Abstract
Chlamydia pneumoniae infection is associated with atherosclerotic heart and vessel disease, but a causal relationship between this pathogen and the disease process has not been established. Recently, it was reported that C. pneumoniae induces human macrophage foam cell formation, a key event in early atheroma development, suggesting a role for the organism in atherogenesis. This study further examines C. pneumoniae-induced foam cell formation in the murine macrophage cell line RAW-264.7. Infected RAW cells accumulated cholesteryl esters when cultured in the presence of low-density lipoprotein in a manner similar to that described for human macrophages. Exposure of C. pneumoniae elementary bodies to periodate, but not elevated temperatures, inhibited cholesteryl ester accumulation, suggesting a role for chlamydial lipopolysaccharide (cLPS) in macrophage foam cell formation. Purified cLPS was found to be sufficient to induce cholesteryl ester accumulation and foam cell formation. Furthermore, the LPS antagonist lipid X inhibited C. pneumoniae and cLPS-induced lipid uptake. These data indicate that cLPS is a C. pneumoniae component that induces macrophage foam cell formation and suggest that infected macrophages chronically exposed to cLPS may accumulate excess cholesterol to contribute to atheroma development.
Atherosclerosis is a chronic, inflammatory disease that results from arterial hemodynamic changes and complex interactions between a variety of lipids, cell types, and their soluble mediators (39, 44). The central cell mediating atheroma development may be the macrophage (32, 48). Atheroma macrophages accumulate excess cholesterol that is esterified and stored in the cytoplasm, and these cholesteryl ester-loaded macrophages, or foam cells, are the hallmark of early fatty streak lesions in atherogenesis (4).
Although multiple risk factors for atherosclerosis have been identified (21, 45, 46), 30 to 40% of disease complications result in the absence of these conditions and may involve previously unrecognized risk factors. Appreciation of atherosclerosis as an inflammatory disease (29, 38, 39) has renewed interest in the role that infectious agents may play in atheroma development. In particular, the obligate intracellular bacterium Chlamydia pneumoniae has been associated with atherosclerosis by seroepidemiology (41), identification of the organism within atheromas by immunohistochemistry (23) and within foam cells by electron microscopy (43), and identification of the C. pneumoniae genome within atheromas by PCR (24). In addition, the organism has been isolated from atheromas (19), and antibiotic treatment trials have shown significant reductions of complications in patients with atherosclerosis (15, 16).
Although ample clinical data exist to suggest a role for C. pneumoniae in atherosclerosis, how the organism may initiate or promote the disease remains unclear. Rabbit and apolipoprotein E-deficient murine animal models have been developed to study atherosclerosis following C. pneumoniae infection (30, 31) and may prove useful to examine mechanisms of disease in vivo. In vitro, C. pneumoniae has been shown to infect and multiply within smooth muscle cells, endothelial cells, and macrophages (12), suggesting that the organism can survive and persist within atheromas. Furthermore, infected mononuclear cells release inflammatory cytokines (22) that may contribute indirectly to atheroma development.
Evidence linking C. pneumoniae infection to events thought to contribute directly to atherogenesis has emerged only recently. C. pneumoniae has been shown to induce human macrophage foam cell formation when cultured in the presence of low-density lipoprotein (LDL) (20), implicating this organism as a causative agent in atherosclerosis. However, detailed characterization of C. pneumoniae-induced foam cell formation by human monocyte-derived macrophages has been difficult due to the widely varying capacity to ingest lipids by monocytes/macrophages isolated from different subjects. In this study, we used the well-characterized murine macrophage cell line RAW-264.7 to further examine the role of C. pneumoniae in foam cell formation by macrophages.
MATERIALS AND METHODS
Reagents.
Cholesterol esterase was purchased from Boehringer Mannheim (Indianapolis, Ind.). Highly purified C. trachomatis chlamydial lipopolysaccharide (cLPS) extracted by the Galanos method (10), as modified by Qureshi et al. (18, 34), was kindly provided by Douglas Golenbock (Boston, Mass.). The purity of cLPS was determined by protein assay, thin-layer chromatography, and high-pressure liquid chromatography (18). Escherichia coli lipid X was synthesized by Peter Stutz (Vienna, Austria) and was a gift from Richard Proctor and Loren Denlinger (Madison, Wis.). All other reagents were purchased from Sigma Chemical Company (St. Louis, Mo.).
Isolation of LDL.
LDL was isolated from normolipidemic donors by density gradient ultracentrifugation as previously described (37), extensively dialyzed against 0.15 M NaCl–0.05% EDTA, and concentrated by centrifugation in Centri/Por concentrators (Spectrum, Houston, Tex.). LDL protein and cholesterol were determined by use of a protein assay (Bio-Rad, Hercules, Calif.) and a total cholesterol test kit (Sigma), respectively. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of LDL on a 4 to 20% gradient gel revealed a single protein (molecular weight, ∼250,000) consistent with LDL apolipoprotein B-100. Agarose gel electrophoresis of LDL showed no increase in relative electrophoretic mobility within 2 weeks, indicating that isolated LDL did not become oxidized (8). Isolated LDL was stored at 4°C and used within 2 weeks of isolation.
Propagation of chlamydiae and growth of macrophages.
C. pneumoniae was purchased from the American Type Culture Collection (ATCC) (Manassas, Va.), propagated in HEp-2 cells (ATCC), and purified by Renografin gradient centrifugation as previously described (6). Propagated organisms were stored at −70°C in sucrose buffer and titered for infectivity as described (20). Titers are reported here as multiplicity of infection (MOI), where one MOI equals one infectious Chlamydia organism/HEp-2 cell.
The murine macrophage cell line RAW-264.7 (ATCC) was grown in RPMI 1640 medium (BioWhittaker, Walkersville, Md.) supplemented with 10% fetal bovine serum (FBS; BioWhittaker), 50 μg of vancomycin per ml, and 10 μg of gentamicin per ml. Cells were maintained in a 37°C, 5% CO2 incubator and split every 2 days by gentle scraping.
Infection and culture of macrophages.
Macrophages were plated at a density of either 4 × 104 cells/well on 96-well trays (0.32-cm2 growth area; Corning Costar Corporation, Cambridge, Mass.) or 2 × 105 cells/well on 24-well trays (1.9-cm2 growth area; Corning) and incubated overnight prior to infection. In some experiments, macrophages were plated overnight at 2 × 104 cells/well on Lab-Tek chamber slides (0.32-cm2 growth area; Nunc Inc., Naperville, Ill.) prior to infection. Macrophages were infected by incubating cells for 2 h with various doses of C. pneumoniae in 50 μl (96-well trays) or 200 μl (24-well trays) of RPMI 1640–10% FBS. Cells then were washed twice with phosphate-buffered saline (PBS; BioWhittaker) and incubated for 24 h in 200 μl (96-well trays) or 500 μl (24-well trays) of RPMI 1640–10% FBS in the presence or absence of heparin (100 U/ml) and/or various doses of LDL. Prior to infection in some experiments, chlamydiae were incubated in RPMI medium for 1 h either in a 100°C water bath or at 37°C with 0.25 M sodium periodate in 0.25 M sodium acetate buffer (pH 5.5). In other experiments, macrophages were cultured with LDL in the presence or absence of cLPS and/or lipid X. All buffers were brought to 37°C before addition to macrophages. Trypan blue staining showed that none of the treatments affected macrophage viability at 24 h (>95% of macrophages were viable).
Quantitation of foam cell formation.
Macrophages were washed twice with PBS, fixed for 15 min in 2% paraformaldehyde, and stained for 15 min in 1% oil red O (in 60% isopropanol). Cells then were washed three times in PBS and examined by light (×200) or dark-field (×400) microscopy (Diaphot 200 or Optiphot microscope; Nikon, Garden City, N.Y.). Cholesteryl ester-laden macrophages were scored by previously established criteria (17, 42).
Quantitation of macrophage cholesteryl ester content.
Macrophage cholesteryl ester content was quantitated by a modification of the method of Gamble et al. (11). Briefly, macrophages were fixed in 0.5% paraformaldehyde, washed three times with PBS, and incubated in 200 μl (24-well trays) of absolute ethanol for 30 min at 4°C to extract cellular lipids. Cholesterol content was determined by incubating 20 μl of ethanol-extracted lipids with 180 μl of assay solution (total cholesterol) (11) or 180 μl of assay solution lacking cholesterol esterase (free cholesterol) for 1 h at 37°C and then measuring fluorescence (HTS-7000 microplate fluorometer; 340-nm excitation, 405-nm emission; Perkin-Elmer, Foster City, Calif.). Total and free cholesterol contents were calculated by using cholesteryl oleate and cholesterol as standards. Cholesteryl ester content was calculated by subtracting free cholesterol from total cholesterol for each sample and is reported as mean ± standard deviation (SD). Lipid-extracted cells were dissolved in 0.1% sodium dodecyl sulfate–0.1 M NaOH for 30 min, and total cell protein was determined by the use of a Lowry protein assay kit (DC Protein Assay; Bio-Rad).
RESULTS
C. pneumoniae induces foam cell formation and cholesteryl ester accumulation by RAW macrophages.
RAW macrophages exposed to C. pneumoniae accumulated cytoplasmic cholesteryl ester droplets in the presence of exogenous LDL (Fig. 1) in a manner similar to that previously observed for human macrophages (20). C. pneumoniae-infected macrophages also accumulated increasing levels of cholesteryl esters when cultured with increasing concentrations of LDL (Fig. 2). Furthermore, infection of macrophages with increasing doses of C. pneumoniae resulted in higher levels of cholesteryl ester formation (Fig. 3). As with human macrophages, RAW macrophages did not accumulate cholesteryl esters when cultured in the presence of heparin (Fig. 3), which binds LDL and prevents its association with a native LDL receptor (13). Heparin also inhibited baseline accumulation of cholesteryl esters by uninfected macrophages cultured with exogenous LDL (Fig. 3). In contrast, C. pneumoniae-induced foam cell formation was not inhibited when cells were cultured with fucoidan, which inhibits modified LDL uptake by class A scavenger receptors (14), or in the presence of the antioxidant butylated hydroxytoluene (not shown).
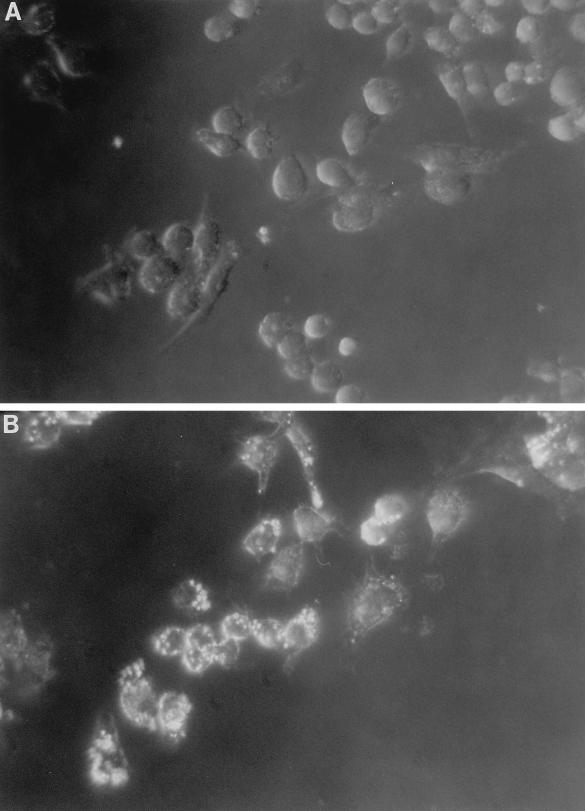
FIG. 1.
Foam cell formation by uninfected (A) and infected (B) RAW macrophages. Cells were infected (MOI = 1) or not infected for 2 h, washed twice, and incubated with 100 μg of LDL per ml for 24 h. Micrographs show oil red O-stained macrophages containing cytoplasmic neutral lipid droplets (magnification, ×400; dark-field microscopy).
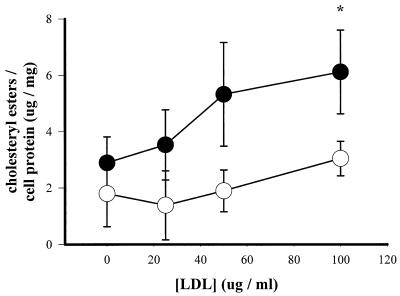
FIG. 2.
Cholesteryl ester levels in uninfected (open circles) and C. pneumoniae-infected (solid circles) macrophages. Cells were infected (MOI = 2) or not infected for 2 h, washed twice, and incubated with various doses of LDL for 24 h. Cholesteryl ester levels were normalized to protein content. Data are representative of three experiments and are reported as means of triplicates with SD. ∗, statistically significant difference compared with infected samples cultured without exogenous LDL, P < 0.05 (t test).
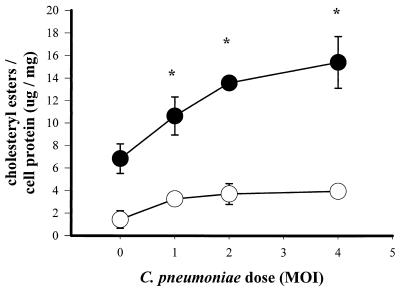
FIG. 3.
Cholesteryl ester levels in heparin-treated (open circles) and untreated (solid circles) macrophages. Cells were infected with C. pneumoniae at various MOIs for 2 h, washed twice, and incubated with 100 μg of LDL per ml for 24 h. Cholesteryl ester levels were normalized to protein content. Data are representative of three experiments and are reported as means of triplicates with SD. ∗, statistically significant difference compared with uninfected, non-heparin-treated control, P < 0.05 (t test).
Exposure of C. pneumoniae to periodate but not elevated temperatures inhibits macrophage foam cell formation.
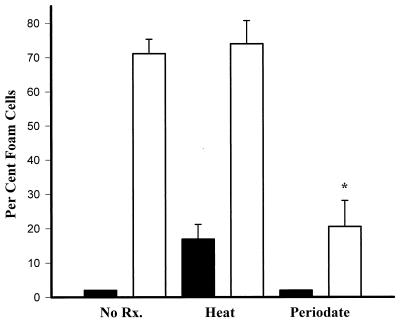
To characterize the C. pneumoniae components involved in inducing macrophage foam cell formation, chlamydiae were exposed to elevated temperatures or periodate before being added to macrophages. Figure 4 shows that incubation of C. pneumoniae for 1 h at 100°C followed by cooling to 37°C did not inhibit the organism’s capacity to induce foam cell formation, although exposure of macrophages to only medium treated in a similar manner did cause ∼18% of macrophages to accumulate neutral lipids. However, C. pneumoniae that was treated for 1 h with 25 mM sodium periodate, which has been shown to oxidize antigenic carbohydrate residues on cLPS (3, 5), exhibited significantly reduced capacity for foam cell formation (Fig. 4). In a separate experiment designed to test if any Chlamydia-secreted products caused induce foam cell formation, chlamydiae were centrifuged and supernatants were added to macrophages in the presence of LDL; Chlamydia supernatants failed to induce macrophage foam cell formation (<10% oil red O-positive cells).
FIG. 4.
Foam cell formation by uninfected (solid bars) and C. pneumoniae-infected (open bars) macrophages. Chlamydiae (MOI = 2) were incubated in RPMI medium for 1 h at 4°C (No Rx.), at 100°C (Heat), or with 0.25 M sodium periodate at 37°C (Periodate) before infection, cultured in the presence of 100 μg of LDL per ml, and stained with oil red O. About 500 cells were counted from each well. Data are representative of three experiments and are reported as means of triplicates with SD. ∗, statistically significant difference compared with untreated, infected and heat-treated, infected samples, P < 0.001.
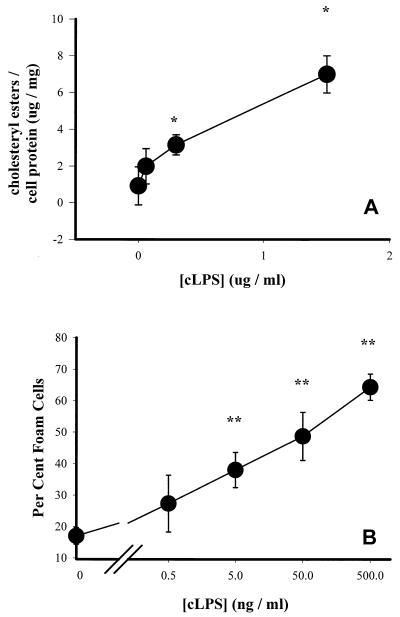
Chlamydial LPS is sufficient to induce macrophage foam cell formation and cholesteryl ester accumulation.
To test whether purified cLPS could induce foam cell formation, macrophages were exposed to cLPS at various doses and incubated in the presence of LDL. The results indicate that cLPS was sufficient to induce cholesteryl ester accumulation (Fig. 5A) and foam cell formation (Fig. 5B).
FIG. 5.
Cholesteryl ester levels in (A) and foam cell formation by (B) cLPS-treated macrophages. Cells were exposed to various concentrations of cLPS for 1 h and cultured in the presence of 100 μg of LDL per ml for 24 h. Data are representative of three experiments and are reported as means of triplicates with SD. ∗, statistically significant difference compared with untreated samples, P < 0.05; ∗∗, statistically significant difference compared with untreated samples, P < 0.005.
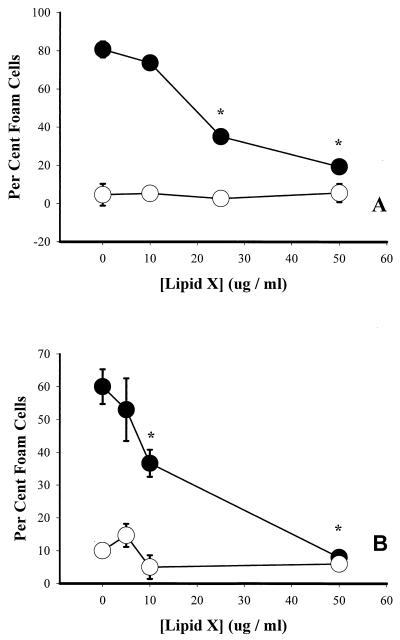
Lipid X inhibits C. pneumoniae- and cLPS-induced foam cell formation.
To determine whether a specific LPS antagonist could inhibit C. pneumoniae-induced macrophage lipid uptake, infected and uninfected macrophages were cultured in the presence of various concentrations of lipid X, a monosaccharide precursor of the lipid A component of LPS (35). Figure 6A shows that lipid X inhibited foam cell formation by C. pneumoniae-infected macrophages in a dose-dependent manner and also inhibited cLPS-induced foam cell formation (Fig. 6B).
FIG. 6.
Effect of lipid X on foam cell formation by C. pneumoniae-infected (A) and cLPS-exposed (B) macrophages. (A) Uninfected (open circles) or infected (MOI = 2; closed circles) cells were washed and incubated with 100 μg of LDL per ml for 20 h in the presence or absence of various concentrations of lipid X. (B) Untreated (open circles) or cLPS-treated (100 ng/ml; closed circles) cells were incubated with 100 μg of LDL per ml for 20 h in the presence or absence of various concentrations of lipid X. Macrophages were fixed and stained with oil red O, and about 500 cells were counted from each well. Data are representative of three experiments and are reported as means of triplicates with SD. ∗, statistically significant difference compared with untreated samples, P < 0.005.
DISCUSSION
Recent appreciation of atherosclerosis as an inflammatory disease (1, 12, 28, 29, 38) has renewed interest in possible infectious etiologies of atherogenesis. Atherosclerosis and chlamydial diseases share some interesting similarities, including the fact that they are chronic, subclinical, and inflammatory in nature (39, 47), with macrophage activation playing a key role in the inflammatory process (40). Formation of macrophage foam cells in the arterial intima is the hallmark of early lesions in atherosclerosis (39), and the pivotal step in foam cell formation is the uptake of excess cholesterol from LDL (4). Recently, C. pneumoniae was shown to induce macrophage foam cell formation in the presence of exogenous LDL (20), suggesting a causal role for the organism in atherogenesis. The present work further characterizes C. pneumoniae-induced foam cell formation by determining the C. pneumoniae component responsible for macrophage lipid accumulation. Chlamydiae incubated for 1 h at 100°C retained the capacity to induce foam cell formation, suggesting that the C. pneumoniae component that induced lipid accumulation was nonproteinaceous and heat stable (Fig. 4). In contrast, chlamydiae treated with periodate, which oxidizes antigenic carbohydrate residues on cLPS, had decreased capacity to induce macrophage foam cell formation (Fig. 4), suggesting that the C. pneumoniae heat-stable component causing macrophage lipid accumulation was cLPS. Indeed, cLPS extracted from chlamydiae was sufficient to induce macrophage foam cell formation and cholesteryl ester accumulation (Fig. 5), and C. pneumoniae- or cLPS-induced lipid uptake was inhibited by the LPS antagonist lipid X (Fig. 6). These data indicate that cLPS is a C. pneumoniae component that induces macrophage foam cell formation and suggest that inhibiting the activity of cLPS in vivo with monoclonal antibodies or LPS analogues may be sufficient to reduce C. pneumoniae-induced foam cell formation and atheroma development.
Persistence of cLPS in the atheroma, either within intact C. pneumoniae-infected cells or in the exogenous subendothelial milieu following cell lysis, may promote atherosclerosis by continual macrophage activation and foam cell formation. Indeed, circulating cLPS-specific immune complexes, which may result from cLPS shedding from the atheroma, have been detected in patients with coronary heart disease (27). Compared to enterobacterial LPS, cLPS is a weak inducer of tumor necrosis factor alpha secretion (18), and the decreased capacity of cLPS to induce an acute inflammatory response may be necessary for chlamydial survival and establishment of chronic infection (2). In contrast, cLPS and enterobacterial LPS may be equally effective in inducing other macrophage activities that are not central to bacterial clearance, such as lipid uptake and foam cell formation. LPSs extracted from E. coli and Salmonella typhimurium have been shown to induce macrophage lipid accumulation when cultured in the presence of native LDL (9, 33), but the relative contribution to foam cell formation of endotoxins isolated from various bacteria is not known. Although endotoxin-mediated foam cell formation is not restricted to cLPS, it is improbable that other gram-negative organisms can invade and survive within the arterial intima to provide a continual source of antigen necessary to induce chronic inflammation and foam cell formation; unlike other gram-negative bacteria, C. pneumoniae has been detected within and isolated from atheromas (7, 25, 36), and it can survive and multiply within all cell types found in the atheroma (12). However, gram-negative septicemia may provide sporadic infiltration of enterobacterial LPS into the arterial intima (26), and therefore a possible role for enterobacterial LPS in foam cell formation in vivo cannot be discounted. Studies are under way to compare the effects of enterobacterial LPS versus cLPS and C. pneumoniae on foam cell formation in vivo in a murine model of atherosclerosis.
ACKNOWLEDGMENTS
We thank Donna Paulnock, Mary Lokuta, and Joy Stuckey for providing advice regarding the RAW-264.7 cells, Charles Schobert and Adam Pleister for helping in propagating C. pneumoniae, Colleen Kane for help with dark-field microscopy, and Loren Denlinger for reviewing the manuscript.
This work was supported in part by NIH grants AI 19782 and AI 34617.
REFERENCES
- 1.Blum A, Miller H. The role of inflammation in atherosclerosis. Isr J Med Sci. 1996;32:1059–1065. [PubMed] [Google Scholar]
- 2.Brade H, Brabetz W, Brade L, Holst O, Lobau S, Mucakova M, Mamat U, Rozalski A, Zych K, Kosma P. Chlamydial lipopolysaccharide. J Endotoxin Res. 1997;4:67–84. [Google Scholar]
- 3.Brade L, Schramek S, Schade U, Brade H. Chemical, biological, and immunochemical properties of the Chlamydia psittaci lipopolysaccharide. Infect Immun. 1986;54:568–574. doi: 10.1128/iai.54.2.568-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown M, Goldstein J. Lipoprotein metabolism in the macrophage. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell H, Hitchcock P. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984;44:306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell L, Brien E O, Cappuccio A, Kuo C, Wang S, Stewart D, Patton D, Cummings P, Grayston J. Detection of Chlamydia pneumoniae TWAR in human coronary atherectomy tissues. J Infect Dis. 1995;172:585–588. doi: 10.1093/infdis/172.2.585. [DOI] [PubMed] [Google Scholar]
- 8.Esterbauer H, Gebicki J, Puhl H, Jurgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- 9.Funk J, Feingold K, Moser A, Grunfeld C. Lipopolysaccharide stimulation of RAW 264.7 macrophages induces lipid accumulation and foam cell formation. Atherosclerosis. 1993;98:67–82. doi: 10.1016/0021-9150(93)90224-i. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Luderitz O, Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969;9:245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 11.Gamble W, Vaughan M, Kruth H, Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978;19:1068–1070. [PubMed] [Google Scholar]
- 12.Gaydos C, Summersgill J, Sahney N, Ramirez J, Quinn T. Replication of Chlamydia pneumoniae in vitro in human macrophages, endothelial cells, and aortic artery smooth muscle cells. Infect Immun. 1996;64:1614–1620. doi: 10.1128/iai.64.5.1614-1620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein J, Basu S, Brunschede G, Brown M. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976;7:85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein J, Hoff H, Ho Y, Basu S, Brown M. Stimulation of cholesteryl ester synthesis in macrophages by extracts of atherosclerotic human aortas and complexes of albumin/cholesteryl esters. Arteriosclerosis. 1981;1:210–226. doi: 10.1161/01.atv.1.3.210. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S, Leatham E, Carrington D, Mendall M, Kaski J, Camm J. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 16.Gurfinkel E, Bozovich G, Daroca A, Beck E, Mautner B. Randomized trial of roxithromycin in non-Q-wave coronary syndromes—Roxis pilot study. Lancet. 1997;350:404–407. doi: 10.1016/s0140-6736(97)07201-2. [DOI] [PubMed] [Google Scholar]
- 17.Huh H, Pearce S, Yesner L, Schindler J, Silverstein R. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 18.Ingalls R, Rice P, Qureshi N, Takayama K, Shin-Lin J, Golenbock D. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson I, Campbell L, Kuo C, Rodriguez D, Lee A, Grayston J. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- 20.Kalayoglu M, Byrne G. Induction of macrophage foam cell formation by Chlamydia pneumoniae. J Infect Dis. 1998;177:725–729. doi: 10.1086/514241. [DOI] [PubMed] [Google Scholar]
- 21.Kannel W, Sytkowski P. Atherosclerosis risk factors. Pharmacol Ther. 1987;32:207–235. doi: 10.1016/0163-7258(87)90075-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaukoranta-Tolvanen S, Teppo A, Kaitinen K, Saikku P, Linnavuori K, Leinonen M. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb Pathog. 1996;21:215–221. doi: 10.1006/mpat.1996.0056. [DOI] [PubMed] [Google Scholar]
- 23.Kuo C, Gown A, Benditt E, Grayston J. Detection of Chlamydia pneumoniae in aortic lesions of atherosclerosis by immunocytochemical stain. Arterioscler Thromb. 1993;13:1501–1504. doi: 10.1161/01.atv.13.10.1501. [DOI] [PubMed] [Google Scholar]
- 24.Kuo C, Grayston J, Campbell L, Goo Y, Wissler R, Benditt E. Chlamydia pneumoniae (TWAR) in coronary arteries of young adults (15–34 years old) Proc Natl Acad Sci USA. 1995;92:6911–6914. doi: 10.1073/pnas.92.15.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo C, Shor A, Campbell L, Fukushi H, Patton D, Grayston T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 26.Liao W. Endotoxin: possible roles in initiation and development of atherosclerosis. J Lab Clin Med. 1996;128:452–460. doi: 10.1016/s0022-2143(96)90042-6. [DOI] [PubMed] [Google Scholar]
- 27.Linnanmaki E, Leinonen M, Mattila K, Nieminen M, Valtonen V, Saikku P. Chlamydia pneumoniae—specific circulating immune complexes in patients with chronic coronary heart disease. Circulation. 1993;87:1130–1134. doi: 10.1161/01.cir.87.4.1130. [DOI] [PubMed] [Google Scholar]
- 28.Maseri A. Inflammation, atherosclerosis, and ischemic events—exploring the hidden side of the moon. N Engl J Med. 1997;336:1014–1016. doi: 10.1056/NEJM199704033361409. [DOI] [PubMed] [Google Scholar]
- 29.Mendall M, Patel P, Ballam L, Strachan D, Northfield T. C-reactive protein and its relation to cardiovascular risk factors—a population based cross sectional study. Br Med J. 1996;312:1061–1065. doi: 10.1136/bmj.312.7038.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moazed T, Kuo C, Grayston J, Campbell L. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 31.Muhlestein J, Anderson J, Hammond E, Zhao L, Trehan S, Schwobe E, Carlquist J. Infection with Chlamydia pneumoniae accelerates the development of atherosclerosis and treatment with azithromycin prevents it in a rabbit model. Circulation. 1998;97:633–636. doi: 10.1161/01.cir.97.7.633. [DOI] [PubMed] [Google Scholar]
- 32.Murakami T, Yamada N. Modification of macrophage function and effects on atherosclerosis. Curr Opin Lipidol. 1996;7:320–323. doi: 10.1097/00041433-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Oiknine J, Aviram M. Increased susceptibility to activation and increased uptake of low density lipoprotein by cholesterol-loaded macrophages. Arterioscler Thromb. 1992;12:745–753. doi: 10.1161/01.atv.12.6.745. [DOI] [PubMed] [Google Scholar]
- 34.Qureshi N, Mascagni P, Ribi E, Takayama K. Monophosphoryl lipid A obtained from lipopolysaccharides of Salmonella minnesota R595. J Biol Chem. 1985;260:5271–5278. [PubMed] [Google Scholar]
- 35.Raetz C. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez J, Ahkee S, Summersgill J, Ganzer B, Ogden L, Quinn T, Goydos C, Bobo L, Hammerschlag M, Roblow P, Lebar W, Grayston J, Campbell L, Patton D, Dean D, Schachter J. Isolation of Chlamydia pneumoniae from the coronary artery of a patient with coronary atherosclerosis. Ann Intern Med. 1996;125:979–982. doi: 10.7326/0003-4819-125-12-199612150-00008. [DOI] [PubMed] [Google Scholar]
- 37.Redgrave T, Roberts D, West C. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65:42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- 38.Ridker P, Cushman M, Stampfer M, Tracy R, Hennekens C. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 39.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 40.Saikku P. Chlamydia pneumoniae infection as a risk factor in acute myocardial infarction. Eur Heart J. 1993;14:62–65. [PubMed] [Google Scholar]
- 41.Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman M-R, Manninen V, Manttari M, Frick M, Huttunen J. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]
- 42.Schaffner T, Taylor K, Bartucci E, Fischer-Dzoga K, Beeson J, Glagov S, Wissler R. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980;100:57–80. [PMC free article] [PubMed] [Google Scholar]
- 43.Shor A, Kuo C, Patton D. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992;82:158–161. [PubMed] [Google Scholar]
- 44.Stehbens W. The pathogenesis of atherosclerosis—a critical evaluation of the evidence. Cardiovasc Pathol. 1997;6:123–153. doi: 10.1016/s1054-8807(96)00090-7. [DOI] [PubMed] [Google Scholar]
- 45.Strong J. Natural history and risk factors for early human atherogenesis. Clin Chem. 1995;41:143–148. [PubMed] [Google Scholar]
- 46.Superko H. New aspects of risk factors for the development of atherosclerosis, including small low-density lipoprotein, homocysteine, and lipoprotein(a) Curr Opin Cardiol. 1995;10:347–354. doi: 10.1097/00001573-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Ward M. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 48.Yokude M, Kita T. The macrophage and its role in atherogenesis. Intern Med. 1995;34:281–283. doi: 10.2169/internalmedicine.34.281. [DOI] [PubMed] [Google Scholar]