Abstract
Malic enzyme was isolated and purified from mature apple fruits (Malus sylvestris, Miller) by utilizing procedures probably applicable to other soluble enzymes in this and similar tissues.
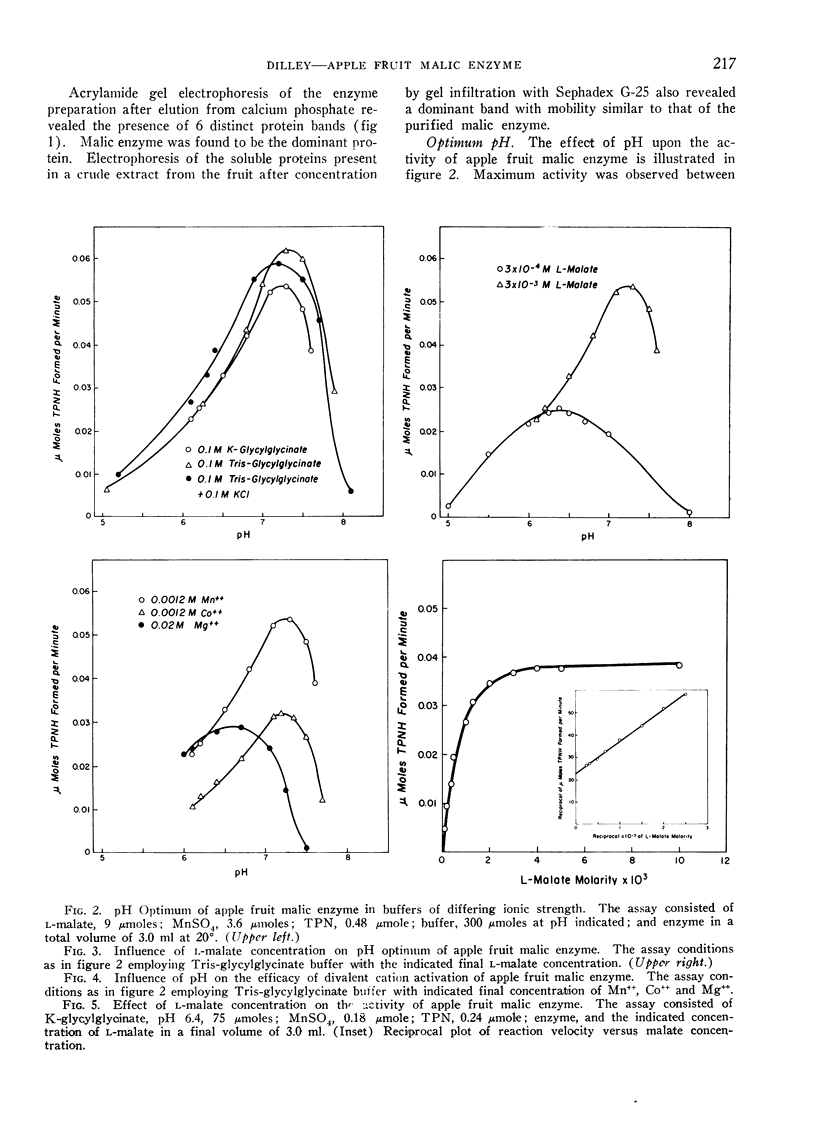
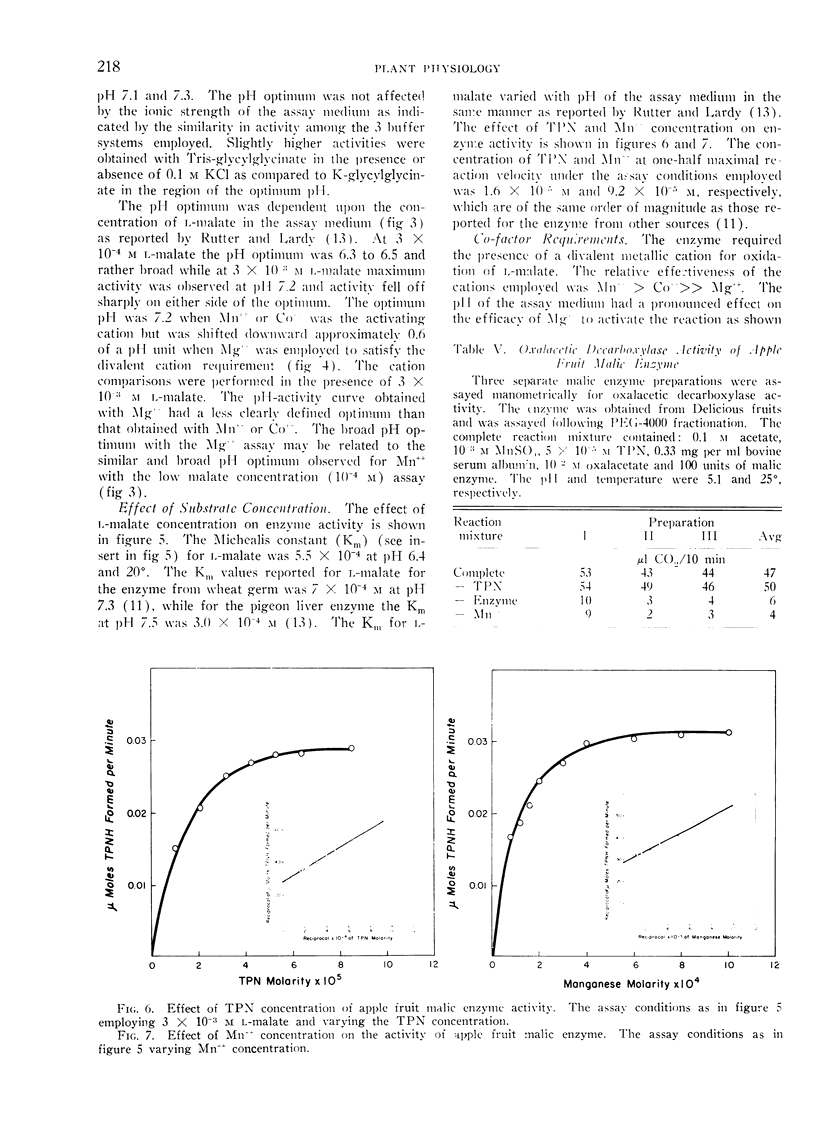
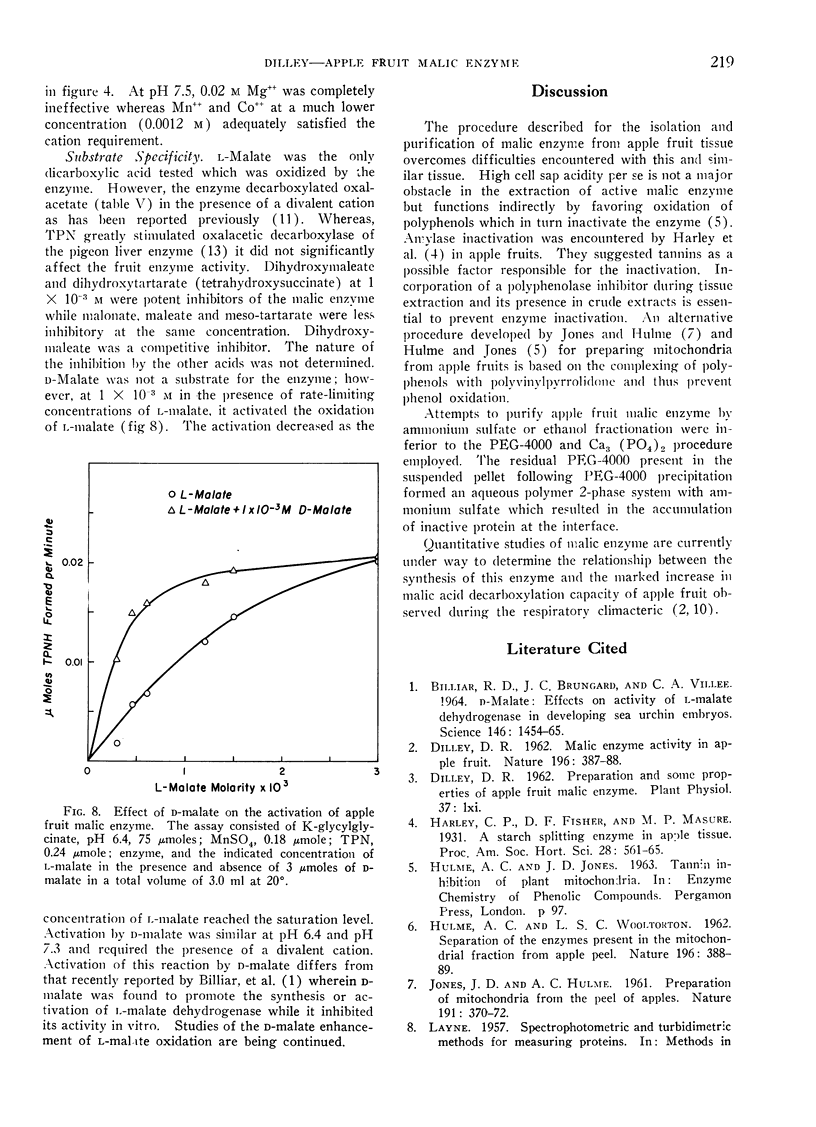
The physical properties of apple fruit malic enzyme are similar to those reported for malic enzyme from other plant and animal sources. It is specific for l-malate, TPN and requires a divalent cation for activity. In contrast to the pigeon liver enzyme, supplemental TPN is not required for oxalacetic decarboxylase activity of the fruit enzyme. The pH optimum of the malic enzyme varied with the l-malate concentration and the nature of the divalent cation present. d-Malate activated the oxidation of l-malate at rate-limiting concentrations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Wang C. H., Doyle W. P., Ramsey J. C. Role of Hexose Monophosphate Pathway in Tomato Catabolism. Plant Physiol. 1962 Jan;37(1):1–7. doi: 10.1104/pp.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]