Abstract
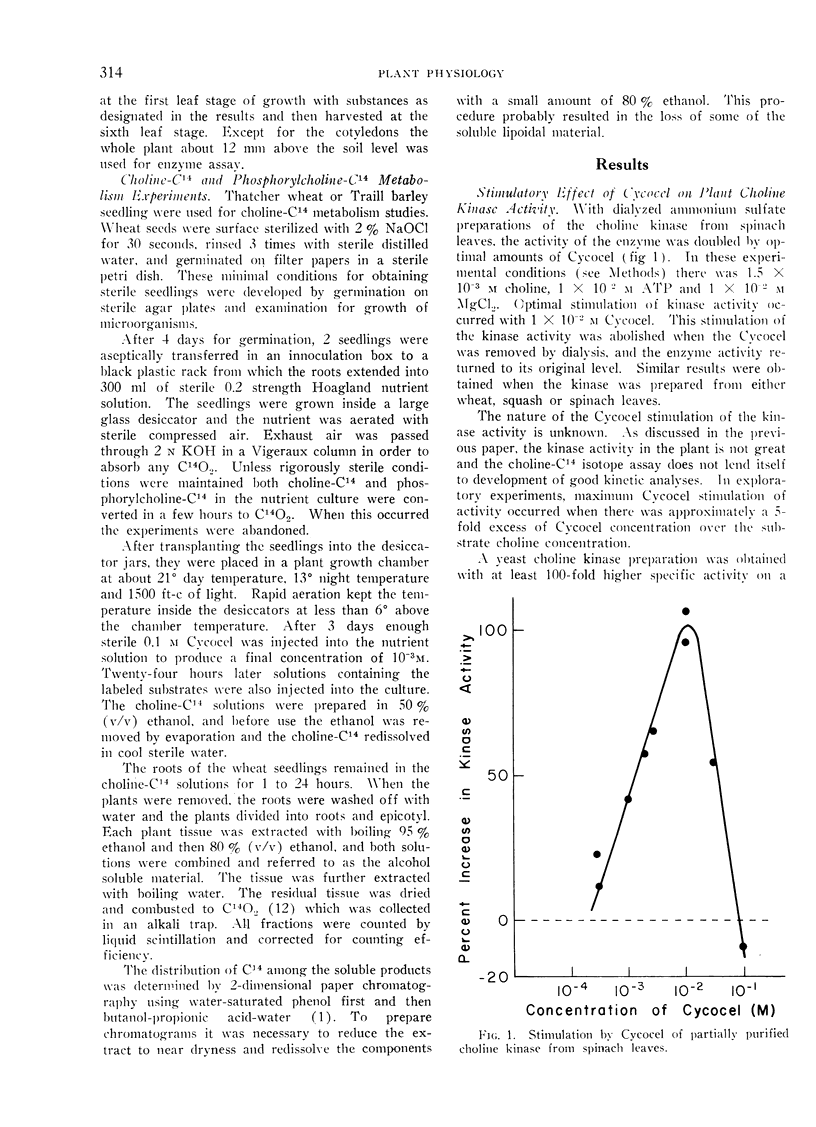
Cycocel stimulated the activity of partial purified choline kinase from spinach or squash leaves, but it inhibited the activity of yeast choline kinase. The activity of different Cycocel analogs on plant growth corresponded to their stimulatory effect on the isolated choline kinase. Cycocel had no effect upon the activity of a plant phosphatase which hydrolyzed phosphorylcholine nor upon adenosine triphosphatase from wheat roots or leaves.
Gibberellin A3 inhibited choline kinase activity and reversed the stimulatory effect of Cycocel on the kinase.
Total choline kinase activity per squash plant was not greatly increased by Cycocel treatment. However, on the basis of fresh weight, total kinase activity was increased by Cycocel treatment. Gibberellin A3 partially reversed these increases. Treatment with Cycocel plus indoleacetic acid resulted in a large increase in choline kinase activity.
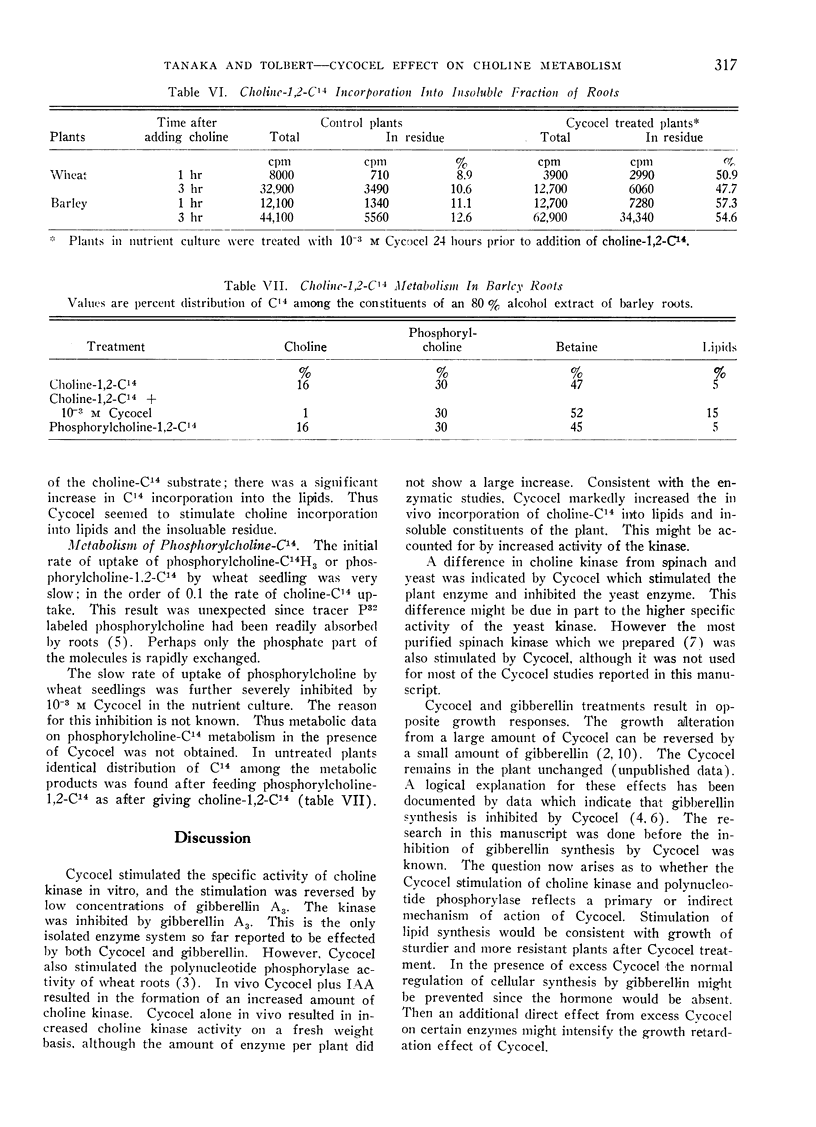
The same distribution of tracer among phosphorylcholine, choline and betaine was observed when either phosphorylcholine-C14 or choline-C14 was fed to barley or wheat roots. Cycocel stimulated the incorporation of choline-C14 into the insoluble fraction and into lipids. Cycocel inhibited phosphorylcholine uptake by roots.
Thus Cycocel stimulated choline kinase activity and the utilization of choline-C14. The effect of Cycocel upon kinase activity in vivo and in vitro was reversed by gibberellin A3.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harada H., Lang A. Effect of some (2-chloroethyl) trimethylammonium chloride analogs and other growth retardants on gibberellin biosynthesis in Fusarium moniliforme. Plant Physiol. 1965 Jan;40(1):176–183. doi: 10.1104/pp.40.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSLER B., CHEN D. DISTRIBUTION, PROPERTIES AND SPECIFICITY OF POLYNUCLEOTIDE PHOSPHORYLASE IN WHEAT ROOTS. Biochim Biophys Acta. 1964 Apr 27;80:533–541. doi: 10.1016/0926-6550(64)90297-x. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E. (2-Chloroethyl) Trimethylammonium Chloride and Related Compounds as Plant Growth Substances. II. Effect on Growth of Wheat. Plant Physiol. 1960 May;35(3):380–385. doi: 10.1104/pp.35.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Wiebe H. Phosphorus and Sulfur Compounds in Plant Xylem Sap. Plant Physiol. 1955 Nov;30(6):499–504. doi: 10.1104/pp.30.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN SLYKE D. D., PLAZIN J., WEISIGER J. R. Reagents for the Van Slyke-Folch wet carbon combustion. J Biol Chem. 1951 Jul;191(1):299–304. [PubMed] [Google Scholar]


