(9):2230–2245. doi: 10.1002/ptr.6690. [DOI] [PubMed] [Google Scholar]
- 49.Bafadam S., Mahmoudabady M., Niazmand S., Rezaee S.A., Soukhtanloo M. Cardioprotective effects of Fenugreek (Trigonella foenum-graceum) seed extract in streptozotocin induced diabetic rats. J. Cardiovasc. Thorac. Res. 2021;13(1):28. doi: 10.34172/jcvtr.2021.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdel-Rahman H., Fathalla S.I., Assayed M.E., Masoad S.R., Nafeaa A.A. Physiological studies on the effect of fenugreek on productive performance of white New-Zealand rabbit does. Food Nutr. Sci. 2016;7:1276. [Google Scholar]
- 51.Hameid A.S.B., Al-Sindi T.A., Allow A.K., Nafie E.M., Alahmad B.E., Faisal G.G. Substantial effect of fenugreek seeds aqueous extract on serum estradiol level in ovarian hyperstimulation syndrome rat model. Oman Med. J. 2019;34(3):238. doi: 10.5001/omj.2019.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khanna A., Thomas J., John F., Maliake B., Krishnakumar I.M. Safety and influence of a novel extract of fenugreek on healthy young women: a randomized, double-blinded, placebo-controlled study. Clin. Phytoscience. 2021;7:1–12. [Google Scholar]
- 53.Al-Tawalbeh D., Bdeir R., Al-Momani J. The use of medicinal herbs to treat male infertility in Jordan: evidence-based review. Int. J. Pharmaceut. Res. Allied Sci. 2023;12(1) [Google Scholar]
- 54.E Sulieman A.M., E Ahmed H., Abdelrahim A.M. The chemical composition of fenugreek (Trigonella foenum-graceum L) and the antimicrobial properties of its seed oil. Gezira J. Eng. Appl. Sci. 2008;3:52–71. [Google Scholar]
- 55.Alenazy R. Antimicrobial activities and biofilm inhibition properties of Trigonella foenum-graecum methanol extracts against multidrug-resistant Staphylococcus aureus and Escherichia coli. Life. 2023;13(3):703. doi: 10.3390/life13030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mansuri A., Chaudhari R., Nasra S., Meghani N., Ranjan S., Kumar A. Development of food-grade antimicrobials of fenugreek oil nanoemulsion—bioactivity and toxicity analysis. Environ. Sci. Pollut. Res. 2023:1–12. doi: 10.1007/s11356-022-19116-y. [DOI] [PubMed] [Google Scholar]
- 57.Yazicioglu N. Sensory analysis of microwave roasted fenugreek and coffee blend. Legume Sci. 2022:e166. [Google Scholar]
- 58.Woolfe M.L., Chaplin M.F., Otchere G. Studies on the mucilages extracted from okra fruits (Hibiscus esculentus L.) and baobab leaves (Adansonia digitata L.) J. Sci. Food Agric. 1977;28:519–529. [Google Scholar]
- 59.Munshi M., Arya P., Kumar P. Physico-chemical analysis and fatty acid profiling of fenugreek (Trigonella foenum-graecum) seed oil using different solvents. J. Oleo Sci. 2020;69(11):1349–1358. doi: 10.5650/jos.ess20137. (M) [DOI] [PubMed] [Google Scholar]
- 60.AACC Approved. 10th ed. American Association of Cereal Chemists, INC. st.; Paul, Minnesota, USA: 2000. Method of the AACC. [Google Scholar]
- 61.Sudha M.L., Srivastava A.K., Vetrimani R., Leelavathi K. Fat replacement in soft dough biscuits: its implications on dough rheology and biscuit quality. J. food engineer. 2007;80:922–930. [Google Scholar]
- 62.American Public Health Association (APHA) first ed. Marvin specked; Washington, DC, USA.: 1976. Compendium Methods for the Microbiological Examination of Foods. [Google Scholar]
- 63.Food and Drug Administration (FDA) ninth ed. AOAC International; Arlington, VA, USA: 2002. Bacteriological Analytical Manual. [Google Scholar]
- 64.Galloway L.D., Burgess R. third ed. Leonard Hill; London: 1952. Applied Mycology and Bacteriology; pp. 54–57. [Google Scholar]
- 65.Frank J.F., Yousef A.E. Am. J. Public Health; Washington, DC: 2004. Thermophilic Bacteria. Standard Methods for the Examination of Dairy Products; pp. 230–231. [Google Scholar]
- 66.Watterson M.J., Kent D.J., Boor K.J., Wiedmann M., Martin N.H. Evaluation of dairy powder products implicates thermophilic sporeformers as the primary organisms of interest. J. Dairy Sci. 2014;97:2487–2497. doi: 10.3168/jds.2013-7363. [DOI] [PubMed] [Google Scholar]
- 67.Mirhosseini H., Amid B.T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res. Int. 2012;46:387–398. [Google Scholar]
- 68.Agrawal R. Dietary Fibers. IntechOpen; 2021. Psyllium: a source of dietary fiber. [Google Scholar]
- 69.Fischer H.M., Nanxiong Y., Ralph R.G.J., Andersond L., Marletta J.A. The gel forming polysaccharide of psyllium husk (P. ovate Forsk) Carbohydr. Res. 2004;339:2009–2017. doi: 10.1016/j.carres.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 70.Raj V., Shim J.J., Lee J. Grafting modification of okra mucilage: recent findings, applications, and future directions. Carbohydr. Polym. 2020;246 doi: 10.1016/j.carbpol.2020.116653. [DOI] [PubMed] [Google Scholar]
- 71.Yuan Q., He Y., Xiang P.Y., Huang Y.J., Cao Z.W., Shen S.W., Zhao L., Zhang Q., Qin W., Wu D.T. Influences of different drying methods on the structural characteristics and multiple bioactivities of polysaccharides from okra (Abelmoschus esculentus) Int. J. Biol. Macromol. 2020;15:1053–1063. doi: 10.1016/j.ijbiomac.2019.10.073. [DOI] [PubMed] [Google Scholar]
- 72.Thaiphanit S., Anprung P. Physicochemical and emulsion properties of edible protein concentrate from coconut (Cocos nucifera L.) processing by-products and the influence of heat treatment. Food Hydrocolloids. 2016;52:756–765. [Google Scholar]
- 73.Zhang Y., Tan C., Abbas S., Eric K., Xia S., Zhang X. Modified SPI improves the emulsion properties and oxidative stability of fish oil microcapsules. Food Hydrocolloids. 2015;51:108–117. [Google Scholar]
- 74.Hsu J.C., U.S. Patent No . 2016. US9498412B2. Method for Producing Microcapsules with a Sun Protection Effect. United States. [Google Scholar]
- 75.Soleimanian Y., Goli S.A.H., Varshosaz J., Sahafi S.M. Formulation and characterization of novel nanostructured lipid carriers made from beeswax, propolis wax and pomegranate seed oil. Food Chem. 2018;244:83–92. doi: 10.1016/j.foodchem.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Tantra R., Schulze P., Quincey P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology. 2010;8(3):279–285. [Google Scholar]
- 77.Jafari S.M., Assadpoor E., He Y., Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008;26(7):816–835. [Google Scholar]
- 78.Chang H.W., Tan T.B., Tan P.Y., Nehdi I.A., Sbihi H.M., Tan C.P. Microencapsulation of fish oil-in-water emulsion using thiol-modified β-lactoglobulin fibrils-chitosan complex. J. Food Eng. 2020;264 doi: 10.1016/j.foodres.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 79.Sulaiman M.G.A.M. Diode laser-induced gum Arabic-G-acrylic acid. MATTER: Int. J. Sci. Technol. 2019;5(3):86–97. [Google Scholar]
- 80.Prieto C., Lagaron J.M. Nanodroplets of docosahexaenoic acid enriched algae oil encapsulated within microparticles ofhydrocolloids by emulsion electrospraying assisted by pressurized gas. Nanomaterials. 2020;10:270. doi: 10.3390/nano10020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fávaro-Trindade C.S., Santana A.D.S., Monterrey-Quintero E.S., Trindade M.A., Netto F.M. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food hydrocoll. 2010;24:336–340. [Google Scholar]
- 82.Su Y.L., Fu Z.Y., Zhang J.Y., Wang W.M., Wang H., Wang Y.C., Zhang Q.J. Microencapsulation of Radix salvia miltiorrhiza nanoparticles by spray-drying. Powder Technol. 2008;184:114–121. [Google Scholar]
- 83.Rocha G.A., Fávaro-Trindade C.S., Grosso C.R.F. Microencapsulation of lycopene by spray drying: characterization, stability and application of microcapsules. Food Bioprod. Process. 2012;90:37–42. [Google Scholar]
- 84.Rai A., Mohanty B., Bhargava R. Supercritical extraction of sunflower oil: a central composite design for extraction variables. Food Chem. 2016;192:647–659. doi: 10.1016/j.foodchem.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 85.Wang L., Wang X., Wang P., Xiao Y., Liu Q. Optimization of supercritical carbon dioxide extraction, physicochemical and cytotoxicity properties of Gynostemma pentaphyllum seed oil: a potential source of conjugated linolenic acids. Sep. Purif. Technol. 2016;159:147–156. [Google Scholar]
- 86.Abdulkarim S., Long K., Lai O., Muhammad S., Ghazali H. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005;93:253–263. [Google Scholar]
- 87.McGaw L.J., Jäger A.K., Van Staden J. Antibacterial effects of fatty acids and related compounds from plants. South Afr. J. Bot. 2002;68:417–423. [Google Scholar]
- 88.Dilika F., Bremner P.D., Meyer J.J.M. Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: a plant used during circumcision rites. Fitoterapia. 2000;71:450–452. doi: 10.1016/s0367-326x(00)00150-7. [DOI] [PubMed] [Google Scholar]
- 89.Lee J.Y., Kim Y.S., Shin D.H. Antimicrobial synergistic effect of linolenic acid and monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002;50:2193–2199. doi: 10.1021/jf011175a. [DOI] [PubMed] [Google Scholar]
- 90.Mishra A., Agarwal M., Bajpai M., Rajani S., Mishra R.P. Plantago psyllium mucilage for sewage and tannery effluent treatment, Iran. Polym. J. 2002;6:381–386. [Google Scholar]
- 91.Li A., Ha Y., Wang F., Li W., Li Q. Determination of thermally induced trans-fatty acids in soybean oil by attenuated total reflectance fourier transform infrared spectroscopy and gas chromatography analysis. J. Agric. Food Chem. 2012;60:10709–10713. doi: 10.1021/jf3033599. [DOI] [PubMed] [Google Scholar]
- 92.Desai K.G.H., Jin Park H. Recent developments in microencapsulation of food ingredients. Dry. Technol. 2005;23:1361–1394. [Google Scholar]
- 93.Kaushik P., Dowling K., Barrow C.J., Adhikari B. Microencapsulation of omega-3 fatty acids: a review of microencapsulation and characterization methods. J. Funct.Foods. 2015;19:868–881. [Google Scholar]
- 94.Zahn S., Pepke F., Rohm H. Effect of inulin as a fat replacer on texture and sensory properties of muffins. Int. J. Food Sci. Technol. 2010;45(12):2531–2537. [Google Scholar]
- 95.Feili R., Zzaman W., Abdullah W.W., Yang T. Physical and sensory analysis of high fiber bread incorporated with jackfruit rind flour. Food Sci. Technol. (N. Y.) 2013;1:30–36. [Google Scholar]
- 96.Kohajdová Z., Karovičová J., Magala M., Kuchtová V. Effect of apple pomace powder addition on farinographic properties of wheat dough and biscuits quality. Chem. Pap. 2014;68:1059–1065. [Google Scholar]
- 97.Beikzadeh S., Peighambardoust S.H., Beikzadeh M., Asghari Javar-Abadi M., Homayouni-Rad A. Effect of psyllium husk on physical, nutritional, sensory, and staling properties of dietary prebiotic sponge cake. Czech J. Food Sci. 2016;34:534–540. [Google Scholar]
- 98.Maache-Rezzoug Z., Bouvier J.M., Allaf K., Patras C. Effect of principal ingredients on rheological behaviour of biscuit dough and on quality of biscuits. J. Food Eng. 1998;35:23–42. [Google Scholar]
- 99.Popov-Raljić J.V., Mastilović J.S., Laličić-Petronijević J.G., Popov V.S. Investigations of bread production with postponed staling applying instrumental measurements of bread crumb color. Sensors. 2009;9:8613–8623. doi: 10.3390/s91108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Purlis E. Browning development in bakery products–A review. J. Food Eng. 2010;99(3):239–249. [Google Scholar]
- 101.Sihombing M., Ananingsih V.K. Encapsulated Curcuma aeruginosa: inhibition method of bitter receptor cells from the perspective of wall formation. Indonesian J. Agric. Res. 2018;1(2):172–178. [Google Scholar]
- 102.Given P.S. Chaman and Hall; New York: 1994. Influence of Fat and Oil–Physicochemical Properties on Cookie and Cracker Manufacture, the Science of Cookie and Cracker Production. [Google Scholar]
- 103.Lucca P.A., Tepper B.J. Fat replacers and the functionality of fat in foods. Trends Food Sci. Technol. 1994;5:12–19. [Google Scholar]
- 104.Chandrasekaran M., Kannathasan K., Venkatesalu V. Antimicrobial activity of fatty acid methyl esters of some members of Chenopodiaceae. Z. Naturforsch., C: J. Biosci. 2008;63(5–6):331–336. doi: 10.1515/znc-2008-5-604. [DOI] [PubMed] [Google Scholar]
- 105.Pacher N., Burtscher J., Johler, Etter S.D., Bender D., Fieseler L., Domig K.J. Ropiness in bread—a re-emerging spoilage phenomenon. Foods. 2022;11:3021. doi: 10.3390/foods11193021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nampuak C., Tongkhao K. Okra mucilage powder: a novel functional ingredient with antioxidant activity and antibacterial mode of action revealed by scanning and transmission electron microscopy. Int. J. Food Sci. Technol. 2020;55:569–577. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated and utilized for analyses of results presented in this manuscript are available from the corresponding author on reasonable requests.