Abstract
This study aimed to investigate the modeling of antimicrobial activity (AA) of nisin and sorbate on Clostridium sporogenes in jar cream cheese (JCC) using the linear regression (LR), multilayer perceptron (MLP) neural network, and reduced error pruning tree (REPTree) methods, in order to prevent the late blowing defect (LBD) in the cheese. Both preservatives used in JCC samples showed AA against C. sporogenes; so that sorbate at all the concentrations used in JCC samples inhibited cracking spoilage during storage period at 35 °C. However, nisin could not inhibit cracking spoilage at concentration of 30 ppm in the samples, and a higher concentration of it was needed. The three models used in this study, followed the similar pattern in both training and validation datasets for nisin and sorbat in JCC. The R2 and root mean square error (RMSE) values of training and validation datasets showed the superiority of the REPTree model compared to the MLP and LR models (conventional methods) in the modeling of AA of nisin and sorbate against C. sporogenes in JCC.
Keywords: Cheese, Clostridium sporogenes, Late blowing defect, Nisin, Sorbate, Modeling
1. Introduction
Dairy industrial factories are looking to increase daily milk reception due to financial aspects. In addition to farming and purchase aspects, the production of dairy products with high shelf-life and variety is important. Among different dairy products, cheese plays a critical role in the dairy field, from an industrial aspect, because of its extended shelf-life and great variety, which leads to high daily milk reception [1]. In general, cheeses can be divided into two categories industrially: processed and unprocessed cheeses. The process is completed by producing the cheese with a different flavor and texture properties and sometimes with a long shelf-life [2]. Cream cheese (CC) is one type of processed cheeses that is very popular, particularly among children. Due to the high-fat content, it can provide a significant amount of energy needed for children. CC is a soft and unripen cheese with a creamy texture and acidic or diacetyl flavor. Generally, CC is produced by the coagulation of cream through acidification with a mesophilic starter culture [3].
Jar cream cheese (JCC) is one type of CCs, which has different production conditions and spreadable texture. JCC is very popular among consumers, especially in the Middle East countries such as Iran, Iraq, and surrounding countries. This popularity may be due to its especial texture, flavor, shelf-life and packaging. All the mentioned properties are depended on the type of production process. For example, the JCC is produced by direct steam contact (steam at 120 °C for 3–5 s) and its special desirable taste is attributed to Millard reaction. The shelf-life is also increased up to 6 months. The shelf-life may be extended by some preservatives [3]. The shelf-life of cheese is important for consumers due to maintaining food safety and security. On the other hand, this subject is also very important for producers to increase the shelf-life of foods on shop shelves and market scope development. The shelf-life of cheese is limited by various factors, including mechanical, chemical, and microbial agents [4]. Unwanted microbial growth in cheeses may lead to spoilage or poising because cheese ingredients and environment may provide a rather suitable medium for spoiling and pathogenic microorganisms, leading to risks to consumer's health. Therefore, microbial growth inhibition in cheeses after production is critical in the dairy industry [5].
Generally, processed cheeses are more susceptible to spoilage that may develop during storage. Late blowing defect (LBD) is a common cause of spoilage in high-pH hard and semi-hard cheeses, which leads to defects in appearance, texture, and flavor. Anaerobic, endospore-forming Gram-positive, gas-producing bacteria are the main microorganisms responsible for the LBD. Spores of these bacteria germinate into vegetative types when suitable growth conditions are provided in the cheese; in this condition, different metabolites are produced through the lactate fermentation, including butyric acid, hydrogen, and carbon dioxide. This produced gas leads to formation of holes and cracks in the cheese, in addition to undesirable odor and flavor [5,6]. Butyric acid fermentation is recognized as the main reason for LBD in high-pH cheeses. The fermentation can be done by Clostridium tyrobutyricum and other Clostridium species, particularly C. butyricum, C. sporogenes, and C. Beijerinckii [7]. The microbial population in the milk used to made processed cheese plays an important role in this spoilage. Hence, bactofugation is recommended to remove bacteria and bacterial spores from contaminated milk before cheese manufacturing in the dairy industry. However, the performance of this process is not perfect; actually, it is relative, and as a result, some spores in the contaminated milk pass through the device [8]. Therefore, the cheese industry has to use some preservatives to inhibit LBD in cheese, such as nitrate [9], sorbate [10], hexamethylenetetramine [5], polyphosphate [4] as the common chemical additives, and nisin [7,11] and lysozyme [12] as biopreservatives. Among these preservatives, nisin and sorbate are the most used types in the cheese industry.
In addition to using extreme thermal conditions, there are several methods to extend the shelf-life of dairy products; for example, natural antimicrobials including essential oils, lactoferrin, lysozyme, lactoperoxidase system, fatty acids and related compounds. Biopreservation means to extend the shelf life of foods by microorganisms and/or their metabolites [13]. Besides, there are non-thermal approaches including high hydrostatic pressure processing [14], high homogenization pressure, pulsed electric fields, high power ultrasound, and irradiation [15].
Nowadays, the importance of applying mathematical modeling and computing in food microbiology has been acknowledged. There are several types of studies on modeling the kinetic of microbial inactivation during thermal and non-thermal food processing. Empirical models (Baranyi and Gompertz) and polynomial models (artificial neural networks, genetic algorithm–artificial neural network, and adaptive neuro-fuzzy inference system) are the major types used to model microbial inactivation [[16], [17], [18]]. However, the use of new models to study the kinetic of non-thermal microbial inactivation and comparing their performance with conventional models are important. To our knowledge, there is no study in the literature concerning the use of computing technology to predict the LBD and the activity of nisin and sorbate in JCC. Hence, the aim of this study was to investigate the effect of nisin and sorbate concentrations on the LBD of JCC using the linear regression (LR), multilayer perceptron neural network (MLP), and reduced error pruning tree (REPTree) methods. The research hypothesis of this study was to investigate the antimicrobial effects of nisin and sorbate on C. sporogenes in JCC, and to determine the optimal concentrations and combinations of these preservatives to prevent cracking spoilage.
2. Material and methods
Culture media and solvents used in this study were all analytical grade and purchased from Merck, Germany. C. sporogenes (PTCC 1651, ATCC 19404) was prepared from Persian Type Culture Collection (PTCC), Iranian Research Organization for Science and Technology (IROST), Tehran, Iran.
2.1. Preparation of microbial suspension
C. sporogenes was inoculated on Reinforced Clostridial Agar (RCA, Scharlau Chemie, Barcelona, Spain) and incubated at 37 °C for 24 h in anaerobic conditions (anaerobic jar 2.5 L-volume, Anaerocult® A Merck Millipore, Germany) to prepare a single colony before storage at 4 °C. The obtained colony was inoculated into Reinforced Clostridial Broth (TSB, Scharlau Chemie, Barcelona, Spain) and incubated according to the above conditions. The working microbial suspension was obtained from this subculture and adjusted at a concentration of 1 × 108 CFU/mL in ringer solution using 0.5 McFarland standard.
2.2. Preparation of cheese samples
JCC was produced through direct steam contact (steaming at 120 °C for 3–5 s) at the Golestan Sabah Dairy Co. in Gonbad Kavoos, Iran. Preservative-free JCC mixture was prepared from the mentioned company and transferred to the microbial laboratory to add sorbate and nisin in clean conditions and during mixing through Thermomixer Blender (TM6, Vorwerk, Germany) at 85 °C. The bacterial suspension (concentration = 1 × 108 CFU/g) was inoculated to each sample. As mentioned in the introduction section, the shelf-life of JCC in ambient temperatures conditions (outside the refrigerator) and even relatively warm environments is important and targeted; hence, the produced samples were stored at 35 °C for 15 days.
2.3. Evaluation of antimicrobial activity
Briefly, 10 g of JCC sample was dissolved in 90 mL peptone water and serial dilutions (10-fold) were prepared from the suspension. Next, 1 mL aliquot from each dilution was pour plated in RCA. After incubation at 37 °C for 48 h under anaerobic conditions, the grown colonies were counted and expressed as log CFU/g. Each JCC sample was plated in triplicate. The survival curve of C. sporogenes for each treatment was plotted [19].
2.4. Moisture content
The moisture content of JCC samples was measured according to AOAC [20]. Briefly, dishes were dried in an oven for 30 min. Each dish was weighed and 3 g JCC sample was put on the dish, and the total weight was measured. The dishes were placed in an oven at 105 °C for 6 h. Afterward, the dishes were cooled down in a desiccator. The cooled dishes were weighed, and the weight loss and finally the moisture content was calculated [20].
2.5. pH variations
The pH of JCC samples was measured by a pH meter (Benchtop pH meters Seven Compact™ S220 Basic, Mettler Toledo). The measurements were carried out in triplicate [19].
2.6. Experimental design
Thirty treatments under full factorial experimental design were employed to study the inhibitory effect of potassium sorbate (0, 250, 500, and 1000 ppm) and nisin (0, 30, 60, and 120 ppm) on C. sporogenes growth in JCC. The upper levels of the used preservative were the highest legal allowed amount. Bacterial enumeration was completed 15 days after production. Duncan's multiple range test was used to determine the significant differences among means, and p-value <0.05 was considered statistically significant. All experiments were performed in triplicate.
2.7. Machine learning modeling
To predict the antimicrobial activity (AA) of nisin and sorbate in JCC, we used three machine-learning methods: LR, MLP, and REPTree. These techniques were chosen because they are suitable for modeling and predicting continuous outcomes, such as the growth rate and lag phase of C. Sporogenes. Moreover, these techniques have been widely used and validated in previous studies on different fields [[21], [22], [23], [24]]. Brief descriptions of these methods are as follows:
LR is a statistical technique that estimates the prediction target using the linear relationship between one independent variable and one dependent variable. LR is the most commonly used estimation method in many fields of science because it has a simple structure that is easy to use and interpret. Artificial neural networks (ANNs) that are among the most powerful and flexible machine learning methods that consist of several processing layers, including the input layer, one or several hidden layer(s), and the output layer. MLP is one of the most common and conventional feed-forward ANNs with a straightforward structure. In a MLP, the input layer receives the independent variables, whereas the values for the output layer are generated based on the dependent (i.e., target) values of the problem being modeled. MLP is a robust classifier in pattern classification problems. MLP is a distributed approach that its output is generated via linear combinations of the outputs of the hidden layer(s) [25]. REPTree is a decision tree classifier that utilizes the principle of calculating information gain with entropy and error minimization of variance [26] for dealing with classification and prediction problems. Using the regression tree approach, REPTree generates several trees in various iterations. Then, it selects the best tree from all the ones generated. This decreases the complexity of the modeling process, particularly for large datasets. The REPTree's pruning mechanism utilizes the backward over-fitting problem based on the post-pruning method to obtain the minimal type of most-accurate tree. The performance of REPTree greatly depends on decreased variance, reduced error pruning techniques, and information gained from entropy [26].
The dataset used in this study consists of 90 samples of JCC, which were inoculated with C. Sporogenes and treated with different levels of nisin and sorbate. The dataset was randomly split into 70 % training and 30 % validation subsets, ensuring that the distribution of the variables was similar in both subsets. For model development, day (1, 2, 3, …, and 15), nisin (0, 30, 60, and 120 ppm), and sorbate (0, 250, 500, and 1000 ppm) concentrations were considered as independent variables. The models were developed using the Weka software ver. 3.8 on a personal computer with a Pentium(R) Dual-Core CPU E5700 @ 3.00 GHz, 4 GB of RAM, a 64-bitoperating system, and the Microsoft Windows 7. For model validation, the coefficient of determination (R2) and root mean squared error (RMSE) were used [27]. These metrics are defined by Eqs (1), (2) [28]:
| (1) |
| (2) |
where Oi are Pi the observed and predicted values, respectively. and are observed and predicted mean values.
3. Results and discussion
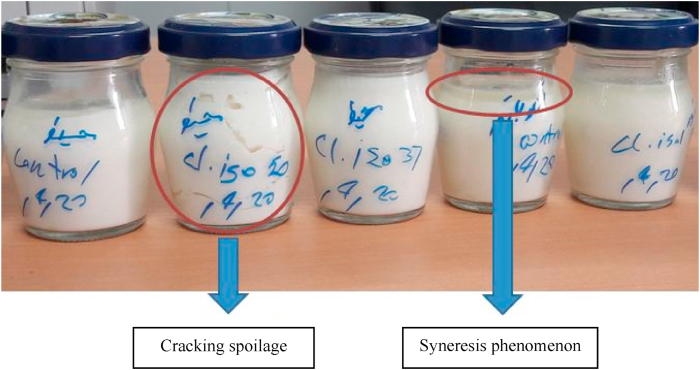
Moisture content and pH value were considered the same in all the JCC samples; these indices were measured at 64 % and 5.5, respectively. Fig. 1 shows the cracking spoilage in JCC in different steps. This spoilage is one type of LBD, which started with the changes in pH value (decreasing in initial steps) then syneresis, and followed by the production of gas and emerging crack, as shown in Fig. 1.
Fig. 1.
The cracking spoilage in jar cream cheese samples.
3.1. Effect of sorbate on C. sporogenes
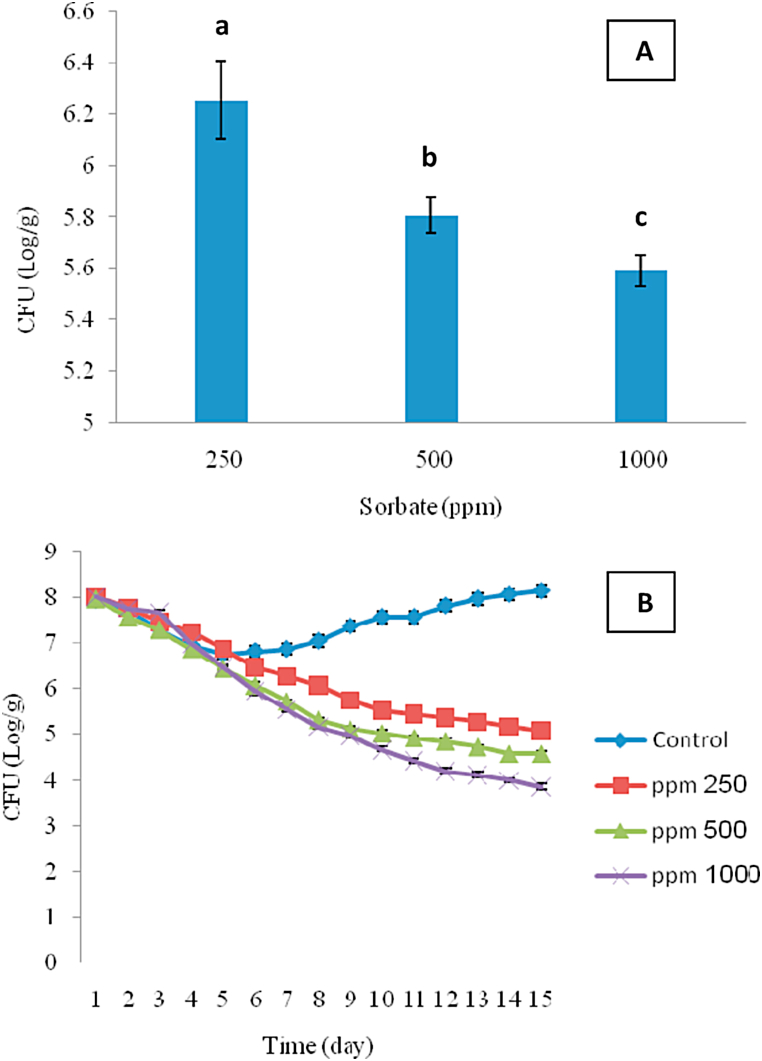
As shown in Fig. 2A, high sorbate concentration (1000 ppm) had significant AA against C. sporogenes (p-value <0.05) by showing a decrease in bacterial count (from 8 to 5.59 CFU/g). The AA of sorbate has been reported in different cheeses [19,29,30], apple juice [31], edible starch films [32], bakery products [33], and fruit juices and purees [34]. The AA mechanisms of sorbate are attributed to the interfering activity on the fatty acid oxidation by feedback inhibition of dehydrogenase enzymes, the interfering activity on the sulfhydryl-containing enzymes in microorganisms, the inhibiting activity on catalase that would cause an increase in toxic hydrogen peroxide within the cell, and the detaching of oxidative phosphorylation by inhibition of enzymes, e.g., sulfhydryl-containing enzymes [35]. Khanipour et al. [6] studied the effects of sodium chloride, potassium sorbate, nisin and lysozyme on the growth probability of C. sporogenes and reported that salt and sorbate were the most effective factors in preventing the growth of C. sporogenes in high-moisture (>95 %) and low-acid conditions. The results showed that salt acts synergistically with sorbate against clostridia, in agreement with the results of Thomas [36] who studied AA of sorbate against C. botulinum in meat slurries.
Fig. 2.
The antimicrobial activity of sorbate on C. sporogenes in jar cream cheese (A) and its effect on the fate of C. sporogenes during storage period at 35 °C (B).
As shown in Fig. 2B, in the control sample, C. sporogenes count was decreased in the initial days of the storage period; this decreasing is probably due to thermal damage of bacteria. However, in the next days of the storage period, C. sporogenes count of the control sample was increased. The increasing microbial population after decreasing could be attributed to the compatibility of microorganisms to the conditions. Unlike the control sample, there was no increase of microbial population in the JCC containing sorbate, so that the lowest C. sporogenes count (3.85 CFU/g) was observed in the JCC sample containing 1000 ppm sorbate after 15 days (Fig. 2B).
3.2. Effect of nisin on C. sporogenes
Fig. 3A shows the AA of nisin against C. sporogenes. As shown, C. sporogenes population decreased (from 8 to 5.98 CFU/g) significantly (p-value <0.05) at higher nisin concentrations (120 ppm). Sharaf et al. [37] evaluated some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. They reported that Lactobacillus helveticus cell-free supernatant; however, displayed lower AA compared with nisin and natamycin, it had both antibacterial and antifungal promising activities. Ávila et al. [38] studied the inhibitory activity of reuterin, nisin, lysozyme and nitrite against vegetative cells and spores of dairy-related Clostridium species. Their results suggested that reuterin and nisin, with a broad inhibitory activity spectrum against Clostridium spp. spores and vegetative cells, might be the best options to control Clostridium growth in dairy products and to prevent associated spoilage, such as LBD of cheese. The AA of nisin has been studied in different types of cheeses [39,40]. The AA mechanisms of nisin are attributed to generating pores in the cell membrane that through leakage of ions and hydrolysis of ATP, results in cell death and interfering cell-wall biosynthesis by specific lipid II interaction [41].
Fig. 3.
The antimicrobial activity of nisin on C. sporogenes in jar cream cheese (A) and its effect on the fate of C. sporogenes during storage period at 35 °C (B).
According to Fig. 3B, C. sporogenes population increased in the JCC containing 30 ppm nisin after an initial decrease in the population, similar to the control sample. However, this was not observed in other samples, which is probably due to the incompatibility of C. sporogenes to these conditions. Indeed, 60 and 120 ppm of nisin showed the bactericidal effect on C. sporogenes in JCC at the final stages of the storage period, revealing that the adaptation of C. sporogenes is dependent on the nisin concentration. This effect was observed for sorbate in all the concentrations used in JCC, as mentioned in the previous section. Antimicrobial resistance of bacteria caused by adaptation is reported by several researchers which observed this issue for Pseudomonas aeruginosa, against siderophore-conjugated antibacterial agents [42], Staphylococcus aureus, against antibacterial agents [43], and S. aureus, against non-antibiotic antibacterial agents (physical stressors, nano-particles, and bacteriophages) [44].
3.3. Modeling results
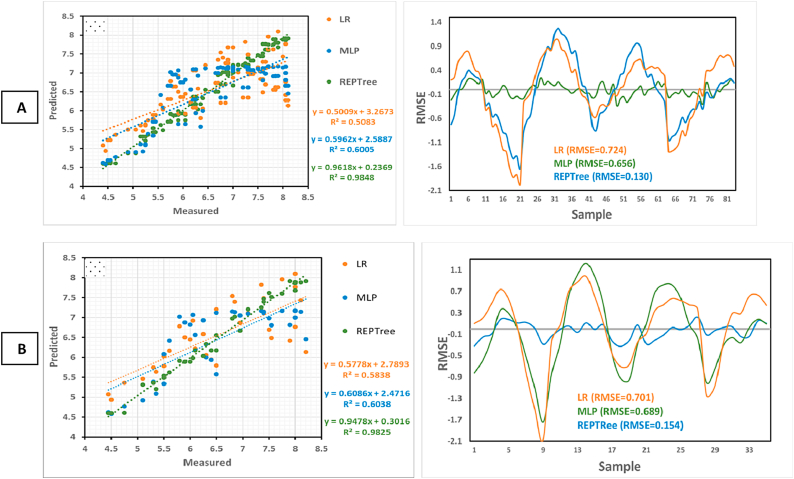
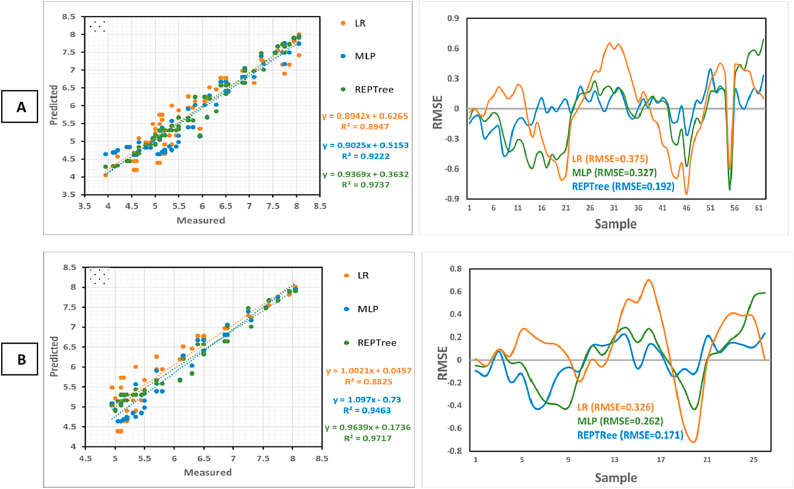
The outcomes of the three models used for the prediction of the LBD preventing activity of nisin and sorbate in JCC are shown in Fig. 4, Fig. 5. The results suggested that the REPTree model is more estimative than the MLP and LR models for both training and validation datasets of nisin. REPTree predicted nisin activity with R2 = 0.98 and RMSE = 0.13 for the training dataset (Fig. 4A) and with R2 = 0.98 and RMSE = 0.15 for the validation dataset (Fig. 4B), which successfully outperformed the other two models. The MLP and LR models with R2 values much lower and RMSE greater than the REPTree were identified as the low-performing models in both training and validation datasets. Similarly, for the sorbate datasets, the R2 values of both training and validation datasets (R2 = 0.97) based on the REPTree model were higher than that of the MLP and LR, indicating that the REPTree model could explain 97 % of the total variability of sorbate (Fig. 5A–B). The results of RMSE for the training dataset (0.192) and validation dataset (0.171) further demonstrated the superiority of the REPTree model over the MLP and LR models.
Fig. 4.
Prediction of nisin activity in jar cream cheese using the training (A) and the validation (B).
Fig. 5.
Prediction of sorbate activity in jar cream cheese using the training (A) and the validation (B).
Overall, the modeling results showed that the three models followed the same pattern in both training and validation datasets for nisin and sorbate. The models achieved higher R2 and lower RMSE in the training datasets compared to the validation datasets. We were expecting this pattern given the previous works reported for various machine-learning modeling projects [45,46]. Since the models are fed by a greater number of samples during the training phase of the modeling, they can better explore the pattern hidden in data and achieve higher values of R2 with lower error rates [[47], [48], [49]]. The advanced REPTree model is resistant to overfitting and insensitive to noise and unbiased error compared to conventional methods, such as MLP and LR [26].
4. Conclusion
Both the preservatives (nisin and sorbate) used in JCC samples showed AA against C. sporogenes. Sorbate at all the concentrations used in JCC samples inhibited cracking spoilage during the 15-day storage period at 35 °C. However, nisin could not inhibit cracking spoilage at concentration of 30 ppm in the samples, so higher levels of nisin were needed (60 and 120 ppm). The three models used in this study, followed a similar pattern in both training and validation datasets for nisin and sorbate. The R2 and RMSE values of training and validation datasets showed the superiority of the REPTree model compared to the MLP and LR models in predicting the AA of nisin and sorbate against C. sporogenes in JCC.
Data availability statement
The data that has been used is confidential.
CRediT authorship contribution statement
Mahmoud Yolmeh: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Seid Mahdi Jafari: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Last author acknowledges the Chinese Ministry of Science and Technology “Belt and Road” Innovative Talent Exchange Foreign Expert Project (Grant Number DL2023003001L).
Contributor Information
Mahmoud Yolmeh, Email: mahmoudyolmeh@gmail.com.
Seid Mahdi Jafari, Email: smjafari@gau.ac.ir.
References
- 1.Jalilzadeh A., Tunçtürk Y., Hesari J. Extension shelf life of cheese: a review. Int. J. Dairy Sci. 2015;10:44–60. doi: 10.3923/ijds.2015.44.60. [DOI] [Google Scholar]
- 2.Kapoor R., Metzger L.E. Process cheese: scientific and technological aspects—a review. Compr. Rev. Food Sci. Food Saf. 2008;7:194–214. doi: 10.1111/j.1541-4337.2008.00040.x. [DOI] [Google Scholar]
- 3.Pombo A.F.W. Cream cheese: historical, manufacturing, and physico-chemical aspects. Int. Dairy J. 2021;117 doi: 10.1016/j.idairyj.2020.104948. [DOI] [Google Scholar]
- 4.Varga L. Use of a long-chain polyphosphate mixture for shelf-life extension of processed cheese spreads. Acta Aliment. 2005;34:493–498. doi: 10.1556/aalim.34.2005.4.16. [DOI] [Google Scholar]
- 5.Oliveira R.B., Margalho L.P., Nascimento J.S., Costa L.E., Portela J.B., Cruz A.G., Sant'Ana A.S. Processed cheese contamination by spore-forming bacteria: a review of sources, routes, fate during processing and control. Trends Food Sci. Technol. 2016;57:11–19. doi: 10.1016/j.tifs.2016.09.008. [DOI] [Google Scholar]
- 6.Khanipour E., Flint S.H., McCarthy O.J., Golding M., Palmer J., Tamplin M. Evaluation of the effects of sodium chloride, potassium sorbate, nisin and lysozyme on the probability of growth of Clostridium sporogenes. Int. J. Food Sci. Technol. 2014;49:1506–1512. doi: 10.1111/ijfs.12446. [DOI] [Google Scholar]
- 7.Ávila M., Gómez‐Torres N., Gaya P., Garde S. Effect of a nisin‐producing lactococcal starter on the late blowing defect of cheese caused by Clostridium tyrobutyricum. Int. J. Food Sci. Technol. 2020;55:3343–3349. doi: 10.1111/ijfs.14598. [DOI] [Google Scholar]
- 8.Júnior J.C.R., Peruzi G.A., Bruzaroski S.R., Tamanini R., Lobo C.M., Alexandrino B., Conti A.C.M., Alfieri A.A., Beloti V. Effect of bactofugation of raw milk on counts and microbial diversity of psychrotrophs. J. Dairy Sci. 2019;102:7794–7799. doi: 10.3168/jds.2018-16148. [DOI] [PubMed] [Google Scholar]
- 9.Genualdi S., Jeong N., DeJager L. Determination of endogenous concentrations of nitrites and nitrates in different types of cheese in the United States: method development and validation using ion chromatography. Food Addit. Contam. 2018;35:615–623. doi: 10.1080/19440049.2018.1426888. [DOI] [PubMed] [Google Scholar]
- 10.Baldissera A.C., De Dea Lindner J., Motta G.E., dos Santos N.N.O., Galvao A.C., Robazza W.D.S. Evaluation of the combined effect of temperature and potassium sorbate on physicochemical and microbial quality of modified atmosphere packaged sliced Mozzarella cheese. J. Food Process. Preserv. 2021;45 doi: 10.1111/jfpp.15136. [DOI] [Google Scholar]
- 11.Hassan H., St-Gelais D., Gomaa A., Fliss I. Impact of nisin and nisin-producing Lactococcus lactis ssp. lactis on Clostridium tyrobutyricum and bacterial ecosystem of cheese matrices. Foods. 2021;10:898. doi: 10.3390/foods10040898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soggiu A., Piras C., Mortera S.L., Alloggio I., Urbani A., Bonizzi L., Roncada P. Unravelling the effect of clostridia spores and lysozyme on microbiota dynamics in Grana Padano cheese: a metaproteomics approach. J. proteomics. 2016;147:21–27. doi: 10.1016/j.jprot.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 13.Yolmeh M., Khomeiri M., Ghaemi E. High-efficiency anti-enterotoxigenic activity of Lactobacillus on staphylococcal enterotoxins biosynthesis. Food Sci. Technol. 2020;125 doi: 10.1016/j.lwt.2020.109266. [DOI] [Google Scholar]
- 14.Evert-Arriagada K., Hernández-Herrero M.M., Guamis B., Trujillo A.J. Commercial application of high-pressure processing for increasing starter-free fresh cheese shelf-life. Food Sci. Technol. 2014;55:498–505. doi: 10.1016/j.lwt.2013.10.030. [DOI] [Google Scholar]
- 15.Meng W., Yang Y., Zhang R., Wu Z., Xiao X. Triboelectric-electromagnetic hybrid generator based self-powered flexible wireless sensing for food monitoring. Chem. Eng. J. 2023;473 doi: 10.1016/j.cej.2023.145465. [DOI] [Google Scholar]
- 16.Soleimanzadeh B., Amoozandeh A., Shoferpour M., Yolmeh M. New approaches to modeling Staphylococcus aureus inactivation by ultrasound. Ann. Microbiol. 2018;68:313–319. doi: 10.1007/s13213-015-1067-4. [DOI] [Google Scholar]
- 17.Wang W., Xu J., Zhang W., Glamuzina B., Zhang X. Optimization and validation of the knowledge-based traceability system for quality control in fish waterless live transportation. Food Control. 2021;122 doi: 10.1016/j.foodcont.2020.107809. [DOI] [Google Scholar]
- 18.Zhang H., Zuo X., Sun B., Wei B., Fu J., Xiao X. Fuzzy-PID-Based atmosphere packaging gas distribution system for fresh food. Appl. Sci. 2023;13:2674. doi: 10.3390/app13042674. [DOI] [Google Scholar]
- 19.Khanipour E., Flint S.H., McCarthy O.J., Palmer J., Golding M., Ratkowsky D.A., Ross T., Tamplin M. Modelling the combined effect of salt, sorbic acid and nisin on the probability of growth of Clostridium sporogenes in high moisture processed cheese analogue. Int. Dairy J. 2016;57:62–71. doi: 10.1016/j.idairyj.2016.02.039. [DOI] [Google Scholar]
- 20.AOAC . 1980. Official Method of Analysis of the Association of Official Analytical chemistS (Washington, DC, USA. [Google Scholar]
- 21.Jaafari A., Pazhouhan I., Bettinger P. Machine learning modeling of forest road construction costs. Forests. 2021;12:1169. doi: 10.3390/f12091169. [DOI] [Google Scholar]
- 22.Luo J., Wang G., Li G., Pesce G. Transport infrastructure connectivity and conflict resolution: a machine learning analysis. Neural Comput. Appl. 2022;34:6585–6601. doi: 10.1007/s00521-021-06015-5. [DOI] [Google Scholar]
- 23.Luo J., Wang Y., Li G. The innovation effect of administrative hierarchy on intercity connection: the machine learning of twin cities. J. Innov. Knowl. 2023;8 doi: 10.1016/j.jik.2022.100293. [DOI] [Google Scholar]
- 24.Yin S., Shan Y., Gao B., Tang S., Han X., Zhang G., Yu B., Guan S. Characterizing and predicting smoldering temperature variations based on non-linear mixed effects models. J. For. Res. 2022:1829–1839. doi: 10.1007/s11676-022-01463-8. [DOI] [Google Scholar]
- 25.Soleimanzadeh B., Hemati L., Yolmeh M., Salehi F. GA‐ANN and ANFIS Models and Salmonella Enteritidis inactivation by ultrasound. J. Food Saf. 2015;35:220–226. doi: 10.1111/jfs.12174. [DOI] [Google Scholar]
- 26.Pham B.T., Jaafari A., Nguyen-Thoi T., Van Phong T., Nguyen H.D., Satyam N., Masroor M., Rehman S., Sajjad H., Sahana M., Le H.V., Prakash I. Ensemble machine learning models based on Reduced Error Pruning Tree for prediction of rainfall-induced landslides. Int. J. Digit. Earth. 2021;14:575–596. doi: 10.1080/17538947.2020.1860145. [DOI] [Google Scholar]
- 27.Yolmeh M., Khomeiri M. Using physical and chemical mutagens for enhanced carotenoid production from Rhodotorula glutinis (PTCC 5256) Biocatal. Agric. Biotechnol. 2016;8:158–166. doi: 10.1016/j.bcab.2016.09.004. [DOI] [Google Scholar]
- 28.Luo Z., Wang H., Li S. Prediction of international roughness index based on stacking fusion model. Sustain. Times. 2022;14:6949. doi: 10.3390/su14126949. [DOI] [Google Scholar]
- 29.Alrabadi N.I., Motasem A.M., Gharaibeh A.A. The antifungal effect of potassium sorbate on Penicillium sp in Labaneh. Am.-Eurasian J. Agric. Environ. Sci. 2013;13:1497–1502. doi: 10.5829/idosi.aejaes.2013.13.11.81265. [DOI] [Google Scholar]
- 30.Marín P., Ginés C., Kochaki P., Jurado M. Effects of water activity on the performance of potassium sorbate and natamycin as preservatives against cheese spoilage moulds. Ir. J. Agric. Food Res. 2017;56:85–92. [Google Scholar]
- 31.Ceylan E., Fung D.Y., Sabah J.R. Antimicrobial activity and synergistic effect of cinnamon with sodium benzoate or potassium sorbate in controlling Escherichia coli O157: H7 in apple juice. J. Food Sci. 2004;69:102–106. doi: 10.1111/j.1365-2621.2004.tb06348.x. [DOI] [Google Scholar]
- 32.Flores S., Haedo A.S., Campos C., Gerschenson L. Antimicrobial performance of potassium sorbate supported in tapioca starch edible films. Eur. Food Res. Technol. 2007;225:375–384. doi: 10.1007/s00217-006-0427-5. [DOI] [Google Scholar]
- 33.Guynot M.E., Ramos A.J., Sanchis V., Marín S. Study of benzoate, propionate, and sorbate salts as mould spoilage inhibitors on intermediate moisture bakery products of low pH (4.5–5.5) Int. J. Food Microbiol. 2005;101:161–168. doi: 10.1016/j.ijfoodmicro.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Schenk M., Ferrario M., Guerrero S. Antimicrobial activity of binary and ternary mixtures of vanillin, citral, and potassium sorbate in laboratory media and fruit purées. Food Bioprocess Technol. 2018;11:324–333. doi: 10.1007/s11947-017-2013-1. [DOI] [Google Scholar]
- 35.Buazzi M.M., Marth E.H. Mechanisms in the inhibition of Listeria monocytogenes by potassium sorbate. Food Microbiol. 1991;8:249–256. doi: 10.1016/0740-0020(91)90057-9. [DOI] [Google Scholar]
- 36.Thomas L.V. In: Encyclopedia of Food Microbiology. Richard K.R., editor. Elsevier; Oxford: 1999. Preservatives/permitted preservatives – sorbicacid; pp. 1769–1776. [Google Scholar]
- 37.Sharaf O.M., Al-Gamal M.S., Ibrahim G.A., Dabiza N.M., Salem S.S., El-Ssayad M.F., Youssef A.M. Evaluation and characterization of some protective culture metabolites in free and nano-chitosan-loaded forms against common contaminants of Egyptian cheese. Carbohydr. Polym. 2019;223 doi: 10.1016/j.carbpol.2019.115094. [DOI] [PubMed] [Google Scholar]
- 38.Ávila M., Gómez-Torres N., Hernández M., Garde S. Inhibitory activity of reuterin, nisin, lysozyme and nitrite against vegetative cells and spores of dairy-related Clostridium species. Int. J. Food Microbiol. 2014;172:70–75. doi: 10.1016/j.ijfoodmicro.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Meira S.M.M., Zehetmeyer G., Scheibel J.M., Werner J.O., Brandelli A. Starch-halloysite nanocomposites containing nisin: characterization and inhibition of Listeria monocytogenes in soft cheese. Food Sci. Technol. 2016;68:226–234. doi: 10.1016/j.lwt.2015.12.006. [DOI] [Google Scholar]
- 40.Soto K.M., Hernández-Iturriaga M., Loarca-Piña G., Luna-Bárcenas G., Mendoza S. Antimicrobial effect of nisin electrospun amaranth: pullulan nanofibers in apple juice and fresh cheese. Int. J. Food Microbial. 2019;295:25–32. doi: 10.1016/j.ijfoodmicro.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 41.de Arauz L.J., Jozala A.F., Mazzola P.G., Penna T.C.V. Nisin biotechnological production and application: a review. Trends Food Sci. Technol. 2009;20:146–154. doi: 10.1016/j.tifs.2009.01.056. [DOI] [Google Scholar]
- 42.Kawada-Matsuo M., Yoshida Y., Nakamura N., Komatsuzawa H. Role of two-component systems in the resistance of Staphylococcus aureus to antibacterial agents. Virulence. 2011;2:427–430. doi: 10.4161/viru.2.5.17711. [DOI] [PubMed] [Google Scholar]
- 43.Tomaras A.P., Crandon J.L., McPherson C.J., Banevicius M.A., Finegan S.M., Irvine R.L., Brown M.F., O'Donnell J.P., Nicolau D.P. Adaptation-based resistance to siderophore-conjugated antibacterial agents by Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013;57:4197–4207. doi: 10.1128/aac.00629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raza S., Matuła K., Karoń S., Paczesny J. Resistance and adaptation of bacteria to non-antibiotic antibacterial agents: physical stressors, nanoparticles, and bacteriophages. Antibiot. 2021;10:435. doi: 10.3390/antibiotics10040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuyen T.T., Jaafari A., Yen H.P.H., Nguyen-Thoi T., Van Phong T., Nguyen H.D., Le H.V., Phuong T.T.M., Nguyen S.H., Prakash I., Pham B.T. Mapping forest fire susceptibility using spatially explicit ensemble models based on the locally weighted learning algorithm. Ecol. Inf. 2021;63 doi: 10.1016/j.ecoinf.2021.101292. [DOI] [Google Scholar]
- 46.Jaafari A., Panahi M., Mafi-Gholami D., Rahmati O., Shahabi H., Shirzadi A., Lee S., Bui D.T., Pradhan B. Swarm intelligence optimization of the group method of data handling using the cuckoo search and whale optimization algorithms to model and predict landslides. Appl. Soft Comput. 2022;116 doi: 10.1016/j.asoc.2021.108254. [DOI] [Google Scholar]
- 47.Tran Q.C., Minh D.D., Jaafari A., Al-Ansari N., Minh D.D., Van D.T., Nguyen D.A., Tran T.H., Ho L.S., Nguyen H.D., Prakash I., Le H.V., Pham B.T. Novel ensemble landslide predictive models based on the Hyperpipes algorithm: a case study in the Nam Dam Commune, Vietnam. Appl. Sci. 2020;10:3710. doi: 10.3390/app10113710. [DOI] [Google Scholar]
- 48.Shabani S., Jaafari A., Bettinger P. Spatial modeling of forest stand susceptibility to logging operations. Environ. Impact Assess. Rev. 2021;89 doi: 10.1016/j.eiar.2021.106601. [DOI] [Google Scholar]
- 49.Adnan R.M., Jaafari A., Mohanavelu A., Kisi O., Elbeltagi A. Novel ensemble forecasting of streamflow using locally weighted learning algorithm. Sustain. Times. 2021;13:5877. doi: 10.3390/su13115877. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.