Abstract
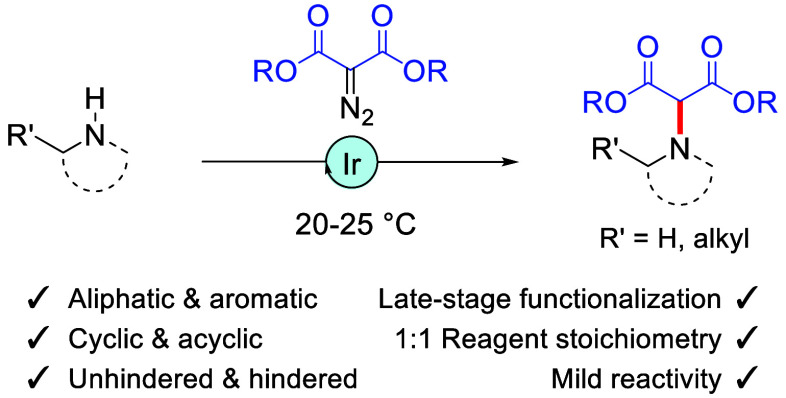
A general N–H insertion reactivity of acceptor–acceptor diazo malonate reagents is reported using [Ir(cod)Cl]2 as catalyst. A large range of amines, primary and secondary, aliphatic and aromatic, is possible. Mild temperatures, perfect substrate/reactant stoichiometry, and good functional group compatibility render the process particularly attractive for the (late-stage) functionalization of amines.
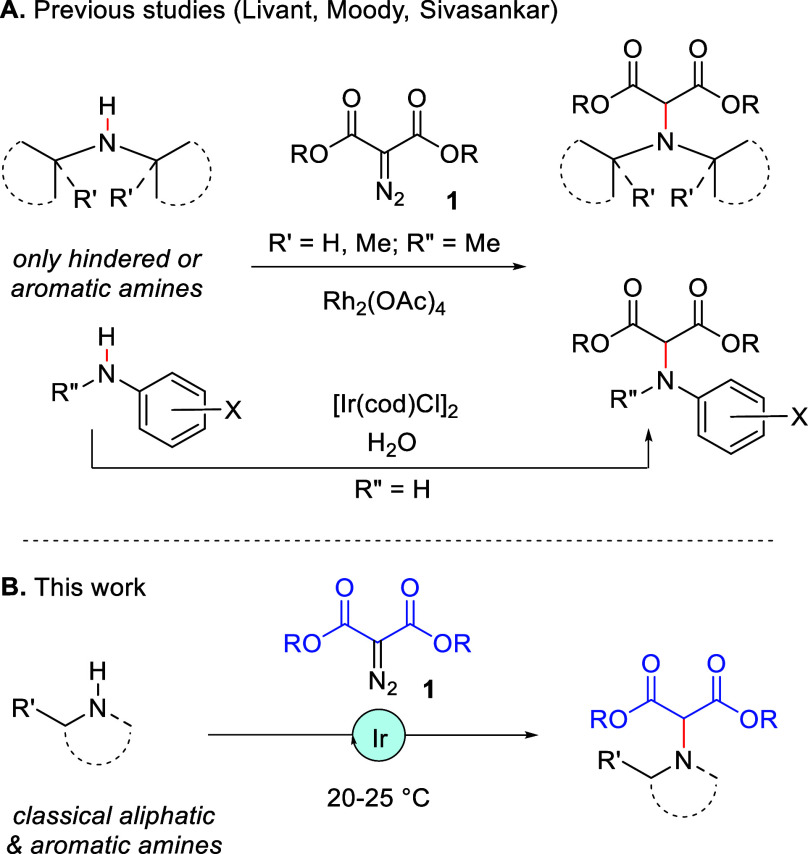
Nitrogen-containing molecules are key to pharmaceutical and medicinal chemistry since >80% of small molecule drugs contain at least one N atom,1 usually present as a heterocycle. Development of methods to access such (cyclic) compounds is thus of academic and industrial importance, and so are processes which allow the easy manipulation of the introduced N atoms.2 In fact, nitrogen is not a trivial atom to handle due to its basicity, nucleophilicity, high polarity, and coordination ability.3 For instance, processes as simple as the synthesis of trisubstituted amines from disubstituted alkyl or aryl precursors are often complex. To achieve such a goal,4 one prominent alternative is the insertion of reactive carbenes into pre-existing N–H bonds.5 Diazo derivatives are classical substrates to generate the necessary divalent intermediates, and their decomposition in the presence of transition-metal catalysts is a recognized strategy to form carbenes.6 Traditionally, different classes of diazo precursors are considered depending on the donor or acceptor nature of the substituents surrounding the central functional group.7 Donor–acceptor diazo reagents are used predominantly in N–H insertions, with much success including for asymmetric variants.8 However, if it becomes necessary to use acceptor–acceptor diazo reactants, one can rely on a few reported studies. In effect, little reactivity is known using diazomalonates 1, despite the overall interest in these more stable diazo reagents,9 and in the resulting N-protected adducts.10 Livant and Moody reported, in the presence of Rh2(OAc)4, N–H insertions of 1 into bulky secondary alkylamines or anilines (Scheme 1).11 These specific substrates are hindered or less basic than regular amines—to avoid catalyst poisoning of the Lewis acidic dirhodium complexes.11a,11d,12 Also, previously, Sivasankar and co-workers reported the room temperature reactivity of diazomalonates 1 and anilines in water under iridium catalysis.13 The method caught our attention for its mildness,14 and we wondered how general the reaction could be in more classical solvent conditions and, importantly, with unhindered primary/secondary (heterocyclic) amines as substrates. Herein, we show that [Ir(cod)Cl]2 (cod = 1,5-cyclooctadiene), but also [CpRu(NCCH3)3][BArF],15 are effective catalysts in nonpolar solvents (Scheme 1). The reactivities of both complexes are compared, and the commercial iridium dimer was overall preferred for its reactivity at room temperature and in the presence of a range of amines, including polyfunctional drugs such as Amoxapine,16 Vortioxetine,17 Pomalidomide,18 or sensitive unsaturated diaza macrocycles.
Scheme 1. N–H Insertions of Diazomalonates 1.
With the goal of achieving NH insertions of diazomalonates into regular (unhindered) aliphatic amines, initial experiments were performed by adding dimethyl diazomalonate 1a (0.5 mmol) to solutions of morpholine 2a (1.0 equiv). Morpholine was chosen to test the (unlikely) competition between nitrogen and oxygen ylide pathways. 1,2-Dichloroethane was selected as solvent, and as a benchmark, the reaction was attempted first with classical Rh2(Oct)4 (1 mol %) as catalyst (Table 1, entry 1). After 15 h at 100 °C, full conversion of diazo 1a was achieved but N–H insertion product 3aa could not be observed, as expected from previous studies.11a,11c,19 This was also the case with Pd(OAc)2 and Pd(acac)2 as catalysts (entries 2 and 3).20 With copper salt Cu(OTf)2 and complex [Cu(CH3CN)4][BF4], adduct 3aa was obtained in 15% and 11% NMR yields, respectively (entries 4 and 5). Then, iridium catalysts were tested. Both dimeric [Ir(cyclooctene)2 Cl]2 and [Ir(cod)Cl]2 gave excellent results under high temperature conditions using a strict 1:1 ratio between 1a and 2a (entries 6 and 7, NMR yields up to 96%), with a preference for the latter complex. [Ir(cod)Cl]2 was thus selected for further studies, and the reaction was performed at different temperatures (20–100 °C range) with effective conversions and yields (entries 7–9).
Table 1. Optimization of Reaction Conditionsa.
| entry | [M] (x mol %) | temp (°C) | yield (%)b |
|---|---|---|---|
| 1 | Rh2(Oct)4 (1) | 100 | NDc |
| 2 | Pd(OAc)2 (2) | 100 | NDc |
| 3 | Pd(acac)2 (2) | 100 | NDc |
| 4 | Cu(OTf)2 (2) | 100 | 15 |
| 5 | [Cu(NCCH3)4][BF4] (2) | 100 | 11 |
| 6 | [Ir(cyclooctene)2Cl]2 (1) | 100 | 80 |
| 7 | [Ir(cod)Cl]2 (1) | 100 | 96 |
| 8 | [Ir(cod)Cl]2 (1) | 60 | 97 |
| 9 | [Ir(cod)Cl]2 (1) | 25 | 96 |
| 10d | [Ir(cod)Cl]2 (1) | 25 | 96 |
| 11 | [CpRu(NCCH3)3][BArF] (2) | 60 | 99 |
| 12d | [CpRu(NCCH3)3][BArF] (2) | 25 | 91 |
Reaction conditions: 1a (0.5 mmol), 2a (0.5 mmol, 1.0 equiv), and [M] (1 or 2 mol %) in 1,2-dichloroethane (1.0 mL) for 15 h, unless otherwise noted.
NMR yield (1H NMR spectroscopy using CH2Br2 as internal reference).
ND = not detected.
Reaction in DCM.
Then, several solvents were tested with general success (see Supporting Information); dichloromethane (DCM) was selected for its practicality (entry 10). In our group, cationic [CpRu(NCCH3)3]+ (Cp = cyclopentadienyl) complexes are known to be also effective catalysts for the decomposition of acceptor–acceptor diazo reagents under mild conditions.15,21 While an excellent result was obtained at 60 °C (99% NMR yield, entry 11), a slightly lower yield was observed at room temperature in DCM (91% NMR yield, entry 12). Considering the effectiveness of the reaction and the commercial availability of [Ir(cod)Cl]2, this complex was thus retained.22
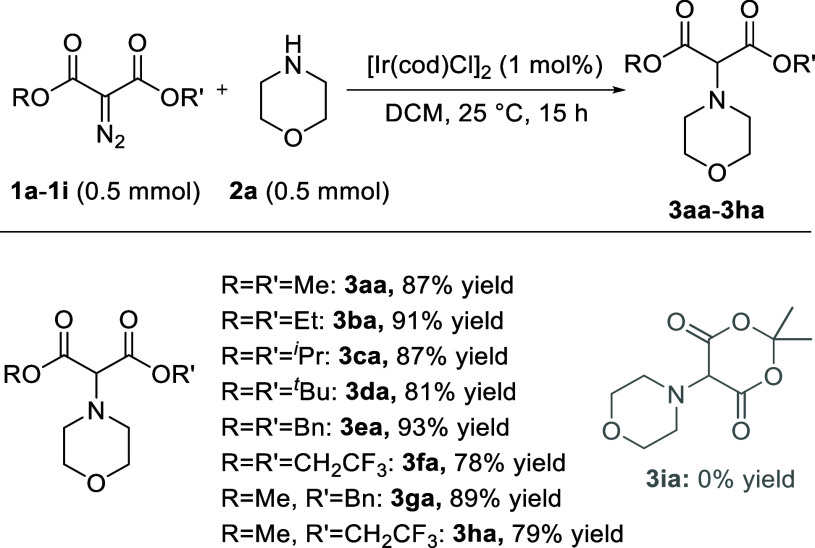
With optimized conditions in hand (Table 1, entry 10), a variety of diazo reagents (1a–h) was tested (0.5 mmol) using morpholine 2a as substrate, and full conversion was always reached after 15 h (Scheme 2). In the ester series from 3aa to 3da, from OMe to OtBu, isolated yields were >80% (up to 91%) with a slight sensitivity to steric hindrance in the case of 3da (81%). Excellent yields were obtained for benzylated 3ea (93%) and 3ga (89%). Clearly, under the Ir-catalysis and in the presence of morpholine, competitive Buchner reactions of 1e and 1g, in favor of products of intramolecular carbene addition onto an aromatic phenyl group, do not seem to happen.23 With 1f and 1h carrying 2,2,2-trifluoroethyl side chains on the ester groups, yields were slightly lower, 78% and 79% for 3fa and 3ha respectively. However, with cyclic 1i, a complete lack of decomposition was noticed; this reagent often presented an orthogonal reactivity in comparison with acyclic derivatives.24
Scheme 2. Diazomalonate Reactivity.
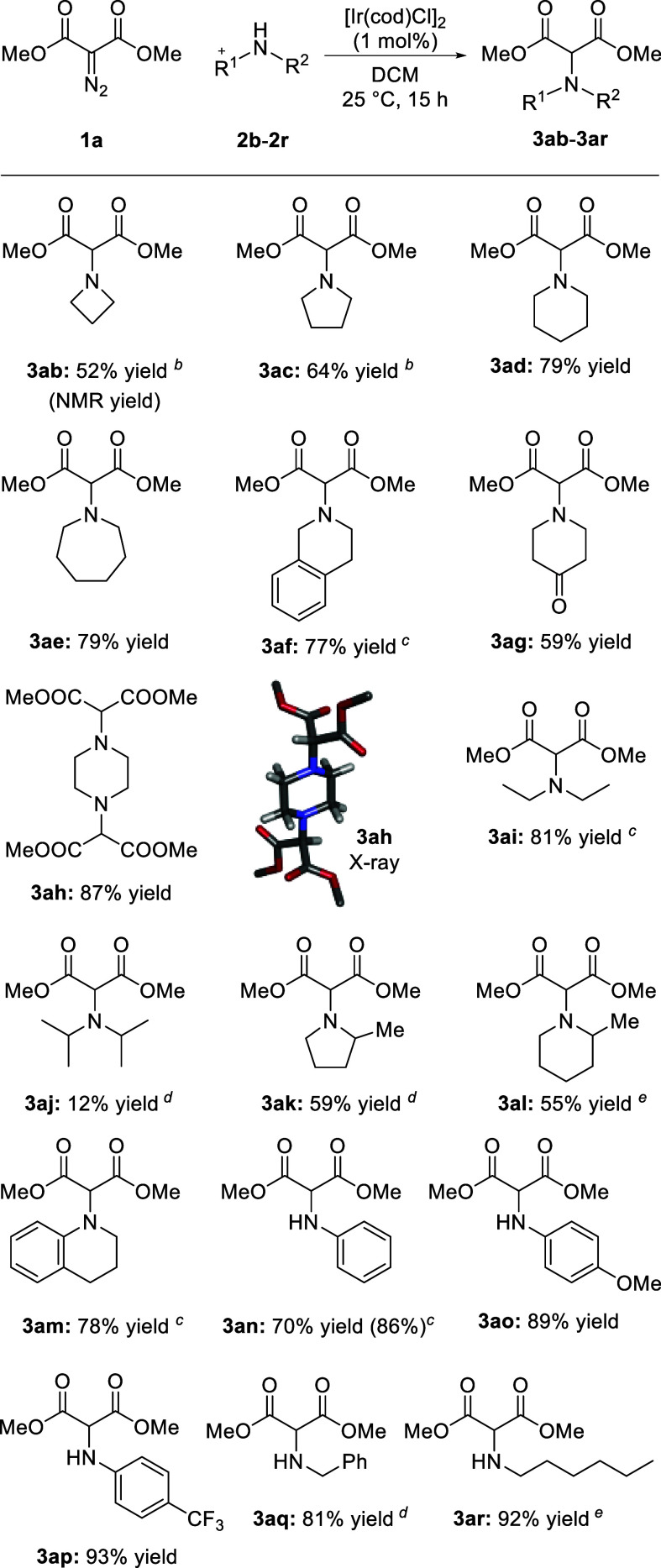
The reaction was then extended to a variety of cyclic, acyclic, and aromatic secondary amines to afford tertiary products 3ab to 3am in yields up to 93% (Scheme 3). Overall, the reaction is general, and few exceptions will be noted (vide infra). Sometimes, an increase in reaction time and temperature was needed for full conversion but a strict 1:1 stoichiometry between diazo reagent and amine moieties could be maintained. Satisfactorily, different ring sizes were amenable, from 4- to 7-membered rings (3ab–3ae, 52–79%). While azetidine 2b reacted to yield volatile 3ab in a subsequently lower yield (52%, NMR), reaction with 2-methylaziridine did not form the expected three-membered azeridine but the 2-(allylamino)malonate adduct instead. With tetrahydroisoquinoline 2f, product 3af was obtained in 77% yield. 4-Oxo-piperidine 2g reacted to afford 3ag in 59% yield; the presence of the ketone moiety perturbs possibly the transformation via competitive formation of a carbonyl ylide intermediate. With piperazine 2h, a double N–H insertion was possible using 2 equiv of diazo. Compound 3ah was prepared in an excellent 87% yield using twice the regular amount of 1 for the 2-fold process. The structure of 3ah was confirmed unambiguously by X-ray analysis (Scheme 3). Acyclic secondary amines 2i and 2j were tested. While the reaction proceeded well to form 3ai in 81% yield, the reactivity of hindered diisopropylamine was poor. It required a higher reaction temperature (60 °C) and adduct 3aj was isolated with only a 12% yield. This led us to test the effect of steric hindrance in the cyclic series with 2-methyl pyrrolidine 2k and piperidine 2l as substrates. The corresponding products were afforded in moderate yields, 3ak (59%) and 3al (55%); the reaction also required a slightly elevated temperature.
Scheme 3. Amine Reactivity.
General conditions: 1a (0.5 mmol), 2b–2r (0.5 mmol, 1.0 equiv), and [Ir(cod)Cl]2 (1 mol %) in DCM (1.0 mL) at 25 °C for 15 h. Isolated yields to the exception of 3ab (NMR).
48 h.
72 h.
60 °C for 36 h.
60 °C for 15 h.
Then, sp2-hybridized nitrogen atoms were considered using secondary tetrahydroquinoline 2m first. Not surprisingly,13 the reaction proceeded well to yield 3am (78%). Next, primary anilines were tested, and good yields were obtained irrespective of electron-neutral, -rich, or -poor character of the N atom (3an–3ap, 86–93%). Good yields could be achieved with aniline, 70% or 86%, after 15 or 72 h of reaction, respectively. Acyclic aliphatic amines 1q and 1r were also used as substrates. Overall, to the exception of a few polyamino or bulky substrates (see Scheme S1), excellent yields were observed for the preparation of mono N–H insertion adducts regardless of the aromatic or aliphatic nature of N-substituents 3an–3ar (81–92%). Finally, gram-scale experiments were performed using dimethyl diazomalonate 1a and morpholine 2a in DCM. After successful attempts under regular conditions, it was found that the catalyst loading could be reduced even to 0.5 mol %, without any impact on the yield (91%) but with an elongated reaction time of 72 h. A mechanistic rationale is proposed in Scheme S2.
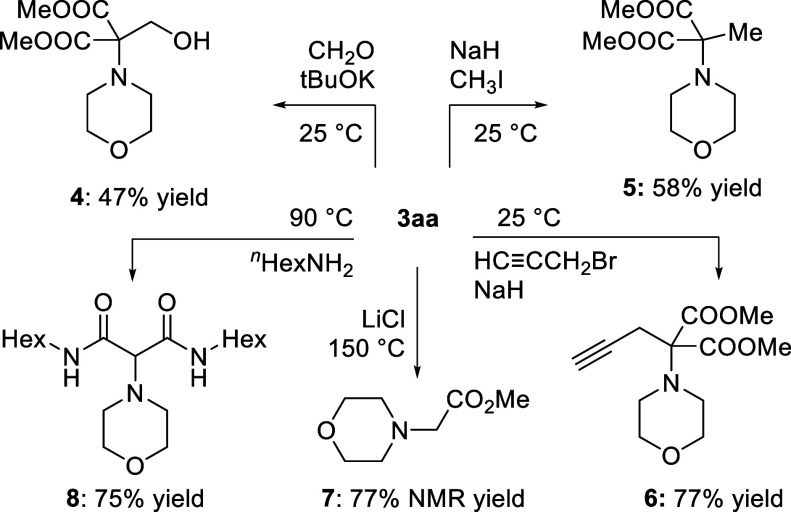
With a larger amount of dimethyl 2-morpholinomalonate 3aa in hand, a series of subsequent reactions was attempted (Scheme 4). Deprotonation of the malonate moiety could be readily achieved with tBuOK and, in the presence of formaldehyde, Knoevenagel adduct 4 was isolated in 47% yield. Alkylations with methyl iodide and propargyl bromide could be realized using NaH as a base to afford 5 (58%) and 6 (77%). Decarboxylative procedure (LiCl, 150 °C) afforded ester 7 (77% NMR yield). Unfortunately, the product was quite volatile and its isolation from DMF low yielding. Of note, this process opens the door to the formation of aminocarboxylate side chains that are useful in many metal binding studies.25 Reaction of 3aa with n-hexylamine, used as a solvent, led to bisamide 8 (75%).
Scheme 4. Post-transformations.
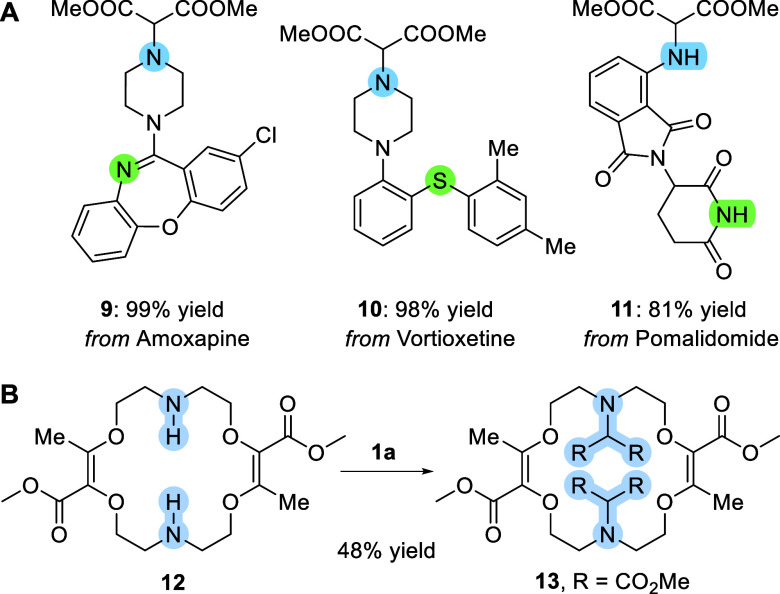
Finally, to demonstrate the utility and mildness of the NH-insertion method, it was studied in the context of late-stage functionalizations with molecules from medical chemistry or our own laboratory (Scheme 5). With Amoxapine and Vortioxetine, molecules used in the treatment of (major) depression, N-protected adducts 9 and 10 were obtained in excellent 99% and 98% yields, respectively. Importantly, side reactions involving ylide formation by the addition of carbene intermediates to the Lewis basic N(sp2) of Amoxapine or the S atom of Vortioxetine did not occur. With Pomalidomide, an anticancer drug, 11 was obtained in 81% by insertion into the primary aniline exclusively; evidence of reactivity on the more acidic imide NH fragment could not be found.
Scheme 5. Late-Stage Reactivity.
A: amines (0.25 mmol), 1a (0.25 mmol, 1.0 equiv), and [Ir(cod)Cl]2 (1 mol %) in DCM (0.5 mL) at 25 °C for 20 h (with Amoxapine and Vortioxetine) or at 60 °C for 72 h (with Pomalidomide). B: 12 (0.1 mmol), 1a (0.3 mmol, 3.0 equiv), and [Ir(cod)Cl]2 (2 mol %) in DCM (0.6 mL) at 60 °C for 72 h.
Previously, in our own group, an original [3 + 6 + 3 + 6] macrocyclization procedure was discovered using α-diazo-β-ketoesters and cyclic ethers as substrates.26 This reaction was extended to the formation of diaza macrocycles with morpholine heterocycles as the starting materials. For instance, using this protocol, bis-NH derivative 12 can be isolated in 68% yield after two steps only.26b In our hand, this derivative was found to be sensitive to both acidic and basic conditions, hence limiting the possibility of functionalization of the N atoms and consequently the introduction of acetic acid side chains. Care was thus taken to investigate the NH-insertion reactivity. Satisfactorily, bis-functionalized 13 was obtained in 48% yield, after an increase in catalyst loading (2 mol %) and diazo reagent (1a, 3.0 equiv), and conditions at 60 °C for 72 h. This unusual difficulty in forming the insertion adducts might be related to conformations of 12 that present, most likely, the two N–H bonds inward, toward the lumen of the macrocycle, thus strongly hindering the accessibility of the heteroatoms.
In conclusion, the overall reactivity demonstrates, including these latest examples, the generality of Ir-catalyzed N–H insertion even in the presence of steric encumbrance. The functional group compatibility, together with the mild reaction temperatures, and usually perfect substrate/reactant stoichiometry render the process particularly attractive for the (late-stage) functionalization of amines.
Acknowledgments
We are grateful for the financial support of this work by the University of Geneva and the Swiss National Science Foundation (JL: 200020-184843 and 200020-207539).
Data Availability Statement
The data underlying this study are openly available at yareta.unige.ch under DOI: 10.26037/yareta:blwwovyd75g5vox7g7i42zvnhm. It will be preserved for 10 years.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.orglett.3c03929.
Synthetic protocols and spectroscopic characterizations; 1H NMR and 13C NMR spectra of new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]; b Conway J. H. Jr.; Rovis T. Regiodivergent Iridium(III)-Catalyzed Diamination of Alkenyl Amides with Secondary Amines: Complementary Access to γ- or δ-Lactams. J. Am. Chem. Soc. 2018, 140, 135–138. 10.1021/jacs.7b11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Trowbridge A.; Walton S. M.; Gaunt M. J. New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev. 2020, 120, 2613–2692. 10.1021/acs.chemrev.9b00462. [DOI] [PubMed] [Google Scholar]; b Heravi M. M.; Zadsirjan V. Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv. 2020, 10, 44247–44311. 10.1039/D0RA09198G. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Cheng Q.-Q.; Zhou Z.; Jiang H.; Siitonen J. H.; Ess D. H.; Zhang X.; Kürti L. Organocatalytic nitrogen transfer to unactivated olefins via transient oxaziridines. Nat. Catal. 2020, 3, 386–392. 10.1038/s41929-020-0430-4. [DOI] [Google Scholar]; d Ricci A.; Bernardi L.. Methodologies in Amine Synthesis: Challenges and Applications; John Wiley & Sons, 2021. [Google Scholar]; e Gasser V. C. M.; Makai S.; Morandi B. The advent of electrophilic hydroxylamine-derived reagents for the direct preparation of unprotected amines. Chem. Commun. 2022, 58, 9991–10003. 10.1039/D2CC02431D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Afagh N. A.; Yudin A. K. Chemoselectivity and the Curious Reactivity Preferences of Functional Groups. Angew. Chem., Int. Ed. 2010, 49, 262–310. 10.1002/anie.200901317. [DOI] [PubMed] [Google Scholar]; b Xu T.; Alper H. Pd-Catalyzed Chemoselective Carbonylation of Aminophenols with Iodoarenes: Alkoxycarbonylation vs Aminocarbonylation. J. Am. Chem. Soc. 2014, 136, 16970–16973. 10.1021/ja508588b. [DOI] [PubMed] [Google Scholar]; c Yamane Y.; Miyazaki K.; Nishikata T. Different Behaviors of a Cu Catalyst in Amine Solvents: Controlling N and O Reactivities of Amide. ACS Catal. 2016, 6, 7418–7425. 10.1021/acscatal.6b02309. [DOI] [Google Scholar]; d Zhou Z.; Kürti L. Electrophilic Amination: An Update. Synlett 2019, 30, 1525–1535. 10.1055/s-0037-1611861. [DOI] [Google Scholar]
- Bermejo-López A.; Raeder M.; Martínez-Castro E.; Martín-Matute B. Selective and quantitative functionalization of unprotected α-amino acids using a recyclable homogeneous catalyst. Chem. 2022, 8, 3302–3323. 10.1016/j.chempr.2022.08.017. [DOI] [Google Scholar]
- Such a transformation belongs to the overall class of X–H carbene insertions (X = C, O, N, S, etc.), see:; Gillingham D.; Fei N. Catalytic X-H insertion reactions based on carbenoids. Chem. Soc. Rev. 2013, 42, 4918–4931. 10.1039/c3cs35496b. [DOI] [PubMed] [Google Scholar]
- Ford A.; Miel H.; Ring A.; Slattery C. N.; Maguire A. R.; McKervey M. A. Modern Organic Synthesis with α-Diazocarbonyl Compounds. Chem. Rev. 2015, 115, 9981–10080. 10.1021/acs.chemrev.5b00121. [DOI] [PubMed] [Google Scholar]
- Zhu D.; Ma J.; Luo K.; Fu H.; Zhang L.; Zhu S. Enantioselective Intramolecular C-H Insertion of Donor and Donor/Donor Carbenes by a Nondiazo Approach. Angew. Chem., Int. Ed. 2016, 55, 8452–8456. 10.1002/anie.201604211. [DOI] [PubMed] [Google Scholar]
- a Su Y.-X.; Huang M.-Y.; Zhu S.-F. Catalytic N-H Insertion Reactions with α-Diazoacetates: An Efficient Method for Enantioselective Amino Acid Synthesis. ChemCatChem 2023, 15, e202300539. 10.1002/cctc.202300539. [DOI] [Google Scholar]; b Arredondo V.; Hiew S. C.; Gutman E. S.; Premachandra I. D. U. A.; Van Vranken D. L. Enantioselective Palladium-Catalyzed Carbene Insertion into the N-H Bonds of Aromatic Heterocycles. Angew. Chem., Int. Ed. 2017, 56, 4156–4159. 10.1002/anie.201611845. [DOI] [PubMed] [Google Scholar]; c Jurberg I. D.; Davies H. M. L. Blue light-promoted photolysis of aryldiazoacetates. Chem. Sci. 2018, 9, 5112–5118. 10.1039/C8SC01165F. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li M.-L.; Yu J.-H.; Li Y.-H.; Zhu S.-F.; Zhou Q.-L. Highly enantioselective carbene insertion into N-H bonds of aliphatic amines. Science 2019, 366, 990–994. 10.1126/science.aaw9939. [DOI] [PubMed] [Google Scholar]; e Shen H.-Q.; Wu B.; Xie H.-P.; Zhou Y.-G. Preparation of Axially Chiral 2,2′-Biimidazole Ligands through Remote Chirality Delivery and Their Application in Asymmetric Carbene Insertion into N-H of Carbazoles. Org. Lett. 2019, 21, 2712–2717. 10.1021/acs.orglett.9b00687. [DOI] [PubMed] [Google Scholar]; f Bera S. S.; Bahukhandi S. B.; Empel C.; Koenigs R. M. Catalyst-controlled site-selective N-H and C3-arylation of carbazole via carbene transfer reactions. Chem. Commun. 2021, 57, 6193–6196. 10.1039/D1CC01863A. [DOI] [PubMed] [Google Scholar]; g He F.; Koenigs R. M. Borane-Catalyzed Carbazolation Reactions of Aryldiazoacetates. Org. Lett. 2021, 23, 5831–5835. 10.1021/acs.orglett.1c01982. [DOI] [PubMed] [Google Scholar]; h Hu S.; Wu J.; Lu Z.; Wang J.; Tao Y.; Jiang M.; Chen F. TfOH-Catalyzed N-H Insertion of α-Substituted-α-Diazoesters with Anilines Provides Access to Unnatural α-Amino Esters. J. Org. Chem. 2021, 86, 3223–3231. 10.1021/acs.joc.0c02588. [DOI] [PubMed] [Google Scholar]; i Liu Z.; Yang Y.; Song Q.; Li L.; Zanoni G.; Liu S.; Xiang M.; Anderson E. A.; Bi X. Chemoselective carbene insertion into the N-H bonds of NH3·H2O. Nat. Commun. 2022, 13, 7649. 10.1038/s41467-022-35394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Zhou Y.; Zhang Y.; Yu C.; Yue X.; Jiang F.; Guo W. Visible-light-mediated catalytic asymmetric synthesis of α-amino esters via free carbene insertion into NH bond. Tetrahedron Lett. 2023, 122, 154496. 10.1016/j.tetlet.2023.154496. [DOI] [Google Scholar]
- Green S. P.; Wheelhouse K. M.; Payne A. D.; Hallett J. P.; Miller P. W.; Bull J. A. Thermal Stability and Explosive Hazard Assessment of Diazo Compounds and Diazo Transfer Reagents. Org. Process Res. Dev. 2020, 24, 67–84. 10.1021/acs.oprd.9b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Dénès F.; Beaufils F.; Renaud P. Thiophenol-Mediated 1,5-Hydrogen Transfer for the Preparation of Pyrrolizidines, Indolizidines, and Related Compounds. Org. Lett. 2007, 9, 4375–4378. 10.1021/ol702017t. [DOI] [PubMed] [Google Scholar]; b Kattamuri P. V.; Yin J.; Siriwongsup S.; Kwon D.-H.; Ess D. H.; Li Q.; Li G.; Yousufuddin M.; Richardson P. F.; Sutton S. C.; et al. Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon-Nitrogen Bond Formation. J. Am. Chem. Soc. 2017, 139, 11184–11196. 10.1021/jacs.7b05279. [DOI] [PubMed] [Google Scholar]
- a Yang M.; Wang X.; Li H.; Livant P. A New Route To Hindered Tertiary Amines. J. Org. Chem. 2001, 66, 6729–6733. 10.1021/jo010583a. [DOI] [PubMed] [Google Scholar]; b Bashford K. E.; Cooper A. L.; Kane P. D.; Moody C. J.; Muthusamy S.; Swann E. N-H Insertion reactions of rhodium carbenoids. Part 3.1 The development of a modified Bischler indole synthesis and a new protecting-group strategy for indoles. J. Chem. Soc., Perkin Trans. 1 2002, 1672–1687. 10.1039/b202666j. [DOI] [Google Scholar]; c Yang M.; Albrecht-Schmitt T.; Cammarata V.; Livant P.; Makhanu D. S.; Sykora R.; Zhu W. Trialkylamines More Planar at Nitrogen Than Triisopropylamine in the Solid State. J. Org. Chem. 2009, 74, 2671–2678. 10.1021/jo802086h. [DOI] [PubMed] [Google Scholar]; d Honey M. A.; Moody C. J. Synthesis of Indoxylic Acid Esters by Rhodium-catalyzed Carbene N-H Insertion and Thermal Cyclization. Aust. J. Chem. 2014, 67, 1211–1216. 10.1071/CH14116. [DOI] [Google Scholar]
- Jie Y.; Livant P.; Li H.; Yang M.; Zhu W.; Cammarata V.; Almond P.; Sullens T.; Qin Y.; Bakker E. An Acyclic Trialkylamine Virtually Planar at Nitrogen. Some Chemical Consequences of Nitrogen Planarity. J. Org. Chem. 2010, 75, 4472–4479. 10.1021/jo100628v. [DOI] [PubMed] [Google Scholar]
- Ramakrishna K.; Sivasankar C. Iridium catalyzed acceptor/acceptor carbene insertion into N-H bonds in water. Org. Biomol. Chem. 2017, 15, 2392–2396. 10.1039/C7OB00177K. [DOI] [PubMed] [Google Scholar]
- In our hands, these aqueous conditions were limiting in terms of substrate scope, as a simple amine like morpholine reacted to give product 3aa in 8% only.
- Achard T.; Egger L.; Tortoreto C.; Guénée L.; Lacour J. Preparation and structural characterization of [CpRu(1,10-phenanthroline)(CH3CN)][X] and precursor complexes (X=PF6, BArF, TRISPHAT-N). Helv. Chim. Acta 2020, 103, e2000190. 10.1002/hlca.202000190. [DOI] [Google Scholar]
- Jue S. G.; Dawson G. W.; Brogden R. N. Amoxapine: A Review of its Pharmacology and Efficacy in Depressed States. Drugs 1982, 24, 1–23. 10.2165/00003495-198224010-00001. [DOI] [PubMed] [Google Scholar]
- Sanchez C.; Asin K. E.; Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacol. Ther. 2015, 145, 43–57. 10.1016/j.pharmthera.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A. A.; Swaika A.; Paulus A.; Kumar S. K.; Mikhael J. R.; Rajkumar S. V.; Dispenzieri A.; Lacy M. Q. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 2013, 3, e143–e143. 10.1038/bcj.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A limited list of Lewis acidic catalysts is available as most metal complexes fail due to an effective poisoning by the nitrogen-containing substrates. It is the case in Livant and Moody’s studies but also in our hands with Rh(II) complexes such as Rh2(Oct)4.
- Kitamura M.; Kisanuki M.; Kanemura K.; Okauchi T. Pd(OAc)2-Catalyzed Macrocyclization of 1,2-Diazonaphthoquinones with Cyclic Ethers. Org. Lett. 2014, 16, 1554–1557. 10.1021/ol500222s. [DOI] [PubMed] [Google Scholar]
- Nikolova Y.; Fabri B.; Moneva Lorente P.; Guarnieri-Ibáñez A.; de Aguirre A.; Soda Y.; Pescitelli G.; Zinna F.; Besnard C.; Guénée L.; et al. Chemo and Regioselective Multiple C(sp2)-H Insertions of Malonate Metal Carbenes for Late-Stage Functionalizations of Azahelicenes. Angew. Chem., Int. Ed. 2022, 61, e202210798.and references therein 10.1002/anie.202210798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synthesis of CpRu complexes is well documented but requires a preparation time (1–2 weeks). Only the BArF salt is bench-stable at 20 °C (ref (15)), but it presents a high molecular weight of 1153. Practically, commercial [Ir(cod)Cl]2 is easier to use despite the elevated price of iridium.
- Moody C. J.; Miah S.; Slawin A. M. Z.; Mansfield D. J.; Richards I. C. Stereocontrol in the intramolecular Buchner reaction of diazoamides and diazoesters. J. Chem. Soc., Perkin Trans. 1 1998, 4067–4076. 10.1039/a807622g. [DOI] [Google Scholar]
- Preliminary experiments indicate a slower decomposition of diazoacetates, diazoarylacetates, and α-diazo-β-ketoesters at 20–25 °C. Products of NH insertion can be obtained at 60 °C in 15 h reaction time.
- Heskamp S.; Raavé R.; Boerman O.; Rijpkema M.; Goncalves V.; Denat F. 89Zr-Immuno-Positron Emission Tomography in Oncology: State-of-the-Art 89Zr Radiochemistry. Bioconjugate Chem. 2017, 28, 2211–2223. 10.1021/acs.bioconjchem.7b00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zeghida W.; Besnard C.; Lacour J. Rhodium(II)-Catalyzed One-Pot Four-Component Synthesis of Functionalized Polyether Macrocycles at High Concentration. Angew. Chem., Int. Ed. 2010, 49, 7253–7256. 10.1002/anie.201003559. [DOI] [PubMed] [Google Scholar]; b Homberg A.; Poggiali D.; Vishe M.; Besnard C.; Guénée L.; Lacour J. One-Step Synthesis of Diaza Macrocycles by Rh(II)-Catalyzed [3 + 6+3 + 6] Condensations of Morpholines and α-Diazo-β-ketoesters. Org. Lett. 2019, 21, 687–691. 10.1021/acs.orglett.8b03875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are openly available at yareta.unige.ch under DOI: 10.26037/yareta:blwwovyd75g5vox7g7i42zvnhm. It will be preserved for 10 years.