Abstract
Superantigens stimulate T-lymphocyte proliferation and cytokine production, but the effects of superantigen exposure on cell function within a complex, highly regulated immune response remain to be determined. In this study, we demonstrate that superantigen exposure significantly alters the murine host response to bacterial antigens in an in vitro coculture system. Two days after exposure to the superantigen staphylococcal enterotoxin B, splenocytes cultured with Streptococcus mutans produced significantly greater amounts of gamma interferon (IFN-γ) and interleukin-12 than did sham-injected controls. The majority of IFN-γ production appeared to be CD8+ T-cell derived since depletion of this cell type dramatically reduced the levels of IFN-γ. To study host cell damage that may occur following superantigen exposure, we analyzed cytotoxicity to “bystander” fibroblast cells cultured with splenocytes in the presence of bacterial antigens. Prior host exposure to staphylococcal enterotoxin B significantly enhanced fibroblast cytotoxicity in the presence of bacteria. Neutralization of IFN-γ decreased the amount of cytotoxicity observed. However, a greater reduction was evident when splenocyte-bacterium cocultures were separated from the bystander cell monolayer via a permeable membrane support. Increased cytotoxicity appears to be primarily dependent upon cell-cell contact. Collectively, these data indicate that overproduction of inflammatory cytokines may alter the activity of cytotoxic immune cells. Superantigen exposure exacerbates cytokine production and lytic cell activity when immune cells encounter bacteria in vitro and comparable activities could possibly occur in vivo.
Superantigen proteins cause excessive stimulation of host immune cells and lead to illness and disease in mammals, including humans (14, 23, 33, 34). Major superantigens are secreted bacterial toxins such as staphylococcal enterotoxins and toxic shock syndrome toxin, although additional ones have been associated with streptococci, pseudomonads, clostridia, and mycoplasmas (5, 7, 9, 22, 40). Other reported superantigens are virally encoded proteins such as the minor lymphocyte-stimulating factor of the mouse mammary tumor virus (8), the Epstein-Barr virus-associated superantigen (21), and possibly the human immunodeficiency virus Nef protein (36, 37, 41).
Superantigens such as staphylococcal enterotoxin B (SEB) differ from conventional antigens in that they do not require processing and presentation with major histocompatibility complex (MHC) on the surface of antigen-presenting cells (APC). Superantigens interact with nonpolymorphic regions of the MHC molecule and specific Vβ regions of the T-cell receptor heterodimer outside of the peptide binding groove (13, 42). This interaction stimulates cellular proliferation and cytokine production, e.g., gamma interferon (IFN-γ), by Vβ-reactive T cells independent of T-cell receptor specificity for conventional antigen (24). Ultimately, many of these T cells become anergic or are deleted (29). However, some of these cells do not remain unresponsive in vivo and appear to be fully functional in vitro when APC present a high concentration of superantigen along with appropriate costimulation (17, 18). In the present study, we address how superantigen-stimulated immune cells respond to bacterial antigenic stimulation in vitro.
We have developed a novel coculture system to address how superantigen exposure alters the host response to native, conventional bacterial antigen (30). In this system, murine splenocytes isolated from BALB/c mice that were previously injected with SEB or saline are cocultured with viable bacterial cells in the presence of spectinomycin, a bacteriostatic antibiotic. The splenocyte pool contains a mixture of immune cell types (e.g., T and B lymphocytes, macrophages, and natural killer cells) and serves as a model for in vivo immune responses. The bacteria do not multiply in the coculture system but presumably retain their native surface antigens. In addition, splenocyte viability is not affected by the presence of the bacteria, allowing the study of bacterium-host cell interaction and responses (11).
In the present studies, we analyzed IFN-γ and interleukin-12 (IL-12) secretion by splenocytes isolated from SEB-pretreated mice that were cultured with gram-positive bacteria. IFN-γ-secreting lymphocytes were identified by flow cytometry utilizing intracellular cytokine staining and cell surface phenotype markers and were confirmed by depletion of this cell type. We compared splenocytes isolated from SEB and phosphate-buffered saline (PBS)-pretreated mice for cytotoxicity and studied the role of cytokine and cell contact in these effects. This is the first study to demonstrate how prior in vivo exposure to superantigen augments cytokine production and cytotoxicity in response to viable bacterial cells in vitro.
MATERIALS AND METHODS
Animals.
Eight- to ten-week-old female BALB/c mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were used in the study. Mice were injected intraperitoneally with 50 μg of SEB (Toxin Technology, Sarasota, Fla.) in 0.2 ml of PBS (pH 7.2) or with 0.2 ml of PBS alone. Splenocyte pools of at least three mice were used for each experiment.
Bacteria and culture conditions.
Streptococcus mutans, which colonizes the oral cavity, was obtained from the American Type Culture Collection (ATCC). In previous coculture studies, S. mutans generated the greatest IFN-γ response. Bacteria grown aerobically at 37°C overnight in brain heart infusion broth (BHI broth; Difco, Detroit, Mich.) were diluted 1:10 in BHI broth and grown to mid-log phase (108 cells ml−1). Cell density was determined by monitoring the optical density at 600 nm (OD600) with a nephelometer flask and spectrophotometer. The cells were centrifuged at 750 × g (Sorvall GLC-1), washed once in 100 mM sodium phosphate buffer (pH 7.2), and resuspended in RPMI 1640 medium supplemented with 10% bovine calf serum, 2 mM l-glutamine, and 25 mM HEPES to a cell density of about 108 cells ml−1.
Cell cocultures.
Cell cocultures were as previously described (30). Briefly, at 2 or 4 days postinjection the mice were euthanized by cervical dislocation. Spleens were aseptically removed, macerated between sterile frosted-end microscope slides, and washed once in RPMI 1640 medium supplemented with 5% calf serum and 60 μg of spectinomycin ml−1. After erythrocyte lysis, the splenocyte numbers were determined with a Coulter Counter (Coulter, Hialeah, Fla.), and 3.75 × 106 splenocytes were added to Falcon 24-well tissue culture plates containing 106 bacteria and supplemented RPMI 1640 medium. The final volume of the coculture was 0.5 ml. Cocultures were incubated at 37°C in a 5% CO2 atmosphere for up to 24 h before the sampling.
IFN-γ and IL-12 detection.
IFN-γ and IL-12 protein levels were quantitated in culture supernatants by sandwich enzyme-linked immunosorbent assay (ELISA) using cytokine antibody pairs according to the manufacturer’s recommendations (PharMingen, Inc., San Diego, Calif.). To detect IFN-γ, purified rat anti-mouse IFN-γ monoclonal antibody (clone R4-6A2) was used as a capture antibody at an optimal concentration of 2 μg ml−1. Biotinylated rat anti-mouse IFN-γ monoclonal antibody (clone XMG1.2) was used as the detection antibody at an optimal concentration of 1 μg ml−1. Recombinant mouse IFN-γ (Biosource International, Camarillo, Calif.) was used for the standard curve determinations. To detect IL-12, purified rat anti-mouse IL-12 (p40/p70, clone C15.6) was used as the capture antibody at an optimal concentration of 4 μg ml−1. Biotinylated rat anti-mouse IL-12 monoclonal antibody (clone C17.8) was used for ELISA detection at an optimal concentration of 2 μg ml−1. Recombinant mouse IL-12 (PharMingen) was used for the standard curve determinations. Plates were developed with avidin-peroxidase working conjugate (diluted 1:300) and the 2,2′-azinobis(3-ethylbenzthiazoline sulfonate) (ABTS) substrate (Sigma, St. Louis, Mo.). A405 was determined by using a Vmax ELISA microtiter plate reader (Molecular Devices Corp., Palo Alto, Calif.).
Monoclonal antibodies.
Monoclonal antibodies for CD90 (Thy-1.2), CD4 (L3T4), CD8a (Ly-2), Vβ 8.1, 8.2, and CD32/CD16 (FcγII/III) were purchased from PharMingen and were used in single and dual immunofluorescence staining for flow cytometry. Biotin-labeled anti-mouse CD8a (Ly-2) was purchased from Caltag Laboratories (Burlingame, Calif.) and used for immunomagnetic depletion experiments. Intracellular cytokine staining was performed with rat anti-mouse IFN-γ (clone XMG1.2) purchased from Caltag Laboratories; this antibody gave optimum results. When applicable, isotype-matched controls were purchased from the same manufacturer. IFN-γ was neutralized with a monoclonal rat anti-mouse IFN-γ (clone XMG1.2; Caltag).
Flow cytometry.
All samples were prepared for flow cytometric analysis as previously described (30). Samples were analyzed with a Becton Dickinson (San Jose, Calif.) FACScan, and the data were acquired by setting a live gate on the mononuclear cell fraction as determined by forward-angle and side-scatter characteristics. Data analysis was performed by using the LYSYS II computer program (Becton Dickinson).
Labeling of intracellular cytokines.
Intracellular IFN-γ was detected as previously described with phycoerythrin-labeled rat anti-mouse IFN-γ (30). Monensin was added to all cultures 6 hours prior to cell harvest to inhibit protein transport, thereby trapping IFN-γ within the cytokine-secreting cell. Without monensin addition, only about 0.6% of the cells contained detectable intracellular cytokine. Isotype-matched, ligand-blocking, and permeability controls were included to verify the specificity and location of IFN-γ labeling. Samples were analyzed with a Becton Dickinson FACScan. Data were acquired by setting a live gate on the T-lymphocyte population, which was approximately 75% Thy-1.2+ as determined by Thy-1.2 fluorescence.
Immunomagnetic depletion.
Biotin-conjugated rat anti-mouse CD8a was bound to streptavidin-labeled M280 magnetic beads at a concentration of 7.5 μg mg−1 of beads as described by the supplier (Dynal, Inc., Lake Success, N.Y.). A 10:1 bead-to-target ratio was used to remove Ly-2+ (CD8a) cells from the splenocyte cell suspensions. The target cell percentage was determined by flow cytometry. The beads were incubated with target cells for 30 min on a bidirectional rotary platform at 4°C; bead-cell conjugates were harvested with a magnetic separator, and the supernatants were collected. The supernatants were subjected to a second round of depletion. To confirm the efficiency of depletion, aliquots of depleted cell suspensions were stained with two separate clones of monoclonal rat anti-mouse Ly-2 antibodies and analyzed by FACScan flow cytometry. Depleted cell suspensions were <3% positive for Ly-2 target cells after two rounds of depletion.
Cytotoxicity assays.
A cytotoxicity detection kit (Boehringer Mannheim, Indianapolis, Ind.) was used to quantitate cell lysis. Target BALB/c 10CrMCA A.2R.1 fibroblast cells were obtained from the ATCC and maintained in RPMI 1640 medium supplemented with 10% bovine calf serum, 25 mM HEPES, 2 mM l-glutamine, and 4.5 g of d-glucose per liter. Splenocyte cell populations were isolated from PBS- or SEB-pretreated mice and used as the effector cell population in the assay. Effector/target cell ratios were optimized for each experiment and cells were cultured with or without bacteria in Corning 96-well tissue culture plates. All cells were suspended in RPMI 1640 without phenol red supplemented with 5% bovine calf serum, 25 mM HEPES, 2 mM l-glutamine, and 60 μg of spectinomycin ml−1. After 18 or 24 h of incubation at 37°C and 3% CO2, the supernatants were collected and analyzed for the relative release of the cytosolic enzyme lactate dehydrogenase (LDH) according to manufacturer’s recommendations. A490 values were obtained by using a 96-well plate reader (Molecular Devices Corp.). The relative LDH release value for each well was calculated as follows, where OD is the OD490: [(ODtarget + effector + bacteria − ODeffector + bacteria − ODtarget + bacteria)/(ODtarget(max) + bacteria − ODtarget + bacteria)] × 100%.
Transwell inserts (Corning Costar Corp., Cambridge, Mass.) and Corning 24-well tissue culture plates were used in the target and effector cell separation experiments.
Statistical analysis.
The data were analyzed by Student’s t test by using SigmaStat statistical analysis software (Jandel Scientific, San Rafael, Calif.).
RESULTS
IFN-γ production increases in 2-day SEB-pretreated splenocyte-bacterium cocultures.
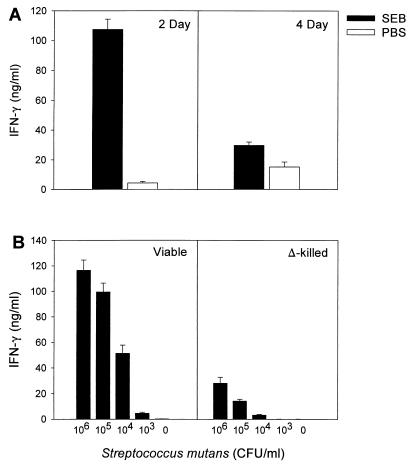
Two days after SEB or PBS pretreatment, splenocytes from SEB-pretreated mice cocultured with S. mutans produced significantly (P ≤ 0.001) greater amounts of IFN-γ than PBS-pretreated splenocytes cultured with S. mutans (Fig. 1A, left panel). Splenocyte cultures without bacteria did not produce elevated IFN-γ levels, indicating that cytokine production occurred in response to bacterial antigens (data not shown). The low IFN-γ levels secreted in PBS-pretreated cocultures indicated that the bacteria do not possess inherent strong superantigen activity like the SEB toxin. Splenocytes isolated 4 days after SEB pretreatment generated lower quantities of IFN-γ than did splenocytes isolated 2 days after SEB injection. Despite the lower level of IFN-γ production, splenocytes isolated 4 days after SEB treatment still yielded a significantly higher IFN-γ level (P ≤ 0.001) in the cocultures than did splenocytes isolated 4 days after PBS pretreatment (Fig. 1A, right panel). Thus, SEB activation of T cells may account for the hyperresponsiveness to bacterial antigens observed 2 days after superantigen exposure, but that response was diminished at 4 days after SEB exposure.
FIG. 1.
IFN-γ production by SEB- or PBS-pretreated splenocytes after a 24-h coculturing with bacteria. (A) IFN-γ produced by spleen cells isolated 2 days or 4 days after SEB or PBS treatment and cocultured with S. mutans. (B) IFN-γ produced by spleen cells isolated 2 days after SEB treatment and cocultured with titrated amounts of viable or heat-killed S. mutans. Each bar represents the mean value of three separate experiments (three mouse spleen pools per experiment) ± the standard error of the mean (SEM).
To determine if the levels of cytokine secreted were dependent on the bacterial load in the culture system, splenocytes isolated 2 days after SEB treatment were cultured with titrated amounts of S. mutans. In the standard coculture, 106 bacteria were mixed with 3.75 × 106 splenocytes, yielding a bacterium/splenocyte ratio of 1:3.75. IFN-γ production under these conditions is shown in Fig. 1B (left panel, leftmost bar). As input bacteria decreased, IFN-γ levels also decreased; the addition of 104 viable bacteria to splenocyte cultures, i.e., a 1:375 ratio of bacteria to splenocyte cells, was needed to produce significant amounts of IFN-γ (P ≤ 0.003; Figure 1B, left panel). Fewer than 103 bacteria did not stimulate cytokine production. The response to heat-killed bacteria was substantially lower (Fig. 1B, right panel). Greater levels of heat-killed bacteria (106) were needed to generate a significant (P ≤ 0.001) IFN-γ response, and even then the cytokine level produced was lower than that observed with viable bacteria. These findings taken together indicate that native bacterial antigens are important for generating the high cytokine response, and the level of the response is related to bacterial load.
CD8+ T cells are responsible for the bulk of IFN-γ production in cocultures.
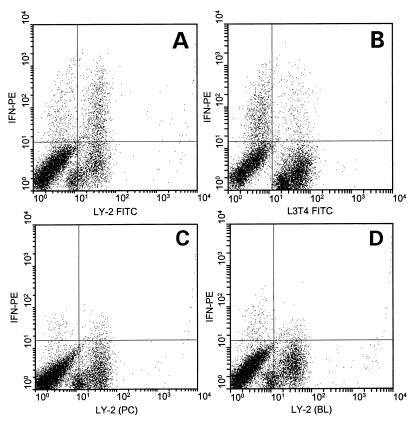
Using in vivo depletion of natural killer (NK) cells with anti-asialo ganglio-n-tetraosylceramide (AAGM1), we have previously shown that about 25% of the IFN-γ in the coculture system is produced by NK cells but that the majority of IFN-γ is generated by T cells (30). To distinguish which T-cell type contributed the majority of IFN-γ, we examined intracellular levels of the cytokine. Splenocytes isolated 2 days after SEB treatment were cocultured with S. mutans and analyzed for intracellular IFN-γ over a period of 6 to 20 h. The percentage of cells containing intracellular IFN-γ increased at 14 h and peaked at 20 h in both Ly-2+ (CD8) and L3T4+ (CD4) T-cell populations (data not shown). Figure 2A shows that about 84% of the IFN-γ-containing cells labeled for the Ly-2 surface antigen, whereas only about 23% stained for the L3T4 antigen (Fig. 2B). Preincubation of permeabilized Ly-2+ stained cells with unlabeled rat anti-IFN-γ prior to phycoerythrin-conjugated rat anti-IFN-γ staining limited the specific labeling of IFN-γ to 0.4% (Fig. 2C), a level similar to the autofluorescence levels. The absence of a permeabilizing agent, saponin, reduced IFN-γ labeling to 0.2% (Fig. 2D), confirming intracellular staining. Approximately four times more CD8+ T cells stained for intracellular IFN-γ than did the CD4+ cells, indicating that CD8+ cells were responsible for more IFN-γ production.
FIG. 2.
Intracellular labeling of IFN-γ in splenocytes isolated 2 days after SEB treatment and cocultured for 20 h with S. mutans. Splenocytes were stained for CD8+ T cells (A) or CD4+ T cells (B) and IFN-γ. Preincubation of the fixed and permeabilized cells with excess unconjugated anti-mouse IFN-γ antibody specifically blocks (BL) cytokine labeling (C), whereas the absence of permeabilization buffer (PC) controls for extracellular cytokine labeling (D). The graphs represent dual labeling for cellular phenotype (x axis) and IFN-γ (y axis). Data were acquired by collecting 10,000 events gated for approximately 75% of the Thy-1.2+ splenocyte fraction, and quadrant markers were set based on isotype-stained controls. The plots are representative of two separate experiments. PE, phycoerythrin; FITC, fluorescein isothiocyanate.
Although we did not directly detect whether the CD4+ and CD8+ T cells that stained for IFN-γ were also Vβ8+, indirect data from other flow cytometry experiments gave insight into the activity of different Vβ-specific T cells (Table 1). When the splenocytes were isolated from the mouse 2 days after SEB treatment, approximately 6.8% of the T cells were CD8+ Vβ8+ and 10.2% of the cells were CD4+ Vβ8+. After 24 h in coculture with S. mutans, the percentages of CD8+ Vβ8+ and CD4+ Vβ8+ T cells both declined to about 4.7%, showing that the Vβ8+ population was being depleted over time. When we compared the percentage of CD8+ T cells containing intracellular IFN-γ (8.3%) to the CD8+ Vβ8+ T cells in the population (4.7%), it was clear that CD8+ T cells with different Vβ specificities must be contributing to the IFN-γ production in the coculture. In contrast, although 4.7% of the CD4+ cells were Vβ8+, the percentage of IFN-γ+ CD4+ cells was only about 2.1%, showing that at most half of the CD4+ Vβ8+ T cells were generating IFN-γ.
TABLE 1.
Analysis of CD8+ and CD4+ T cells 2 days after SEB treatment
| Markers detected | % of T-cell populationa at:
|
|
|---|---|---|
| 0 hb | 20 to 24 hc | |
| Ly-2+ + Vβ8+ | 6.8 ± 0.5 | 4.7 ± 0.1 |
| Ly-2+ + IFN-γ+ | ND | 8.3 ± 0.1 |
| L3T4+ + Vβ8+ | 10.2 ± 0.4 | 4.7 ± 0.1 |
| L3T4+ + IFN-γ+ | ND | 2.1 ± 0.2 |
Live gate set on T cells; 20,000 events were recorded. ND, not done.
Ly-2 (CD8+), L3T4 (CD4+), and Vβ8 markers were measured on splenocyte cells immediately after isolation from mice pretreated with SEB 2 days before.
Ly-2 (CD8+), L3T4 (CD4+), and IFN-γ were measured for splenocytes from SEB-pretreated mice after 20 to 24 h of coculture with 106 S. mutans.
CD8+ cell depletion decreases IFN-γ levels.
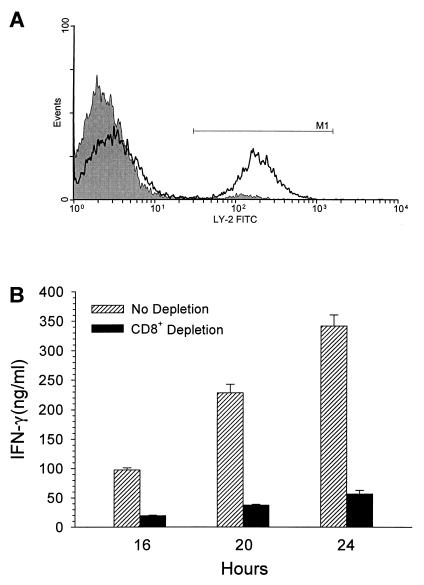
To confirm the CD8+ T-cell contribution to IFN-γ production, we removed these cells from the splenocyte pool prior to coculture. Splenocytes isolated 2 days after SEB treatment were depleted of Ly-2+ cells prior to culturing with S. mutans for 16, 20, and 24 h. In a scatter plot of a nondepleted population gated to contain approximately 75% of the T cells, 33.8% of the cells were Ly-2+ (data not shown). After two rounds of depletion, Ly-2+ cells decreased to 3.0% of the T-cell population (Fig. 3A, gray histogram) compared to the nondepleted population (Fig. 3A, overlay) and remained at <4% after 20 h of coculture with bacteria (data not shown). After 16, 20, and 24 h of coculturing, supernatants were collected and analyzed for IFN-γ concentrations. Removal of Ly-2+ cells, which represented about 84% of the T cells staining intracellularly for IFN-γ, resulted in an approximately 85% reduction in IFN-γ levels generated in response to bacteria (Fig. 3B). The remainder of the secreted IFN-γ in the CD8+-depleted cocultures was probably generated by CD4+ T cells and NK cells.
FIG. 3.
CD8+ T-cell depletion and IFN-γ production. (A) A representative histogram shows CD8+ T-cell labeling prior to (black overlay) and after (gray histogram) depletion of this cell type from splenocytes isolated 2 days after SEB treatment. (B) CD8+ T-cell-depleted and nondepleted splenocytes were cultured for 16 to 24 h with S. mutans and analyzed for IFN-γ production. Each bar represents the mean value of three separate experiments ± the SEM.
SEB pretreatment primes IL-12 production in response to bacterial antigens.
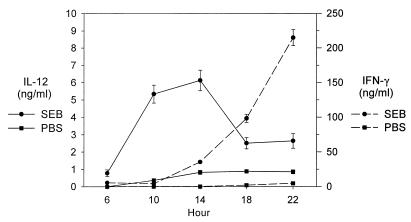
Since IFN-γ production is stimulated by IL-12 (1), we examined the levels of this cytokine in our coculture system. Supernatants from cocultures with splenocytes 2 days after SEB and PBS pretreatment were collected and analyzed for IL-12 and IFN-γ production over a 6- to 24-h culture period. Figure 4 shows that IL-12 production preceded IFN-γ appearance in the coculture. Only SEB-pretreated splenocytes cocultured with bacteria resulted in rapid production of IL-12, which began at 4 h and peaked at 14 h (6.5 ng ml−1). PBS-pretreated splenocytes only produced about 1 ng of IL-12 ml−1. After IL-12 production, a rapid increase in IFN-γ was observed from 14 to 22 h in cocultures with splenocytes from SEB-pretreated mice. Neutralization of IL-12 in culture decreases IFN-γ levels (data not shown). No IL-12 or IFN-γ was detected in any cultures without bacteria added (data not shown).
FIG. 4.
Time course of IL-12 and IFN-γ production. Supernatants were collected from SEB- or PBS-pretreated splenocyte cocultures after 6 to 22 h and analyzed for IL-12 and IFN-γ production. Each datum point represents the mean of two separate experiments ± the SEM.
SEB pretreatment augments cytotoxic effects on bystander cells.
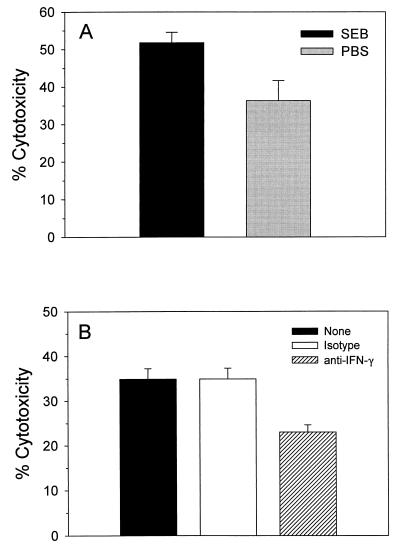
To assess host cell damage that may occur following superantigen exposure, we analyzed cytotoxicity to “bystander” fibroblast cells when cultured with splenocytes in the presence of bacterial antigens. PBS- or SEB-pretreated splenocytes were cultured for 24 h with a BALB/c fibroblast line. SEB-pretreated splenocytes in the presence of bacterial cells showed a statistically significant (P = 0.028) 35% increase in cytotoxicity of fibroblasts compared to those levels observed with PBS-pretreated splenocytes (Fig. 5A). Levels of cytotoxicity were proportional to the amount of bacteria added per well (data not shown). Maximal cytotoxicity was attained with 105 bacteria per well. The level of cytotoxicity observed with PBS-pretreated splenocytes reflects potential tissue damage as a result of an inflammatory response to a large bolus of bacteria. Superantigen exposure appears to enhance the cytotoxic effects on bystander cells in the presence of bacterial antigens.
FIG. 5.
Cytotoxicity of BALB/c fibroblast cells and effect of IFN-γ neutralization. A.2R.1 fibroblast cells (targets) were seeded at a density of 2.0 × 104 cells per well in 96-well plates and allowed to adhere for 10 h at 37°C and 5% CO2. SEB- and PBS-pretreated splenocytes (effectors) were added at a concentration of 105 cells per well with or without bacteria at 105 cells per well. (A) After 24 h the LDH levels in culture supernatants were determined and the relative target LDH release was calculated as described in Materials and Methods. (B) A titrated concentration of neutralizing rat anti-mouse IFN-γ (0.01 μg ml−1) or isotype control was added to cultures at 0 h. After 18 h the LDH levels in the culture supernatants were determined as described above. Each bar represents the mean value for three separate experiments ± the SEM.
In order to characterize whether the SEB-enhanced cytotoxicity is dependent upon IFN-γ production, we neutralized IFN-γ activity with titrated amounts of anti-IFN-γ and measured the cytotoxicity after an 18-h culturing of SEB-pretreated splenocytes with bacterial cells. As shown in Fig. 5B, neutralization of IFN-γ resulted in a 10% net decrease in cytotoxicity levels compared to cultures with an isotype control. IFN-γ appears to augment cytotoxic effects on bystander cells. Since splenocytes from PBS-pretreated mice did not yield appreciable IFN-γ in the cocultures, we presumed that this cytokine did not play the same role in causing cytotoxicity in these cocultures.
Cytotoxicity is dependent upon cell-to-cell contact.
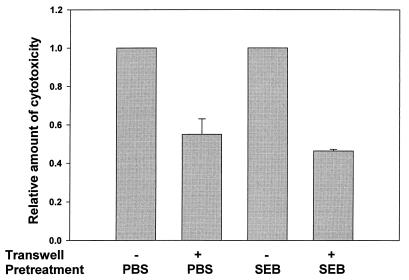
To assess whether cell-cell contact also contributes to the observed cytotoxic response, we added a permeable support membrane (Transwell) to prevent splenocyte and bacterial cell contact with the fibroblast monolayer. As shown in Fig. 6, blocking the splenocyte cell contact with the fibroblast monolayer reduced cytotoxic levels in both PBS- and SEB-pretreated cultures. In cocultures containing the Transwell inserts, a greater reduction in the percent cytotoxicity was observed with the superantigen-activated splenocytes than with the PBS-pretreated splenocytes (2.2-fold versus 1.8-fold). Although this difference is slight, these data suggest an important role for direct cell-to-cell contact in mediating cytotoxicity in the coculture system; however, in cultures containing SEB-pretreated splenocytes, a slightly higher amount of cytotoxicity seems to be dependent on these cellular interactions.
FIG. 6.
Effect of soluble factors on the cytotoxicity of BALB/c fibroblast cells. A.2R.1 cells (target) were seeded at a density of 2.0 × 105 cells per well in 24-well plates and allowed to adhere for 10 h at 37°C and 5% CO2. SEB- and PBS-pretreated splenocytes (effectors) were added at a concentration of 1.5 × 106 cells per well with or without bacteria at 105 cells per well. Parallel cultures contained permeable membrane inserts to separate the effector and bacterium cells from the target cells. After 24 h the LDH levels in the culture supernatants were determined, and the relative target LDH release was calculated as described in Materials and Methods. Each bar represents the mean value for three separate experiments ± the SEM.
DISCUSSION
Upon exposure to the superantigen SEB, immune cells, especially T lymphocytes, undergo significant functional alterations. The response mechanisms of superantigen-exposed T cells to conventional and self antigens is not clearly established; however, this response involves cytokine production, e.g., IL-2, IFN-γ, and tumor necrosis factor alpha (TNF-α) (34), and/or lytic activity of these cells. Previously, we described a novel method for coculturing splenocytes with viable bacteria (30). The splenocyte system had been used earlier by Bigley and coworkers as an in vitro model that accurately reflects in vivo immune responses to viral infection (11). This system allows the study of complex host-bacterium interactions and the superantigen-altered host response. In the present study, we further characterize the in vitro immune response to bacterial antigens after in vivo exposure to SEB.
Our results indicate that superantigen exposure exacerbates splenocyte cytokine production in the presence of bacteria. A significant amount of IFN-γ is produced only when bacteria are cultured with splenocytes from SEB-pretreated mice. This response is evident 2 days after exposure to the superantigen and increases with exposure to increasing numbers (≥104) of viable bacteria. This heightened response of SEB-pretreated cells follows the kinetics of SEB stimulation of T lymphocytes in vivo. Exposure to SEB results in increased Vβ8+ proliferation at 2 days postinjection; by 4 days these cells are unresponsive to further SEB stimulation or have been deleted (25).
In the coculture system, a majority of the IFN-γ produced in response to bacterial antigens is generated by CD8+ T cells; some of these cells must have non-Vβ8 specificities. SEB activates both CD4+ and CD8+ cells (19, 32) and can interact with both class I and class II molecules on APC (2, 16). Presentation of exogenous antigen does not appear to be limited to class II presentation since macrophages transfer antigens from phagosomes into the cytosol and endogenous and exogenous antigens can use a final common pathway for class I presentation (26). It is also possible that the bacteria in the coculture adhere to eukaryotic cells and are possibly internalized, which might lead to class I presentation of bacterial antigens. In our coculture system, a possible scenario would involve SEB activation of CD8+ cells in vivo that respond in vitro to MHC class I molecules complexed with bacterial peptides, resulting in IFN-γ production. Alternatively, CD8+ T cells may be primed to produce IFN-γ as a result of cytokine-mediated activation. Recently, Coppola and Blackman (10) demonstrated that CD8+ memory T cells do respond to superantigen stimulation by proliferating and developing effector lytic function. In addition, SEB stimulates indirect secondary activation of non-Vβ8 reactive T cells (3).
Superantigen exposure may generate a hyperresponsive state in which antigen-specific cells respond to conventional (bacterial) antigen in the coculture system, thereby resulting in excessive cytokine production, effector function, and bystander cell activation. While some studies indicate that superantigen exposure contributes to a loss in memory cell function toward a conventional antigen (4), others maintain that memory T cells are tolerant to SEB exposure (27). Also, prior exposure to SEB does not appear to render cells unresponsive to further superantigen exposure if appropriate antigen presentation and costimulation are provided (17, 18); these cells maintain the ability to secrete IFN-γ (15).
IFN-γ is a major activator of macrophages, leading to the production of TNF-α, IL-1, and IL-12 (12). It is interesting to note that IL-12 production in the coculture precedes the dramatic increase in IFN-γ by 8 to 10 h and also that IL-12 is required to achieve the higher levels of IFN-γ. Since NK cells contribute to IFN-γ in the coculture system (30), it is possible that early IFN-γ synthesis by these cells leads to the stimulation of IL-12 production. Although we do not know if the response of SEB-pretreated mice to bacteria in vivo would be the same, the splenocyte culture system has previously been used to predict the in vivo immune response accurately (11).
APC, such as macrophages and dendritic cells, are the primary producers of IL-12 in vivo (38). In the coculture system, we do not presently know which APC is responsible for IL-12 production but we are currently investigating this question. Our coculture data suggest a regulatory effect of IL-12 in directing production of the striking levels of IFN-γ seen in SEB-pretreated splenocyte-bacterium cultures, an effect which resembles a T-helper 1 (Th1) response activated by APC (38). In vivo, SEB stimulates Th1 cytokine production, which contributes to the shock syndrome occurring upon SEB rechallenge (15). Administration of a neutralizing monoclonal antibody against IFN-γ before that of SEB counteracts the weight loss and hypoglycemia associated with SEB treatment (31). Variations in cytokine secretion stimulated by bacterial infection may possibly elicit a Th1/Th2 imbalance, favoring domination of one type of response over the other. Th1 skewing by SEB observed in vivo may account for IFN-γ production by CD8+ T cells in our cocultures.
We examined the potential cytotoxic effects generated by immune cells from superantigen-exposed mice when the cells encounter bacteria. SEB restimulation in the presence of costimulus-competent APC can revert anergy into responsiveness (18). This shift to a responsive state may depend on CD4+ T-cell cytokine release and subsequent stimulation of cytokine-producing CD8+ T cells (20). In addition, lytic effector function does not appear to be compromised by SEB exposure (35). Utilizing a coculture system, we examined the cumulative results of superantigen stimulation of Vβ–T-cell-specific and bystander cell activation on host tissue destruction. SEB is cleared from the host in a matter of hours, yet the effects on T cells continue beyond 2 days after the exposure (39). This indicates that a local exposure to superantigen could result in systemic effects, particularly at sites of bacterial cell colonization. In this work, we have shown that SEB exacerbates the splenocyte cytotoxicity of compatible fibroblast cells only in the presence of bacteria. One scenario that would explain the enhanced cytotoxicity is as follows: upon encountering presented bacterial antigens, a superantigen-altered T cell would secrete IFN-γ and IL-2, further stimulating T-cell effector functions and cytokine production and activating macrophages to produce IL-12, TNF-α, nitric oxide, and oxygen intermediates (6, 28). The activated macrophage would in turn stimulate more T cells to respond. This overproduction of cytokines may contribute to host tissue destruction at the site of bacterial colonization. In contrast, a typical interaction of T cells and APC would stimulate cytokine cascades to clear the invading bacteria, but the additional secretion of regulatory cytokines would be available to downregulate the response. Although we have no evidence for downregulatory effects in PBS-pretreated cocultures, we do not observe the high levels of IFN-γ in these cocultures that are seen in SEB-pretreated cocultures. Neutralization of IFN-γ leads to a reduction in observed cytotoxicity, suggesting an involvement of cytokine-mediated lytic activities. Separation of splenocytes and bacteria from fibroblast cells by way of a porous membrane demonstrates that cell-cell contact is a key component for these cytotoxic effects. Cytotoxicity is observed in the cocultures even with splenocytes from PBS-pretreated mice, but it should be noted that the magnitude of the response is greater in the SEB-treated cultures. It is possible that the bacteria actually are tightly associated with the fibroblast cells and stimulate a cytotoxic response. Overproduction of inflammatory cytokines in these cultures could enhance the activity of the cytotoxic immune cells.
It is clear from this study that SEB exposure plays a significant role in directing an inflammatory response. The heightened cytokine levels observed when SEB-pretreated splenocytes were cultured with bacteria favors a proinflammatory response, one possibly contributing to tissue destruction in vivo. We do not know if other superantigens would stimulate the same response; however, since the mechanism of activity seems to be conserved for different superantigens, similar results might be expected if other superantigens were used. These data give insight into how superantigens may contribute to many inflammatory disease processes.
REFERENCES
- 1.Aste-Amezaga M, D’Andrea A, Kubin M, Trinchieri G. Cooperation of natural killer cell stimulatory factor/interleukin-12 with other stimuli in the induction of cytokines and cytotoxic cell-associated molecules in human T and NK cells. Cell Immunol. 1994;156:480–492. doi: 10.1006/cimm.1994.1192. [DOI] [PubMed] [Google Scholar]
- 2.Avery A, Markowitz J, Grusby M, Glimcher L, Cantor H. Activation of T cells by superantigen in class II-negative mice. J Immunol. 1994;153:4853–4861. [PubMed] [Google Scholar]
- 3.Bell S, Vroegop S, Buxser S. Early activation and cell trafficking induced by staphylococcal enterotoxin B: effects of high- versus low-dose challenge on induction of anergy. Cell Immunol. 1994;154:440–452. doi: 10.1006/cimm.1994.1090. [DOI] [PubMed] [Google Scholar]
- 4.Bell S, Buxser S. Staphylococcal enterotoxin B modulates Vβ8 TCR-associated T-cell memory against conventional antigen. Cell Immunol. 1995;160:58–64. doi: 10.1016/0008-8749(95)80009-8. [DOI] [PubMed] [Google Scholar]
- 5.Bowness P, Moss P, Tranter H, Bell J, McMichael A. Clostridium perfringens enterotoxin is a superantigen reactive with human T cell receptors V beta 6.9 and Vβ 22. J Exp Med. 1992;176:893–896. doi: 10.1084/jem.176.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chensue S, Ruth J, Warmington K, Lincoln P, Kunkel S. In vivo regulation of macrophage IL-12 production during type I and type 2 cytokine-mediated granuloma formation. J Immunol. 1995;155:3546–3551. [PubMed] [Google Scholar]
- 7.Choi Y, Lafferty J, Clements J, Todd J, Gelfand E, Kappler J, Marrack P, Kotsin B. Selective expansion of T cells expressing Vβ2 in toxic shock syndrome. J Exp Med. 1990;172:981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi Y, Kappler J, Marrack P. A superantigen encoded in the open reading frame of the 3′ long terminal repeat of mouse mammary tumour virus. Nature. 1991;350:203–207. doi: 10.1038/350203a0. [DOI] [PubMed] [Google Scholar]
- 9.Cole B, Daynes R, Ward J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. J Immunol. 1981;127:1931–1936. [PubMed] [Google Scholar]
- 10.Coppola M, Blackman M. Bacterial superantigens reactivate antigen-specific CD8+ memory T cells. Int Immunol. 1997;9:1393–1403. doi: 10.1093/intimm/9.9.1393. [DOI] [PubMed] [Google Scholar]
- 11.Curiel R, Mason K, Dryden T, Bigley N. Cytokines produced early in picornavirus infection reflect resistance or susceptibility to disease, J. Interferon Cytokine Res. 1998;18:587–596. doi: 10.1089/jir.1998.18.587. [DOI] [PubMed] [Google Scholar]
- 12.Farrar M, Schreiber R. The molecular cell biology of interferon-γ and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- 13.Fleischer B, Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. J Exp Med. 1988;167:1697–1701. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleischer B. Superantigens produced by infectious pathogens: molecular mechanism of action and biological significance. Int J Clin Lab Res. 1994;24:193–197. doi: 10.1007/BF02592461. [DOI] [PubMed] [Google Scholar]
- 15.Florquin S, Amraoui S, Goldman M. T cells made deficient in interleukin-2 production by exposure to staphylococcal enterotoxin B in vivo are primed for interferon gamma and interleukin-10 secretion. Eur J Immunol. 1995;25:1148–1153. doi: 10.1002/eji.1830250503. [DOI] [PubMed] [Google Scholar]
- 16.Haffner A, Zepter K, Elmets C. Major histocompatibility complex class I molecule serves as a ligand for presentation of the superantigen staphylococcal enterotoxin B to T cells. Proc Natl Acad Sci USA. 1996;93:3037–3042. doi: 10.1073/pnas.93.7.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heeg K, Gaus H, Griese D, Bendigs S, Miethke T, Wagner H. Superantigen-reactive T cells that display an anergic phenotype in vitro appear functional in vivo. Int Immunol. 1995;7:105–114. doi: 10.1093/intimm/7.1.105. [DOI] [PubMed] [Google Scholar]
- 18.Heeg K, Wagner H. Induction of responsiveness in superantigen-induced anergic T cells. Role of ligand density and costimulatory signals. J Immunol. 1995;155:83–92. [PubMed] [Google Scholar]
- 19.Herrmann T, Baschieri S, Lees R, MacDonald H. In vivo responses of CD4+ and CD8+ cells to bacterial superantigens. Eur J Immunol. 1992;22:1935–1938. doi: 10.1002/eji.1830220739. [DOI] [PubMed] [Google Scholar]
- 20.Hoiden I, Moller G. CD8+ cells are the main producers of IL10 and IFN gamma after superantigen stimulation. Scand J Immunol. 1996;44:501–505. doi: 10.1046/j.1365-3083.1996.d01-339.x. [DOI] [PubMed] [Google Scholar]
- 21.Huber B, Hsu P, Sutkowski N. Virus-encoded superantigens. Microbiol Rev. 1996;60:473–482. doi: 10.1128/mr.60.3.473-482.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson H, Russell J, Pontzer C. Staphylococcal enterotoxin microbial superantigens. FASEB J. 1991;5:2706–2712. doi: 10.1096/fasebj.5.12.1916093. [DOI] [PubMed] [Google Scholar]
- 23.Johnson H, Russell J, Pontzer C. Superantigens in human disease. Sci Am. 1992;266:92–101. doi: 10.1038/scientificamerican0492-92. [DOI] [PubMed] [Google Scholar]
- 24.Kappler J, Kotzin B, Herron L, Gelfand E, Bigler R, Boylston A, Carrel S, Posnett D, Choi Y, Marrack P. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 25.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature. 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 26.Kovacsovics-Bankowski M, Rock K. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 27.Lee W, Vitetta E. Memory T cells are anergic to the superantigen staphylococcal enterotoxin B. J Exp Med. 1992;176:575–579. doi: 10.1084/jem.176.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Seguin R, Keller K, Chadee K. Tumor necrosis factor alpha augments nitric oxide-dependent macrophage cytotoxicity against Entamoeba histolytica by enhanced expression of the nitric oxide synthase gene. Infect Immun. 1994;62:1534–1541. doi: 10.1128/iai.62.5.1534-1541.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litton M, Sander B, Murphy E, O’Garra A, Abrams J. Early expression of cytokines in lymph nodes after treatment in vivo with Staphylococcus enterotoxin B. J Immunol Methods. 1994;175:47–58. doi: 10.1016/0022-1759(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 30.Mason K M, Bigley N J, Fink P S. Development of a novel in vitro coculture system for studying host response to native bacterial antigens. J Immunol Methods. 1998;211:147–158. doi: 10.1016/s0022-1759(97)00200-7. [DOI] [PubMed] [Google Scholar]
- 31.Matthys P, Mitera T, Heremans H, Van Damme J, Billiau A. Anti-gamma interferon and anti-interleukin-10 antibodies affect staphylococcal enterotoxin B-induced weight loss, hypoglycemia, and cytokine release in d-galactosamine-sensitized and unsensitized mice. Infect Immun. 1995;63:1158–1164. doi: 10.1128/iai.63.4.1158-1164.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miethke T, Bendigs S, Bader P, Wagner H, Heeg K. Murine CD4+ and CD8+ T cells are activated by the superantigen (SA) staphylococcal enterotoxin B (SEB) and exhibit MHC-unrestricted cytotoxicity. Zentbl Bakteriol. 1991;275:264–268. doi: 10.1016/s0934-8840(11)80074-5. [DOI] [PubMed] [Google Scholar]
- 33.Murray D, Ohlendorf D, Schlievert P. Staphylococcal and streptococcal superantigens: their role in human diseases. ASM News. 1995;61:229–235. [Google Scholar]
- 34.Sawitzke A, Knudtson K, Cole B. Bacterial superantigens in disease. In: Roth J, Bolin C, Brogden K, Minion F, Wannemuehler M, editors. Virulence mechanisms of bacterial pathogens. Washington, D.C: ASM Press; 1995. pp. 145–169. [Google Scholar]
- 35.Sundstedt A, Dohlsten M, Hedlund G, Hoiden I, Bjorklund M, Kalland T. Superantigens anergize cytokine production but not cytotoxicity in vivo. Immunology. 1994;82:117–125. [PMC free article] [PubMed] [Google Scholar]
- 36.Torres B, Johnson H. Identification of an HIV-1 Nef peptide that binds to HLA class II antigens. Biochem Biophys Res Commun. 1994;200:1059–1065. doi: 10.1006/bbrc.1994.1557. [DOI] [PubMed] [Google Scholar]
- 37.Torres B, Tanabe T, Yamamoto J, Johnson H. HIV encodes for its own CD4 T-cell superantigen mitogen. Biochem Biophys Res Commun. 1996;225:672–678. doi: 10.1006/bbrc.1996.1228. [DOI] [PubMed] [Google Scholar]
- 38.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 39.Vabulas R, Bittlingmaier R, Heeg K, Hermann W, Miethke T. Rapid clearance of the bacterial superantigen staphylococcal enterotoxin B in vivo. Infect Immun. 1996;64:4567–4573. doi: 10.1128/iai.64.11.4567-4573.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watanabe-Ohnishi R, Aelion J, LeGros L, Tomai M, Sokurenko E, Newton D, Takahara J, Irino S, Rashed S, Kotb M. Characterization of unique human TCR Vβ specificities for a family of streptococcal superantigens represented by rheumatogenic serotypes of M protein. J Immunol. 1994;152:2066–2073. [PubMed] [Google Scholar]
- 41.Weber G, Cantor H. HIV glycoprotein as a superantigen. A mechanism of autoimmunity and implications for a vaccination strategy. Med Hypotheses. 1993;41:247–250. doi: 10.1016/0306-9877(93)90241-h. [DOI] [PubMed] [Google Scholar]
- 42.White J, Herman A, Pullen A, Kubo R, Kappler J, Marrack P. The Vβ-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]