The use of fetoscopic endoluminal tracheal occlusion in severe left-sided congenital diaphragmatic hernia may improve morbidity but did not reduce overall mortality in a North American cohort.
Abstract
OBJECTIVE:
To report the outcomes of fetoscopic endoluminal tracheal occlusion in a multicenter North American cohort of patients with isolated, left-sided congenital diaphragmatic hernia (CDH) and to compare neonatal mortality and morbidity in patients with severe left-sided congenital diaphragmatic hernia who underwent fetoscopic endoluminal tracheal occlusion with those expectantly managed.
METHODS:
We analyzed data from 10 centers in the NAFTNet (North American Fetal Therapy Network) FETO (Fetoscopic Endoluminal Tracheal Occlusion) Consortium registry, collected between November 1, 2008, and December 31, 2020. In addition to reporting procedure-related surgical outcomes of fetoscopic endoluminal tracheal occlusion, we performed a comparative analysis of fetoscopic endoluminal tracheal occlusion compared with contemporaneous expectantly managed patients.
RESULTS:
Fetoscopic endoluminal tracheal occlusion was successfully performed in 87 of 89 patients (97.8%). Six-month survival in patients with severe left-sided congenital diaphragmatic hernia did not differ significantly between patients who underwent fetoscopic endoluminal tracheal occlusion and those managed expectantly (69.8% vs 58.1%, P=.30). Patients who underwent fetoscopic endoluminal tracheal occlusion had higher rates of preterm prelabor rupture of membranes (54.0% vs 14.3%, P<.001), earlier gestational age at delivery (median 35.0 weeks vs 38.3 weeks, P<.001), and lower birth weights (mean 2,487 g vs 2,857 g, P=.001). On subanalysis, in patients for whom all recorded observed-to-expected lung/head ratio measurements were below 25%, patients with fetoscopic endoluminal tracheal occlusion required fewer days of extracorporeal membrane oxygenation (ECMO) (median 9.0 days vs 17.0 days, P=.014).
CONCLUSION:
In this cohort, fetoscopic endoluminal tracheal occlusion was successfully implemented across several North American fetal therapy centers. Although survival was similar among patients undergoing fetoscopic endoluminal tracheal occlusion and those expectantly managed, fetoscopic endoluminal tracheal occlusion in North American centers may reduce morbidity, as suggested by fewer days of ECMO in those patients with persistently reduced lung volumes (observed-to-expected lung/head ratio below 25%).
The prevalence of congenital diaphragmatic hernia has been reported as 1–4 cases per 10,000 live births, of which 30–50% are not isolated.1,2 Poor outcomes in infants with severe pulmonary hypoplasia and hypertension attributable to congenital diaphragmatic hernia drove the development of fetal interventions for this condition.3,4 Fetoscopic endoluminal tracheal occlusion has been proposed as a minimally invasive method to improve fetal lung growth and infant survival.5,6
The lack of robust evidence supporting fetoscopic endoluminal tracheal occlusion for congenital diaphragmatic hernia has precluded its adoption as a standard of care. The TOTAL (Tracheal Occlusion to Accelerate Lung Growth) trial is the largest randomized controlled trial to evaluate outcomes of fetoscopic endoluminal tracheal occlusion compared with expectant management for isolated left-sided congenital diaphragmatic hernia.7–9 This trial, conducted predominantly in European centers, reported better survival with fetoscopic endoluminal tracheal occlusion in patients with severe left-sided congenital diaphragmatic hernia.7 These findings remain controversial, however. Some argue that they are not generalizable to centers where postnatal management includes centralized care and liberal use of extracorporeal membrane oxygenation (ECMO).10 Others contend that outcomes for severe left-sided congenital diaphragmatic hernia have improved in North American centers over the past decade, so fetoscopic endoluminal tracheal occlusion may provide less of a survival benefit over expectant management in these centers.11 Therefore, further investigation of fetoscopic endoluminal tracheal occlusion outcomes in different care settings is warranted.
In this study, we 1) report procedural outcomes in a North American cohort of patients with prenatally diagnosed left-sided congenital diaphragmatic hernia treated with fetoscopic endoluminal tracheal occlusion and 2) compare neonatal mortality and morbidity in patients with severe left-sided congenital diaphragmatic hernia who underwent fetoscopic endoluminal tracheal occlusion with those who were expectantly managed.
METHODS
This was a collaborative study performed at 10 centers of the NAFTNet (North American Fetal Therapy Network). Principal investigators at each center had performed more than 36 fetoscopies a year, and centers had an active pediatric management program.10 Principal investigator training included observation and participation in 15 procedures at established fetoscopic endoluminal tracheal occlusion centers. All centers had institutional or ethics board approval and, in the United States, operated under an investigational device exemption by the Food and Drug Administration.
In 2015, NAFTNet established the FETO (Fetoscopic Endoluminal Tracheal Occlusion) Consortium Registry, a prospective REDCap-based registry for patients with congenital diaphragmatic hernia managed at NAFTNet FETO centers. This registry includes data collected prospectively from November 2008 to December 2020. Seven of the NAFTNet centers performing fetoscopic endoluminal tracheal occlusion were enrolled in a prospective feasibility study initiated in 2015 under a shared investigational device exemption with the purpose of participating in the TOTAL trial. Fetoscopic endoluminal tracheal occlusions were also performed before the establishment of the FETO Consortium Registry in two centers operating under separate investigational device exemptions and at a Canadian center that obtained research ethics board FETO approval in 2010. These prospectively collected data were entered retrospectively into the FETO Consortium Registry after 2015. The registry also includes 14 cases that have been reported previously in two feasibility studies and in the TOTAL trial.7–9,12,13
Centers submitted data on singleton pregnancies between November 1, 2008, and December 31, 2020, that met the inclusion criteria for fetoscopic endoluminal tracheal occlusion: any isolated severe or moderate-sized, left-sided congenital diaphragmatic hernia diagnosed after 18 weeks of gestation with a normal karyotype or chromosomal microarray. Patients were offered expectant management or fetoscopic endoluminal tracheal occlusion, according to preference. Lung volumes were measured after 20 0/7 weeks of gestation with ultrasound trace of the observed-to-expected lung/head circumference ratio, longest axis, or midclavicular anterior–posterior techniques. An observed-to-expected lung/head ratio below 25% was defined as severe and 25–34.9% as moderate.7,8 Patient demographics, prenatal imaging, antenatal operative, delivery, neonatal variables, postnatal repair, and neonatal intensive care unit course were extracted from the medical record. Per protocol, patient race was extracted from the medical record and included for comparison with previous trials. Procedural data for the entire cohort were analyzed separately and in addition to the comparative analysis, which was limited to patients with isolated severe congenital diaphragmatic hernia.
Fetoscopic endoluminal tracheal occlusion for left-sided congenital diaphragmatic hernia was performed between 27 0/7 and 29 6/7 weeks of gestation for severe cases and between 30 0/7 and 31 6/7 weeks of gestation for moderate cases, per the study protocol. Patients with a later diagnosis of severe left-sided congenital diaphragmatic hernia with fetoscopic endoluminal tracheal occlusion after 29 6/7 weeks of gestation and those who were expectantly managed with initial assessment after 29 6/7 weeks were included in an intention-to-treat analysis. Apart from 15 cases at two institutions using the NFocus Goldvalve balloon, the BALT GOLDBAL2 system was used for fetoscopic endoluminal tracheal occlusion (www.balt.fr). Fetoscopic endoluminal tracheal occlusion removal was performed between 34 0/7 and 34 6/7 weeks of gestation by fetoscopy, percutaneous ultrasound-guided needle puncture, or bronchoscopy at ex utero intrapartum treatment (EXIT) or after umbilical cord clamping and transection.
Among the entire cohort in the registry, fetoscopic endoluminal tracheal occlusion procedural outcomes measured included gestational age at insertion and removal, procedure success, anesthesia, removal technique, duration of occlusion, delivery within 24 hours of removal, preterm prelabor rupture of membranes (PPROM) within 3 weeks of fetoscopic endoluminal tracheal occlusion, and gestational age at delivery. Among a cohort of patients with isolated severe congenital diaphragmatic hernia, comparative analysis of 6-month survival in fetoscopic endoluminal tracheal occlusion compared with expectant management was performed. Secondary outcomes included incidence of PPROM before 37 weeks of gestation; gestational age at PPROM and delivery; neonatal birth weight; congenital diaphragmatic hernia repair details; ECMO use and duration; neonatal intensive care unit stay; rates of intraventricular hemorrhage, sepsis, necrotizing enterocolitis, and periventricular leukomalacia; and fetoscopic endoluminal tracheal occlusion operative data.
Statistical analysis was performed with R 4.1.0.14 Data are reported as number (percent), mean±SD, or medians and interquartile ranges or ranges (minimum–maximum), as appropriate. Appropriate parametric and non-parametric tests were used to analyze continuous data. Categorical data were analyzed using χ2 or Fisher exact tests, as appropriate. P<.05 indicated significance.
RESULTS
A total of 447 patients were entered into the registry. Eighty-nine underwent fetoscopic endoluminal tracheal occlusion, and 358 were managed expectantly. Appendix 2, available online at http://links.lww.com/AOG/D534, lists 6-month survival among severe cases, ECMO use, and total case volume per center. Appendix 3, available online at http://links.lww.com/AOG/D534, lists procedural details of all fetoscopic endoluminal tracheal occlusion, severe and moderate, for all 10 centers.
The median gestational age at fetoscopic endoluminal tracheal occlusion was 28.7 weeks (range 26.3–31.4 weeks) in the severe group and 28.9 weeks (range 25.7–31.4 weeks) in the moderate group. In the severe group, two patients had fetoscopic endoluminal tracheal occlusion placed after 29 6/7 weeks of gestation; in the moderate group, 13 had fetoscopic endoluminal tracheal occlusion placed at or before 29 6/7 weeks. There were two failed fetoscopic endoluminal tracheal occlusion placements: one attributable to inability to enter the trachea and one with the balloon malpositioned within the right mainstem bronchus. In all other cases (n=87), the balloon was placed successfully at the first procedure.
Fetoscopic endoluminal tracheal occlusion removal in severe and moderate cases occurred at a median gestational age of 34.0 weeks (range 30.6–37.6 weeks) and 34.0 weeks (range 29.4–36 weeks), respectively. Removal was fetoscopic (n=53, 61.6%), percutaneous ultrasound-guided puncture (n=16, 18.6%), or at EXIT (n=15, 16.3%). Five patients required EXIT after failed fetoscopic removal (n=4) or ultrasound-guided puncture (n=1). Median latency from fetoscopic endoluminal tracheal occlusion placement until removal was 36 days (range 14–63 days) in the severe and 31 days (5–63 days) in the moderate group. Twelve patients (13.5%) experienced PPROM within 3 weeks of fetoscopic endoluminal tracheal occlusion placement. In 24 patients (27.9%), delivery occurred within 24 hours of balloon removal.
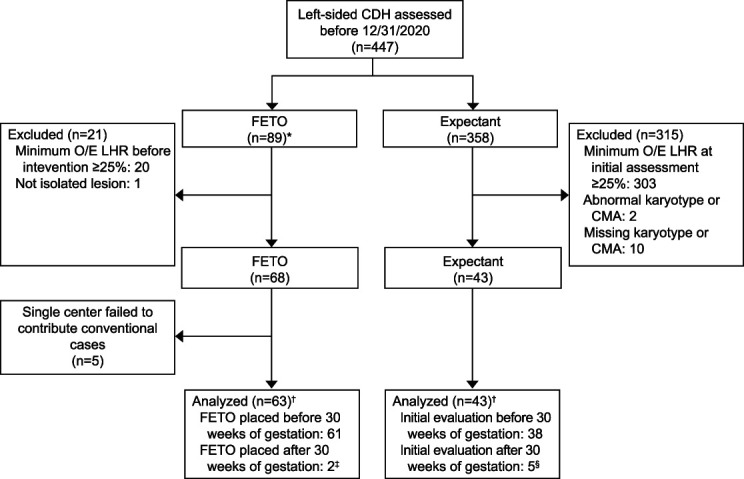
Among 447 patients in the registry, we excluded 323 with observed-to-expected lung/head ratio of 25% or higher (fetoscopic endoluminal tracheal occlusion n=20, expectant n=303), one fetoscopic endoluminal tracheal occlusion case with anomalies, and 12 expectantly managed patients with abnormal or missing genetic results (Fig. 1). One center contributed nine fetoscopic endoluminal tracheal occlusion cases and no expectantly managed cases; these were removed from comparative analysis but are included in fetoscopic endoluminal tracheal occlusion procedural details (Appendix 3, http://links.lww.com/AOG/D534).7,8,15,16 The final analysis included 63 fetuses (59.4%) with severe left-sided congenital diaphragmatic hernia who underwent fetoscopic endoluminal tracheal occlusion and 43 (40.6%) managed expectantly. Two patients with a late diagnosis of severe left-sided congenital diaphragmatic hernia had balloons placed after 29 6/7 weeks of gestation (30.3 and 31.4 weeks), and four expectantly managed patients had initial evaluation after 29 6/7 weeks (31.9–34.4 weeks) and are included in an intention-to-treat analysis (Fig. 1).
Fig. 1. Flowchart demonstrating patient inclusion and exclusion for the comparative analysis. *Patients for whom procedural data for entire cohort is included (data presented in Table 1). †Patients included for comparative analysis (data presented in Table 2). ‡Two patients had balloon placement after 30 weeks of gestation (31 3/7 and 30 2/7 weeks). §Five patients had initial evaluation after 30 weeks of gestation (31 6/7, 32 1/7, 32 3/7, 32 4/7, and 34 3/7 weeks). CDH, congenital diaphragmatic hernia; O/E LHR, observed-to-expected lung area/head circumference ratio; FETO, fetoscopic endoluminal tracheal occlusion; CMA, chromosomal microarray.
Bergh. Fetoscopic Endoluminal Tracheal Occlusion for Diaphragmatic Hernia. Obstet Gynecol 2024.
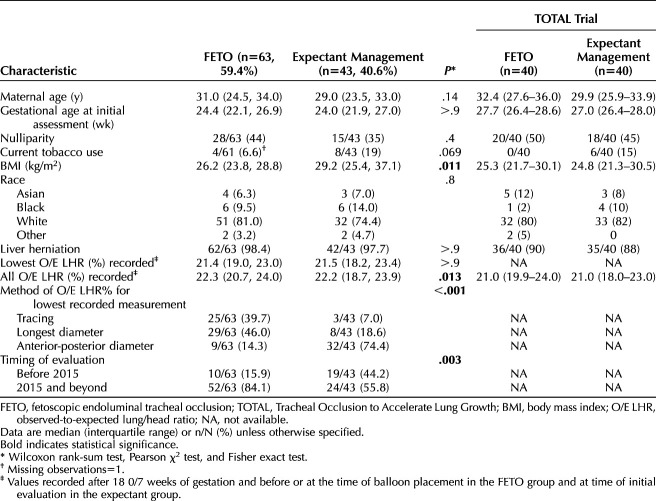
Table 1 lists demographics of fetoscopic endoluminal tracheal occlusion and expectantly managed patients with severe congenital diaphragmatic hernia. There were no differences in maternal age, gestational age at initial assessment, nulliparity, smoking status, or race. Patients with fetoscopic endoluminal tracheal occlusion had significantly lower body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) than expectantly managed patients (median 26.2 vs 29.2, P=.011). Observed-to-expected lung/head ratio values were comparable between groups (median lowest observed-to-expected lung/head ratio in fetoscopic endoluminal tracheal occlusion vs expectant 21.4% vs 21.5%, P>.9; median of all recorded observed-to-expected lung/head ratio in fetoscopic endoluminal tracheal occlusion vs expectant 22.3% vs 22.2%, P=.2). Lung size evaluation with ultrasound-derived observed-to-expected lung/head ratio for the lowest recorded measurement was performed most often by longest diameter and tracing methods in fetoscopic endoluminal tracheal occlusion cases (46.0% and 39.7% respectively), whereas the anterior–posterior measurement was most frequent in expectant cases (74.4%, P<.001). Significantly more fetoscopic endoluminal tracheal occlusion cases than expectantly managed cases were entered prospectively (84.1% vs 55.6%, P=.003). Demographic results for the fetoscopic endoluminal tracheal occlusion and expectant management cases from the severe arm of the TOTAL trial are included for comparison.7 In patients who underwent fetoscopic endoluminal tracheal occlusion, all observed-to-expected lung/head ratio values were recorded after 20 0/7 weeks of gestation up to the time of balloon placement; for expectantly managed patients, the initially recorded observed-to-expected lung/head ratios are shown.
Table 1.
Comparative Analysis of Fetoscopic Endoluminal Tracheal Occlusion and Expectant Management of Severe Congenital Diaphragmatic Hernia, Baseline Characteristics
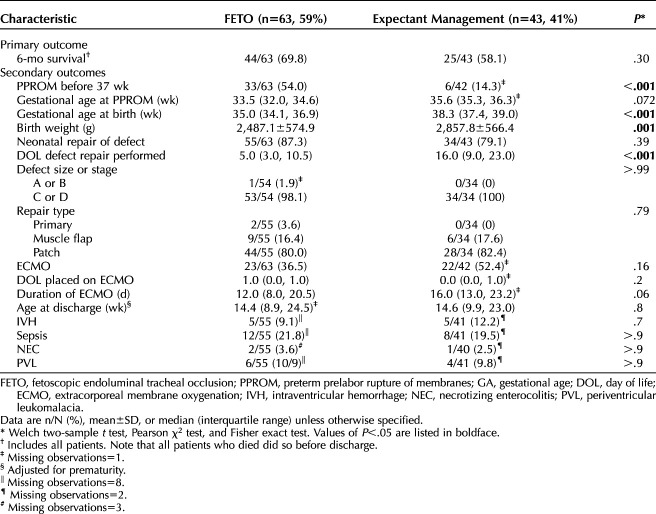
The primary and secondary outcomes of the comparative analysis are presented in Table 2. Although 6-month survival was higher in the fetoscopic endoluminal tracheal occlusion group than the expectantly managed group (69.8% vs 58.1%, P=.30) (Table 2), this did not reach statistical significance. Patients who underwent fetoscopic endoluminal tracheal occlusion had more PPROM before 37 weeks of gestation (54.0% vs 14.3%, P<.001), delivered earlier (35.0 weeks vs 38.3 weeks, P<.001), and had lower birth weight (2,487 g vs 2,857 g, P=.001). There were no differences in rates of neonatal defect repair, defect size or stage, or repair type. Neonates who underwent fetoscopic endoluminal tracheal occlusion had congenital diaphragmatic hernia repair earlier than expectantly managed neonate (5 days of life vs 16.0 days of life, P<.001). There was no difference in use, timing of initiation, or duration of ECMO or in adjusted age at discharge between groups. There were no differences in the incidence of intraventricular hemorrhage, sepsis, necrotizing enterocolitis, or periventricular leukomalacia.
Table 2.
Comparative Analysis of Fetoscopic Endoluminal Tracheal Occlusion and Expectant Management, Outcomes
Appendix 4, available online at http://links.lww.com/AOG/D534, presents a subanalysis of patients for whom all recorded observed-to-expected lung/head ratio measurements were below 25% and were obtained before 30 weeks of gestation with fetoscopic endoluminal tracheal occlusion performed before 30 weeks. This subset contained 43 patients with fetoscopic endoluminal tracheal occlusion (55.1%) and 35 expectantly managed patients (44.9%). The duration of ECMO was significantly shorter in patients with fetoscopic endoluminal tracheal occlusion than in expectantly managed patients (median 9 days vs 17 days, P=.014).
DISCUSSION
In this multicenter report of patients with isolated severe left-sided congenital diaphragmatic hernia managed with fetoscopic endoluminal tracheal occlusion or expectant care in North America, fetoscopic endoluminal tracheal occlusion placement and removal had a success rate and safety profile consistent with published literature. Although 6-month survival was higher in the fetoscopic endoluminal tracheal occlusion group than in those managed expectantly, this difference was not statistically significant. Fetoscopic endoluminal tracheal occlusion patients had significantly more PPROM, earlier gestational age at delivery, and lower birth weight without increased neonatal morbidity than expectantly managed patients. In patients with all observed-to-expected lung/head ratio measurements below 25% and fetoscopic endoluminal tracheal occlusion performed before 30 weeks of gestation, fetoscopic endoluminal tracheal occlusion required fewer ECMO days than expectant management.
In the TOTAL trials, fetoscopic endoluminal tracheal occlusion was performed at 12 centers with training metrics comparable with those of our study. Postnatal congenital diaphragmatic hernia management occurred in 46 pediatric surgical programs with varying case volumes and followed European consensus guidelines.7,8,17,18 The TOTAL trial demonstrated a more than 2.5-fold increase in survival to discharge for patients with isolated severe left-sided congenital diaphragmatic hernia after fetoscopic endoluminal tracheal occlusion compared with expectantly managed patients (40% vs 15%). However, our study cohort had a 75% higher survival rate after fetoscopic endoluminal tracheal occlusion than TOTAL and more than 280% higher survival with expectant management. Given this lower background mortality for expectantly managed patients, fetoscopic endoluminal tracheal occlusion in North America may not offer the survival benefit demonstrated in the TOTAL trial.
In our cohort, neonatal management differed from that in TOTAL in that ECMO was used more frequently for patients with severe left-sided congenital diaphragmatic hernia after fetoscopic endoluminal tracheal occlusion (37% vs 5% in TOTAL) and in those expectantly managed (52% vs 29% in TOTAL). In our cohort, 87.3% of patients with fetoscopic endoluminal tracheal occlusion and 79.1% of expectantly managed patients underwent surgical repair compared with 53% and 37% of patients in the corresponding TOTAL trial arms. In both studies, surgical repair occurred earlier after fetoscopic endoluminal tracheal occlusion than expectant management but was performed later in TOTAL, perhaps reflecting a more aggressive congenital diaphragmatic hernia management approach in North America.10 The North American care setting also differed from the TOTAL setting in that all participating centers fulfilled prescribed metrics for both prenatal and postnatal congenital diaphragmatic hernia care. The transition to postnatal care in such an integrated setting may offer significant survival benefit after fetoscopic endoluminal tracheal occlusion compared with postnatal management at a separate neonatal center.19
The nonsignificant survival benefit after fetoscopic endoluminal tracheal occlusion for patients managed in North American care settings raises the question of whether fetoscopic endoluminal tracheal occlusion improves morbidity. Recent reports suggest that in severe left-sided congenital diaphragmatic hernia, fetoscopic endoluminal tracheal occlusion was associated with a greater likelihood of early discharge, faster resolution of pulmonary hypertension and lower bronchodilator use between 1 and 5 years of life.20,21 We cannot report the effects of fetoscopic endoluminal tracheal occlusion on pulmonary hypertension because of inconsistent reporting among centers of postnatal echocardiogram findings, discharge medications, and 6-month pulmonary hypertension. However, our subanalysis finding of shortened ECMO duration after fetoscopic endoluminal tracheal occlusion is consistent with the anticipated pulmonary benefits.
The primary limitation of this study is that it was not randomized and was a cohort study not powered to demonstrate efficacy. Our data were also entered into the registry both retrospectively and prospectively, with significant heterogeneity among centers. Registry data span about 10 years, and among patients in the comparative analysis, significantly more fetoscopic endoluminal tracheal occlusions were performed after 2015 (n=52) than before (n=10, P=.003). The reason was rapid adoption of fetoscopic endoluminal tracheal occlusion after 2015 approval of a shared investigational device exemption, although this was unlikely to affect outcomes because 98.1% and 100% of patients in the fetoscopic endoluminal tracheal occlusion and expectant management groups respectively had a C or D type lesion on postnatal evaluation (P>.99). The data also do not include long-term outcomes, nor was postnatal care standardized. Accordingly, we cannot report long-term morbidity relevant to ECMO use,22 which was significantly affected by fetoscopic endoluminal tracheal occlusion on subanalysis. Additional measures of pulmonary hypertension and cardiac function not captured in this study might explain why this group required less ECMO. Finally, the majority of expectantly managed patients in the subanalysis had only a single observed-to-expected lung/head ratio available for review. It is possible that expectantly managed patients in this subanalysis actually were less severe cases than reported according to our exclusion criteria. However, the previously stated finding of either type C or D lesion at birth in almost the entire comparative analysis cohort suggests that the severity was similar between groups.
The main strength of this study is our ability to report a rare outcome in two contemporaneously managed groups through multicenter collaboration. This is the largest reported North American cohort of patients with congenital diaphragmatic hernia treated with fetoscopic endoluminal tracheal occlusion or expectantly managed, with more fetoscopic endoluminal tracheal occlusion cases than were included in the TOTAL trial. Thus, it represents real-world clinical experience across centers and adds important data regarding the implementation, safety, and success of fetoscopic endoluminal tracheal occlusion in North America.
Our study indicates that fetoscopic endoluminal tracheal occlusion can be performed safely in severe isolated left-sided congenital diaphragmatic hernia at North American centers with experienced pediatric programs. Fetoscopic endoluminal tracheal occlusion demonstrated a trend toward improved survival that did not reach statistical significance. Although obstetric complications were higher with fetoscopic endoluminal tracheal occlusion, these did not appear to negatively affect survival, despite delivery 3 weeks earlier than those managed expectantly. When considered together with lower ECMO requirements in the most severe cases on subanalysis, this suggests that fetoscopic endoluminal tracheal occlusion may help decrease pediatric morbidity and possibly mortality in programs with high survival rates. This emphasizes the need for further research and monitoring of long-term outcomes in patients undergoing fetoscopic endoluminal tracheal occlusion to determine its role in alleviating disease severity and morbidity in survivors, particularly in North America.
Footnotes
Financial Disclosure Eric Bergh disclosed that the NFocus Goldvalve balloon is no longer commercially available, and he receives royalties from UpToDate. Dr. Cromblehome disclosed that the Balt GOLDBAL2 balloon and catheter and Storz fetoscope are used under an investigational device exemption from the Food and Drug Administration, which are not otherwise available for use in the United States. Ahmet A. Baschat disclosed that the instrumentation was provided in-kind by Karl Storz, and he receives royalties from UpToDate. Holly Hedrick receives royalties from UpToDate. The other authors did not report any potential conflicts of interest.
Presented in part at the 2023 Society for Maternal-Fetal Medicine Annual Pregnancy Meeting, February 6–11, 2023, San Francisco, California.
A list of members of the NAFTNet FETO Consortium can be found in Appendix 1, available online at http://links.lww.com/AOG/D534.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/D535.
Figure.
No available caption
REFERENCES
- 1.Dott MM, Wong LY, Rasmussen SA. Population-based study of congenital diaphragmatic hernia: risk factors and survival in metropolitan Atlanta, 1968-1999. Birth Defects Res A Clin Mol Teratol 2003;67:261–7. doi: 10.1002/bdra.10039 [DOI] [PubMed] [Google Scholar]
- 2.Montalva L, Lauriti G, Zani A. Congenital heart disease associated with congenital diaphragmatic hernia: a systematic review on incidence, prenatal diagnosis, management, and outcome. J Pediatr Surg 2019;54:909–19. doi: 10.1016/j.jpedsurg.2019.01.018 [DOI] [PubMed] [Google Scholar]
- 3.Harrison MR, Adzick NS, Flake AW, Jennings RW, Estes JM, MacGillivray TE, et al. Correction of congenital diaphragmatic hernia in utero, VI: hard-earned lessons. J Pediatr Surg 1993;28:1411–7. doi: 10.1016/s0022-3468(05)80338-0 [DOI] [PubMed] [Google Scholar]
- 4.Harrison MR, Mychaliska GB, Albanese CT, Jennings RW, Farrell JA, Hawgood S, et al. Correction of congenital diaphragmatic hernia in utero, IX: fetuses with poor prognosis (liver herniation and low lung-to-head ratio) can be saved by fetoscopic temporary tracheal occlusion. J Pediatr Surg 1998;33:1017–22. doi: 10.1016/s0022-3468(98)90524-3 [DOI] [PubMed] [Google Scholar]
- 5.Deprest J, Gratacos E, Nicolaides KH, FETO Task Group. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound Obstet Gynecol 2004;24:121–6. doi: 10.1002/uog.1711 [DOI] [PubMed] [Google Scholar]
- 6.Peralta CF, Sbragia L, Bennini JR, de Fátima Assunção Braga A, Sampaio Rousselet M, Machado Rosa IR, et al. Fetoscopic endotracheal occlusion for severe isolated diaphragmatic hernia: initial experience from a single clinic in Brazil. Fetal Diagn Ther 2011;29:71–7. doi: 10.1159/000314617 [DOI] [PubMed] [Google Scholar]
- 7.Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med 2021;385:107–18. doi: 10.1056/NEJMoa2027030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deprest JA, Benachi A, Gratacos E, Nicolaides KH, Berg C, Persico N, et al. Randomized trial of fetal surgery for moderate left diaphragmatic hernia. N Engl J Med 2021;385:119–29. doi: 10.1056/NEJMoa2026983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Calster B, Benachi A, Nicolaides KH, Gratacos E, Berg C, Persico N, et al. The randomized Tracheal Occlusion to Accelerate Lung growth (TOTAL) – trials on fetal surgery for congenital diaphragmatic hernia: reanalysis using pooled data. Am J Obstet Gynecol 2022;226:560.e1–24. doi: 10.1016/j.ajog.2021.11.1351 [DOI] [PubMed] [Google Scholar]
- 10.Gien J, Kinsella JP, Behrendt NJ, Zaretsky MV, Galan HL, Liechty KW. Improved survival for infants with severe congenital diaphragmatic hernia. J Perinatol 2022;42:1189–94. doi: 10.1038/s41372-022-01397-3 [DOI] [PubMed] [Google Scholar]
- 11.Stolar CJH, Wilson JM, Losty PD, Flake AW. Fetal surgery for moderate and severe CDH – the TOTAL trials. J Pediatr Surg 2022;57:552–3. doi: 10.1016/j.jpedsurg.2021.09.034 [DOI] [PubMed] [Google Scholar]
- 12.Baschat AA, Rosner M, Millard SE, Murphy JD, Blakemore KJ, Keiser AM, et al. Single-center outcome of fetoscopic tracheal balloon occlusion for severe congenital diaphragmatic hernia. Obstet Gynecol 2020;135:511–21. doi: 10.1097/AOG.0000000000003692 [DOI] [PubMed] [Google Scholar]
- 13.Belfort MA, Olutoye OO, Cass DL, Olutoye OA, Cassady CI, Mehollin-Ray AR, et al. Feasibility and outcomes of fetoscopic tracheal occlusion for severe left diaphragmatic hernia. Obstet Gynecol 2017;129:20–9. doi: 10.1097/AOG.0000000000001749 [DOI] [PubMed] [Google Scholar]
- 14.R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing; 2021. [Google Scholar]
- 15.Persico N, Fabietti I, Ciralli F, Gentilino V, D’Ambrosi F, Boito S, et al. Fetoscopic endoluminal tracheal occlusion in fetuses with severe diaphragmatic hernia: a three-year single-center experience. Fetal Diagn Ther 2017;41:215–9. doi: 10.1159/000448096 [DOI] [PubMed] [Google Scholar]
- 16.Jani JC, Nicolaides KH, Gratacós E, Valencia CM, Doné E, Martinez JM, et al. Severe diaphragmatic hernia treated by fetal endoscopic tracheal occlusion. Ultrasound Obstet Gynecol 2009;34:304–10. doi: 10.1002/uog.6450 [DOI] [PubMed] [Google Scholar]
- 17.Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus. Neonatology 2010;98:354–64. doi: 10.1159/000320622 [DOI] [PubMed] [Google Scholar]
- 18.Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium consensus – 2015 update. Neonatology 2016;110:66–74. doi: 10.1159/000444210 [DOI] [PubMed] [Google Scholar]
- 19.Sferra SR, Miller JL, Cortes M S, Belfort MA, Cruz-Martínez R, Kunisaki SM, et al. Postnatal care setting and survival after fetoscopic tracheal occlusion for severe congenital diaphragmatic hernia: a systematic review and meta-analysis. J Pediatr Surg 2022;57:819–25. doi: 10.1016/j.jpedsurg.2022.05.011 [DOI] [PubMed] [Google Scholar]
- 20.Style CC, Olutoye OO, Belfort MA, Ayres NA, Cruz SM, Lau PE, et al. Fetal endoscopic tracheal occlusion reduces pulmonary hypertension in severe congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 2019;54:752–8. doi: 10.1002/uog.20216 [DOI] [PubMed] [Google Scholar]
- 21.Sferra SR, Nies MK, Miller JL, Garcia AV, Hodgman EI, Penikis AB, et al. . Morbidity in children after fetoscopic endoluminal tracheal occlusion for severe congenital diaphragmatic hernia: results from a multidisciplinary clinic. J Pediatr Surg 2023;58:14–9. doi: 10.1016/j.jpedsurg.2022.09.042 [DOI] [PubMed] [Google Scholar]
- 22.de Munck S, van der Cammen-van Zijp MHM, Zanen-van den Adel TPL, Wijnen RMH, Cochius-den Otter SCM, van Haren NEM, et al. Persisting motor function problems in school-aged survivors of congenital diaphragmatic hernia. Front Pediatr 2021;9:729054. doi: 10.3389/fped.2021.729054 [DOI] [PMC free article] [PubMed] [Google Scholar]