Abstract
Lipopolysaccharide (LPS) is cleared from the blood mainly by the liver. The Kupffer cells are primarily responsible for this clearance; liver endothelial and parenchymal cells contribute to a lesser extent. Although several binding sites have been described, only CD14 is known to be involved in LPS signalling. Among the other LPS binding sites that have been identified are scavenger receptors. Scavenger receptor class A (SR-A) types I and II are expressed in the liver on endothelial cells and Kupffer cells, and a 95-kDa receptor, identified as macrosialin, is expressed on Kupffer cells. In this study, we examined the role of scavenger receptors in the binding of LPS by the liver in vivo and in vitro. Fucoidin, a scavenger receptor ligand, significantly reduced the clearance of 125I-LPS from the serum and decreased the liver uptake of 125I-LPS about 40%. Within the liver, the in vivo binding of 125I-LPS to Kupffer and liver endothelial cells was decreased 72 and 71%, respectively, while the binding of 125I-LPS to liver parenchymal cells increased 34% upon fucoidin preinjection. Poly(I) inhibited the binding of 125I-LPS to Kupffer and endothelial cells in vitro 73 and 78%, respectively, while poly(A) had no effect. LPS inhibited the binding of acetylated low-density lipoprotein (acLDL) to Kupffer and liver endothelial cells 40 and 55%, respectively, and the binding of oxidized LDL (oxLDL) to Kupffer and liver endothelial cells 65 and 61%, respectively. oxLDL and acLDL did not significantly inhibit the binding of LPS to these cells. We conclude that on both endothelial cells and Kupffer cells, LPS binds mainly to scavenger receptors, but SR-A and macrosialin contribute to a limited extent to the binding of LPS.
Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, causes the same clinical features as can be observed in patients with sepsis (24). LPS administered to an organism is cleared from the blood mainly by the liver (10, 12). Kupffer cells, and to a lesser extent liver endothelial cells and liver parenchymal cells, are responsible for the clearance of LPS from the blood (25, 29, 36, 49). Upon binding of LPS to Kupffer cells, LPS is taken up and processed (11). Uptake of LPS by Kupffer cells leads to the release of mediators, such as tumor necrosis factor alpha (TNF-α), interleukin-1α (IL-1α), IL-1β, and IL-6, by those cells (4, 6), which have profound effects on the physiological and metabolic state of the liver and body.
Several binding sites for LPS have been described, but the binding of LPS to these sites does not necessarily lead to a biological response of the target cells. Wright and Jong showed that the CD11-CD18 complex on human macrophages was able to bind LPS (45). This receptor consists of a CD18 β chain and a CD11a, -b, or -c α chain. CD11-CD18 recognizes the lipid A part of LPS and may contribute to the clearance of LPS from the circulation. Monocyte-derived macrophages of which the CD11/CD18 receptors are blocked show a reduced binding of erythrocyte-bound LPS and Escherichia coli bacteria (45). In addition, polymorphonuclear leukocytes of CD18-deficient patients are significantly less able than control cells to bind LPS (46). However, leukocytes obtained from patients who lack CD18 are able to respond to LPS in the same way as leukocytes from healthy humans (44), and treatment of leukocytes with an antibody against CD18 does not reduce the LPS-induced release of TNF-α by these cells (47). Until recently, no role in cellular activation by LPS was known for the CD18 receptor, but Ingalls and Golenbock described an activation of the transcription factor NF-κB upon stimulation of CD11c-CD18-transfected CHO cells with LPS (18).
In the circulation, LPS may bind via lipid A to the LPS binding protein (LBP), which is an acute-phase protein produced by parenchymal cells (37, 38, 48). Binding of LPS by LBP is followed by binding of this complex to CD14 (47). Although it is known that CD14 mediates LPS-induced cell activation (40, 41, 47), the exact mechanism is not fully understood. Since CD14 is a glycosylphosphatidylinositol (GPI)-anchored membrane protein (17, 33), direct communication with the interior of the cell is not possible, indicating that an additional protein(s) is required for signalling. This protein has not been identified. Furthermore, LBP may also transfer LPS to a soluble form of CD14, forming a complex that binds to non-CD14-bearing cells and can provoke a cellular response in these cells (13, 26). A heptose-specific receptor for LPS is located on liver parenchymal cells (23). A metabolic response upon binding of LPS to this receptor has not been reported.
Another group of receptors known to bind LPS are the scavenger receptors. Scavenger receptors are reported to bind modified forms of low-density lipoprotein (LDL) (reviewed in reference 34), as well as lipid IVA (the bioactive precursor of lipid A) and LPS (15). Binding of lipid A or lipid IVA does not result in a metabolic response but may be important in the clearance and detoxification of LPS in animals. Liver cells express at least two kinds of scavenger receptors. In the liver, the 220-kDa scavenger receptor, known as scavenger receptor class A (SR-A), is expressed predominantly on endothelial cells but also present on Kupffer cells. Two types of SR-A (type I and type II), which are equally efficient in the binding and uptake of acetylated LDL (acLDL), have been described (19, 28). In addition to SR-A, Kupffer cells express a 95-kDa scavenger receptor that is specific for oxidized LDL (oxLDL) (7, 8), identified as macrosialin, the mouse equivalent of CD68 (43).
In this study, we examined the role of scavenger receptors on Kupffer and liver endothelial cells in the binding of LPS.
MATERIALS AND METHODS
Chemicals.
125I, sodium salt, was obtained from Amersham, Buckinghamshire, England; collagenase type IV, bovine serum albumin (BSA) fraction V, fucoidin, poly(I), and poly(A) were from Sigma, St. Louis, Mo. LPS from Salmonella minnesota R595 (Re-LPS) was obtained as a lyophilized powder from List Biological Laboratories Inc., Campbell, Calif. Dulbecco’s modified Eagle’s medium (DMEM) was from GIBCO, Irvine, Scotland. Nycodenz was from Nycomed A/S, Oslo, Norway; phospholipase C was from Boehringer GmbH, Mannheim, Germany. All other reagents were of analytical grade.
Isolation and modification of LDLs.
Blood from healthy volunteers was collected into K3-EDTA (1 mg/ml)-containing tubes. Plasma was separated from the blood within 1 h, and butylated hydroxytoluene (40 μM) in dimethylsulfoxide (Me2SO; 0.1%, vol/vol) was added. LDL (density of 1.019 to 1.063 g/ml) was isolated by density gradient ultracentrifugation as described by Redgrave et al. (27). Immediately after isolation, 40 μM butylated hydroxytoluene was added to the LDL, except for the LDL used for oxidative modification. Acetylation of LDL was done as described by Basu et al. (1a). Cu2+ oxidation of LDL was performed as described earlier (42). Lipoproteins were dialyzed against Tris buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.2 mM EDTA) and saturated with nitrogen. acLDL and oxLDL were radiolabelled by the method of McFarlane (21) as modified by Bilheimer et al. (2).
Labelling of LPS.
LPS was labelled by the method of Ulevitch (39). LPS was derivatized with p-OH methylbenzimidate and dialyzed against phosphate-buffered saline. The modified LPS was subsequently iodinated by using chloramine T. The reaction was stopped by the addition of sodium metabisulfite. Free Na125I was removed by dialysis against phosphate-buffered saline for 72 h. The labelling method did not alter the biological activity of LPS as determined by the method of Ulevitch (39).
Serum decay, liver uptake, and organ distribution.
Male Wistar rats (220 to 350 g) were anesthetized by intraperitoneal injection of sodium pentobarbital (15 mg/kg of body weight). The abdomens were opened, and 125I-LPS was injected in the inferior vena cava with or without a preinjection of fucoidin 1 min prior to 125I-LPS injection. At the indicated times, blood samples of 300 μl were withdrawn from the inferior vena cava and allowed to clot for 30 min. The samples were centrifuged for 3 min at 10,000 × g, and serum samples of 100 μl were counted for radioactivity.
At the indicated times, liver lobules were tied off and removed. The liver lobules were weighed, and radioactivity was determined. Radioactivity was corrected for radioactivity in serum determined to be present at the time of sampling.
Cell distribution.
Rats were anesthetized by intraperitoneal injection of sodium pentobarbital (15 mg/kg of body weight). The abdomens were opened, and 125I-LPS was injected in the inferior vena cava with or without a preinjection of fucoidin 1 min prior to 125I-LPS injection. After 15 min, the liver was perfused with Hanks’ buffer containing 1.6 g of HEPES/liter at 8°C. After 8 min of perfusion, a lobule was tied off and removed for determination of total liver uptake. The liver was subsequently perfused with collagenase (0.05%, wt/vol) at 8°C, and parenchymal cells were separated from nonparenchymal cells by differential centrifugation. The liver residue was further digested by stirring with pronase (0.25%, wt/vol) at 8°C, and the endothelial cells and Kupffer cells were separated by centrifugal elutriation as described before (22). The contribution of the various liver cell types to total liver uptake was calculated as described earlier (22).
Binding studies.
Endothelial and Kupffer liver cells were isolated from male Wistar rats (250 to 300 g) by collagenase (type IV, 0.05%) perfusion of the liver by the method of Seglen (31) and subsequent counterflow centrifugation as described in detail elsewhere (20). Kupffer and endothelial cells were more than 95% pure, as judged by peroxidase staining for 20 min at 37°C in Tris buffer (pH 7.4) containing 0.1% (wt/vol) 3,3′diaminobenzidine, 7% (wt/vol) sucrose, and 0.3% H2O2.
Freshly isolated cells were incubated with 125I-LPS, in the presence or absence of a competitor, in DMEM containing 2% (wt/vol) BSA (total volume of 0.5 ml) at 4°C for 2 h. A circulating lab shaker (Adolf Kühner AG, Switzerland) at 150 rpm was used. After incubation, the cells were washed twice with 1 ml of medium containing 50 mM Tris-HCl, 0.15 M NaCl, 5 mM CaCl2, and 0.2% BSA (pH 7.4) and once with 1 ml of medium without BSA. Cells were resuspended in 0.5 ml of H2O, and radioactivity was counted in a Packard gamma counter. Protein was then determined by the Lowry method, using BSA as the standard.
For displacement studies, the binding of 125I-LPS without competitors was taken as 100% binding. For saturation studies, maximal binding of LPS to the different cell types was determined by computerized nonlinear graph fitting according to a single-site binding model (20).
Statistical analysis.
Statistical significance was determined by using a two-way analysis of variance or a two-tailed Student t test. Data are expressed as means, and errors represent the variation between data points (when n = 2) or standard deviation (SD) (n ≥ 3).
RESULTS
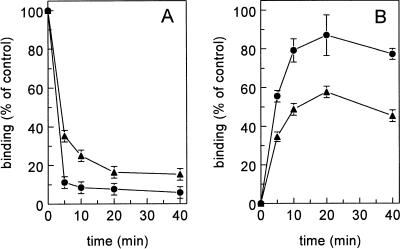
To identify the role of scavenger receptors in the metabolism of LPS, we determined the effect of fucoidin, a well-described inhibitor of scavenger receptors, on the uptake of LPS by the liver. In vivo, the serum decay of 125I-LPS in rats that received a preinjection of fucoidin was less than that in control rats (P < 0.0001) (Fig. 1A). Upon injection of 125I-LPS, a biphasic serum decay was observed, and approximately 90% of the LPS was cleared in the first phase (half-life of <2 min). In control rats, 5.8% ± 1.2% of the injected radioactivity was found in the serum 40 min after injection of 125I-LPS. After preinjection of fucoidin, approximately 65% was cleared from the serum in the first 5 min, and 15.2% ± 1.3% of the radioactivity remained in the serum until 40 min after injection. In control rats, maximally 82.0% ± 10.0% of the injected dose (ID) was recovered in the liver at 20 min after injection (Fig. 1B). At 40 min after injection, still 77.3% ± 2.8% of the injected radioactivity was found in the liver. After preinjection of fucoidin, the radioactivity found in the liver at 20 min after injection was 57.6% ± 2.5% of the ID, which is a 34% reduction compared to control rats. At 40 min after injection, fucoidin reduced the maximum radioactivity in the liver to 45.4% ± 0.4% of the ID, a decrease of 41% (P < 0.01).
FIG. 1.
Serum decay and liver uptake of 125I-LPS. Rats were injected with 125I-LPS without (•) or with (▴) a preinjection of fucoidin (15 mg/kg). At the indicated times, levels of radioactivity in serum (A) and liver (B) were determined. Data are means of two experiments ± variation.
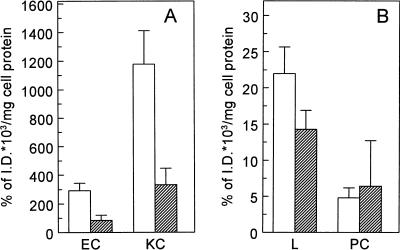
To determine the role of the various liver cell types in the liver uptake of 125I-LPS, we determined the liver cell distribution of 125I-LPS. In control rats, Kupffer cells showed a specific binding of 1,179% ± 233% of the ID × 103/mg of cell protein. Preinjection of rats with fucoidin decreased the binding of 125I-LPS to Kupffer cells 72%, to 331% ± 116% of the ID × 103/mg of cell protein (P < 0.005) (Fig. 2A). The specific binding of 125I-LPS to endothelial cells in control rats was 292% ± 52% of the ID × 103/mg of cell protein, and that in fucoidin-treated rats was 84% ± 37% of the ID × 103/mg of cell protein, a reduction of 71% (P < 0.005) (Fig. 2A). Although the absolute binding of 125I-LPS to both Kupffer and endothelial cells decreased after preinjection with fucoidin, no effect of fucoidin on the specific binding of 125I-LPS to the parenchymal cells was observed (Fig. 2B).
FIG. 2.
Cell distribution of 125I-LPS. Rats were injected with 125I-LPS without (open bars) or with (hatched bars) a preinjection of fucoidin (15 mg/kg). Fifteen minutes after 125I-LPS injection, the liver was perfused at 8°C, cells were isolated, and binding of 125I-LPS to the different liver cell types was determined. (A) Binding to endothelial cells (EC) and Kupffer cells (KC); (B) binding to liver tissue (L) and parenchymal cells (PC). Data are means of three experiments ± SD.
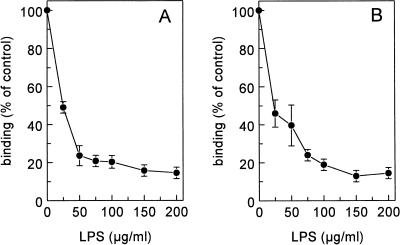
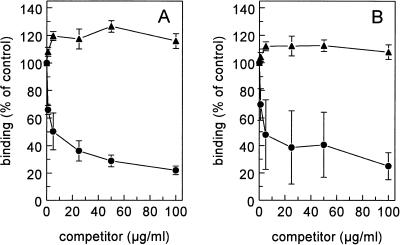
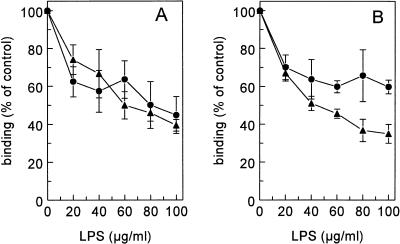
The influence of various scavenger receptor ligands on the binding of 125I-LPS to Kupffer and liver endothelial cells was tested on freshly isolated liver cells. Unlabelled LPS inhibited the binding of 125I-LPS to endothelial and Kupffer cells 85% ± 2% and 86% ± 1%, respectively, indicating that specific binding sites were involved in the interaction of LPS with those cells (Fig. 3). LPS displaced itself with a Ki of 6 μg/ml on endothelial cells and a Ki of 12.7 μg/ml on Kupffer cells. Scavenger receptors discriminate between polynucleotides: poly(I) is a scavenger receptor ligand, while poly(A) is not. Therefore, we evaluated the influence of poly(I) and poly(A) on the binding of LPS to isolated liver endothelial and Kupffer cells. Poly(I) inhibited the binding of 125I-LPS to endothelial and Kupffer cells 78% ± 3% and 75% ± 10%, respectively. Poly(I) displaced LPS with a Ki of 1.9 μg/ml on endothelial cells and a Ki of 2.0 μg/ml on Kupffer cells. Poly(A) had no significant effect on the binding of LPS to both cell types (Fig. 4).
FIG. 3.
Binding of 125I-LPS to liver endothelial and Kupffer cells. Liver endothelial cells (A) and Kupffer cells (B) were isolated as described in Materials and Methods. Cells were incubated in DMEM–2% BSA (pH 7.4) for 2 h at 4°C with 5 μg of 125I-LPS per ml and increasing amounts of unlabelled LPS. Cells were then washed, and radioactivity was counted. Data are means of three experiments ± SD.
FIG. 4.
Effects of poly(I) and poly(A) on the binding of 125I-LPS. Liver endothelial cells (A) and Kupffer cells (B) were isolated as described in Materials and Methods. Cells were incubated in DMEM–2% BSA (pH 7.4) for 2 h at 4°C with 5 μg of 125I-LPS per ml and increasing amounts of poly(I) (•) or poly(A) (▴). Cells were then washed, and radioactivity was counted. Data are means of three experiments ± SD.
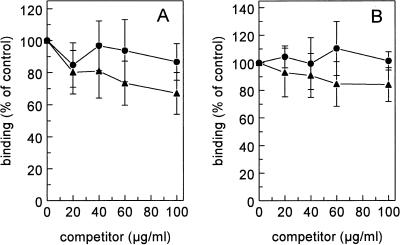
Because lipid IVA is described as a ligand for SR-A on endothelial cells and macrophages, we performed competition experiments using radioiodinated acLDL and oxLDL as ligands and LPS as a competitor. LPS inhibited the binding of 125I-acLDL to endothelial cells 55% ± 10% and 125I-acLDL binding to Kupffer cells 40% ± 4% (Fig. 5). Displacement of 125I-acLDL by LPS on endothelial and Kupffer cells took place with Ki values of 11.1 and 3.4 μg/ml, respectively. Binding of 125I-oxLDL to endothelial and Kupffer cells was reduced by LPS 61% ± 3% and 65% ± 5%, respectively (Fig. 5). LPS displaced 125I-oxLDL on endothelial and Kupffer cells with Ki values of 18.4 and 6.9 μg/ml, respectively. We also tested whether modified lipoproteins could inhibit binding of LPS to liver endothelial or Kupffer cells. oxLDL and acLDL were not able to inhibit binding of 125I-LPS to endothelial or Kupffer cells (Fig. 6).
FIG. 5.
Displacement of 125I-oxLDL and 125I-acLDL by LPS. Liver endothelial cells (A) and Kupffer cells (B) were isolated as described in Materials and Methods. Cells were incubated in DMEM–2% BSA (pH 7.4) for 2 h at 4°C with 5 μg of 125I-acLDL (•) or 125I-oxLDL (▴) per ml and increasing amounts of LPS. Cells were then washed, and radioactivity was counted. Data are means of three experiments ± SD.
FIG. 6.
Effects of acLDL and oxLDL on the binding of 125I-LPS. Liver endothelial cells (A) and Kupffer cells (B) were isolated as described in Materials and Methods. Cells were incubated in DMEM–2% BSA (pH 7.4) for 2 h at 4°C with 5 μg of 125I-LPS per ml and increasing amounts of acLDL (•) or oxLDL (▴). Cells were then washed, and radioactivity was counted. Data are means of three experiments ± SD.
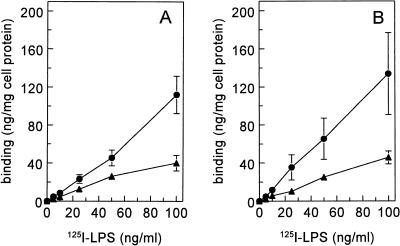
To compare the total number of binding sites for LPS with the number of binding sites on the 220- and 95 kDa receptors, we determined the maximal binding of 125I-LPS to liver endothelial and Kupffer cells. Cells were incubated with increasing amounts of 125I-LPS. Nonspecific binding was determined with an excess of unlabelled LPS. Maximal binding of 125I-LPS to endothelial cells was 6,701 ± 1,566 ng/mg of cell protein, and that to Kupffer cells was 7,786 ± 807 ng/mg of cell protein.
To estimate whether the contribution of poly(I)-sensitive sites to the binding of LPS is the same at different LPS concentrations, cells were incubated with an increasing amount of 125I-LPS and an excess of poly(I) (Fig. 7). To determine the percentage of poly(I)-sensitive LPS binding sites, we compared the difference between binding of 125I-LPS in the absence and in the presence of poly(I). The contribution of poly(I)-sensitive sites to the binding of LPS by endothelial and Kupffer cells remained constant up to an LPS concentration of 100 ng/ml (Fig. 7). When the amount of LPS was further increased, the contribution of poly(I)-sensitive binding sites increased (results not shown).
FIG. 7.
Contribution of poly(I)-sensitive binding sites to the binding of LPS. Cells were isolated as described in Materials and Methods. Endothelial cells (A) and Kupffer cells (B) were incubated with increasing amounts of 125I-LPS, without (•) or with (▴) an excess of poly(I) (200 μg/ml) for 2 h at 4°C.
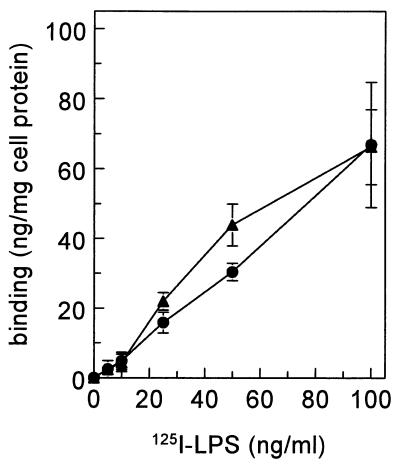
To estimate the contribution of CD14 to the binding of LPS by Kupffer cells, Kupffer cells were treated with phospholipase C prior to binding of 125I-LPS. CD14 is a GPI-anchored protein. GPI-linked proteins were specifically removed from the cell membrane by phospholipase C. Treatment of Kupffer cells with phospholipase C, however, had no significant effect on the total binding of 125I-LPS (Fig. 8).
FIG. 8.
Effect of phospholipase C on the binding of 125I-LPS to Kupffer cells. Isolated Kupffer cells were not treated (•) or were incubated with phospholipase C (3 U/ml) at 37°C for 30 min (▴) and subsequently incubated with 5 μg of 125I-LPS per ml at 4°C for 2 h.
DISCUSSION
The scavenger receptor was first recognized as a receptor that played an important role in the uptake of modified lipoproteins by cultured macrophages, resulting in an accumulation of cholesterol esters and the subsequent transformation of the macrophages into foam cells (3, 14). Macrophage-derived foam cells are an important feature of early atherosclerotic lesions (34). Kodama et al. identified and cloned two types of the scavenger receptor, SR-A types I and II (19). Modified lipoproteins injected into an organism are recognized by the liver (22, 42). Within the liver, acLDL is preferentially associated with endothelial cells, whereas oxLDL is associated mainly with Kupffer cells (42). Kupffer cells have an additional scavenger receptor different from SR-A. This scavenger receptor, with a molecular mass of 95 kDa, is a specific binding site for oxLDL (7, 8) and was recently identified as macrosialin (43).
Besides having a role in the development of atherosclerosis, scavenger receptors may also be involved in the course of sepsis. It has been demonstrated that lipid IVA is a scavenger receptor ligand (15). Recently, it was demonstrated that although SR-A knockout mice are less prone to develop atherosclerotic lesions, they are more susceptible to infections with Listeria monocytogenes or LPS-induced shock (16, 35). The present study describes the role of scavenger receptors on liver endothelial and Kupffer cells in the binding of LPS. Scavenger receptors are known to bind negatively charged compounds. We tested the ability of the scavenger receptor ligand fucoidin to inhibit the uptake of LPS by the liver in vivo. Serum decay of radiolabelled LPS was inhibited by fucoidin. In addition, fucoidin reduced the liver uptake of radiolabelled LPS. The decreased binding of LPS to the liver was due to a reduced binding to the liver endothelial and Kupffer cells. These cells are known to express scavenger receptors on their surface (8, 9). The binding of LPS to isolated liver endothelial and Kupffer cells was markedly inhibited by poly(I), while poly(A) had no effect. This specificity in inhibition is in accordance with the binding of poly(I) and poly(A) to SR-A types I and II (3). The modified lipoproteins acLDL and oxLDL did not significantly inhibit the binding of LPS to isolated liver endothelial and Kupffer cells, although LPS can bind to SR-A (15). In the reciprocal experiments, LPS was able to inhibit binding of radiolabelled acLDL and oxLDL to liver endothelial and Kupffer cells, indicating that LPS does bind to SR-A. On Kupffer cells, LPS inhibited binding of acLDL approximately 40%, representing binding to SR-A. Binding of oxLDL was reduced by LPS approximately 65%. Macrosialin on Kupffer cells is oxLDL specific, and this receptor does not bind acLDL (7). According to this concept, binding of oxLDL to Kupffer cells is a result of binding to SR-A and macrosialin. On Kupffer cells, relatively more oxLDL than acLDL could be replaced by LPS, indicating that some of the binding of oxLDL to Kupffer cells was binding to an oxLDL-specific binding site, presumably macrosialin. Since LPS could displace the binding of oxLDL to Kupffer cells, LPS may also bind to macrosialin.
To compare the total number of binding sites for LPS with the total number of binding sites for acLDL and oxLDL, we estimated the maximal binding of LPS to endothelial and Kupffer cells in nanograms per milligram of cell protein. Since the concentration of LPS that we used was higher than the critical micelle concentration (30), we assume that the LPS in our experiments is present in the form of micelles. From the molecular mass of Re-LPS used in our experiments (2.9 kDa) and the average size of micelles formed by Re-LPS in aqueous solutions (2,000 kDa) (32), the levels of binding of LPS to endothelial and Kupffer cells were calculated to be 2,310,000 and 2,680,000 fmol/mg of cell protein, respectively. Maximal binding of acLDL and oxLDL to liver endothelial and Kupffer cells was determined previously in this laboratory (7). Maximal levels of binding of acLDL and oxLDL to Kupffer cells were 45 and 779 ng/mg of cell protein, respectively (7), which correspond to 86.5 fmol of acLDL/mg of cell protein and 46.9 fmol of oxLDL/mg of cell protein. Maximal levels of binding of acLDL and oxLDL to endothelial cells were 156 and 803 ng/mg of cell protein, respectively (7), which correspond to 300 fmol of acLDL/mg of cell protein and 48 fmol of oxLDL/mg of cell protein. From these data the percentages of binding sites of oxLDL and acLDL relative to total binding sites of LPS can be calculated. Assuming that LPS binds as a micelle, binding of acLDL and binding of oxLDL to Kupffer cells are approximately 2.2 and 1.2%, respectively, of total LPS binding. Binding of acLDL and binding of oxLDL to liver endothelial cells are 9.0 and 1.43%, respectively, of the total binding of LPS to these cells. The phenomenon that LPS inhibits binding of modified lipoproteins to liver endothelial and Kupffer cells but the same modified lipoproteins do not inhibit the binding of LPS to those cells may therefore be explained by the large number of binding sites for LPS compared to the limited number for acLDL and oxLDL. The data confirm that LPS can bind to SR-A types I and II and that there is an additional interaction with macrosialin. However, the quantitative role of these receptors in the removal of LPS is limited, and clearly additional receptors with scavenger receptor-like properties are important for the removal of LPS from the blood. It is not known whether LPS indeed binds as a micelle. If LPS binds as a monomeric compound or a micelle that is smaller than 2,000 kDa, then the total number of LPS binding sites increases, resulting in an even more limited role for SR-A and macrosialin in the binding and liver uptake of LPS.
The lack of reciprocal inhibition of acLDL and LPS that we have observed was also demonstrated by Shnyra and Lindberg for cultured liver endothelial and Kupffer cells (32). They did not determine the effect of oxLDL on the binding of LPS. Inhibition of oxLDL binding to Kupffer cells by LPS as we found is somewhat surprising, because previous experiments showed that lipid A, a component of LPS, was not able to inhibit binding of oxLDL to isolated Kupffer cells (8). The LPS used in our experiments (Re-LPS) differs from lipid A by the presence of 2-keto-3-deoxyoctonic acid sugars, which are linked to one of the glucosamines of the lipid A moiety. Experiments by Shnyra and Lindberg showed that the acidic 2-keto-3-deoxyoctonic acid sugars and the phosphate groups of glucosamine play a role in the binding of Re-LPS to liver cells (32). Our results indicate that those carbohydrate and phosphate groups may be important for binding of LPS to macrosialin on Kupffer cells. Scavenger receptors of class B (SR-BI and CD36) are not likely to be involved in the binding of LPS. SR-B, which in the liver is expressed mainly on parenchymal cells, is not sensitive to fucoidin or poly(I) (1, 10a), whereas CD36 is not expressed at all in the liver (1). This finding indicates that the polyanion-sensitive binding of LPS in the liver is not likely to be mediated by class B scavenger receptors.
Cavaillon and colleagues found that fucoidin, a scavenger receptor ligand, was able to induce the release of TNF-α and IL-6 by isolated human monocytes (5). The responsible receptor appeared to be the CD14 molecule, as assessed with anti-CD14 antibodies. The removal of CD14 by phosphatidylinositol (PI)-specific phospholipase C (PI-PLC) treatment could not be monitored adequately in our experiments, since an anti-rat CD14 is not available. We have, however, shown that treatment of Kupffer cells with PI-PLC efficiently removes other PI-linked receptors, such as the urokinase receptor, from Kupffer cells (42a). The fact that treatment of Kupffer cells with PI-PLC had no effect on the binding of LPS strongly indicated that CD14 was quantitatively not involved in the binding of LPS to Kupffer or endothelial cells.
In our study, we identified binding sites for LPS that behave like scavenger receptors, yet their binding characteristics did not fully correspond to those of SR-A (type I and type II) on liver endothelial and Kupffer cells or those of macrosialin on Kupffer cells. In addition, we found that LPS binds to an oxLDL-specific binding site on Kupffer cells, which may represent macrosialin. The binding of LPS to scavenger receptors may be considered a noninflammatory way to remove LPS from the body, since it was shown by Hampton et al. (15) that the binding of lipid A to SR-A did not lead to cellular activation. More recently, it was shown that mice that were SR-A deficient were more sensitive to LPS, indicating that the SR-A contributed to the noninflammatory removal of LPS (16). Further experiments should concentrate on the nature of these new receptors and explore their role in the metabolism and pathophysiology of LPS.
ACKNOWLEDGMENTS
This study was supported by the Netherlands Organisation for Scientific Research, Council for Medical Research, Medical Sciences grant 902-23-139.
REFERENCES
- 1.Acton S L, Scherer P E, Lodish H F, Krieger M. Expression cloning of SR-BI, a cd36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 1a.Basu S K, Goldstein J L, Anderson R G W, Brown M S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci USA. 1976;73:3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilheimer D W, Eisenberg S, Levy R I. On the apoprotein composition of human plasma very low density lipoprotein subfractions. Biochim Biophys Acta. 1972;260:212–221. doi: 10.1016/0005-2760(72)90045-8. [DOI] [PubMed] [Google Scholar]
- 3.Brown M S, Goldstein J L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 4.Callery M P, Mangino M J, Kamei T, Flye M W. Interleukin-6 production by endotoxin-stimulated Kupffer cells is regulated by prostaglandin E2. J Surg Res. 1990;48:523–527. doi: 10.1016/0022-4804(90)90224-p. [DOI] [PubMed] [Google Scholar]
- 5.Cavaillon J-M, Marie C, Caroff M, Ledur A, Godard I, Poulain D, Fitting C, Haeffner-Cavaillon N. CD14/LPS receptor exhibits lectin-like properties. J Endotoxin Res. 1996;3:471–480. [Google Scholar]
- 6.Chensue S W, Terebuh P D, Remick D G, Scales W E, Kunkel S L. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia: kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;1338:395–402. [PMC free article] [PubMed] [Google Scholar]
- 7.de Rijke Y B, Biessen E A L, Vogelezang C J M, van Berkel T J C. Binding characteristics of scavenger receptors on liver endothelial and Kupffer cells for low-density lipoproteins. Biochem J. 1994;304:69–73. doi: 10.1042/bj3040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Rijke Y B, van Berkel T J C. Rat liver Kupffer and endothelial cells express different binding proteins for modified low density lipoproteins. J Biol Chem. 1994;269:824–827. [PubMed] [Google Scholar]
- 9.Dresel H A, Friedrich E, Via D P, Sinn H, Ziegler R, Schletter G. Binding of acetylated low density lipoprotein and maleylated bovine serum albumin to the rat liver: one or two receptors? EMBO J. 1987;6:319–326. doi: 10.1002/j.1460-2075.1987.tb04757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Echtenacher B, Falk W, Männel D N, Krammer P H. Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J Immunol. 1990;145:3762–3766. [PubMed] [Google Scholar]
- 10a.Fluiter K, van der Westhuijzen R, van Berkel T J C. In vitro regulation of scavenger receptor BI and the selective uptake of high density lipoprotein cholesteryl esters in rat liver parenchymal and Kupffer cells. J Biol Chem. 1998;273:8434–8438. doi: 10.1074/jbc.273.14.8434. [DOI] [PubMed] [Google Scholar]
- 11.Fox E S, Thomas P, Broitman S A. Uptake and modification of 125I-lipopolysaccharide by isolated rat Kupffer cells. Hepatology. 1988;8:1550–1554. doi: 10.1002/hep.1840080613. [DOI] [PubMed] [Google Scholar]
- 12.Freudenberg M A, Freudenberg N, Galanos C. Time course of cellular distribution of endotoxin in liver, lungs and kidneys of rats. Br J Exp Pathol. 1982;63:56–65. [PMC free article] [PubMed] [Google Scholar]
- 13.Frey E A, Miller D S, Gullstein-Jahr T, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein J L, Ho Y K, Basu S K, Brown M S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampton R Y, Golenbock D T, Penman M, Krieger M, Raetz C R H. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–352. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 16.Haworth R, Platt N, Keshav S, Hughes D, Darley E, Suzuki H, Kurihara Y, Kodama T, Gordon S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–1439. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haziot A, Chen S, Ferrero E, Low M G, Silber R, Goyert S M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 18.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper J, Bakkeren H F, Biessen E A L, van Berkel T J C. Characterisation of the interaction of galactose-terminated particles with rat Kupffer cells. Biochem J. 1994;299:285–290. doi: 10.1042/bj2990285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarlane A S. Efficient trace-labelling of proteins with iodine. Nature. 1958;812:53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- 22.Nagelkerke J F, Barto K P, van Berkel T J C. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer and parenchymal cells. J Biol Chem. 1983;258:12221–12227. [PubMed] [Google Scholar]
- 23.Parent J B. Membrane receptors on rat hepatocytes for the inner core region of bacterial lipopolysaccharide. J Biol Chem. 1990;265:3455–3461. [PubMed] [Google Scholar]
- 24.Parillo J E. Pathogenetic mechanisms of septic shock. N Engl J Med. 1993;328:1471–1477. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 25.Praaning-van Dalen D P, Brouwer A, Knook D L. Clearance capacity of rat liver Kupffer, endothelial, and parenchymal cells. Gastroenterology. 1981;81:1036–1044. [PubMed] [Google Scholar]
- 26.Pugin J, Schürer-Maly C-C, Leturcq D J, Moriarty A M, Ulevitch R J, Tobias P S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA. 1993;90:2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redgrave T G, Roberts D C K, West C E. Separation of plasma lipoproteins by density-gradient ultracentrifugation. Anal Biochem. 1975;65:42–49. doi: 10.1016/0003-2697(75)90488-1. [DOI] [PubMed] [Google Scholar]
- 28.Rohrer L, Freeman M, Kodama T, Penman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 29.Ruiter D J, van der Meulen J, Brouwer A, Hummel M J R, Mauw B J, van der Ploeg J C M, Wisse E. Uptake by liver cells of endotoxin following its intravenous injection. Lab Invest. 1981;45:38–45. [PubMed] [Google Scholar]
- 30.Schromm A B, Brandenburg K, Rietschel E T, Seydel U. Do endotoxin aggregates intercalate into phospholipid membranes in a nonspecific, hydrophobic manner? J Endotoxin Res. 1995;2:313–323. [Google Scholar]
- 31.Seglen P O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 32.Shnyra A, Lindberg A A. Scavenger receptor pathway for lipopolysaccharide binding to Kupffer and endothelial liver cells in vitro. Infect Immun. 1995;63:865–873. doi: 10.1128/iai.63.3.865-873.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons D L, Tan S, Tenen D G, Nicholson-Weller A, Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284–289. [PubMed] [Google Scholar]
- 34.Steinberg D. Metabolism of lipoproteins and their role in the pathogenesis of atherosclerosis. Atheroscler Rev. 1988;18:1–23. [Google Scholar]
- 35.Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, Takashima Y, Kawabe Y, Cynshi O, Wada Y, Honda M, Kurihara H, Aburatani H, Doi T, Matsumoto A, Azuma S, Noda T, Toyoda Y, Itakura H, Yazaki Y, Horiuchi S, Takahashi K, Kruijt J K, van Berkel T J C, Steinbrecher U P, Ishibashi S, Maeda N, Gordon S, Kodama T. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 36.Takeuchi M, Nakashima Y, Miura Y, Nakagawa K, Uragoh K, Iwanaga S, Hori Y, Sueishi K. The localization of lipopolysaccharide in an endotoxemic rat liver and its relation to sinusoidal thrombogenesis: light and electron microscopic studies. Pathol Res Pract. 1994;190:1123–1133. doi: 10.1016/S0344-0338(11)80438-3. [DOI] [PubMed] [Google Scholar]
- 37.Tobias P S, Soldau K, Ulevitch R J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobias P S, Soldau K, Ulevitch R J. Identification of a lipid A binding site in the acute phase reactant lipopolysaccharide binding protein. J Biol Chem. 1989;264:10867–10871. [PubMed] [Google Scholar]
- 39.Ulevitch R J. The preparation and characterization of a radioiodinated bacterial lipopolysaccharide. Immunochemistry. 1978;15:157–164. doi: 10.1016/0161-5890(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 40.Ulevitch R J, Tobias P S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Opin Immunol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 41.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 42.van Berkel T J C, de Rijke Y B, Kruijt J K. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoproteins in rats. J Biol Chem. 1991;266:2282–2289. [PubMed] [Google Scholar]
- 42a.Van der Kaaden M E, Rijken D C, Kruyt J K, van Berkel Th J C, Kuiper J. The role of LRP in the plasma clearance of recombinant single chain u-PA. Thromb Haemostasis. 1997;77:710–717. [PubMed] [Google Scholar]
- 43.van Velzen A G, da Silva R P, Gordon S, van Berkel T J C. Characterization of a receptor for oxidized low-density lipoproteins on rat Kupffer cells: similarity to macrosialin. Biochem J. 1997;322:411–415. doi: 10.1042/bj3220411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright S D, Detmers P A, Aida Y, Adamowski R, Anderson D, Chad Z, Kabbash L G, Pabst M J. CD18-deficient cells respond to lipopolysaccharide in vitro. J Immunol. 1990;144:2566–2571. [PubMed] [Google Scholar]
- 45.Wright S D, Jong M T C. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright S D, Levin S M, Jong M T C, Chad Z, Kabbash L G. CR3 (CD11b/CD18) expresses one binding site for Arg-Gly-Asp-containing peptides and a second site for bacterial lipopolysaccharide. J Exp Med. 1989;169:175–183. doi: 10.1084/jem.169.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 48.Wright S D, Tobias P S, Ulevitch R J, Ramos R A. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasui M, Nakao A, Yuuki T, Harada A, Nonami T, Takagi H. Immunohistochemical detection of endotoxin in endotoxemic rats. Hepatogastroenterology. 1995;42:683–690. [PubMed] [Google Scholar]