Abstract
The genetic basis of isoniazid (INH) resistance remains unknown for a significant proportion of clinical isolates. To identify genes which might confer resistance by detoxifying or sequestering INH, we transformed the Escherichia coli oxyR mutant, which is relatively sensitive to INH, with a Mycobacterium tuberculosis plasmid library and selected for INH-resistant clones. Three genes were identified and called ceo for their ability to complement the Escherichia coli oxyR mutant. ceoA was the previously identified M. tuberculosis glf gene, which encodes a 399-amino-acid NAD+- and flavin adenine dinucleotide-requiring enzyme responsible for catalyzing the conversion of UDP-galactopyranose to UDP-galactofuranose. The proteins encoded by the ceoBC pair were homologous with one another and with the N terminus of the potassium uptake regulatory protein TrkA. Each of the three Ceo proteins contains a motif common to NAD+ binding pockets. Overexpression of the M. tuberculosis glf gene by placing it under the control of the hsp60 promoter on a multicopy plasmid in Mycobacterium bovis BCG produced a strain for which the INH MIC was increased 50% compared to that for the control strains, while similar overexpression of the ceoBC pair had no effect on INH susceptibility in BCG. Mycobacterial extracts containing the overexpressed Glf protein did not bind radiolabeled INH directly, suggesting a more complex mechanism than the binding of unmodified INH. Our results support the hypothesis that upregulated mycobacterial proteins such as Glf may contribute to INH resistance in M. tuberculosis by binding a modified form of INH or by sequestering a factor such as NAD+ required for INH activity.
Since its discovery as an antimycobacterial drug, isonicotinic acid hydrazide (isoniazid [INH]) has been one of the first-line antibiotics in the treatment of tuberculosis. Because of its unique toxicity for tuberculocus mycobacteria, it has long been believed that insights into the mechanism of action of INH, as well as into the organism’s mechanisms of resistance, might provide clues to the human pathogenicity of Mycobacterium tuberculosis complex bacilli (2). Another strong impetus for investigating the mode of action of this drug has been the emergence of resistance to INH and other key antimycobacterial agents (11). Drug resistance poses a significant threat to our ability to control the estimated 8 million new cases of tuberculosis that occur annually.
It appears that M. tuberculosis may become resistant to INH by a variety of genetic changes. Alteration or loss of the katG gene encoding a catalase-peroxidase enzyme is clearly associated with INH resistance in a high proportion of clinical isolates (48, 49). DNA sequencing or PCR-single-strand conformational polymorphism analyses of INH-resistant strains have demonstrated katG alterations in as many as 97% (21) or as few as 18% (27) of strains. Other large studies indicate that about 50 to 75% of INH-resistant M. tuberculosis isolates contain at least one mutation in the katG gene locus (12, 20, 31). To account for the remaining fraction of strains with normal katG genes, other INH resistance genes must exist.
Regulators of katG, such as OxyR, a redox-sensitive protein which activates katG transcription following oxidative stress in gram-negative bacilli, were hypothesized to participate in INH resistance. However, M. tuberculosis complex species contain a defective, vestigial remnant of the oxyR gene in spite of the fact that other nontuberculous mycobacteria contain close oxyR homologues (6, 35). Another candidate, the alkyl hydroperoxidase gene (ahpC), whose expression is OxyR dependent in enteric bacilli, has also been investigated as an INH resistance gene (8, 50). While upregulatory mutations in the ahpC gene of M. tuberculosis are associated with virulence in katG mutant strains, there are conflicting reports as to whether overexpression of ahpC leads to INH resistance (13, 36, 43). Finally, mutations in inhA, which encodes a fatty acyl enoyl reductase that is a component of fatty acid synthase type II systems, have been shown to cause INH resistance in Mycobacterium smegmatis (1, 7). DNA sequencing of the inhA locus of INH-resistant M. tuberculosis strains has unmasked mutations in the 5′ noncoding sequences upstream of inhA, and it has been suggested that these substitutions may correlate with INH resistance (21, 31). Other studies have challenged the role of inhA in INH resistance in M. tuberculosis altogether (18). In the current model of INH action, KatG is believed to convert INH to an activated form that subsequently inhibits InhA or another enzyme involved in mycolic acid biosynthesis. In view of these uncertainties it is not surprising that most surveys of INH-resistant strains have documented that a high percentage of resistance (25 to 50%) remains unaccounted for by katG mutations or other putative resistance loci, such as inhA or ahpC.
Recently, Escherichia coli oxyR mutants were shown to be moderately sensitive to INH (29, 30). This sensitivity was exacerbated by oxidants such as hydrogen peroxide, suggesting that a reduced ability to detoxify reactive oxygen intermediates was associated with susceptibility to INH. Since a major mechanism of drug resistance is the acquisition or upregulation of detoxification enzymes, we postulated that some strains of M. tuberculosis might become INH resistant by overexpressing genes that modify or sequester the drug. In the present study we took advantage of the INH sensitivity of the E. coli oxyR mutant to select for M. tuberculosis gene products that might play such a role.
MATERIALS AND METHODS
Strains, plasmids, and culture conditions.
The bacterial strains and plasmids used here are listed in Table 1. E. coli strains were cultured on Luria-Bertani (LB) agar or broth with or without selective antibiotics. Mycobacterial strains were cultured in Middlebrook 7H9 broth or 7H10 agar (Difco) supplemented with albumin-dextrose complex, Tween 80, and glycerol according to the specifications of the manufacturers and with 50 μg of cycloheximide (Sigma) per ml. The concentrations of selective antibiotics for the pNBV1 vector were 200 μg of hygromycin for E. coli and 50 μg of hygromycin for mycobacteria per ml. The kanamycin concentration used for the pMV261 vector was 25 μg/ml for both E. coli and mycobacteria.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | φ80lacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) supE44 thi-1 gyrA96 | 33 |
| TA4112 | oxyRΔ3 [oxyΔ(oxyRbtuB)3] (derived from RK4936) | 42 |
| M. bovis BCG | Pasteur strain | ATCC 35734 |
| M. smegmatis mc26 1-2 C | Transformable variant of mc26 | 47 |
| Plasmids | ||
| pUC18 and 19 | High-copy-number E. coli plasmids | 33 |
| pMV261 | E. coli-mycobacterial shuttle vector with hsp60 promoter | 38 |
| pCJ5 | pUC18 containing the original 1,778-bp M. tuberculosis ceoA (glf) genomic sequence | This study |
| pCJ6 | pUC18 containing the original 1,692-bp M. tuberculosis ceoBC genomic sequence | This study |
| pCJ5-10 | pMV261 containing the ORF of glf (1,197 bp) cloned in frame with the hsp60 promoter | This study |
| pCJ6-10 | pMV261 containing the ORF of ceoB (681 bp) cloned in frame with the hsp60 promoter | This study |
| pCJ6-11 | pMV261 containing a 947-bp ceoBC sequence cloned in frame with the hsp60 promoter | This study |
Screening for INH-resistant TA4112 transformed with a library of M. tuberculosis DNA.
Genomic DNA from M. tuberculosis H37Rv was sheared by nebulization, and size-selected fragments ranging from 1.9 to 2.1 kb were gel purified, end repaired, and cloned into phosphatase-treated pUC18 according to the method developed by H. O. Smith and proven to give highly random insert distributions that are suitable for shotgun genomic sequencing projects (9). Characterization of the library showed that over 98% of transformants contained cloned single inserts. Electrocompetent E. coli TA4112 (oxyR deletion [see Table 1]) was electroporated with 0.5 μg of the library DNA in a 0.1-cm cuvette at 1.8 kV. Ampicillin-resistant transformants were selected on LB agar plates containing 400 μg of INH per ml, 400 μg of INH per ml–50 μM H2O2, or 400 μg of INH per ml–200 μM H2O2.
INH resistance assay for E. coli strains.
Tenfold dilutions of overnight cultures of TA4112 harboring recombinant pUC18 clones were made in LB broth, and 10 μl samples of each dilution were spotted onto LB agar plates containing ampicillin and various concentrations of INH or H2O2. We included H2O2 along with INH in our selection protocol since it suppressed the growth of minimally INH resistant colonies and produced a much cleaner background. After an overnight incubation at 37°C, the colonies in each spot were counted (up to 50 colonies could be counted accurately). For those spots that had more than 50 colonies the growth was scored in percentage scales compared to untreated controls. The minimal bactericidal concentration (MBC) was the average concentration at which fewer than 10 colonies survived (at least a fivefold reduction in CFU). This method was previously shown to correlate well with classical agar dilution assays (4).
DNA sequencing.
Recombinant plasmids of interest were end sequenced by double-stranded dye terminator methods with the M13 forward and reverse primers. To acquire internal sequence information, XhoI, HaeIII, and Sau3AI digests of the plasmid inserts were cloned into pUC18 or pUC19 and sequenced with the M13 forward and reverse primers. Primer walking was performed to complete the sequences. The sequences were aligned with the AssemblyALIGN software program (Oxford Molecular Group).
Cloning glf and ceoB into overexpression vectors.
To clone the glf open reading frame (ORF) into the mycobacterial overexpression vector pMV261, PCR amplification with the primers MtbrfbP1 (5′-CTGCAGCAACCGCTCGTTTTGACCTTTTCG) and MtbrfbP2 (5′-GAATTCGTTGACTCCTCGAGGTAC) and directional cloning with PstI and EcoRI were used. Similar strategies were used to construct pMV261-based expression vectors for ceoB and ceoBC with the primer pair MtbtrkAP1 (5′-GGATCCAATGCGGGTGGTTGTGATG) and MtbtrkAP2 (5′-GAATTCATGTCGTGTCCGTTTTCC) and the primer pair MtbtrkAP1 and MtbtrkAP3 (5′-CAGGCGTCGTTGAACAGC), respectively. The junctions between the hsp60 promoter and the beginning of the coding sequence of ceo genes were verified by DNA sequencing.
Transformation of mycobacteria.
Electrocompetent M. smegmatis and BCG were prepared by washing exponentially growing bacteria in sterile ice-chilled deionized water and storing them in 10% glycerol at −80°C until use. Plasmid DNA (100 to 500 ng) was mixed with 50 μl of competent cells on ice and electroporated at 1.8 kV in a 1-mm cuvette. The mycobacteria were rescued in 1 ml of Middlebrook 7H9 broth supplemented with 10% albumin-dextrose complex and 50 μg of cycloheximide per ml at 37°C for 2 to 4 h for M. smegmatis and overnight for BCG. The bacterial suspension was then plated in fractions onto Middlebrook 7H10 agar plates containing kanamycin. The plates were incubated at 37°C in 5% CO2 until the colonies were visible on the plates. The presence of the appropriate plasmid was confirmed in kanamycin-resistant transformants by colony PCR.
INH susceptibility testing of recombinant BCG strains.
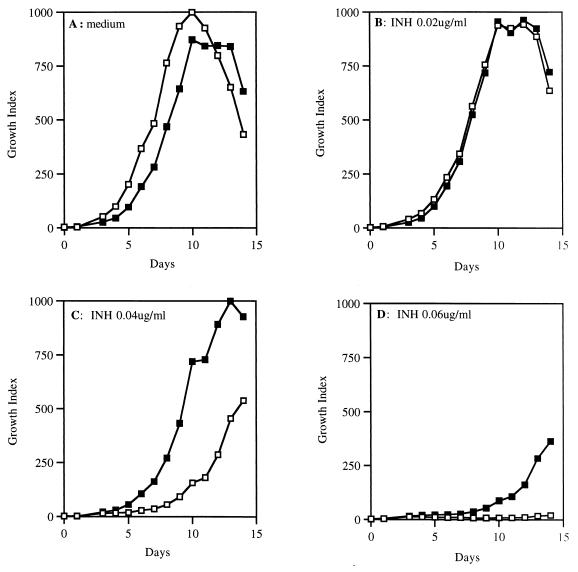
BCG harboring glf, ceoB, and ceoBC overexpression plasmids or a control plasmid grown on 7H10 agar plates were suspended in phosphate-buffered saline. Clumps were dispersed by vigorous vortexing in the presence of 3-mm glass beads. The remaining unbreakable clumps were removed by gravity. The clump-free suspension was adjusted to the optical density of McFarland standard no. 1. BACTEC bottles (12B medium; Becton Dickinson) were first inoculated with 0.1 ml of INH at different concentrations and 0.1 ml of kanamycin (for transformed BCG only) to a final concentration of 50 μg/ml. Then 0.1 ml of BCG suspension (McFarland standard no. 1) was inoculated into these bottles. The controls included diluted (1:100) and undiluted inocula in the absence of INH. The growth indices (GI) of these bottles were read daily at approximately same time each day for 14 days. Growth of the bacilli in a particular bottle was indicated if positive changes in GI from day to day continued during the incubation period, whereas no growth was noted when there were negative or no changes in GI (see Fig. 4). The INH MICs for the strains tested were defined as the lowest concentrations at which no growth was observed.
FIG. 4.
Overexpression of Glf confers INH resistance on BCG. By using the BACTEC radiometric assay, the growth of BCG harboring either control plasmid pMV261 (□) or the Glf overexpression plasmid pCJ5-10 (■) in the presence of 0 (A), 0.02 (B), 0.04 (C), or 0.06 (D) μg of INH per ml was evaluated daily over a period of 2 weeks.
In vitro INH binding assay.
Cell lysates (100 μg of total protein) from M. smegmatis carrying either pMV261 or pCJ5-10 were mixed with 5 μCi of 14C-labeled INH (specific activity, 59 mCi/mmol; AIDS Research and Reference Reagent Program, National Institutes of Health) in a 100-μl reaction mixture. After 30 min at 4°C, the mixture was passed over a Sephadex G-25 (Pharmacia) gel filtration column (1.0 by 20.0 cm) that had been prewashed and equilibrated with 10 mM Tris-HCl (pH 8.0) at 4°C. The flow rate was ca. 0.15 ml per min. Fractions (0.5 ml) were collected and assayed for radioactivity by scintillation counting, and the protein content was determined by the Coomassie blue protein assay (Pierce).
The sequences reported here have been deposited in the GenBank database (accession numbers AF026540 and AF026541).
RESULTS
Selection of clones which complement the E. coli oxyR mutant to INH resistance.
A highly random, size-selected M. tuberculosis genomic DNA library constructed in pUC18 was used to transform the INH-sensitive E. coli oxyR mutant TA4112. An estimated 24,000 ampicillin-resistant transformants were generated and evaluated in the selection for genes that conferred INH resistance on the E. coli oxyR mutant. With the average insert size in this library being 2.0 kb and a narrow distribution range of insert sizes (>99% of inserts were 1.9 to 2.1 kb), our selection tested the equivalent of approximately 4.8 × 107 bp of DNA, more than 10 times the size of the M. tuberculosis genome. Seven clones were selected on INH-H2O2, three of which showed a consistent phenotype upon retransformation into TA4112. Of these three clones, two nonidentical plasmids were derived for further study: pCJ5 (identified twice) and pCJ6. For TA4112 harboring pCJ5 and pCJ6, the MBCs of both INH and H2O2 are listed in Table 2. Each clone conferred at least a twofold increase in resistance to INH alone. In contrast, both TA4112 transformants were more sensitive to the H2O2 alone compared to the same strain harboring empty pUC19. This observation suggests that the effect conferred by pCJ5 and pCJ6 was specific to INH and that the recombinant plasmids did not encode peroxide-detoxifying proteins such as KatG.
TABLE 2.
MBCs of INH and hydrogen peroxide for E. coli TA4112 harboring glf and ceoBC
| Plasmid | MBC of:
|
|
|---|---|---|
| INH (μg/ml) | H2O2 (μM) | |
| pUC19 | 200 | 200 |
| pCJ5 | >400 | 150 |
| pCJ6 | >400 | 150 |
DNA sequencing identifies two M. tuberculosis genes that complement the E. coli oxyR mutant: glf and ceoBC.
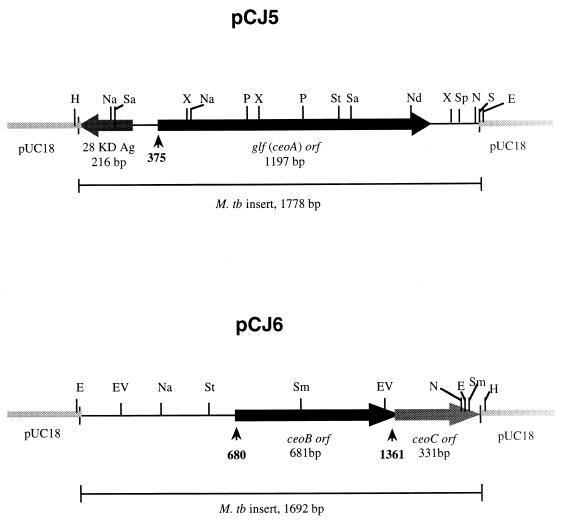
The inserts carried in pCJ5 and pCJ6 were sequenced. pCJ5 contained a 1,778-bp insert, and pCJ6 carried a 1,692-bp insert (Fig. 1). ORFs in the sequences were evaluated for their adherence to known mycobacterial codon usage, for the presence of appropriate translational start signals, and for their homology to other genes in the database.
FIG. 1.
ORF and restriction analysis of cloned DNA containing the ceo genes identified in pCJ5 and pCJ6. The black-boxed regions with arrowheads represent the ORFs of interest. The gray-boxed region with an arrowhead indicates the incomplete ORF of ceoC in pCJ6. The small arrows under each ORF indicate the positions of the initiation codons for ceo ORFs. The abbreviations for the restriction enzymes are as follows: E, EcoRI; EV, EcoRV; H, HindIII; N, NheI; Na, NarI; Nd, NdeI; P, PvuII; S, SalI; Sa, SacII; Sm, SmaI; Sp, SphI; St, StyI; and X, XhoI.
The 5′-terminal 216 bp of the 28-kDa antigen gene was present at one end of pCJ5, matching its previously reported sequences (3, 16). A 1,197-bp ORF was present in the center of the cloned DNA and was transcribed divergently from the 28-kDa antigen gene. The gene was originally named ceoA for complementing E. coli oxyR; later, when it became apparent that ceoA was identical to the recently identified M. tuberculosis glf gene encoding UDP-galactopyranose mutase, we adopted the glf nomenclature (40). The apparent start codon for the glf gene is ATG, and 11 bp upstream of the ATG a reasonable ribosome binding site (5′-AAGG-3′) appears.
In pCJ6 two tandem ORFs were identified that appear to be translationally coupled since the stop codon of the first overlaps the start codon of the second. We have named this gene pair ceoBC. Only the 5′-terminal 331 bp of ceoC is contained in clone pCJ6. The deduced protein sequences of CeoB and CeoC share significant homology to one another, with 30% amino acid identity over 110 residues. The apparent start codon of ceoB is ATG, and it is preceded by a ribosome binding site (5′-GAGAGGA-3′) spaced 6 bp upstream.
M. tuberculosis Glf and CeoBC share amino acid sequence homology to Glf and TrkA, respectively, and each contains an N-terminal NAD+ binding motif.
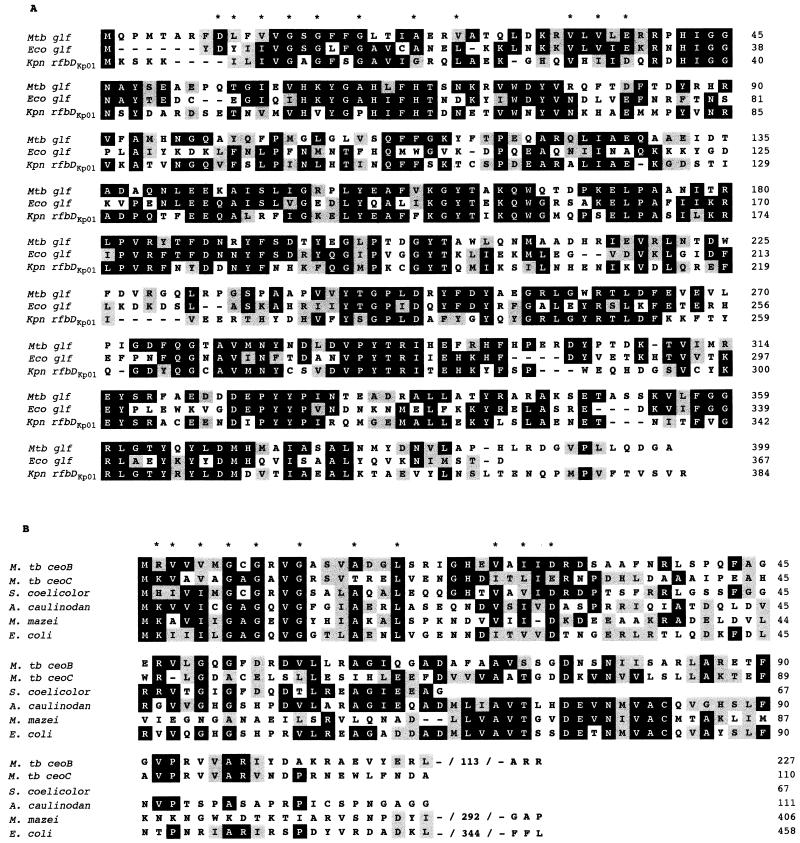
The M. tuberculosis glf gene encodes a deduced protein of 399 amino acids that is identical to the M. tuberculosis glf gene product identified by Weston et al. (40) and shows 43% amino acid identity to gene products from the rfb gene clusters of Klebsiella pneumoniae (39) and E. coli (37, 46), which participate in lipopolysaccharide O-antigen biosynthesis (Fig. 2A). At the N terminus of these proteins a classical NAD+ binding motif appears (45). The enzymes encoded by these genes have been biochemically characterized as the UDP-galactopyranose mutases that convert UDP-galactopyranose to UDP-galactofuranose (15, 23, 40).
FIG. 2.
Amino acid sequence alignments among Glf and RfbD homologues (A) and among CeoB, CeoC, and TrkA homologues (B). Identical amino acid residues are grouped in black boxes, and conserved residues are indicated in gray. Those amino acids involved in the NAD+ binding motif are identified with asterisks. The alignment was performed by the method of Hein (10) and is available in the DNAStar software package.
CeoB shares amino acid sequence homology with the N-terminal sequence of the TrkA protein family, with 52% identity with Streptomyces coelicolor TrkA and 25% identity with E. coli TrkA (Fig. 2B). TrkA is an essential component of the Trk protein complex that is responsible for the constitutive K+ uptake system in E. coli (5, 34). The same NAD+ binding motif found in the Glf family is also found in the CeoB and TrkA proteins of various species, including gram-negative bacteria (22, 24), S. coelicolor (41), Azorhizobium caulinodans (25), and archaebacteria (17). Part of a second gene, which we have named ceoC, is present immediately after ceoB starting at nucleotide position 1361, and it overlaps ceoB by one nucleotide. The CeoC fragment has 30% amino acid identity with CeoB and 24% identity with the TrkA proteins of S. coelicolor and E. coli (Fig. 2B).
Overexpression of the M. tuberculosis glf gene results in INH resistance in BCG.
Given that both glf and ceoB have NAD+ binding motifs in their primary amino acid sequences and that INH is a nicotinamide analogue, we hypothesized that overexpression of these NAD+ binding proteins might sequester or modify INH in vivo, leading to INH resistance. To test our hypothesis directly, plasmids overexpressing glf and ceoB were constructed and introduced into M. bovis BCG.
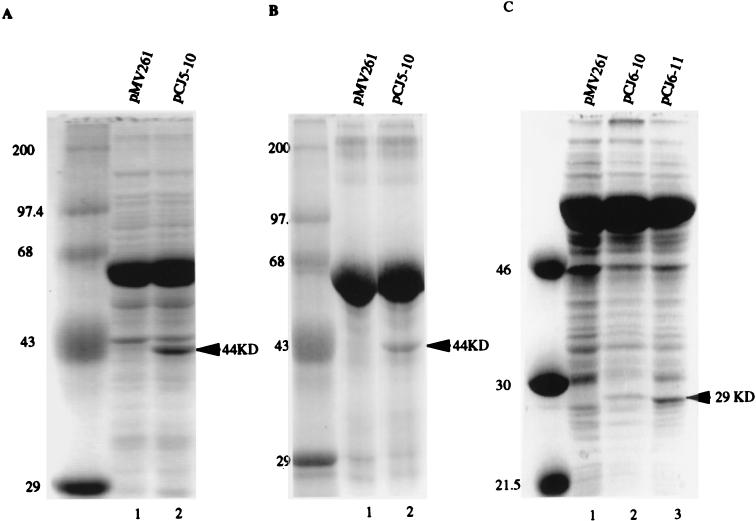
The coding sequences of the M. tuberculosis glf, ceoB, and ceoBC genes were placed under the control of the hsp60 promoter, which is strongly expressed in mycobacteria, to yield pCJ5-10, pCJ6-10, and pCJ6-11, respectively. Figure 3 shows the levels of Glf and CeoB expression in M. smegmatis and M. bovis BCG harboring these plasmids. A 44-kDa protein consistent with Glf was observed only in the extracts of pCJ5-10-transformed M. smegmatis (Fig. 3A, lane 2) and BCG (Fig. 3B, lane 2). A unique 29-kDa protein consistent with CeoB was found in both pCJ6-10- and pCJ6-11-transformed M. smegmatis extracts (Fig. 3C, lanes 2 and 3). The sizes of both proteins agreed with the predicted masses of 45 and 28 kDa for the M. tuberculosis Glf and CeoB proteins, respectively. Surprisingly, pCJ6-11, in which both CeoB and the CeoC protein fragment are expressed, appears to give significantly higher levels of the CeoB protein (Fig. 3C, lane 3) than does pCJ6-10, which does not contain CeoC-encoding sequences (Fig. 3C, lane 2).
FIG. 3.
Overexpression of the glf gene in M. smegmatis (A) and BCG (B) and of the ceoB gene in M. smegmatis (C). Whole bacterial SDS lysates equivalent to 10 mg of bacilli (wet weight) or 30 μg of partially purified protein extracts from mycobacteria harboring the glf (pCJ5-10), ceoB (pCJ6-10), or ceoBC (pCJ6-11) overexpression plasmids or the control plasmid (pMV261) were analyzed. Proteins were stained with Coomassie blue after SDS-polyacrylamide electrophoresis. The numbers on the left of each image indicate the sizes of the molecular weight standards, and the arrows on the right indicate the apparent molecular weights of Glf and CeoB.
To assess whether the overexpression of M. tuberculosis Glf or CeoB could lead to INH resistance in mycobacteria, we determined the INH MICs of BCG clones harboring pCJ5-10, pCJ6-10, and pCJ6-11 by using the BACTEC radiometric method. Figure 4 shows a panel of BACTEC growth indices for BCG–pCJ5-10 (recombinant glf overexpressor) and BCG-pMV261 (vector control) in which matched inocula of each strain were monitored daily for growth in the presence of different concentrations of INH. As can be seen, the growth of BCG-pMV261 (vector control) was inhibited by INH concentrations of 0.04 and 0.06 μg/ml, while BCG–pCJ5-10 continued to grow exponentially at these concentrations. At a 0.08-μg/ml concentration of INH, both BCG strains did not grow (data not shown). The 50% increase in the INH MIC for BCG overexpressing glf was consistently observed and reproduced with another recombinant BCG isolate. No significant differences in catalase activity were detected among these BCG strains. Thus, the overexpression of glf in BCG resulted in low-level INH resistance.
The same analysis with BCG-pCJ6-10 (recombinant ceoB overexpressor) and pCJ6-11 (recombinant ceoBC overexpressor) did not reveal changes in the INH susceptibility compared with the BCG control strain. Similarly, glf and ceoBC overexpression in M. smegmatis did not produce a significant increase in the INH MIC for this species. Since the INH MIC for M. smegmatis is higher (5 μg/ml) than for BCG (0.02 μg/ml), it is possible that the low sensitivity of M. smegmatis masked the effects of Glf and CeoBC even when they were overexpressed.
Glf-containing extracts do not reveal INH binding in vitro.
To evaluate whether the M. tuberculosis Glf protein might be acting as an INH-sequestering protein, we tested its ability to bind to INH in vitro. Cell extracts were prepared from M. smegmatis strains overexpressing the Glf protein from pCJ5-10, and the extracts were mixed with 14C-labeled INH. The mixtures were then passed through a Sephadex G-25 column to separate the proteins from unbound INH. The elution profiles for total protein and radiolabeled INH obtained from the control extract and the Glf overexpression extract were very similar. No radioactive INH was found to be associated with the protein fractions in the Glf overexpression extract and, in view of the fact that the Glf protein constitutes at least 1% of the soluble protein of this strain (Fig. 3A), our assay should have detected stoichiometric INH binding to Glf. One possible explanation for this observation could be that Glf might only bind to activated INH. It is also possible that certain components were absent in our assay, such as Mn2+ and NADH, which were used in the in vitro INH binding assay for InhA (28). To determine whether Glf interacts with activated INH in vivo, we cultured the M. smegmatis strain overexpressing the M. tuberculosis glf gene in the presence of radiolabeled INH. Cell lysates were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide electrophoresis, and the protein-associated radioactivity was detected by phosphorimaging. This analysis did not reveal in vivo binding of radiolabeled INH to the Glf protein expressed in M. smegmatis (data not shown). On the basis of this experiment we cannot exclude the possibility that in association with other mycobacterial proteins, perhaps BCG or M. tuberculosis-specific proteins, Glf does in fact bind INH or its activated intermediate. However, it is more likely that Glf sequesters a factor required for INH activity or that Glf modifies INH but is a relatively poor INH-binding protein.
DISCUSSION
Artificially overexpressing the glf gene in M. bovis BCG led to a small but reproducible change of the INH MIC from 0.06 to 0.08 μg/ml. Hence, the overexpression of the M. tuberculosis glf gene produces a strain that is almost twice as resistant to INH as the parent strain but which is still narrowly within the susceptibility range adopted by most clinical laboratories (0.1 μg/ml in BACTEC 12B medium). A twofold increase in the MIC has also been observed when the ahpC gene was overexpressed in M. tuberculosis H37Rv (13).
Low-level mycobacterial resistance to INH may be important clinically. Humans metabolize INH by hepatic N-acetylation, and population-based studies have shown that individuals are either rapid or slow INH acetylators (26). By 6 h after a 4-mg/kg oral dose of INH, rapid acetylators have an INH concentration in serum of <0.2 μg/ml. In view of the growing trend towards the twice-weekly administration of INH, rapid acetylators infected with M. tuberculosis strains with low-level INH resistance may experience prolonged intervals of subtherapeutic drug levels. Low-level resistance mutations might predispose to high-level resistance mutations. By permitting a larger portion of the bacillary population to survive an initial exposure to INH, such mutations might in effect “buy time” for classical katG mutations to arise.
The M. tuberculosis glf gene which we found in this study encodes the Glf enzyme that catalyzes the conversion of UDP-galactopyranose to UDP-galactofuranose (40). The latter is the substrate for the biosynthesis of arabinogalactan, an essential cell wall component of mycobacteria. The enzyme’s unique substrate specificity and pivotal role in arabinogalactan biosynthesis have made Glf a target for developing new antimycobacterial agents. Our results suggest that Glf may also participate in INH resistance. Although our binding assay did not detect Glf sequestration or covalent linkage to INH, it remains possible that Glf is an INH binding protein in the presence of appropriate cofactors or with the activated form of INH.
Alternatively, Glf may confer relative INH resistance by sequestering a cofactor for INH action such as NAD+ or NADH. An interaction between INH and NAD+ has been proposed (44) and is supported by a considerable amount of biochemical and genetic literature. Quemard et al. (28) have shown that binding of the radiolabeled, activated INH to InhA only occurred in the presence of NADH, and another biochemical study revealed that NAD+ or NADH was required for the inhibition of InhA (14). A recent study has shown that InhA binds NADH and that the binary enzyme-nucleotide complex is the target for activated INH, which causes covalent attachment of NAD+ to InhA (32). Mutations in the NADH binding domain of InhA reduce the affinity of NADH binding to the enzyme and confer resistance to INH (7, 28).
Further evidence suggesting the importance of NADH in INH resistance came from a study by Miesel et al. (19). Temperature-sensitive, INH-resistant mutants of M. smegmatis were found to have mutations in the ndh gene (type II) encoding NADH dehydrogenase (Ndh). Genes encoding Ndh and malate dehydrogenase (Mdh), another enzyme that utilizes NADH, from M. tuberculosis complemented the mutant phenotypes. These investigators propose that the intracellular ratio of NADH to NAD+ can influence the activation of INH by KatG or the interaction of INH with its target InhA. Thus, Glf, which requires reduced flavin adenine dinucleotide and either NADH or NADPH for activity (40), may cause INH resistance by influencing the NADH/NAD+ ratio.
While it is clear that katG mutations play an important role in INH resistance, the resistance mechanisms involved in catalase-positive, INH-resistant strains have not been fully explained. Further studies of glf expression in clinical isolates will be necessary to determine if the findings described here play a significant role in human drug-resistant tuberculosis.
ACKNOWLEDGMENTS
We thank G. Storz and J. Rosner for strains; B. Dougherty, R. Stern, and M. McNeil for helpful suggestions; and H. O. Smith for advice and assistance with the DNA library preparation.
This work was supported by NIH grant AI36973, a Young Investigator Matching Grant from the National Foundation for Infectious Diseases, and an equipment grant from Becton Dickinson and Co. P.C. was supported by a Feinstone Fellowship and by NIH training grant AI07417.
REFERENCES
- 1.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um K S, Wilson T, Collins D, de Lisle G, Jacobs W R., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263:227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 2.Barry C E, III, Mdluli K. Drug sensitivity and environmental adaptation of mycobacterial cell wall components. Trends Microbiol. 1996;4:275–281. doi: 10.1016/0966-842x(96)10031-7. [DOI] [PubMed] [Google Scholar]
- 3.Bigi F, Alito A, Fisanotti J C, Romano M I, Cataldi A. Characterization of a novel Mycobacterium bovis secreted antigen containing PGLTS repeats. Infect Immun. 1995;63:2581–2586. doi: 10.1128/iai.63.7.2581-2586.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishai W R, Howard N S, Winkelstein J A, Smith H O. Characterization and virulence analysis of catalase mutants of Haemophilus influenzae. Infect Immun. 1994;62:4855–4860. doi: 10.1128/iai.62.11.4855-4860.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossemeyer D, Borchard A, Dosch D C, Helmer G C, Epstein W, Booth I R, Bakker E P. K+-transport protein TrkA of Escherichia coli is a peripheral membrane protein that requires other trk gene products for attachment to the cytoplasmic membrane. J Biol Chem. 1989;264:16403–16410. [PubMed] [Google Scholar]
- 6.Deretic V, Philipp W, Dhandayuthapani S, Mudd M H, Curcic R, Garbe T, Heym B, Via L E, Cole S T. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995;17:889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- 7.Dessen A, Quemard A, Blanchard J S, Jacobs W R, Jr, Sacchettini J C. Crystal structure and function of the isoniazid target of Mycobacterium tuberculosis. Science. 1995;267:1638–1641. doi: 10.1126/science.7886450. [DOI] [PubMed] [Google Scholar]
- 8.Dhandayuthapani S, Zhang Y, Mudd M H, Deretic V. Oxidative stress response and its role in sensitivity to isoniazid in mycobacteria: characterization and inducibility of ahpC by peroxides in Mycobacterium smegmatis and lack of expression in M. aurum and M. tuberculosis. J Bacteriol. 1996;178:3641–3649. doi: 10.1128/jb.178.12.3641-3649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 10.Hein J. Unified approach to alignment and phylogenies. Methods Enzymol. 1990;183:626–645. doi: 10.1016/0076-6879(90)83041-7. [DOI] [PubMed] [Google Scholar]
- 11.Heym B, Honore N, Truffor-Pernod C, Banerjee A, Schurra C, Jacobs W R, Jr, van Embden J D A, Grosset J H, Cole S T. Implications of multidrug resistance for the future of short-course chemotherapy of tuberculosis: a molecular study. Lancet. 1994;344:293–298. doi: 10.1016/s0140-6736(94)91338-2. [DOI] [PubMed] [Google Scholar]
- 12.Heym B, Alzari P M, Honore N, Cole S T. Missense mutations in the catalase-peroxidase gene, katG, are associated with isoniazid resistance in Mycobacterium tuberculosis. Mol Microbiol. 1995;15:235–245. doi: 10.1111/j.1365-2958.1995.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 13.Heym B, Stavropoulos E, Honoré N, Domenech P, Saint-Joanis B, Wilson T M, Collins D M, Colston M J, Cole S T. Effects of overexpression of the alkyl hydroperoxide reductase AhpC on the virulence and isoniazid resistance of Mycobacterium tuberculosis. Infect Immun. 1997;65:1395–1401. doi: 10.1128/iai.65.4.1395-1401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsson K, King D S, Schultz P G. Studies on the mechanism of action of isoniazid and ethionamide in the chemotherapy of tuberculosis. J Am Chem Soc. 1995;117:5009–5010. [Google Scholar]
- 15.Kîplin R, Brisson J-R, Whitfield C. UDP-galactofuranose precursor required for formation of the lipopolysaccharide O antigen of Klebsiella pneumoniae serotype O1 is synthesized by the product of the rfbDKP01 gene. J Biol Chem. 1997;272:4121–4128. doi: 10.1074/jbc.272.7.4121. [DOI] [PubMed] [Google Scholar]
- 16.Lim E M, Rauzier J, Timm J, Torrea G, Murray A, Gicquel B, Portnoi D. Identification of Mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J Bacteriol. 1995;177:59–65. doi: 10.1128/jb.177.1.59-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macario A J L, Dugan C B, de Macario E C. An archaeal trkA homologue near dnaK and dnaJ. Biochim Biophys Acta. 1993;1216:495–498. doi: 10.1016/0167-4781(93)90022-6. [DOI] [PubMed] [Google Scholar]
- 18.Mdluli K, Sherman D R, Hickey M J, Kreiswirth B N, Morris S, Stover C K, Barry C E., III Biochemical and genetic data suggest that InhA is not the primary target for activated isoniazid in Mycobacterium tuberculosis. J Infect Dis. 1996;174:1085–1090. doi: 10.1093/infdis/174.5.1085. [DOI] [PubMed] [Google Scholar]
- 19.Miesel L, Weisbrod T R, Marcinkeviciene J A, Bittman R, Jacobs W R., Jr NADH dehydrogenase defects confer isoniazid resistance and conditional lethality in Mycobacterium smegmatis. J Bacteriol. 1998;180:2459–2467. doi: 10.1128/jb.180.9.2459-2467.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris S, Bai G H, Suffys P, Portillo-Gomez L, Fairchok M, Rouse D. Molecular mechanisms of multiple drug resistance in clinical isolates of Mycobacterium tuberculosis. J Infect Dis. 1995;171:954–960. doi: 10.1093/infdis/171.4.954. [DOI] [PubMed] [Google Scholar]
- 21.Musser J M, Kapur V, Williams D L, Kreiswirth B N, van Soolingen D, van Embden J D A. Characterization of the catalase-peroxidase gene (katG) and inhA locus in isoniazid-resistant and -susceptible strains of Mycobacterium tuberculosis. J Infect Dis. 1996;173:196–202. doi: 10.1093/infdis/173.1.196. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Matsuba Y, Yamamuro N, Booth I R, Unemoto T. Cloning and sequencing of a K+ transport gene (trkA) from the marine bacterium Vibrio alginolyticus. Biochim Biophys Acta. 1994;1219:701–705. doi: 10.1016/0167-4781(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 23.Nassau P M, Martin S L, Brown R E, Weston A, Monsey D, McNeil M R, Duncan K. Galactofuranose biosynthesis in Escherichia coli K-12: identification and cloning of UDP-galactopyranose mutase. J Bacteriol. 1996;178:1047–1052. doi: 10.1128/jb.178.4.1047-1052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parra-Lopez C, Lin R, Aspedon A, Groisman E A. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J. 1994;13:3964–3972. doi: 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlowski K, Klosse U, de Bruijn F J. Characterization of a novel Azorhizobium caulinodans ORS571 two-component regulatory system, NtrY/NtrX, involved in nitrogen fixation and metabolism. Mol Gen Genet. 1991;231:124–138. doi: 10.1007/BF00293830. [DOI] [PubMed] [Google Scholar]
- 26.Pratt W B. Chemotherapy of infection. New York, N.Y: Oxford University Press; 1977. Chemotherapy of tuberculosis; pp. 231–262. [Google Scholar]
- 27.Pretorius G S, van Helden P D, Sirgel F, Eisenach K D, Victor T C. Mutation in katG gene sequences in isoniazid-resistant clinical isolates of Mycobacterium tuberculosis are rare. Antimicrob Agents Chemother. 1995;39:2276–2281. doi: 10.1128/aac.39.10.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quemard A, Dessen A, Sugantino M, Jacobs W R, Jr, Sacchettini J C, Blanchard J S. Binding of catalase-peroxidase-activated isoniazid to wild-type and mutant Mycobacterium tuberculosis enoyl-ACP reductase. J Am Chem Soc. 1996;118:1561–1562. [Google Scholar]
- 29.Rosner J L. Susceptibilities of oxyR regulon mutants of Escherichia coli and Salmonella typhimurium to isoniazid. Antimicrob Agents Chemother. 1993;37:2251–2253. doi: 10.1128/aac.37.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosner J L, Storz G. Effects of peroxides on susceptibilities of Escherichia coli and Mycobacterium smegmatis to isoniazid. Antimicrob Agents Chemother. 1994;38:1829–1833. doi: 10.1128/aac.38.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouse D A, Li Z, Bai G H, Morris S L. Characterization of the katG and inhA genes of isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:2472–2477. doi: 10.1128/aac.39.11.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rozwarski D A, Grant G A, Barton D H R, Jacobs W R, Jr, Sacchettini J C. Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis. Science. 1998;279:98–102. doi: 10.1126/science.279.5347.98. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schlösser A, Hamann A, Bossemeyer D, Schneider E, Bakker E P. NAD+ binding to the Escherichia coli K+-uptake protein TrkA and sequence similarity between TrkA and domains of a family of dehydrogenases suggest a role for NAD+ in bacterial transport. Mol Microbiol. 1993;9:533–543. doi: 10.1111/j.1365-2958.1993.tb01714.x. [DOI] [PubMed] [Google Scholar]
- 35.Sherman D R, Sabo P J, Hickey M J, Arain T M, Mahairas G G, Yuan Y, Barry III D E, Stover C K. Disparate responses to oxidative stress in saprophytic and pathogenic mycobacteria. Proc Natl Acad Sci USA. 1995;92:6625–6629. doi: 10.1073/pnas.92.14.6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman D R, Mdluli K, Hickey M J, Arain T M, Morris S L, Barry III C E, Stover C K. Compensatory ahpC gene expression in isoniazid-resistant Mycobacterium tuberculosis. Science. 1996;272:1641–1643. doi: 10.1126/science.272.5268.1641. [DOI] [PubMed] [Google Scholar]
- 37.Stevenson G, Neal B, Liu D, Hobbs M, Packerm N H, Batley M, Redmond J W, Lindquist L, Reeves P. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J Bacteriol. 1994;176:4144–4156. doi: 10.1128/jb.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Jr, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 39.Szabo M, Bronner D, Whitfield C. Relationships between rfb gene clusters required for biosynthesis of identical d-galactose-containing O antigens in Klebsiella pneumoniae serotype O1 and Serratia marcescens serotype O16. J Bacteriol. 1995;177:1544–1553. doi: 10.1128/jb.177.6.1544-1553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weston A, Stern R J, Lee R E, Nassau P M, Monsey D, Martin S L, Scherman M S, Besra G S, Duncan K, McNeil M. Biosynthetic origin of mycobacterial cell wall galactofuranosyl residues. Tubercle Lung Dis. 1998;78:123–131. doi: 10.1016/s0962-8479(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 41.Takano E, Gramajo H C, Strauch E, Andres N, White J, Bibb M J. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2) Mol Microbiol. 1992;6:2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 42.Tartaglia L A, Storz G, Ames B N. Identification and molecular analysis of oxyR-regulated promoters important for the bacterial adaptation to oxidative stress. J Mol Biol. 1989;210:709–719. doi: 10.1016/0022-2836(89)90104-6. [DOI] [PubMed] [Google Scholar]
- 43.Wilson T M, Collins D M. ahpC, a gene involved in isoniazid resistance of the Mycobacterium tuberculosis complex. Mol Microbiol. 1996;19:1025–1034. doi: 10.1046/j.1365-2958.1996.449980.x. [DOI] [PubMed] [Google Scholar]
- 44.Winder F G. Mode of action of the antimycobacterial agents and associated aspects of the molecular biology of the mycobacteria. In: Ratledge C, Stanford J, editors. The biology of the mycobacteria. New York, N.Y: Academic Press, Inc.; 1982. pp. 353–438. [Google Scholar]
- 45.Wirenga R K, Terpstra P, Hol W G J. Prediction of the occurrence of the ADP-binding βαβ-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 46.Yao Z, Valvano M A. Genetic analysis of the O-specific lipopolysaccharide biosynthesis region (rfb) of Escherichia coli K-12 W3110: identification of genes that confer group 6 specificity to Shigella flexneri serotypes Y and 4a. J Bacteriol. 1994;176:4133–4143. doi: 10.1128/jb.176.13.4133-4143.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Lathigra R, Garbe T, Catty D, Young D. Genetic analysis of superoxide dismutase, the 23-kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991;5:381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358:591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Garbe T, Young D. Transformation with katG restores isoniazid-sensitivity in Mycobacterium tuberculosis isolates resistant to a range of drug concentrations. Mol Microbiol. 1993;8:521–534. doi: 10.1111/j.1365-2958.1993.tb01596.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Dhandayuthapani S, Deretic V. Molecular basis for the exquisite sensitivity of Mycobacterium tuberculosis to isoniazid. Proc Natl Acad Sci USA. 1996;93:13212–13216. doi: 10.1073/pnas.93.23.13212. [DOI] [PMC free article] [PubMed] [Google Scholar]