Abstract
Sensory processing dysfunction not only affects most individuals with autism spectrum disorder (ASD), but at least 5% of children without ASD also experience dysfunctional sensory processing. Our understanding of the relationship between sensory dysfunction and resting state brain activity is still emerging. This study compared long-range resting state functional connectivity of neural oscillatory behavior in children aged 8–12 years with autism spectrum disorder (ASD; N=18), those with sensory processing dysfunction (SPD; N=18) who do not meet ASD criteria, and typically developing control participants (TDC; N=24) using magnetoencephalography (MEG). Functional connectivity analyses were performed in the alpha and beta frequency bands, which are known to be implicated in sensory information processing. Group differences in functional connectivity and associations between sensory abilities and functional connectivity were examined. Distinct patterns of functional connectivity differences between ASD and SPD groups were found only in the beta band, but not in the alpha band. In both alpha and beta bands, ASD and SPD cohorts differed from the TDC cohort. Somatosensory cortical beta-band functional connectivity was associated with tactile processing abilities, while higher-order auditory cortical alpha-band functional connectivity was associated with auditory processing abilities. These findings demonstrate distinct long-range neural synchrony alterations in SPD and ASD that are associated with sensory processing abilities. Neural synchrony measures could serve as potential sensitive biomarkers for ASD and SPD.
Introduction
Sensory dysfunction is estimated to impact at least 70% of individuals with Autism Spectrum Disorders (ASD; Adamson, Hare, & Graham, 2006; Al-Heizan, AlAbdulwahab, Kachanathu, & Natho, 2015; Greenspan & Wieder, 1997; Mayes & Calhoun, 1999; Tomcheck & Dunn, 2007), and with its recognition as a core symptom in DSM-5 (American Psychiatric Association 2013), there is a rapidly growing body of research focused on understanding the causes and impact of sensory dysfunction in ASD. This line of research can be advanced not only by studying sensory dysfunction in individuals with ASD and other clinical populations, but also through examination of the estimated >5% of non-autistic individuals who experience clinically significant sensory processing dysfunction (SPD) (Ahn et al. 2004). Yet, despite the impairment in adaptive functioning associated with SPD, the absence of a recognized categorical diagnosis limits access to resources for research and treatment in affected individuals. Nevertheless, biological differences, such as white matter abnormalities (Chang et al. 2014; Owen et al. 2013) and cortical response latencies (Demopoulos et al. 2017), have been identified in children with SPD and these measurable structural and physiologic differences have been associated with sensory processing behaviors (Chang et al., 2016). While some features of sensory dysfunction may be shared among children with SPD and those with ASD, such as tactile processing deficits (Demopoulos, Brandes-Aitken, et al. 2015), some domains of sensory dysfunction may identify important distinctions between these populations. For example, auditory processing abnormalities have been identified as distinguishing ASD from SPD groups in both behavioral tasks and neural response latencies (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015). Understanding these similarities and differences in sensory processing dysfunction among children with and without ASD can not only help delineate the sensory dysfunction that is specific to ASD, but it can also heighten our understanding of sensory information processing more broadly and guide treatment strategies.
Because differences in resting state oscillatory activity can be indicative of functional pathology (Papanicolaou 2009), there has been extensive research examining differences in resting state brain activity in individuals with and without ASD diagnoses. While previous sensory processing research has focused on differences in performance-based measures of, and neural responses to, sensory processing (Chang et al., 2014; Demopoulos et al., 2015, 2017), our understanding of the relationship between sensory dysfunction and resting state brain activity is still emerging. This study will be the first to use using silently acquired recording via magnetoencephalography (MEG) to examine whole brain functional connectivity during rest in participants with ASD, SPD, and typically developing children (TDC). The goal of this study is to identify relevant differences in whole brain functional connectivity that may be associated with sensory dysfunction. Concurrent examination of these three groups offers two key benefits. First, it will add to the emerging literature identifying the shared and distinct patterns of neural activity in children with ASD and SPD. Second, it will allow us to examine differences in functional connectivity and behavioral measures of sensory discrimination in affected children. Prior research has suggested that auditory and tactile processing are particularly impacted in children with ASD (Fernandez-Andres et al. 2015), and that auditory processing has been associated with the communication impairments that are core to ASD (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015; Demopoulos, Hopkins, et al. 2015; Edgar et al. 2013, 2014; Lerner, McPartland, and Morris 2013; Oram-Cardy et al. 2005; Oram Cardy et al. 2008; Roberts et al. 2011, 2012, 2019, 2008, 2010). As such, we also examine associations between functional connectivity and performance-based measures of auditory and tactile processing and verbal abilities.
Our functional connectivity analyses were performed in the alpha and beta frequency bands, which are known to be implicated in sensory information processing. Specifically, these frequency bands have been associated with sensory gating (Buchholz, Jensen, and Medendorp 2014) and direction of sensory attention in the auditory and visual cortex for alpha (Foxe and Snyder 2011) and in the somatosensory cortex for beta (Bauer, Kennett, and Driver 2012; van Ede, Jensen, and Maris 2010). Further, the role of alpha activity in states of psychological distress has been widely studied (Adolph and Margraf 2017; Boutcher and Landers 1998; Demerdzieva and Pop-Jordanova 2015; Fingelkurts et al. 2007; Knyazev, Savostyanov, and Levin 2006; Mennella, Patron, and Palomba 2017; Smith, Zambrano-Vazquez, and Allen 2016), and may be relevant to differences in psychological response to sensory input in our clinical groups.
Prior research has demonstrated that both children with SPD and ASD were impaired on behavioral and neural measures of tactile processing, but only the ASD group demonstrated auditory dysfunction (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015). This work is consistent with structural findings that children with ASD and SPD demonstrate decreased connectivity in parieto-occipital tracts, but connectivity in temporal tracts was only reduced in the ASD group (Chang et al., 2014). Thus, given these shared and divergent sensory findings between children with ASD and SPD, and given that alpha and beta connectivity has been associated with sensory gating and sensory attention in these frequency bands (Buchholz et al. 2014; Foxe and Snyder 2011), we hypothesize that similar shared and divergent MEG-derived findings of resting state functional connectivity in the alpha and beta ranges will be identified between children with ASD, SPD, and TDC participants. In addition, based on work implicating alpha oscillations in the direction of auditory attention (Bauer et al. 2012) and evidence of somatosensory cortex beta band modulation in advance of tactile stimuli (van Ede et al. 2010), we also hypothesize that alpha connectivity will be associated with auditory processing and beta connectivity will be associated with tactile processing. To test these hypotheses, these frequency bands were subjected to source space reconstruction for analysis of differences in long-range neural synchrony and associations with sensory processing abilities.
Methods
Participants
Participants were 60 boys aged 8–12 years (ASD N=18; SPD N=18; typically developing controls (TDC) N=24) who were recruited from the UCSF Sensory Neurodevelopmental and Autism Program (SNAP) participant registry and website, UCSF SNAP clinic, and local online parent groups. Experimental protocols were approved by the UCSF IRB and carried out in accordance with those approved procedures. Participants provided their written assent and written informed consent was obtained from parents or legal guardians prior to enrollment. Consent and assent procedures were witnessed by a member of the study team. Participants were recruited between 5/22/2003 and 10/26/2015. All participants who were taking medication were on a stable dose for at least six weeks prior to testing as reported in our previously published studies that recruited from this pool of participants (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015). Specifically, in the TDC group one participant regularly used an antihistamine and a leukotriene inhibitor for seasonal allergies as well as melatonin for sleep. Another TDC participant regularly used steroid medications paired with a bronchodilator as needed for asthma and allergies and omeprazole for reflux. A third TDC participant regularly used methylphenidate for attention. In the SPD group, one participant was prescribed lisdexamfetamine, sertraline, and valproic acid for inattention and challenging behavior, and four others were taking stimulants (amphetamine/dextroamphetamine and methylphenidate) for inattention. One additional SPD participant was taking nonstimulant medication (atomoxetine) for inattention and montelukast for allergies, and another was taking steroid medication for asthma. In the ASD group, one participant was taking a chelation agent (DMSA), another participant was taking escitalopram for anxiety, and a third was taking guanfacine and methylphenidate for calming and inattention.
Inclusion/exclusion criteria and diagnostic classification followed the criteria utilized in previous studies (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015). Specifically, exclusion criteria included (1) bipolar disorder, psychotic disorder, or other neurological disorder or injury, and (2) a score of 70 or below on the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV; Wechsler, 2003) Perceptual Reasoning Index (PRI). The PRI rather than the Full Scale Intelligence Quotient (FSIQ) was utilized for exclusion criteria because verbal abilities (represented in the Verbal Comprehension Index and incorporated into the FSIQ) were examined as an outcome measure in this study. Specifically, those with prior clinical diagnosis of ASD and those scoring ≥15 on the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003), regardless of previous diagnostic status, were evaluated with the Autism Diagnostic Inventory-Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994) and the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1989). Diagnostic cutoffs on both of these measures were met for participants in the ASD group, who also met DSM-IV-TR criteria for Autistic Disorder, confirmed by a pediatric neurologist (EJM). SPD participants were previously diagnosed with SPD by a community occupational therapist. Inclusion criteria for this group were included (1) SCQ score <15 and (2) a score in the “Definite Difference” range in one or more of the auditory, visual, oral/olfactory, tactile, vestibular, or multisensory processing domains of the Sensory Profile (Dunn 1999). All SCQ and Sensory Profile scores for the TDC group were not in clinical ranges. Demographic characteristics of the study sample are presented in Table 1. [Table 1]
Table 1.
Group Characteristics (M ± SD [range])
| ASD | SPD | TDC | Statistics | |
|---|---|---|---|---|
|
| ||||
| Age | 9.88 ± 1.32 [8.13–12.00] | 9.94 ± 1.29 [8.28–12.08] | 10.18 ± .1.13 [8.18–11.94] | F(2,57) = .36 |
| FSIQ | 96.94 ± 13.54 ac [71–121] | 109.39 ± 11.35 [89–131] | 114.92 ± 9.31 [97–135] | F(2,57)=13.20*** |
| PRI | 103.17 ± 8.56 d [94–123] | 113.11 ± 11.63 [92–131] | 111.00 ± 12.29 [89–129] | F(2,57)=4.09* |
| VCI | 98.56 ± 21.81 bd [59–140] | 113.11 ± 14.63 [83–136] | 118.46 ± 13.08 [93–144] | F(2,57)=7.65** |
| Sensory Profile Total Score | 135.78 ± 16.92 ac [102–160] | 119.11 ± 17.38 a [74–145] | 172.04 ± 10.38 [145–187] | F(2,57)=71.12*** |
| Ethnicity (N) | ||||
| Caucasian | 10 | 12 | 17 | |
| Asian | 4 | 1 | 1 | |
| Multiracial | 4 | 3 | 4 | |
| Hispanic | 0 | 1 | 0 | |
| Unknown | 0 | 1 | 2 | |
| Handedness | ||||
| Right | 15 | 17 | 20 | |
| Left | 1 | 1 | 2 | |
| Ambidextrous | 2 | 0 | 1 | |
| Unknown | 0 | 0 | 1 | |
p < .05
p < .01
p < .001
Significantly different from TDC at p<.001 following Bonferroni correction for multiple comparisons
Significantly different from TDC at p<.01 following Bonferroni correction for multiple comparisons
Significantly different from SPD at p<.01 following Bonferroni correction for multiple comparisons
Significantly different from SPD at p<.05 following Bonferroni correction for multiple comparisons
Measures
Tactile Processing.
Tactile processing measures were assessed according to previously published procedures (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015). Tactile form discrimination was assessed using the Van Boven Domes task (Van Boven & Johnson, 1994) and quantified by the lowest grating size of passed trials. Tactile proprioception was measured according to the total score of the right and left hand scores of the graphesthesia subtest of the Sensory Integration Praxis Tests (Ayres 1989).
Auditory Processing.
Auditory processing also was assessed according to previously published procedures (Demopoulos, Brandes-Aitken, et al. 2015; Demopoulos et al., 2017) via the Acoustic (AI) and Acoustic-Linguistic Index (ALI) of the Differential Screening Test for Processing (DSTP; Richard & Ferre, 2006). The AI is derived from performance on measures of dichotic listening, temporal sequencing, and auditory filtering skills. The ALI assesses auditory processing skills associated with language via tasks focused on phonic and phonemic manipulation.
Verbal Abilities.
Because auditory processing dysfunction has been repeatedly associated with weaker verbal abilities in children with ASD (Demopoulos et al. 2017; Edgar et al. 2013; Oram-Cardy et al. 2005; Roberts et al. 2011; Russo et al. 2009; Schmidt et al. 2009), we also assessed for associations between functional connectivity and verbal abilities in the ASD group using established protocols for our assessment of verbal abilities (Demopoulos et al. 2017; Demopoulos, Brandes-Aitken, et al. 2015). The Linguistic Index (LI) of the DSTP was used to evaluate semantic and pragmatic aspects of language. The VCI of the WISC-IV (Wechsler, 2003) was used to index verbal intellectual abilities.
Magnetic Resonance Image (MRI) Acquisition and Processing.
Structural MRIs were acquired for co-registration with MEG functional data on a 3T Siemens MRI scanner at the UCSF Neuroscience Imaging Center. T1-weighted images were spatially normalized to the standard Montreal Neurological Institute template brain using 5mm voxels in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Normalization results were manually verified in all participants.
Magnetoencephalographic Image Acquisition and Processing.
Methods for acquisition and processing of MEG data follow protocols similar to those used in prior research employing these imaginary coherence metrics (Demopoulos et al. 2020; Ranasinghe et al. 2017). Specifically, MEG data were acquired at a 1200 Hz sampling rate using a 275-channel CTF System whole-head biomagnetometer (MEG International Services Ltd., Coqiotlam, BC, Canada). Fiducial coils were placed at the nasion and bilateral peri-auricular points to localize the head to the sensor array. These localizations were utilized for coregistration to the T1-weighted MRI and generation of a head shape. Four minutes of continuous recording was collected from each subject while awake with eyes closed in a supine position. While keeping eyes closed can increase alpha in resting state activity, it also serves to control visual stimulation and because this procedure was implemented for all participants, this would not confound group contrasts. As such, we elected to use an eyes closed approach, as has been used in many previous studies of resting state activity in children with ASD (Berman et al. 2015; Brodski-Guerniero et al. 2018; Cornew et al. 2012; Edgar et al. 2019; Edgar, Heiken, et al. 2015; Green et al. 2020, 2022; Port et al. 2019). Based on previous studies demonstrating reliable results from 60 second segments of MEG resting state data (Guggisberg et al. 2008; Hinkley 2010; Hinkley et al. 2011), we selected a 60-second artifact-free epoch. Artifact rejection criteria were signal amplitude >10pT or visual evidence of movement or muscle contractions.
A whole brain lead field was computed according to a spatially normalized MRI with a 10mm voxel size. The Neurodynamic Utility Tool for MEG (NUTMEG; http://nutmeg.berkeley.edu; Dalal et al., 2011) was used for source-space reconstruction and functional connectivity analyses. Source-space was reconstructed from filtered sensor (fourth-order Butterworth filter of 1–20 Hz). A linear combination of spatial weighting and sensor data matrices were used to estimate each voxel’s amplitudes (Hinkley et al., 2011).
Following source space reconstruction, functional connectivity analysis was performed by computing imaginary coherence. The imaginary coherence approach excludes zero- or π-phase-lag-connectivity to eliminate neural synchrony attributable to volume spread (Nolte et al. 2004). This approach has been documented as a reliable method for estimating long-range neural synchrony (Engel et al. 2013; Guggisberg et al. 2008; Martino et al. 2011; Nolte et al. 2004), and has been shown to reduce overestimation (Guggisberg et al. 2008; Martino et al. 2011; Nolte et al. 2004). Imaginary coherence values were transformed to Fisher’s Z prior to calculating associations between each voxel and all other voxels. These associations were averaged within each voxel to derive voxel wise global connectivity values for group contrasts in the alpha and beta frequency bands. Correlations also were performed between behavioral measures and global connectivity values at each voxel for the combined group study sample. All voxel-wise results with uncorrected p < 0.05 were further subjected to a 5% False Discovery Rate multiple comparisons correction (Benjamini and Hochberg 1995) and a 5-voxel cluster correction.
Missing Data.
Data from the sensory battery tasks are missing for some participants because these tasks were added to the protocol after these participants were enrolled. Thus, these data can be considered missing at random. DSTP data was available for 17 ASD participants, 17 TDC participants, and 11 SPD participants. Van Boven Domes were administered to 16 participants in the ASD group, 16 in the TDC group, and 11 in the SPD group. Graphesthesia was administered to 17 ASD participants, 15 TDC participants, and 11 SPD participants.
Results
Group Contrasts in Alpha Connectivity.
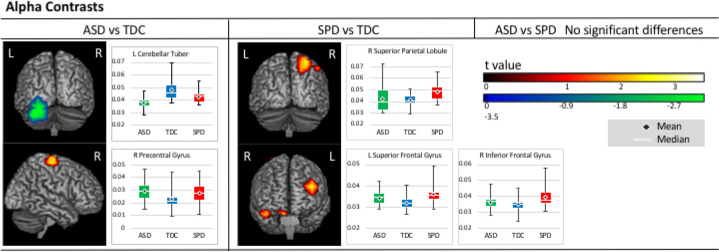
Group contrasts in alpha coherence indicated that, relative to TDC participants, the ASD group showed reduced connectivity in the left fusiform and inferior occipital gyri and cerebellum and increased connectivity in the right pre- and postcentral gyri. No significant differences were identified between the ASD and SPD groups; however, the SPD group showed increased connectivity compared to TDC participants in the left middle and superior frontal gyri and in the right inferior frontal gyrus, precuneus, and inferior and superior parietal lobules. Alpha contrast results are presented in Figure 1 and summarized in Table 2.
Figure 1.
Alpha Contrasts. Areas of significantly increased (warm) and reduced (cool) alpha connectivity are presented on figures for each pairwise contrast. Accompanying boxplots are presented for each cluster showing imaginary coherence values for all groups at the voxel within that cluster that demonstrated the greatest pairwise difference.
Table 2.
Summary of Group Contrast Results
| Group | Band | Regions | Direction of Difference |
|---|---|---|---|
| ASD vs. TDC | α | left fusiform and inferior occipital gyri and cerebellum | Decreased |
| right pre- and postcentral gyri | Increased | ||
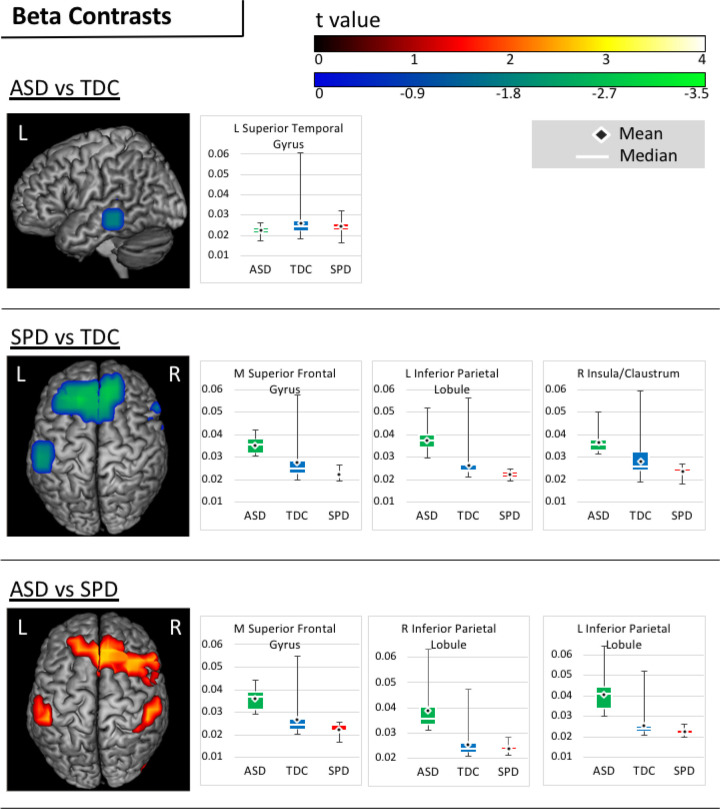
| β | left middle and inferior temporal gyri | Decreased | |
| SPD vs. TDC | α | left middle and superior frontal gyri and in the right inferior frontal gyrus, precuneus, and inferior and superior parietal lobules | Increased |
| β | bilaterally in the superior and middle frontal gyri, insula and putamen, as well as in the left inferior frontal gyrus, cingulate gyrus, caudate body, pre- and postcentral gyri, and inferior parietal lobule, and the right superior temporal gyrus, lentiform nucleus, globus pallidus, and caudate | Decreased | |
| ASD vs. SPD | α | no significant differences | N/A |
| β | right cingulate, middle frontal, and precentral gyri, and bilaterally in the superior and medial frontal gyri, the postcentral gyrus, the inferior parietal lobule, and in the supramarginal gyrus | Increased |
Correlations Between Alpha Connectivity and Sensory Processing/Verbal Abilities.
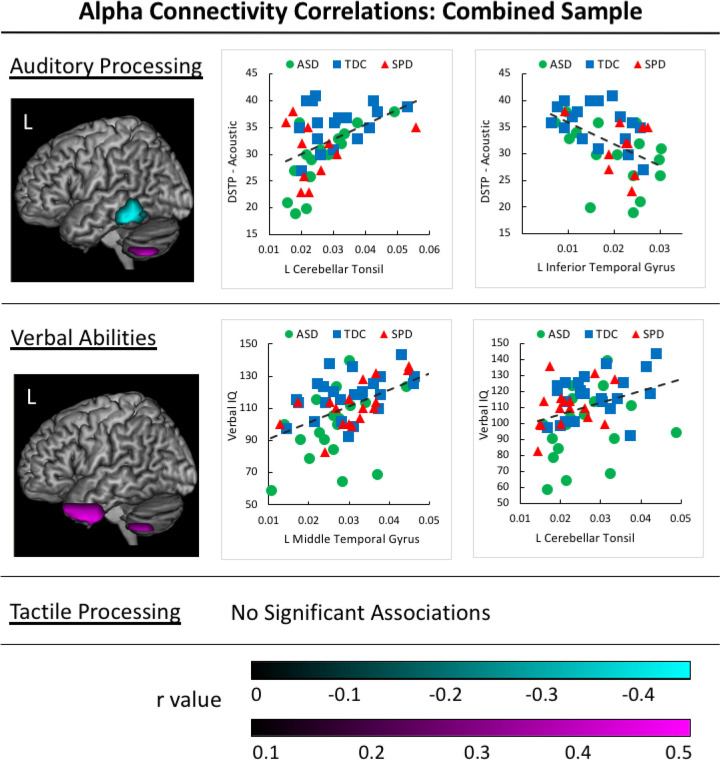
Correlation analyses were performed on all study participants combined across groups to examine the relations between functional connectivity and the range of sensory processing and verbal abilities in our sample. No significant associations were identified between tactile processing performance and measures of alpha coherence; however, significant associations were identified between measures of alpha coherence and auditory processing performance. Specifically, scores on the DSTP Acoustic scale were positively associated with alpha coherence in the left cerebellar tonsil and negatively associated with alpha coherence in the left inferior and middle temporal gyri. A significant positive association also was identified between VIQ and alpha coherence in the left uncus, cerebellar tonsil, and anterior superior, middle, and inferior temporal gyri (Figure 2). A summary of correlation results is presented in Table 3.
Figure 2.
Alpha Correlations in the Combined Participant Sample. Positive associations between auditory processing/verbal abilities and alpha connectivity values are identified in magenta clusters for the sample of all participants in the study. Negative associations between auditory processing and imaginary coherence values are identified in cyan clusters. Corresponding scatterplots are presented for the voxel with the greatest correlation value within each cluster, with groups identified by color and shape (ASD group = yellow circle, SPD group = green triangle, and TDC group = grey square).
Table 3.
Summary of Correlation Results for the Combine Groups Sample
| Band | Domain | Task | Regions | Direction of Correlation |
|---|---|---|---|---|
| α | tactile | no significant associations | N/A | |
| auditory | DSTP Acoustic Scale | left cerebellar tonsil | + | |
| left inferior and middle temporal gyri | − | |||
| verbal | VIQ | left uncus, cerebellar tonsil, and anterior superior, middle, and inferior temporal gyri | + | |
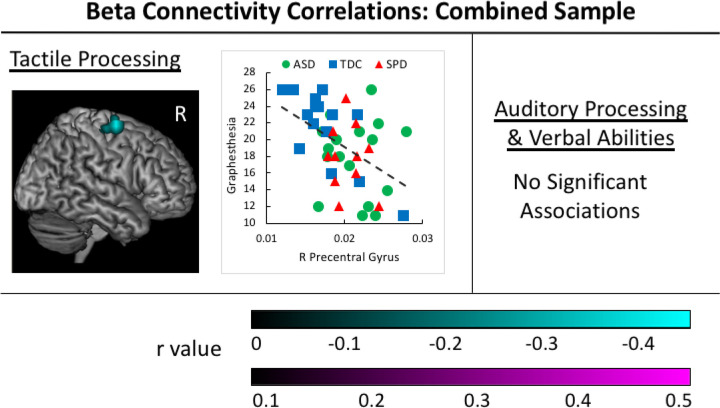
| β | tactile | Graphesthesia | right precentral gyrus | − |
| auditory | no significant associations | N/A | ||
| verbal | no significant associations | N/A |
Group Contrasts in Beta Connectivity.
Group contrasts in beta coherence indicated that, relative to TDC participants, the ASD group showed reduced connectivity in the left middle and inferior temporal gyri. Relative to SPD participants, however, the ASD group showed a pattern of increased beta connectivity in the right cingulate, middle frontal, and precentral gyri, and bilaterally in the superior and medial frontal gyri, the postcentral gyrus, the inferior parietal lobule, and in the supramarginal gyrus. Finally, when compared to TDC participants, the SPD group demonstrated a pattern of reduced beta connectivity bilaterally in the superior and middle frontal gyri, insula and putamen, as well as in the left inferior frontal gyrus, cingulate gyrus, caudate body, pre- and postcentral gyri, and inferior parietal lobule, and in the right superior temporal gyrus, lentiform nucleus, globus pallidus, and caudate. Beta contrasts are presented in Figure 3 and summarized in Table 2.
Figure 3.
Beta Contrasts. Areas of significantly increased (warm) and reduced (cool) beta connectivity are presented on figures for each pairwise contrast. Accompanying boxplots are presented for each cluster showing imaginary coherence values for all groups at the voxel within that cluster that demonstrated the greatest pairwise difference.
Correlations Between Beta Connectivity and Sensory Processing.
A significant negative association was identified between beta coherence in the right precentral gyrus and performance on the graphesthesia task (Figure 4). No significant associations were identified between beta coherence and measures of auditory processing or verbal abilities in the combined groups sample. A summary of correlation results is presented in Table 3.
Figure 4.
Beta Correlations in the Combined Participant Sample. Negative associations between tactile processing abilities and beta connectivity values are identified in the cyan cluster for the sample of all participants in the study. The corresponding scatterplot is presented for the voxel with the greatest correlation value within each cluster, with groups identified by color and shape (ASD group = yellow circle, SPD group = green triangle, and TDC group = grey square).
Discussion
This study used two methods to investigate associations between direct assessment of auditory and tactile sensory processing and resting state functional connectivity in the brain. First, we examined differences between groups that would allow us to isolate the sensory processing dysfunction that presents as part of an ASD from that which manifests in the absence of the other defining features of ASD. Second, we directly examined associations between functional connectivity and auditory and tactile processing and verbal abilities in a combined participant sample including all three groups, allowing us to examine the distribution of these variables across children with a range of sensory functioning.
Group Contrasts in Functional Connectivity
ASD vs TDC Contrasts.
Relative to the TDC group, participants with ASD showed increased alpha connectivity in the right sensorimotor cortex and decreased connectivity in left posterior fusiform, occipital, and cerebellar regions. Notably, increased alpha power (Edgar, Heiken, et al. 2015) in a similar region in the right medial sensorimotor cortex, and increased alpha to low-gamma phase amplitude coupling in this central midline region (Port et al. 2019) has been reported in prior ASD samples. The present results also recapitulate our previous structural findings in children with ASD, in which we reported decreased structural connectivity in the inferior fronto-occipital fasciculus and the fusiform-hippocampus and fusiform-amygdala tracts (Chang et al., 2014). Our findings of increased cerebellar connectivity are also consistent with considerable prior research implicating the cerebellum in the pathology of ASD. Specifically, cerebellar anomalies, including abnormal anatomy, neurotransmission, oxidative stress, neuroinflammation, and cerebellar motor and cognitive deficits are among the most replicated findings in individuals with ASD (Fatemi et al. 2012).
In the beta range, the ASD group demonstrated decreased beta connectivity in left temporal regions relative to TDC participants. Stronger beta connectivity in TDC relative to ASD participants in temporal regions has been demonstrated in prior work (Kitzbichler et al. 2015). Beta power in the auditory cortex has been hypothesized to be involved in auditory-motor communication (Fujioka et al. 2009) and recent work has demonstrated increases in sensorimotor low beta power in response to perceived self-produced vocal errors on an altered auditory feedback speech paradigm (Franken et al. 2018). The decreased beta connectivity in the left auditory cortex demonstrated in the present study may reflect under-recruitment of this area needed for auditory processing and auditory motor communication in participants with ASD.
SPD vs TDC Contrasts.
The SPD group differed from the TDC group via increased alpha connectivity in bilateral frontal and right posterior parietal regions and reduced beta connectivity in left parietal and medial and right frontal regions. These differences in functional connectivity identified in these regions may be associated with the impairments in visuomotor skills and attention previously reported in the SPD population (Brandes-Aitken et al., 2018). In fact, prior work examining diffusion imaging in children with SPD identified associations between visuomotor and cognitive control abilities and structural connectivity in regions of the superior longitudinal fasciculus that run adjacent to the parietal regions identified in this study (Brandes-Aitken et al., 2019).
ASD vs SPD Alpha and Beta Contrasts.
Notably, the ASD and SPD groups did not show significant differences in alpha connectivity. In fact, it was beta connectivity that distinguished these two groups. Specifically, the SPD group showed a pattern of reduced beta connectivity relative to both the TDC and ASD groups in bilateral and medial frontal and left parietal regions. Taken together, these findings suggest that decreased beta connectivity in medial frontal and parietal regions may be involved in, or a response to, the sensory disturbance experienced by children with SPD. Beta activity has been previously reported to be associated with somatosensory gating and attention (Bauer et al. 2012; Buchholz et al. 2014; van Ede et al. 2010). Our previous work has demonstrated common tactile processing deficits in both ASD and SPD groups (Demopoulos, Brandes-Aitken, et al. 2015), although when MEG-acquired somatosensory latencies were compared between these groups, the SPD group demonstrated an intermediate latency and did not significantly differ from TDC or ASD participants (Demopoulos et al. 2017). These previous results, in conjunction with the present finding that beta activity distinguished the ASD and SPD groups in the bilateral somatosensory cortex, may suggest that the pathology underlying tactile dysfunction in these two groups is divergent.
Combined Groups Correlation Results.
When correlation analyses were performed on all participants combined into one group, alpha connectivity was positively associated with auditory and verbal abilities, whereas beta connectivity was negatively associated with tactile processing. Specifically, there was a common area of positive correlation between left cerebellar alpha connectivity and both auditory processing and verbal abilities; however, an additional positive association was identified between left anterior temporal alpha connectivity and verbal abilities. Previous work has identified an association between increased anterior temporal alpha power and autism symptomatology measured via the SRS total score (Cornew et al. 2012). whereas an additional negative association was identified between posterior temporal alpha connectivity and auditory processing. Taken together, these findings may suggest that increased cerebellar alpha recruitment may be utilized to address auditory processing weakness that affects not only basic auditory processing abilities, a deficit that is common in individuals with ASD (Abdeltawwab and Baz 2015; Alcántara et al. 2004; Demopoulos et al. 2017; Demopoulos, Hopkins, et al. 2015; Demopoulos and Lewine 2016; DePape et al. 2012; Edgar et al. 2013, 2014; Edgar, Fisk IV, et al. 2015; Gage, Siegel, Callen, et al. 2003; Gage, Siegel, and Roberts 2003; Hitoglou et al. 2010; Järvinen-Pasley and Heaton 2007; Kargas et al. 2015; Oram-Cardy et al. 2005; Oram Cardy et al. 2005; Tecchio et al. 2003; Tomcheck and Dunn 2007), but also verbal abilities. Indeed, prior work has demonstrated links between cortical auditory processing abnormalities and verbal abilities (Berman et al. 2016; Demopoulos et al. 2017; Edgar et al. 2013; Oram-Cardy et al. 2005; Oram Cardy et al. 2008; Roberts et al. 2011, 2012; Schmidt et al. 2009). With regard to beta connectivity, increases in the right somatosensory cortex were associated with poorer performance on the graphesthesia task. Examination of the scatterplot distribution suggests that somatosensory processing limitations may drive the graphesthesia impairments demonstrated in the two clinical groups. Correlation results were consistent with our hypothesis that beta connectivity would be associated with tactile processing and alpha connectivity would be associated with auditory processing. This is consistent with prior work in which alpha oscillations were associated with direction of auditory attention (Bauer et al. 2012) and somatosensory cortex beta band modulation was reported in advance of tactile stimuli (van Ede et al. 2010).
Limitations and Future Directions
Several limitations of the present study must be acknowledged. First, the participant sample was restricted to males between the ages of 8–12 years. Prior studies examining resting state neural oscillatory behavior have also restricted analyses to males given the high prevalence of ASD in males and sex differences in peak alpha frequency (Edgar et al. 2019; Green et al. 2022; Manyukhina et al. 2022). While these restrictions result in more homogenous groups and minimize confounds of sex and age differences in neurobiology, they also create limitations for the generalizability of these results to females and children and adolescents outside the age range studies. Future research is necessary to understand the applicability of these findings across ages and sexes. This study also included only children with a nonverbal IQ>70, which limits the generalizability of these results to lower functioning individuals. Further, this study focused on only two frequency bands (alpha and beta) and only two sensory domains, auditory and tactile processing. While prior research suggests that these domains may be the most severely impacted in individuals with ASD (Fernandez-Andres et al. 2015), sensory dysfunction is heterogeneous in its presentation among individuals with and without ASD, and understanding neurobiological factors associated with dysfunction in other sensory domains also will be important to inform treatment development. Finally, this study focused on specific aspects of sensory processing (e.g., discrimination, temporal processing, etc.), but did not incorporate measures of sensory responsivity or sensory seeking behavior. Further, this work only focused on two frequency bands, alpha and beta. Future studies could expand upon this work to examine relations between sensory processing dysfunction and functional connectivity in other frequency bands, as gamma oscillatory behavior has been associated with multisensory communication (Misselhorn et al. 2019) and sensory sensitivity (Manyukhina et al. 2021). Future studies are needed to characterize differences in functional connectivity that may account for these heterogeneous sensory responses or behaviors in children with ASD and SPD.
Conclusions
This study was the first to use MEG to examine participants with ASD and SPD in relation to neurotypical children to identify relevant differences in resting state whole brain functional connectivity that may be associated with sensory dysfunction. This study design allowed us to identify both shared and distinct patterns of neural activity in two groups affected by sensory dysfunction. Specifically, both clinical groups were distinguished from the TDC group by patterns of functional connectivity differences in the alpha and beta bands, whereas the clinical groups were only distinguished from each other on measures of beta connectivity. Associations between functional connectivity and behavior identified that sensorimotor regions were associated with tactile processing performance and temporal and cerebellar regions were associated with auditory processing and language abilities. These results suggest that resting state differences in oscillatory brain activity in the alpha and beta frequencies is associated with the sensory dysfunction that characterizes children with ASD and SPD.
Acknowledgments:
CD was funded in part by National Institutes of Health grants (K23DC016637-01A1, R01DC019167-01A1) Autism Speaks CAPD Pilot award 11637, and UCSF Weill Institute for Neurosciences Weill Award for Clinical Neuroscience Research (2016038). EJM was funded by NIH grant K23MH083890, the Wallace Research Foundation and crowdfunding support to the UCSF Sensory Neurodevelopment & Autism Program. SSN was funded in part by National Institutes of Health grants (R01NS100440, R01DC176960, R01DC017091, R01AG062196), UCOP-MRP-17-454755, and the US Department of Defense grant (W81XWH-13-1-0494).
Footnotes
The authors have no competing interests to declare.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- Abdeltawwab Mohamed Moustafa, and Baz Hemmat. 2015. “Automatic Pre-Attentive Auditory Responses: MMN to Tone Burst Frequency Changes in Autistic School-Age Children.” The Journal of International Advanced Otology 11(1):36–41. doi: 10.5152/iao.2014.438. [DOI] [PubMed] [Google Scholar]
- Adamson Amanda, Hare Anne O., and Graham Catriona. 2006. “Impairments in Sensory Modulation in Children with Autistic Spectrum Disorder.” British Journal of Occupational Therapy 69(8):357–64. [Google Scholar]
- Adolph Dirk, and Margraf Jürgen. 2017. “The Differential Relationship between Trait Anxiety, Depression, and Resting Frontal α-Asymmetry.” Journal of Neural Transmission 124(3):379–86. doi: 10.1007/s00702-016-1664-9. [DOI] [PubMed] [Google Scholar]
- Ahn R. R., Miller L. J., Milberger S., and McIntosh D. N.. 2004. “Prevalence of Parents’ Perceptions of Sensory Processing Disorders among Kindergarten Children.” Am J Occup Ther 58(3):287–93. [DOI] [PubMed] [Google Scholar]
- Al-Heizan Mohammed O., AlAbdulwahab Sami S., Kachanathu Shaji John, and Natho Mohan. 2015. “Sensory Processing Dysfunction among Saudi Children with and without Autism.” Journal of Physical Therapy Science 27(5):1313–16. doi: 10.1589/jpts.27.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcántara José I., Weisblatt Emma J. L., Moore Brian C. J., and Bolton Patrick F.. 2004. “Speech-in-Noise Perception in High-Functioning Individuals with Autism or Asperger’s Syndrome.” Journal of Child Psychology and Psychiatry, and Allied Disciplines 45(6):1107–14. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). American Psychiatric Association. [Google Scholar]
- Anon. n.d. “Van Boven Domes.” [Google Scholar]
- Ayres Jean. 1989. Sensory Integration and Praxis Tests (SIPT). Los Angeles: Western Psychological Services. [Google Scholar]
- Bauer M., Kennett S., and Driver J.. 2012. “Attentional Selection of Location and Modality in Vision and Touch Modulates Low-Frequency Activity in Associated Sensory Cortices.” Journal of Neurophysiology 107(9):2342–51. doi: 10.1152/jn.00973.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., and Hochberg Y.. 1995. “Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing.” Journal of Royal Statistical Society 57:289–300. [Google Scholar]
- Berman Jeffrey I., Edgar James C., Blaskey Lisa, Kuschner Emily S., Levy Susan E., Ku Matthew, Dell John, and Roberts Timothy P. L.. 2016. “Multimodal Diffusion-MRI and MEG Assessment of Auditory and Language System Development in Autism Spectrum Disorder.” Frontiers in Neuroanatomy 10(March). doi: 10.3389/fnana.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JI, Liu S., Bloy L., Blaskey L., Roberts T., and Edgar JC. 2015. “Alpha-to-Gamma Phase-Amplitude Coupling Methods and Application to Autism Spectrum Disorder.” Brain Connectivity 5(2):89–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutcher S. H., and Landers D. M.. 1998. “The Effects of Vigorous Exercise on Anxiety, Heart Rate, and Alpha Activity of Runners and Nonrunners.” Psychophysiology 25(6):696–702. [DOI] [PubMed] [Google Scholar]
- Van Boven R. W., and Johnson K. O.. 1994. “The Limit of Tactile Spatial Resolution in Humans: Grating Orientation Discrimination at the Lip, Tongue, and Finger.” Neurology 44(12):2361–66. [DOI] [PubMed] [Google Scholar]
- Brandes-Aitken A., Anguera J. A., Rolle C. E., Desai S. S., Demopoulos C., Skinner S. N., Gazzaley A., and Marco E. J.. 2018. “Characterizing Cognitive and Visuomotor Control in Children With Sensory Processing Dysfunction and Autism Spectrum Disorders.” Neuropsychology 32(2):148–60. doi: 10.1037/neu0000404. [DOI] [PubMed] [Google Scholar]
- Brandes-Aitken A., Anguera J.A., Chang Y., Demopoulos C., Owen J.P., Gazzaley A., Mukherjee P., and Marco E.J. 2019. “White Matter Microstructure Associations of Cognitive and Visuomotor Control in Children : A Sensory Processing Perspective.” Frontiers in Integrative Neuroscience 12(January):1–12. doi: 10.3389/fnint.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodski-Guerniero A., Naumer MJ, Moliadze V., Chan J., Althen H., Ferreira-Santos F., Lizier JT, Schlitt S., Kitzerow J., Schütz M., Langer A., Kaiser J., Freitag CM, and Wibral M.. 2018. “Predictable Information in Neural Signals during Resting State Is Reduced in Autism Spectrum Disorder.” Human Brain Mapping 39(8):3227–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz Verena N., Jensen Ole, and Medendorp W. Pieter. 2014. “Different Roles of Alpha and Beta Band Oscillations in Anticipatory Sensorimotor Gating.” Frontiers in Human Neuroscience 8(June):1–9. doi: 10.3389/fnhum.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Yi-Shin, Owen Julia P., Desai Shivani S., Hill Susanna S., Arnett Anne B., Harris Julia, Marco Elysa J., and Mukherjee Pratik. 2014. “Autism and Sensory Processing Disorders: Shared White Matter Disruption in Sensory Pathways but Divergent Connectivity in Social-Emotional Pathways.” PloS One 9(7):e103038. doi: 10.1371/journal.pone.0103038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Yi Shin, Gratiot Mathilde, Owen Julia P., Anne Brandes-Aitken Shivani S. Desai, Hill Susanna S., Arnett Anne B., Harris Julia, Marco Elysa J., and Mukherjee Pratik. 2016. “White Matter Microstructure Is Associated with Auditory and Tactile Processing in Children with and without Sensory Processing Disorder.” Frontiers in Neuroanatomy 9:1–16. doi: 10.3389/fnana.2015.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornew Lauren, Roberts Timothy P. L., Blaskey Lisa, and Edgar J. Christopher. 2012. “Resting-State Oscillatory Activity in Autism Spectrum Disorders.” Journal of Autism and Developmental Disorders 42(9):1884–94. doi: 10.1007/s10803-011-1431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal Sarang S., Zumer Johanna M., Guggisberg Adrian G., Trumpis Michael, Wong Daniel D. E., Sekihara Kensuke, and Nagarajan Srikantan S.. 2011. “MEG/EEG Source Reconstruction, Statistical Evaluation, and Visualization with NUTMEG.” Computational Intelligence and Neuroscience 2011. doi: 10.1155/2011/758973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerdzieva A., and Pop-Jordanova N.. 2015. “Relation between Frontal Alpha Asymmetry and Anxiety in Young Patients with Generalized Anxiety Disorder.” Pril (Makedon Akad Nauk Umet Odd Med Nauki) 36(2):157–77. [DOI] [PubMed] [Google Scholar]
- Demopoulos C., Aitkens A., Findley A., Mizuiri D., Honma S., Desai S. S., Antovich A. D., Yu N., Hill S. S., Nagarajan S., and Marco E. J.. 2017. “Magnetoencephalographic Imaging of Auditory and Somatosensory Cortical Responses in Children with Autism and Sensory Processing Dysfunction.” Frontiers in Human Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos Carly, Brandes-Aitken Annie N., Desai Shivani S., Hill Susanna S., Antovich Ashley D., Harris Julia, and Marco Elysa J.. 2015. “Shared and Divergent Auditory and Tactile Processing in Children with Autism and Children with Sensory Processing Dysfunction Relative to Typically Developing Peers.” Journal of the International Neuropsychological Society 21(6):444–54. doi: 10.1017/S1355617715000387. [DOI] [PubMed] [Google Scholar]
- Demopoulos Carly, Duong Xuan, Hinkley Leighton B., Ranasinghe Kamalini G., Mizuiri Danielle, Garrett Coleman, Honma Susanne, Henderson-Sabes Jennifer, Findlay Anne, Racine-Belkoura Caroline, Cheung Steven W., and Nagarajan Srikantan S.. 2020. “Global Resting-State Functional Connectivity of Neural Oscillations in Tinnitus with and without Hearing Loss.” Human Brain Mapping 41(10):2846–61. doi: 10.1002/hbm.24981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos Carly, Hopkins Joyce, Kopald Brandon E. B. E., Paulson Kim, Doyle Lauren, Andrews W. E. Whitney E., and Lewine Jeffrey David J. D.. 2015. “Deficits in Auditory Processing Contribute to Impairments in Vocal Affect Recognition in Autism Spectrum Disorders: A MEG Study.” Neuropsychology 29(6):895–908. doi: 10.1037/neu0000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos Carly, and Lewine Jeffrey David J. D.. 2016. “Audiometric Profiles in Autism Spectrum Disorders: Does Subclinical Hearing Loss Impact Communication?” Autism Research 9(1):107–20. doi: 10.1002/aur.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demopoulos Carly, Yu Nina, Tripp Jennifer, Mota Nayara, Brandes-Aitken Anne N., Desai S. S. Shivani S., Hill Susanna S., Antovich A. D. Ashley D., Harris Julia, Honma Susanne, Mizuiri Danielle, Nagarajan Srikantan S., Marco E. J. Elysa J., Aitkens A., Findley A., Mizuiri Danielle, Honma Susanne, Desai S. S. Shivani S., Antovich A. D. Ashley D., Yu Nina, Hill Susanna S., Nagarajan Srikantan S., and Marco E. J. Elysa J.. 2017. “Magnetoencephalographic Imaging of Auditory and Somatosensory Cortical Responses in Children with Autism and Sensory Processing Dysfunction.” Frontiers in Human Neuroscience 11(May):1–15. doi: 10.3389/fnhum.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePape Anne-Marie R., Hall Geoffrey B. C., Tillmann Barbara, and Trainor Laurel J.. 2012. “Auditory Processing in High-Functioning Adolescents with Autism Spectrum Disorder.” PLoS ONE 7(9):e44084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn Winifred. 1999. Sensory Profile User’s Manual. San Antonio, TX: Psychological Corporation. [Google Scholar]
- van Ede Freek, Jensen Ole, and Maris Eric. 2010. “Tactile Expectation Modulates Pre-Stimulus Α-Band Oscillations in Human Sensorimotor Cortex.” NeuroImage 51(2):867–76. doi: 10.1016/j.neuroimage.2010.02.053. [DOI] [PubMed] [Google Scholar]
- Edgar J. Christopher, Dipiero Marissa, McBride Emma, Green Heather L., Berman Jeffrey, Ku Matthew, Liu Song, Blaskey Lisa, Kuschner Emily, Airey Megan, Ross Judith L., Bloy Luke, Kim Mina, Koppers Simon, Gaetz William, Schultz Robert T., and Roberts Timothy P. L.. 2019. “Abnormal Maturation of the Resting-State Peak Alpha Frequency in Children with Autism Spectrum Disorder.” Human Brain Mapping 40(11):3288–98. doi: 10.1002/hbm.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J. Christopher, Fisk Charles L. IV, Berman Jeffrey I., Chudnovskaya Darina, Liu Song, Pandey Juhi, Herrington John D., Port Russell G., Schultz Robert T., and Roberts Timothy P. L.. 2015. “Auditory Encoding Abnormalities in Children with Autism Spectrum Disorder Suggest Delayed Development of Auditory Cortex.” Molecular Autism 6(1):69. doi: 10.1186/s13229-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J. Christopher, Heiken Kory, Chen Yu Han, Herrington John D., Chow Vivian, Liu Song, Bloy Luke, Huang Mingxiong, Pandey Juhi, Cannon Katelyn M., Qasmieh Saba, Levy Susan E., Schultz Robert T., and Roberts Timothy P. L.. 2015. “Resting-State Alpha in Autism Spectrum Disorder and Alpha Associations with Thalamic Volume.” Journal of Autism and Developmental Disorders 45(3):795–804. doi: 10.1007/s10803-014-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J., Khan Sarah Y., Blaskey Lisa, Chow Vivian Y., Rey Michael, Gaetz William, Cannon Katelyn M., Monroe Justin F., Cornew Lauren, Qasmieh Saba, Liu Song, Welsh John P., Levy Susan E., and Roberts Timothy P. L.. 2013. “Neuromagnetic Oscillations Predict Evoked-Response Latency Delays and Core Language Deficits in Autism Spectrum Disorders.” Journal of Autism and Developmental Disorders. doi: 10.1007/s10803-013-1904-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar J. Christopher, Lanza Matthew R., Daina Aleksandra B., Monroe Justin F., Khan Sarah Y., Blaskey Lisa, Cannon Katelyn M., Jenkins Julian, Qasmieh Saba, Levy Susan E., and Roberts Timothy P. L.. 2014. “Missing and Delayed Auditory Responses in Young and Older Children with Autism Spectrum Disorders.” Frontiers in Human Neuroscience 8(June):1–13. doi: 10.3389/fnhum.2014.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel Andreas K., Gerloff Christian, Hilgetag Claus C., and Nolte Guido. 2013. “Intrinsic Coupling Modes: Multiscale Interactions in Ongoing Brain Activity.” Neuron 80(4):867–6. doi: 10.1016/j.neuron.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Fatemi S. Hossein, Aldinger Kimberly A., Ashwood Paul, Bauman Margaret L., Blaha Charles D., Blatt Gene J., Chauhan Abha, Chauhan Ved, Dager Stephen R., Dickson Price E., Estes Annette M., Goldowitz Dan, Heck Detlef H., Kemper Thomas L., King Bryan H., Martin Loren A., Millen Kathleen J., Mittleman Guy, Mosconi Matthew W., Persico Antonio M., Sweeney John A., Webb Sara J., and Welsh John P.. 2012. “Consensus Paper: Pathological Role of the Cerebellum in Autism.” Cerebellum 11(3):777–807. doi: 10.1007/s12311-012-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Andres M. Inmaculada, Pastor-Cerezuela Gemma, Sanz-Cervera Pilar, and Tarraga-Mingues Raul. 2015. “A Comparative Study of Sensory Processing in Children with and without Autism Spectrum Disorder in the Home and Classroom Environments.” Research in Developmental Disabilities 38:202–12. doi: 10.1016/j.ridd.2014.12.034. [DOI] [PubMed] [Google Scholar]
- Fingelkurts Andrew A., Fingelkurts Alexander A., Rytsälä Heikki, Suominen Kirsi, Isometsä Erkki, and Kähkönen Seppo. 2007. “Impaired Functional Connectivity at EEG Alpha and Theta Frequency Bands in Major Depression.” Human Brain Mapping 28(3):247–61. doi: 10.1002/hbm.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe John J., and Snyder Adam C.. 2011. “The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention.” Frontiers in Psychology 2(JUL):1–13. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken Matthias K., Eisner Frank, Acheson Daniel J., McQueen James M., Hagoort Peter, and Schoffelen Jan Mathijs. 2018. “Self-Monitoring in the Cerebral Cortex: Neural Responses to Small Pitch Shifts in Auditory Feedback during Speech Production.” NeuroImage 179(January):326–36. doi: 10.1016/j.neuroimage.2018.06.061. [DOI] [PubMed] [Google Scholar]
- Fujioka Takako, Trainor Laurel J., Large Edward W., and Ross Bernhard. 2009. “Beta and Gamma Rhythms in Human Auditory Cortex during Musical Beat Processing.” Annals of the New York Academy of Sciences 1169:89–92. doi: 10.1111/j.1749-6632.2009.04779.x. [DOI] [PubMed] [Google Scholar]
- Gage Nicole M., Siegel Bryna, Callen Melanie, and Roberts Timothy. 2003. “Cortical Sound Processing in Children with Autism Disorder : An MEG Investigation.” Neuroreport 14(16):2047–51. doi: 10.1097/01.wnr.0000090030.46087. [DOI] [PubMed] [Google Scholar]
- Gage Nicole M., Siegel Bryna, and Roberts Timothy P. L.. 2003. “Cortical Auditory System Maturational Abnormalities in Children with Autism Disorder: An MEG Investigation.” Developmental Brain Research 144(2):201–9. [DOI] [PubMed] [Google Scholar]
- Green Heather L., Dipiero Marissa, Koppers Simon, Berman Jeffrey I., Bloy Luke, Liu Song, McBride Emma, Ku Matthew, Blaskey Lisa, Kuschner Emily, Airey Megan, Kim Mina, Konka Kimberly, Roberts Timothy P. L., and Edgar J. Christopher. 2022. “Peak Alpha Frequency and Thalamic Structure in Children with Typical Development and Autism Spectrum Disorder.” Journal of Autism and Developmental Disorders 52(1):103–12. doi: 10.1007/s10803-021-04926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green Heather L., Edgar J. Christopher, Matsuzaki Junko, and Roberts Timothy P. L.. 2020. “Magnetoencephalography Research in Pediatric Autism Spectrum Disorder.” Neuroimaging Clinics of North America 30(2):193–203. doi: 10.1016/j.nic.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan Stanley I., and Wieder Serena. 1997. “Developmental Patterns and Outcomes in Infants and Children with Disorders in Relating and Communicating: A Chart Review of 200 Cases of Children with Autistic Spectrum Diagnoses.” The Journal of Developmental and Learning Disorders 1(1):1–38. [Google Scholar]
- Guggisberg Adrian G., Honma Susanne M., Findlay Anne M., Dalal Sarang S., Kirsch Heidi E., Berger Mitchel S., and Nagarajan Srikantan S.. 2008. “Mapping Functional Connectivity in Patients with Brain Lesions.” Annals of Neurology 63(2):193–203. doi: 10.1002/ana.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley Leighton. 2010. “Cognitive Impairments in Schizophrenia as Assessed through Activation and Connectivity Measures of Magnetoencephalography (MEG) Data.” Frontiers in Human Neuroscience 3(November). doi: 10.3389/neuro.09.073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley Leighton, Vinogradov Sophia, Guggisberg Adrian G., Fisher Melissa, Findlay Anne M., and Nagarajan Srikantan S.. 2011. “Clinical Symptoms and Alpha Band Resting-State Functional Connectivity Imaging in Patients with Schizophrenia: Implications for Novel Approaches to Treatment.” Biological Psychiatry 70(12):1134–42. doi: 10.1016/j.biopsych.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitoglou Magdalini, Ververi Athina, Antoniadis Alexandros, and Zafeiriou Dimitrios I.. 2010. “Childhood Autism and Auditory System Abnormalities.” Pediatric Neurology 42(5):309–14. doi: 10.1016/j.pediatrneurol.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Järvinen-Pasley Anna, and Heaton Pamela. 2007. “Evidence for Reduced Domain-Specificity in Auditory Processing in Autism.” Developmental Science 10(6):786–93. doi: 10.1111/j.1467-7687.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Kargas Niko, Lopez Beatriz, Reddy Vasudevi, Morris Paul, López Beatriz, Reddy Vasudevi, and Morris Paul. 2015. “The Relationship between Auditory Processing and Restricted, Repetitive Behaviors in Adults with Autism Spectrum Disorders.” Journal of Autism and Developmental Disorders 45(3):658–68. doi: 10.1007/s10803-014-2219-2. [DOI] [PubMed] [Google Scholar]
- Kitzbichler Manfred G., Khan Sheraz, Ganesan Santosh, Vangel Mark G., Herbert Martha R., Hämäläinen Matti S., and Kenet Tal. 2015. “Altered Development and Multifaceted Band-Specific Abnormalities of Resting State Networks in Autism.” Biological Psychiatry 77(9):794–804. doi: 10.1016/j.biopsych.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyazev Gennady G., Savostyanov Alexander N., and Levin Evgenij A.. 2006. “Alpha Synchronization and Anxiety: Implications for Inhibition vs. Alertness Hypotheses.” International Journal of Psychophysiology 59(2):151–58. doi: 10.1016/j.ijpsycho.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Lerner Matthew D., McPartland James C., and Morris James P.. 2013. “Multimodal Emotion Processing in Autism Spectrum Disorders: An Event-Related Potential Study.” Developmental Cognitive Neuroscience 3:11–21. doi: 10.1016/j.dcn.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C., Rutter M., and Le Couteur A.. 1994. “Autism Diagnostic Interview-Revised: A Revised Version of a Diagnostic Interview for Caregivers of Individuals with Possible Pervasive Developmental Disorders.” Journal of Autism and Developmental Disorders 24(5):659–85. [DOI] [PubMed] [Google Scholar]
- Lord C., Rutter M., Goode S., Heemsbergen J., Jordan H., Mawhood L., and Schopler E.. 1989. “Autism Diagnostic Observation Schedule: A Standardized Observation of Communicative and Social Behavior.” Journal of Autism and Developmental Disorders 19(2):185–212. [DOI] [PubMed] [Google Scholar]
- Manyukhina Viktoriya O., Prokofyev Andrey O., Galuta Ilia A., Goiaeva Dzerassa E., Obukhova Tatiana S., Schneiderman Justin F., Altukhov Dmitrii I., Stroganova Tatiana A., and Orekhova Elena V.. 2022. “Globally Elevated Excitation–Inhibition Ratio in Children with Autism Spectrum Disorder and below-Average Intelligence.” Molecular Autism 13(1):1–14. doi: 10.1186/s13229-022-00498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manyukhina Viktoriya O., Rostovtseva Ekaterina N., Prokofyev Andrey O., Obukhova Tatiana S., Schneiderman Justin F., Stroganova Tatiana A., and Orekhova Elena V.. 2021. “Visual Gamma Oscillations Predict Sensory Sensitivity in Females as They Do in Males.” Scientific Reports 11(1):1–10. doi: 10.1038/s41598-021-91381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino Juan, Honma Susanne M., Findlay Anne M., Guggisberg Adrian G., Owen Julia P., Kirsch Heidi E., Berger Mitchel S., and Nagarajan Srikantan S.. 2011. “Resting Functional Connectivity in Patients with Brain Tumors in Eloquent Areas.” Annals of Neurology 69(3):521–32. doi: 10.1002/ana.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes Susan Dickerson, and Calhoun Susan L.. 1999. “Symptoms of Autism in Young Children and Correspondence with the DSM.” Infants and Young Children 12(2):90–97. [Google Scholar]
- Mennella Rocco, Patron Elisabetta, and Palomba Daniela. 2017. “Frontal Alpha Asymmetry Neurofeedback for the Reduction of Negative Affect and Anxiety.” Behaviour Research and Therapy 92:32–40. doi: 10.1016/j.brat.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Misselhorn Jonas, Schwab Bettina C., Schneider Till R., and Engel Andreas K.. 2019. “Synchronization of Sensory Gamma Oscillations Promotes Multisensory Communication.” ENeuro 6(5). doi: 10.1523/ENEURO.0101-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte Guido, Bai Ou, Wheaton Lewis, Mari Zoltan, Vorbach Sherry, and Hallett Mark. 2004. “Identifying True Brain Interaction from EEG Data Using the Imaginary Part of Coherency.” Clinical Neurophysiology 115(10):2292–2307. doi: 10.1016/j.clinph.2004.04.029. [DOI] [PubMed] [Google Scholar]
- Oram-Cardy Janis E., Flagg C. A. Elissa J., Roberts Wendy, Brian Jessica, and Roberts Timothy P. L.. 2005. “Magnetoencephalography Identifies Rapid Temporal Processing Deficit in Autism and Language Impairment.” Neuroreport 16(4):329–32. [DOI] [PubMed] [Google Scholar]
- Oram Cardy Janis E., Flagg Elissa J., Roberts Wendy, and Roberts Timothy P. L.. 2005. “Delayed Mismatch Field for Speech and Non-Speech Sounds in Children with Autism.” Neuroreport 16(5):521–25. [DOI] [PubMed] [Google Scholar]
- Oram Cardy Janis E., Flagg Elissa J., Roberts Wendy, and Roberts Timothy P. L.. 2008. “Auditory Evoked Fields Predict Language Ability and Impairment in Children.” International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology 68(2):170–75. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Owen Julia P., Marco Elysa J., Desai Shivani, Fourie Emily, Harris Julia, Hill Susanna S., Arnett Anne B., and Mukherjee Pratik. 2013. “Abnormal White Matter Microstructure in Children with Sensory Processing Disorders.” NeuroImage. Clinical 2:844–53. doi: 10.1016/j.nicl.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou Andrew C. 2009. Clinical Magnetoencephalography and Magnetic Source Imaging. New York: Cambridge University Press. [Google Scholar]
- Port Russell G., Dipiero Marissa A., Ku Matthew, Liu Song, Blaskey Lisa, Kuschner Emily S., Edgar J. Christopher, Roberts Timothy P. L., and Berman Jeffrey I.. 2019. “Children with Autism Spectrum Disorder Demonstrate Regionally Specific Altered Resting-State Phase-Amplitude Coupling.” Brain Connectivity 9(5):425–36. doi: 10.1089/brain.2018.0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe Kamalini G., Hinkley Leighton B., Beagle Alexander J., Mizuiri Danielle, Honma Susanne M., Welch Ariane E., Hubbard Isabel, Mandelli Maria Luisa, Miller Zachary A., Garrett Coleman, La Alice, Boxer Adam L., Houde John F., Miller Bruce L., Vossel Keith A., Gorno-tempini Maria Luisa, and Nagarajan Srikantan S.. 2017. “Distinct Spatiotemporal Patterns of Neuronal Functional Connectivity in Primary Progressive Aphasia Variants.” Brain 140:2737–51. doi: 10.1093/brain/awx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard G. J., and Ferre J. M.. 2006. Differential Screening Test for Processing. East Moline, IL: Linguisystems, Inc. [Google Scholar]
- Roberts Timothy P. L., Cannon Katelyn M., Tavabi Kambiz, Blaskey Lisa, Khan Sarah Y., Monroe Justin F., Qasmieh Saba, Levy Susan E., and Edgar J. Christopher. 2011. “Auditory Magnetic Mismatch Field Latency: A Biomarker for Language Impairment in Autism.” Biological Psychiatry 70(3):263–69. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Timothy P. L., Heiken Kory, Kahn Sarah Y., Qasmieh Saba, Blaskey Lisa, Solot Cynthia, Parker William Andrew, Verma Ragini, and Edgar James Christopher. 2012. “Delayed Magnetic Mismatch Negativity Field, but Not Auditory M100 Response, in Specific Language Impairment.” Neuroreport 23(8):463–68. doi: 10.1097/WNR.0b013e32835202b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Timothy P. L., Khan Sarah Y., Rey Mike, Monroe Justin F., Cannon Katelyn, Woldoff Sarah, Qasmieh Saba, Gandal Mike, Schmidt Gwen L., Deborah M., Levy Susan E., and Edgar J. Christopher. 2010. “MEG Detection of Delayed Auditory Evoked Responses in Autism Spectrum Disorders: Towards an Imaging Biomarker for Autism.” Autism Research 3(1):8–18. doi: 10.1002/aur.111.MEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Timothy P. L., Matsuzaki Junko, Blaskey Lisa, Bloy Luke, Edgar J. Christopher, Kim Mina, Ku Matthew, Kuschner Emily S., and Embick David. 2019. “Delayed M50/M100 Evoked Response Component Latency in Minimally Verbal/Nonverbal Children Who Have Autism Spectrum Disorder.” Molecular Autism 10(1):1–11. doi: 10.1186/s13229-019-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts Timothy P. L., Schmidt Gwen L., Egeth Marc, Blaskey Lisa, Rey Michael M., Edgar J. Christopher, and Levy Susan E.. 2008. “Electrophysiological Signatures: Magnetoencephalographic Studies of the Neural Correlates of Language Impairment in Autism Spectrum Disorders.” International Journal of Psychophysiology : Official Journal of the International Organization of Psychophysiology 68(2):149–60. doi: 10.1016/j.ijpsycho.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo Nicole, Zecker Steven, Trommer Barbara, Chen Julia, and Kraus Nina. 2009. “Effects of Background Noise on Cortical Encoding of Speech in Autism Spectrum Disorders.” Journal of Autism and Developmental Disorders 39:1185–96. doi: 10.1007/s10803-009-0737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M., Bailey A., and Lord C.. 2003. SCQ: Social Communication Questionnaire. Los Angeles: Western Psychological Services. [Google Scholar]
- Schmidt Gwenda L., Rey Michael M., Cardy Janis E. Oram, and Roberts Timothy P. L.. 2009. “Absence of M100 Source Asymmetry in Autism Associated with Language Functioning.” Neuroreport 20(11):1037–41. doi: 10.1097/WNR.0b013e32832e0ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Ezra E., Zambrano-Vazquez Laura, and Allen John J. B.. 2016. “Patterns of Alpha Asymmetry in Those with Elevated Worry, Trait Anxiety, and Obsessive-Compulsive Symptoms: A Test of the Worry and Avoidance Models of Alpha Asymmetry.” Neuropsychologia 85:118–26. doi: 10.1016/j.neuropsychologia.2016.03.010. [DOI] [PubMed] [Google Scholar]
- Tecchio Franca, Benassi Francesca, Zappasodi Filippo, Gialloreti Leonardo Emberti, Palermo Mark, Seri Stefano, and Rossini Paolo Maria. 2003. “Auditory Sensory Processing in Autism: A Magnetoencephalographic Study.” Biological Psychiatry 54(6):647–54. doi: 10.1016/S0006-3223(03)00295-6. [DOI] [PubMed] [Google Scholar]
- Tomcheck S., and Dunn W.. 2007. “Sensory Processing in Children with and without Autism: A Comparative Study Using the Short Sensory Profile.” The American Journal of Occupational Therapy 61(2):190–200. [DOI] [PubMed] [Google Scholar]
- Wechsler David. 2003. Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV). San Antonio, TX: Pearson Assessments. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.