Abstract
Latent sensitization is a model of chronic pain in which a persistent state of pain hypersensitivity is suppressed by opioid receptors, as evidenced by the ability of opioid antagonists to induce a period of mechanical allodynia. Our objective was to determine if substance P and its neurokinin 1 receptor (NK1R) mediate the maintenance of latent sensitization. Latent sensitization was induced by injecting rats in the hindpaw with complete Freund’s adjuvant (CFA), or by tibial spared nerve injury (SNI). When responses to von Frey filaments returned to baseline (day 28), the rats were injected intrathecally with saline or the NK1R antagonist RP67580, followed 15 min later by intrathecal naltrexone. In both pain models, the saline-injected rats developed allodynia for 2 h after naltrexone, but not the RP67580-injected rats. Saline or RP67580 were injected daily for two more days. Five days later (day 35), naltrexone was injected intrathecally. Again, the saline-injected rats, but not the RP67580-injected rats, developed allodynia in response to naltrexone. To determine if there is sustained activation of NK1Rs during latent sensitization, NK1R internalization was measured in lamina I neurons in rats injected in the paw with saline or CFA, and then injected intrathecally with saline or naltrexone on day 28. The rats injected with CFA had a small amount of NK1R internalization that was significantly higher than in the saline-injected rats. Naltrexone increased NK1R internalization in the CFA-injected rats but nor in the saline-injected rats. Therefore, sustained activation of NK1Rs maintains pain hypersensitivity during latent sensitization.
Keywords: inflammation, nerve injury, neurokinin-1 receptor, primary afferent, substance P
1. Introduction
The latent sensitization model of chronic pain shows that certain injuries put the organism in a long-term state of pain hypersensitivity that is continuously suppressed by opioid and α2A adrenergic receptors (Marvizon et al., 2015; Taylor and Corder, 2014). This shows that chronic pain is a dysregulation of pain pathways so that they stay sensitized after the initial injury is healed. In animals with latent sensitization, opioid receptor antagonists induce episodes of mechanical allodynia and thermal hyperalgesia (Campillo et al., 2011; Corder et al., 2013; Walwyn et al., 2016), demonstrating the coexistence of pain hypersensitivity and its suppression by opioid receptors. Furthermore, mice with genetic deletion of μ-opioid receptors (MORs) do not fully recover from hyperalgesia and no longer respond to MORs antagonists with an episode of allodynia. This occurs both when the MORs deletion is global (Walwyn et al., 2016) or targeted to nociceptive afferents (Severino et al., 2018), indicating that the MORs that suppress the hypersensitivity are located in these afferents.
Different stimuli can induce latent sensitization, including inflammation (Corder et al., 2013; Walwyn et al., 2016), nerve injury (Solway et al., 2011), plantar incision (Campillo et al., 2011; Rivat et al., 2009) or opioids (Rivat et al., 2007; Rivat et al., 2009). However, it is unclear if these represent different types of latent sensitization with different mechanisms.
We propose that the initiation, expression and maintenance of latent sensitization are mediated by different signals. Its initiation would be due to temporary signals evoked by the triggering injury. For example, enhancement by BDNF of NMDA receptor-induced substance P release lasts only 7 days after nerve injury (Chen et al., 2014). Its maintenance would require signals able to endure for long periods of time, such as synaptic plasticity (Doiron et al., 2011; Ikeda et al., 2003; Klein et al., 2004), changes in neuronal excitability (Rivera-Arconada and Lopez-Garcia, 2010), or microglia activation (Huang et al., 2017; McMahon and Malcangio, 2009; Zhou et al., 2019). Its expression would be mediated by short-lived signals that are required for pain hypersensitivity and are recruited by the maintenance mechanisms, but that are not able to persist for long periods of time. Drugs that inhibit the maintenance of latent sensitization should permanently eliminate the allodynia induced by opioid antagonists because they would interrupt the self-perpetuation of the sensitization. In contrast, drugs that inhibit the expression of latent sensitization would eliminate it only while the drugs are present. In this study, we used this criterion to determine whether neurokinin 1 receptors (NK1Rs) for substance P mediate the expression or the maintenance of latent sensitization.
NK1Rs in dorsal horn neurons are involved in central sensitization (Laird et al., 2001; Xu et al., 1992) and long-term potentiation of the first order synapses of nociceptors (Ikeda et al., 2003; Liu and Sandkuhler, 1998). Substance P release is mediated by NMDA receptors (Marvizon et al., 1997), adenylyl cyclase and protein kinase A (Chen et al., 2018a), which are also required for latent sensitization (Celerier et al., 2000; Corder et al., 2013; Fu et al., 2019). Conversely, substance P release is inhibited by the presynaptic MORs (Chen et al., 2018a) that suppress pain hypersensitivity in latent sensitization (Severino et al., 2018). We hypothesized that NK1Rs mediate the maintenance of latent sensitization and tested this hypothesis by determining whether an NK1R antagonist eliminated latent sensitization. We also determined if NK1Rs are tonically activated by substance P during latent sensitization and if this activation is increased by a MOR antagonist.
2. Material and methods
2.1. Animals
Male adult (2–4 months old) Sprague-Dawley rats (Envigo, Indianapolis, IN) were used in the study. The Institutional Animal Care and Use Committee of the Veteran Affairs Greater Los Angeles Healthcare System approved all animal procedures, which abide by the Guide for the Care and Use of Laboratory Animals, National Institutes of Health of the United States of America.
2.2. Chemicals
Naltrexone (NTX) and RP67580 were from Tocris Bioscience (Minneapolis, MN). Complete Freund’s adjuvant (CFA) and other reagents were from Sigma-Aldrich (St. Louis, MO). RP67580 was dissolved at 10 mM in DMSO and then diluted to 30 μM in saline (final DMSO was 0.3%). NTX was dissolved in saline. CFA was injected undiluted.
2.3. CFA injections
Rats were injected subcutaneously in one hindpaw with 50 μl undiluted CFA, using a 26 gauge needle and a 50 μl Hamilton syringe. The needle was inserted in the middle of the paw, near the base of the third toe, at an oblique angle from the heel. It was held for 15 s and then withdrawn.
2.4. Tibial spared nerve injury (SNI)
This is a variation of the SNI model in which the tibial nerve is spared instead of the sural nerve (Shields et al., 2003). This decreases the period of mechanical allodynia to less than 30 days (Marvizon et al., 2019; Solway et al., 2011). Rats were anesthetized with isoflurane (2–3 % in O2) and prepared for surgery. The skin was cut in the dorsal upper thigh. The muscle was separated to expose the trifurcation of the sciatic nerve. The common peroneal and sural nerves were ligated and cut on both sides of the suture to remove 2–3 mm of each nerve. The muscle was sutured in a continuous pattern. The cut in the skin was closed using a subcuticular intradermal pattern. Rats were given daily injections of the analgesic carprofen for 3 days. Endpoint criteria were motor weakness or signs of paresis, but this did not occur in any of the rats.
2.5. Intrathecal (i.th.) injections
Intrathecal catheters were inserted through the lumbar vertebrae (Storkson et al., 1996; Walwyn et al., 2016). Rats were anesthetized with isoflurane (2–3 % in O2). Skin and muscle were cut and a 20G needle was inserted between the L5 and L6 vertebrae to puncture the dura mater. The catheter (20 mm of PE-5 tube heat-fused to 150 mm of PE-10 tube) was inserted into the subdural space and pushed rostrally to place its tip at L5-L6, then its PE-10 portion was inserted under the skin of the back and brought out at the head. The skin was sutured. The catheter was filled with saline and heat-sealed. Rats were housed separately. The presence of motor weakness or paresis were criteria for euthanasia, but they did not occur in any of the rats.
Intrathecal injections consisted of 10 μl of injectate followed by 10 μl of saline. Solutions were preloaded into a PE-10 tube and delivered in 1 min. The correct position of the catheter was confirmed postmortem. Three rats lost the catheter after day 28 and were excluded from the measures on day 35.
The dose of NTX, 2.6 nmol i.th., was chosen from our previous studies (Corder et al., 2013; Taylor and Corder, 2014; Walwyn et al., 2016). The dose of the NK1R antagonist RP-67580, 0.3 nmol i.th., was calculated based on its Ki of 4.16 nM to displace [3H]substance P binding to the NK1R (Garret et al., 1991). The i.th. dose was obtained by multiplying the Ki by 30 to get a saturating concentration and then dividing by 500 nM/nmol, the ratio between the EC50s for NMDA to induce substance P release in spinal cord slices, 258 nM (Chen et al., 2010), and by i.th. injection in vivo, 0.49 nmol (Chen et al., 2014).
2.6. Measures of mechanical hyperalgesia
Mechanical allodynia was measured with von Frey filaments (Touch-Test) using the two-out-of-three method (Chen et al., 2018b; Jarahi et al., 2014; Kingery et al., 2000; Michot et al., 2012; Walwyn et al., 2016). Measures were done by putting the rats in acrylic enclosures on an elevated metal grid (IITC Life Science Inc., CA), to which the rats were habituated for 30 min, daily for 3 days. A series of von Frey filaments were applied in ascending order to the plantar surface of the hindpaw for a maximum of 3 s. A withdrawal response was counted if the hindpaw was completely removed from the grid. Each filament was applied three times. The minimal value that caused at least two responses was recorded as the paw withdrawal threshold (PWT). The cut-off threshold was the 15 g filament.
2.7. Timeline
The timeline of the experiments to study the effect of the NK1R antagonist RP67580 on latent sensitization is shown in Fig. 1. The rats were habituated to the von Frey enclosures for 3 days. On day −7, the i.th. catheters were implanted. On days −4 and 0, baseline von Frey measures were obtained. On day 0, rats were injected with CFA or underwent SNI. On days 3 and 21, von Frey measures were taken to follow the mechanical allodynia produced by CFA or SNI. On day 28, a baseline von Frey measure was taken, the rats were injected i.th. with vehicle or RP67580, and 15 min later they were injected i.th. with NTX. For the following 2 h, von Frey measures were taken at 15, 30, 60, 120 min after NTX. On days 29 and 30, the rats received daily i.th. injections of vehicle or RP67580. On day 35, a baseline von Frey measure was taken, NTX was injected i.th., and von Frey measures were taken for 2 h at the same time points as before.
Figure 1. Timeline.
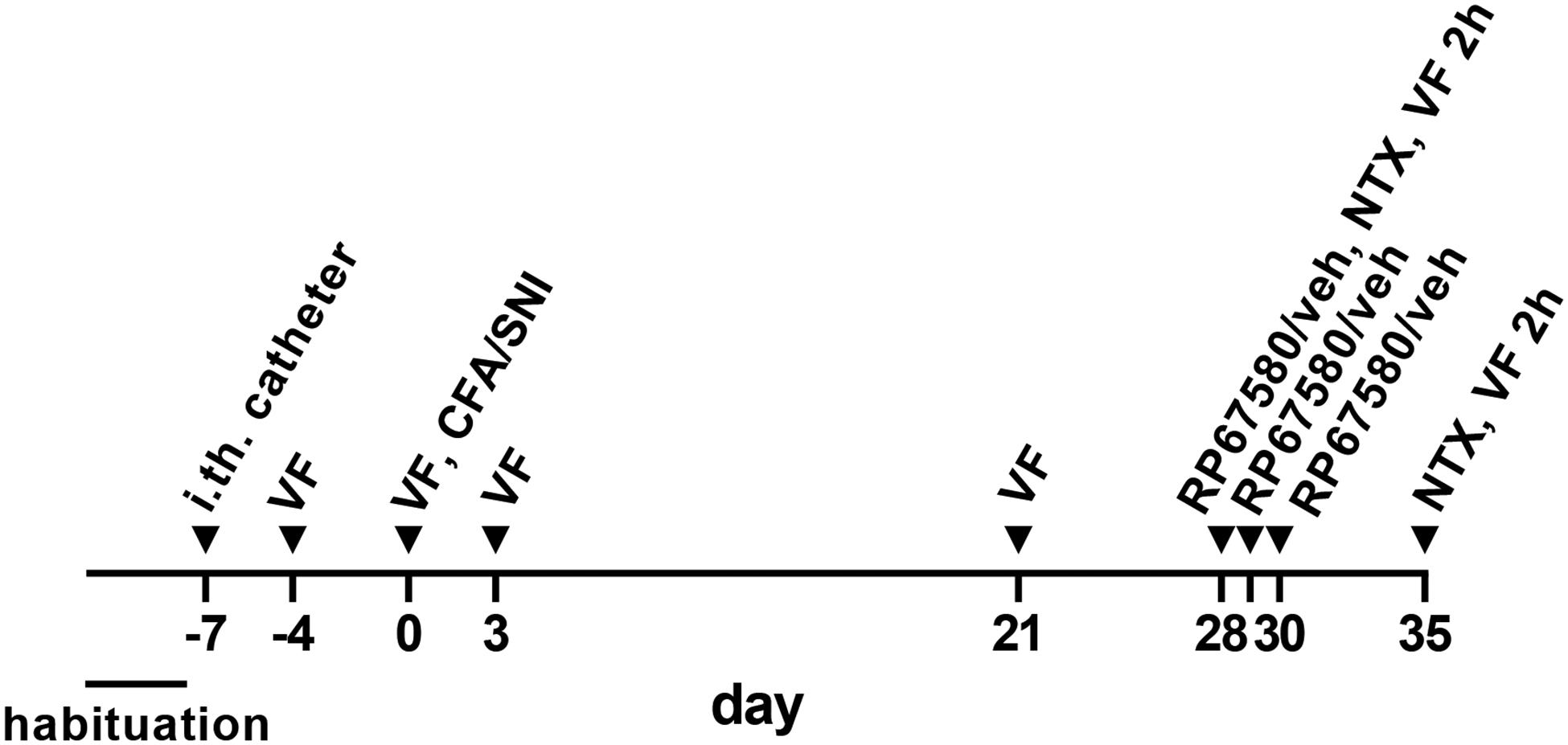
Habituation to the von Frey enclosure: 30 min daily for 3 days. Intrathecal (i.th.) catheter implantation. von Frey measures, single (VF) or repeated for 2 h (VF 2h). Complete Freund’s adjuvant (CFA, 50 μl s.c.) injected in the paw. Spared nerve injury (SNI). RP67580, 0.3 nmol i.th. in 10 μl. Vehicle (veh), 10 μl i.th. Naltrexone (NTX) 2.6 nmol i.th. in 10 μl.
2.8. Immunohistochemistry
The NK1R antiserum (AB5060, EDM Millipore, Billerica, MA) was raised in rabbit against amino acids 385–407 at the C-terminus of the rat NK1R. This and similar antiserums raised against the same epitope of the NK1R had been fully characterized (Allen et al., 1999; Chen et al., 2018a; Chen et al., 2014; Grady et al., 1996; Honore et al., 1999; Mantyh et al., 1995; Marvizon et al., 2003). They all produce the same staining pattern in the rat spinal cord.
NK1R immunohistochemistry was done as described (Adelson et al., 2009; Marvizon et al., 2003). Rats were killed with pentobarbital (100 mg/kg) and fixed by aortic perfusion of 100 ml 0.1 M sodium phosphate (pH 7.4) containing 0.01% heparin, followed by 400 ml of ice-cold fixative (4% paraformaldehyde, 0.18% picric acid in phosphate buffer). Lumbar spinal cord segments (L4-L5) were post-fixed, cryoprotected, frozen and sectioned at 25 μm using a cryostat. Sections were washed four times and then incubated overnight at room temperature with the NK1R antiserum diluted 1:3000 in phosphate-buffered saline containing 0.3% Triton X-100, 0.001% thimerosal and 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA). After three washes, the secondary antibody (1:2000, Alexa Fluor 488 goat anti-rabbit, Invitrogen-Molecular Probes, Eugene, OR) was applied at for 2 h at room temperature. Sections were washed four more times, mounted on glass slides, and coverslipped with Prolong Gold (Invitrogen-Molecular Probes, Eugene, OR).
2.9. Quantification of NK1R internalization
NK1R internalization was used as a measure in situ of substance P release, a method that has been extensively used and validated (Adelson et al., 2009; Allen et al., 1997; Honore et al., 1999; Mantyh et al., 1995; Marvizon et al., 2003). NK1R neurons in lamina I with or without internalization were visually counted using a Zeiss Axio-Imager A1 (Carl Zeiss, Inc., Thornwood, NY) fluorescence microscope with a 63x (1.40 numerical aperture) objective. The criterion for having internalization was the presence in the neuronal soma of ten or more NK1R endosomes. The person counting the neurons (Dr. Chen) was blinded to the treatment of the rats. Four sections per rat were used, counting all NK1R neurons in lamina I in each section. Results were expressed as the percentage of the NK1R neurons in lamina I with NK1R internalization.
2.10. Confocal microscopy and image processing
Confocal images were acquired using a Zeiss LSM 900 confocal microscope (Carl Zeiss, Inc., Thornwood, NY) with an objective of 63x oil (numerical aperture 1.4). Excitation light for the Alexa Fluor 488 fluorophore (emission peak 519 nm) was provided by the 488 nm line of a diode laser. The pinhole was 1.0 Airy unit: 44 μm. Images were acquired as confocal stacks of 1024×1024 pixels. The separation between confocal sections was 0.23 μm, as determined by the microscope software using the Nyquist formula. Each section was averaged 4 times to reduce noise. Photomultiplier gain and offset were adjusted to avoid pixel saturation. Imaris 6.1.5 (Bitplane AG, Zurich, Switzerland) was used to crop the images in three dimensions, taking 6 optical sections through the middle of each cell. A two-dimension projection was imported into Adobe Photoshop 5.5 (Adobe Systems Inc., Mountain View, CA), which was used to compose the figure and to add text.
2.11. Sample size, randomization and blinding
The reduce the number of animals used, the CFA and SNI control groups were shared with another study on the effect of the Src family kinase inhibitor PP2 on latent sensitization, which was given at the same times as the NK1R antagonist in the present study (Chen and Marvizón, 2020). The target sample size was n = 7–8 rats per group based on a power analysis. Three rats in the SNI-control group were excluded from measures on day 35 because they had lost the i.th. catheter. Rats were randomly assigned to treatment and drugs. Blinding procedure: solutions of vehicle, PP2 and RP67580 were prepared and randomly coded by Dr. Marvizon. Dr. Chen injected the coded drugs i.th. and performed the von Frey measures. Dr. Marvizon analyzed the data and un-blinded the results.
2.12. Data analysis
Data were analyzed using Prism 8.3 (GraphPad Software, San Diego, CA) and expressed as mean ± standard error of the mean. Statistical significance was set at 0.05. Statistical analyses consisted of repeated-measures two-way ANOVA followed by Holm-Sidak’s post-hoc tests, or three-way ANOVA.
3. Results
3.1. Effect of the NK1R antagonist on CFA-induced latent sensitization
We studied the effect of the NK1R antagonist RP67580 on the latent sensitization induced by injecting 50 μl CFA in one hindpaw, a model of inflammatory chronic pain. CFA produced strong mechanical allodynia in the ipsilateral hindpaw on day 3, which decreased to near baseline values by days 28–35 (Fig. 2A, D). There was no allodynia in the contralateral hindpaw.
Figure 2. CFA-induced LS, effect of RP67580 on NTX-induced allodynia.
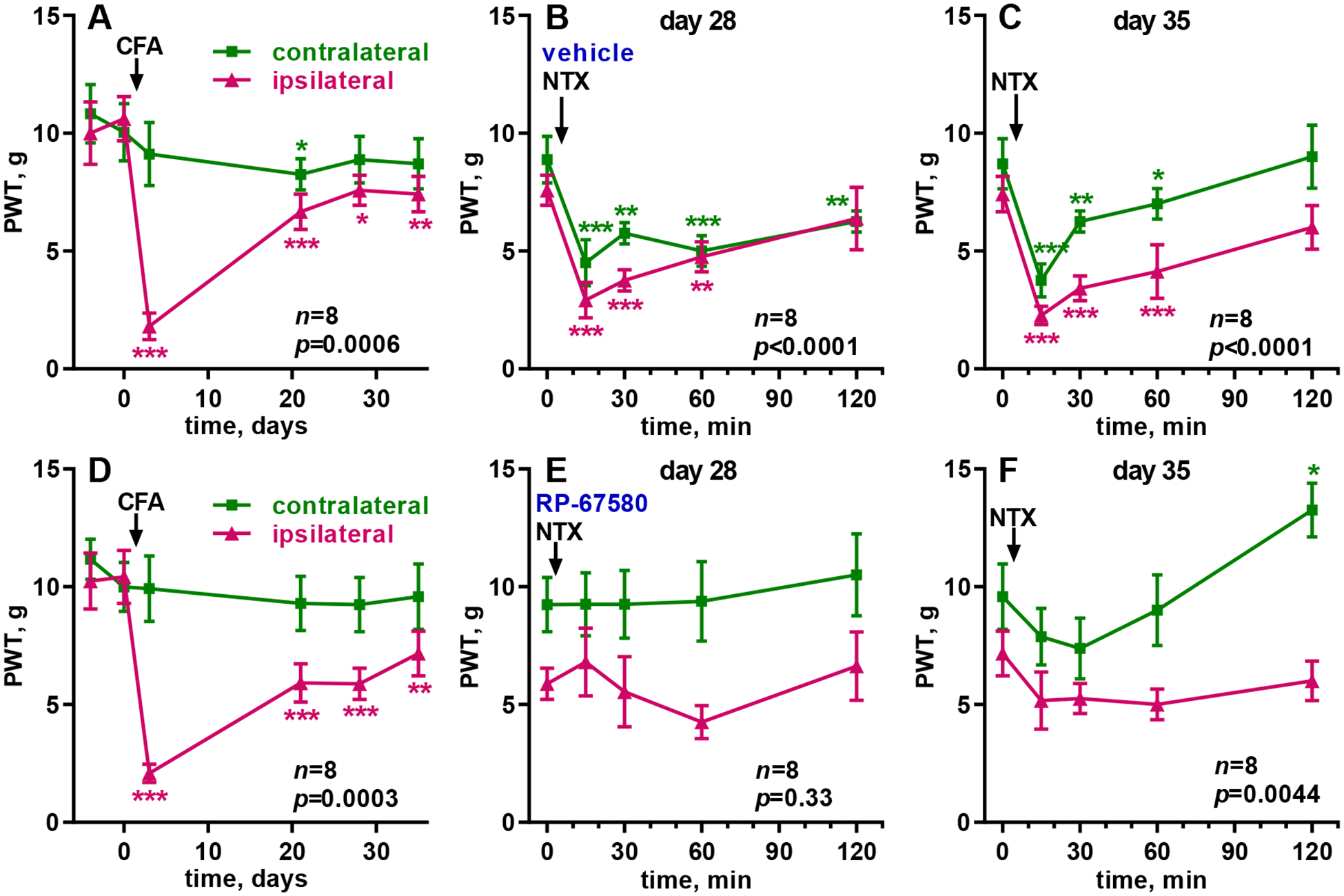
Paw withdrawal thresholds (PWT, von Frey) were measured in the ipsilateral (‘ipsi’) and contralateral (‘contra’) hindpaws. A, D: CFA (50 μl) was in injected in the hindpaw of eight rats at the time indicated by the arrow. B, E: On day 28, 10 μl of vehicle (0.3% DMSO in saline, B) or RP67580 (0.3 nmol in 0.3% DMSO, E) were injected i.th.; NTX (2.6 nmol in 10 μl i.th.) was injected 15 min later (arrow). The following two days, vehicle or RP67580 were injected daily. C, F: On day 35, NTX (2.6 nmol in 10 μl i.th.) was injected again (arrow). Each panel was analyzed by RM (both variables) 2-way ANOVA; overall p values for ‘time’ (effect of CFA or NTX) are given in each panel. Holm-Sidak’s post-hoc tests comparing to day −4 or 0 min: * p<0.05, ** p<0.01, *** p<0.001.
To study the role of NK1Rs on the expression of latent sensitization, we determined if RP67580 suppressed the allodynia induced by NTX given shortly afterward. Thus, on day 28, rats were injected i.th. with vehicle (control) or RP67580 (0.3 nmol) followed 15 min later by i.th. NTX (2.6 nmol). In the control group, NTX induced bilateral allodynia in the hindpaws, which peaked at 15 min and lasted more than 2 h (Fig. 2B), whereas after RP67580 the NTX-induced allodynia was eliminated (Fig. 2E).
To study the role of NK1Rs on the maintenance of latent sensitization, rats were injected with vehicle or RP67580 daily for two more days and then with NTX (2.6 nmol i.th.) on day 35 (Fig. 1). NTX produced bilateral allodynia in the control rats (Fig. 2C) but not in the rats injected with RP67580 (Fig. 2F). In addition, 2 h after NTX there was an unexpected decrease in responses to the von Frey filaments in the contralateral paw of the rats injected with RP67580.
Results were analyzed by 2-way ANOVA, repeated-measures for the two variables, time and side (Table 1). The variable ‘time’ indicates whether there was a significant effect of CFA or NTX. The variable ‘side’ indicates whether the effects were ipsilateral or bilateral. The effect of CFA over time was significant in both groups (A and D), and a significant effect of ‘side’ and ‘time x side’ showed that the effect of CFA was limited to the ipsilateral paw. On day 28, the effect of NTX over time was significant in the control rats (B) but not in the rats injected with RP67580 (E). On day 35, the effect of NTX over time was significant in the controls (C) and also in the RP67580-injected rats (F), but the later was due to a decrease in allodynia at the 2 h time point and not to the expected increase in allodynia at 15 min and 30 min (Fig. 2F). After the injections of NTX, ‘time x side’ was not significant in the controls, indicating that NTX produced allodynia bilaterally. In the RP67580-injected rats (F), ‘time x side’ was significant on day 35 because the decrease in allodynia at 2 h occurred only in the contralateral paw (Fig. 2F).
Table 1 -.
Statistical analyses of data in Fig. 2: CFA-induced latent sensitization
| group | Fig. 2 panel | n | data analyzed | time | side | time x side |
|---|---|---|---|---|---|---|
| control | A | 8 | CFA in the paw, days −4 to 35 |
p = 0.0006 F(5, 35) = 5.7 |
p = 0.0386 F(1, 16) = 45 |
p < 0.0001 F(5, 35) = 11 |
| B | 8 | day 28: vehicle i.th. then NTX i.th. |
p < 0.0001 F(4, 28) = 10.3 |
p = 0.203 F(1, 7) = 1.97 |
p = 0.305 F(4, 28) = 1.27 |
|
| C | 8 | day 35: NTX i.th. |
p < 0.0001 F(4, 28) = 18 |
p = 0.051 F(1, 7) = 5.5 |
p = 0.273 F(4, 28) = 1.36 |
|
| RP67580 | D | 8 | CFA in the paw, days −4 to 35 |
p = 0.0003 F(5, 35) = 6.33 |
p = 0.0027 F(1, 7) = 20 |
p < 0.0001 F(5, 35) = 8.9 |
| E | 8 | day 28: RP67580 i.th. then NTX i.th. |
p = 0.330 F(4, 28) = 1.20 |
p = 0.015 F(1, 7) = 10.3 |
p = 0.56 F(4, 28) = 0.75 |
|
| F | 8 | day 35: NTX i.th. |
p = 0.0044 F(4, 28) = 4.8 |
p = 0.0089 F(1, 7) = 12.9 |
p = 0.0175 F(4, 28) = 3.59 |
Data in the panels of Fig. 2 were analyzed by 2-way ANOVA, repeated-measures by both variables: time of von Frey measures and side (ipsilateral vs. contralateral). n is the sample size.
3.2. Effect of the NK1R antagonist on tibial SNI-induced latent sensitization
We also studied the effect of the NK1R antagonist on the latent sensitization induced by tibial SNI, a model of neuropathic pain. To get a faster return to baseline responses, SNI was induced by sparing the tibial nerve (Shields et al., 2003; Solway et al., 2011) instead of the most common procedure of sparing the sural nerve (Marvizon et al., 2019). SNI produced strong mechanical allodynia in the ipsilateral hindpaw on day 3, which went back to baseline values by day 28 (Fig. 3A, D). There was no allodynia in the contralateral hindpaw.
Figure 3. Tibial SNI-induced LS, effect of RP67580 on NTX-induced allodynia.
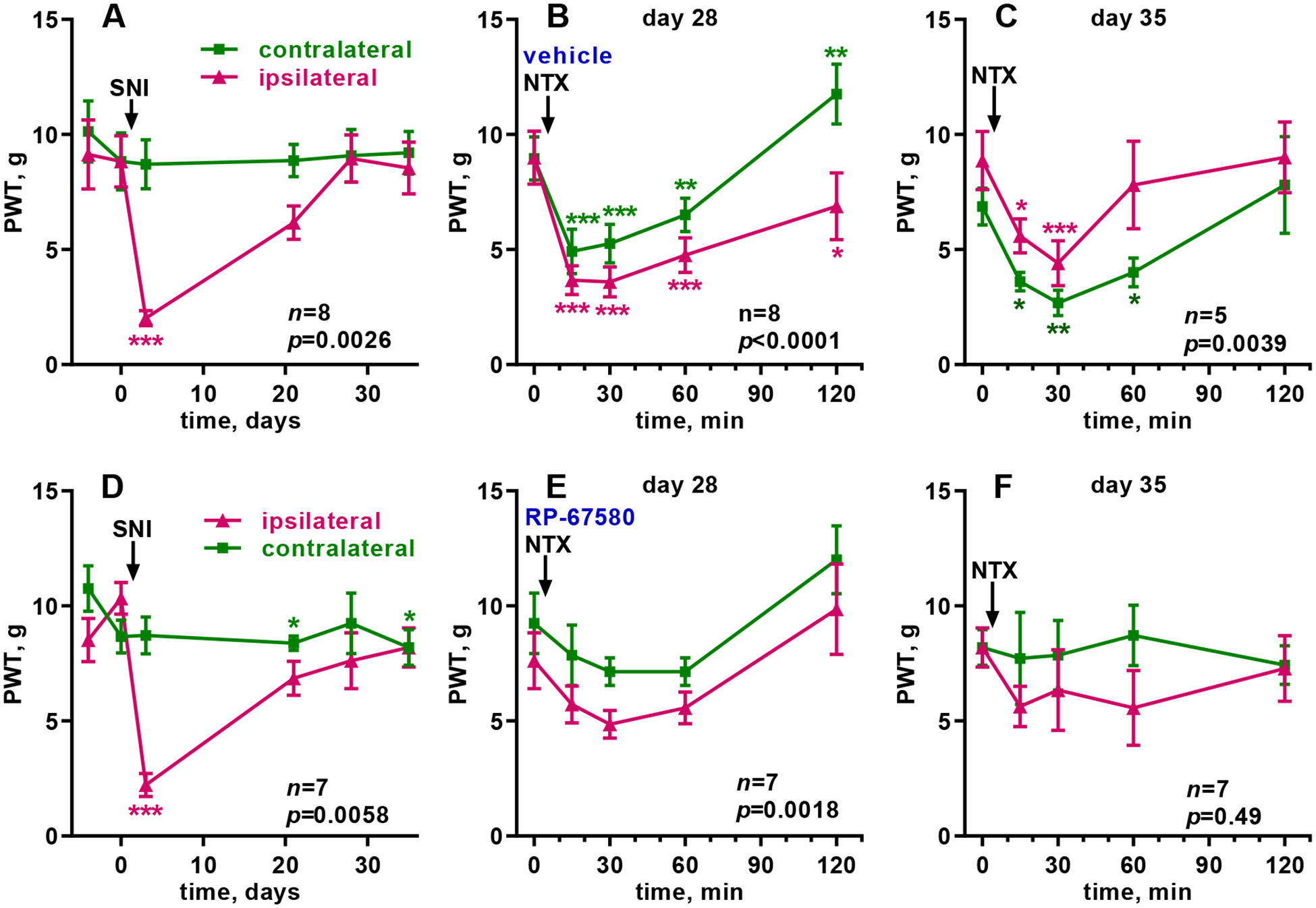
Paw withdrawal thresholds (PWT, von Frey) were measured in the ipsilateral (‘ipsi’) and contralateral (‘contra’) hindpaws. A, D: Tibial SNI was performed in one hindpaw of eight (A-C) or seven (D-F) rats at the time indicated by the arrow. B, E: On day 28, 10 μl of vehicle (0.3% DMSO in saline, B) or RP67580 (0.3 nmol in 0.3% DMSO, E) were injected i.th.; NTX (2.6 nmol in 10 μl i.th.) was injected 15 min later (arrow). The following two days, vehicle or RP67580 were injected daily. C, F: On day 35, NTX (2.6 nmol in 10 μl i.th.) was injected again (arrow). Each panel was analyzed by RM (both variables) 2-way ANOVA; overall p values for ‘time’ (effect of CFA or NTX) are given in each panel. Holm-Sidak’s post-hoc tests comparing to day −4 or 0 min: * p<0.05, ** p<0.01, *** p<0.001.
To study the role of NK1Rs on the expression of SNI-induced latent sensitization we determined if RP67580 suppressed the allodynia induced by NTX given shortly afterward. On day 28, rats were injected i.th. with vehicle (control) or RP67580 (0.3 nmol), and 15 min later with NTX (2.6 nmol). In the control group, NTX induced bilateral allodynia in the hindpaws, which peaked at 15 min and lasted 2 h (Fig. 3B). After RP67580, the NTX-induced allodynia was largely eliminated (Fig. 3E).
To study the role of NK1Rs on the maintenance of SNI-induced latent sensitization, rats were injected i.th. with vehicle or RP67580 daily for two more days and then with NTX (2.6 nmol i.th.) on day 35 (Fig. 1). NTX produced bilateral allodynia in the control rats (Fig. 3C) but not in the rats injected with RP67580 (Fig. 3F).
Results were analyzed by repeated-measures 2-way ANOVA (Table 2). The effect of SNI over time was significant in both groups (A and D), and a significant effect of ‘side’ and ‘time x side’ showed that the effect of SNI was limited to the ipsilateral paw. On day 28, the effect of NTX over time was significant in the control rats (B) and also in the RP67580-injected rats (E), but the later seems to be due both to a small increase in allodynia at 30 min and to a decrease in allodynia at 2 h, both of which were not statistically significant in the Holm-Sidak’s post-hoc test (Fig. 3E). On day 35, the effect of NTX over time was significant in the controls (C) but not in the rats injected with RP67580 (F). After the injections of NTX, ‘time x side’ was not significant in the RP67580-injected rats (E, F), indicating a lack of effect of NTX in either paw. In the control rats, the effect of ‘time x side’ was significant on day 28 due to a decrease in allodynia in the contralateral paw at 2 h (Fig. 3B). The effect of ‘time x side’ was not significant in the control rats on day 35 (Fig. 3C).
Table 2 -.
Statistical analyses of data in Fig. 3: SNI-induced latent sensitization
| group | Fig. 3 panel | n | data analyzed | time | side | time x side |
|---|---|---|---|---|---|---|
| control | A | 8 | SNI, days −4 to 35 |
p = 0.0026 F(5, 35) = 4.58 |
p = 0.0017 F(1, 7) = 24 |
p < 0.0001 F(5, 29) = 8.3 |
| B | 8 | day 28: vehicle i.th. then NTX i.th. |
p < 0.0001 F(4, 28) = 16 |
p = 0.0085 F(1, 7) = 13 |
p = 0.0042 F(4, 28) = 4.8 |
|
| C | 5 | day 35: NTX i.th. |
p = 0.0039 F(4, 16) = 6.0 |
p = 0.063 F(1, 4) = 27 |
p = 0.403 F(4, 16) = 1.07 |
|
| RP67580 | D | 7 | SNI, days −4 to 35 |
p = 0.0058 F(5, 30) = 4.12 |
p = 0.0006 F(1, 6) = 42 |
p < 0.0001 F(5, 30) = 9.4 |
| E | 7 | day 28: RP67580 i.th. then NTX i.th. |
p = 0.0018 F(4, 24) = 5.9 |
p = 0.0084 F(1, 6) = 14.9 |
p = 0.98 F(4, 24) = 0.08 |
|
| F | 7 | day 35: NTX i.th. |
p = 0.49 F(4, 24) = 0.88 |
p = 0.14 F(1, 6) = 2.88 |
p = 0.52 F(4, 24) =0.82 |
Data in the panels of Fig. 3 were analyzed by two-way ANOVA, repeated-measures by both variables: time of von Frey measures and side (ipsilateral vs. contralateral). Panel A was analyzed using mixed-effects analysis because a few baseline data were missing. n is the sample size. In row C, 3 of the 8 rats were excluded because they lost the i.th. catheter.
3.3. Substance P release and NK1R activation during latent sensitization
If latent sensitization is maintained by NK1Rs in dorsal horn neurons, then these receptors should be activated by a sustained release of their agonists substance P and neurokinin A. This should result in increased basal NK1R internalization in lamina I neurons in rats with latent sensitization. Substance P release from primary afferents is inhibited by MORs (Chen et al., 2018a; Kondo et al., 2005), which are constitutively active during latent sensitization (Corder et al., 2013; Walwyn et al., 2016). Therefore, we predicted that an i.th. injection of NTX would increase NK1R internalization in rats with latent sensitization.
To test these two predictions, we injected rats in the hindpaw with 50 μl CFA to induce latent sensitization. Control rats were injected with 50 μl saline. The saline-injected rats developed a small amount of bilateral mechanical allodynia on day 7 (Fig. 4A), as indicated by a significant effect of the variable time (Table 3A) without significant differences between the hindpaws (‘side’). In contrast, the CFA-injected rats showed strong allodynia ipsilaterally that gradually returned to baseline levels and no allodynia contralaterally (Fig. 4B), as indicated by a significant effect of CFA over time, significant differences between the hindpaws (‘side’) and significant interaction between these variables (Table 3B). On day 28, the control and CFA-injected groups were divided into two sub-groups: one that received i.th. saline and another that received i.th. NTX (2.6 nmol). Rats were killed and fixed 40 min after the i.th. injection, and lumbar (L4-L5) segments of the spinal cord were processed for NK1R immunohistochemistry.
Figure 4. NK1R internalization during latent sensitization.
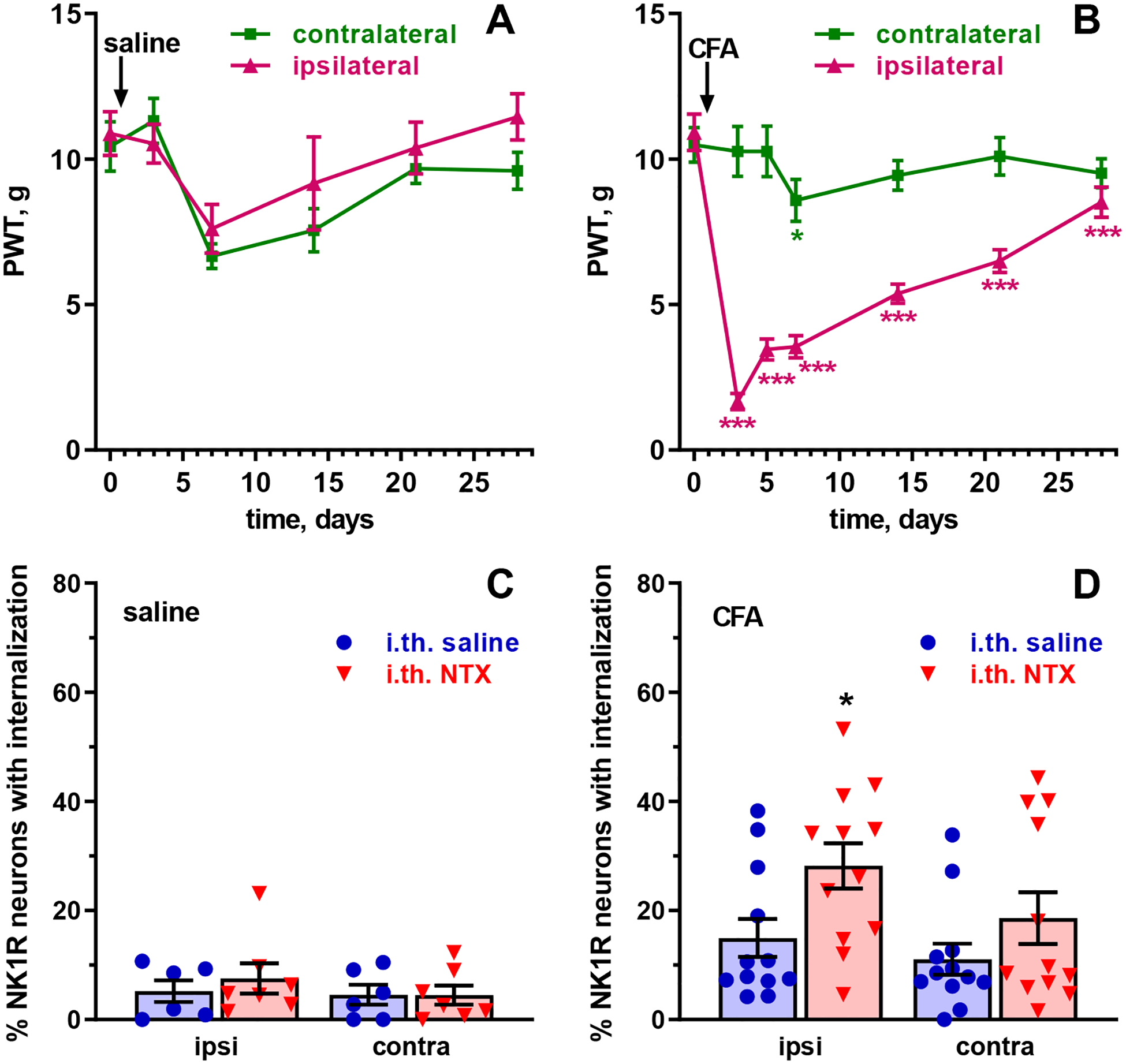
Rats were injected in one hindpaw with 50 μl saline (A, n=14 rats) or CFA (B, n=24 rats). Paw withdrawal thresholds (PWT, von Frey filaments) were measured in the ipsilateral and contralateral hindpaws at the times indicated. On day 28, rats injected in the paw with saline (C, n=6 or 7 rats/group) or CFA (D, n=12 rats/group) received 10 μl intrathecal saline or NTX (2.6 nmol). Rats were killed 40 min later and NK1R internalization was measured in lamina I neurons of the L4-L5 dorsal horns ipsilateral and contralateral to the injected paw. Analyses are shown in Tables 3 and 4. Holm-Sidak’s post-hoc tests comparing to time 0 (A, B) or i.th. saline (C, D): * p<0.05, *** p<0.001.
Negligible NK1R internalization was found in the control rats without latent sensitization, regardless of whether they received i.th. saline or i.th. NTX (Fig. 4C). In contrast, rats with CFA-induced latent sensitization showed low but significant amounts of NK1R internalization even after i.th. saline, which increased in the rats that received i.th. NTX (Fig. 4D). A three-way ANOVA of the combined data of panels C and D of Fig. 4 (Table 4) yielded significant effects for the variables CFA and NTX, showing that both the CFA injection in the paw and i.th. NTX resulted in significant increases in NK1R internalization.
Table 4 -.
| CFA | NTX | side | NTX x CFA | NTX x side | CFA x side | NTX x CFA x side | |
| p | < 0.0001 | 0.0425 | 0.126 | 0.100 | 0.470 | 0.383 | 0.769 |
| F(1, 66) | 21 | 4.28 | 2.39 | 2.78 | 0.526 | 0.768 | 0.087 |
Fig. 5 shows confocal images of lamina I NK1R neurons from the L4-L5 spinal segments of rats injected with CFA in the paw. Fig. 5 shows a large neuron in central lamina I ipsilateral to the paw injected with CFA in a rat that received i.th. saline. This neuron shows no NK1R internalization, with all NK1R immunoreactivity at the cell surface and no endosomes. Fig. 5 B and C show two neurons in medial lamina I (near the dorsal funiculus) contralateral to the paw injected with CFA in a rat that received i.th. NTX. In both neurons NK1R immunoreactivity has disappeared from the cell surface and is present in numerous endosomes outside the nucleus. Fig. 5 C shows a neuron in central lamina I ipsilateral to the paw injected with CFA in a rat that received i.th. NTX. This neuron was considered with NK1R internalization due to the presence of more than 10 endosomes, although NK1R immunoreactivity is also present at the cell surface. Other examples of NK1R neurons with and without internalization can be found in our previous reports (Adelson et al., 2009; Chen et al., 2014; Marvizon et al., 1997; Zhang et al., 2010).
Figure 5. Confocal microscope images of NK1R neurons in lamina I of rats with latent sensitization.
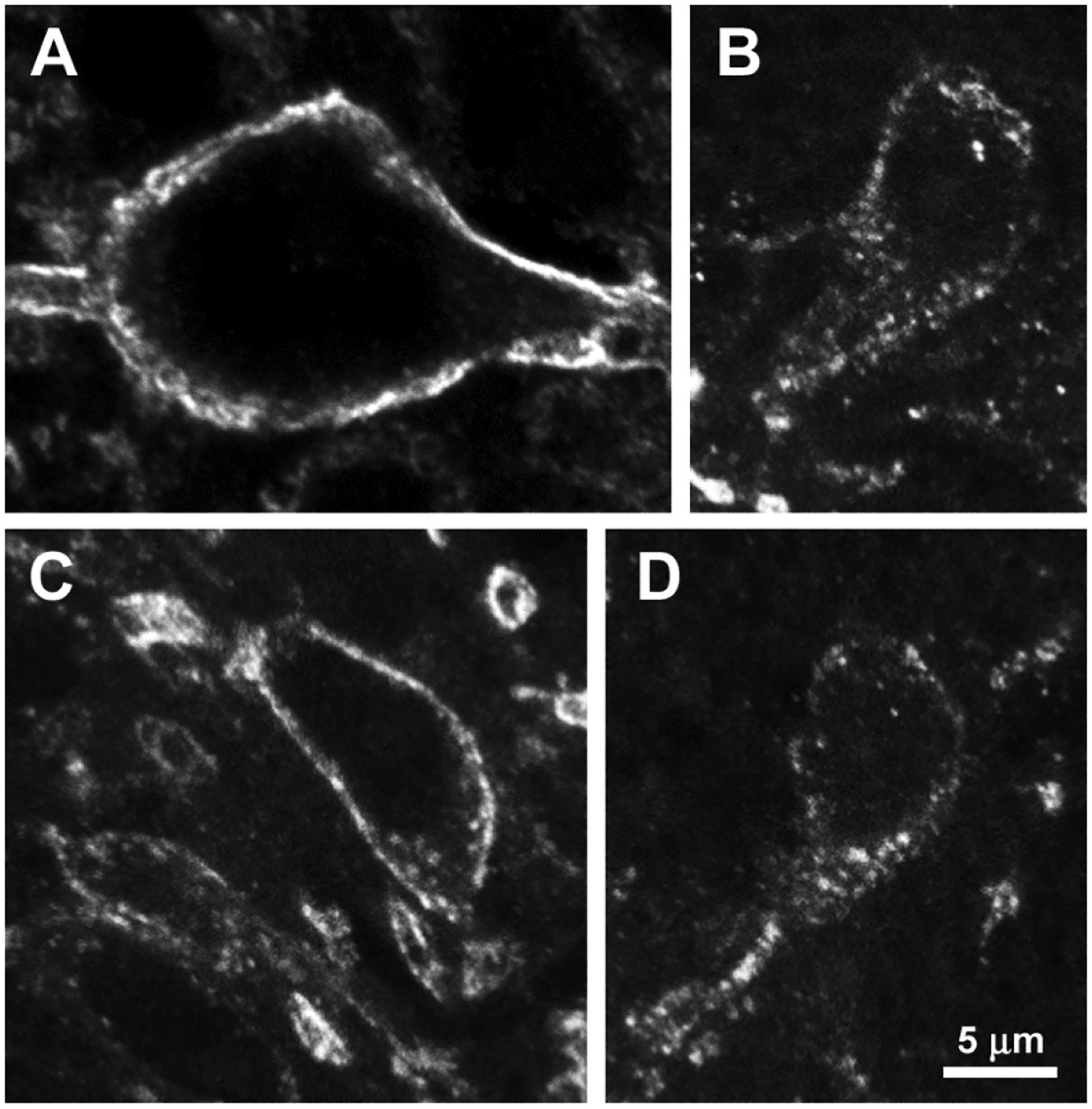
Images were taken from the L4-L5 spinal segments of rats from the experiment in Figure 4. Images are 6 optical sections separated 230 nm, taken with a 63x objective. Voxel size is 99 × 99 × 230 nm. All panels are at the same scale; scale bar is 5 μm. Rats were injected in one hindpaw with 50 μl CFA on day 0. On day 28 received 10 μl intrathecal saline (A) or 2.6 nmol NTX (B-D) and were fixed 40 min later for NK1R immunohistochemistry. A: Neuron in central lamina I ipsilateral to paw injection of CFA after intrathecal saline; there is no NK1R internalization. B-D: Two neurons in medial lamina I contralateral to paw injection of CFA after intrathecal NTX; there is extensive NK1R internalization and almost no NK1Rs at the cell surface. C: Neuron in the central lamina I ipsilateral to paw injection of CFA after intrathecal NTX; there are many NK1R endosomes and also NK1Rs at the cell surface.
These results confirmed our two predictions and strongly support the ideas that 1) there is sustained substance P release during latent sensitization and 2) this substance P release is partially, but not completely, suppressed by MORs.
4. Discussion
4.1. NK1Rs mediate the maintenance of latent sensitization
We postulate that different signals mediate the initiation, the maintenance and the expression of latent sensitization. Our premise is that, since hyperalgesia is continuously suppressed by opioid receptors during latent sensitization (Corder et al., 2013; Severino et al., 2018; Walwyn et al., 2016), if a compound eliminates NTX-induced allodynia after several days then it blocks the maintenance of latent sensitization. In contrast, inhibitors of mechanisms that mediate the expression of latent sensitization would inhibit NTX-induced allodynia only while the inhibitor is present.
We found that the NK1R antagonist RP67580 eliminated the allodynia induced by NTX not only when NTX was given shortly afterward, but also when NTX was given one week later. We used three daily i.th. injections of SR67580 and did not assess if a single injection of SR67580 would achieve the same effect. Also, it is possible that NTX-induced allodynia returns after one week. Still, our findings provide a strong indication that NK1Rs are involved in the maintenance of latent sensitization. In a parallel study, we used the same criteria to show that a Src family kinase also maintains latent sensitization (Chen and Marvizón, 2020).
Our results are consistent with a previous study (Rivat et al., 2009) showing that selective elimination of NK1R-expressing neurons in the dorsal horn with substance P-saporin decreased the mechanical allodynia and thermal hyperalgesia induced by paw incision or by several injections of the opioid fentanyl, two stimuli that induce latent sensitization (Campillo et al., 2011; Lian et al., 2010; Rivat et al., 2007). However, Rivat et al. (2009) studied only the initial period of hyperalgesia induced by incision or fentanyl (up to day 11) and not the reinstatement of hyperalgesia by NTX, the hallmark of latent sensitization.
4.2. Mechanism
Neurons with NK1Rs in lamina I of the dorsal horn are key components of the dorsal horn circuitry that modulates pain in rodents (Gutierrez-Mecinas et al., 2016; Haring et al., 2018; Polgar et al., 2013; Sathyamurthy et al., 2018; Todd, 2017; Yasaka et al., 2014) because many of them send axons to the parabrachial nucleus, the brainstem and the thalamus (Polgár et al., 2010; Ruscheweyh et al., 2004; Todd et al., 2000). These neurons receive direct synapses from peptidergic C/Aδ fibers (Todd et al., 2000; Torsney and MacDermott, 2006), and these synapses are an important locus of pain regulation (Li et al., 2018). NK1R neurons also receive synapses from vertical cells, which serve as a hub to integrate signals from excitatory and inhibitory interneurons of the dorsal horn (Kato et al., 2009; Peirs and Seal, 2016; Peirs et al., 2015; Yasaka et al., 2010; Yasaka et al., 2014). Activation of NK1Rs in dorsal horn neurons produces slow depolarizations, increases excitability (Murase and Randic, 1984; Murase et al., 1986), increases NMDA receptor currents (Rusin et al., 1993; Rusin et al., 1992), and induces long-term potentiation of their synapses with primary afferents (Ikeda et al., 2003; Liu and Sandkuhler, 1997; Liu and Sandkuhler, 1998). Therefore, sensitization of NK1R neurons in lamina I would increase the gain of pain signals sent to the brain, which can explain the pain hypersensitivity during latent sensitization and why interrupting these signals can eliminate latent sensitization.
4.3. NK1Rs are involved in both inflammatory and neuropathic pain
The NK1R antagonist RP67580 eliminated latent sensitization induced by either CFA or tibial SNI, indicating that NK1Rs maintain latent sensitization induced by inflammation or nerve injury. Previous studies have shown that NK1R expression in dorsal horn neurons increases after inflammation induced by CFA, arthritis and nerve injury (Abbadie et al., 1997; Allen et al., 1999; Honore et al., 1999; Krause et al., 1995). Cahill and Coderre (2002) showed that the NK1R antagonist L-732,138, given i.th. daily for 4 days, inhibited the induction of mechanical and cold allodynia produced by sciatic nerve constriction. L-732,138 also decreased allodynia when given at days 8–12 after nerve injury, but its effect was gone by day 16. Hence, NK1Rs do not seem to maintain allodynia induced by nerve constriction, at least at these early time points. This suggests that initial phase of allodynia induced by nerve injury is mediated by mechanisms different from those that maintain latent sensitization.
4.4. Sustained substance P release and NK1R activation during latent sensitization
If latent sensitization is maintained by the continuous activation of NK1Rs this should be driven by a sustained release of substance P, since there is no evidence for NK1R constitutive activity. Indeed, we found that NK1R internalization is higher in rats with latent sensitization than in control rats, indicating that there is increased activation of NK1Rs during latent sensitization. However, the amount of NK1R internalization was quite low, ~15%, showing that a few active NK1Rs or NK1R-expressing neurons are sufficient to maintain hypersensitivity. This suggests that a complete blockade of NK1Rs by an antagonist is necessary to eliminate latent sensitization. NTX increased the number of neurons with NK1R internalization to ~30% in the CFA-injected rats, consistent with the idea that during latent sensitization MORs in primary afferents suppress hyperalgesia (Severino et al., 2018; Walwyn et al., 2016) by inhibiting the release of substance P and other neurotransmitters.
These findings are consistent with previous studies. Thus, NK1Rs are tonically active during the second phase of the formalin test (Henry et al., 1999). Nerve injury and CFA injection in the paw or the joint increases the expression of NK1Rs and their internalization after electrical stimulation of primary afferents (Abbadie et al., 1997; Allen et al., 1999; Honore et al., 1999).
4.5. Failure of NK1R antagonists in clinical trials
Any report on the anti-hyperalgesic effect of NK1R antagonists inevitably elicits the criticism that these antagonists have failed as analgesics in clinical trials. Nevertheless, the evidence presented in this study and previous work clearly shows that NK1Rs in dorsal horn neurons play a key role in pain modulation in rodents. Latent sensitization is not just a change in nociceptive thresholds but an aversive state, as demonstrated using the grimace scale and conditioned place preference after NTX injection in mice with latent sensitization (Corder et al., 2013). This discrepancy between animal studies and clinical trials was examined in two brief reviews 20 years ago (Hill, 2000; Urban and Fox, 2000), but no further clinical trials on NK1R antagonists as analgesics were performed. There may be species differences in the role of NK1Rs between rodents and humans, which would need to be studied. However, NK1R immunoreactive neurons in the human spinal cord are similar to the ones in the rat (Ding et al., 1999; Todd et al., 2000), so it is likely that NK1R dorsal horn neurons are also projection neurons in humans.
Hill (2000) suggested that the analgesic effects of NK1R antagonists could be due to their supraspinal effects on stress (Rupniak and Kramer, 2017). Indeed, stress induces hyperalgesia in rats with latent sensitization (Chen et al., 2018b; Rivat et al., 2007). However, in this study both RP67580 and NTX were given intrathecally, so it is unlikely that they had supraspinal effects. This volume of intrathecal injection precludes the injectate from reaching the brain (Yaksh and Rudy, 1976).
Another possibility is that the clinical trials with NK1R antagonists may not have detected the effects on chronic pain studied here. One of these clinical trials (Dionne et al., 1998) studied the effect of the NK1R antagonist CP-99,994 on pain measured up to 4 h after a dental extraction, which is not a measure of chronic pain. Nevertheless, CP-99,994 produced significant analgesia at 90 min. Three other clinical trials studied the effect of the NK1R antagonist lanepitant. The effect of lanepitant on migraine (Goldstein et al., 2001a) was close to statistical significance (p=0.065). Lanepitant did not decrease pain in osteoarthritis (Goldstein et al., 2000). Osteoarthritis is a chronic pain condition but, unlike latent sensitization, it involves a persistent peripheral injury. Lanepitant also failed to alleviate diabetic neuropathy (Goldstein et al., 2001b), perhaps because it is mechanistically different from nerve transection. In all three studies, lanepitant was administered orally and it is unclear whether it can cross the blood-brain barrier. NK1R antagonists have important effects in the brain (Rupniak and Kramer, 2017) that might counteract their effects on the spinal cord.
The latent sensitization model shows that the chronic pain state is radically different from normal pain transmission. The goal, then, is not so much to reduce pain but to reset the organism to the normal state. This study indicates that NK1R antagonists may be able to do this and our strategy may be useful to design any future clinical trials.
4.6. Conclusions
Activation of NK1Rs in lamina I neurons of the dorsal horn maintains pain hypersensitivity in the latent sensitization model of chronic pain. This is true for latent sensitization initiated by either inflammation or nerve injury.
Table 3 -.
Statistical analyses of von Frey data in Fig. 4
| group | Fig. 4 panel | n | time | side | time x side |
|---|---|---|---|---|---|
| saline | A | 8 |
p = 0.0012 F(3, 42) = 6.4 |
p = 0.957 F(1, 14) = 0.003 |
p = 0.178 F(3, 42) = 1.7 |
| CFA | B | 18 |
p < 0.0001 F(6, 136) = 22 |
p < 0.0001 F(1, 34) = 51 |
p < 0.0001 F(6, 136) = 21 |
Data in panels A and B of Fig. 4 were analyzed by mixed-effects model, repeated-measures by the variable ‘side’. n is the sample size.
Acknowledgements
Funding:
This work was supported by the Rehabilitation Research & Development Service, Department of Veterans Affairs [grant number 1I01-RX001646A] and the National Institute of Drug Abuse [grant number R01-DA033059].
Abbreviations:
- ANOVA
analysis of variance
- CFA
complete Freund’s adjuvant
- EC50
dose that produces half the effect
- i.th.
intrathecal
- Ki
equilibrium inhibition constant
- MOR
μ opioid receptor
- NTX
naltrexone
- NK1R
neurokinin 1 receptor
- NMDA
N-methyl-D-aspartate
- PE
polyethylene
- PWT
paw withdrawal threshold
- RP67580
(3aR,7aR)-Octahydro-2-[1-imino-2-(2-methoxyphenyl)ethyl]-7,7-diphenyl-4H-isoindol
- SNI
spared nerve injury
Footnotes
This study was done under the umbrella of the following UCLA institutes: Brain Research Institute, Center for the Study of Opioid Receptors and Drugs of Abuse, CURE: Digestive Diseases Research Center, and the Oppenheimer Family Center for Neurobiology of Stress and Resilience.
Declarations of interest: none.
References
- Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI, 1997. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J. Neurosci 17, 8049–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelson DW, Lao L, Zhang G, Kim W, Marvizón JC, 2009. Substance P release and neurokinin 1 receptor activation in the rat spinal cord increases with the firing frequency of C-fibers. Neuroscience 161, 538–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA, 1999. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J. Neurophysiol 81, 1379–1390. [DOI] [PubMed] [Google Scholar]
- Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW, 1997. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J. Neurosci 17, 5921–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Coderre TJ, 2002. Attenuation of hyperalgesia in a rat model of neuropathic pain after intrathecal pre- or post-treatment with a neurokinin-1 antagonist. Pain 95, 277–285. [DOI] [PubMed] [Google Scholar]
- Campillo A, Cabanero D, Romero A, Garcia-Nogales P, Puig MM, 2011. Delayed postoperative latent pain sensitization revealed by the systemic administration of opioid antagonists in mice. Eur. J. Pharmacol 657, 89–96. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G, 2000. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology 92, 465–472. [DOI] [PubMed] [Google Scholar]
- Chen W, Ennes HS, McRoberts JA, Marvizon JC, 2018a. Mechanisms of mu-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord. Neuropharmacology 128, 255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Marvizón JC, 2020. A Src family kinase maintains latent sensitization in rats, a model of inflammatory and neuropathic pain. Brain Res 1746, 146999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Taché Y, Marvizon JCG, 2018b. Corticotropin Releasing Factor in the brain and blocking spinal descending signals induce hyperalgesia in the latent sensitization model of chronic pain. Neuroscience 381, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Walwyn W, Ennes H, Kim H, McRoberts JA, Marvizon JC, 2014. BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci 39, 1439–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC, 2010. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience 166, 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder G, Doolen S, Donahue RR, Winter MK, Jutras BL, He Y, Hu X, Wieskopf JS, Mogil JS, Storm DR, Wang ZJ, McCarson KE, Taylor BK, 2013. Constitutive mu-opioid receptor activity leads to long-term endogenous analgesia and dependence. Science 341, 1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YQ, Zheng HX, Wang DS, Xu JQ, Gong LW, Lü Y, Qin BZ, Shi J, Li HL, Li JS, Shigemoto R, Kaneko T, Mizuno N, 1999. The distribution of substance P receptor (NK1)-like immunoreactive neurons in the newborn and adult human spinal cord. Neurosci Lett 266, 133–136. [DOI] [PubMed] [Google Scholar]
- Dionne RA, Max MB, Gordon SM, Parada S, Sang C, Gracely RH, Sethna NF, MacLean DB, 1998. The substance P receptor antagonist CP-99,994 reduces acute postoperative pain. Clin Pharmacol Ther 64, 562–568. [DOI] [PubMed] [Google Scholar]
- Doiron B, Zhao Y, Tzounopoulos T, 2011. Combined LTP and LTD of Modulatory Inputs Controls Neuronal Processing of Primary Sensory Inputs. The Journal of Neuroscience 31, 10579–10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Nelson TS, Santos DF, Doolen S, Gutierrez JJP, Ye N, Zhou J, B, K. T, 2019. An NPY Y1 receptor antagonist unmasks latent sensitization and reveals the contribution of protein kinase A and Epac to chronic inflammatory pain. Pain 160, 1754–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garret C, Carruette A, Fardin V, Moussaoui S, Peyronel JF, Blanchard JC, Laduron PM, 1991. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci U S A 88, 10208–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DJ, Offen WW, Klein EG, Phebus LA, Hipskind P, Johnson KW, Ryan RE Jr., 2001a. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia 21, 102–106. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wang O, Gitter BD, Iyengar S, 2001b. Dose-response study of the analgesic effect of lanepitant in patients with painful diabetic neuropathy. Clinical neuropharmacology 24, 16–22. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Wang O, Todd LE, Gitter BD, DeBrota DJ, Iyengar S, 2000. Study of the analgesic effect of lanepitant in patients with osteoarthritis pain. Clin Pharmacol Ther 67, 419–426. [DOI] [PubMed] [Google Scholar]
- Grady EF, Baluk P, Bohm S, Gamp PD, Wong H, Payan DG, Ansel J, Portbury AL, Furness JB, McDonald DM, Bunnett NW, 1996. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci 16, 6975–6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Furuta T, Watanabe M, Todd AJ, 2016. A quantitative study of neurochemically defined excitatory interneuron populations in laminae I-III of the mouse spinal cord. Mol Pain 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Zeisel A, Hochgerner H, Rinwa P, Jakobsson JET, Lonnerberg P, La Manno G, Sharma N, Borgius L, Kiehn O, Lagerstrom MC, Linnarsson S, Ernfors P, 2018. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat Neurosci 21, 869–880. [DOI] [PubMed] [Google Scholar]
- Henry JL, Yashpal K, Pitcher GM, Chabot J, Coderre TJ, 1999. Evidence for tonic activation of NK-1 receptors during the second phase of the formalin test in the rat. J. Neurosci 19, 6588–6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R, 2000. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol.Sci 21, 244–246. [DOI] [PubMed] [Google Scholar]
- Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW, 1999. Spinal cord substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J. Neurosci 19, 7670–7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Li Y, Zhong X, Hu Y, Liu P, Zhao Y, Deng Z, Liu X, Liu S, Zhong Y, 2017. Src-family kinases activation in spinal microglia contributes to central sensitization and chronic pain after lumbar disc herniation. Mol Pain 13, 1744806917733637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J, 2003. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science 299, 1237–1240. [DOI] [PubMed] [Google Scholar]
- Jarahi M, Sheibani V, Safakhah HA, Torkmandi H, Rashidy-Pour A, 2014. Effects of progesterone on neuropathic pain responses in an experimental animal model for peripheral neuropathy in the rat: a behavioral and electrophysiological study. Neuroscience 256, 403–411. [DOI] [PubMed] [Google Scholar]
- Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji R-R, Strassman AM, 2009. Organization of Intralaminar and Translaminar Neuronal Connectivity in the Superficial Spinal Dorsal Horn. J. Neurosci 29, 5088–5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingery WS, Guo TZ, Davies MF, Limbird L, Maze M, 2000. The alpha(2A) adrenoceptor and the sympathetic postganglionic neuron contribute to the development of neuropathic heat hyperalgesia in mice. Pain 85, 345–358. [DOI] [PubMed] [Google Scholar]
- Klein T, Magerl W, Hopf HC, Sandkuhler J, Treede RD, 2004. Perceptual correlates of nociceptive long-term potentiation and long-term depression in humans. J Neurosci 24, 964–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL, 2005. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J. Neurosci 25, 3651–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause JE, DiMaggio DA, McCarson KE, 1995. Alterations in neurokinin 1 receptor gene expression in models of pain and inflammation. Can J Physiol Pharmacol 73, 854–859. [DOI] [PubMed] [Google Scholar]
- Laird JM, Roza C, De Felipe C, Hunt SP, Cervero F, 2001. Role of central and peripheral tachykinin NK1 receptors in capsaicin-induced pain and hyperalgesia in mice. Pain 90, 97–103. [DOI] [PubMed] [Google Scholar]
- Li J, Serafin E, Baccei ML, 2018. Prostaglandin signaling governs spike timing-dependent plasticity at sensory synapses onto mouse spinal projection neurons. J Neurosci 38, 6628–6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian B, Vera-Portocarrero L, King T, Ossipov MH, Porreca F, 2010. Opioid-induced latent sensitization in a model of non-inflammatory viscerosomatic hypersensitivity. Brain Res. 1358, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sandkuhler J, 1997. Characterization of long-term potentiation of C-fiber-evoked potentials in spinal dorsal horn of adult rat: essential role of NK1 and NK2 receptors. J Neurophysiol 78, 1973–1982. [DOI] [PubMed] [Google Scholar]
- Liu XG, Sandkuhler J, 1998. Activation of spinal N-methyl-D-aspartate or neurokinin receptors induces long-term potentiation of spinal C-fibre-evoked potentials. Neuroscience 86, 1209–1216. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, DeMaster E, Malhotra A, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE, 1995. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science 268, 1629–1632. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Chen W, Fu W, Taylor BK, 2019. Neuropeptide Y release in the rat spinal cord measured with Y1 receptor internalization is increased after nerve injury. Neuropharmacology 158, 107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA, 1997. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J. Neurosci 17, 8129–8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Walwyn W, Minasyan A, Chen W, Taylor BK, 2015. Latent sensitization: a model for stress-sensitive chronic pain. Curr Protoc Neurosci 71, 9 50 51–59 50 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, Wang X, Matsuka Y, Neubert JK, Spigelman I, 2003. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience 118, 535–545. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Malcangio M, 2009. Current challenges in glia-pain biology. Neuron 64, 46–54. [DOI] [PubMed] [Google Scholar]
- Michot B, Bourgoin S, Viguier F, Hamon M, Kayser V, 2012. Differential effects of calcitonin gene-related peptide receptor blockade by olcegepant on mechanical allodynia induced by ligation of the infraorbital nerve vs the sciatic nerve in the rat. Pain 153, 1939–1948. [DOI] [PubMed] [Google Scholar]
- Murase K, Randic M, 1984. Actions of substance P on rat spinal dorsal horn neurones. J Physiol 346, 203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M, 1986. Substance P augments a persistent slow inward calcium-sensitive current in voltage-clamped spinal dorsal horn neurons of the rat. Brain Res 365, 369–376. [DOI] [PubMed] [Google Scholar]
- Peirs C, Seal R, 2016. Neural circuits for pain: Recent advances and current views. Science 354, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirs C, Williams SP, Zhao X, Walsh CE, Gedeon JY, Cagle NE, Goldring AC, Hioki H, Liu Z, Marell PS, Seal RP, 2015. Dorsal Horn Circuits for Persistent Mechanical Pain. Neuron 87, 797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár E, Al Ghamdi KS, Todd AJ, 2010. Two populations of neurokinin 1 receptor-expressing projection neurons in lamina I of the rat spinal cord that differ in AMPA receptor subunit composition and density of excitatory synaptic input. Neuroscience 167, 1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgar E, Sardella TC, Tiong SY, Locke S, Watanabe M, Todd AJ, 2013. Functional differences between neurochemically defined populations of inhibitory interneurons in the rat spinal dorsal horn. Pain 154, 2606–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C, Laboureyras E, Laulin JP, Le Roy C, Richebe P, Simonnet G, 2007. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology 32, 2217–2228. [DOI] [PubMed] [Google Scholar]
- Rivat C, Vera-Portocarrero LP, Ibrahim MM, Mata HP, Stagg NJ, De Felice M, Porreca F, Malan TP, 2009. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur J Neurosci 29, 727–737. [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada I, Lopez-Garcia JA, 2010. Changes in Membrane Excitability and Potassium Currents in Sensitized Dorsal Horn Neurons of Mice Pups. J. Neurosci 30, 5376–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupniak NMJ, Kramer MS, 2017. NK1 receptor antagonists for depression: Why a validated concept was abandoned. Journal of affective disorders 223, 121–125. [DOI] [PubMed] [Google Scholar]
- Ruscheweyh R, Ikeda H, Heinke B, Sandkuhler J, 2004. Distinctive membrane and discharge properties of rat spinal lamina I projection neurones in vitro. J Physiol 555, 527–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusin KI, Bleakman D, Chard PS, Randic M, Miller RJ, 1993. Tachykinins potentiate N-methyl-D-aspartate responses in acutely isolated neurons from the dorsal horn. J Neurochem 60, 952–960. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Ryu PD, Randic M, 1992. Modulation of excitatory amino acid responses in rat dorsal horn neurons by tachykinins. J Neurophysiol 68, 265–286. [DOI] [PubMed] [Google Scholar]
- Sathyamurthy A, Johnson KR, Matson KJE, Dobrott CI, Li L, Ryba AR, Bergman TB, Kelly MC, Kelley MW, Levine AJ, 2018. Massively Parallel Single Nucleus Transcriptional Profiling Defines Spinal Cord Neurons and Their Activity during Behavior. Cell Rep 22, 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severino A, Chen W, Hakimian JK, Kieffer BL, Gaveriaux-Ruff C, Walwyn W, Marvizon JCG, 2018. Mu-opioid receptors in nociceptive afferents produce a sustained suppression of hyperalgesia in chronic pain. Pain 159, 1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Eckert WA 3rd, Basbaum AI, 2003. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. The journal of pain: official journal of the American Pain Society 4, 465–470. [DOI] [PubMed] [Google Scholar]
- Solway B, Bose SC, Corder G, Donahue RR, Taylor BK, 2011. Tonic inhibition of chronic pain by neuropeptide Y. Proc Natl Acad Sci U S A 108, 7224–7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storkson RV, Kjorsvik A, Tjolsen A, Hole K, 1996. Lumbar catheterization of the spinal subarachnoid space in the rat. J Neurosci Methods 65, 167–172. [DOI] [PubMed] [Google Scholar]
- Taylor BK, Corder G, 2014. Endogenous Analgesia, Dependence, and Latent Pain Sensitization. Curr Top Behav Neurosci 20, 283–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, 2017. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol Pain 13, 1744806917693003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd AJ, McGill MM, Shehab SA, 2000. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem. Eur. J. Neurosci 12, 689–700. [DOI] [PubMed] [Google Scholar]
- Torsney C, MacDermott AB, 2006. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci 26, 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban LA, Fox AJ, 2000. NK1 receptor antagonists--are they really without effect in the pain clinic? Trends Pharmacol Sci. 21, 462–464. [DOI] [PubMed] [Google Scholar]
- Walwyn W, Chen W, Kim H, Minasyan A, Ennes H, McRoberts JA, Marvizon JC, 2016. Sustained suppression of hyperalgesia during latent sensitization by μ, δ and κ opioid receptors and α2A adrenergic receptors - role of constitutive activity. J Neurosci 36, 204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XJ, Dalsgaard CJ, Wiesenfeld-Hallin Z, 1992. Spinal substance P and N-methyl-D-aspartate receptors are coactivated in the induction of Central Sensitization of the nociceptive flexor reflex. Neuroscience 51, 641–648. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA, 1976. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 17, 1031–1036. [DOI] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ, 2010. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain 151, 475–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Polgar E, Watanabe M, Kumamoto E, Riddell JS, Todd AJ, 2014. A putative relay circuit providing low-threshold mechanoreceptive input to lamina I projection neurons via vertical cells in lamina II of the rat dorsal horn. Mol Pain 10, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Chen W, Marvizon JC, 2010. Src family kinases mediate the inhibition of substance P release in the rat spinal cord by μ-opioid receptors and GABAB receptors, but not α2 adrenergic receptors. Eur. J. Neurosci 32, 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L-J, Peng J-Y, Xu Y-N, Zeng W-J, Zhang J, Wei X, Mai C-L, Lin Z-J, Liu Y, Murugan M, Eyo U, Umpierre A, Xin W-J, Chen T, Li M, Wang H, Richardson J, Tan Z, Liu X, Wu L-J, 2019. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Reports 27, 3844–3859.e3846. [DOI] [PMC free article] [PubMed] [Google Scholar]


