Abstract
Auditory-motor and visual-motor networks are often coupled in daily activities, such as when listening to music and dancing; but these networks are known to be highly malleable as a function of sensory input. Thus, congenital deafness may modify neural activities within the connections between the motor, auditory, and visual cortices. Here, we investigated whether the cortical responses of children with cochlear implants (CI) to a simple and repetitive motor task would differ from that of children with typical hearing (TH) and we sought to understand whether this response related to their language development. Participants were 75 school-aged children, including 50 with CI (with varying language abilities) and 25 controls with TH. We used functional near-infrared spectroscopy (fNIRS) to record cortical responses over the whole brain, as children squeezed the back triggers of a joystick that vibrated or not with the squeeze. Motor cortex activity was reflected by an increase in oxygenated hemoglobin concentration (HbO) and a decrease in deoxygenated hemoglobin concentration (HbR) in all children, irrespective of their hearing status. Unexpectedly, the visual cortex (supposedly an irrelevant region) was deactivated in this task, particularly for children with CI who had good language skills when compared to those with CI who had language delays. Presence or absence of vibrotactile feedback made no difference in cortical activation. These findings support the potential of fNIRS to examine cognitive functions related to language in children with CI.
Keywords: cochlear implant, auditory-motor coupling, visuo-motor coupling, cortical activity changes
Introduction
Imitation and action learning are inborn abilities that help babies interact with external signals in real-time and build the neural basis for sensorimotor synchronization through their first months of life. Experiences like being rocked or listening to a rhythmic song stimulate babies’ sensory organs and help them connect sound to movement (Laland et al., 2016; Oztop et al., 2006; Repp & Su, 2013). Step by step and during the first year, babies become experts in beat perception, categorization of rhythms, and synchronization of body movements with auditory perception (Hannon et al., 2017). This entrainment and rhythmic coordination of movements toward external stimuli plays a key role in shaping the foundation of auditory-motor coupling, which is a prerequisite for higher cognitive functions and, most specifically, for speech performance (Chen et al., 2006; Kasdan et al., 2022; Lehmann et al., 2016; Puschmann et al., 2021; Zatorre et al., 2007).
A complicated neural network spreading from subcortical and cortical regions underlies auditory-motor interaction (Cannon & Patel, 2021; Nozaradan et al., 2018; Zatorre et al., 2007). More specifically, for speech performance, this auditory-motor interplay has been understood in terms of a dual stream model in which speech processing involves ventral and dorsal pathways that form the bases for speech recognition and production, respectively (Hickok & Poeppel, 2007). Structures in the superior and middle portions of the temporal lobe cooperate to form the ventral pathway, bilaterally, while the dorsal stream is more dominant in the left hemisphere and includes structures in the posterior temporal lobe, parietal operculum, and posterior frontal lobe. This dorsal pathway plays a prominent role in beat detection, auditory-motor integration, and most specifically in creating frontal lobe articulatory representations of speech signals (Hickok, 2022). From this neurological angle, it makes sense that this dorsal stream activity would be relevant to speech and communication disorders.
The interface between motor actions and auditory information in the dorsal pathway has some role in beat perception within the context of other sensory modalities like vision and proprioception. Such evidence emphasizes the importance of processing sensory stimuli independent of their modality, and pinpoints functional organization with respect to the stimuli attributes (Araneda et al., 2017; Karabanov et al., 2009; Su, 2014). Indeed, multisensory integration generates a comprehensive profile of the external world (Dionne-Dostie et al., 2015). The organizational balance between neighbouring brain regions involved in multisensory integration can be altered when one sensory modality is impaired or absent. Intra-modal changes then occur within different layers of the affected sensory cortex, but changes across modalities may also occur. This latter phenomenon is referred to as cross-modal neuroplasticity. The part of the cortex that is no longer exposed to sensation from the impaired modality becomes sensitive to other (intact) sensory modalities (Bavelier & Neville, 2002; Voss & Zatorre, 2012). One common cause of cross-modal plasticity - of particular interest here is the lack of auditory stimulation (Kral & Pallas, 2011).
Hearing loss is accompanied by substantial alterations in the structure and functional connections of the auditory cortex (Dell Ducas et al., 2021; Manno et al., 2021; Shiell et al., 2015; Wallace et al., 2020). The so-called takeover of the auditory cortex by the visual sense is one of the most notable, or rather most studied, phenomena (Campbell & Sharma, 2014; Wang et al., 2019). This phenomenon is highly adaptive (Voss et al., 2010) and has attracted numerous neuroimaging and electrophysiological studies revealing superior visual functioning among people with severe hearing loss, relative to those with typical hearing (TH) on tasks like visual speech comprehension (Lyxell & Holmberg, 2000), visual motion detection (Bottari et al., 2014), recognition of communicative gestures (Simon et al., 2020), and sign language (Capek et al., 2008; Fine et al., 2005). Considering the cooperation of three functional modalities (e.g., auditory, vision, and motor) in this multimodal network, the auditory cortex might be less connected to the motor cortex in children with severe hearing loss than in TH controls, and the visual cortex might be more connected with both motor and speech cortices (Shi et al., 2016).
Restoration of sensory stimuli might partially reverse the changes first associated with sensory impairment (although this is not always true for the visual sense; e.g., see Mowad et al., 2020). Within this context, children with cochlear implants (CI) provide an important opportunity to study how restoration of hearing might reverse changes induced during auditory deprivation (Kral et al., 2019). Children implanted early in life might be particularly prone to such reversal changes, depending on the strategies followed by a given child and their family in terms of language development (some being more prone to visual language at home, with others emphasizing oral communications). According to the sensorimotor coupling model of speech development (Westermann & Miranda, 2004), we hypothesized that at least some children with CI would bear similarities to children without hearing in that the functional connectivity between auditory and motor cortices would be weakened compared to controls with TH. In contrast, connectivity between their visual and motor cortices would be strengthened compared to children with TH.
Of note, however, coupling between two brain regions is not necessarily positive. In a purely motor task (devoid of speech content), neither the auditory nor the visual cortex would be expected to contribute to squeezing a joystick. Thus, the prediction of a weaker auditory-motor coupling could translate into a weaker deactivation of the auditory cortex; and, similarly, the prediction of a stronger visual-motor coupling could translate into a stronger deactivation of the visual cortex as the motor cortex activates. If changes in the multimodal integration network are related to language and communication, we would further hypothesize that the quality of coupling (auditory-motor or visual-motor) would differ for children with good versus poor language skills, with the latter group activating a network pattern more similar to individuals without hearing (non-implanted).
For the reader who is naïve to CI science, we should emphasize that outcomes with these devices are vastly heterogeneous. While CI technology has been effective in restoring hearing and allowing communication in quiet environments, there have been many cases in which users of these devices gained little benefit in important aspects of daily life. Concerning pediatric CI users, the research emphasis has often been on academic performance (Marschark et al., 2007; Wilson Ottley et al., 2023) and some children have continued to struggle despite early implantation (Ching et al., 2018; Dettman et al., 2016; Geers et al., 2017; Wolfe et al., 2021). The extent to which these differences stem from variable multimodal integration, language processing difficulties, or functional activation/deactivation of various brain regions has remained unclear.
Clinically, a better understanding is needed. One recent research team (Choi et al., 2020) evaluated the academic performance of 6-17-year-old children who had early implantation and at least five years of hearing experience with CIs. These children still had difficulties understanding abstract concepts in science and social sciences, and they showed problems in speech perception in noisy or group environments. Although assistive hearing technologies may enhance children’s speech perception and academic performance, they do not guarantee age-appropriate or grade-level abilities. Neuroimaging technologies may help researchers better understand the source of these discrepancies.
Functional near-infrared spectroscopy (fNIRS) is a safe, practical, and informative neuroimaging tool that can depict patterns of cortical activation and deactivation in pediatric CI recipients (Saliba et al., 2016). Like functional magnetic resonance imaging (fMRI), fNIRS relies on blood-oxygen-level-dependent (BOLD) signals. In fMRI, brain activation is inferred from an increase in blood flow (i.e., local oxygenation), and, hence, a reduction in the relative proportion of deoxy-hemoglobin. With less deoxy-hemoglobin, the fMRI or fNIRS signal rises above baseline and is detectable. BOLD activation has direct links to neuronal activation. On the other hand, deactivation happens when oxy-hemoglobin decreases, causing a net increase in deoxy-hemoglobin (Frankenstein et al., 2003).
Unlike fMRI, which only detects changes in deoxy-hemoglobin (most specifically in venous blood), fNIRS directly estimates the level of both oxy- and deoxy-hemoglobin in arterial and venous blood. Therefore, despite certain technical limitations (see Discussion section of this paper), fNIRS has an advantage over fMRI for examining the exact nature of neurovascular coupling. Since hemodynamic responses are dynamic interactions between vascular systems that cooperate to deliver and extract oxygen, imaging that does not provide information about the arterial side of this interplay can lead to misinterpretation; recording both oxy- and deoxy-hemoglobin depicts neurovascular coupling more precisely (Tam & Zouridakis, 2014). We took advantage of this technique with measurements over the whole brain, even though our analysis focused on certain brain regions of interest (ROI), namely motor, auditory, and visual cortices.
Method
Participants
Our study took place in Oklahoma City at Hearts for Hearing (https://heartsforhearing.org/). Seventy-five children between 7 and 18 years old were selected from patient records: 50 with CIs (CI group- Tables 1-A and 2-A) and 25 controls with typical hearing and language development (TH group). Details about inclusion/exclusion criteria, participants’ demographic characteristics, hearing experience, and device use are provided in Wolfe et al. (2021), a study that reported exclusively on audiological outcomes. In the present study, CI recipients were divided into two groups based on their language skills: 26 children had age-appropriate language skills (referred to as Typical Language, or TL group) and 24 had language delays (referred to as Low Language, or LL group). Language skills were assessed through the Clinical Evaluation of Language Fundamentals - Fifth Edition (CELF; Wiig et al., 2013). The CELF included an age-based assessment of Receptive Language Index, Expressive Language Index, Core language Score, Language Structure and Content.
All children with CIs had received implants before four years of age, and they were all properly fitted (aided thresholds <30 dB HL) and communicated primarily through spoken language. The three groups (CI with TL, CI with LL, and TH) were matched by sex but not by chronological age. While this age difference was not intended, it may have conferred a maturational advantage to the CI with LL group compared to the CI with TL group.
We explained the study rationale to each child and their parents, after which the parents provided their informed consent. Note that the full study from which this experiment was drawn was more extensive than that described in this article in that several other tasks were also conducted: a low-level visual task (checkerboard), a low-level auditory task (oddball design), a phonological task (words and sudo-words), audio-visual integration, emotional processing (a 10-min video from the movie Despicable Me), and a 7-min resting-state recording. As the entire protocol was lengthy, testing was conducted at different times separated by a large time break. Participants were compensated financially ($25/hour) for their entire participation. The study protocol was approved by the Western Institutional Review Board and all the ethical considerations in research were respected (reference #20190882).
Protocol
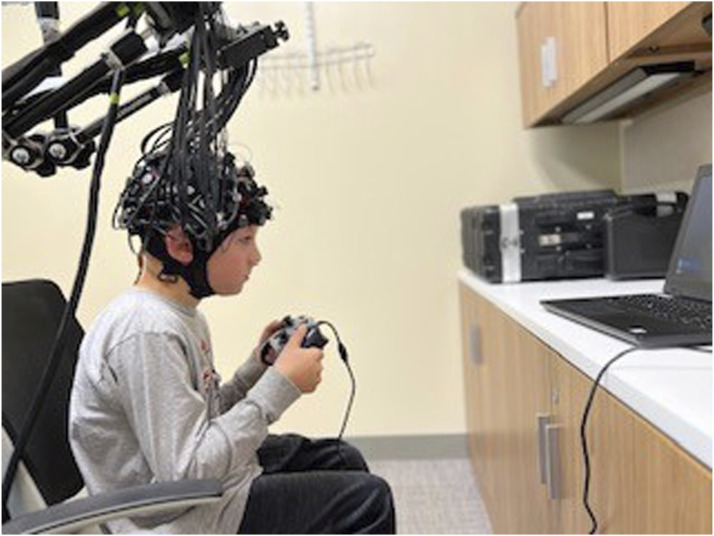
Children sat in front of a monitor and held a joystick. The response protocol involved completing a block design task with 10 motor events that were 15 seconds long, alternating with 10 rest periods of 15 seconds. In each event, the word “squeeze” was displayed every second on a laptop that was placed one meter in front of the children; and the children were instructed to squeeze the two back triggers of the joystick each time they saw the cue (Figure 1). In half of the blocks, the joystick also vibrated with the squeeze, allowing us to probe both somatosensory and motor processing. This experimental session lasted 5 minutes. It was coded in PsychoPy (https://www.psychopy.org/) and included triggers at the onset of each event.
Figure 1.
Experimental Protocol Depicting a Child Squeezing the Joystick When Instructed by a Monitor to Do So, in a 15-s Block-Design.
Note. No sound was presented in this study, and all children with CIs turned their devices off. CI: Cochlear Implant.
Apparatus
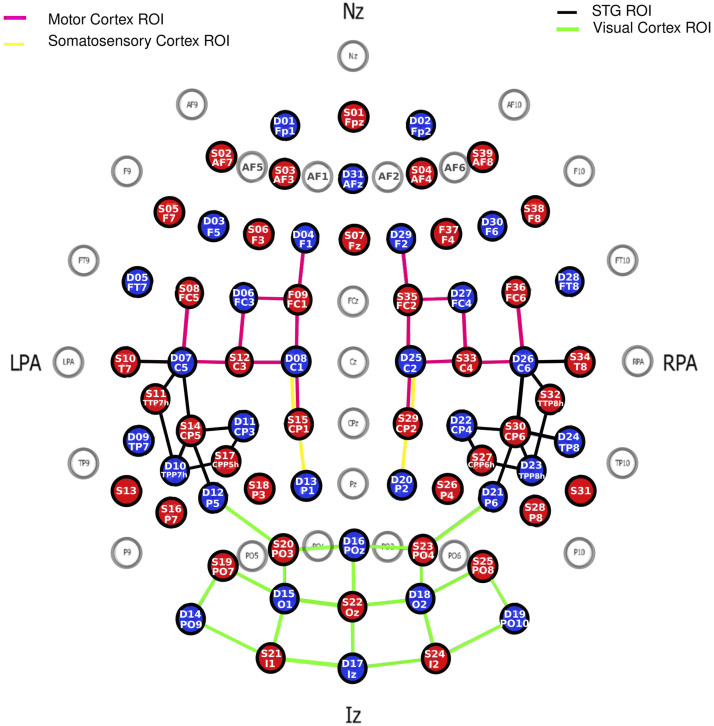
We recorded continuous fNIRS using 39 LED sources and 31 detectors from the NIRScout system developed by NIRx Medical Technologies (LLC, USA). The theoretical montage (Figure 2) resulted in a total of 122 channels, with no short channel. Each source emitted light at two wavelengths of 760 and 850 nm. An EasyCap (EASYCAP GmbH, Germany) was used to hold the sources and detectors, and their position was registered using the Structure Sensor Pro application by Occipital Inc. (https://structure.io/structure-sensor-pro. with three fiducials (nasion and left/right pre-auricular point) and later digitized using the FieldTrip Matlab toolbox (Oostenveld et al., 2011). Out of the 122 channels, the source-detector distance was on average 29.9 mm (SD = 6.5 mm). Four ROIs were selected.
Figure 2.
fNIRS Montage with 39 Sources (red) and 31 Detectors (Blue) Making Up a Total of 122 Channels.
Note. Only channels targeting 4 ROIs (Motor, Somatosensory, Visual, and Auditory Cortices) are shown. ROI: Region of Interest.
Data Analysis
We analyzed data using the NIRS toolbox (Santosa et al., 2018) running in MATLAB. First, the entire recording was trimmed 5 seconds before the first trigger and 5 seconds after the last trigger. Second, oversaturated channels were replaced with high-variance noise. Third, bad channels were flagged if their standard deviation over the trimmed signals (averaged over the two wavelengths) exceeded 15%. These bad channels reflected major alterations in the signal caused by environmental noise or physiological artifacts. There were, on average, 15.0 (SD = 13.7), 13.9 (SD = 11.3), and 7.3 (SD = 8.9) bad channels out of 122, in each group respectively. This number of bad channels differed significantly across groups, F (2,72) = 3.3, p = .043, as data obtained from TH children tended to be cleaner than data obtained from children with CI (although none of the pairwise comparisons reached significance, p > .056). This difference may have occurred because the presence of the coil may have been detrimental to scalp-to-optode contact. All flagged channels were linearly interpolated from adjacent good channels. Fourth, signals were converted to optical density (Huppert et al., 2009). Fifth, motion artifacts in these data were corrected using Temporal Derivative Distribution Repair (TDDR) that were first projected onto a principal components analysis (PCA) space before returning to the optical density space (Fishburn et al., 2019). Sixth, optical density signals were converted into changes in oxygenated hemoglobin concentration (HbO) and deoxygenated hemoglobin concentration (HbR) using the modified Beer-Lambert Law and using the source-detector distances calculated from the digitized montage specific to each child. The partial path length factors were set at 7.25 and 6.38 for the 760 and 850 nm wavelengths, respectively. Seventh, the hemoglobin signals were band-pass filtered between .01 and .25 Hz to limit low-frequency drift and cardiac oscillations. Eighth and finally, the hemoglobin signals were passed through a PCA, and the first component was systematically removed to reduce systemic physiological components. These participant-level statistics were performed by the AR_IRLS function of the NIRS toolbox which provided beta weights for each channel. These beta weights (just like in the fMRI field) represented the weight of the regressors (i.e., squeeze vs. rest) obtained when fitting the canonical hemodynamic response function to the time course of the recording, using ordinary least square fit conducted independently for HbO and HbR signals.
The statistical maps of t-statistics contrasting betas in squeeze versus rest were projected on the digitized montage averaged across all 75 children. But to address our hypotheses more directly, we isolated four brain ROIs (Figure 2): (a) the motor ROI was defined by 16 channels that overlapped (according to the Talairach atlas) in different proportions (between 14.2% and 94.4%) with either the primary motor cortex or the pre-motor and supplementary motor cortex; (b) the somatosensory ROI was defined by only four channels that overlapped between 14.4% and 87.0% with the primary somatosensory cortex or the somatosensory association cortex; (c) the auditory ROI was defined by 18 channels that overlapped between 20.7% and 32.1% with the superior temporal gyrus (STG) bilaterally; and (d) the visual ROI was defined by 20 channels that overlapped between 28.4% and 99.4% with either the primary visual cortex or the visual association cortex. In each ROI, a weighted average was calculated over all relevant channels with weights taken directly from how much each one overlapped with the ROI in question. Group averages of HbO and HbR waveforms across the ten blocks of the motor/vibrotactile task were calculated after baseline correction (using 5 seconds prior to the event onset).
We conducted analyses of variance (ANOVAs) with one between-subject factor (groups LL, TL, and TH) in each ROI on the weighted beta average of each ROI for HbO, HbR, and the difference (referred to as hemoglobin difference, HbDiff), for the comparison between the motor task (squeeze) versus rest period. When appropriate, pairwise comparisons were run with Bonferroni corrections to further explore group differences. In the absence of group differences, simple t-tests were conducted on the entire sample (of 75 children) to determine whether a given ROI was activated or deactivated when on the task as compared to rest. Pearson correlational analyses were systematically conducted to examine the relationships between HbDiff and (a) chronological age, (b) age at implantation, and (c) the CELF score. Each attempt was conducted independently (not in a stepwise fashion) for selected ROIs, and Bonferroni corrections were applied to adjust for the inflation of type-I error. Finally, all these analyses were reiterated for brain activity with or without joystick vibration (both being extracted from a common rest baseline).
Results
Motor Cortex
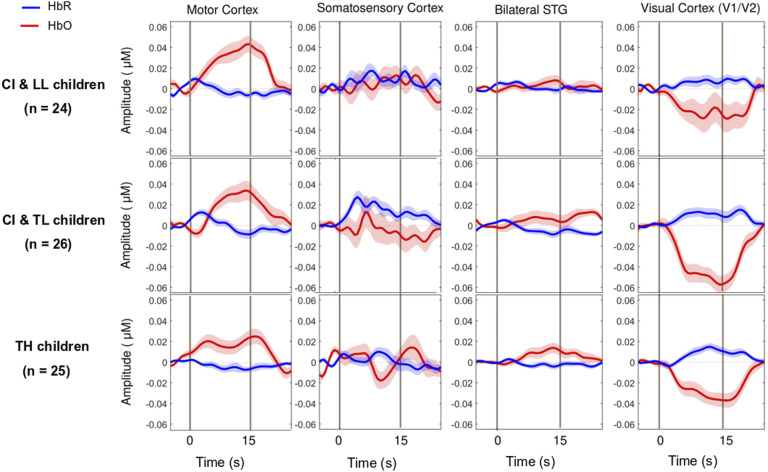
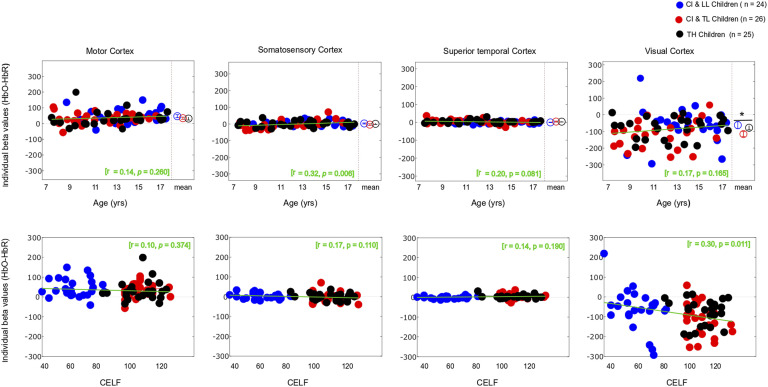
As expected, the motor task elicited a strong response from the motor cortex (Figures 3 and 4-left panels) in all groups, and there was no significant main effect for Group differences. The entire population exhibited a significant increase in HbO, a significant decrease in HbR, and a significant increase in HbDiff during the 15-s squeeze compared to the 15-s rest period (Table 1- top). In other words, all children engaged their motor cortex on this task (and fNIRS successfully revealed this engagement), but children engaged in the task, irrespective of their hearing/language status. Individual beta weights (HbDiff) illustrated that there were no relationships to chronological age or to language outcomes (Figure 5, left panels). Among the children with CI, these values did not relate to their age at implantation [p = .291] (not shown).
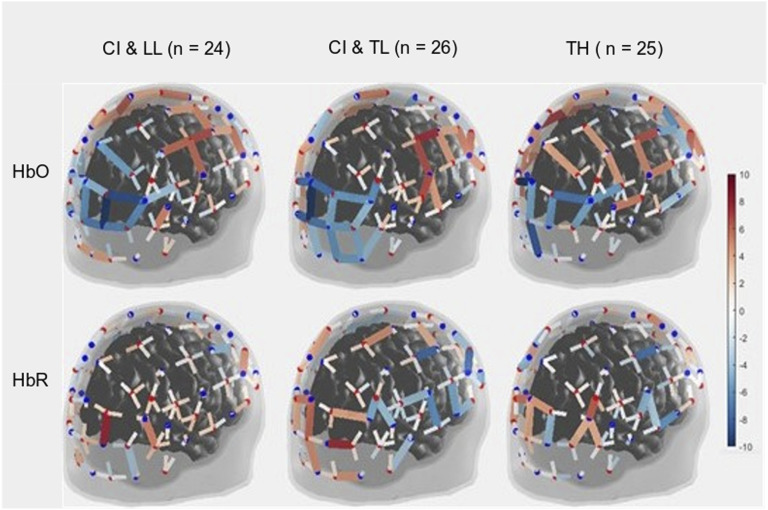
Figure 3.
Three-Dimensional Map of t-Statistic Values on the Beta Weights Obtained for the Effect of the Squeeze versus Rest Periods in Each Group, for Oxygenated Hemoglobin (Top) and Deoxygenated Hemoglobin (Bottom).
Note. CI & LL: Cochlear Implant and Low Language, CI & TL: Cochlear Implant and Typical Language, TH: Typical Hearing.
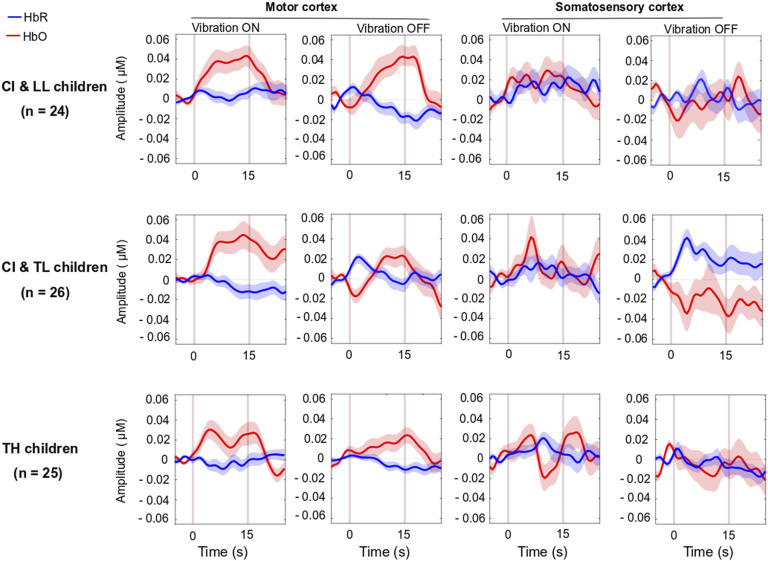
Figure 4.
Group-Averaged Event-Related Changes in Oxygenated and Deoxygenated Hemoglobin (HbO & HbR, respectively) Occurring in the Motor Cortex (Most Left), Somatosensory Cortex (Middle Left), Superior Temporal Cortices Bilaterally (Middle Right), and Visual Cortex (Most Right).
Note. CI & LL: Cochlear Implant and Low Language, CI & TL: Cochlear Implant and Typical Language, TH: Typical Hearing.
Table 1.
Results of the Between-Subject ANOVA (Across CI With LL, CI With TL, and TH Groups) and the One-Sample t-Tests (Against 0, i.e., No Change in the Cortical Activity) on Beta Weights Captured Over Selected Brain Regions of Interest.
| ROI | Chromophore | F Statistics | p Value | η2 | t Statistics | p Value | Cohen’s d | |||
|---|---|---|---|---|---|---|---|---|---|---|
| df | Error df | F | df | t | ||||||
| Motor cortex | HbO | 2 | 72 | 1.3 | .282 | .035 | 74 | +8.4 | <.001 | .967 |
| HbR | 2 | 72 | 0.1 | .885 | .003 | 74 | −2.6 | .010 | .118 | |
| HbDiff | 2 | 72 | 0.9 | .429 | .023 | 74 | +7.4 | <.001 | .135 | |
| Somatosensory cortex | HbO | 2 | 72 | 0.1 | .416 | .024 | 74 | +1.0 | .337 | .112 |
| HbR | 2 | 72 | 0.7 | .519 | .018 | 74 | +2.1 | .036 | .117 | |
| HbDiff | 2 | 72 | 0.8 | .445 | .022 | 74 | −0.2 | .864 | .115 | |
| STG | HbO | 2 | 72 | 0.6 | .526 | .018 | 74 | +1.1 | .290 | .123 |
| HbR | 2 | 72 | 1.0 | .364 | .028 | 74 | −1.0 | .310 | .118 | |
| HbDiff | 2 | 72 | 0.8 | .454 | .022 | 74 | +1.3 | .212 | .145 | |
| Visual cortex | HbO | 2 | 72 | 3.9 | .025 | .098 | 74 | −8.3 | <.001 | −.954 |
| HbR | 2 | 72 | 0.1 | .890 | .003 | 74 | +3.3 | .001 | .384 | |
| HbDiff | 2 | 72 | 2.6 | .079 | .068 | 74 | −8.5 | <.001 | −.984 | |
Figure 5.
Individual Beta Weights (HbDiff) Obtained in the Motor Cortex (Most Left), Somatosensory Cortex (Middle Left), Superior Temporal Cortices Bilaterally (Middle Right), and Visual Cortex (Most Right), as a Function of the Child’s Chronological Age (Top) and Their Language Skills (Bottom).
Note. HbDiff: Hemoglobin difference (i.e., HbO-HbR), CI & LL: Cochlear Implant and Low Language, CI & TL: Cochlear Implant and Typical Language, TH: Typical Hearing.
Somatosensory Cortex
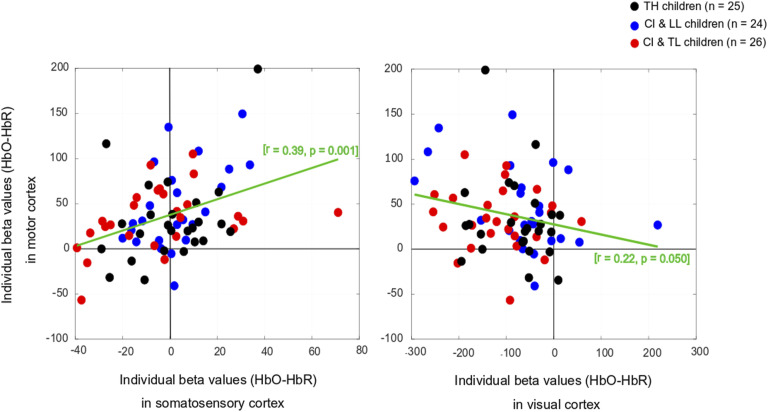
There was no significant main effect of Group on somatosensory cortical activation (Table 1- middle top). Across the entire population, there was no significant change in HbO, but there was a modest increase in HbR and no change in HbDiff during the 15-s squeeze compared to the 15-s rest period. If anything, the somatosensory cortex seemed to have been deactivated, irrespective of hearing status (Figure 4, middle left). However, we caution this interpretation since somatosensory cortical activity was positively correlated (across all participants) with motor cortex activity, as illustrated in Figure 6 (left panel). Individual beta weights were correlated with chronological age; younger children tended to show deactivation while older children tended to show activation of their somatosensory cortex. Yet, there was no relationship between HbDiff and language skills (Figure 5, middle left). Within the CI group, there was no relationship between HbDiff in this ROI and age at implantation [p = .822] (not shown).
Figure 6.
Individual Beta Weights (HbDiff) Obtained in the Motor Cortex versus Somatosensory (left) or Visual (right) Cortex.
Note. HbDiff: Hemoglobin difference (i.e., HbO-HbR), CI & LL: Cochlear Implant and Low Language, CI & TL: Cochlear Implant and Typical Language, TH: Typical Hearing.
Bilateral Superior Temporal Gyrus
Regarding activation of the bilateral STG, there was no significant main effect across the three groups of study (Table 1- middle bottom). The entire population exhibited no significant change in HbO, HbR, or HbDiff during the 15-s squeeze compared to the 15-s rest periods (Figure 4, middle right). Put differently, there was no change in the activity of the STG, and activity in these regions was not significantly correlated with activity in the motor cortex [p = .874] (not shown). Individual beta weights for brain activity in this ROI did not depend on chronological age or language skills (Figure 5, middle right). Among children with CIs, age at implantation had no significant role in brain activity in this ROI [p = .136] (not shown).
Visual Cortex
There was a significant main effect of Group for HbO, but not for HbR in the visual cortex, and there was only a trend toward group significance for HbDiff (Table 1- bottom). The group difference in HbO was driven by larger deactivation of the visual cortex in the CI with TL group versus the CI with LL group [p = .025, 95% CI [5.03,105.0]], while the other two comparisons (i.e., CI with TL vs. TH; and CI with LL vs. TH) were not significant [p > .106]. The entire population exhibited a significant decrease in HbO, a significant increase in HbR, and a significant decrease in HbDiff during the 15-s squeeze compared to the 15-s rest period (Figure 4, right panels). In other words, children deactivated their visual cortex to perform the motor task and, among children with CIs, this behaviour was exacerbated among those with better language skills. Note that this deactivation tended to be inversely related to activity in the motor cortex, as illustrated in Figure 6 (right panel). Individual beta weights did not reveal any relationship between brain activation and age; but interestingly, there was a negative correlation between visual cortex activation and CELF scores (Figure 5, right panels). Specifically, the more a child deactivated their visual cortex during this task, the better their language skills. Age at implantation had no role in the degree of visual cortex deactivation [p = .260] (not shown).
Vibrotactile Information
In the analyses above, we disregarded whether the joystick vibrated or not, as the child squeezed the triggers. However, the presence of the vibration across the ten events alternated, yielding a set of five events in which the joystick vibrated in response to the child’s action and five events in which the joystick did not vibrate. There was no group difference in brain activation in any of the four brain region activations (motor, somatosensory, STG, and visual cortex) for HbO, HbR, or HbDiff [p > .085 across all cases] when comparing events with and without vibration. Pooled data across all children revealed no vibration effect on brain activation in any ROI, [p > .202], except that there was a significant difference in HbR in the visual cortex [t (74) = −2.1, p = .044]. This means that there was a more pronounced deactivation of the visual cortex when the joystick vibrated than when it did not (Figure 7).
Figure 7.
Same as Figure 4 in motor and somatosensory cortex but split by the two conditions where the joystick vibrated or not, as the child squeezed on the back triggers.
Note. CI & LL: Cochlear implant and low language, CI & TL: Cochlear implant and typical Language, TH: Typical hearing, HbO: Oxygenated hemoglobin, HbR: Deoxygenated hemoglobin.
Discussion
Along with other reports on the same pediatric population (Deroche et al., 2023; Koirala et al., 2023; Wolfe et al., 2021), we explored motor cortex activity and its association with visual and auditory networks in children with CIs. We found an increase in HbO and a decrease in HbR in the motor cortex of all participants, irrespective of their hearing and language status. Meanwhile the visual cortex was strongly deactivated, with this finding more prominent among those CI users who had typical language than CI users who with low language profile; the pattern seen in CI users who had typical language was similar to children with TH. Thus, in addition to motor task activation of the motor cortex, a deactivation of supposedly irrelevant brain regions was detectable by fNIRS in children with better language functions.
No Differential Activity in the Motor Cortex, but Better Disengagement of the Visual Cortex
Recent investigators (Chen et al., 2017; Fullerton et al., 2023; Paul et al., 2022) have questioned the classical view (Lee et al., 2001) that cross-modal plasticity negatively affects speech performances of participants with CI. Cortical reorganization following CI may not always prevent the auditory cortex from responding to auditory stimuli (Land et al., 2016). Quite to the contrary, some individuals with CI may integrate multisensory information very effectively (Rouger et al., 2007) and cross-modal plasticity may strengthen their communication skills, especially those children who are familiar with sign language (see Beckers et al., 2023 for a comprehensive review on adult CI users). This is an unsettled on-going debate with many ramifications (Anderson et al., 2017b; Stropahl et al., 2016).
Perhaps closer to findings in this study, Chen et al. (2017) recruited 40 adult CI users and controls and separately measured fNIRS for circular checkerboard patterns as visual stimuli, and for words, reverse words, and tones as auditory stimuli. They found that intramodal connectivity within visual and within auditory areas was weaker in adult CI users than in their matched TH peers. In contrast, cross-modal connectivity between visual and auditory areas was stronger for these CI users, and this was beneficial to their speech recognition scores. They concluded that this strong cross-modal connectivity, which happened irrespective of the stimulus modality, was a result of concurrent processing of auditory and visual stimuli in both brain areas.
In a similar fashion, albeit with some methodological differences in experimental design and stimuli, Fullerton et al. (2023) used fNIRS to explore functional connectivity and cross-modal brain activation between visual and auditory cortices in fourteen post-lingual adult CI users. They tested these participants with speech and non-speech auditory stimuli and examined task-related differences in the evoked-related brain activity in auditory and visual cortices. Coordinated activity within the speech network was observed from both auditory and visual cortices; so, the authors concluded that such multimodal processing must be beneficial to the listening skills of CI users.
Anderson et al. (2017a) used fNIRS to examine changes between auditory and visual cortices in participants with CI, focusing on brain activations in some specific regions prior to and within six months of the implantation. Remarkably, they found that change patterns in brain activation were related to more successful speech understanding in CIs. Auditory cortex activation with visual stimuli was associated with adaptive benefits for these participants, as it seemed to promote auditory recovery after CI. While this claim was advanced for adult recipients, Mushtaq et al. (2020) applied this strategy to children with CI who mostly (16 out of 19) tested well on a phonetic perception task. While they found no difference in CI users relative to TH controls in processing auditory stimuli, the two groups differed in their ability to process visual speech stimuli such that children with CI then exhibited greater STG activity than TH controls. These authors concluded that cross-modal plasticity for processing auditory and visual speech stimuli is a synergistic process; they rejected the idea that visual language stimuli prevent the auditory cortex from responding to oral language.
From this multimodal perspective, we reasoned in this study that the motor cortex could also play a strong role for communication (and not just speech production) in daily life, especially for young children who are still amidst multifaceted development (Glennon et al., 2020). We hypothesized that in response to holding and squeezing a joystick, participants with CI would show weakened auditory-motor connectivity than TH controls (similar to findings in children with early-onset hearing loss without CI - Shi et al., 2016). However, this hypothesis was not supported. Our CI participants’ superior temporal cortices were simply not engaged (or disengaged) in this task. Presumably, such an association might exist in other tasks more closely related to speech (e.g., vocal production, tapping to an auditory beat, or perhaps writing). We also found mixed evidence for our hypothesis of stronger visual-motor connectivity in these participants than in TH controls. Indeed, the visual cortex of CI users was largely deactivated in an inverse relationship to motor cortex activity, particularly for CI users who had typical language functioning (CI with TL group). Thus, at least some children with CI (similar to children with early-onset hearing loss without CI in Shi et al., 2016) exhibited strong visual-motor coupling. The surprising aspect of this finding was that our CI with LL group did not exhibit as much visual deactivation as our CI with TL group (and yet they had a longer period of auditory deprivation). Exactly why this inverse coupling was not achieved by children in the CI with LL group remains unclear. Being able to visualize these cortical changes prior to (i.e., induced by the deprivation) and after implantation (i.e., induced by the restoration of auditory stimulation) would greatly help in this interpretation.
In the somatosensory cortex, there were no changes in HbO, but there was a modest HbR increase across all our participants. In other words, the somatosensory cortex tended to be deactivated irrespective of hearing or language skills status. These task-induced deactivations have been previously observed in studies using positron emission tomography scan (PET) (Haxby et al., 1994; Kawashima et al., 1995; Sadato et al., 1996; Shulman et al., 1997) and fMRI (Jäncke et al., 2000; Jorge et al., 2018; Morita et al., 2019, 2021; Newton et al., 2005; Shulman et al., 2007; Weisser et al., 2005) but their interpretation remains elusive. Yet, our findings warn that deactivation of brain ROIs may hold more predictive power than using specific tasks to direct sensory stimuli toward an expected brain ROI (here motor tasks directed toward the motor cortex). This finding generally calls for more fNIRS studies recording the activity of the whole brain rather than specific ROIs, even for seemingly low-level tasks.
The Meaning of Brain Region Activation or Deactivation
The exact mechanisms behind deactivation of brain regions, known as a negative BOLD response, are unclear (Hayes & Huxtable, 2012; He et al., 2022). To date, several mechanisms have been proposed. One is that deactivation acts as a filter (i.e., neuronal suppression) for behavioural relevance of a brain region to a specific task. When target objects in the task require shifts of attention, behavioural relevance is increased. Thus, deactivation widely occurs through many parts of sensory cortices and prevents attention shifts toward irrelevant cues, thereby enhancing target detection to maintain optimal performance). Considering, for example, the motor task used in this study, the visual and perhaps somatosensory cortices may have been deactivated to minimize brain processing of unimportant visual cues (or somatosensory joystick vibrations) to redirect cognitive resources toward the required motor activity. Notably, in a task that involves such targets, there is a direct relationship between the amount of deactivation and the performance such that the greater the deactivation of non-relevant cortical regions, the better the target detection (Shulman et al., 2007). In the current study, we could not observe this link because our motor task was devoid of any goal-related behaviour (e.g., earning points by squeezing more strongly or at a particular time). Yet, we still found a link between deactivation and the longer-term purpose of language development (as measured by the CELF), leading to speculation that the deactivation we observed in the visual cortex reflects a filtering process whose purpose was to better allocate cognitive resources.
Ecological Impact
Auditory-motor and visual-motor networks are often coupled in daily activities such as listening to music and dancing, helping us extract fundamental aspects of music, like rhythm and possibly aspects of melody. Coupling between auditory and motor modalities enhances attention, memory formation, and retrieval, as this multimodal learning has been shown to strengthen cognitive reserve, creating alternate neural pathways (Brown & Palmer, 2012; Mitterová et al., 2021). Although individuals with CI have difficulties decoding spectro-temporal cues to perceive melody (Jiam & Limb, 2020), they can still exploit CI technology to move to a beat (Phillips-Silver et al., 2015). Most relevant to this research, when children with CI listen and dance to music, active engagement of movement to auditory stimuli enhances learning and memory, as seen by improved song identification when moving, as compared to passive listening (Vongpaisal et al., 2016). Moreover, children with CIs were able to synchronize their body movements to the temporal pattern of music, generally a means of heightening pleasure with music (Janata et al., 2012; Matthews et al., 2020). Such observations highlight the importance of multimodal sensory networks other than those associated with speech (e.g., Kim & Zatorre, 2010). In audiology, we often take speech perception or production as a benchmark for evaluating CI outcomes, but there are many other human activities that require good auditory-motor and visual-motor coupling. Our study is another step towards better understanding innovative ways that patients with CI leverage multimodal integration.
Limitations and Directions for Further Study
We relied in this study on the assumption that fNIRS measurements could reliably reflect activation of the four brain ROIs we investigated, as confirmed recently by Lawrence et al. (2021). However, there are some common concerns about this assumption. First, fNIRS is arguably a newer and less reliable technique than fMRI. Perhaps as many as one third of the children we studied displayed little activity in either HbO or HbR (Figure 5), which has also occurred in other studies, leading investigators to have sometimes used an irrelevant task to first ensure that a BOLD-like response is measurable in a given participant (Cui et al., 2011; Sato et al., 2013). But this approach is less than ideal when examining special populations for which there are few participants. Investigators should pursue further developments in fNIRS technology to make it less dependent on skin pigmentation or melanin levels (Couch et al., 2015; Matas et al., 2002; Wassenaar & Van den Brand, 2005) and more reliable on an individual basis. Second, the “banana shaped photon path” of fNIRS limits its application to cortical regions that are relatively close to the scalp, narrowing the types of research questions that can be addressed with fNIRS (Harrison et al., 2021; Pinti et al., 2020). We easily captured activation/deactivation in the motor, somatosensory, and visual cortices, but one might question whether fNIRS could record activity from the primary auditory cortex (A1) located deep in the sylvian fissure. Our current view is that brain activation changes that occur in response to sensory deprivation have often involved broader structures (e.g., auditory association areas in visual tasks), making it likely that activity from the whole auditory cortex (but probably not A1 exclusively) may be captured by fNIRS (see reviews by Harrison & Hartley, 2019; Saliba et al., 2016). Third, one might question whether our motor task was too simplistic to observe anything useful. Squeezing the triggers of a joystick with both hands should generate activity in the motor cortex, and had there been group differences in that region, we should have detected them with this task. However, the fact that the task had little to do with speech or auditory-motor synchronization may be why there were no significant informative changes in STG activation between groups. We recommend replicating this work with a motor task that is more closely linked to language-related functions. Finally, a strength of fNIRS (e.g., over fMRI) is its ability to deal with motion artifacts (depending on the equipment/system) such as head and jaw movements resulting from speaking or singing. Such tasks would have enormous rehabilitation potential, opening new neuroimaging questions previously impossible to address in this population such as using altered feedback designs (Alemi et al., 2020, 2021) to explore the extent to which children with CI can correct for errors they detect in their vocal productions.
Conclusion
Severe hearing loss, especially early in life, is known to weaken auditory-motor coupling and reinforce visual-motor coupling. In the present study, we showed that hearing loss in children with CIs with good language skills was associated with stronger visual-motor coupling than was the case for children with CIs who had weaker language aptitudes. We suggest that this finding hints at a general adaptive strategy to allocate as few cognitive resources as possible to the task at hand, sparing attentional systems from irrelevant visual information. This interpretation is in line with what “Resource-rational Models” suggest when making realistic assumptions about the behaviour of the brain in cognitive tasks (Lieder & Griffiths, 2020).
Acknowledgements
We thank the Oberkotter Foundation for providing funding for this research program and are deeply grateful to all the children who participated.
Author Biographies
Razieh Alemi is a Postdoctoral Research Fellow in the Psychology Department at Concordia University, Montreal. She holds B.Sc and M.Sc degrees in Speech and Language Pathology and earned her Ph.D. in Neuroscience from Tehran University of Medical Sciences (TUMS) in Iran. With a background as a research assistant and Postdoctoral Research Fellow in the Otolaryngology–Head and Neck Surgery department at McGill University, Razieh has amassed several years of valuable experience in her field. Her primary research focus lies in investigating the impact of hearing loss and its restoration through cochlear implants on the brain plasticity of affected individuals.
Jace Wolfe, Ph.D., is the Senior Vice President of Innovation at the Oberkotter Foundation. He is author of the textbook entitled “Cochlear Implants: Audiologic Management and Considerations for Implantable Hearing Devices,” and he is co-editor (with Carol Flexer, Jane Madell, and Erin Schafer) of the textbooks “Pediatric Audiology: Diagnosis, Technology, and Management, Third Edition” and “Pediatric Audiology Casebook, Second Edition.” He is also a co-author of the textbook entitled “Programming Cochlear Implants, Third Edition,” and he has published over 130 book chapters and articles in peer-reviewed and trade journals. His areas of interest are pediatric hearing healthcare, pediatric amplification and cochlear implantation, personal remote microphone technology, and signal processing for children.
Sara Neumann, Au.D., is a clinical and research audiologist and the Manager of Audiology Research at Hearts for Hearing in Oklahoma City, Oklahoma. She specializes in pediatric audiology and cochlear implants for children and adults and is responsible for conducting research studies evaluating clinically relevant outcomes with hearing aids, cochlear implants, remote microphone technology, and treatment for single-sided deafness. She has co-authored several articles and textbook chapters with Dr. Jace Wolfe on pediatric amplification, implantable hearing devices, and cochlear implant programming. She has a B.S. in Deaf Education from Northern Illinois University (2003) and six years of experience as a deaf educator and as an early intervention provider. She obtained her Doctorate of Audiology (Au.D.) from Illinois State University in 2012 after completing her 4th-year externship at Hearts for Hearing and has been at Hearts for Hearing as an audiologist since 2013 and as the Manager of Audiology Research since 2018.
Jacy Manning Au.D., Ph.D. is a clinical research audiologist at Hearts for Hearing, a non-profit hearing clinic in Oklahoma City, Oklahoma. Her research interests involve the use of brain imaging techniques combined with behavioral testing to investigate neural correlates of speech perception in individuals with cochlear implants. Her current research is focused on determining if phonetic categorization skills predict speech perception outcomes in quiet and noise in adult CI users. Identification of biomarkers associated with successful speech and language outcomes in CI recipients will allow for earlier and more effective clinical interventions. Other research projects she is working on are focused on determining appropriate test batteries and treatment for APD.
Lindsay Hanna is Chief Administrative Officer, a Speech-Language Pathologist, and Certified Listening and Spoken Language Specialist (Cert AVT) with Hearts for Hearing in Oklahoma City, Oklahoma. Lindsay has oversight and responsibility for managing daily administrative operations with a focus on better healthcare value and quality, including the improvement of clinical outcomes, patient experience, patient safety, costs, revenue, productivity, efficiency, employee and physician satisfaction, and process reliability. Mrs. Hanna formerly provided listening and spoken language services to children and adults with hearing loss for almost two decades and served as President of the Oklahoma Speech Language and Hearing Association, alternate delegate to the Medical Advisory Committee for the Oklahoma Healthcare Authority, and State Advocate for Reimbursement for the American Speech-Language-Hearing Association (ASHA). She is a member of Zero to Three.
Will Towler graduated in 2018 from Oklahoma State University with a B.S. in Psychology and a pre-medical minor. Throughout his undergraduate studies, he worked in multiple psychology laboratories, spent 2 years as an assistant in a brain imaging laboratory, and became a Fleming Scholar at the Oklahoma Medical Research Foundation in 2017. After earning his degree in 2018, he became the first full-time research employee at the Hearts for Hearing Foundation in Oklahoma City as a research assistant and later a coordinator. Will is a 3-time published research author and has experience in the fields of psychology, audiology, microphone/speaker technology, hearing aids, cochlear implants, various forms of brain imaging, and the COVID-19 virus. Currently, Will works as the Manager of Special Events and Grants at the Oklahoma Center for Nonprofits.
Caleb Wilson is a currently resident physician within the Department of Otolaryngology–Head and Neck Surgery at Wake Forest Baptist Hospital in Winston-Salem, North Carolina. An Oklahoma native, he is originally from Tulsa and completed a bachelor’s degree at Oklahoma State University in Biochemistry and Molecular Biology and a medical degree from the University of Oklahoma.
Alexander Bien, M.D., completed a fellowship in otology, neurotology, and skull base surgery at the House Ear Clinic in Los Angeles, California. He is board-certified in Otolaryngology–Head and Neck Surgery and sub-certified in Neurotology. Dr. Bien treats medical and surgical diseases of the ear and lateral skull base in adult and pediatric patients. His interests include surgery for chronic ear disease and cholesteatoma, cochlear implant surgery, middle ear reconstruction, stapedectomy, and lateral skull base tumors.
Sharon Miller is an Assistant Professor in the Department of Audiology and Speech-Language Pathology at the University of North Texas. She earned her Ph.D. and M.A. degrees in Speech-Language-Hearing Science and Audiology from The University of Minnesota-Twin Cities and has a B.S. in Communication Disorders from Northwestern University. Her research program examines the cortical mechanisms underlying successful speech perception outcomes in persons with hearing loss and other communication disorders. This translational work aims to develop objective electrophysiological tests that are sensitive to individual patient differences in speech perception, allowing for more targeted rehabilitation protocols for persons across varying ages and communication abilities.
Erin Schafer is a Professor and the Department Chair of the Department of Audiology & Speech-Language Pathology at the University of North Texas, where she has been a faculty member since 2005. She received her Ph.D. in Communication Sciences and Disorders from the University of Texas at Dallas. Her research programs focus on the assessment and (re)habilitation of adults and children with hearing loss and auditory disorders.
Jessica Gemignani is a Marie Skodowska-Curie Postdoctoral Fellow at the University of Padua, Italy. She received her degree in biomedical engineering from the University of Pisa in 2014 and her PhD in neurotechnology from Technical University of Berlin in 2019. Her research focuses on the development and validation of methods for the analysis of multivariate neuroimaging data acquired on newborns and infants, particularly in the field of language acquisition.
Nabin Koirala completed his Ph.D. in Neuroscience from Johannes Gutenberg University focusing on understanding brain pathophysiology involved in different neurological and neuropsychological disorders and developing disease-specific brain biomarkers. He is currently working as a research faculty at Yale School of Medicine on exploring the neural correlates of speech, hearing, and language-related disorders with the use of multi-modal brain imaging and electrophysiological data and utilizing the state-of-the-art frameworks of complex network algorithms and machine learning approaches.
Vincent L. Gracco's research focuses on the neuroscience of human communication using multiple neuroimaging modalities and physiological techniques. Current research areas focus on the development and mechanisms of sensorimotor control for spoken language, sensorimotor dysfunctions associated with stuttering and other speech motor disorders, neuroplasticity and sensorimotor learning, and the neural changes following cochlear implantation in children.
Mickael Deroche, Ph.D. is an Assistant Professor in the Psychology Department of Concordia University, where he has been a faculty member since 2019. His laboratory focuses on hearing and cognition, from audiological work with hearing-impaired individuals to cognitive work on language and communication, and on both ends of the lifespan.
Appendix.
Table 1-A.
Demographic Information for the Cochlear Implant Users in the Low Language Group.
| Subject | CI Side | Age (y) | Age at First HA (mo) | Age at First CI (mo) | CELF | Sound Processor R/L |
|---|---|---|---|---|---|---|
| 1A | Seq Bil | 15.4 | 19 | 26 | 58 | Nuc CP1000/CP1000 |
| 2A | Seq Bil | 11.3 | 2 | 15 | 75 | Nuc CP1000/CP1000 |
| 3A | Seq Bil | 16.0 | 24 | 48 | 84 | Nuc Freedom/CP910 |
| 4A | Seq Bil | 10.9 | 24 | 26 | 76 | Nuc CP910/CP910 |
| 5A | Seq Bil | 16.7 | 24 | 48 | 58 | Nuc CP1000/CP1000 |
| 6A | Seq Bil | 16.7 | 24 | 48 | 61 | Nuc CP1000/CP1000 |
| 7A | Seq Bil | 17.0 | 29 | 33 | 52 | Nuc CP1000/CP910 |
| 8A | Seq Bil | 17.5 | 29 | 50 | 50 | Nuc CP1000/CP1000 |
| 9A | Left | 8.7 | 10 | 13 | 73 | NA/Nuc CP1000 |
| 10A | Seq Bil | 14.1 | 19 | 22 | 45 | Nuc CP1000/CP1000 |
| 11A | Seq Bil | 15.9 | 12 | 14 | 77 | Nuc CP910/CP910 |
| 12A | Right | 11.6 | 31 | 39 | 57 | Nuc CP950/NA |
| 13A | Seq Bil | 17.1 | 17 | 21 | 75 | Nuc CP1000/Naida Q90 |
| 14A | Seq Bil | 14.7 | 8 | 20 | 61 | Nuc CP910/CP910 |
| 15A | Seq Bil | 12.9 | 19 | 24 | 85 | Nuc CP1000/CP1000 |
| 16A | Seq Bil | 13.5 | 2 | 13 | 57 | Nuc CP1000/CP1000 |
| 17A | Seq Bil | 12.2 | 13 | 16 | 76 | Nuc CP1000/CP1000 |
| 18A | Seq Bil | 9.8 | 22 | 26 | 40 | Nuc CP1000/CP1000 |
| 19A | Seq Bil | 13.0 | 33 | 40 | 67 | Nuc CP1000/CP1000 |
| 20A | Seq Bil | 13.2 | 2 | 15 | 45 | Nuc CP910/CP910 |
| 21A | Seq Bil | 16.6 | 1 | 32 | 62 | Nuc CP1000/CP 1000 |
| 22A | Seq Bil | 14.1 | 24 | 24 | 70 | NaidaQ70/NaidaQ70 |
| 23A | Right | 10.1 | 21 | 33 | 62 | Nuc CP910/NA |
| 24A | Right | 14.1 | 4 | 15 | 73 | Nuc CP910/NA |
| Mean (SD) | 13.9 (2.6) | 17.2 (10.0) | 27.5 (12.3) | 64.1 (12.5) |
Abbreviations: Bil, bilateral; CELF, Clinical Evaluation of Language Fundamentals- Fifth edition standard score; CI, cochlear implant; HA, hearing aid; L, left ear; Nuc, Nucleus; R, right ear; Seq, sequential.
Table 2-A.
Demographic Information for the Cochlear Implant Users in the Typical Language Group.
| Subject | CI Side | Age (y) | Age at First HA (mo) | Age at First CI (mo) | CELF | Sound Processor R/L |
|---|---|---|---|---|---|---|
| 1B | Seq Bil | 13.4 | 12 | 25 | 108 | Nuc CP1000/CP1000 |
| 2B | Seq Bil | 14.8 | 3 | 13 | 100 | Nuc CP910/CP910 |
| 3B | Sim Bil | 10.3 | 9 | 17 | 100 | Nuc CP950/CP950 |
| 4B | Seq Bil | 10.0 | 1.5 | 32 | 100 | Nuc CP910/CP910 |
| 5B | Seq Bil | 12.5 | 16 | 40 | 116 | Nuc CP910/CP800 |
| 6B | Seq Bil | 13.1 | 13 | 17 | 120 | Sonnet 2/Sonnet 2 |
| 7B | Seq Bil | 7.50 | 1 | 13 | 111 | Nuc CP910/CP910 |
| 8B | Seq Bil | 8.00 | 4 | 41 | 133 | Nuc CP910/CP910 |
| 9B | Seq Bil | 9.60 | 26 | 30 | 107 | Nuc CP1000/CP1000 |
| 10B | Seq Bil | 8.70 | 1 | 14 | 120 | Nuc CP1000/CP1000 |
| 11B | Sim Bil | 12.5 | 15 | 28 | 103 | Naida Q70/Naida Q70 |
| 12B | Seq Bil | 7.50 | 1 | 12 | 111 | Nuc CP1000/CP1000 |
| 13B | Seq Bil | 9.60 | 16 | 20 | 117 | Nuc CP910/CP910 |
| 14B | Seq Bil | 8.30 | 28 | 30 | 100 | Nuc CP910/CP910 |
| 15B | Sim Bil | 12.5 | 1 | 10 | 102 | Nuc CP1000/CP1000 |
| 16B | Seq Bil | 9.30 | 3 | 10 | 120 | Nuc CP1000/CP1000 |
| 17B | Seq Bil | 14.5 | 3 | 10 | 108 | Nuc CP1000/CP1000 |
| 18B | Seq Bil | 11.2 | 3 | 13 | 100 | Nuc CP910/CP910 |
| 19B | Seq Bil | 15.3 | 2 | 35 | 106 | Nuc CP910/CP910 |
| 20B | Sim Bil | 10.4 | 2 | 14 | 111 | Nuc CP910/CP910 |
| 21B | Seq Bil | 14.3 | 1.5 | 13 | 120 | Nuc CP950/CP950 |
| 22B | Seq Bil | 16.0 | 2 | 12 | 132 | NaidaQ70/NaidaQ70 |
| 23B | Seq Bil | 16.9 | 1 | 22 | 100 | Nuc CP1000/CP1000 |
| 24B | Seq Bil | 14.0 | 10 | 34 | 106 | Nuc CP910/CP910 |
| 25B | Sim Bil | 8.00 | .75 | 9 | 109 | Nuc CP1000/CP1000 |
| 26B | Sim Bil | 11.2 | 12 | 15 | 111 | Nuc CP910/CP910 |
| Mean (SD) | 11.5 (2.8) | 7.2 (7.9) | 20.3 (10.1) | 110.4 (9.5) |
Abbreviations: Bil, bilateral; CELF, Clinical Evaluation of Language Fundamentals - Fifth edition standard score; CI, cochlear implant; HA, hearing aid; L, left ear; Nuc, Nucleus; R, right ear; Seq, sequential; Sim, simultaneous.
Footnotes
Author Contributions: MD, JW, and VG developed the rationale of the research program. MD coded the experimental interface, analyzed the data, generated figures, and wrote up the core of the manuscript with RA. In Oklahoma, JW, SN, JM, LH, WT collected all the fNIRS data. CW, AB, SM, and ES helped with subject recruitment. JG helped with data analysis. All authors contributed to manuscript editing.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Razieh Alemi https://orcid.org/0000-0002-9788-5305
Alexander Bien https://orcid.org/0000-0001-9544-5296
References
- Alemi R., Lehmann A., Deroche M. (2020). Adaptation to pitch-altered feedback is independent of one’s own voice pitch sensitivity. Scientific Reports, 10(1), 16860. 10.1038/s41598-020-73932-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemi R., Lehmann A., Deroche M. (2021). Changes in spoken and sung productions following adaptation to pitch-shifted auditory feedback. Journal of Voice, 37(3), 466.e1–466.e15. 10.1016/j.jvoice.2021.02.016 [DOI] [PubMed] [Google Scholar]
- Anderson C. A., Lazard D. S., Hartley D. E. (2017. b). Plasticity in bilateral superior temporal cortex: Effects of deafness and cochlear implantation on auditory and visual speech processing. Hearing Research, 343(January), 138–149. 10.1016/j.heares.2016.07.013 [DOI] [PubMed] [Google Scholar]
- Anderson C. A., Wiggins I. M., Kitterick P. T., Hartley D. E. H. (2017. a). Adaptive benefit of cross-modal plasticity following cochlear implantation in deaf adults. Proceedings of the National Academy of Sciences, 114(38), 10256–10261. 10.1073/pnas.1704785114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R., Renier L., Ebner-Karestinos D., Dricot L., De Volder A. G. (2017). Hearing, feeling or seeing a beat recruits a supramodal network in the auditory dorsal stream. European Journal of Neuroscience, 45(11), 1439–1450. 10.1111/ejn.13349 [DOI] [PubMed] [Google Scholar]
- Bavelier D., Neville H. J. (2002). Cross-modal plasticity: Where and how? Nature Reviews Neuroscience, 3(6), 443–452. 10.1038/nrn848 [DOI] [PubMed] [Google Scholar]
- Beckers L., Tromp N., Philips B., Mylanus E., Huinck W. (2023). Exploring neurocognitive factors and brain activation in adult cochlear implant recipients associated with speech perception outcomes–A scoping review. Frontiers in Neuroscience, 17, 1046669. 10.17605/OSF.IO/Z3G7W [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottari D., Heimler B., Caclin A., Dalmolin A., Giard M.-H., Pavani F. (2014). Visual change detection recruits auditory cortices in early deafness. NeuroImage, 94(1 July), 172–184. 10.1016/j.neuroimage.2014.02.031. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Palmer C. (2012). Auditory–motor learning influences auditory memory for music. Memory & Cognition, 40(4), 567–578. 10.3758/s13421-011-0177-x [DOI] [PubMed] [Google Scholar]
- Campbell J., Sharma A. (2014). Cross-modal re-organization in adults with early stage hearing loss. PLoS One, 9(2), e90594. 10.1371/journal.pone.0090594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. J., Patel A. D. (2021). How beat perception co-opts motor neurophysiology. Trends in Cognitive Sciences, 25(2), 137–150. 10.1016/j.tics.2020.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capek C. M., Macsweeney M., Woll B., Waters D., McGuire P. K., David A. S., Brammer M. J., Campbell R. (2008). Cortical circuits for silent speechreading in deaf and hearing people. Neuropsychologia, 46(5), 1233–1241. 10.1016/j.neuropsychologia.2007.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Zatorre R. J., Penhune V. B. (2006). Interactions between auditory and dorsal premotor cortex during synchronization to musical rhythms. NeuroImage, 32(4), 1771–1781. 10.1016/j.neuroimage.2006.04.207 [DOI] [PubMed] [Google Scholar]
- Chen L. C., Puschmann S., Debener S. (2017). Increased cross-modal functional connectivity in cochlear implant users. Scientific Reports, 7(1), 10043. 10.1038/s41598-017-10792-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching T. Y. C., Dillon H., Leigh G., Cupples L. (2018). Learning from the longitudinal outcomes of children with hearing impairment (LOCHI) study: Summary of 5-year findings and implications. International Journal of Audiology, 57(sup2), S105–S111. 10.1080/14992027.2017.1385865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. E., Hong S. H., Moon I. J. (2020). Academic performance, communication, and psychosocial development of prelingual deaf children with cochlear implants in mainstream schools. Journal of audiology & otology, 24(2), 61–70. https://doi.org/10.7874%2Fjao.2019.00346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch L., Roskosky M., Freedman B., Shuler M. (2015). Effect of skin pigmentation on near infrared spectroscopy. American Journal of Analytical Chemistry, 6(12), 911–916. [Google Scholar]
- Cui X., Bray S., Bryant D. M., Glover G. H., Reiss A. L. (2011). A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage, 54(4), 2808–2821. 10.1016/j.neuroimage.2010.10.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell Ducas K., Senra Filho A. C. da S., Silva P. H. R., Secchinato K. F., Leoni R. F., Santos A. C. (2021). Functional and structural brain connectivity in congenital deafness. Brain Structure and Function, 226(4), 1323–1333. 10.1007/s00429-021-02243-6 [DOI] [PubMed] [Google Scholar]
- Deroche M., Wolfe J., Neumann S., Manning J., Towler W., Alemi R., Bien A., Koirala N., Hanna L., Henry L., Gracco V. L. (2023). Auditory evoked response to an oddball paradigm in children wearing cochlear implants. Clinical Neurophysiology, 149(1), 133–145. 10.1016/j.clinph.2023.02.179 [DOI] [PubMed] [Google Scholar]
- Dettman S. J., Dowell R. C., Choo D., Arnott W., Abrahams Y., Davis A., Dornan D., Cowan R. (2016). Long-term communication outcomes for children receiving cochlear implants younger than 12 months: A multicenter study. Otology & Neurotology, 37(2), e82–e95. 10.1097/MAO.0000000000000915 [DOI] [PubMed] [Google Scholar]
- Dionne-Dostie E., Paquette N., Lassonde M., Gallagher A. (2015). Multisensory integration and child neurodevelopment. Brain Sciences, 5(1), 32–57. 10.3390/brainsci5010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine I., Finney E. M., Boynton G. M., Dobkins K. R. (2005). Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. Journal of Cognitive Neuroscience, 17(10), 1621–1637. 10.1162/089892905774597173 [DOI] [PubMed] [Google Scholar]
- Fishburn F. A., Ludlum R. S., Vaidya C. J., Medvedev A. V. (2019). Temporal derivative distribution repair (TDDR): A motion correction method for fNIRS. NeuroImage, 184(January), 171–179. 10.1016/j.neuroimage.2018.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenstein U., Wennerberg A., Richter W., Bernstein C., Morden D., Rémy F., Mcintyre M. (2003). Activation and deactivation in blood oxygenation level dependent functional magnetic resonance imaging. Concepts in Magnetic Resonance Part A: An Educational Journal, 16(1), 63–70. 10.1002/cmr.a.10054 [DOI] [Google Scholar]
- Fullerton A. M., Vickers D. A., Luke R., Billing A. N., McAlpine D., Hernandez-Perez H., Peelle J. E., Monaghan J. J., McMahon C. M. (2023). Cross-modal functional connectivity supports speech understanding in cochlear implant users. Cerebral Cortex, 33(7), 3350–3371. 10.1093/cercor/bhac277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geers A. E., Mitchell C. M., Warner-Czyz A., Wang N. Y., Eisenberg L. S., CDaCI Investigative Team . (2017). Early sign language exposure and cochlear implantation benefits. Pediatrics, 140(1), e20163489. 10.1542/peds.2016-3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon E., Svirsky M. A., Froemke R. C. (2020). Auditory cortical plasticity in cochlear implant users. Current Opinion in Neurobiology, 60(1), 108–114. 10.1016/j.conb.2019.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon E. E., Schachner A., Nave-Blodgett J. E. (2017). Babies know bad dancing when they see it: Older but not younger infants discriminate between synchronous and asynchronous audiovisual musical displays. Journal of Experimental Child Psychology, 159(5), 159–174. 10.1016/j.jecp.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Harrison S., Hartley D. (2019). Shedding light on the human auditory cortex: A review of the advances in near infrared spectroscopy (NIRS). Reports in Medical Imaging, 12, 31–42. 10.2147/RMI.S174633 [DOI] [Google Scholar]
- Harrison S. C., Lawrence R., Hoare D. J., Wiggins I. M., Hartley D. E. (2021). Use of functional near-infrared spectroscopy to predict and measure cochlear implant outcomes: A scoping review. Brain Sciences, 11(11), 1439. 10.3390/brainsci11111439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J. V., Horwitz B., Ungerleider L. G., Maisog J. M., Pietrini P., Grady C. L. (1994). The functional organization of human extrastriate cortex: A PET-rCBF study of selective attention to faces and locations. Journal of Neuroscience, 14(11), 6336–6353. 10.1523/JNEUROSCI.14-11-06336.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes D. J., Huxtable A. G. (2012). Interpreting deactivations in neuroimaging. Frontiers in Psychology, 3, 27. 10.3389/fpsyg.2012.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Ettehadi N., Shmuel A., Razlighi Q. R. (2022). Evidence suggesting common mechanisms underlie contralateral and ipsilateral negative BOLD responses in the human visual cortex. NeuroImage, 262(November), 119440. 10.1016/j.neuroimage.2022.119440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G. (2022). The dual stream model of speech and language processing. Handbook of Clinical Neurology, 185, 57–69. 10.1016/B978-0-12-823384-9.00003-7 [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. (2007). The cortical organization of speech processing. Nature Reviews Neuroscience, 8(5), 393–402. 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Huppert T. J., Diamond S. G., Franceschini M. A., Boas D. A. (2009). HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Applied Optics, 48(10), D280–D298. 10.1364/AO.48.00D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata P., Tomic S. T., Haberman J. M. (2012). Sensorimotor coupling in music and the psychology of the groove. Journal of Experimental Psychology: General, 141(1), 54–75. https://psycnet.apa.org/doi/10.1037/a0024208 [DOI] [PubMed] [Google Scholar]
- Jäncke L., Loose R., Lutz K., Specht K., Shah N. J. (2000). Cortical activations during paced finger-tapping applying visual and auditory pacing stimuli. Cognitive Brain Research, 10(1–2), 51–66. 10.1016/S0926-6410(00)00022-7 [DOI] [PubMed] [Google Scholar]
- Jiam N. T., Limb C. (2020). Music perception and training for pediatric cochlear implant users. Expert Review of Medical Devices, 17(11), 1193–1206. 10.1080/17434440.2020.1841628 [DOI] [PubMed] [Google Scholar]
- Jorge J., Figueiredo P., Gruetter R., van der Zwaag W. (2018). Mapping and characterization of positive and negative BOLD responses to visual stimulation in multiple brain regions at 7T. Human Brain Mapping, 39(6), 2426–2441. 10.1002/hbm.24012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A., Blom Ö., Forsman L., Ullén F. (2009). The dorsal auditory pathway is involved in performance of both visual and auditory rhythms. NeuroImage, 44(2), 480–488. 10.1016/j.neuroimage.2008.08.047 [DOI] [PubMed] [Google Scholar]
- Kasdan A. V., Burgess A. N., Pizzagalli F., Scartozzi A., Chern A., Kotz S. A., Wilson S. M., Gordon R. L. (2022). Identifying a brain network for musical rhythm: A functional neuroimaging meta-analysis and systematic review. Neuroscience & Biobehavioral Reviews, 136(3), 104588. 10.1016/j.neubiorev.2022.104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima R., O’Sullivan B. T., Roland P. E. (1995). Positron-emission tomography studies of cross-modality inhibition in selective attentional tasks: Closing the“ mind’s eye”. Proceedings of the National Academy of Sciences, 92(13), 5969–5972. 10.1073/pnas.92.13.5969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. K., Zatorre R. J. (2010). Can you hear shapes you touch? Experimental Brain Research, 202(May), 747–754. 10.1007/s00221-010-2178-6. [DOI] [PubMed] [Google Scholar]
- Koirala N., Deroche M. L., Wolfe J., Neumann S., Bien A. G., Doan D., Goldbeck M., Muthuraman M., Gracco V. L. (2023). Dynamic networks differentiate the language ability of children with cochlear implants. Frontiers in Neuroscience, 17, 1141886. 10.3389/fnins.2023.1141886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral A., Dorman M. F., Wilson B. S. (2019). Neuronal development of hearing and language: Cochlear implants and critical periods. Annual Review of Neuroscience, 42(1), 47–65. 10.1146/annurev-neuro-080317-061513 [DOI] [PubMed] [Google Scholar]
- Kral A., Pallas S. L. (2011). Development of the auditory cortex. In The auditory cortex (pp. 443–463). Springer US. [Google Scholar]
- Laland K., Wilkins C., Clayton N. (2016). The evolution of dance. Current Biology, 26(1). R5–R9. [DOI] [PubMed] [Google Scholar]
- Land R., Baumhoff P., Tillein J., Lomber S. G., Hubka P., Kral A. (2016). Cross-modal plasticity in higher-order auditory cortex of congenitally deaf cats does not limit auditory responsiveness to cochlear implants. Journal of Neuroscience, 36(23), 6175–6185. DOI: 10.1523/JNEUROSCI.0046-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. J., Wiggins I. M., Hodgson J. C., Hartley D. E. (2021). Evaluating cortical responses to speech in children: A functional near-infrared spectroscopy (fNIRS) study. Hearing Research, 401(2), 108155. 10.1016/j.heares.2020.108155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. S., Lee J. S., Oh S. H., Kim S.-K., K im J.-W., Chung J.-K., Lee M. C., Kim C. S. (2001). Cross-modal plasticity and cochlear implants. Nature, 409(6817), 149–150. 10.1038/35051653 [DOI] [PubMed] [Google Scholar]
- Lehmann A., Arias D. J., Schönwiesner M. (2016). Tracing the neural basis of auditory entrainment. Neuroscience, 337(19 November), 306–314. 10.1016/j.neuroscience.2016.09.011. [DOI] [PubMed] [Google Scholar]
- Lieder F., Griffiths T. L. (2020). Resource-rational analysis: Understanding human cognition as the optimal use of limited computational resources. Behavioral and Brain Sciences, 43, e1. 10.1017/S0140525X1900061X [DOI] [PubMed] [Google Scholar]
- Lyxell B., Holmberg I. (2000). Visual speechreading and cognitive performance in hearing‐impaired and normal hearing children (11‐14 years). British Journal of Educational Psychology, 70(4), 505–518. 10.1348/000709900158272 [DOI] [PubMed] [Google Scholar]
- Manno F. A. M., Rodríguez-Cruces R., Kumar R., Ratnanather J. T., Lau C. (2021). Hearing loss impacts gray and white matter across the lifespan: Systematic review, meta-analysis and meta-regression. NeuroImage, 231(46), 117826. 10.1016/j.neuroimage.2021.117826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschark M., Rhoten C., Fabich M. (2007). Effects of cochlear implants on children's reading and academic achievement. Journal of Deaf Studies and Deaf Education, 12(3), 269–282. 10.1093/deafed/enm013 [DOI] [PubMed] [Google Scholar]
- Matas A., Sowa M. G., Taylor G., Mantsch H. H. (2002). Melanin as a confounding factor in near infrared spectroscopy of skin. Vibrational Spectroscopy, 28(1): 45–52. 10.1016/S0924-2031(01)00144-8 [DOI] [Google Scholar]
- Matthews T. E., Witek M. A. G., Lund T., Vuust P., Penhune V. B. (2020). The sensation of groove engages motor and reward networks. NeuroImage, 214(1 July), 116768. 10.1016/j.neuroimage.2020.116768. [DOI] [PubMed] [Google Scholar]
- Mitterová K., Lamoš M., Mareček R., Pupíková M., Šimko P., Grmela R., Skotáková A., Vaculíková P., Rektorová I. (2021). Dynamic functional connectivity signifies the joint impact of dance intervention and cognitive reserve. Frontiers in Aging Neuroscience, 13, 724094. 10.3389/fnagi.2021.724094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Asada M., Naito E. (2019). Developmental changes in task‐induced brain deactivation in humans revealed by a motor task. Developmental Neurobiology, 79(6), 536–558. 10.1002/dneu.22701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita T., Asada M., Naito E. (2021). Examination of the development and aging of brain deactivation using a unimanual motor task. Advanced Robotics, 35(13–14), 842–857. 10.1080/01691864.2021.1886168 [DOI] [Google Scholar]
- Mowad T. G., Willett A. E., Mahmoudian M., Lipin M., Heinecke A., Maguire A. M., Ashtari M. (2020). Compensatory cross-modal plasticity persists after sight restoration. Frontiers in Neuroscience, 14, 291. 10.3389/fnins.2020.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq F., Wiggins I. M., Kitterick P. T., Anderson C. A., Hartley D. E. H. (2020). The benefit of cross-modal reorganization on speech perception in pediatric cochlear implant recipients revealed using functional near-infrared spectroscopy. Frontiers in Human Neuroscience, 14, 308. 10.3389/fnhum.2020.00308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton J. M., Sunderland A., Gowland P. A. (2005). fMRI signal decreases in ipsilateral primary motor cortex during unilateral hand movements are related to duration and side of movement. NeuroImage, 24(4), 1080–1087. 10.1016/j.neuroimage.2004.10.003 [DOI] [PubMed] [Google Scholar]
- Nozaradan S., Schönwiesner M., Keller P. E., Lenc T., Lehmann A. (2018). Neural bases of rhythmic entrainment in humans: Critical transformation between cortical and lower‐level representations of auditory rhythm. European Journal of Neuroscience, 47(4), 321–332. 10.1111/ejn.13826 [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. (2011). FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011(1), 1–9. 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztop E., Kawato M., Arbib M. (2006). Mirror neurons and imitation: A computationally guided review. Neural Networks, 19(3), 254–271. 10.1016/j.neunet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Paul B. T., Bajin M. D., Uzelac M., Chen J., Le T., Lin V., Dimitrijevic A. (2022). Evidence of visual crossmodal reorganization positively relates to speech outcomes in cochlear implant users. Scientific Reports, 12(1), 17749. 10.1038/s41598-022-22117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Silver J., Toiviainen P., Gosselin N., Turgeon C., Lepore F., Peretz I. (2015). Cochlear implant users move in time to the beat of drum music. Hearing Research, 321(7), 25–34. 10.1016/j.heares.2014.12.007 [DOI] [PubMed] [Google Scholar]
- Pinti P., Tachtsidis I., Hamilton A., Hirsch J., Aichelburg C., Gilbert S., Burgess P. W. (2020). The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience, Annals of the New York Academy of Sciences, 1464(1), 5–29. 10.1111/nyas.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puschmann S., Regev M., Baillet S., Zatorre R. J. (2021). MEG intersubject phase locking of stimulus-driven activity during naturalistic speech listening correlates with musical training. Journal of Neuroscience, 41(12), 2713–2722. 10.1523/JNEUROSCI.0932-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repp B. H., Su Y.-H. (2013). Sensorimotor synchronization: A review of recent research (2006–2012). Psychonomic Bulletin & Review, 20(3), 403–452. 10.3758/s13423-012-0371-2 [DOI] [PubMed] [Google Scholar]
- Rouger J., Lagleyre S., Fraysse B., Deneve S., Deguine O., Barone P. (2007). Evidence that cochlear-implanted deaf patients are better multisensory integrators. Proceedings of the National Academy of Sciences, 104(17), 7295–7300. 10.1073/pnas.0609419104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N., Pascual-Leone A., Grafman J., Ibañez V., Deiber M.-P., Dold G., Hallett M. (1996). Activation of the primary visual cortex by Braille reading in blind subjects. Nature, 380(6574), 526–528. 10.1038/380526a0 [DOI] [PubMed] [Google Scholar]
- Saliba J., Bortfeld H., Levitin D. J., Oghalai J. S. (2016). Functional near-infrared spectroscopy for neuroimaging in cochlear implant recipients. Hearing Research, 338, 64–75. 10.1016/j.heares.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santosa H., Zhai X., Fishburn F., Huppert T. (2018). The NIRS brain AnalyzIR toolbox. Algorithms, 11(5), 73. 10.3390/a11050073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Yahata N., Funane T., Takizawa R., Katura T., Atsumori H., Nishimura Y., Kinoshita A., Kiguchi M., Koizumi H., Fukuda M., Kasai K. (2013). A NIRS–fMRI investigation of prefrontal cortex activity during a working memory task. NeuroImage, 83(December), 158–173. 10.1016/j.neuroimage.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Shi B., Yang L.-Z., Liu Y., Zhao S.-L., Wang Y., Gu F., Yang Z., Zhou Y., Zhang P., Zhang X. (2016). Early-onset hearing loss reorganizes the visual and auditory network in children without cochlear implantation. NeuroReport, 27(3), 197–202. 10.1097/WNR.0000000000000524 [DOI] [PubMed] [Google Scholar]
- Shiell M. M., Champoux F., Zatorre R. J. (2015). Reorganization of auditory cortex in early-deaf people: Functional connectivity and relationship to hearing aid use. Journal of Cognitive Neuroscience, 27(1), 150–163. 10.1162/jocn_a_00683 [DOI] [PubMed] [Google Scholar]
- Shulman G. L., Astafiev S. V., McAvoy M. P., d’Avossa G., Corbetta M. (2007). Right TPJ deactivation during visual search: Functional significance and support for a filter hypothesis. Cerebral Cortex, 17(11), 2625–2633. 10.1093/cercor/bhl170 [DOI] [PubMed] [Google Scholar]
- Shulman G. L., Corbetta M., Buckner R. L., Fiez J. A., Miezin F. M., Raichle M. E., Petersen S. E. (1997). Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. Journal of Cognitive Neuroscience, 9(5), 624–647. 10.1162/jocn.1997.9.5.624 [DOI] [PubMed] [Google Scholar]
- Simon M., Lazzouni L., Campbell E., Delcenserie A., Muise-Hennessey A., Newman A. J., Champoux F., Lepore F. (2020). Enhancement of visual biological motion recognition in early-deaf adults: Functional and behavioral correlates. PLoS One, 15(8), Article e0236800. 10.1371/journal.pone.0236800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stropahl M., Chen L. C., Debener S. (2016). Cortical reorganization in postlingually deaf cochlear implant users: Intra-modal and cross-modal considerations. Hearing Research, 343(January), 128–137. 10.1016/j.heares.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Su Y.-H. (2014). Visual enhancement of auditory beat perception across auditory interference levels. Brain and Cognition, 90(October), 19–31. 10.1016/j.bandc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Tam N. D., Zouridakis G. (2014). Temporal decoupling of oxy-and deoxy-hemoglobin hemodynamic responses detected by functional near-infrared spectroscopy (fNIRS). Journal of Biomedical Engineering and Medical Imaging, 1(2), 18–28. 10.14738/jbemi.12.146 [DOI] [Google Scholar]
- Vongpaisal T., Caruso D., Yuan Z. (2016). Dance movements enhance song learning in deaf children with cochlear implants. Frontiers in Psychology, 7(320), 835. 10.3389/fpsyg.2016.00835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss P., Collignon O., Lassonde M., Lepore F. (2010). Adaptation to sensory loss. Wiley Interdisciplinary Reviews: Cognitive Science, 1(3), 308–328. 10.1002/wcs.13 [DOI] [PubMed] [Google Scholar]
- Voss P., Zatorre R. J. (2012). Organization and reorganization of sensory-deprived cortex. Current Biology, 22(5), R168–R173. 10.1016/j.cub.2012.01.030 [DOI] [PubMed] [Google Scholar]
- Wallace M. T., Woynaroski T. G., Stevenson R. A. (2020). Multisensory integration as a window into orderly and disrupted cognition and communication. Annual Review of Psychology, 71(1), 193–219. 10.1146/annurev-psych-010419-051112 [DOI] [PubMed] [Google Scholar]
- Wang S., Chen B., Yu Y., Yang H., Cui W., Li J., Fan G. G. (2019). Alterations of structural and functional connectivity in profound sensorineural hearing loss infants within an early sensitive period: A combined dti and fMRI study. Developmental Cognitive Neuroscience, 38(August), 100654. 10.1016/j.dcn.2019.100654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenaar E. B., Van den Brand J. G. H. (2005). Reliability of near-infrared spectroscopy in people with dark skin pigmentation. Journal of Clinical Monitoring and Computing, 19(3), 195–199. 10.1007/s10877-005-1655-0 [DOI] [PubMed] [Google Scholar]
- Weisser V., Stilla R., Peltier S., Hu X., Sathian K. (2005). Short-term visual deprivation alters neural processing of tactile form. Experimental Brain Research, 166(3), 572–582. 10.1007/s00221-005-2397-4 [DOI] [PubMed] [Google Scholar]
- Westermann G., Miranda E. R. (2004). A new model of sensorimotor coupling in the development of speech. Brain and Language, 89(2), 393–400. 10.1016/S0093-934X(03)00345-6 [DOI] [PubMed] [Google Scholar]
- Wiig E. H., Secord W. A., Semel E. (2013). Clinical evaluation of language fundamentals: CELF-5. Pearson Psychcorp. [Google Scholar]
- Wilson Ottley S. M., Ouellette M., Mellon N. K., Caverly C., Mitchell C. M., Adams Costa E. (2023). Language and academic outcomes of children with cochlear implants in an inclusive setting: Evidence from 18 years of data. Cochlear Implants International, 24(3), 130–143. 10.1080/14670100.2022.2154427 [DOI] [PubMed] [Google Scholar]
- Wolfe J., Deroche M., Neumann S., Hanna L., Towler W., Wilson C., Bien A. G., Miller S., Schafer E. C., Gracco V. (2021). Factors associated with speech-recognition performance in school-aged children with cochlear implants and early auditory-verbal intervention. Journal of the American Academy of Audiology, 32(07), 433–444. 10.1055/s-0041-1730413 [DOI] [PubMed] [Google Scholar]
- Zatorre R. J., Chen J. L., Penhune V. B. (2007). When the brain plays music: Auditory–motor interactions in music perception and production. Nature Reviews Neuroscience, 8(7), 547–558. 10.1038/nrn2152 [DOI] [PubMed] [Google Scholar]