Abstract
CD36 is a transmembrane glycoprotein receptor for oxidized low density lipoprotein (LDL) and other endogenous danger signals and promotes athero-thrombotic processes. CD36 has been shown to associate physically with other transmembrane proteins, including integrins, tetraspanins, and toll-like receptors, which modulate CD36-mediated cell signaling. The CD36 N-terminal transmembrane domain (nTMD) contains a GXXXG sequence motif that mediates protein-protein interactions in many membrane proteins. We thus hypothesized that the nTMD is involved in CD36 interactions with other membrane proteins. CD36 interactions with partner cell surface proteins on murine peritoneal macrophages were detected with an immunofluorescence-based proximity ligation cross linking assay (PLA) and confirmed by immunoprecipitation/immunoblot. Prior to performing these assays, cells were incubated with a synthetic 29 amino acid peptide containing the 22 amino acid of CD36 nTMD or a control peptide in which the glycine residues in GXXXG motif were replaced by valines. In functional experiments, macrophages were preincubated with peptides and then treated with oxLDL to assess LDL uptake, foam cell formation, ROS formation and cell migration. CD36 nTMD peptide treated cells compared to untreated or control peptide treated cells showed decreased CD36 surface associations with tetraspanin CD9 and ameliorated pathologically important CD36 mediated responses to oxLDL, including uptake of DiI-labeled oxLDL, foam cell formation, ROS generation, and inhibition of migration.
Introduction
CD36 is a class B scavenger receptor highly expressed on the surface of macrophages, platelets, microvascular endothelial cells, adipocytes, myocytes, and some epithelial cells. It has 2 transmembrane domains and 2 very short intra-cytoplasmic domains, both of which are acylated and neither of which contain scaffolding or signaling domains.1 CD36 promotes athero-thrombosis via interaction with endogenous “danger signals” including oxidized phospholipids within oxidized LDL particles (oxLDL),2 advanced glycated proteins,3 cell-derived microvesicles,4 and MRP8/14, a member of the S100A family of pro-inflammatory calcium binding proteins.5
CD36 interaction with its ligands mediates many cell-specific responses. For example, in endothelial cells CD36 inhibits angiogenesis induced by growth factors and promotes apoptosis. In macrophages, CD36 promotes oxLDL uptake, activation of PPARγ transcriptional pathways, reactive oxygen species (ROS) generation and inflammatory responses and inhibits migration. In platelets, CD36 promotes activation, aggregation, and procoagulant function. CD36 is also known at Fatty Acid Translocase (FAT) and in adipocytes and myocytes, it participates in trafficking of long chain free fatty acids across the plasma membrane for storage or metabolism (reviewed in1,6). The mechanism by which CD36 transmits pro-inflammatory, prothrombotic, and internalization signals remain incompletely defined. Experimental evidence suggests that in many cases, CD36 functions in partnership with other plasma membrane proteins, including tetraspanins,7 integrins,8 toll-like receptors,9,10 and sodium/potassium ATPase (NKA)11,12 to affect its complex biological functions. However, the structural basis of these protein-protein interactions is not known.
In silico analysis revealed that the CD36 N-terminal transmembrane domain (nTMD) amino acid sequence is highly conserved, with 100% identity among human, mouse and bovine genomes, while the C-terminal transmembrane domain shows only 73%–82% identity, suggesting that the nTMD may serve important functions. This α–helical domain contains a GXXXGXXXG sequence which has been shown to function as a protein-protein interaction domain in other membrane proteins.13,14 We thus hypothesized that the GXXXG motif within the nTMD is required for CD36 interactions with other membrane proteins and that interruption of protein-protein interactions mediated by this motif would compromise CD36 signaling functions. In this study we focused on CD36 interactions with the tetraspanin CD9 because of data from our lab7 and others15 showing that CD9 and its related tetraspanin CD81 contain a transmembrane GXXXG motif and modulate function of type 2 scavenger receptors CD36 and SR-B1, perhaps by facilitating localization in specific membrane microdomains.
We utilized a synthetic peptide containing the CD36 nTMD sequence flanked by 3 highly charged amino acids to provide stop transfer signals that facilitate insertion and retention of the α–helical peptide into the plasma membrane. Previous studies and our data showed that amphipathic peptides such as these rapidly insert in a random orientation into lipid bilayers and outer cell plasma membranes. The nTMD peptide, when incubated with mouse peritoneal macrophages, had no effect on CD36 homo-dimerization, but specifically inhibited CD36 associations with CD9, and attenuated oxLDL mediated CD36 pro-atherogenic functions.
Materials and Methods
Animals, antibodies and other reagents.
All mouse studies were approved by the Institutional Animal Care and Use Committee of Medical College of Wisconsin and conform to ethical guidelines. Peritoneal macrophages were obtained by lavage 4d after injection with thioglycollate and adherent cells were maintained in culture. Cell culture reagents were from Invitrogen, CA, USA. Rabbit monoclonal anti-CD9 IgG was from BD Biosciences, CA, USA. Mouse monoclonal anti-mouse CD36 IgA was prepared as previously described.16 DiI labeled acetylated LDL was purchased from Cell Applications, CA, USA. Green Fluorescent Polymer Microspheres were purchased from Thermo scientific, CA, USA. LDL was isolated from human plasma as previously described17 and oxidized by Cu2SO4 or with a myeloperoxidase based system as previously described.2 In some experiments LDL was exposed to all elements of the system except the oxidant to create control non-oxidized LDL. All chemicals were obtained from Sigma (St. Louis, MO, USA) unless otherwise indicated.
In vitro studies with human monocyte-derived macrophages.
The human studies reported in this manuscript used cells (buffy coats) purchased from a commercial vendor (Versiti Blood Center of Wisconsin, Milwaukee, WI). The investigators have no access to any information that could connect these samples to a specific human donor and no records are maintained to connect these commercial products to an individual donor. These studies therefore do not meet the criteria set by NIH or our IRB for human subjects research. Monocytes were isolated from the buffy coats and differentiated to macrophages by incubation in X-VIVO 10 hematopoietic media (Lonza) supplemented with 5% human serum (Sigma), 100 U/mL penicillin, and 100 μg/mL streptomycin (Sigma) at 37°C in a humidified incubator with 5% CO2 for 5 days.
Synthetic peptides
A peptide containing the sequence of CD36 nTMDP - NH2-KKWGLIAGAVIGAVLAVFGGILMPVGKKK-COOH was synthesized by Dr. Li’s lab or the Protein Synthesis Core Facility of Versiti Blood Research Institute. Peptides were synthesized using modified FMOC protocols on a Liberty-1 (CEM) peptide synthesizer using universal NovaTag resin as the solid support. C- or N-terminal amine labeling was done with the fluorescent dye 5(6)-carboxyfluorescein (FAM, SE mixed isomer, Anaspec). Resins from the synthesis reactions were split and labeled on either the N-terminus or the C-terminus by capping the final amine position, removing the Mtt protecting group and coupling the label on the exposed amine. In this way, the same synthesis resulted in both N and C terminal labeled products.18 A control peptide substituting valines for glycines in the second GXXXG motif NH2-KKWGLIAVAVIVAVLAVFGGILMPVGKKK-COOH was also synthesized. In some studies the labeled peptides were added to macrophage culture medium without FBS for timed points so that peptide insertion into the plasma membrane could be documented by confocal microscopy and flow cytometry.
Immunoprecipitation and immunoblot.
Mouse monoclonal anti-mouse CD36 IgA was coupled to NHS-activated agarose beads (GE life sciences, NJ, USA) according to manufacturer’s instruction. Peritoneal macrophages were treated with dithiobis-succinimidylpropionate and then lysed by 1% CHAPS (3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate hydrate) in buffer made up of 50 mM Tris-HCl (pH7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, and a broad spectrum protease inhibitor cocktail (Roche Applied Science, IN, USA). Lysates were centrifuged at 12,000 g for 10 minutes and the supernatants containing 750 μg protein were incubated with antibody beads rotating overnight at 4°C. After extensive washing, beads were boiled in SDS-PAGE loading buffer, run on SDS-PAGE and analyzed by immunoblot after transfer to PVDF membrane (BioRad, CA, USA) with specific antibodies using chemi-luminescence based detection systems (Thermo scientific, IL, USA or GE life sciences, Pittsburgh, PA, USA).
oxLDL uptake and foam cell formation.
Peritoneal macrophages from C57BL/6 mice were seeded on coverslips and cultured in RPMI 1640 medium supplied with 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in a 5% CO2 incubator. Attached cells were pretreated with CD36 nTMD or control peptide (final concentration 5 μM) for 60 minutes at 37°C and then incubated in serum-free medium with DiI-labeled oxLDL (10 μg/mL) for timed points at 37°C. For fluorescence microscopy, cells were then fixed in 4% formaldehyde and counterstained with 4′, 6-diamidino-2-phenylindole (DAPI). Images were obtained using a laser confocal fluorescence microscope. For flow cytometry, macrophages were incubated in serum-free medium containing DiIoxLDL (10 μg/mL) at 37°C for 25 minutes after peptide treatment. After washing, cells were harvested then analyzed. To assess foam cell formation, cells were cultured in complete medium (2% FBS) with 50 μg/mL unlabeled oxLDL for 16 hours and then fixed with 4% formaldehyde and stained with Oil Red O. Foam cells were counted and the foam cell/total cell ratio was calculated. For intracellular cholesterol assay data, peptide pre-treated cells were cultured in complete medium (2% FBS) containing 50 μg/mL oxLDL for 16 hours then cholesterol and protein concentrations in whole cell lysates were measured by Amplex Red Cholesterol Assay Kit (Thermo scientific, IL, USA) or Protein Assay reagents (BioRad, CA, USA).
Immunofluorescence cross-linking assay (proximity ligation assay, PLA).
Fixed peritoneal macrophages were incubated with rabbit anti-CD9 and mouse anti-CD36 monoclonal antibodies. Coverslips were then washed and incubated with species specific secondary antibodies (Duolink; Olink, Inc) conjugated to unique DNA strands that serve as templates for hybridization of specific oligonucleotides. The oligonucleotides were then added as per the manufacturer’s protocol along with a ligase to form a circular template. The anchored template was then amplified and detected using complementary fluorescently labeled probes. Distinct bright red fluorescent dots representing single-molecule protein interaction events were visualized using a laser confocal fluorescence microscope as previously described.7
Quantification of reactive oxygen species (ROS).
Peritoneal macrophages adherent on 12 well plate were treated with peptides as above, washed once with warm HBSS/Ca/Mg, then incubated in 25 μM carboxy-H2DCFDA (Molecular Probe, MA, USA) 30 minutes at 37°C. After washing, the cells were exposed to LDL or oxLDL (50 μg/mL), and fluorescence assessed at a timed points from 0 to 75 minutes (Ex/Em = 495/529).
Cell migration assay
Macrophages were pretreated with the peptides as above and then allowed to migrate for 15 hours at 37°C in the presence of oxLDL (50 μg/mL), native LDL (50 μg/mL), or serum-free media, using a modified Boyden Chamber with 5.0 μm pore polycarbonate membrane transwell inserts. Migrated cells were fixed with 4% paraformaldehyde, stained with DAPI, and counted in random fields under fluorescence microscopy as previously described.19
Statistical analysis
In vitro assays were performed in quadruplicate cultures. All experiments were done using macrophages from at least 3 mice for each group. All numerical results are expressed as mean ± SEM. Statistical differences were determined by 1-way Anova.
Results
CD36 nTMD peptide interrupts CD36 interaction with CD9
We first used fluorescently tagged peptides to demonstrate that the CD36nTMD and control peptides inserted rapidly into macrophage plasma membranes. Supplementary Fig 1A shows by flow cytometry that surface fluorescence rapidly increased after addition of FAM-labeled peptides (5μM), plateauing at 45−60 minutes. Supplementary Fig 1B shows representative high power confocal images of single cells. Surface fluorescence was maintained over longer time periods, although the signal was attenuated to ~43% of maximum after 18 hours (Supplementary Fig 1C). Supplementary Fig 1D shows representative fluorescence microscopy images taken at 1 and 18 hours. Membrane localization of FAM-labeled peptides was confirmed by showing fluorescence overlap with anti-F4/80, a well-validated macrophage membrane marker (Supplementary Fig 1E).
We next used 2 different previously validated assays for association of CD36 with CD9 and other membrane proteins, co-immunoprecipitation and proximity ligation assay (PLA),7 and found that incubation of cells with a synthetic CD36nTMD peptide containing the GXXXG domain, but not a control peptide substituting valines for 2 critical glycines, effectively interrupted CD36 interactions with CD9, a tetraspanin that also contains a transmembrane GXXXG domain. Fig 1A shows the PLA assays in which bright red fluorescent dots in a rim pattern indicated protein-protein interactions at 40 nM distance or less. In these studies, cells incubated with the control peptide showed a PLA signal indistinguishable from cells incubated without peptides, while a PLA signal was very weak in cells incubated with the CD36nTMD peptide. These data were quantified by counting the bright fluorescent dots in 10 cells from each group. The cells incubated with CD36nTMD peptide showed 90% fewer dots than those incubated with the control peptide. (P< 0.0001), These results were then validated using a co-immunoprecipitation approach. Fig 1B shows that anti-CD36 IgA precipitated both CD36 and CD9 from untreated cells and cells treated with the control peptide, but with cells incubated with CD36nTMD only CD36 was precipitated. Together these studies strongly suggest that the nTMD peptide specifically blocks membrane associations of CD36 with potential transmembrane signaling partners.
Fig 1.
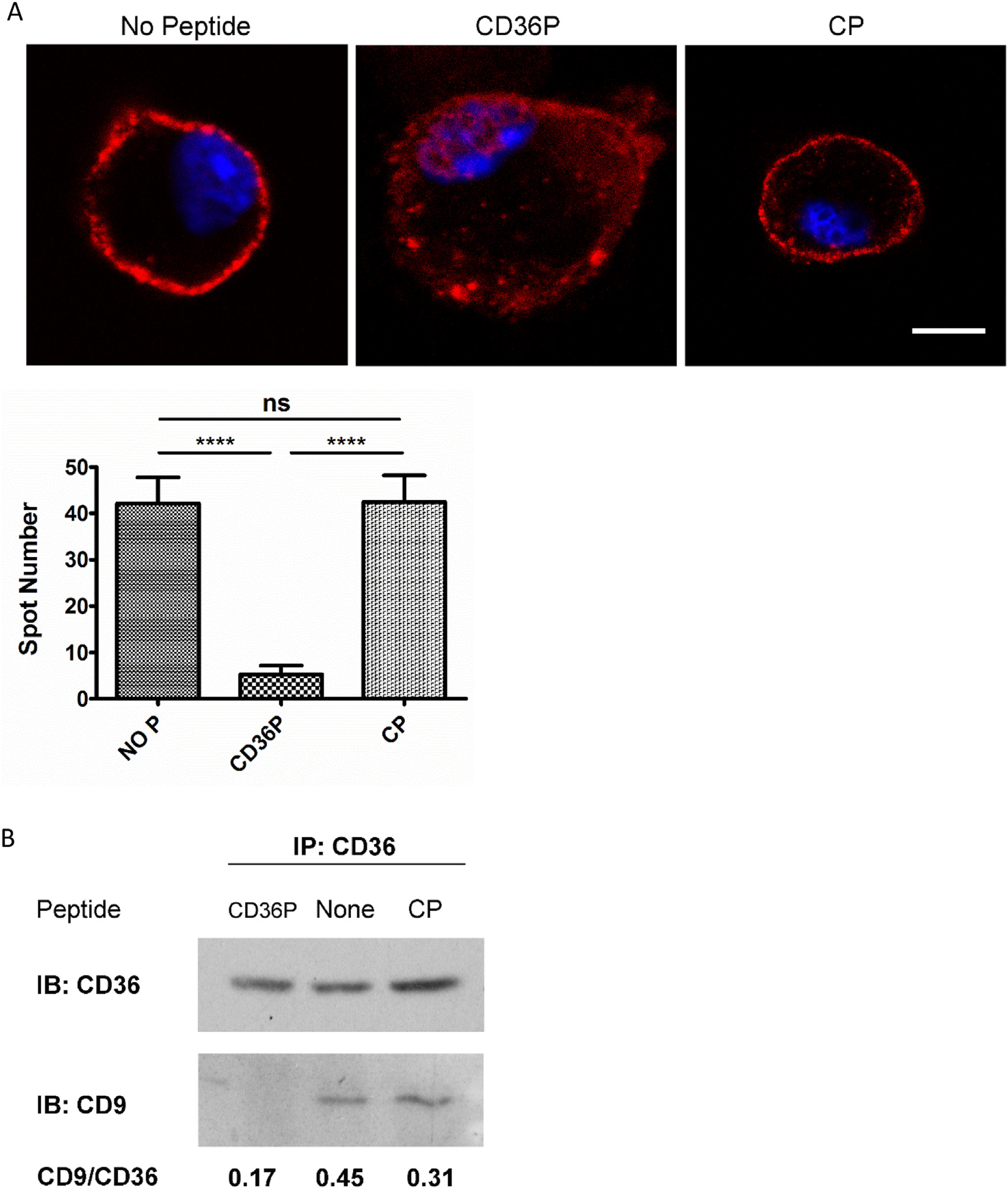
CD36 interactions with CD9 on mouse macrophages are inhibited by CD36nTMD peptide. A. Peritoneal macrophages from C57BL/6 mice were incubated with CD36P or the CP (5 μM) for one hour prior to detection of protein-protein interactions of CD36 with CD9 using the Proximity Ligation Cross-linking Assay and specific mouse anti-CD36 IgA and rabbit anti-CD9 IgG antibodies. Non-treated macrophages were used as an additional control. Bright red fluorescent dots in a rim pattern are indicative of protein-protein associations within a distance of 40 nM or less. Bar scale: 5 μM. The bar graph shows quantification of dots in the PLA assay of macrophages treated by CD36P, CP or no treatment control (n = 10). B. Lysates from cells treated as in Panel A were immunoprecipitated with monoclonal anti-CD36 IgA, run on SDS-PAGE gel and subjected to immunoblot using anti-CD9 or anti-CD36 antibodies. Ratio of CD9:CD36 band densities are shown below the blot. Ns = non-significant (P > 0.05); *: P < 0.05; **: P < 0.01; *** P < 0.001; **** P < 0.0001. CD36P indicates CD36nTMD peptide; CP indicates Control Peptide.
Published studies from other groups suggest that CD36 may form homo-dimers on the cell surface, and that dimerization is mediated by covalent interactions between extracellular domains.20 We thus hypothesized that the nTMD peptide would not impact homo-dimerization. A modest level of CD36 homo-dimer formation was detected by both PLA and immunoblot after native polyacrylamide gel electrophoresis; and as hypothesized, treatment of cells with the CD36 nTMD peptide did not reduce homo-dimer formation (Fig 2).
Fig 2.
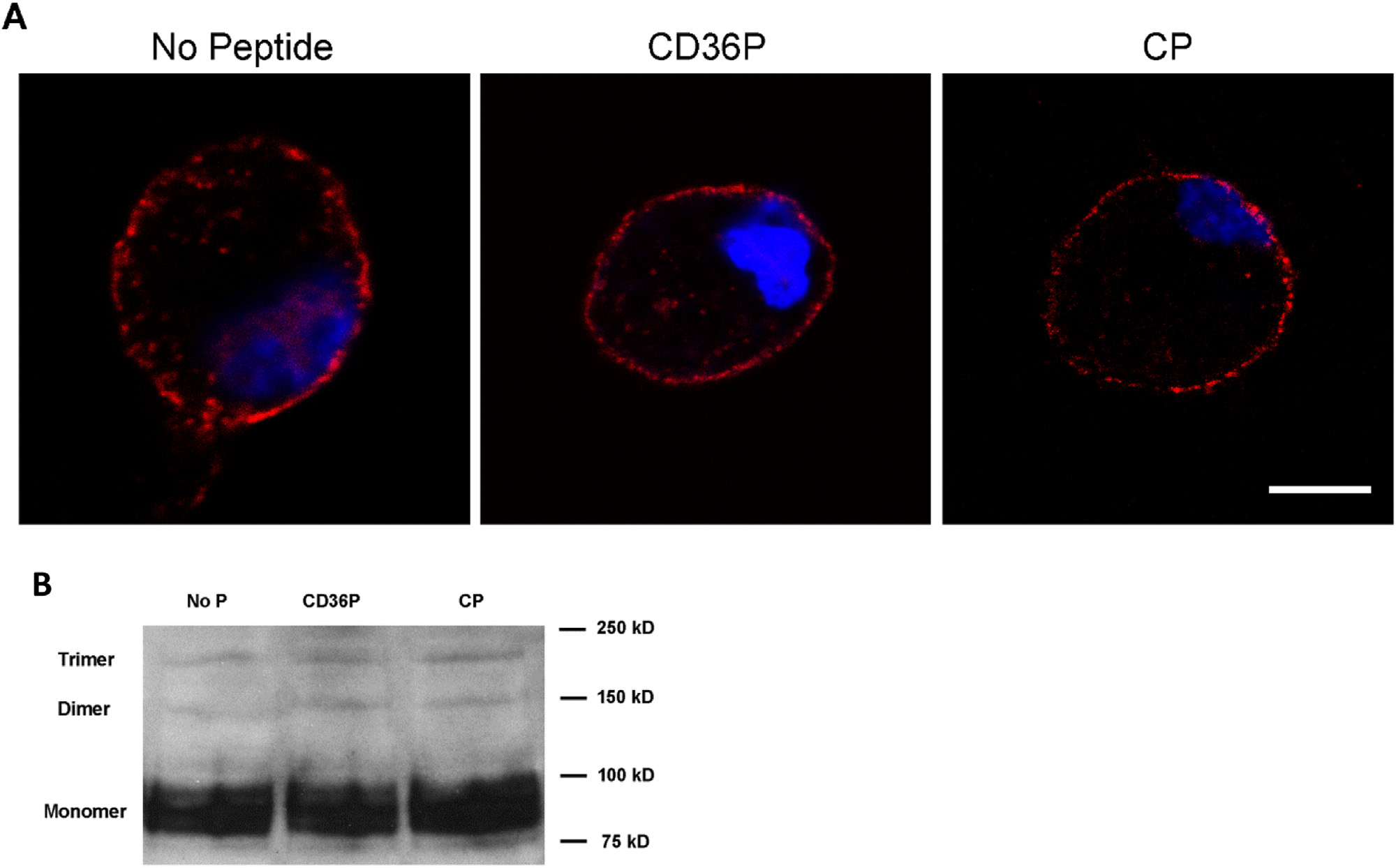
CD36 homodimer formation is not impacted by transmembrane peptides. Macrophages from C57BL/6 mice were incubated with CD36P or CP (5 μM), or media alone for 1hr. A. CD36 dimer formation was detected by PLA as described in Fig 1A except the primary antibodies were a mixture of equal amount of biotin-labeled or FITC-labeled anti-mouse CD36 IgA and anti-biotin and anti-FITC were used as secondary PLA antibodies. Bar scale: 5 μM. B. CD36 monomer/dimers/trimer were detected in cells treated as above with CD36P or CP by western blot after native PAGE gel electrophoresis.
CD36nTMD peptide inhibits macrophage oxLDL uptake and foam cell formation without impacting oxLDL binding
To determine whether interruption of CD36 interactions with other membrane proteins influences CD36-mediated biological functions we treated wide type C57BL/6 macrophages with the CD36nTMD peptide and then incubated them at 37°C with DiI-oxLDL. As shown in Fig 3A, DiI-oxLDL uptake measured by flow cytometry at 45 minutes was inhibited by more than 40% by the CD36nTMD peptide but not the control peptide. Fig 3B shows fluorescence microscopy image of the cells, confirming the findings from flow cytometry.
Fig 3.
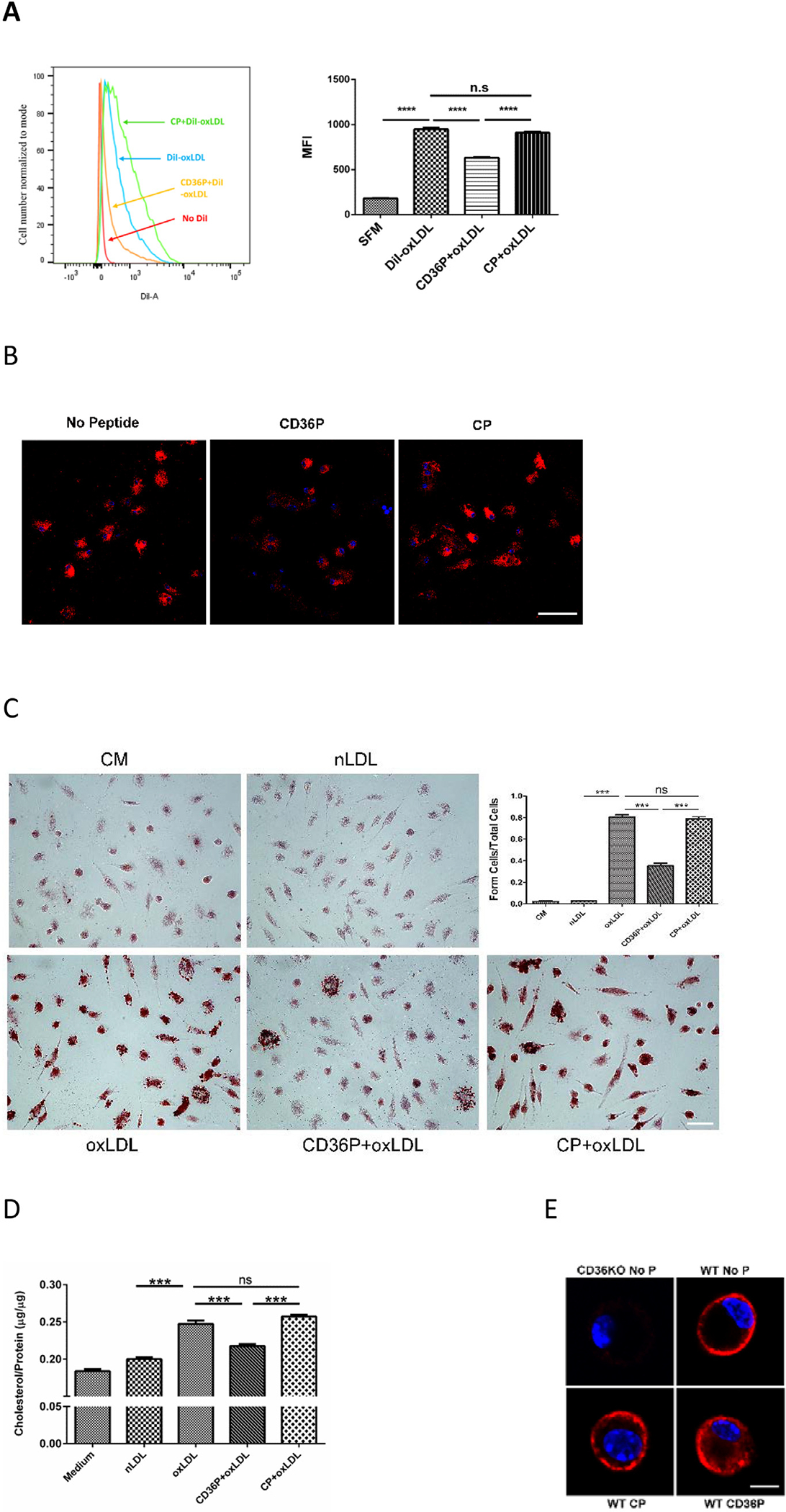
CD36P inhibits DiI-oxLDL uptake and oxLDL-induced foam cell formation, without influencing oxLDL binding to the macrophage surface. Macrophages from C57BL/6 mice were incubated with CD36P or control peptide (5 μM) for one hour and then with serum-free medium containing DiI-oxLDL (10μg/ml) at 37°C for 45 minutes. Cells were then analyzed by flow cytometry (A) or fluorescence microscopy (B) (Bar scale: 20 μM) to assess DiI uptake. The bar graph in panel A shows Mean Fluorescence intensity (n = 3). (C) Macrophages treated with peptides as above were then cultured in complete medium (2% FBS) containing oxLDL (50 μg/mL) or native LDL for 16 hours. Cells were then stained with Oil red O to detect neutral lipids and lipid droplets were counted to quantify foam cell formation. Bar scale: 20 μM. The bar graph shows the ratio of foam cells to total cells in the different treatment groups (n = 5). (D) Macrophages treated as in panel C were lysed and cholesterol content quantified using a cholesterol analysis kit (n = 4). (E) DiI-oxLDL binding was assessed in cells treated with peptides as above and then incubated in serum-free medium with DiI-oxLDL (10μg/ml) at 4°C for 60 minutes to allow binding but not internalization. Representative images of single cells are shown. CD36 null macrophages (CD36KO) were used to show that DiI binding was CD36 dependent (upper left image). Bar scale: 5 μM. In all panels, ns = non-significant, *** indicates P < 0.001, **** P < 0.0001.
To examine the impact of CD36nTMD peptide on longer term lipid uptake we assessed foam cell formation by Oil Red O staining after 16hours incubation with oxLDL, and as shown in Fig 3C, the CD36nTMD peptide decreased foam cell formation by ~60% (P< 0.001) while the control peptide had no effect. To validate these data, total cellular cholesterol levels of the different groups were measured biochemically and showed that CD36nTMDP treatment decreased the oxLDL-induced increase in cellular cholesterol by 50% compared to controls. (Fig 3D).
Neither CD36nTMD peptide nor control peptide influenced binding of oxLDL to the macrophage surface as assessed by cell associated DiI fluorescence after incubation of cells with DiI-oxLDL at 4°C for 45 minutes (Fig 3E). Macrophages from CD36 null mice were used as control in these studies (Fig 3E; upper left panel) demonstrating that the DiI-oxLDL binding was CD36 dependent. These studies are consistent with the hypothesis that the CD36nTMD peptide inhibits CD36 signaling functions downstream of oxLDL binding that are necessary for oxLDL uptake and foam cell formation.
To show that the effect of the CD36nTMD peptide was not due to non-specific disruption of plasma membrane functions we showed that the CD36nTMD peptide did not influence CD36-independent macrophage membrane functions. Specifically, neither DiI-labeled acetylated LDL uptake at 24 hours which is mediated by scavenger receptor A-1, nor phagocytosis of Fluorescent Polymer Microspheres, which is receptor independent, were inhibited by the peptides (Supplementary Fig 2).
To show potential human translational significance of these studies we assessed the impact of the CD36nTMD peptide on oxLDL uptake by human monocyte-derived macrophages. Fig 4A shows that the human cells took up DiI labeled oxLDL and that uptake was inhibited by ~50% by pretreating the cells with the CD36nTMD peptide, while no inhibition was seen with the control peptide (P< 0.01). Similarly, the CD36nTMD inhibited foam cell formation in these cells (Figure 4B).
Fig 4.
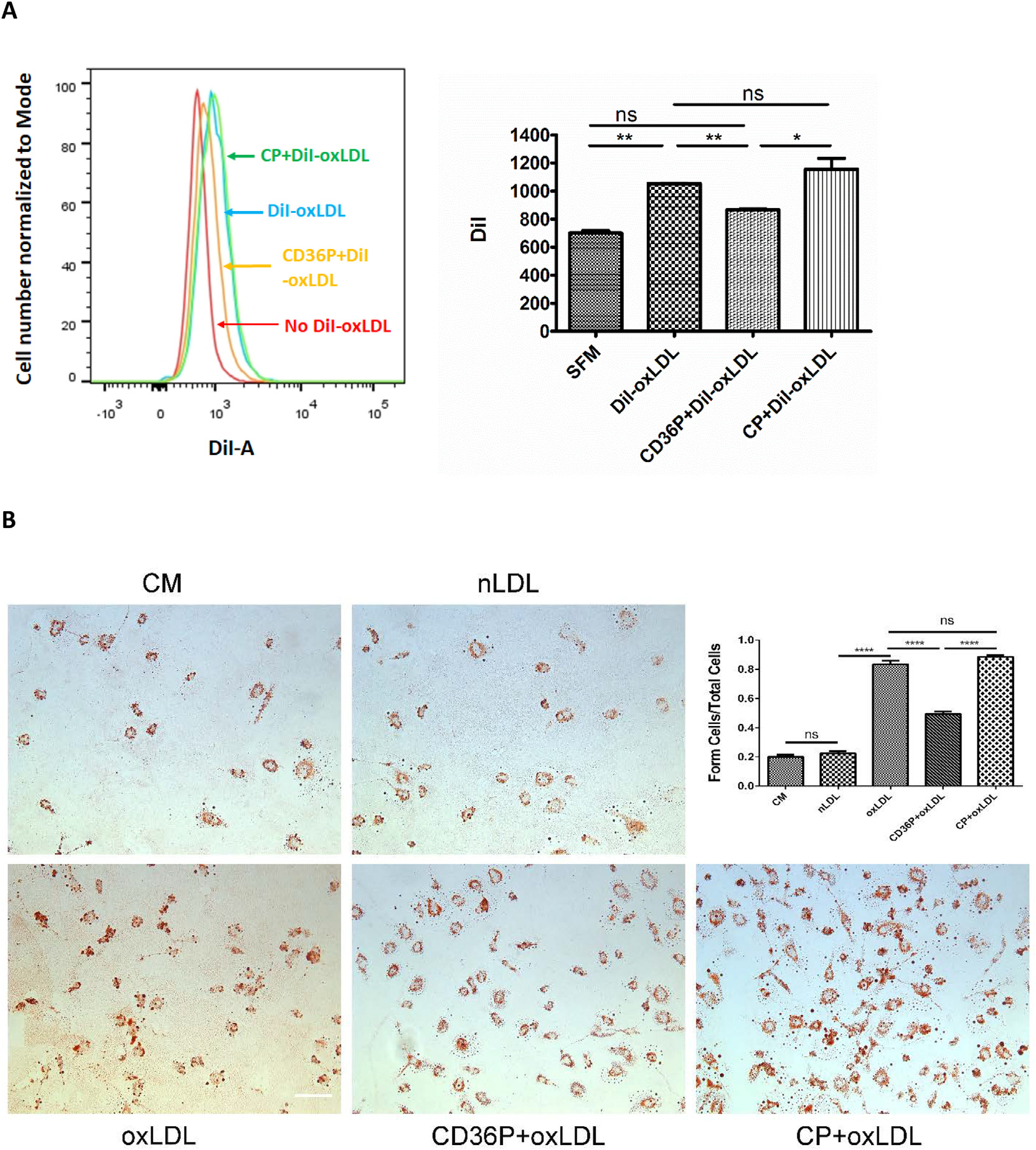
CD36P inhibits DiI-oxLDL uptake and oxLDL-induced foam cell formation in human peripheral blood monocyte (PBMC) derived macrophages. Human PBMC derived macrophages were incubated with CD36nTMD Peptide or control peptide (5 μM) in serum free medium for one hour. After washing, serum-free medium containing DiI-oxLDL (10 μg/mL) was added and incubated at 37°C for 30min. Cells were then harvested and analyzed by flow cytometry. The bar graph in panel A shows mean fluorescence intensity (n = 3). (B) Macrophages treated with peptides as above were then cultured in complete medium (1% human serum) containing oxLDL (50 μg/mL) or native LDL for 16 hours. Cells were then stained with Oil red O to detect neutral lipids and lipid droplets were counted to quantify foam cell formation. Bar scale: 20 μM. The bar graph shows the ratio of foam cells to total cells in the different treatment groups (n = 3). In all panels, ns= non-significant, (P > 0.05); *: P < 0.05; **: P < 0.01; *** indicates P < 0.001, **** P < 0.0001.
CD36 nTMD inhibits oxLDL-induced ROS accumulation in macrophages
Our lab and others have shown that intracellular generation of ROS is an important downstream signaling event in oxLDL/CD36-mediated atherothrombotic responses in macrophages,19 platelets,21 and vascular cells.22 Using a fluorescence probe for intracellular ROS we found that the CD36nTMD peptide significantly inhibited oxLDL-induced ROS generation in mouse peritoneal macrophages (P< 0.01). The level of fluorescence was not different from that seen in cells exposed to native LDL. The control peptide had no impact on ROS generation (Fig 5).
Fig 5.
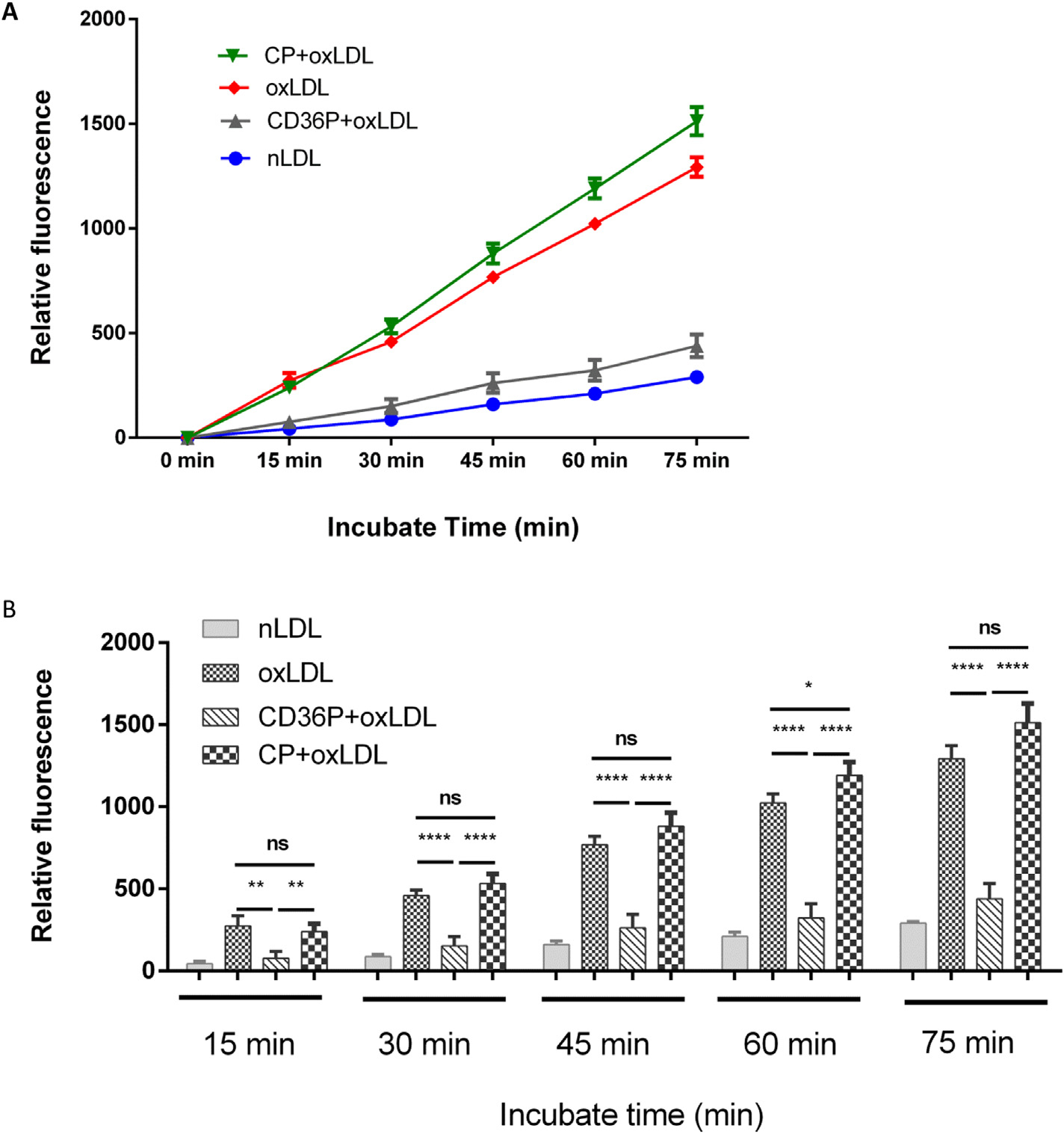
CD36P inhibits oxLDL-induced macrophage generation of reactive oxygen species (ROS). Macrophages seeded in 12 well plates (5 × 105 cells/well) were incubated with CD36P or CP (5 μM) for 1hr and then loaded with carboxy-H2DCFDA. Native LDL or oxLDL (50 μg/mL) were then added in HBSS/Ca/Mg buffer and fluorescence (excitation at 495nm and emission at 529 nm) was measured at timed points from 0 to 75 minutes by a fluorescent plate reader. The bar graphs show the ROS generation at different time points in different groups (n = 3). Ns = non-significant (P > 0.05); *: P < 0.05; **: P < 0.01; *** P < 0.001; **** P < 0.0001.
CD36-nTMD abrogates oxLDL-mediated inhibition of macrophage migration
Inhibition of macrophage migration by oxLDL has been proposed to contribute to macrophage retention in atherosclerotic plaque. Our laboratory reported that this inhibitory effect on macrophage migration was mediated by oxLDL/CD36 signaling and required ROS generation.19 We therefore tested the role of CD36 interactions with other membrane proteins on this process using a modified Boyden Chamber assay. As shown in Figure 6, oxLDL, but not native LDL inhibited macrophage migration. Addition of the CD36-nTMD peptide, but not the control peptide blocked this inhibitory effect, restoring migration to that seen in control conditions or in the presence of native LDL.
Fig 6.
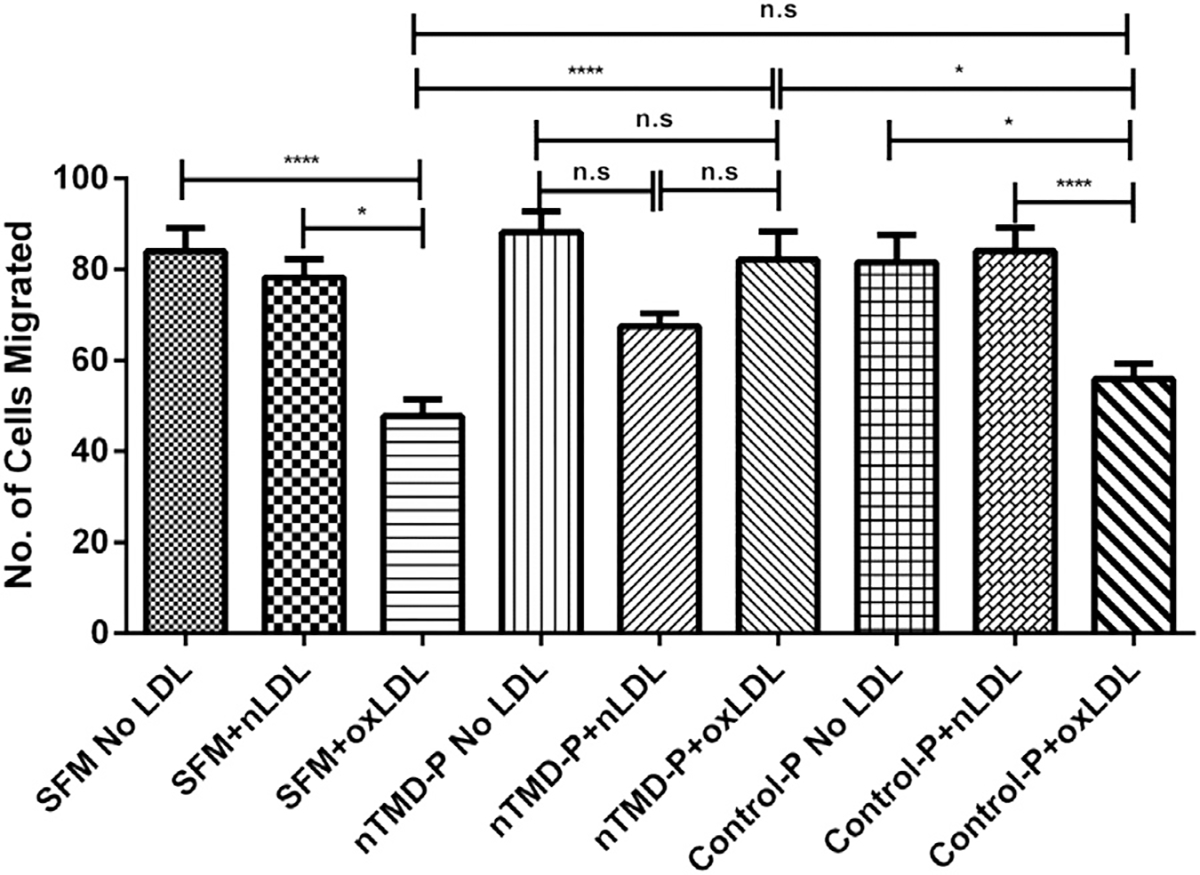
CD36P blunts oxLDL-induced inhibition of macrophage migration. Macrophages from C57BL/6 macrophages were incubated with CD36P or CP (5 μM) for 1hr. Cells were then placed in media containing native LDL or oxLDL (50 μg/mL) and migration was measured in a modified Boyden Chamber assay. After 15 hours the membranes were fixed in paraformaldehyde, cells were stained with DAPI, and the number of cells migrated was counted from random fields under fluorescence microscopy. Ns = non-significant (P > 0.05); *: P < 0.05; **: P < 0.01; *** P < 0.001; **** P < 0.0001 (n = 3).
DISCUSSION
As a widely expressed, multi-ligand, cell surface scavenger receptor, CD36 mediates cellular responses to endogenous and exogenous danger signals, including oxLDL, AGE-proteins, and microbial surface components.1 CD36 interactions with its ligands results in ligand internalization as well as initiation of intracellular signaling cascades that are pro-inflammatory,11,12 prothrombotic,4,5,23 and atherogenic.16,17 While many downstream components of CD36 signaling cascades have been identified,6 including specific src-family kinases, MAP kinases, and vavfamily guanine nucleotide exchange factors, the mechanisms by which CD36 initiates signaling are not well understood.
Although it was reported that the C-terminal domain of CD36 is necessary for its signaling24 and can interact with the downstream cytoplasmic signaling partners, many studies have now shown that CD36 co-immunoprecipitates from lysates of platelets, macrophages, and other cells with a surprising range of membrane proteins, including integrins, tetraspanins,7 toll-like receptors (TLR),9,10 caveolins,25 and sodium-potassium ATPase (NKA).11,12 We and others have shown that inhibiting or knocking down integrins, TLRs, NKA, or CD9 tetraspanin can blunt CD36-mediated cellular responses.9,10,26 These findings suggest that CD36 interaction with “partners” on the cell surface may be critically important for the initiation of signal transduction. Thus defining molecular mechanisms mediating CD36 cell surface protein-protein interactions is important to understand how CD36 signals and may lead to identification of novel targets for athero-inflammatory and thrombotic disorders.
Based on the observation that the CD36nTMDP containing the GXXXG protein interaction motif is highly conserved in vertebrate evolution, we hypothesized that specific CD36 associations with other membrane receptors may be mediated by this domain and may be essential for signal initiation.
We found that a short α-helical amphipathic synthetic peptide containing the CD36 N-terminal transmembrane domain (CD36nTMD), including the GXXXGXXXG sequence, efficiently inserted into the cell surface membrane of macrophages and interrupted CD36 interactions with the tetraspanin CD9, but did not interrupt CD36 homo-dimerization.
CD36-oxLDL interaction plays a key role in oxLDL uptake by macrophage and foam cell formation in atherosclerosis. Our study shows that the CD36nTMD mimic, but not a control peptide substituting valines for glycines, inhibited oxLDL uptake and foam cell formation. Furthermore, intracellular ROS generation and an important downstream functional consequence of CD36 activation, migration inhibition, was also inhibited by the CD36nTMD mimic. Since CD36nTMD mimic did not block oxLDL binding to macrophages, these studies strongly suggest that reduction of oxLDL uptake, foam cell formation, and migration inhibition by CD36nTMD mimic were due to interruption of CD36-partner protein interactions and subsequent downstream signaling.
While these studies do not directly identify roles for specific CD36 protein partners, they support the hypothesis that CD36 signaling requires cell surface partners, and that these partners interact via specific interactions of transmembrane domains. Interestingly, the studies also suggest that CD36 homo-dimerization is not mediated by the N-terminal transmembrane domain and may not be essential for signal transduction. Lastly, the ability to interrupt CD36 protein-protein interactions and downstream functional CD36 signals with a synthetic peptide suggests that a therapeutic strategy could be developed to blunt pro-inflammatory, prothrombotic, and atherogenic signaling mediated by CD36 interactions with endogenous danger signals generated in high risk situations, such as diabetes and hyperlipidemia.
Supplementary Material
At A Glance Commentary.
Wenxin, H et al.,
Background
CD36 interacts with other cell membrane proteins which facilitate its signaling functions. The GXXXG motif in its n-terminal transmembrane domain is a potential protein interaction domain and is highly conserved. We thus hypothesized that this domain mediates CD36 interactions with other membrane proteins in macrophages and plays an important role in CD36 pro-atherogenic functions.
Translational Significance
A synthetic peptide representing the CD36 n-terminal transmembrane domain inserts into macrophage surface membrane and interrupts CD36 interactions with its tetraspanin partner, CD9, leading to inhibition of oxidized LDL uptake and foam cell formation, as well as oxLDL-induced ROS formation and inhibition of cell migration. These data suggest that CD36 interactions with other membrane proteins could be targeted as a potential therapeutic strategy for atherosclerosis.
ACKNOWLEDGEMENTS
Conflicts of Interest: The outstanding technical support of Trudy Holyst and the Versiti Blood Research Institute Peptide Synthesis Core is gratefully acknowledged. All authors have read the journal’s authorship agreement and the manuscript has been reviewed by and approved by all named authors. All authors have read the journal’s policy on disclosure of potential conflicts of interest and report no conflicts. No editorial support for preparation of the manuscript was obtained.
This work was supported by NIH R01HL142152 (RLS), P01HL087018 (RLS), NIH R01HL164460 (RLS and YC), NIH R01HL153397 (RLS and YC) and an American Heart Association Scientist Development Grant 17SDG33661117 (YC).
Abbreviations:
- LDL
low density lipoprotein
- oxLDL
oxidized low density lipoprotein
- nTMD
N-terminal transmembrane domain
- ROS
reactive oxygen species
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- MAPK
mitogen activated protein kinase
- PLA
proximity ligation assay
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.trsl.2022.10.005.
References
- 1.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009;2:re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Podrez EA, Poliakov E, Shen Z, et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J Biol Chem 2002;277:38503–16. [DOI] [PubMed] [Google Scholar]
- 3.Ohgami N, Nagai R, Ikemoto M, et al. CD36, serves as a receptor for advanced glycation endproducts (AGE). J Diabetes Complications 2002;16:56–9. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh A, Li W, Febbraio M, et al. Platelet CD36 mediates interactions with endothelial cell-derived microparticles and contributes to thrombosis in mice. J Clin Invest 2008;118:1934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Fang C, Gao H, et al. Platelet-derived S100 family member myeloid-related protein-14 regulates thrombosis. J Clin Invest 2014;124:2160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Silverstein RL. CD36 signaling in vascular redox stress. Free Radic Biol Med 2019;136:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang W, Febbraio M, Silverstein RL. CD9 tetraspanin interacts with CD36 on the surface of macrophages: a possible regulatory influence on uptake of oxidized low density lipoprotein. PLoS One 2011;6:e29092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao WM, Vasile E, Lane WS, Lawler J. CD36 associates with CD9 and integrins on human blood platelets. Blood 2001;97:1689–96. [DOI] [PubMed] [Google Scholar]
- 9.Erdman LK, Cosio G, Helmers AJ, et al. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J Immunol 2009;183:6452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab 2010;12:467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy DJ, Chen Y, Huang W, et al. CD36 and Na/K-ATPase-alpha1 form a pro-inflammatory signaling loop in kidney. Hypertension 2013;61:216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Kennedy DJ, Ramakrishnan DP, et al. Oxidized LDL-bound CD36 recruits an Na(+)/K(+)-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci Signal 2015;8:ra91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teese MG, Langosch D. Role of GxxxG motifs in transmembrane domain interactions. Biochemistry 2015;54:5125–35. [DOI] [PubMed] [Google Scholar]
- 14.Russ WP, Engelman DM. The GxxxG motif: a framework for transmembrane helix-helix association. J Mol Biol 2000;296:911–9. [DOI] [PubMed] [Google Scholar]
- 15.Bartosch B, Vitelli A, Granier C, et al. Cell entry of hepatitis C virus requires a set of coreceptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J Biol Chem 2003;278:41624–30. [DOI] [PubMed] [Google Scholar]
- 16.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 2000;105:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahaman SO, Lennon DJ, Febbraio M, et al. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab 2006;4:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mo X, Lu N, Padilla A, Lopez JA, Li R. The transmembrane domain of glycoprotein Ibbeta is critical to efficient expression of glycoprotein Ib-IX complex in the plasma membrane. J Biol Chem 2006;281:23050–9. [DOI] [PubMed] [Google Scholar]
- 19.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest 2009;119:136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thorne RF, Meldrum CJ, Harris SJ, et al. CD36 forms covalently associated dimers and multimers in platelets and transfected COS-7 cells. Biochem Biophys Res Commun 1997;240:812–8. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, Cooley BC, Li W, et al. Platelet CD36 promotes thrombosis by activating redox sensor ERK5 in hyperlipidemic conditions. Blood 2017;129:2917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Febbraio M, Reddy SP, et al. CD36 participates in a signaling pathway that regulates ROS formation in murine VSMCs. J Clin Invest 2010;120:3996–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med 2007;13:1086–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malaud E, Hourton D, Giroux LM, et al. The terminal six amino-acids of the carboxy cytoplasmic tail of CD36 contain a functional domain implicated in the binding and capture of oxidized low-density lipoprotein. Biochem J 2002;364:507–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattern HM, Raikar LS, Hardin CD. The effect of caveolin-1 (Cav-1) on fatty acid uptake and CD36 localization and lipotoxicity in vascular smooth muscle (VSM) cells. Int J Physiol Pathophysiol Pharmacol 2009;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 26.Finnemann SC, Silverstein RL. Differential roles of CD36 and alphavbeta5 integrin in photoreceptor phagocytosis by the retinal pigment epithelium. J Exp Med 2001;194:1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


