Abstract
Background
Nutritional anemia is a significant public health concern worldwide, particularly affecting young adults and children in Saudi Arabia, where inadequate nutrition is considered a primary contributing factor. This study aims to (i) examine the levels of serum iron, folate, and vitamin B12 in young adult students, with a focus on identifying any deficiencies and their association with anemia; (ii) explore the prevalence of mixed-deficiency anemia resulting from deficiencies in serum iron, folate, and vitamin B12 (iii) explore how sociodemographic characteristics and dietary habits influence serum iron, folate, and vitamin B12 levels.
Materials and Methods
This cross-sectional study encompassed 158 young adult students at Jazan University, Saudi Arabia. Blood samples were collected following a comprehensive questionnaire addressing sociodemographic and health characteristics. These samples were analyzed for complete blood count, serum iron, folate, and vitamin B12 levels.
Results
The findings of this study revealed a significant decrease in serum iron levels, with 70.6% of males and 88% in females exhibiting reduced level. Additionally, low levels of folate were observed in 4% of the study population, while deficiency in vitamin B12 was found in 2.2% of the study population. However, the simultaneous presence of low serum iron levels along with deficiencies in folate or vitamin B12 was not observed in the study participants.
Conclusion
The study indicates that there is a high incidence of low serum iron and ferritin levels among university students in Saudi Arabia, which poses a considerable public health concern. Conversely, the prevalence of folate and vitamin B12 deficiencies among the students was comparatively low, and notably, there were no cases where these deficiencies were observed alongside iron deficiency.
Keywords: anemia, folate, vitamin B12, serum iron, iron deficiency
Introduction
Nutritional anemia is a prevalent health burden that affects individuals across all ages, with higher vulnerability observed among young children, women, and older adults.1,2 Anemia is characterized by a low number of healthy circulating red blood cells (RBCs) or insufficient amount of hemoglobin (Hb) to carry oxygen.2,3 Micronutrients, such as vitamins (A, B, C) and minerals (iron, zinc, iodine, calcium), play diverse and essential roles in the body, including proper growth, development, and blood formation.4 Inadequate intake of these micronutrients, particularly iron, folate, and vitamin B12, is considered the primary contributing factor to anemia.5 A recent study conducted in 2021 estimated that anemia influences approximately 1.92 billion individuals worldwide, with prevalence rate of 24.3%. Iron deficiency (ID) is the most common cause of nutritional anemia, followed by deficiencies in folate and vitamin B12.2,6,7 Developing nations experience the greatest impact, characterized by a prevalence exceeding 60% and estimated rates of 47.4%, 41.8%, and 30.2% among young children, pregnant women, and women of reproductive age, respectively.6,8
A nationwide study conducted in four regions of Saudi Arabia unveiled a significant prevalence of anemia, with rates ranging from 16.5% to 41.3% among children, 7.2% to 16.5% among adult males, and 10.8% to 23.5% among adult females.9 Remarkably, a recent study documented that 67% of young female students encountered either iron deficiency anemia (IDA) or ID without anemia.10 Moreover, a cohort study conducted on 1312 adults enrolled in a premarital screening program reported an incidence rate of 7.23% for acquired anemia in Jazan region.11 This observation further underscores the burden of nutritional anemia in the Jazan region.
Micronutrients, specifically vitamin B12 and folate, play essential roles in DNA synthesis, cell division, and the production of RBCs.12 Insufficient levels of these micronutrients can lead to impaired DNA synthesis, which in turn can contribute to the development of macrocytic anemia, a condition that is relatively less frequently reported in Saudi Arabia. A retrospective study conducted in Makkah hospitals investigated the occurrence of macrocytic anemia in relation to folate and vitamin B12 deficiencies among male and female patients aged over 15 years. The study found that deficiencies in folate and vitamin B12 were linked with a 2% incidence of macrocytic anemia in this population. Additionally, another study carried out in Asir Central Hospital in Abha City reported a 1.6% incidence of only folate deficiency.13 Moreover, there is a significant occurrence of subclinical inadequacy and deficiency in vitamin B12 within specific societies, with estimates suggesting rates as high as 60%.14–18 In Saudi Arabia, especially in Jazan region, data is remain limited regarding nutritional anemia. Hence, further studies are required to determine the prevalence of nutritional anemia caused by deficiencies in serum iron, folate, and vitamin B12. Furthermore, it is crucial for these investigations to explore additional variables including sociodemographic factors and dietary patterns to obtain a more comprehensive understanding of the situation. Consequently, the objectives of this study encompassed three aspects: (i) determining the prevalence of nutritional anemia resulting from deficiencies in serum iron, folate, and vitamin B12 among young male and female students, (ii) investigating the co-occurrence of low serum iron levels with deficiencies in folate and/or vitamin B12, and (iii) assessing the relationship between sociodemographic characteristics, dietary intakes, and circulating levels of iron, folate, and vitamin B12.
Materials and Methods
Study Design
A cross-sectional study was undertaken on a sample consisting of 158 young adult students (68 males and 90 females), ranging in age from 18 to 25 years, who were enrolled at the Faculty of Applied Medical Sciences, Jazan University. The participants included in the study exhibited no apparent signs of illness. The survey questionnaire was employed to collect sociodemographic information, including age, weight, height, marital status, and personal history of any chronic diseases. The selection of participants was randomized, with exclusion criteria applied for individuals with known chronic conditions and pregnant females.
Ethical Consideration
The present study underwent a thorough review and received approval from the Standing Scientific Research Ethics Committee of Jazan University, with the reference number REC-44/06/478. The study was conducted in strict adherence to the principles outlined in the Declaration of Helsinki.
Sample Size and Collection
The sample size was determined based on a previously established method.10 Venous blood samples were obtained from all participants using ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes for complete blood count (CBC) analysis, and plain tubes were used for serum iron, serum vitamin B12, and folate analyses.
Complete Blood Count
Sysmex XN–1000 (Japan) was used for complete blood count analysis. The CBC report included red blood cells (RBC, x1012/L), hemoglobin (Hb, g/dL), hematocrit (Hct, %), and RBC indices, including mean corpuscular volume (MCV, fL), mean corpuscular hemoglobin (MCH, pg), mean corpuscular hemoglobin concentration (MCHC, g/dL), red cell distribution width (RDW, %), white blood cell count (WBC, x109/L), differential leukocyte count, and platelet count (109/L).
Biochemical Analysis
The levels of iron, ferritin, folate, and vitamin B12, were determined by analyzing serum samples. The measurements of ferritin, folate, and Vitamin B12 were performed using a Maglumi 600 Chemiluminescence Immunoassay system (China), while iron levels were determined using a HumaStar 200 chemistry analyzer (Germany).
Reference Values
Anemia was defined as hemoglobin (Hb) <12.0 g/dL in females and <13.0 g/dL in males.19 MCV was used to classify anemia morphologically. MCV greater than 100 fL, along with low Hb levels, indicated macrocytic anemia, while MCV less than 80 fL, coupled with low Hblevels, indicated microcytic anemia. The local reference values for serum iron were 80–180 μg/dL in males and 60–160 μg/dL in females.10 Ferritin levels ranged from 24 to 336 μg/L in male and 11 to 307 μg/L in female.20 Folate levels ranged from 3.1 to 17.5 ng/mL and vitamin B12 levels ranged from 211 to 950 pg/mL.21 Values below the normal reference intervals were considered low (deficiency), while values above the upper limit were considered high.
Statistical Analysis
Data from the current study were analyzed using the GraphPad Prism software (version 8.0; San Diego, CA, USA). Unless otherwise stated, the results are presented as mean ± standard deviation (SD). CBC parameters, folate, vitamin B12, serum iron and ferritin values were analyzed using independent unpaired Student’s t-test for normally distributed and Mann–Whitney for non-normally distributed. The chi-square test was used for demographic data analysis and Pearson correlation test was used for correlation study. P-values less than 0.05 were considered statistically significant.
Results
Sociodemographic and Health Characteristics
The sociodemographic and health characteristics of the study participants (68 males and 90 females) are presented in Table 1. The participants exclusively consisted of Saudi students enrolled at the College of Applied Medical Sciences, Jazan University. Although the mean age of the male group was comparable to the female group (p > 0.05), the weight (kg), height (cm), and BMI were significantly higher in the male group as compared to the female group (p < 0.001) (Table 1). All male participants were single, with 75% being non-smokers, whereas 77.8% of the female participants were single, and 95.6% were non-smokers. The nutritional habits displayed some variations between the male and female participants (Table 1). There was a significant difference between male and female in nutritional habits (p < 0.05) expect for daily milk consumption and participants following a diet (p > 0.05) (Table 1).
Table 1.
Socio-Demographic, Health Characteristics and Nutritional Habits of the Study Participants (Male = 68 and Female = 90).
| Variables | Male (n = 68) | Female (n = 90) | P value | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age/year | 22.4±2.4 | 21.9±1.9 | 0.097 | |
| Weight/kg | 71.7±23.2 | 54.2±17.2 | <0.0001 | |
| Height/cm | 171.0±5.0 | 153.7±6.2 | <0.0001 | |
| BMI | 25.5±5.3 | 22.6±4.9 | 0.0005 | |
| Marital status | Single | 68 (100) | 70 (77.8) | <0.0001 |
| Married | 0 (0) | 20 (22.2) | ||
| Smoking | Yes | 17 (25) | 4 (4.4) | <0.0001 |
| No | 51 (75) | 86 (95.6) | ||
| Vitamin supplementation | Yes | 3 (4.4) | 19 (21.1) | 0.009 |
| No | 65 (95.6) | 71 (78.9) | ||
| Exercise (3 /week) | Yes | 28 (41.2) | 9 (10) | <0.0001 |
| No | 40 (58.8) | 81 (90) | ||
| Regular meals (3 meals/day) | Yes | 28 (41.2) | 21 (23) | <0.0001 |
| No | 40 (58.8) | 69 (77) | ||
| Fast food (5 time/week) | Yes | 57 (83.8) | 86 (95.6) | 0.046 |
| No | 11 (16.2) | 4 (4.4) | ||
| On diet | Yes | 3 (4.4) | 8 (8.9) | 0.292 |
| No | 65 (95.6) | 82 (91.1) | ||
| Consumption of red meat | Yes | 58 (85.3) | 28 (31.1) | <0.0001 |
| No | 10 (14.7) | 62 (68.9) | ||
| Daily drink milk | Yes | 48 (70.6) | 61 (67.8) | 0.536 |
| No | 20 (29.4) | 29 (32.2) | ||
| Fruit consumption | Yes | 34 (50) | 17 (18.9) | <0.0001 |
| No | 34 (50) | 73 (81.1) | ||
| Vegetable consumption | Yes | 39 (57.3) | 19 (21.1) | <0.0001 |
| No | 29 (42.7) | 71 (78.9) | ||
Comparative Analysis of Vitamin B12, Folate, and Serum Iron Levels in Males and Females
The study compared the serum levels of iron, folate, and vitamin B12 between males and females (Table 2). Serum iron and ferritin levels were significantly lower in females compared to males (p < 0.0001). However, the levels of folate were comparable between the male and female (p > 0.05), while vitamin B12 levels were significantly higher in females than in males (p < 0.01).
Table 2.
Comparison of Mean Levels of Folate, Vitamin B12 and Serum Iron Between Young Adult Males (n = 68) and Young Adult Females (n = 90).
| Parameters | Male (n = 68) | Female (n = 90) | P value |
|---|---|---|---|
| Folate (ng/mL) | 10.7±4.6 | 9.7±5.1 | 0.2051 |
| Vitamin B12 (pg/mL) | 555±347 | 692±243 | 0.0040 |
| Serum iron (µg/dL) | 65±29 | 40±34 | <0.0001 |
| Serum ferritin (µg/L) | 71.3±53 | 26±23 | <0.0001 |
Gender-Based Distribution of Folate Levels in the Study Population
Serum Iron and Ferritin Levels
Among males, 70.6% and 8.8% exhibited significantly low levels of serum iron and ferritin, respectively. In the female population, 88% and 21% had significantly low levels of serum iron and ferritin, respectively (Table 3 and Figure 1).
Table 3.
Levels of Folate, Vitamin B12, Serum Iron and Serum Ferritin in Female and Male Population According to the Normal Range.
| Parameters | Male (n=68) | % Prevalence of deficiency | Female (n=90) | % Prevalence of deficiency | ||||
|---|---|---|---|---|---|---|---|---|
| Low | Normal | P value | Low | Normal | P value | |||
| Folate (ng/mL) | 0 | 10.7±4.1 | NA | 0 | 1.5±0.6 | 4.9±1.5 | NA | 3% |
| (n = 0) | (n = 68) | (n = 2) | (n = 88) | |||||
| Vitamin B12 (pg/mL) | 208±2 | 570±348 | <0.0001 | 4% | 0 | 224±114 | NA | 0 |
| (n = 3) | (n = 65) | (n = 0) | (n = 90) | |||||
| Serum iron (µg/dL) | 50.1±14.8 | 102.1±14.4 | <0.0001 | 70.6% | 28.2±18.9 | 99.1±36.5 | <0.0001 | 88% |
| (n = 48) | (n = 20) | (n = 79) | (n = 11) | |||||
| Serum ferritin (µg/L) | 12.3±4 | 78.5±52 | <0.0001 | 8.8% | 7.3±2.6 | 58.1±40.4 | <0.0001 | 21% |
| (n = 6) | (n = 62) | (n = 19) | (n = 71) | |||||
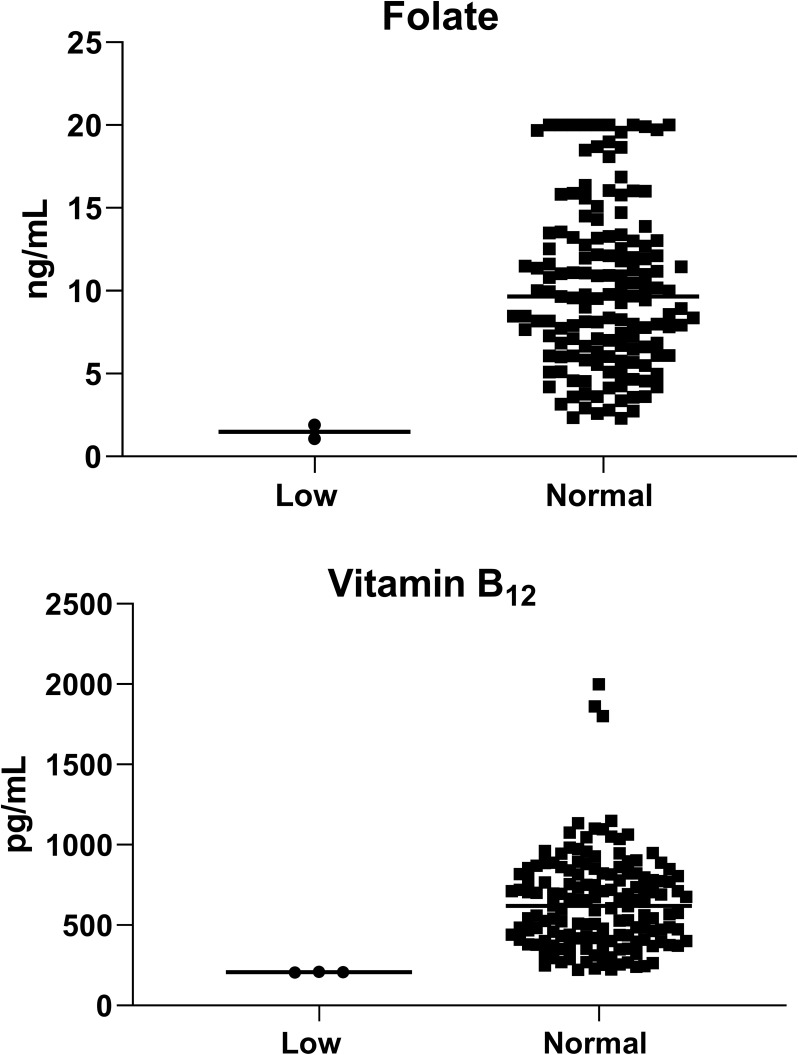
Figure 1.
Levels of folate, and vitamin B12 among the study population according to the normal range (total = 158; male = 68 and female = 90).
Folate Levels
In the male population, all study participants had normal folate levels, while in the female population, 97% (n = 88) had normal folate levels, and 2% (n = 2) had low levels (Table 3 and Figure 1).
Vitamin B12 Levels
In the male population, 96% of the study participants had normal levels of vitamin B12, while 4% had low levels. In contrast, all female in the study population had normal levels of vitamin B12 (Table 3 and Figure 1).
Co-Existence of Low Serum Iron Levels with Folate and/or Vitamin B12
The data report no co-existence of low serum iron levels with folate and/or vitamin B12 (data not shown).
Anemia Among Study Participants
The CBC data for both male and female participants are presented in Table 4. The female population was divided into two groups based on mean Hb concentration: anemic (Hb < 12.0 g/dL) and normal (Hb > 12.0 g/dL), following WHO criteria for anemia.10 51.1% of the female participants (n = 46) were classified as anemic, with a mean Hb concentration of 10.4 ± 1.3 g/dL, while the remaining 49.9% had normal Hb levels, with a mean Hb concentration of 13.1 ± 0.8g/dL (p < 0.01). Notably, CBC parameters in anemic females were significantly lower than those in normal females (p < 0.01), except for the RDW (Table 4). Both anemic and normal females exhibited significantly low mean levels of serum iron and ferritin (Table 4; p > 0.05). The MCV data indicated the presence of microcytic anemia in the female group (Table 4). Surprisingly, anemic females had significantly higher levels of vitamin B12 than normal females (722 ± 263 vs 660 ± 239; p < 0.05), while no significant difference in folate levels were observed (9.6 ± 5.3 vs 9.8 ± 4.7; p > 0.05) (Table 4).
Table 4.
Red Blood Cell Parameters, Serum Iron, Vitamin B12 and Folic Acid of Male and Female Populations
| Parameters | Male (n = 68) | Female (90) | ||
|---|---|---|---|---|
| Normal (Hb > 13.0g/dL) (n=68) | Normal (Hb>12.0g/dL) (n=44) | Anemic (Hb<12.0g/dL) (n=46) | P value | |
| RBCs (x1012/L) | 5.4±0.4 | 4.8± 0.5 | 4.6±0.5 | 0.0055 |
| Hgb (g/dL) | 15.3± 1.0 | 13.1 ± 0.8 | 10.4±1.3 | <0.0001 |
| Hct (%) | 45.9± 3.5 | 37.1± 2.8 | 31.3± 4.2 | <0.0001 |
| MCV (fL) | 85.0±4.5 | 77.3±5.6 | 68.8±9.0 | <0.0001 |
| MCH (pg) | 28.5± 2.2 | 27.3±2.3 | 22.9± 3.5 | <0.0001 |
| MCHC (g/dL) | 33.3±1.4 | 35.3±1.4 | 33.1±2.3 | <0.0001 |
| RDW (%) | 17.3± 3.4 | 21.1±2.8 | 21±3.3 | 0.7573 |
| Folic acid (ng/mL) | 10.9±4.8 | 9.6±5.3 | 9.8±4.7 | 0.4759 |
| Vitamin B12 (pg/mL) | 810± 538 | 660±239 | 722±263 | <0.0001 |
| Serum iron (µg/dL) | 65±29 | 47±39 | 36±27 | 0.1220 |
| Serum ferritin (µg/L) | 71.3±53 | 48±39 | 18± 19 | <0.0001 |
Abbreviations: RBC, red cell count; Hb, hemoglobin; Hct, hematocrit; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; RDW; red cell distribution width.
In contrast, all male participants were non anemic (Table 4). Among the male population, 67.6% (n = 46) exhibited low mean levels of serum iron, while 32.4% (n = 22) had normal levels (50 ± 15 vs 101 ± 18; p < 0.0001) (Supplementary Table 1). The CBC parameters were comparable between the male population with low serum iron and those with normal levels, except for the RDW, MCH, and MCHC, which were significantly higher in the iron deficient male population (Supplementary Table 1). Although the study indicated a low incidence rate of low folate and vitamin B12 levels, macrocytic anemia was not observed.
Correlation Between Study Parameters (Hemoglobin, Vitamin B12, Folate, Serum Iron, Ferritin, Sociodemographic Characteristics, and Nutritional Habits)
In the male population, a significant positive correlation was observed between vitamin B12 and folate levels (R = 0.25, p < 0.044). However, no significant correlations were found between vitamin B12 and serum iron, ferritin, or hemoglobin levels. Folate, on the other hand, showed a significant positive correlation with serum iron. Additionally, a negative correlation was observed between folic acid and age in the male population only (Supplementary Table 2).
In the female population, a significant positive correlation was found between vitamin B12 and folate levels (R = 0.22, p < 0.044). Serum iron, on the other hand, showed a significant negative correlation with hemoglobin levels and low red meat consumption (Supplementary Table 2).
Discussion
The current study reported significantly low serum iron levels among young adult males (70.6%) and female (88%), while folate and vitamin B12 deficiencies were only found in 2.2% and 4% of participants, respectively. Low serum iron and ferritin levels were significantly associated with low Hb levels (p < 0.05) and a high incidence rate of microcytosis (51.1%) in female population, contributing to the development of ID and IDA, which was not observed in the male cohort. These findings are consistent with previous studies conducted in the Jazan region10 and in other parts of Saudi Arabia.9–11,22–34 The high prevalence of ID and IDA among young and childbearing females in the Saudi community has been attributed to nutritional habits, including skipping breakfast and low consumption of red meat.10,22,26,35,36 In the current study, the female population showed strong association between low serum iron and low red-meat consumption. Indeed, low consumption of red meat (less than 2 times per week) had been found to be associated with the development of IDA among female students in Saudi Arabia.32 Other factor including menstrual cycle in females has been linked to the development of IDA.22,36 Furthermore, previous studies has attributed the high incidence of low iron levels and ID to irregular meals,10,26,35,36 which is very high among males and females in the current study.
To the best of our knowledge, this study represents the first investigation into folate and vitamin B12 levels among young male and female students in Jazan region. The current study revealed noteworthy gender differences. Folate deficiency was more prevalent in females (2.2%) compared to males (0%), while vitamin B12 deficiency was more frequent in males (4%) than in females (0%). The findings regarding folate levels were in line with a study conducted in Abha city (2.8%). However, there was a disparity in the findings concerning vitamin B12 levels, as the Abha city study reported a considerably higher prevalence of 17.2% for vitamin B12 deficiency, which did not align with our results.13 In our study, no case of vitamin B12 deficiency was reported in the female population. This finding contradicts a study conducted at King Saud University in Riyadh, where 6% of childbearing females were found to have vitamin B12 deficiency.
Comparatively, the prevalence of folate and vitamin B12 deficiencies in our study was lower than in some other Middle Eastern countries.37 For instance, in Iran, the prevalence of folate and vitamin B12 deficiencies was reported to be 16.8% and 6.1%, respectively.37 Globally, the rate of folate and vitamin B12 deficiencies varies widely, ranging from 2.5 to >20% depending on factors such as age, sex, and ethnic background.13–16,18,37,38 Therefore, the relatively low incidence of folate and vitamin B12 deficiencies in Saudi Arabia may be attributed to the high consumption of animal products and fortified wheat flour, which are rich in folate and vitamin B12.39
In addition to dietary factors, several other reasons contribute for folate and vitamin B12 deficiencies and the development of anemia in young women. These include repeated pregnancies, adherence to strict vegetarian diets, the use of certain medications, and various clinical conditions.40 Low folate levels has been linked to many pathological conditions such as cancer and cardiovascular disorders.41 Conversely, sufficient folate intake is associated with health benefits, including a reduced risk of cardiovascular diseases, lower cancer incidence, and a decreased likelihood of neural tube defects.42 Adequate folate levels particularly critical in pregnant women to prevent fetal neural tube defects. Folate deficiency is not uncommon among women of childbearing, especially during conception.41,42 Vitamin B12 deficiency is likewise associated with a range of severe health issues, including anemia, neurological complications, and metabolic disorders.43
Several studies had linked folate and vitamin B12 deficiencies to the development of low incidence of macrocytic anemia in Saudi Arabia.13,44 Macrocytic anemia was observed in 3.2% of adult patients in Abha city (involving 614 patients)13 and in 2% of adult patients in Makkah city (involving 21,524 patients).45 The current study did not find any association between low levels of folate and vitamin B12 with the occurrence of macrocytic anemia. Data regarding vitamin B12 and folic deficiency and the prevalence of macrocytic anemia among young adults in the Jazan region are scarce, with only a few studies conducted in Saudi Arabia.13,33,45–49 Notably, no prior studies from the Jazan region have specifically investigated the prevalence of macrocytic anemia among young male and female students. As a result, the authors suggest a low occurrence of macrocytic in this demographic. This assertion is supported by two studies conducted in Jazan that focused on young male and female students (n = 134), as well as data from adults who underwent premarital screening (n = 1312), which did not identify any cases of macrocytic anemia.10,11 Nationally, various studies have explored RBCs abnormalities and high MCV values in different regions of Saudi Arabia, including Taibah, Riyadh, Asser and Alghat.33,47–49 The findings of the current study align with those reported in the Asser region, where no cases of anemia related to high MCV were documented.48 Additionally, the prevalence of macrocytic anemia in Taibah and Riyadh was found to be low, at 0.4% and 0.7%, respectively.33,47 However, it is worth noting that a recent study demonstrated a high prevalence of macrocytic anemia, which contradicts the findings of our study.49 The observed discrepancy in the prevalence of folate and vitamin B12 deficiencies between studies could be attributed to variations in the populations studied and differences in sample sizes. Our research focused on healthy young adults, while the Alsagaby (2022) study included patients who had visited Prince Nasser bin Saad Al-Sudairy Hospital over a period of 12 months.49 The presence of macrocytosis, as indicated by a higher MCV, was evident in elderly patients but not in younger individuals. Moreover, our study had a smaller sample size than those conducted in Abha and Makkah. It is worth mentioning that Saudi Arabia has implemented folate food fortification, which could contribute to the relatively low incidence of folate deficiency and its limited role in the development of macrocytic anemia.39 This contrasts with the findings in different countries.50,51
In contrast to folate and vitamin B12 deficiencies, the study population exhibited low iron levels. It is important to note that the current study did not identify a significant coexistence of low levels of either folate or vitamin B12 with low levels of serum iron. Iron deficiency stands as one of the most prevalent common clinical ailments.52 Factors such as increased iron demand and menstrual blood loss are the leading etiological contributors to ID.52
The present study, like many others, has certain limitations, including a relatively small sample size. Additionally, some participants were taken vitamin supplementation, which could potentially influence the results; however, this is consistent with practices in other studies. Moreover, this study focused on young adults, and other groups such as children, pregnant women, and the elderly were not assessed.
In summation, prevalence of low serum iron remains a subject of concern and interest, whereas macrocytic anemia, particularly attributed to deficiencies in folate and vitamin B12, were not observed. While our study confirms the presence of these deficiencies, it does not reveal a direct association with macrocytic anemia, evoking intriguing avenues for future exploration. To advance our understanding, additional comprehensive studies encompassing diverse age groups, including children, pregnant women, and the elderly, are warranted. These inquiries should encompass a broad spectrum of sociodemographic variables and dietary habits and hold the key to addressing these challenges and enhancing the nutritional well-being of young adults in Saudi Arabia.
Funding Statement
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia, for funding this research work through project number ISP22-1.
Ethical Approval
All procedures performed in the current studies involving human participants were approved by the Scientific Research Ethics Committee (REC-44/06/478), Jazan University and was conducted in accordance with the Declaration of Helsinki.
Informed Consent
Informed consent was obtained from all study participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interest.
References
- 1.Hercberg S, Rouaud C. Nutritional anaemia. Child Trop. 1981;133:1–36. [PubMed] [Google Scholar]
- 2.Naeim F. Disorder of red blood cells: anemias. Hematop Morphol Immunoph Cytogenet Mol Approaches. 2008;529–565. doi: 10.1016/B978-0-12-370607-2.00023-5 [DOI] [Google Scholar]
- 3.Stoltzfus RJ. Defining iron-deficiency anemia in public health terms: a time for reflection. J Nutr. 2001;131(2):565S–567S. doi: 10.1093/jn/131.2.565s [DOI] [PubMed] [Google Scholar]
- 4.Ritchie H, Roser M. Micronutrient deficiency; 2017. Available from: https://ourworldindata.org/micronutrient-deficiency. Accessed February 01, 2024.
- 5.Chowdhury S, Kazmi S. Chakraborty P pratim. Universal health coverage ‑ There is more to it than meets the eye. J Fam Med Prim Care. 2017;6(2):169–170. doi: 10.4103/jfmpc.jfmpc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bill F, Foundation MG. Prevalence, years lived with disability, and trends in anaemia burden by severity and cause, 1990–2021: findings from the global burden of disease study 2021. Lancet Haematol. 2023;10(9):e713–e734. doi: 10.1016/S2352-3026(23)00160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotecha PV. Nutritional anemia in young children with focus on Asia and India. Indian J Community Med. 2011;36(1):8–16. doi: 10.4103/0970-0218.80786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo Costa E, de Paula Ayres-Silva J. Global profile of anemia during pregnancy versus country income overview: 19 years estimative (2000–2019). Ann Hematol. 2023;102(8):2025–2031. doi: 10.1007/s00277-023-05279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Hazmi MA, Warsy AS. Normal reference values for hematological parameters, red cell indices, HB A2 and HB F from early childhood through adolescence in Saudis. Ann Saudi Med. 2001;21(3–4):165–169. doi: 10.5144/0256-4947.2001.165 [DOI] [PubMed] [Google Scholar]
- 10.Hamali HA, Mobarki AA, Saboor M, et al. Prevalence of anemia among Jazan university students. Int J Gen Med. 2020;13:765–770. doi: 10.2147/IJGM.S275702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamali HA, Saboor M. Undiagnosed hemoglobinopathies: a potential threat to the premarital screening program. Pak J Med Sci. 2019;35(6):1611–1615. doi: 10.12669/pjms.35.6.976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary F, Samman S. Vitamin B12 in health and disease. Nutrients. 2010;2(3):299–316. doi: 10.3390/nu2030299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhaldy HY, Alqahtani M, Alamri ZS, et al. Clinical appropriateness of serum folate ordering pattern in a tertiary care hospital in Saudi Arabia. Saudi Pharm J. 2020;28(8):905–910. doi: 10.1016/j.jsps.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aparicio-Ugarriza R, Palacios G, Alder M, González-Gross M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med. 2015;53(8):1149–1159. doi: 10.1515/cclm-2014-0784 [DOI] [PubMed] [Google Scholar]
- 15.Palacios G, Sola R, Barrios L, Pietrzik K, Castillo MJ, González-Gross M. Algorithm for the early diagnosis of vitamin B12 deficiency in elderly people. Nutr Hosp. 2013;28(5):1447–1452. doi: 10.3305/nh.2013.28.5.6821 [DOI] [PubMed] [Google Scholar]
- 16.Bailey RL, Carmel R, Green R, et al. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011;94(2):552–561. doi: 10.3945/ajcn.111.015222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quay TAW, Schroder TH, Jeruszka-Bielak M, et al. High prevalence of suboptimal vitamin B12 status in young adult women of South Asian and European ethnicity. Appl Physiol Nutr Metab. 2015;40(12):1279–1286. doi: 10.1139/apnm-2015-0200 [DOI] [PubMed] [Google Scholar]
- 18.Green R, Allen LH, Bjørke-Monsen A-L, et al. Vitamin B(12) deficiency. Nat Rev Dis Primers. 2017;3:17040. doi: 10.1038/nrdp.2017.40 [DOI] [PubMed] [Google Scholar]
- 19.Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309LP–1316 . doi: 10.1136/gut.2010.228874 [DOI] [PubMed] [Google Scholar]
- 20.Shekunov J, de Groen PC, Lindor NM, et al. Hereditary hyperferritinemia-cataract syndrome in two large multigenerational American families. J AAPOS off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2011;15(4):356–361. doi: 10.1016/j.jaapos.2011.03.020 [DOI] [PubMed] [Google Scholar]
- 21.Al-Musharaf S, Aljuraiban GS, Danish Hussain S, Alnaami AM, Saravanan P, Al-Daghri N. Low serum vitamin B12 levels are associated with adverse lipid profiles in apparently healthy young Saudi women. Nutrients. 2020;12:8. doi: 10.3390/nu12082395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abalkhail B, Shawky S. Prevalence of daily breakfast intake, iron deficiency anemia and awareness of being anemic among Saudi school students. Int J Food Sci Nutr. 2002;53:519–528. doi: 10.1080/09637480220164370 [DOI] [PubMed] [Google Scholar]
- 23.Hanafi MI, Abdallah AR, Zaky A. Study of hemoglobin level and body mass index among preparatory year female students at Taibah University, Kingdom of Saudi Arabia. J Taibah Univ Med Sci. 2013;8(3):160–166. doi: 10.1016/j.jtumed.2013.04.004 [DOI] [Google Scholar]
- 24.Alquaiz AM, Gad Mohamed A, Khoja TAM, et al. Prevalence of anemia and associated factors in child bearing age women in Riyadh, Saudi Arabia. J Nutr Metab. 2013. doi: 10.1155/2013/636585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993-2005: WHO global database on anaemia. WHO Glob Database Anaemia. 2008;1–4. doi: 10.1017/S1368980008002401 [DOI] [Google Scholar]
- 26.Al-Quaiz JM. Iron deficiency anemia: a study of risk factors. Saudi Med J. 2001;22(6):490–496. [PubMed] [Google Scholar]
- 27.Owaidah T, Al-Numair N, Al-Suliman A, et al. Iron deficiency and iron deficiency anemia are common epidemiological conditions in Saudi Arabia: report of the national epidemiological survey. Anemia. 2020;2020:6642568. doi: 10.1155/2020/6642568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Sharbatti SS, Al-Ward NJ, Al-Timimi DJ, et al. Anemia among adolescents. Saudi Med J. 2003;24:189–194. [PubMed] [Google Scholar]
- 29.Al-Jamea L, Woodman A, Elnagi EA, et al. Prevalence of Iron-deficiency anemia and its associated risk factors in female undergraduate students at prince sultan military college of health sciences. J Appl Hematol. 2019;10(4):126–133. doi: 10.4103/joah.joah_44_19 [DOI] [Google Scholar]
- 30.AlSheikh MH. Prevalence of risk factors of iron-deficiency anemia in Saudi female medical students. Saudi J Heal Sci. 2018;7(3):148–152. doi: 10.4103/sjhs.sjhs [DOI] [Google Scholar]
- 31.Alswailem A, Alahmed S, Alshehri M. The prevalence of iron deficiency anemia and its associated risk factors among a sample of females in Riyadh, Saudi Arabia. Egypt J Hosp Med. 2018;72(6):462. [Google Scholar]
- 32.Alzaheb RA, Al-Amer O. The prevalence of iron deficiency anemia and its associated risk factors among a sample of female university students in Tabuk, Saudi Arabia. Clin Med Insights Women’s Heal. 2017. doi: 10.1177/1179562x17745088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Hassan N. The prevalence of iron deficiency anemia in a Saudi University female students. J Microsc Ultrastruct. 2015;3(1):25–28. doi: 10.1016/j.jmau.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alquaiz AJMA, Khoja TAM, Alsharif A, et al. Prevalence and correlates of anaemia in adolescents in Riyadh city, Kingdom of Saudi Arabia. Public Heal Nutr. 2015;18(17):3192–3200. doi: 10.1017/S1368980015001214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AlFaris N, ALTamimi J, AlKehayez N, et al. Prevalence of anemia and associated risk factors among non-pregnant women in Riyadh, Saudi Arabia: a cross-sectional study. Int J Gen Med. 2021;14:765–777. doi: 10.2147/IJGM.S299450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almasmoum HA, Iqbal MS, Aljaadi A, et al. Prevalence of undiagnosed iron deficiency anemia and associated factors among female undergraduate medical students in Makkah, Saudi Arabia. Cureus. 2023;15(12):e50046. doi: 10.7759/cureus.50046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azimi S, Faramarzi E, Sarbakhsh P, Ostadrahimi A, Somi MH, Ghayour M. Folate and vitamin B(12) status and their relation to hematological indices in healthy adults of Iranians: azar cohort study. Nutr Health. 2019;25(1):29–36. doi: 10.1177/0260106018815392 [DOI] [PubMed] [Google Scholar]
- 38.Rogers LM, Cordero AM, Pfeiffer CM, et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci. 2018;1431(1):35–57. doi: 10.1111/nyas.13963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aljaadi AM, Alsunaid FF, Abdulrahim M, Almehmadi NH, Alzaben AS. The status of B vitamin in Saudi Adults: a review. Curr Res Nutr Food Sci. 2023;11(3):894–909. doi: 10.12944/CRNFSJ.11.3.01 [DOI] [Google Scholar]
- 40.Hwalla N, Al Dhaheri AS, Radwan H, et al. The prevalence of micronutrient deficiencies and inadequacies in the middle east and approaches to interventions. Nutrients. 2017;9(3). doi: 10.3390/nu9030229 [DOI] [Google Scholar]
- 41.Sauer J, Mason JB, Choi S-W. Too much folate: a risk factor for cancer and cardiovascular disease? Curr Opin Clin Nutr Metab Care. 2009;12(1):30–36. doi: 10.1097/MCO.0b013e32831cec62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Am Soc Prev Oncol. 2006;15(2):189–193. doi: 10.1158/1055-9965.EPI-152CO [DOI] [PubMed] [Google Scholar]
- 43.Sobczyńska-Malefora A, Delvin E, McCaddon A, Ahmadi KR, Harrington DJ. Vitamin B(12) status in health and disease: a critical review. Diagnosis of deficiency and insufficiency - clinical and laboratory pitfalls. Crit Rev Clin Lab Sci. 2021;58(6):399–429. doi: 10.1080/10408363.2021.1885339 [DOI] [PubMed] [Google Scholar]
- 44.Chan Y-M, Bailey R, O’Connor DL. Folate. Adv Nutr. 2013;4(1):123–125. doi: 10.3945/an.112.003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arbaeen AF, Iqbal MS. Anemia burden among hospital attendees in Makkah, Saudi Arabia. Anemia. 2022;2022:4709119. doi: 10.1155/2022/4709119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Musharaf S, McTernan PG, Hussain SD, et al. Prevalence and indicators of vitamin B12 Insufficiency among young women of childbearing age. Int J Environ Res Public Health. 2020;18(1). doi: 10.3390/ijerph18010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elsayid M, Al-Qahtani A, Alanazi A, Qureshi S. Determination of the most common morphological patterns of anemia among Saudi anemic patients attending King Abdul-aziz Medical City-Riyadh. Int J Med Public Heal. 2015. doi: 10.4103/2230-8598.165957 [DOI] [Google Scholar]
- 48.Hammad MA. Evaluation of prevalence and pattern of anemia – a hospital based study in Aseer Province, Saudi Arabia. J Exp Med Surg Res Cercet. 2013;2(13):32–35. [Google Scholar]
- 49.Alsagaby SA. A comprehensive study on abnormalities associated with red blood cells in Saudi adult patients. Int J Health Sci. 2022;16(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- 50.Shojania AM, von Kuster K. Ordering folate assays is no longer justified for investigation of anemias, in folic acid fortified countries. BMC Res Notes. 2010;3:22. doi: 10.1186/1756-0500-3-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown RD, Langshaw MR, Uhr EJ, Gibson JN, Joshua DE. The impact of mandatory fortification of flour with folic acid on the blood folate levels of an Australian population. Med J Aust. 2011;194(2):65–67. doi: 10.5694/j.1326-5377.2011.tb04169.x [DOI] [PubMed] [Google Scholar]
- 52.Saboor M, Zehra A, Hamali HA, Mobarki AA. Revisiting iron metabolism, iron homeostasis and iron deficiency anemia. Clin Lab. 2021;67(3). doi: 10.7754/Clin.Lab.2020.200742 [DOI] [PubMed] [Google Scholar]