Abstract
Antimicrobial resistance (AMR) has emerged as one of the most serious worldwide public health issues of the twenty-first century. The expeditious rise of AMR has urged the development of new, natural effective therapeutic strategies against drug-resistant pathogens. Endophytic fungi, which inhabit distinctive environments like endosymbiotic relationships with plants, are gaining interest as alternative reservoirs for novel compounds that exhibit a broad range of chemical diversity and unique modes of action by releasing a variety of secondary metabolites with antimicrobial properties. The objective of the current research was to isolate and identify endophytic fungal species from leaves of Tradescantia pallida and to investigate their antagonistic effects on Multi-Drug-Resistant human pathogens. Endophytic fungus TPL11 and TPL14 showed maximum inhibition in agar plug and agar well diffusion assay. The ethyl acetate crude extract effectively suppressed growth of MRSA (Methicillin-resistant Staphylococcus aureus) ATCC 43300,700699 strains and VRE (Vancomycin-resistant Enterococcus) with the Inhibition zone of 22 ± 0.05, 23 ± 0.11 and 24 ± 0.11 mm respectively with minimum inhibitory concentration (MIC) of 3.125 µg/mL. Whereas TPL11 fungus revealed antibiosis of 22 ± 0.05 and 21 ± 0.15 mm against MRSA(ATCC 43300,700699) and 24 ± 0.05 mm for VRE with MIC of 6.25,3.125 and 1.56 μg/mL respectively. The MIC (Minimum inhibitory concentration) index further confirmed that both the extracts were bacteriostatic against MRSA and bactericidal against VRE. The isolates TPL11 and TPL14 were identified as Fusarium oxysporum and Nigrospora sphaerica by 18S rRNA internal transcribed spacer (ITS) sequencing. To our insight, it is the first report to reveal the presence of F.oxysporum and N.sphaerica in T.pallida and their antibacterial activity.
Keywords: Endophytic fungi, Nigrospora sphaerica, Fusarium oxysporum, MDR, Human pathogens, Antibiotic resistance, Pharmacology
1. Introduction
Antibiotic overuse has aided the outbreaks of drug-resistant bacterial infections, particularly the multiple drug-resistant strains, and a growing percentage of drug-unresponsive infectious disease agents are posing a significant threat to the global healthcare system (Janes et al., 2007). Infections triggered by antibiotic-resistant S.aureus represent an important threat to the health of people around the world. Methicillin-resistant S.aureus, drug-resistant Streptococcus pneumonia, mono to multiple drug-resistant M. tuberculosis, and VRE species are the most common Gram-positive pathogens that cause infections (Ratnaweera et al., 2018). Many human disorders have been attributed to Staphylococci, including osteomyelitis, periodontitis, endocarditis, chronic wound infection, and implanted device infection (Sadrati et al., 2023). MDR and XDR-TB are developing worldwide problems that are problematic to identify, costly to treat and result in varied outcomes. The organisms S. aureus, A. baumanii, K. pneumoniae, P. aeruginosa, Enterobacter sp. and E. faecium are the primary pathogens in most US hospitals (Deshmukh et al., 2015). Despite intensive attempts to suppress them, resistant microbial infections remain prevalent, triggering death and disability, continuing to pose a severe danger to human health (Ratnaweera et al., 2018). There are several methods for developing novel antimicrobial chemicals that are biologically active. The most conventional measures in the world of natural products is the research of lesser-known microorganism species and genera (Sadrati et al., 2023).
Medicinal plants play a key role in treating several human diseases, as they are the potential sources of biologically active molecules. The bioactive molecules comprise simple alkaloids, naphthopyrone glucosides, saponins, terpenes, anthraquinones (emodin, chrysophanol and physcion) and steroids (carpesterol, ecdysterone and β-sitosterol). Either plants or some microbial consortium that resides asymptomatically within the certain medicinal plant parts synthesizes these active molecules and this microbial community is referred to as endophytes (Yadav et al., 2021). Medicinal plants are the well-known reservoir of endophytic fungi with pharmaceutically important novel metabolites. The structures of the compounds from fungal endophytes are unique with interesting biological properties, thereby offering a wider scope for different clinical applications (Santos et al., 2015). Endophytes are microbial entities that inhabit the host plant tissues at any stage of their life cycle and perform different ecological relationships without causing any without causing any adverse effects on the host organism/or host plant (Silva et al., 2018). Endophytic fungi are the least considered microorganisms, but they have attracted attention owing high biological diversity and aptitude to synthesize new pharmacological substances (Sadrati et al., 2023). They are eminent cherished homes of an extensive variety of bioactive substances with cytotoxic, antimalarial, antimicrobial, antioxidant, antiviral and anticancer actions. About 80 % of endophytic fungi are responsible for biologically active product productions that have herbicidal and antimicrobial properties (Mousa et al., 2021). Fungal endophytes have recently gained impetus, due to their immense potential to produce numerous medicinally important metabolites. Hence, exploring endophytic fungi from different plant species would provide plenty of opportunities to discover new potential bioactive metabolites (Dhayanithy et al., 2019).
Tradescantia pallida (Rose) D. R. Hunt var. purpurea, an ornamental plant from the Commelinaceae family, generally acknowledged as Purple Heart or wandering jew. It is a shade-tolerant low-growing tetraploid plant that can grow well in different soil conditions. It also holds tough resistance to insects and parasites thereby colonizing rapidly in different environments. They are broadly scattered in tropical and subtropical sections and are ordinarily grown as ornamental, hanging, or ground-covering plants (Tan et al., 2014). In all Brazilian states, the genus Tradescantia was largely used as an ornament as they can grow and propagate easily and are highly resistant to environmental factors and climatic conditions (Cabral et al., 2022). They can eliminate unstable organic impurities from the air effectively (Tan et al., 2014). Traditionally, T.pallida was used as an anti-toxic and anti-inflammatory property and in Taiwanese traditional medicine, it is used to improve blood circulation (Bokhari et al., 2020). The leaves were used as dyes and they acted as analgesics against joint pain and rheumatism. It has been reported that the plant exhibits various biological activities like antioxidant, antimicrobial and anticancer properties (Cabral et al., 2022).
It is also reported to have antagonistic activity against gram-positive and negative bacteria (Kamiya et al., 2019). The chloroform leaf extract obtained from T.pallida purpurea has demonstrated significant efficacy against pathogens affecting Labeo rohita fish. Additionally, natural colorants such as anthocyanin and annatto derived from T. pallida are associated with numerous health advantages (Bokhari et al., 2020). Biosynthesized silver nanoparticles with T.pallida extract (TpAgNPs) exhibited cytotoxic activity on the rhabdomyosarcoma cell line. TpAgNPs revealed significant antibacterial activity against Pseudomonas aeruginosa with MIC of 64 μg/mL and antifungal activity against Candida parapsilosis. Further, the plant extract had effective antioxidant potentials of 77.2 ± 1.0 % and 45.1 ± 0.5 % free radical scavenging activity (Shahzadi et al., 2022). In Malaysia, the Ayta communities of Porac Pampanga utilize this plant to treat sore eyes (Ragragio et al., 2013). T. pallida has also been recognized as a viable alternative for in situ mutagenesis testing (Suyama et al., 2002). Ethanol extract showed beneficial hepatoprotective activity against CCl4-induced liver damage in rats (Villanueva-Toledo et al., 2002). Hexane extract obtained from aerial parts of T. pallida displayed significant antifungal activity against Rhizopus stolonifera, Sclerotinia sclerotiorum, and Penicillium digitatum. It also revealed cytotoxic activity against human tumor cell lines such as MCF‐7, fibroblasts, glioblastoma and HeLa (Cabral et al., 2022). The aqueous extract of T. pallida was reported to exhibit insecticidal properties (Rocha et al., 2022).
Hence, the present study describes the isolation, characterization and screening of endophytic fungi isolated from the ornamental plant, Tradescantia pallida. Further, the antimicrobial activity of the fungal extracts will tested against multi-drug resistant human pathogens. In this way, we provide a new resource that efficiently produces antibacterial agents from endophytic fungi that we obtained from T. pallida.
2. Materials and methods
2.1. General experimental measures
Potato dextrose agar (PDA), broth (PDB), Muller Hinton agar (MHA), broth (MHB), Tryptic soy agar (TSA) and broth (TSB) were acquired from Hi-Media (Mumbai, India). Glassware was purchased from Borosil and the solvents used for the analysis and experiments were purchased from SRL.
2.2. Procurement of test microorganisms
The resistant bacterial pathogens utilized were Methicillin-resistant Staphylococcus aureus-MRSA (ATCC 43300; 700699), Staphylococcus aureus (ATCC 25923), S.aureus (MTCC 3160), Vancomycin-resistant Enterococci-VRE (ATCC 51299) and Enterococcus fecalis. The cultures were acquired from American type culture collection (ATCC) and Microbial type culture collection (MTCC; Chandigarh, India). Test pathogens sub-cultured in Tryptic soy agar and Brain heart infusion agar respectively for further use.
2.3. Plant sampling, authentication and isolation of endophytic fungi
To isolate endophytic fungi, a healthy Tradescantia pallida plant was collected from the Vellore district (12°56′17.3″N 79°09′24.5″E), Tamilnadu, India in November (2020). The samples were collected aseptically in a clean polyethylene cover and handled within 8 hrs. The specimens were authenticated by the botanist from Dr. P. Jayaraman, (Director), Plant Anatomy Research Center, Chennai. Samplings were cleaned first with distilled H2O (5 mins) to eradicate adhered soil particles and surface-adhering microorganisms. Then sterilized aseptically by sequential immersion of samples into 70 % ethanol (C2H5OH) (1 min), 35 % Sodium hypochlorite (NaOCl) for 30 s followed by double distilled water. The segments were cut into 2 cm long with a sterile scalpel blade and placed on a Potato Dextrose Agar medium supplement with 50 mg/L chloramphenicol to prevent the growth of bacteria. In addition, incubated for 3–7 days at 27 ± 1 °C. The emerging endophytic fungi from the explants were isolated, purified and maintained by continuous sub-culturing. Explants and last rinsed aliquot were considered as a control measure. The media dish impregnated with 50 µl of the last rinsed H2O with no growth of microbes determines the success ratio of the surface sterilization method (Potshangbam et al., 2022).
2.4. Preservation of endophytic fungal isolates
The pure entities were transferred individually to PDA slants and in a 15 % sterilized glycerol (v/v) solution. The growth was observed for 3 to 7 days and the slants at −4°C and glycerol stock at −20 °C were preserved for further study (Ibrahim et al., 2021).
2.5. Susceptibility test: Primary evaluation through agar plug diffusion technique
The pure cultures derived from T.pallida were first screened for antibacterial properties by agar Plug diffusion standard protocol. The agar plugs were made from the 7-day-grown pure fungal cultures. Then the pure culture plugs were positioned onto the Muller Hinton agar medium previously seeded by respective bacterial pathogens using a sterile cotton swab. The plates were refrigerated overnight at 4 °C to facilitate diffusion of metabolites, followed by 16–18 hrs incubation at 37 °C for the bacterial growth. The potential fungal isolates were selected based on the zone of inhibition observed against the test pathogens for secondary screening (Sadrati et al., 2020).
2.6. Characterization of the potential isolate
2.6.1. Morphological characterization
The fungal isolates were grown on a PDA medium and morphological examinations were carried out after the isolates mycelium occupied the entire plates. Characteristics comprise microscopic and macroscopic characterization. The shape, textures, pigmentation, colony morphology and characteristics of spores were categorized under macroscopic inspections. Microscopic was done by the Lacto phenol cotton blue staining technique and images were taken at 100x (Manganyi et al., 2018).
2.6.2. Scanning electron microscopy (SEM) analysis
The spore surface morphology and hyphae configuration were observed under a Scanning electron microscope. To study the morphology of test bacteria upon treatment with fungal crude extract, the samples were analyzed under SEM (EVO/18 Research, Carl Zeiss) (Santra et al., 2022).
2.6.3. Cultural characterization
The potential isolates were grown on assorted commercially available media such as Potato dextrose agar, Sabouraud Dextrose Agar, Malt extract agar (MEA), Czapek Dox agar (CDA) and Corn meal agar purchased from Hi Media, India for optimization of media for maximal growth and bioactivity.
2.6.4. Phylogenetic investigation
The fungal endophyte that exhibited the greatest antibiosis was classified molecularly with 18S rRNA ITS sequencing. The genomic DNA was isolated in the laboratory. Universal primers ITS1(5′TCCGTAGGTGAACCTGCGG3′) and ITS4 (5′ TCCTCCGCTTATTGATATGC 3′) were chosen, PCR amplification was processed with thermal cycling conditions of initial denaturation for 2 mins at 95 °C, followed by 25 cycles denaturation at 95 °C of 30 secs, primer annealing for 30 secs at 55 °C, first extension for 1 min and last extension (72 °C) for 5 mins. ABI PRISM® was used to achieve sequencing outcomes. Results from BLAST were matched with NCBI Genbank to find out the identities of the isolates (Settu et al., 2023).
2.7. Analysis of the antagonistic activity of crude extract
2.7.1. Optimization of ideal culture medium for the production of antibacterial substances
The purified potent 7 days old fungal cultures that were selected in the previous step, were cultivated on PDA, SDA, MEA, CDA, CMA and incubated for 3 weeks at room temperature under a consistent state in 500 mL volume conical flask. Post incubation, culture broth was drained by Whatman filter paper No. 1 (Z240079) to section mycelium and filtrate. The obtained filtrate was extracted with equivalent measurements of multiple polarity solvents such as Hexane, Dichloromethane (DCM), Ethyl acetate (EA) and Butanol with a Borosil separating funnel. The mycelial mat was extracted with methanol. Using a (Model: RE100-Pro rotatory evaporator, the solvent extracts are condensed. Derived metabolites were directly used to assess the in-vitro assays (Supaphon et al., 2013).
2.7.2. Secondary antibiosis screening by Kirby-Bauer method
The inhibitory effect of the obtained extracts from the selected fungus was tested for the antibacterial properties by the Kirby Bauer disc diffusion method (Katoch et al., 2017). The screening was carried out against human pathogenic bacteria. Muller Hinton agar served as the basal medium. Bacterial turbidity was normalized with standard Mc Farland (0.5) before carrying out the microbial assay. Agar dish seeded with pre-prepared inoculum of trial microbes. Subsequently, sterile discs about 6 mm comprising the testing extract of the chosen concentration (25, 50, 75, 100 µg/mL) were placed onto the bacteria-seeded plate. DMSO (5 %) was used as a negative control to detect the effect of solvent. Commercial antibiotics Oxacillin (1mcg) and Vancomycin disc (30 mg) as positive control. Petri dishes were incubated at 37 °C for 16–18 hrs and the inhibition zone was measured. The test was carried out in independent triplicates.
2.7.3. Evaluation of minimum inhibitory and bactericidal concentration
Broth micro-dilution susceptibility assay was executed to find out the MIC and MBC of endophytic fungal extract. In a sterile 2 mL 96 well plate, 100 µL of sterilized MH broth was added. The test samples of the extracts were prepared at 1 mg/ml concentration and 100 µL was added to the first well. Followed by two-fold serial dilutions were made resulting in different concentrations ranging from 50 to 0.78 µg/mL. To the wells, 10 µL of pre-prepared bacterial inoculum was added. The well with MHB alone serves as positive control and the well with bacteria and MHB serves as negative control and reference drug controls. Petri dishes were incubated for 16–18 hrs at 35 ± 2 °C. The lowermost concentration at which no observable bacteria in the wells following incubation was recorded as the MIC value. Further, the concentration showing a complete absence of visible bacterial growth in the MIC assay was determined. About 50 µL was subcultured onto a Muller Hinton agar medium and incubated for 18 to 24 hrs at 37 °C. The lowermost extract concentration showing a full absence of bacterial growth was recorded as the MBC (Valle et al., 2016).
2.7.4. MIC index of the extracts
To validate the effectiveness and the outcome of whether ethyl acetate extract is bactericidal or bacteriostatic, MIC index was assessed by dividing MBC by MIC value. If the index is lesser than or equal to 4, then it is considered bactericidal and bacteriostatic if it is greater than 4 (Sadrati et al., 2020).
2.7.5. Synergistic activity of the extracts
Interactive inhibition of synergy between the fungal extract and antibiotics was measured using the chequerboard method following the protocol of Omokhua et al., 2019. A synergistic combination was performed using antibiotics and the fungal extract to which the bacterial strains were resistant. Antibiotics and crude concentration began at their MIC and were serially diluted at two-fold rates. Combination effect was estimated by valuing the FICI of individual combinations and the average values were measured.
Fungal extract FIC = MIC of fungal extract in combination with antibiotic/ Fungal extract MIC alone.
Antibiotic FIC = MIC of antibiotics combined with fungal extract/antibiotics MIC only
FICI = Fungal extract FIC + Antibiotic FIC
FICI ≤ 0.5 was used to define synergy. A FICI between 0.5 and –4 specifies the absence of interaction between agents. FIC greater than 4 designates that there is an antagonistic reaction between agents.
2.8. Mycochemical screening
The phytochemical analysis of ethyl acetate crude extract was carried out using a standard protocol (Sharma et al., 2016). The test was done to determine the existence of secondary metabolites such as phenols, alkaloids, steroids, terpenoids and tannins in the fungal extract.
2.9. Gas chromatography and mass spectrometry analysis
Perkin Elmer Clarus 680 with MS Clarus 600 (electron ionization) fitted out with elite-5MS capillary medium was carried out to assess the crude extracts obtained from F.oxysporum and N.sphaerica. The crude diluted with the same solvent was studied. The oven's initial temperature was 55° for 3 mins and the ramp program was 10 mins to 300 °C for 6 mins. Helium with a 1 mL/min continuous flow rate is used as carrier gas. The mass transfer line and temperature are fixed at 240 °C. Turbo version 5.4.2 software was used for analysis. About 25 min were required to complete GC. On comparing the component's spectrum with the known spectrum database saved in the NIST (National Institute of Standards and Technology) library, the constituent's structures were determined. Area peak percentage (%) analysis was done to determine the measurable percentage of assessed biological compounds present in the fungal crude extract at the respective retention time. (Settu et al., 2023).
2.10. FTIR analysis of fungal extract
The dried fungal extracts were subjected to FTIR instrumentation study by IR Affinity model, Shimadzu FT-IR. At 4000–400/cm range, the chemical constituent’s functional groups were recorded. The chemical bonds of a molecule are determined using the infrared absorption spectrum. According to the annotated spectrum, chemical bonds present in the extract take up a certain light wavelength (Segaran et al., 2020).
2.11. Statistical assessment
Results are stated as a mean of triplicate determination ± standard deviation for experiments done in vitro. Statistically, the data were calculated by Version 9.5.1 GraphPad Prism software.
3. Results
3.1. Ethno-medicinal investigation of selected plant
In the current investigation, the ornamental plant Transcantia pallida was investigated for fungal endophytes and has tremendous importance in traditional medicine despite being one of the ornamental plants. Previous research has shown that plant extracts have anti-inflammatory, antimicrobial and antioxidant effects. As a result, the major purpose of this research was to find an effective endophytic fungus with antibacterial action.
3.2. Isolation of endophytic fungi from Transcantia pallida
In the present study, fourteen (n = 14) morphologically distinct fungi were isolated from 14 mature leaves obtained from the same plant. Among the four different growth media used, PDA media was found to be excellent for the development and yield of fungus.
3.3. Identification of potent endophytic fungi
Based on the bioactivity, the most potent endophytic fungi TPL11 and TPL14 isolated from the surface-sterilized leaves were identified by the morphological appearance on solid medium and microstructure variation under SEM using CLSI guidelines (Fig. 1). The TPL11 isolate was white, velvety, raised in the center and brownish yellow on reverse whereas TPL14 isolate was white and cottony. Further confirmed by molecular technique and identified successfully as Fusarium oxysporum and Nigrospora sphaerica by 18S rRNA ITS gene sequencing and was confirmed through the neighbor-joining tree (Fig. 2). Further deposited in GenBank accession numbers (ON076556) and (ON248210).
Fig. 1.
Characterization and identification of endophytic fungus from T. pallida: (a) Morphology of TPL11 on PDA medium, (b) LCB staining at 100x magnification, (c) SEM Spore morphology at 3.00KX of TPL11 isolate; (a’) Morphology of TPL14 on PDA medium, (b’) LCB staining at 100x magnification, (c’) SEM Spore morphology at 1.50KX of TPL14 isolate.
Fig. 2.
Phylogenetic tree derived from the neighbor-joining analysis of endophytic fungus: (a) TPL11 identified as Fusarium oxysporum; (b) TPL14 identified as Nigrospora sphaerica isolated from the leaf region of T. pallida.
3.4. Identification of optimal growth media and antibacterial potential of F.oxysporum and N.sphaerica
The antibacterial potential of the most active endophytic fungal extracts obtained from strain F.oxysporum (TPL11) and N.sphaerica (TPL14) was examined using different growth media in liquid fermentation. The results obtained revealed that only the PDB medium displayed a varied range of growth inhibition against the tested pathogens at 100 µg/mL concentration as verified by a recorded zone of inhibition (Table 1). The delicate activity was witnessed in PDB medium followed by SDB and MEA.
Table 1.
Antibacterial significance inhibition of microbes with ethyl acetate extract obtained from F.oxysporum and N.sphaerica isolated from T.pallida cultivated in different growth media.
| Culture growth medium | Concentration 100 (µg/mL); ZOI (mm) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
MRSA (ATCC 43300) |
MRSA (ATCC700699) |
S.aureus (ATCC 25923) |
S.aureus (MTCC 3160) |
VRE (ATCC 51299) |
E.fecalis |
|||||||
| TPL11 | TPL14 | TPL11 | TPL14 | TPL11 | TPL14 | TPL11 | TPL14 | TPL11 | TPL14 | TPL11 | TPL14 | |
| Potato Dextrose Broth | 22 | 22 | 21 | 3 | 22 | 20 | 15 | 18.5 | 24 | 24 | 20 | 19 |
| Czapek Dox Broth | 16.2 | – | 14 | – | 19 | 15 | 12.6 | 14 | – | – | 18 | 16 |
| Sabouraud Dextrose Broth | 18 | 17 | 15 | 13 | 17.5 | 15.7 | 18 | 13.6 | 19 | 18 | 21.5 | 19 |
| Corn Meal Agar | – | 12 | – | 14 | 15.2 | 17 | 15 | 12.5 | 18 | – | 14 | – |
| Malt extract agar | 15 | 12 | 15 | 17 | 18 | 14 | 21 | 12 | 19 | 18 | 15.8 | 13 |
3.5. Inhibitory effect of endophytic fungi on resistant pathogen
3.5.1. Screening for potential isolates
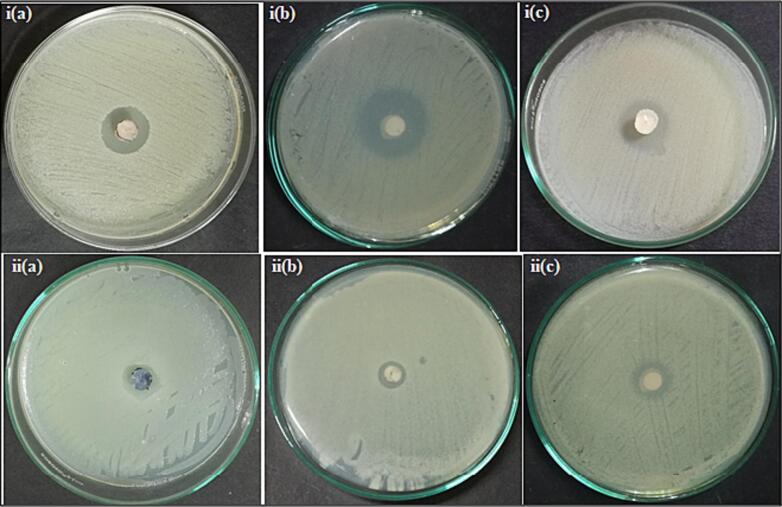
The results obtained revealed that the tested isolates showed a varying range of antibacterial effects against the tested bacterial pathogens. Among the 14 fungal isolates, TPL11 and TPL14 displayed significant antagonistic activity against the test pathogens (Fig. 3) by the agar plug diffusion method (primary screening) followed, it was confirmed with the disc diffusion technique (secondary screening) with reference to CLSI (2021) recommendations. These findings revealed that F.oxysporum and N.sphaerica isolated from those associated with T.pallida have a range of notable disease-suppressing properties.
Fig. 3.
Inhibition of resistant pathogens by endophytic fungus F.oxysporum against i(a) ATCC 43300, i(b) ATCC 700699, i(c) ATCC 51299 and endophytic fungus N.sphaerica against ii(a) ATCC 43300, ii(b) ATCC 700699, ii(c) ATCC 51299 strain by Agar plug diffusion method.
3.5.2. Antibiotic sensitivity assay
The studied endophytic fungal samples confirmed varying levels of inhibition against the tested pathogen Table 2, Fig. 4. The results display clearly that the tested strain was susceptible with a substantial variance (P < 0.05) in mean inhibitory zone diameter on different solvent crude extracts. F.oxysporum ethyl acetate extract exhibited maximum activity at a concentration of 100 (µg/mL) against MRSA-ATCC 43,300 and VRE with ZOI of 22 ± 0.05 and 24 ± 0.05 mm respectively. Against S.auerus ATCC 25923 the ZOI was 19 ± 0.15 mm. Ethyl acetate extract of N.sphaerica showed significant activity against MRSA-ATCC 43,300 and ATCC 700699 with inhibition zones of 22 ± 0.05 and 23 ± 0.11 mm. Whereas it showed ZOI of 24 ± 0.11 and 19 ± 0.2 mm against VRE and E.fecalis which is also maximum in activity to positive control Oxacillin (7 ± 0.05 mm). DCM and Hexane exhibited minimum activity whereas butanol crude extract of both fungi exhibited an inhibition zone, similar to that of Oxacillin (7 mm). No zone was observed in negative control. The positive control had no zone of inhibition around the disc showing resistance against MRSA (ATCC 700699) and VRE. This indicates that the ethyl acetate extract of both the fungus was more efficiently suppressing the growth of resistant pathogens with varying potency than the commercially available Oxacillin antibiotic. Further, the negative control (DMSO) has not shown any ZOI, indicating the solvent used to dissolve the extracts does not affect the test pathogens.
Table 2.
Anti- Multiple Drug Resistant (MDR) bacterial activity of ethyl acetate crude extracts.
| Extracts | Endophytic Fungi |
Concentrations (µg/mL) |
MRSA (ATCC 43300) | MRSA (ATCC 700699) | S.aureus (ATCC 25923) | S.aureus (MTCC 3160) | VRE (ATCC 51299) |
E.fecalis |
|---|---|---|---|---|---|---|---|---|
| Ethyl acetate | F.oxysporum | 25 | 6 ± 0.15 | – | 17 ± 0.1 | 8 ± 0.4 | – | 11 ± 0.5 |
| 50 | 13 ± 0.12 | 15 ± 0.1 | 18.5 ± 0.2 | 10 ± 0.25 | 17 ± 0.12 | 13 ± 0.1 | ||
| 75 | 16 ± 0.15 | 19 ± 0.05 | 20 ± 0.1 | 12 ± 0.2 | 21 ± 0.1 | 17 ± 0.1 | ||
| 100 | 22 ± 0.05 | 21 ± 0.15 | 22 ± 0.06 | 15 ± 0.5 | 24 ± 0.05 | 20 ± 0.05 | ||
| N.sphaerica | 25 | 14 ± 0.11 | – | 15 ± 0.5 | 12 ± 0.1 | – | – | |
| 50 | 18 ± 0.05 | 19 ± 0.05 | 17 ± 0.1 | 14 ± 0.25 | 18 ± 0.05 | 14.5 ± 0.5 | ||
| 75 | 20 ± 0.1 | 20 ± 0.05 | 18.2 ± 0.15 | 17 ± 0.4 | 21 ± 0.11 | 17 ± 0.2 | ||
| 100 | 22 ± 0.05 | 23 ± 0.11 | 20 ± 0.06 | 18.5 ± 0.5 | 24 ± 0.11 | 19 ± 0.2 | ||
| Dichloromethane | F.oxysporum | 25 | – | – | 14 ± 0.2 | 7 ± 0.5 | – | 7 ± 0.5 |
| 50 | 10 ± 0.2 | – | 17 ± 0.2 | 10 ± 0.25 | – | 12 ± 0.1 | ||
| 75 | 11.5 ± 0.5 | 17 ± 0.05 | 19 ± 0.15 | 12.5 ± 0.5 | 15 ± 0.05 | 18 ± 0.1 | ||
| 100 | 13 ± 0.01 | 19 ± 0.02 | 21 ± 0.07 | 19 ± 0.1 | 18 ± 0.2 | 20 ± 0.05 | ||
| N.sphaerica | 25 | – | – | 12 ± 0.2 | 7 ± 0.5 | – | 10 ± 0.2 | |
| 50 | 14 ± 0.03 | – | 15 ± 0.2 | 10 ± 0.25 | 17 ± 0.05 | 12.5 ± 0.5 | ||
| 75 | 16 ± 0.01 | 14.6 ± 0.05 | 19 ± 0.15 | 12.5 ± 0.5 | 18 ± 0.1 | 16 ± 0.2 | ||
| 100 | 18.5 ± 0.02 | 17 ± 0.12 | 22 ± 0.07 | 15.9 ± 0.1 | 20 ± 0.1 | 17.2 ± 0.2 |
*Values are reported as Mean ± SD, n = 3 inhibition percentage; significant differences (p < 0.05) from the control; ‘-’ denotes no antibacterial activity.
Fig. 4.
Anti-Multiple Drug Resistant (MDR) bacterial activity of F.oxysporum (TPL11) against (A) MRSA ATCC 43300, (B) MRSA ATCC 700699 and (C) VRE ATCC 51299; N.sphaerica (TPL14) against (A’) MRSA ATCC 43300, (B’) MRSA ATCC 700699 and (C’) VRE ATCC 51299 by disc diffusion method at different concentrations (25, 50, 75, 100 µg/mL) after 16–18 hrs incubation.
3.5.3. Bacteriostatic and bactericidal effects of fungal extracts
The extracts attained on the PDB filtrate were exposed to a microdilution trial to decide the MIC and MBC. The ethyl acetate extract of N.sphaerica was more active against MRSA (ATCC 43300, 700699) with the MIC of 3.125 and 3.125 µg/mL compared to extract of F.oxysporum (6.25 and 3.125 µg/mL) followed by S.auerus and VRE. As shown in Table 3, the MBC values ranged from 6.25 µg/mL to 25 µg/mL. Although all extracts demonstrated antibacterial activity, results of the disc diffusion, MIC and MBC values suggest that the ethyl acetate extract provided the highest antagonistic activity against the tested strains followed by DCM, hexane and butanol. The least potent was butanol extract. All the extracts were bactericidal for all the tested resistant strains. The MIC verified for individual antibiotics was also shown in Table 4.
Table 3.
Minimum Bacteriostatic and Bactericidal Concentration of ethyl acetate extract.
| Bacterial Pathogens | Concentration (µg/mL) |
|||||
|---|---|---|---|---|---|---|
|
MIC |
MBC |
MIC Index |
||||
| F.oxysporum | N.sphaerica | F.oxysporum | N.sphaerica | F.oxysporum | N.sphaerica | |
| MRSA (ATCC 43300) |
6.25 | 3.125 | 25 | 12.5 | 4 | 4 |
| MRSA (ATCC 700699) |
3.125 | 3.125 | 12.5 | 12.5 | 4 | 4 |
| S.aureus (ATCC 25923) | 6.25 | 0.78 | 25 | 6.25 | 4 | 8 |
| S.aureus (MTCC 3160) | 3.125 | 6.25 | 12.5 | 12.5 | 4 | 2 |
| VRE (ATCC 56299) | 1.56 | 3.125 | 12.5 | 25 | 8 | 8 |
| E.faecalis | 3.125 | 3.125 | 25 | 25 | 8 | 8 |
MIC index ≤ 4 is bactericidal and > 4 is bacteriostatic.
Table 4.
Synergistic antibacterial effect of ethyl acetate fungal crude extract of F.oxysporum and N.sphaerica with conventional antibiotics against human pathogens.
| Bacterial strains | Antibiotics | MIC (μg/mL) |
FICI |
Interpretation |
|||
|---|---|---|---|---|---|---|---|
|
Extract |
Antibiotic |
||||||
| Alone | Combination | Alone | Combination | ||||
| F.oxysporum | |||||||
| MRSA (ATCC 43300) | Oxacillin | 6.25 | 3.12 | 50 | 3.12 | 0.1 | Synergistic |
| Tetracycline | 3.12 | 25 | 6.25 | 0.7 | Indifference | ||
| Chloramphenicol | 0.78 | 25 | 12.5 | 0.6 | Indifference | ||
| Vancomycin | 0.19 | 6.25 | 0.78 | 0.1 | Synergistic | ||
| MRSA (ATCC 700699) | Oxacillin | 3.12 | 1.56 | 50 | 3.12 | 0.5 | Synergistic |
| Tetracycline | 1.56 | 25 | 0.78 | 0.5 | Synergistic | ||
| Chloramphenicol | 1.56 | 25 | 1.56 | 0.5 | Synergistic | ||
| Vancomycin | 0.39 | 6.25 | 1.56 | 0.3 | Synergistic | ||
| VRE (ATCC 51299) |
Oxacillin | 1.56 | 0.78 | 25 | 0.39 | 0.5 | Synergistic |
| Tetracycline | 0.78 | 25 | 3.12 | 0.6 | Indifference | ||
| Chloramphenicol | 0.78 | 50 | 0.78 | 0.5 | Synergistic | ||
| Vancomycin | 0.19 | 50 | 1.56 | 0.1 | Synergistic | ||
| N.sphaerica | |||||||
| MRSA (ATCC 43300) | Oxacillin | 3.12 | 1.56 | 50 | 3.12 | 0.5 | Synergistic |
| Tetracycline | 1.56 | 25 | 6.25 | 0.7 | Indifference | ||
| Chloramphenicol | 1.56 | 25 | 12.5 | 1 | Indifference | ||
| Vancomycin | 0.39 | 6.25 | 0.78 | 0.2 | Synergistic | ||
| MRSA (ATCC 700699) | Oxacillin | 3.12 | 0.78 | 50 | 3.12 | 0.3 | Synergistic |
| Tetracycline | 1.56 | 25 | 0.78 | 0.5 | Synergistic | ||
| Chloramphenicol | 1.56 | 25 | 1.56 | 0.5 | Synergistic | ||
| Vancomycin | 0.78 | 6.25 | 1.56 | 0.4 | Synergistic | ||
| VRE (ATCC 51299) |
Oxacillin | 3.12 | 0.78 | 25 | 3.12 | 0.3 | Synergistic |
| Tetracycline | 1.56 | 50 | 0.78 | 0.5 | Synergistic | ||
| Chloramphenicol | 1.56 | 50 | 1.56 | 0.5 | Synergistic | ||
| Vancomycin | 0.78 | 12.5 | 0.39 | 0.2 | Synergistic | ||
FICI ≤ 0.5: synergy, FICI > 0.5 to 4: indifference, FICI > 4: antagonism.
3.5.4. Checkerboard assays
The combination of ethyl acetate crude with a variety of classes of antibiotics was evaluated against the bacterial pathogens. The fractional inhibitory concentration index (FICI) exploration by the checkerboard assay exhibited values below 0.5 (FICI ≤ 0.5), which indicates a synergistic effect. As shown in Table 4, it was observed that against MRSA and VRE strains there is a significant decrease in drug MICs when the ethyl acetate crude extract is merged with the antibiotics reaching a 2–3 times fold reduction. For all combinations tested, ethyl acetate crude extract of F.oxysporum combined with Vancomycin declined the MIC value among all the tested combinations from 6.25 to 0.19 μg/mL and from 1.56 to 0.19 μg/mL against MRSA (ATCC 43300) and VRE respectively with FICI of 0.1 indicates synergisim. Whereas, N.sphaerica ethyl acetate was highly active when it was combined with Vancomycin resulting in MIC from 3.12 to 0.39 μg/mL against MRSA (ATCC 43300) with FICI of 0.2 indicating synergistic.
3.6. Mycochemical screening
Phytochemical analysis of ethyl acetate extracts exposed the existence of phenols, alkaloids, tannin, steroids and terpenoids. Entirely these classes of composites have been stated as antimicrobial antibacterial activity of endophytic fungus.
3.7. Fungal extract analysis by GC–MS
The potent endophytic fungi TPL11 and TPL14 ethyl acetate crude were exposed to GC–MS to identify volatile compounds, alcohols, long-chain hydrocarbons, ketones, etc. Chemical complexes were recognized based on molecular mass, structures and formula and retention time by comparing mass spectra with the MS spectral database (NIST library). The peak range represented a quantifiable proportion of predicted chemicals in the extract. The chromatogram and area percentage analysis obtained are shown in Fig. 5, Fig. 6. The major compound present in TPL11 extract were heptacosanoic acid, methyl ester (RT 18.99, 2.9 %), pterin-6-Carboxylic acid (RT 19.34, 3.9 %), paredrine tms (RT 28.03, 5.4 %). Where, 1,2-benzenedicarboxylic acid, butyl 2-ethylhexyle (RT 19.41, 12.4 %), 8-heptadecene (RT 19.76, 14.5 %), 1,2-benzenedicarboxylic acid, butyl octyl ester (RT 20.34, 2.2 %), 1-docosene (RT 21.64, 6.7 %) were the major compound present in the TPL14 crude extracts These compounds were known to exhibit a major role in the anti-pathogenic activity. Area peak percentage (%) analysis represents a measurable % of estimated biological compounds ie., it determines an approximate concentration of an analyte present in the fungal crude extract at the respective retention time (RT).
Fig. 5.
Gas Chromatography-Mass Spectroscopy ethyl acetate crude extract (i) Chromatogram profile of endophytic fungus F.oxysporum TPL11 and (ii) Chromatogram profile of endophytic fungus N.sphaerica TPL14.
Fig. 6.
Area percentage analysis (i) chemical constituents present in ethyl acetate extract of F.oxysporum TPL11 and (ii) chemical constituents present in ethyl acetate extract of N.sphaerica TPL14.
3.8. Spectrum analysis
F.oxysporum FTIR revealed different peaks corresponding to various functional groups. A peak at 3334.68 cm−1 specifies the existence of an alcohol (O–H stretch). The peak at 2972.79 and 2930.62 cm−1 exposed the existence of the alkanes group (C–H stretch), the peak at 1751.54 cm−1 relates to the carboxylic acid group (C = O stretch). The peaks 1598.14 and 1450.10 cm−1 were alkane (C–H stretch). Peak 1055.62 and 1008.60 cm−1 was the existence of esters (C-O stretch). Peak 887.12 and 815.10 cm−1 correspond to alkene stretch (C–H). A peak at 521.16 cm−1 was due to the halide (C–H stretch) presence. Whereas, N. sphaerica revealed peaks at 3295.81 (alcohol), 2957.31 (alkanes), 2917.32 (alkanes), 2856.01 (alkanes), 1590.01 cm−1 (alkane), 1459.99 cm−1 (alkane), 1377.30 cm−1 (alkane), 1316.68 cm−1 (alkane), 1267.26 cm−1 (alkane), 1074.54 (esters), 1032.70 cm−1 (esters), 590.25 cm−1 (halide).
4. Discussion
Endophytes are now recognized to be capable of manufacturing chemicals, antibiotics, and a variety of other biotechnologically interesting compounds, in addition to supporting plant protection. Endophytic microbe’s ability to create a variety of secondary metabolites makes them an intriguing source in the hunt for novel antimicrobial agents, and their use in the food and cosmetics industries can contribute to a variety of biotechnological applications (Silva et al., 2022). Endophytic fungi have been identified as a source of new secondary metabolites with biologically beneficial attributes. Numerous investigations have been undertaken to assess the antibacterial potential of fungal extracts (Avinash et al., 2015).
Previously a study reported that phomalactone produced from N. sphaerica of Adiantum philippense showed an efficient inhibitory impact against E. coli and Xanthomonas campestris in a disc diffusion investigation, with a considerable MIC at 150 g/ml concentration (Ramesha et al., 2020). After 12 h of treatment with ethyl acetate extract, N. sphaerica cultivated from old leaves of Swietenia macrophylla showed a spectacular growth-killing profile on MRSA, resulting in cell membrane rupture and cell death (Ibrahim et al., 2015). A novel chemical, nigronapthaphenyl, was discovered by bioactive N. sphaerica ethyl acetate extract of Bruguiera gymnorrhyza plant and showed significant antagonistic effects against B.cereus TISTR 688 with MIC of 2 μg/mL and B. subtilis TISTR 088 with MIC of 4 μg/mL. It also showed anticancer activity against the HCT 116 colon cancer cell line, with an IC50 of 9.62 ± 0.5 μM. The chemical was also anti-inflammatory and anti-diabetic (Ukwatta et al., 2019). Lactones, pyrones, nigrosporolides, diterpenes, nigrosporins, nigrosporolides, diketopiperazines, and epoxydons have all been new antimicrobial secondary substances reported to be harvested by N. sphaerica (Chutulo et al., 2020).
Similarly, a study reported that F.oxysporum from Psidium guava leaves inhibited the tested bacteria in an extensive spectrum of zones ranging from 6.67 to 0.58 mm to 22–1 mm, with MIC values ranging from 0.156 to 5.0 mg/ml and MBC values ranging from 0.625 to 10.0 mg/ml (Chutulo et al., 2020). With ZOI ranging from 17 to 9 mm, the culture supernatant of F.oxysporum 01 from Catharanthus roseus displayed a considerable antibacterial impact on S. aureus, V.parahaemolyticus, P.aeruginosa, E.coli, and Serratia marcescens. It also showed antioxidant and anticancer properties in Hep-G2 and MCF-7 cells. (Tu et al., 2021). The chemicals obtained from an ethyl acetate extract of Fusarium sp. (internal strain 3042) were colletochlorin B, 4,5-dihydrodechloroascochlorin, llicicolin B, colletorin B, ascochlorin, and 4,5-dihydroascochlorin. Against Chlorella fusca, F.oxysporum, Ustilago violacea, the compound colletochlorin, llicicolin B and Colletorin B demonstrated antimicrobial action. Similarly, 4,5-dihydroascochlorin showed an antibacterial on Bacillus megaterium growth and 4,5-dihydrodechloroascochlorin showed very strong antifungal activity towards Eurotium repens growth (Hussain et al., 2015). The strain BH-3 derived from Lilium lancifolium had an inhibitor impact against Leuc. Mesenteroides (Liu et al., 2012). Ethyl acetate extract of F. oxyporum was isolated from the bark of Cinnamomum kanehirae, resulting in the identification of two novel compounds, a new oxysporidinone analog and a new 3-hydroxyl-2-piperidinone derivative and known compounds, fusarinolic acid, gibepyrone D, fusaruside, beauvericin, (−)-4,6′-anhydrooxysporidinone, cerevisterol, (2S,2′R,3R,3′E,4E,8E)-1-O-D-glucopyranosyl-2-N-(2′-hydroxy-3′-octadecenoyl)-3-hydroxy-9-methyl-4,8-sphingadienine. Beauvericin displayed anticancer impact on human cell line A549, PANC-1 and C-3 by MTT method. Further, it demonstrated a substantial antibacterial effect on MRSA with MIC of 3.125 μg/mL and B. subtilis (ATCC 66333) (Wang et al., 2011).
Inhibitors were found for the first time in crude extracts of endophytic fungus obtained from T. pallida, establishing the framework for future investigation. More studies into the bioactive chemicals of the extracts should be conducted to gain a better understanding of the potential of natural inhibitors. According to the study's findings, the compounds detected in the extracts can be used as antibacterial agents to treat a variety of microbial illnesses. However, more study is needed to analyze all of the costs and benefits of hiding fungal endophytes in a range of environmental settings to increase the use and proficiency of endophytes in pharmaceutics.
5. Conclusion
In conclusion, our findings revealed various endophytic fungi from different tissues of the T.pallida plant. This approach is the foremost report of F.oxysporum and N. sphaerica from the leaves of T.pallida and their antibacterial investigation. Agar plug diffusion, disc diffusion and MIC displayed a broad range of activity. Thereby it indicates both the fungal endophytes from the plant serve as potent producers of antibacterial secondary metabolites. It is also evident that they can serve as potential antibacterial mediators against a wide range of clinically important bacterial pathogens. More studies into the bioactive chemicals of the extracts should be conducted to gain a superior understanding of the potential activities of natural inhibitors. The chemicals found in the extracts, according to the study's findings, can be employed in therapeutic remedies to protect plants and eukaryotic models. Additional experiments on the isolation of pure compounds from the ethyl acetate crude extract may generate new novel natural bioactive compounds.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank the Vellore Institute of Technology for providing the lab facility to carry out this study.
Contributor Information
Ranjitha Dhevi V. Sundar, Email: ranjithadhevi.vs2018@vitstudent.ac.in.
Sathiavelu Arunachalam, Email: asathiavelu@vit.ac.in.
References
- Avinash K.S., Ashwini H.S., Babu H.N.R., Krishnamurthy Y.L. Antimicrobial Potential of Crude Extract of Curvularia lunata, an Endophytic Fungi Isolated from Cymbopogon caesius. J. Mycol. 2015;1–4 doi: 10.1155/2015/185821. [DOI] [Google Scholar]
- Bokhari S.S., Yasmeen R., Manzoor R., Rafi U., Qureshi A.W. Antibacterial effect of Tradescantia pallida purpurea against fish (Labeo rohita) pathogens. Pak J Med Sci. 2020;2(1) doi: 10.52229/pbmj.v1i2.44. [DOI] [Google Scholar]
- Cabral, F.V., Fernandes, C.C., Dias, A.L.B.D., A.B., Squarisi, I.S., Tavares, D.C., Crotti, A.E.M., Moreira, F.F., Miranda, M.L.D., 2022. Hexane Extract from Tradescantia pallida (Rose) D.R. Hunt (Commelinaceae): Its Volatile Constituents and in vitro Antifungal and Cytotoxic Activities. Braz. Arch. Biol. Technol. 65, e22210621. https://doi.org/10.1590/1678-4324-2022210621.
- Chutulo E.C., Chalannavar R.K. Antimicrobial activity of Fusarium oxysporum, endophytic fungus, isolated from Psidium guajava L. (White fruit) Int J Pharm Sci Res. 2020;11(11):5844–5855. doi: 10.13040/IJPSR.0975-8232. [DOI] [Google Scholar]
- Deshmukh S.K., Verekar S.A., Bhave S.V. Endophytic fungi: a reservoir of antibacterials. Front. Microbiol. 2015;5:1–43. doi: 10.3389/fmicb.2014.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhayanithy G., Subban K., Chelliah J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019;19:22. doi: 10.1186/s12866-019-1386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain H., Drogies K., Al-Harrasi A., Hassan Z., Shah A., Rana U.A., Green I.R., Draeger S., Schulz B., Krohn K. Antimicrobial constituents from endophytic fungus Fusarium sp. Asian Pac J Trop Dis. 2015;5(3):186–189. doi: 10.1016/S2222-1808(14)60650-2. [DOI] [Google Scholar]
- Ibrahim D., Lee C.C., Yenn T.W., Zakaria L., Lim Sheh-Hong L. Effect of the Extract of Endophytic fungus, Nigrospora sphaerica CL-OP 30, Against the Growth of Methicillin-Resistant Staphylococcus aureus (MRSA) and Klebsiella pneumonia cells. Trop. J. Pharm. Res. 2015;14(11):2091–2097. doi: 10.4314/tjpr.v14i11.20. [DOI] [Google Scholar]
- Ibrahim M., Oyebanji E., Fowora M., Aiyeolemi A., Orabuchi C., Akinnawo B., Adekunle A.A. Extracts of endophytic fungi from leaves of selected Nigerian ethnomedicinal plants exhibited antioxidant activity. BMC Complement Med Ther. 2021;21:1–13. doi: 10.1186/s12906-021-03269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes, D., Kreft, S., Jurc, M., Seme, K., S ˇ trukelj, B., 2007. Antibacterial Activity in Higher Fungi (Mushrooms) and Endophytic Fungi from Slovenia. Pharm. Biol. 45 (9), 700–706. DOI: 10.1080/13880200701575189.
- Kamiya M., Mori T., Nomura M., Inagaki T., Nonogaki T., Nagatsu A., Yamagishi Y., Mikamo H., Ikeda Y. Tradescantia pallida extract inhibits biofilm formation in Pseudomonas aeruginosa. J. Med. Sci. 2019;81:439–452. doi: 10.18999/nagjms.81.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoch M., Phull S., Vaid S., Singh S. Diversity, Phylogeny, anticancer and antimicrobial potential of fungal endophytes associated with Monarda citriodora L. BMC Microbiol. 2017;17:44. doi: 10.1186/s12866-017-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.L., Huang K.H., Zhou J.Z., Meng L., Wang Y., Zhang L.X. Identification and antibacterial characteristics of an endophytic fungus Fusarium oxysporum from Lilium lancifolium. Lett. Appl. Microbiol. 2012;399–406 doi: 10.1111/j.1472-765X.2012.03306.x. [DOI] [PubMed] [Google Scholar]
- Manganyi M.C., Regnier T., Kumar A., Bezuidenhout C.C., Ateba C.N. Biodiversity and antibacterial screening of endophytic fungi isolated from Pelargonium sidoides. S. Afr. J. Bot. 2018;116:192–199. doi: 10.1016/j.sajb.2018.03.016. [DOI] [Google Scholar]
- Mousa A.A.A., Mohamed H., Hassane A.M.A., Abo-Dahab N.F. Antimicrobial and cytotoxic potential of an endophytic fungus Alternaria tenuissima AUMC14342 isolated from Artemisia judaica L. growing in Saudi Arabia. J King Saud Univ Sci. 2021;33 doi: 10.1016/j.jksus.2021.101462. [DOI] [Google Scholar]
- Omokhua A.G., Ondua M., van Staden J., McGaw L.J. Synergistic activity of extracts of three South African alien invasive weeds combined with conventional antibiotics against selected opportunistic pathogens. S. Afr. J. Bot. 2019;124:251–257. doi: 10.1016/j.sajb.2019.05.023. [DOI] [Google Scholar]
- Ragragio E.M., Zayas C.N., Obico J.J.A. Useful plants of selected Ayta communities from Porac, Pampanga, Twenty years after the eruption of Mt. Pinatubo. Philipp J. Sci. 2013;142:169–181. [Google Scholar]
- Ramesha K.P., Mohana N.C., Nuthan B.R., Rakshith D., Satish S. Antimicrobial metabolite profiling of Nigrospora sphaerica from Adiantum philippense L. J. Genet. Eng. Biotechnol. 2020;18:66. doi: 10.1186/s43141-020-00080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaweera P.B., Walgama R.C., Jayasundera K.U., Herath S.D., Abira S., Williams D.E., Andersen R.J., de Silva E.D. Antibacterial activities of endophytic fungi isolated from six Sri Lankan plants of the family Cyperaceae. Bangladesh J. Pharmacol. 2018;13:264–272. doi: 10.3329/bjp.v13i3.36716. [DOI] [Google Scholar]
- Rocha, A.d.N., Souza, S.A.d., Fioratti, C.A.G., Mauad, J.R.C., Mauad, M., Mussury, R.M., 2022. Tradescantia pallida (Commelinaceae) Promotes Reductions in Plutella xylostella (Lepidoptera: Plutellidae) Populations. Agronomy, 12, 1-10. https://doi.org/10.3390/agronomy12112646.
- Sadrati N., Zerroug A., Demirel R., Harzallah D. Antimicrobial activity of secondary metabolites produced by Aspergillus neobridgeri isolated from Pistacia lentiscus against multi-drug resistant bacteria. Bangladesh J Pharmacol. 2020;2020(15):82–95. doi: 10.3329/bjp.v15i3.40923. [DOI] [Google Scholar]
- Sadrati N., Zerroug A., Demirel R., Harzallah D. Anti-multidrug-resistant Staphylococcus aureus and anti-dermatophyte activities of secondary metabolites of the endophytic fungus Penicillium brevicompactum ANT13 associated with the Algerian endemic plant Abies numidica. Arch. Microbiol. 2023;205:110. doi: 10.1007/s00203-023-03452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos I.P.D., Silva L.C.N., Silva M.V.D., Araújo J.M.D., Cavalcanti M.D.S., Lima V.L.D.M. Antibacterial activity of endophytic fungi from leaves of Indigofera suffruticosa Miller (Fabaceae) Front. Microbiol. 2015;6:350. doi: 10.3389/fmicb.2015.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra H.K., Banerjee D. Bioactivity study and metabolic profiling of Colletotrichum alatae LCS1, an endophyte of club moss Lycopodium clavatum L. PLoS ONE. 2022;17(4):e0267302. doi: 10.1371/journal.pone.0267302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segaran G., Anitha K.P.G.U., Sathiavelu M. Evaluation of antioxidant, total phenol and phytoconstituents of Culvularia sp., a fungal endophyte of Boerhaavia diffusa L. Res. J. Biotechnol. 2020;15(6):117–122. [Google Scholar]
- Settu S., Arunachalam S. Anti-diabetic effect of Daldinia eschscholtzii and Phomopsis mangiferae isolated from Bryophyllum pinnatum. Bangladesh J Pharmacol. 2023;18:58–71. doi: 10.3329/bjp.v18i2.65836. [DOI] [Google Scholar]
- Shahzadi I., Aziz Shah S.M., Shah M.M., Ismail T., Fatima N., Siddique M., Waheed U., Baig A., Ayaz A. Antioxidant, Cytotoxic, and Antimicrobial Potential of Silver Nanoparticles Synthesized using Tradescantia pallida Extract. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.907551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Pramanik A., Agrawal P.K. Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglecta BAB-5510 isolated from leaves of Cupressus torulosa D. Don. 2016;3 Biotech. 6:210. doi: 10.1007/s13205-016-0518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D.P.D., Cardoso M.S., Macedo A.J. Endophytic Fungi as a Source of Antibacterial Compounds—A Focus on Gram-Negative Bacteria. Antibiotics. 2022;11:1509. doi: 10.3390/antibiotics11111509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva F.D.A., Liotti R.G., Boleti A.P.D.A., Reis E.D.M., Passos M.B.S., Santos E.L.D.S., Sampaio O.M., Januário A.H., Branco C.L.B., Silva G.F.D., Mendonca E.A.F.D., Soares M.A. Diversity of cultivable fungal endophytes in Paullinia cupana (Mart.) Ducke and bioactivity of their secondary metabolites. PLoS ONE. 2018;13(4):e0195874. doi: 10.1371/journal.pone.0195874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supaphon P., Phongpaichit S., Rukachaisirikul V., Sakayaroj J. Antimicrobial Potential of Endophytic Fungi Derived from Three Seagrass Species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS ONE. 2013;8(8):e72520. doi: 10.1371/journal.pone.0072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama F., Guimarães E.T., Lobo D.-J.-A., Rodrigues G.S., Domingos M., Alves E.S., Carvalho H.A., Saldiva P.H.N. Tradescantia bioassay for X-ray mutagenesis Pollen mother cells of Tradescantia clone 4430 and Tradescantia pallida var. purpurea are equally sensitive to the clastogenic effects of X-rays. Braz. J. Med. Biol. Res. 2002;35:127–129. doi: 10.1590/s0100-879x2002000100018. [DOI] [PubMed] [Google Scholar]
- Tan J.B.L., Yap W.J., Tan S.Y., Lim Y.Y., Lee S.M. Antioxidant Content, Antioxidant Activity, and Antibacterial Activity of Five Plants from the Commelinaceae Family. Antioxid. 2014;3:758–769. doi: 10.3390/antiox3040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu N.H.K., Thuy N.T.T., Vinh D.T.T., Anh N.P.Q., Ly H.D. Detection of Antimicrobial, Antioxidant and Cytotoxicity Activities of Fusarium oxysporum F01 Isolated from Catharanthus roseus Collected in Vietnam. J. Pure Appl. Microbiol. 2021;15(3):1643–1654. doi: 10.22207/JPAM.15.3.61. [DOI] [Google Scholar]
- Ukwatta K.M., Lawrence J.L., Wijayarathna C.D. The study of antimicrobial, anti-cancer, antiinflammatory and α-glucosidase inhibitory activities of Nigronapthaphenyl, isolated from an extract of Nigrospora sphaerica. Mycol. 2019;10(4):222–228. doi: 10.1080/21501203.2019.1620892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle D.L., Jr., Cabrera E.C., Puzon J.J.M., Rivera W.L. Antimicrobial Activities of Methanol, Ethanol and Supercritical CO2 Extracts of Philippine Piper betle L. on Clinical Isolates of Gram Positive and Gram Negative Bacteria with Transferable Multiple Drug Resistance. PLoS ONE. 2016;11(1):e0146349. doi: 10.1371/journal.pone.0146349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Li S., Zhao F., Dai H., Bao L., Ding R., Gao H., Zhang L., Wen H., Liu H. Chemical constituents from endophytic fungus Fusarium oxysporum. Fitoterapia. 2011;82:777–781. doi: 10.1016/j.fitote.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Yadav G., Meena M. Bioprospecting of endophytes in medicinal plants of Thar Desert: An attractive resource for biopharmaceuticals. Biotechnol. Rep. 2021;30:e00629. doi: 10.1016/j.btre.2021.e00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- Potshangbam M., Devi S.I., Sahoo D., Strobel G.A. Functional Characterization of Endophytic Fungal Community Associated with Oryza sativa L. and Zea mays L. Front. Microbiol. 2017;8(325) doi: 10.3389/fmicb.2017.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva-Toledo J.R., Chale-Dzul J., Castillo-Bautista C., Olivera-Castillo L., Rangel-Méndez J.A., Graniel-Sabido M.J., Moo-Puc R.E. Hepatoprotective effect of an ethanol extract of Tradescantia pallida against CCl4-induced liver damage in rats. S. Afr. J. Bot. 2020;135:444–450. doi: 10.1016/j.sajb.2020.09.031. [DOI] [Google Scholar]