Abstract
Six Gardnerella vaginalis strains were examined for the ability to utilize various iron-containing compounds as iron sources. In a plate bioassay, all six strains acquired iron from ferrous chloride, ferric chloride, ferrous sulfate, ferric ammonium citrate, ferrous ammonium sulfate, bovine and equine hemin, bovine catalase, and equine, bovine, rabbit, and human hemoglobin. All six strains also acquired iron from human lactoferrin, but not from human transferrin, as determined by a liquid broth growth assay. Siderophore production was detected in eight G. vaginalis strains by the chrome azurol S universal chemical assay. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the cytoplasmic membrane proteins isolated from G. vaginalis 594 grown under iron-replete and iron-restricted conditions revealed several iron-regulated proteins ranging in molecular mass from 33 to 94 kDa. These results indicate that G. vaginalis may acquire iron from iron salts and host iron compounds.
Gardnerella vaginalis is a fastidious, beta-hemolytic, nonmotile, unencapsulated, rod-shaped bacterium (6). Originally named Haemophilus vaginalis by Gardner and Dukes (17), the organism was renamed Corynebacterium vaginale by Zinnemann and Turner (84) on the basis of a Gram stain reaction and cell morphology. Subsequent extensive taxonomic studies using biochemical and DNA hybridization assays and electron microscopy led to the assignment of this bacterium to its current taxonomic designation (23, 58). Although G. vaginalis cells stain gram variable, several studies indicate that G. vaginalis possesses a gram-positive cell wall (28, 54, 62, 65). Furthermore, a recent study which analyzed G. vaginalis proteins, fatty acids, and 16S rRNA gene sequences supported the current taxonomic designation of G. vaginalis and indicated that this bacterium was closely related to the genus Bifidobacterium (82).
G. vaginalis is the predominant microorganism associated with bacterial vaginosis (BV), a common disorder which occurs primarily in women of reproductive age and is characterized by (i) the presence of a milky or gray homogeneous discharge, (ii) an amine (fishy) odor, (iii) the presence of vaginal epithelial “clue cells,” and (iv) an increase in the pH of the vagina to >4.5 (13, 49, 77). It is also characterized by a shift in the microbiological flora of the lower vagina, where the Lactobacillus-predominant flora is replaced by a number of different microorganisms, including Mobiluncus spp., Peptostreptococcus spp., Prevotella spp., Bacteroides spp., and Mycoplasma hominis (2, 13, 31, 32, 77, 78). Although it is found at low concentrations in healthy subjects, G. vaginalis is found in higher concentrations in BV patients. Recent studies have suggested that BV is a significant risk factor for upper genital tract infections (14, 15, 19, 33, 40, 53, 57) in pregnant women, which can result in adverse outcomes of pregnancy, including preterm delivery and low birth weight of infants (22, 34, 47, 50), premature rupture of membranes (48), premature labor (35, 63), and impaired fetal development (18). Furthermore, a study by Sewankambo et al. suggests that BV may increase susceptibility to infection by human immunodeficiency virus (75). However, with the exception of evidence for a commensal relationship between G. vaginalis and Prevotella bivia (61), very little is known about the interactions between the microorganisms associated with BV or the contributions of G. vaginalis and the other microorganisms to the establishment of BV or upper genital tract infections.
In addition to being associated with BV, G. vaginalis has been detected in intrauterine infections (6, 39, 46), intraamniotic and chorioamniotic infections (19, 20, 33, 40, 52), and pelvic inflammatory disease (14, 15). G. vaginalis has also been isolated in cases of urinary-tract infection and bladder infection (41, 76), and G. vaginalis bacteremia has also been documented (37). However, there is little information concerning the pathogenic mechanisms of G. vaginalis. G. vaginalis secretes a 60-kDa hemolysin which lyses human erythrocytes, neutrophils, and endothelial cells and thus is a potential virulence factor (7, 64). Studies have also indicated that pili and an exopolysaccharide coat are involved in the adherence of G. vaginalis to vaginal epithelial cells and erythrocytes (5, 74), although their specific roles in the establishment of G. vaginalis infection remain unclear.
Iron is an essential growth factor required by virtually all living cells. Furthermore, the acquisition of iron plays an important role in the virulence potential of many bacterial pathogens (24, 44). There are many examples of bacterial virulence factors regulated by iron levels, some of which are directly or indirectly involved in iron acquisition, including toxins, hemolysins, and high-affinity iron uptake systems (42, 44, 83). However, free iron is found in limited amounts in the human body and is sequestered in compounds such as ferritin, heme, and hemoglobin or bound by high-affinity iron-binding proteins such as lactoferrin and transferrin (56, 83). As a result, bacteria have developed high-affinity mechanisms to obtain this essential nutrient. One mechanism is the secretion of high-affinity iron chelators, known as siderophores, which remove iron from carrier molecules and then are bound by outer-surface receptors for import of the iron or iron-siderophore complex into the bacterial cell (9, 44, 45). Another mechanism is the use of cell surface receptors to directly bind iron-containing compounds such as heme, hemoglobin, heme-hemopexin, lactoferrin, and transferrin (44, 56, 83). A third mechanism is the production of hemolysins or cytolysins which lyse host cells, presumably resulting in the release of iron-containing compounds (44). Many bacteria possess more than one iron acquisition system and/or obtain iron from more than one source, presumably to ensure the acquisition of this essential nutrient. For example, Haemophilus ducreyi, a sexually transmitted pathogen which does not produce siderophores, can utilize several heme-containing compounds as iron sources, but not lactoferrin or transferrin (43). H. ducreyi expresses a protein, designated HgbA, which has been shown to bind hemoglobin directly (12). Vibrio vulnificus, which can obtain iron from a number of sources, uses a siderophore-mediated mechanism to acquire iron from transferrin (45).
Virtually nothing is known about iron acquisition by G. vaginalis. It is not known what host iron-containing compounds G. vaginalis may potentially use as a source of iron. Furthermore, although the lysis of host cells by the 60-kDa hemolysin may be one mechanism by which G. vaginalis obtains iron, it is not known if G. vaginalis has the potential to sequester iron by other mechanisms, such as the production of siderophores. In this study, the ability of G. vaginalis strains to utilize various iron-containing compounds as iron sources was examined. The ability of this organism to produce and excrete siderophores was also examined by a universal chemical assay for siderophore production. Finally, experiments were performed to determine if G. vaginalis possesses iron-regulated proteins.
Bacterial strains, reagents, media, and growth conditions.
The bacterial strains used in this study are listed in Table 1. G. vaginalis strains were routinely grown on human blood bilayer-Tween (HBT) agar plates (81) obtained from BBL Microbiology Systems (Cockeysville, Md.) or basal medium (58) agar plates supplemented with 0.3% starch. Depending on the experiment, proteose-maltose-dextrose (PMD) or proteose-starch-dextrose (PSD) medium (11) was also used. All G. vaginalis cultures were incubated at 37°C under an atmosphere of 5% CO2. Permanent frozen stocks were stored at −75°C in Proteose Peptone 3 (Difco, Detroit, Mich.) broth with 50% glycerol. All eight G. vaginalis strains were colistin and nalidixic acid resistant and beta-hemolytic when cultured on HBT plates, catalase negative, and hydrogen peroxide sensitive. No beta-hemolysis was detected when the G. vaginalis strains were cultured on Columbia-colistin-nalidixic acid (BBL Microbiology Systems) agar plates containing 5% sheep blood. Escherichia coli strains were routinely cultured on Luria-Bertani medium (67) at 37°C. All iron-containing compounds, apo-transferrin, the iron chelators 2,2′-dipyridyl and deferoxamine mesylate, Chelex-100 chelating resin, chrome azurol S dye, and piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; free acid) were purchased from Sigma Chemical Company (St. Louis, Mo.) or from Fluka Chemical Corporation (Milwaukee, Wis.). All iron-compound solutions were freshly prepared by dissolving the compounds in distilled water (dH2O) followed by filter sterilization, with the exception of hemin, which was dissolved in a solution of 0.02 N sodium hydroxide prior to filter sterilization.
TABLE 1.
Bacterial strains
| Strain | Description or relevant genotype | Source or reference |
|---|---|---|
| G. vaginalis | ||
| 594 | G. vaginalis type strain; ATCC 14018 | ATCCa |
| 317 | Clinical isolate; ATCC 14019 | ATCC |
| AmMS 117 | Clinical isolate; ATCC 49145 | ATCC |
| OCH1 | Clinical isolate | E. D. Sledge |
| OCH2 | Clinical isolate | E. D. Sledge |
| OCH3 | Clinical isolate | E. D. Sledge |
| OCH4 | Clinical isolate | E. D. Sledge |
| OCH5 | Clinical isolate | E. D. Sledge |
| E. coli | ||
| HB101 | Δ(gpt-proA) leuB6 supE44 ara-14 galK2 lacY1 Δ(mcrC0mrr) rpsL20 xyl-5 mtl-1 recA13 ent+ | Lab stock |
| RW193 | proC leu trp thi entA | 73 |
| H1780 | araD139 Δ(argF-lac)U169 rpsL150 relA1 rbsR flB5301 deoC1 ptsF25 fiu::λplacMu fur ent+ | 27 |
ATCC, American Type Culture Collection.
Iron source utilization assays.
For the plate bioassay, fresh overnight cultures of the G. vaginalis strains grown on PSD plates were suspended at a concentration of approximately 108 CFU/ml in PSD broth made low in iron by treatment with Chelex-100. Chelex-100 treatment consisted of stirring 10 g of resin/100 ml of broth for 4 to 6 h prior to autoclaving. Chelex-100-treated medium was supplemented with 0.1 mM magnesium sulfate, 0.1 mM calcium chloride, and 10 μM zinc chloride. Fifty microliters of the cell suspension was spread onto PSD agar plates containing 100 μM deferoxamine mesylate or 100 μM 2,2′-dipyridyl. Following drying, sterile filter disks (7 mm) were placed onto the agar plates and the various iron sources (10 μl of each, except for hemin [2.5 μl]) were spotted onto the filters. After drying, the plates were incubated at 37°C under an atmosphere of 5% CO2 for 24 to 48 h and were then examined for bacterial growth around the filters. Bacterial growth around the filter disk indicated that the cells could utilize the iron source. The following iron sources were used at the concentrations indicated: ferrous chloride (FeCl2), 1 mg/ml; ferric chloride (FeCl3), 1 mg/ml; ferrous ammonium sulfate, 1 mg/ml; ferrous sulfate (FeSO4), 1 mg/ml; ferric ammonium citrate, 1 mg/ml; hemin (bovine and equine), 1 mM; catalase (bovine), 85 μM; hemoglobin (equine, human, rabbit, and bovine), 80 μM; apo-transferrin and iron-loaded transferrin (33 or 98% iron-saturated), 125 μM; and iron-loaded lactoferrin (90% saturated), 125 μM. All assays were performed in triplicate. The optimal minimal concentration of the iron chelators which inhibited G. vaginalis growth in the plate bioassay was determined by titration experiments in which G. vaginalis cells were inoculated onto PSD agar plates containing varying concentrations (50, 100, 150, or 200 μM) of deferoxamine mesylate or 2,2′-dipyridyl. From these experiments, it was determined that 100 μM was the optimal minimal concentration of the iron chelators which inhibited G. vaginalis growth.
For the liquid broth assay, fresh G. vaginalis cultures were suspended (approximately 107 CFU/ml) in Chelex-100-treated PMD. After 4 to 6 h, the cultures were diluted 1:100 in fresh Chelex-100-treated PMD broth supplemented with catalase or hemoglobin (all sources) to a final concentration of 250 μg/ml. Apo-transferrin, iron-loaded human transferrin (33 or 98% iron-saturated), or iron-loaded lactoferrin was added to a final concentration of 250 or 500 μg/ml. Stock solutions of the iron-saturated transferrin and lactoferrin were filtered by using a Microcon-30 Microconcentrator (Millipore, Bedford, Mass.) in order to remove any excess free iron. Bovine and equine hemin were added to a final concentration of 5 μg/ml, and the iron salts were added to a final concentration of 10 μg/ml. As a control, the G. vaginalis strains were diluted 1:100 in Chelex-100-treated broth containing no added iron-compound supplements. The cultures were monitored for bacterial growth (increase in turbidity) after incubation at 37°C under an atmosphere of 5% CO2 for 24 to 36 h.
Acquisition of iron from iron-containing compounds by G. vaginalis.
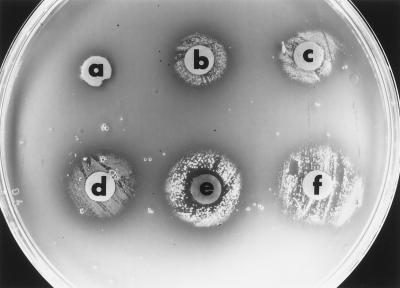
G. vaginalis inhabits an environment where it can potentially be exposed to a variety of iron-containing compounds, including heme, lactoferrin, and hemoglobin. However, it is not known which iron-containing compounds G. vaginalis can utilize as potential iron sources. To determine this, six strains were examined by a plate bioassay for their abilities to utilize a variety of compounds, including host iron compounds, as sources of iron. An example of the plate bioassay using PSD plates containing 100 μM deferoxamine mesylate is shown in Fig. 1. Growth of G. vaginalis 594 was detected around the filter disks inoculated with ferrous chloride, ferric chloride, hemoglobin, catalase, or hemin. No growth was detected around the control disk inoculated with sterile dH2O.
FIG. 1.
Utilization of iron sources by G. vaginalis 594 as determined by the plate bioassay. G. vaginalis cells were inoculated onto a PSD plate containing 100 μM deferoxamine mesylate as described in Materials and Methods. Filter disks were spotted with dH2O (a), ferric chloride (b), ferrous chloride (c), catalase (d), bovine hemin (e), or bovine hemoglobin (f).
The abilities of six G. vaginalis strains to utilize various iron-containing compounds as iron sources are summarized in Table 2. All the strains examined, including the clinical isolates, were capable of utilizing ferrous chloride, ferric chloride, ferrous ammonium sulfate, ferrous sulfate, ferric ammonium citrate, hemin (bovine and equine), catalase (bovine), and hemoglobin (human, equine, rabbit, and bovine), as determined by the plate bioassay. Similar results were observed when PSD agar plates containing 100 μM 2,2′-dipyridyl were used in the bioassay (data not shown). Furthermore, all six strains were able to utilize these compounds as iron sources in the liquid broth growth assay. No growth was detected around the disks inoculated with human apo-transferrin, iron-loaded human transferrin, or iron-loaded human lactoferrin. However, the G. vaginalis strains were able to grow in Chelex-100-treated PMD broth supplemented with iron-loaded lactoferrin (Table 2). No growth was detected in liquid broth assays using apo-transferrin or iron-loaded transferrin (33 and 98% saturated) (Table 2), indicating that the G. vaginalis strains could utilize lactoferrin but not transferrin as an iron source. No growth was detected in liquid cultures which did not contain an iron source.
TABLE 2.
Utilization of iron sources by G. vaginalis
| G. vaginalis strain | Utilization of the following iron sourcea:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeCl2 | FeCl3 | FeSO4 | FAS | FAC | eHm | bHm | bHg | hHg | eHg | rHg | Cat | Lf | Tf | aTf | dH2O | |
| 594 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − |
| 317 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − |
| AmMS 117 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − |
| OCH1 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − |
| OCH2 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − |
| OCH3 | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − |
FAS, ferrous ammonium sulfate; FAC, ferric ammonium citrate; eHm, equine hemin; bHm, bovine hemin; bHg, bovine hemoglobin; hHg, human hemoglobin; eHg, equine hemoglobin; rHb, rabbit hemoglobin; Cat, catalase; Lf, human lactoferrin; Tf, human transferrin; aTf, apo-transferrin. +, ability to utilize the iron source; −, inability to use the iron source.
Detection of siderophore production.
Many bacteria produce siderophores as a mechanism for acquiring iron from the environment. To determine if G. vaginalis produces siderophores, the chrome azurol S universal assay for the detection of siderophores was used (73). The advantage of this assay is that it can detect siderophores based on their affinity for iron and not on their specific chemical structures (73). Briefly, fresh overnight cultures of bacterial cells grown on PSD agar plates were patched onto agar plates containing chrome azurol S. Siderophore production, as indicated by the presence of orange halos around the patches, was determined after incubation for 18 to 24 h at 37°C under an atmosphere of 5% CO2. E. coli strains which were either proficient or defective in siderophore production were used as controls. All assays were performed in triplicate. All eight of the G. vaginalis strains tested produced siderophores, as indicated by the presence of yellow-orange halos around the cell patches (Table 3). Siderophore production was also detected for E. coli HB101, which is wild type for siderophore production, and E. coli H1780 (27), which contains a fur mutation resulting in the derepression of siderophore production (Table 3). Siderophore production was not detected for E. coli RW193 (73), an entA mutant deficient in siderophore production (Table 3).
TABLE 3.
Siderophore production by G. vaginalis
| Strain |
Siderophore productiona |
|---|---|
| G. vaginalis | |
| 594 | + |
| 317 | + |
| AmMS 117 | + |
| OCH1 | + |
| OCH2 | + |
| OCH3 | + |
| OCH4 | + |
| OCH5 | + |
| E. coli | |
| H1780 fur | + |
| HB101 | + |
| RW193 entA | − |
+, siderophore production; −, no siderophore production.
Protein profile of the membrane fraction of G. vaginalis 594.
It has been demonstrated in many bacteria that the synthesis of some proteins is iron regulated such that, characteristically, the expression of these proteins is induced under iron-limiting conditions. To determine if G. vaginalis expressed iron-regulated proteins, the membrane fraction of G. vaginalis 594 cells grown under iron-replete conditions (PMD medium) or iron-restrictive conditions (PMD medium with 100 μM 2,2′-dipyridyl) was isolated and proteins contained within this fraction were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The membrane fraction of G. vaginalis 594 was isolated by using the method for isolating the membrane fraction of Streptomyces spp. (21). Briefly, G. vaginalis 594 cells were harvested after growth for 24 h on either iron-replete (PMD agar plates) or iron-restricted (PMD agar plates with 100 μM 2,2′-dipyridyl) medium. Harvested cells were resuspended in a 50 mM Tris (pH 8)–10% sucrose buffer solution containing 0.2 mM dithiothreitol (DTT), lysozyme (10 mg/ml), RNase A (0.2 mg/ml), and DNase I (0.2 mg/ml) and then incubated for 1 h at 37°C to form protoplasts. Protoplast formation was monitored microscopically. Following sedimentation, the protoplasts were resuspended in buffer (50 mM Tris [pH 8]–0.2 mM DTT–0.2 M KCl–0.1 mM phenylmethylsulfonyl fluoride [PMSF]) and lysed by mild sonication. Following filtration through a 0.45 μm-pore-size filter to remove unbroken cells, the membrane fraction was harvested by centrifugation (at 260,000 × g) and resuspended in 50 mM Tris–0.1 mM PMSF. Protein concentrations were determined by using the dotMETRIC protein assay kit (Gene Technology, St. Louis, Mo.) according to the manufacturer’s instructions. Membrane proteins were separated by SDS-PAGE as described elsewhere (67) by using a 5% (wt/vol) stacking and a 10% (wt/vol) separating gel. Following electrophoresis, the proteins were visualized by silver staining (67). Comparison of the protein profile of cells grown in iron-replete conditions (Fig. 2, lane 1) with the protein profile of cells grown in iron-restricted conditions (Fig. 2, lane 2) revealed several iron-regulated proteins whose molecular masses ranged from 33 to 94 kDa.
FIG. 2.
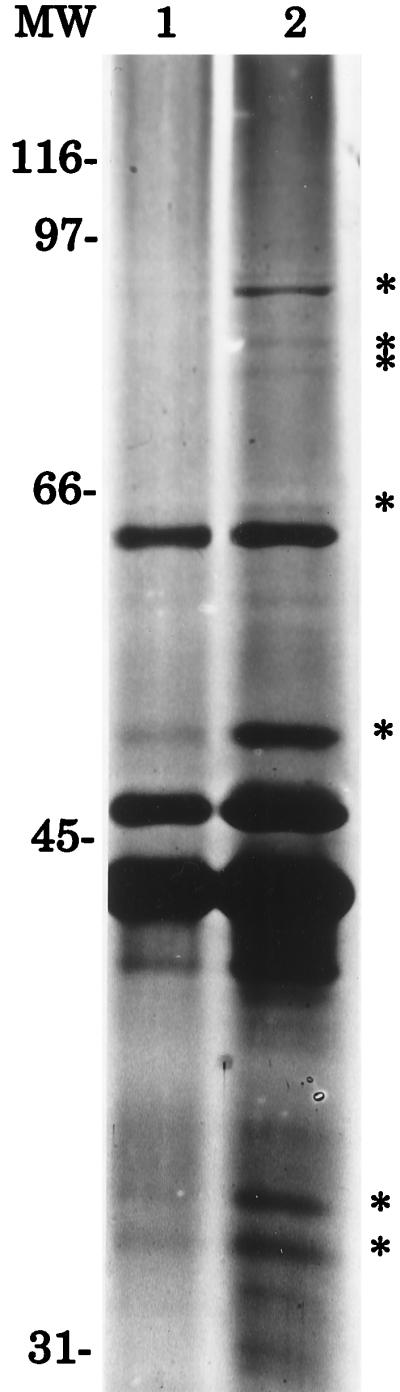
Iron-regulated proteins of G. vaginalis 594. Equal amounts of protein (50 μg) were loaded onto each lane. Membrane protein profiles of G. vaginalis 594 cells grown under iron-replete (lane 1) and iron-restrictive (lane 2) conditions are shown. MW, molecular weight markers (in thousands).
G. vaginalis inhabits an environment in which a number of potential iron sources may be available. Lactoferrin, an extracellular iron-binding glycoprotein, can be found on mucosal surfaces, including those of the urogenital tract. A second extracellular iron-binding glycoprotein, transferrin, is found in blood serum. Intracellular hemoglobin is found in erythrocytes and is presumably released upon lysis of erythrocytes by the G. vaginalis hemolysin. However, it is not known what iron sources G. vaginalis utilizes in vivo. This study demonstrated that G. vaginalis can utilize a number of mammalian iron sources in vitro, including hemoglobin (human, equine, bovine, and rabbit) and human lactoferrin. Bovine and equine hemin, as well as bovine catalase, were also used as iron sources in vitro. Whether G. vaginalis can utilize the human counterparts of hemin and catalase is not known. The utilization of hemoglobin and hemin derived from equine sources is consistent with the isolation of G. vaginalis from horses (30, 66). Also, the utilization of rabbit hemoglobin is consistent with the growth of G. vaginalis in rabbits used in an experimental animal model to study the effects of G. vaginalis infection (16). It is not clear why G. vaginalis was able to utilize lactoferrin in a liquid medium but not on the agar plates. It is possible that the iron chelators somehow interfere with the ability of G. vaginalis to utilize this compound on a solid medium. Control experiments indicated that Neisseria meningitidis OLOL1 could utilize human transferrin and lactoferrin when assayed by a plate bioassay (data not shown). However, results similar to ours have been reported for Corynebacterium sp. strains that were able to utilize transferrin as an iron source in a liquid broth medium but were not able to utilize transferrin in a plate bioassay (69).
In many bacteria, the response to low iron levels results in the expression not only of gene products involved in iron acquisition, such as siderophores, but also cell products unrelated to iron uptake, such as bacterial toxins and virulence factors (44). In E. coli, this coordinated regulation is mediated by the Fur protein (44). When complexed with iron, the Fur protein binds to operator sequences of iron-regulated promoters to control their expression. Under low-iron conditions, the Fur protein does not bind the promoter, resulting in the expression of iron-regulated genes (44). Homologs of the fur gene have been identified in other gram-negative bacteria, including Neisseria gonorrhoeae (4), N. meningitidis (80), and Pseudomonas aeruginosa (60). In the gram-positive bacterium Corynebacterium diphtheriae, regulation of the diphtheria toxin, siderophore production, and the IRP1 protein is mediated by the DtxR protein and iron (70, 71). Like the Fur protein, the DtxR protein is an iron-dependent repressor of gene expression. Homologs of the dtxR gene have been found in other gram-positive bacteria, including Brevibacterium lactofermentum, Streptomyces lividans, and Streptomyces pilosus (26, 55). Results from this study showed an increase in the expression of several proteins when G. vaginalis 594 was grown under iron-restrictive conditions, suggesting that G. vaginalis possesses iron-regulated proteins. The function(s) of these proteins is not known. It is possible that one or more of these proteins are involved in some aspect of iron acquisition, such as siderophore-mediated iron acquisition, or function as receptors that directly bind iron-containing compounds. It is also possible that some of these proteins represent iron-regulated virulence factors of G. vaginalis. Further studies will be required to determine the functions of G. vaginalis iron-regulated proteins and to determine if this response to iron-restrictive conditions is mediated by a DtxR homolog.
Most of what is known about bacterial iron acquisition is derived from studies examining this process in gram-negative bacteria. Siderophore-mediated iron uptake systems, as well as outer membrane receptors involved in the direct binding of iron-containing compounds, have been identified in many gram-negative bacteria (9, 44, 56, 83). Furthermore, genes encoding proteins presumed to be involved in the transport of iron or heme from the outer membrane into the periplasm, such as the tonB and exbB genes, have also been identified in a number of gram-negative bacteria (36, 59). Finally, periplasmic-binding-protein-dependent iron transport systems have been described in several gram-negative bacteria (3, 8, 68).
In contrast, there is less known about iron acquisition and transport in gram-positive bacteria. Siderophore production has been detected in a number of gram-positive bacteria; the siderophore-mediated iron uptake system of Bacillus subtilis is the best studied, and genes encoding proteins putatively involved in B. subtilis iron transport have been identified (25, 72). However, information about non-siderophore-mediated iron acquisition systems and the utilization of heme and heme-containing compounds as iron sources in gram-positive bacteria is limited. Transferrin-binding proteins have been identified in Listeria monocytogenes and Staphylococcus spp. (29, 51). L. monocytogenes also synthesizes an iron reductase that is involved in iron acquisition (1, 38). Although C. diphtheriae uses siderophores to acquire iron from transferrin, the acquisition of iron from heme and hemoglobin is not siderophore mediated (69). Instead, the utilization of these compounds requires, at least in part, the hmuO gene, which encodes a predicted protein possessing some homology to eukaryotic heme oxygenases (69). Streptococcus pneumoniae can utilize heme and hemoglobin as iron sources (79), but the mechanism(s) by which it can utilize these compounds remains unknown. Our results in this study, obtained by a universal assay to detect siderophores, demonstrated that several G. vaginalis strains were able to produce siderophores, suggesting that this may be one mechanism by which G. vaginalis can acquire iron. However, it is not yet clear from which sources G. vaginalis acquires iron via a siderophore-mediated system. Furthermore, preliminary studies from our laboratory (10) also indicate that G. vaginalis can directly bind iron-containing compounds, including heme, hemoglobin, and catalase, suggesting that G. vaginalis may utilize a direct binding mechanism in addition to a siderophore-mediated mechanism to obtain iron. Taken together, the results from this study and other studies indicate that G. vaginalis can acquire iron from several different sources and can potentially use three different mechanisms to obtain this essential nutrient.
Additional studies will be required in order to further understand iron acquisition by G. vaginalis and to determine the contribution of this process to the ability of this bacterium to cause infection. Furthermore, with the observations that G. vaginalis may potentially use three different mechanisms to acquire iron from a number of sources, such studies may also provide information about the mechanisms, particularly non-siderophore-mediated mechanisms, used by gram-positive pathogens to obtain iron. Studies to further characterize the direct binding of iron-containing compounds by G. vaginalis are in progress. The information obtained from this work will be useful towards identifying cell components involved in iron uptake by this organism.
Acknowledgments
We thank Gregg Pettis and Alan Biel for critical reading of the manuscript and E. David Sledge, Klaus Hantke, and Mark Coy for bacterial strains. We also thank Michael Schmitt for helpful suggestions.
P.D. and R.C. were supported in part by Howard Hughes Medical Institute Undergraduate Research Fellowships through the LSU College of Basic Sciences. P.D. was also supported in part by the LSU Ronald E. McNair Program. This work was supported by a grant from the Joe W. and Dorothy Dorsett Brown Foundation.
REFERENCES
- 1.Adams T J, Vartivarian S, Cowart R E. Iron acquisition systems in Listeria monocytogenes. Infect Immun. 1990;58:2715–2718. doi: 10.1128/iai.58.8.2715-2718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsel R, Totten P A, Spiegel C A, Chen K C S, Eschenbach D, Holmes K K. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 3.Angerer A, Gaisser S, Braun V. Nucleotide sequences of the sfuA, sfuB, and sfuC genes of Serratia marcescens suggest a periplasmic-binding-protein-dependent iron transport mechanism. J Bacteriol. 1990;172:572–578. doi: 10.1128/jb.172.2.572-578.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berish S A, Subbarao S, Chen C-Y, Trees D L, Morse S A. Identification and cloning of a fur homolog from Neisseria gonorrhoeae. Infect Immun. 1993;61:4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boustouller Y L, Johnson A P, Taylor-Robinson D. Pili on Gardnerella vaginalis studied by electron microscopy. J Med Microbiol. 1987;23:327–329. doi: 10.1099/00222615-23-4-327. [DOI] [PubMed] [Google Scholar]
- 6.Catlin B W. Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin Microbiol Rev. 1992;5:213–237. doi: 10.1128/cmr.5.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauci S, Monte R, Ropele M, Missero C, Not T, Quadrifoglio F, Menestrina G. Pore-forming and haemolytic properties of the Gardnerella vaginalis cytolysin. Mol Microbiol. 1993;9:1143–1155. doi: 10.1111/j.1365-2958.1993.tb01244.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen C Y, Berish S A, Morse S A, Mietzner T A. The ferric iron-binding protein of pathogenic Neisseria spp. functions as a periplasmic transport protein in iron acquisition from human transferrin. Mol Microbiol. 1993;10:311–318. doi: 10.1111/j.1365-2958.1993.tb01957.x. [DOI] [PubMed] [Google Scholar]
- 9.Crosa J H. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol Rev. 1989;53:517–530. doi: 10.1128/mr.53.4.517-530.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duhon P, Chandler R, Mercer T, Land C B, Jarosik G P. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Initial studies on iron acquisition by Gardnerella vaginalis, abstr. D-110; p. 227. [Google Scholar]
- 11.Dunkelberg W E, McVeigh I. Growth requirements of Haemophilus vaginalis. Antonie Leeuwehoek. 1969;35:129–145. doi: 10.1007/BF02219124. [DOI] [PubMed] [Google Scholar]
- 12.Elkins C. Identification and purification of a conserved heme-regulated hemoglobin-binding outer membrane protein from Haemophilus ducreyi. Infect Immun. 1995;63:1241–1245. doi: 10.1128/iai.63.4.1241-1245.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eschenbach D A. History and review of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:441–445. doi: 10.1016/0002-9378(93)90337-i. [DOI] [PubMed] [Google Scholar]
- 14.Eschenbach D A, Buchanan T M, Pollock H M, Forsyth P S, Alexander E R, Lin J-S, Wang S-P, Wentworth B B, McCormack W M, Holmes K K. Polymicrobial etiology of pelvic inflammatory disease. N Engl J Med. 1975;293:166–171. doi: 10.1056/NEJM197507242930403. [DOI] [PubMed] [Google Scholar]
- 15.Faro S, Martens M, Maccato M, Hammill H, Pearlman M. Vaginal flora and pelvic inflammatory disease. Am J Obstet Gynecol. 1993;169:470–473. doi: 10.1016/0002-9378(93)90344-i. [DOI] [PubMed] [Google Scholar]
- 16.Field N T, Newton E R, Kagan-Hallet K, Peairs W A. Perinatal effects of Gardnerella vaginalis deciduitis in the rabbit. Am J Obstet Gynecol. 1993;168:988–994. doi: 10.1016/s0002-9378(12)90858-3. [DOI] [PubMed] [Google Scholar]
- 17.Gardner H L, Dukes C H. Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified as “nonspecific” vaginitis. Am J Obstet Gynecol. 1955;69:962–976. [PubMed] [Google Scholar]
- 18.Germain M, Krohn M A, Hillier S L, Eschenbach D A. Genital flora in pregnancy and its association with intrauterine growth and retardation. J Clin Microbiol. 1994;32:2162–2168. doi: 10.1128/jcm.32.9.2162-2168.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbs R S. Chorioamnionitis and bacterial vaginosis. Am J Obstet Gynecol. 1993;169:460–462. doi: 10.1016/0002-9378(93)90341-f. [DOI] [PubMed] [Google Scholar]
- 20.Gibbs R S, Weiner M H, Walmer K, St. Clair P J. Microbiologic and serologic studies of Gardnerella vaginalis in intra-amniotic infection. Obstet Gynecol. 1987;70:187–190. [PubMed] [Google Scholar]
- 21.Gramajo H C, White J, Hutchinson C R, Bibb M J. Overproduction and localization of components of the polyketide synthase of Streptomyces glaucescens involved in the production of the antibiotic tetracenomycin C. J Bacteriol. 1991;173:6475–6483. doi: 10.1128/jb.173.20.6475-6483.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gravett M G, Hummel D, Eschenbach D A, Holmes K K. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstet Gynecol. 1986;67:229–237. doi: 10.1097/00006250-198602000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Greenwood J R, Pickett M J. Transfer of Haemophilus vaginalis Gardner and Dukes to a new genus, Gardnerella: G. vaginalis (Gardner and Dukes) comb. nov. Int J Syst Bacteriol. 1980;30:170–178. [Google Scholar]
- 24.Griffiths E. Iron and bacterial virulence—a brief overview. Biol Metals. 1991;4:7–13. doi: 10.1007/BF01135551. [DOI] [PubMed] [Google Scholar]
- 25.Grossman T H, Tuckman M, Ellestad S, Osburne M S. Isolation and characterization of Bacillus subtilis genes involved in siderophore biosynthesis: relationship between B. subtilis sfp0 and Escherichia coli entD genes. J Bacteriol. 1993;175:6203–6211. doi: 10.1128/jb.175.19.6203-6211.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Günter-Seeboth K, Schupp T. Cloning and sequence analysis of the Corynebacterium diphtheriae dtxR homologue from Streptomyces lividans and S. pilosus encoding a putative iron repressor protein. Gene. 1995;166:117–119. doi: 10.1016/0378-1119(95)00628-7. [DOI] [PubMed] [Google Scholar]
- 27.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 28.Harper J J, Davis G H G. Cell wall analysis of Gardnerella vaginalis (Haemophilus vaginalis) Int J Syst Bacteriol. 1982;32:48–50. [Google Scholar]
- 29.Hartford T, O’Brien S, Andrew P W, Jones D, Roberts I S. Utilization of transferrin-bound iron by Listeria monocytogenes. FEMS Microbiol Lett. 1993;108:311–318. doi: 10.1111/j.1574-6968.1993.tb06121.x. [DOI] [PubMed] [Google Scholar]
- 30.Higgins R, Messier S, Bada R. Isolation of Gardnerella vaginalis from the genital tract of six mares. Can Vet J. 1992;33:745–746. [PMC free article] [PubMed] [Google Scholar]
- 31.Hill G B. The microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:450–454. doi: 10.1016/0002-9378(93)90339-k. [DOI] [PubMed] [Google Scholar]
- 32.Hillier S L. Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:455–459. doi: 10.1016/0002-9378(93)90340-o. [DOI] [PubMed] [Google Scholar]
- 33.Hillier S L, Martius J, Krohn M, Kiviat N, Holmes K K, Eschenbach D A. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 34.Hillier S L, Nugent R P, Eschenbach D A, Krohn M A, Gibbs R S, Martin D H, Cotch M F, Edelman R, Pastorek II J G, Rao A V, McNellis D, Regan J A, Carey J C, Klebanoff M A. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N Engl J Med. 1995;333:1737–1742. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- 35.Holst E, Goffeng A R, Andersch B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol. 1994;32:176–186. doi: 10.1128/jcm.32.1.176-186.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jarosik G P, Sanders J D, Cope L D, Muller-Eberhard U, Hansen E J. A functional tonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson A P, Boustouller Y L. Extra-vaginal infection caused by Gardnerella vaginalis. Epidemiol Infect. 1987;98:131–137. doi: 10.1017/s0950268800061835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson W, Varner L, Poch M. Acquisition of iron by Legionella pneumophila: role of iron reductase. Infect Immun. 1991;59:2376–2381. doi: 10.1128/iai.59.7.2376-2381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kristiansen F V, Øster S, Frost L, Boustouller Y, Korsager B, Møller B R. Isolation of Gardnerella vaginalis in pure culture from the uterine cavity of patients with irregular bleedings. Br J Obstet Gynaecol. 1987;94:979–984. doi: 10.1111/j.1471-0528.1987.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 40.Krohn M A, Hillier S L, Nugent R P, Cotch M F, Carey J C, Gibbs R S, Eschenbach D A the Vaginal Infection and Prematurity Group. The genital flora of women with intraamniotic infection. J Infect Dis. 1995;171:1475–1480. doi: 10.1093/infdis/171.6.1475. [DOI] [PubMed] [Google Scholar]
- 41.Lam M H, Birch D F, Fairley K F. Prevalence of Gardnerella vaginalis in the urinary tract. J Clin Microbiol. 1988;26:1130–1133. doi: 10.1128/jcm.26.6.1130-1133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee B C. Quelling the red menace: haem capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 43.Lee B C. Iron sources for Haemophilus ducreyi. J Med Microbiol. 1991;34:317–322. doi: 10.1099/00222615-34-6-317. [DOI] [PubMed] [Google Scholar]
- 44.Litwin C M, Calderwood S B. Role of iron in the regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Litwin C M, Rayback T W, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996;64:2834–2838. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malone B H, Schreiber M, Schneider N J, Holdeman L V. Obligately anaerobic strains of Corynebacterium vaginale (Haemophilus vaginalis) J Clin Microbiol. 1975;2:272–275. doi: 10.1128/jcm.2.3.272-275.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald H M, O’Loughlin J A, Jolley P T, Vigneswaran R, McDonald P J. Changes in vaginal flora during pregnancy and association with preterm birth. J Infect Dis. 1994;170:724–728. doi: 10.1093/infdis/170.3.724. [DOI] [PubMed] [Google Scholar]
- 48.McGregor J A, French J I, Seo K. Premature rupture of membranes and bacterial vaginosis. Am J Obstet Gynecol. 1993;169:463–466. doi: 10.1016/0002-9378(93)90342-g. [DOI] [PubMed] [Google Scholar]
- 49.Mead P B. Epidemiology of bacterial vaginosis. Am J Obstet Gynecol. 1993;169:446–449. doi: 10.1016/0002-9378(93)90338-j. [DOI] [PubMed] [Google Scholar]
- 50.Meis P J, Goldenberg R L, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams J D, Thom E, Andrews W W the National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. The preterm prediction study: significance of vaginal infections. Am J Obstet Gynecol. 1995;173:1231–1235. doi: 10.1016/0002-9378(95)91360-2. [DOI] [PubMed] [Google Scholar]
- 51.Modun B, Kendall D, Williams P. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect Immun. 1994;62:3850–3858. doi: 10.1128/iai.62.9.3850-3858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moran D J, Payne A. Subclinical intra-amniotic infection with Gardnerella vaginalis associated with preterm delivery. Br J Obstet Gynaecol. 1989;96:489–490. doi: 10.1111/j.1471-0528.1989.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 53.Newton E R, Piper J, Peairs W. Bacterial vaginosis and intraamniotic infection. Am J Obstet Gynecol. 1997;176:672–677. doi: 10.1016/s0002-9378(97)70568-4. [DOI] [PubMed] [Google Scholar]
- 54.O’Donnell A G, Minnikin D E, Goodfellow M, Piot P. Fatty acid, polar lipid and wall amino acid composition of Gardnerella vaginalis. Arch Microbiol. 1984;138:68–71. doi: 10.1007/BF00425410. [DOI] [PubMed] [Google Scholar]
- 55.Oguiza J A, Tao X, Marcos A T, Martín J F, Murphy J R. Molecular cloning, DNA sequence analysis, and characterization of the Corynebacterium diphtheriae dtxR homolog from Brevibacterium lactofermentum. J Bacteriol. 1995;177:465–467. doi: 10.1128/jb.177.2.465-467.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otto B R, Verweij-van Vught A M J J, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 57.Peipert J F, Montagno A B, Cooper A S, Sung C J. Bacterial vaginosis as a risk factor for upper genital tract infection. Am J Obstet Gynecol. 1997;177:1184–1187. doi: 10.1016/s0002-9378(97)70038-3. [DOI] [PubMed] [Google Scholar]
- 58.Piot P, Van Dyck E, Goodfellow M, Falkow S. A taxonomic study of Gardnerella vaginalis (Haemophilus vaginalis) Gardner and Dukes 1955. J Gen Microbiol. 1980;119:373–396. doi: 10.1099/00221287-119-2-373. [DOI] [PubMed] [Google Scholar]
- 59.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 60.Prince R W, Cox C D, Vasil M L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pybus V, Onderdonk A B. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis. 1997;175:406–413. doi: 10.1093/infdis/175.2.406. [DOI] [PubMed] [Google Scholar]
- 62.Reyn A, Birch-Anderson A, Lapage S P. An electron microscope study of thin sections of Haemophilus vaginalis (Gardner and Dukes) and some possibly related species. Can J Microbiol. 1966;12:1125–1136. doi: 10.1139/m66-154. [DOI] [PubMed] [Google Scholar]
- 63.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell M C, Cotton D B. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086–1091. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 64.Rottini G, Dobrina A, Forgiarini O, Nardon E, Amirante G A, Patriarca P. Identification and partial characterization of a cytolytic toxin produced by Gardnerella vaginalis. Infect Immun. 1990;58:3751–3758. doi: 10.1128/iai.58.11.3751-3758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadhu K, Domingue P A G, Chow A W, Nelligan J, Cheng N, Costerton J W. Gardnerella vaginalis has a gram-positive cell-wall ultrastructure and lacks classical cell-wall lipopolysaccharide. J Med Microbiol. 1989;29:229–235. doi: 10.1099/00222615-29-3-229. [DOI] [PubMed] [Google Scholar]
- 66.Salmon S A, Walker R D, Carleton C L, Shah S, Robinson B E. Characterization of Gardnerella vaginalis and G. vaginalis-like organisms from the reproductive tract of the mare. J Clin Microbiol. 1991;29:1157–1161. doi: 10.1128/jcm.29.6.1157-1161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sambrook J, Fritsch E F, Maniatis T, editors. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 68.Sanders J D, Cope L D, Hansen E J. Identification of a locus involved in the utilization of iron by Haemophilus influenzae. Infect Immun. 1994;62:4515–4525. doi: 10.1128/iai.62.10.4515-4525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schmitt M P. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J Bacteriol. 1997;179:838–845. doi: 10.1128/jb.179.3.838-845.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitt M P, Holmes R K. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect Immun. 1991;59:1899–1904. doi: 10.1128/iai.59.6.1899-1904.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmitt M P, Talley B G, Holmes R K. Characterization of lipoprotein IRP1 from Corynebacterium diphtheriae, which is regulated by the diphtheria toxin repressor (DtxR) and iron. Infect Immun. 1997;65:5364–5367. doi: 10.1128/iai.65.12.5364-5367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schneider R, Hantke K. Iron-hydroxymate uptake systems in Bacillus subtilis: identification of a lipoprotein as a part of a binding protein-dependent transport system. Mol Microbiol. 1993;8:111–121. doi: 10.1111/j.1365-2958.1993.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 73.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 74.Scott T G, Curran B, Smyth C J. Electron microscopy of adhesive interactions between Gardnerella vaginalis and vaginal epithelial cells, McCoy cells, and human red blood cells. J Gen Microbiol. 1989;135:475–480. doi: 10.1099/00221287-135-3-475. [DOI] [PubMed] [Google Scholar]
- 75.Sewankambo N, Gray R H, Wawer M J, Paxton L, McNaim D, Wabwire-Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier S L, Rabe L, Gaydos C A, Quinn T C, Konde-Lule J. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet. 1997;350:546–550. doi: 10.1016/s0140-6736(97)01063-5. [DOI] [PubMed] [Google Scholar]
- 76.Smith S M, Ogbara T, Eng R H K. Involvement of Gardnerella vaginalis in urinary tract infections in men. J Clin Microbiol. 1992;30:1575–1577. doi: 10.1128/jcm.30.6.1575-1577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Spiegel C A. Bacterial vaginosis. Clin Microbiol Rev. 1991;4:484–502. doi: 10.1128/cmr.4.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spiegel C A, Amsel R, Eschenbach D, Schoenknecht F, Holmes K K. Anaerobic bacteria in nonspecific vaginitis. N Engl J Med. 1980;303:601–607. doi: 10.1056/NEJM198009113031102. [DOI] [PubMed] [Google Scholar]
- 79.Tai S S, Lee C, Winter R E. Hemin utilization is related to virulence of Streptococcus pneumoniae. Infect Immun. 1993;61:5401–5405. doi: 10.1128/iai.61.12.5401-5405.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thomas C E, Sparling P F. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol Microbiol. 1994;11:725–737. doi: 10.1111/j.1365-2958.1994.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 81.Totten P A, Amsel R, Hale J, Piot P, Holmes K K. Selective differential human blood bilayer media for isolation of Gardnerella (Haemophilus) vaginalis. J Clin Microbiol. 1982;15:141–147. doi: 10.1128/jcm.15.1.141-147.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Esbroeck M, Vandamme P, Falsen E, Vancanneyt M, Moore E, Pot B, Gavini F, Kersters K, Goossens H. Polyphasic approach to the classification and identification of Gardnerella vaginalis and the unidentified Gardnerella vaginalis-like coryneforms present in bacterial vaginosis. Int J Syst Bacteriol. 1996;46:675–682. doi: 10.1099/00207713-46-3-675. [DOI] [PubMed] [Google Scholar]
- 83.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 84.Zinnemann K, Turner G C. The taxonomic position of ’Haemophilus vaginalis’ (Corynebacterium vaginale) J Pathol Bacteriol. 1963;85:213–219. [Google Scholar]