Abstract
Salt sensitivity concerns blood pressure alterations after a change in salt intake (sodium chloride). The heart is a pump, and vessels are tubes; sodium can affect both. A high salt intake increases cardiac output, promotes vascular dysfunction and capillary rarefaction, and chronically leads to increased systemic vascular resistance. More recent findings suggest that sodium also acts as an important second messenger regulating energy metabolism and cellular functions. Besides endothelial cells and fibroblasts, sodium also affects innate and adaptive immunometabolism, immune cell function, and influences certain microbes and microbiota-derived metabolites. We propose the idea that the definition of salt sensitivity should be expanded beyond high blood pressure to cellular and molecular salt sensitivity.
Keywords: macrophages, mitochondria, salts, sodium, T lymphocytes
WHITHER SODIUM?
Earlier, all was clear.1 The intracellular composition (muscle for instance) exhibited a sodium concentration of ±10 mmol/L, potassium concentration of ±160 mmol/L, magnesium concentration of ±35 mmol/L, etc. Anions were protein and phosphate. In the extracellular plasma water, the corresponding concentrations were sodium, ±151 mmol/L and potassium, ±4.3 mmol/L. In humans, the electrolytes were distributed within the (about 40 L) total body water; two-thirds were inside cells and one-third outside cells. Sodium was mostly exchangeable and extracellular although some was deposited in bone, the fate of which was largely unclear but not so exchangeable. By the 1970s, the regulation of these constituents had been well worked out and balance concepts were sufficiently accepted to support pressure natriuresis of blood pressure regulation and our understanding of salt and water balance.2 These ideas, and the supportive body of evidence, served us clinically well and were convincing. A veritable clinical tool was the knowledge generated by Edelman et al3 and Birkenfeld et al.4 They studied the relationship between plasma sodium, exchangeable sodium, exchangeable potassium, and total body water. Their insights showed us that by calculating effective free water clearance, we clinicians could always predict whose plasma sodium was going up or down and even at what rate the changes would occur. As a result, we had a fairly clear understanding of total body water, electrolyte contents, plasma concentrations, and subsequent speculations on blood pressure regulation. Osmotic forces, being what they are, would dictate that gain of exchangeable components would increase water and vice versa. That these ideas (a standard model if you will) could have immunologic implications never occurred to us, and we did not need anything else.
Standard models work until the mathematical infrastructure becomes wobbly. Titze et al5 studied only 3 people but those for weeks over time, in a Mars-flight simulation study. The subjects resided in a capsule over weeks. The sodium and all components of the diet were known. The investigators’ findings that sodium accumulation ran largely independent of water accumulation (or vice versa) was unexpected and stressed the standard model. How do we fit these findings into our understanding of human physiology?
The next task was to discover where the not-so-exchangeable sodium could be hiding. This experiment required carcass ashing and electrolyte measurements with atomic absorption spectroscopy.6 The skin proved to be an osmotically inactive, sodium accumulation site, an area that was also influenced by extremes of dietary salt intake or kidney function. Humans carry around about 5 kg of skin, and skin is the largest reservoir of extracellular fluid in the body. Skin is particularly rich in glycosaminoglycans, which because of their strong negative charge, in particular for sulphated glycosaminoglycans, became a prime candidate for a sodium interaction. Support for a role of glycosaminoglycans, in particular, sulphated glycosaminoglycans, was found in initial studies.7,8 However, this notion has been contested in 2 more recent rodent studies showing either no change or a reduction in glycosaminoglycans in animals receiving a high-salt diet (HSD) or in the deoxycorticosterone acetate and high-salt diet (DOCA)-salt model, respectively.9,10 Moreover, no indication of Na+ binding to glycosaminoglycans was identified.10 Thus, follow-up studies have to address this issue and to explain the discrepancies. Does sodium accumulation apply to man?
The development of magnetic resonance imaging for sodium allowed to visualize and quantify the tissue sodium content in humans.11 Of note, sodium content appeared to be higher in skin than in muscle for men, while women tended to have higher muscle sodium.12 Interestingly, hypertensive patients, patients with end-stage renal disease, as well as patients with autoimmune disease display an increased sodium content in this third space.13 Sodium stores seem to be dynamic, since SGLT-2 inhibition in patients with type 2 diabetes,14 diuretic treatment in patients with acute heart failure,15 successful treatment of patients with primary aldosteronism,11 hemodialysis,16 and after kidney transplantation17 show reduced tissue sodium content. In addition, an experimental HSD study in humans triggered cutaneous Na+ deposition as assessed by chemical analysis of punch biopsies.18 These findings indicate that stored sodium can be mobilized. However, whether or not the sodium is readily exchangeable to immediately satisfy the Edelman equation is another issue.
These largely descriptive findings were enhanced by additional human balance studies in another Mars-simulation study. However, in this study, dietary salt intake could be manipulated. The subjects ingested diets differing only in 3 levels of sodium content. The investigators confirmed results from the earlier Mars-flight simulation study and found rhythmic sodium excretory and retention patterns that are independent of blood pressure or body water and occur independently of salt intake.19 The idea that sodium can accumulate in skin and skeletal muscle is now generally accepted,20,21 while how sodium influences blood pressure is not.21 Taken together, these hosts of findings suggested that the standard model requires revision. More importantly, what local molecular mechanisms could help us further?
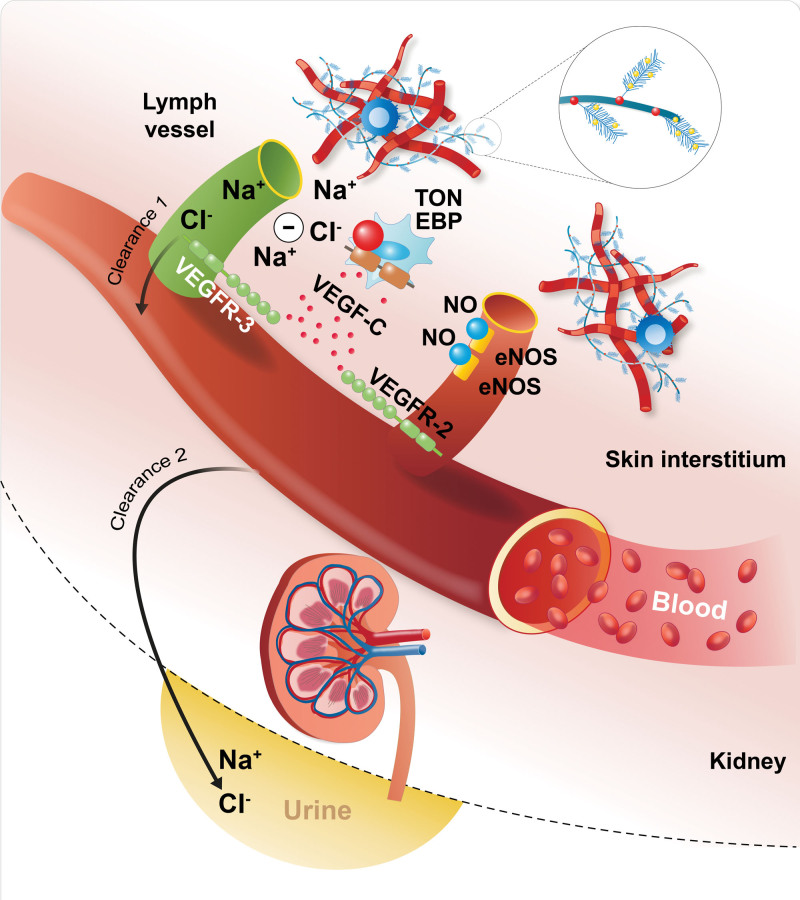
The answers lay in basic research. Parallel to the above reports, the Titze group focused on addressing the mechanisms involved. They were aware that TonEBP (tonicity-responsive enhancer-binding protein; NFAT5 [nuclear factor of activated T-cells 5])—a rel-like protein that activates transcription in response to hypertonicity—can detect even minimal changes in osmolarity.22 HSD led to interstitial hypertonic sodium accumulation in skin, resulting in increased density and hyperplasia of the lymph-capillary network. Mechanistically, the NFAT5–VEGF-C (vascular endothelial growth factor C) axis in mononuclear phagocytes infiltrating the interstitium of the skin was the underlying mechanism for these effects on skin lymphatics. Mononuclear phagocyte depletion or VEGF-C trapping by soluble VEGFR-3 (vascular endothelial growth factor receptor-3) blocked VEGF-C signaling, augmented interstitial hypertonic volume retention, decreased endothelial NO synthase expression, and elevated blood pressure in response to HSD.23 These preclinical findings were the first to connect immune cells with salt balance, which was also observed in humans in later follow-up studies.24,25 Obvious calls were issued to confirm these results that were based on the uncomfortable assumption that differences in osmolality can exist between cell surfaces in a nanometer range. Thus, the experiments were repeated. The subsequent results demonstrated that the skin harbors a hypertonic fluid compartment in which mononuclear phagocytes modify local cutaneous electrolyte clearance via NFAT5 and VEGF-C/VEGFR-3–mediated modulation of cutaneous lymphatic capillary function. Thereby, mononuclear phagocytes carry out a homeostatic and blood pressure–regulatory function (Figure 1).26 In this study, several confirmatory methods were used to investigate the osmolar differences at the cellular level.26,27 Subsequently, these ideas were translated to an infectious disease and inflammatory skin diseases, incorporating both magnetic resonance imaging for sodium and molecular data.13,28–30 Most recently, a link between inflammation, metabolic dysfunction in obesity, and tissue sodium levels was established.31 These ideas, particularly those concerning osmolar gradients in the nanometer range, understandably caused skepticism.32 However, as discussed earlier, the methods available at the present time did not give sufficient detailed spatial information to allow resolving these questions. Methodological differences could be responsible. For instance, the precision ashing of tissues and atomic absorption spectroscopy (rats) to measure cation concentrations was not performed in all studies. These methods require expensive and not distributable facilities. Recent data from the Pravenec laboratory, which measured such precautions, supported the notion that sodium accumulation and blood capillary rarefaction in the skin predisposes to hypertension in a rat model.27 Nonetheless, we argue that immune cells are involved in electrolyte metabolism, even at the most local of levels. We are convinced that this argument is compelling.
Figure 1.
A schematic diagram of how local (nanometer domain) sodium gradients influence osmotic sensors, effectors, and immune cells. eNOS indicates endothelial NO synthase; TonEBP, tonicity-responsive enhancer-binding protein; VEGF-C, vascular endothelial growth factor; VEGFR-2, vascular endothelial growth factor receptor-2; and VEGFR-3, vascular endothelial growth factor receptor-3.
SODIUM SHIFTS THE IMMUNE CELL BALANCE TOWARD INFLAMMATION
Immune cells patrol throughout our body, change location from gut or bone marrow to the circulation, spleen, lymph nodes, and to target organs to fulfill their diverse functions. On their way, they sense and integrate diverse environmental signals, translate them into intracellular information to permit adaption to a particular inflammatory environment for the maintenance of immune homeostasis. In the 1990s, investigators aimed to study the mechanism of saturated potassium iodide to treat infections. They used increases in NaCl as a control and found that increases by 40 mM, which is similar to increases found in skin stores upon dietary and inflammatory stimuli, could provoke proinflammatory responses in peripheral blood mononuclear cells.33 These findings were among the first studies to show that ionic signals are able to profoundly impact immune cell responses. Since then, the literature clearly demonstrates that high-salt conditions favor proinflammatory macrophage and T helper 17 (Th17) cell activation and curtail the regulatory and anti-inflammatory activation of these cells.34,35 In macrophages, the NCLX (Na+/Ca2+ exchanger) is critically involved in sensing ionic sodium balance changes36 and triggers enhanced activation of osmoprotective signaling pathways, such as NFAT5. Of utmost interest, these high salt responses contain profound metabolic adaption of macrophages, which includes triggering increased stabilization of hypoxia-inducible factor 1α and autophagy.37 This process helps to fight infections.27,36–40 Increased local sodium conditions, therefore, represent a local microenvironmental cue, which can strengthen barrier surfaces.27,40,41
In 2013, 2 landmark studies42,43 connected high salt intake as a potential environmental factor contributing to experimental autoimmunity. These investigations showed that high NaCl conditions could boost the differentiation of naive CD4+ T cells in humans and mice into pathogenic Th17 cells. IL (interleukin)-17A and IL-23R (IL-23 receptor) expression under Th17 differentiation conditions was enhanced. This regulatory mechanism involved p38/MAPK (mitogen-activated protein kinase), NFAT5, and SGK1.34 SGK1 inactivates FOXO1 (forkhead box O1), thereby promoting enhanced RORγt (retinoic acid receptor-related orphan receptor gamma t) and IL-23R expression.43 In vivo, an HSD increases the number of Th17 cells in gut-associated lymphoid tissue and the central nervous system, resulting in exacerbating experimental autoimmune encephalomyelitis (EAE), a mouse model mimicking many aspects of multiple sclerosis.42,43 CD4+ T cell–specific SGK1 (serum/glucocorticoid regulated kinase 1) deletion in mice leads to resistance to EAE, owing to a defect in maintaining the Th17 phenotype.43 Both in vitro and in vivo evidence in EAE suggests that Th17 activation by high NaCl is not mediated through dendritic cells but rather through a separate pathway or direct impact on Th17 cells.44 Besides the NFAT5 axis, high salt also affects SGK1 via the upstream long noncoding RNA (Lnc)-SGK1.45 Furthermore, aside from its pivotal role in T cells, SGK1 in dendritic cells is important for salt-sensitive hypertension, vascular function, and renal inflammation.46 Translating these experimental findings to humans, a 10-day HSD (15 g NaCl/day, about 300 mmol Na+/day) in healthy nonsmoking male volunteers resulted in increased Th17 population in the peripheral blood, which was reversed after switching the men to normal-salt diet.47 Further, also a rather moderate 14-day dietary high-salt challenge (6 g of NaCl/day in addition to accustomed diets) increased circulating Th17 frequencies.48 Of note, in humans, potassium also affects high salt–induced Th17 polarization by a thus far unknown mechanism.49 Circulating IL-17 levels were significantly increased after switching from 3 to 18 g of NaCl for 7 days, while the addition of 4.5 g KCl to the HSD completely reversed the IL-17 levels.49 A dichotomous role for NaCl on Th17-like cells has also been reported, where the impact of NaCl on CD4+ T cells may be context dependent and could differ depending on stimulation and cytokine environment.50
IL-17 plays a critical role in infections, autoimmune diseases, and inflammatory diseases. Of note, hypertensive humans show significantly increased circulating IL-17 levels.51 Physiologically, IL-17 is not only involved in host defense but also may promote inflammatory tissue damage. IL-17A knockout mice initially increase the blood pressure similarly to controls upon angiotensin II infusion, whereas over time, they develop a less hypertensive state.51 In addition, human prototype IL-17–related diseases such as psoriasis, periodontal disease, and rheumatoid arthritis are also associated with hypertension.52–54
High salt is further linked to an increased risk of cerebrovascular diseases and dementia. A recent study focusing on the gut-brain axis described how high salt initiates a Th17 response in the gut resulting in a marked increase in plasma IL-17, which subsequently suppresses resting cerebral blood flow and endothelial function, leading to neurovascular dysregulation and cognitive impairment.55 Endothelial dysfunction and cognitive impairment are mediated via inhibitory Rho kinase–dependent phosphorylation of endothelial NO synthase with reduced NO production of cerebral endothelial cells.55
Lupus-prone MRL/lpr mice under HSD showed a significant increase in the ratio of splenic Th1/Th2 and Th17/Regulatory T (Treg) cells, accompanied with reduced survival.56,57 In BALB/c mice, HSD induced tubular proteinuria and a profibrotic phenotype with increased renal cortical Th1 and Th17 and reduced Treg cells.58 HSD also primed follicular helper T-cell differentiation and promoted autoimmunity by hydroxytransferase TET2 (Ten-Eleven Translocation 2)-induced DNA demethylation. In vitro, TET2 silencing reduced NaCl-induced follicular helper T-cell polarization. In models of colitis, several reports have established the relationship between high sodium and Th17 cells. In human intestinal lamina propria mononuclear cells, IL-17A, IL-23R, TNF-α, and RORγt significantly increased after NaCl exposure.59 Mice on HSD developed severe 2,4,6-trinitrobenzenesulfonic acid (TNBS)- or dextran sulfate sodium (DSS)-induced colitis compared with control mice, which could be ameliorated by p38 MAPK inhibition.59 Besides Th17 cells, IL-17–producing type 3 innate lymphoid cells may also be affected by HSD in this context. When mice were fed an HSD, an intestinal inflammatory response associated with increased IL-23 production, neutrophil mobilization, and frequency of IL-17–producing type 3 innate lymphoid cells in the colon was observed. Since intestinal inflammation was not observed in IL-17 knockout mice but albeit to a lesser degree in recombination-activating genes (RAG)-deficient mice, which lack B and T cells but have ILC, IL-17 production by both type 3 innate lymphoid cells and Th17 cells likely contributes to the inflammatory response.60 Altogether, these data suggest that a high salt intake may prime various T-cell subsets, promote IL-17 production with consequences for the pathogenesis of cardiovascular diseases (CVDs) and autoimmune diseases. Whether or not reducing salt intake to currently recommended levels would reduce this state of affairs remains to be seen.
SODIUM AFFECTS THE MICROBIOME AND INTESTINAL INFLAMMATION
Recent research has further been focusing on indirect roles of NaCl regulating immunity through changing the gut microbiota.34 High salt intake impacts the gut microbiota in mice and humans, particularly by depleting Lactobacillus spp.48,61–63 Along with changes in the gut microbiota, HSD increases the frequency of Th17 cells in the intestines, spleen, and spinal cord along with the blood pressure and aggravated disease courses of EAE mice.48 Replenishment of live Lactobacillus murinus to HSD-fed mice ameliorated the HSD-mediated exacerbation of EAE and salt-sensitive hypertension by regulating Th17 cells, likely through increased levels of the bacterial tryptophan metabolite indole-3 lactic acid. Mechanistically, indole-3 lactic acid suppressed Th17 cell polarization, suggesting that tryptophan metabolites of bacterial origin may act as inhibitors of the high salt–induced Th17 increase of the host.48 Concordantly, a meta-analysis demonstrated that probiotics (mostly containing Lactobacillus spp.) may reduce blood pressure in hypertensive subjects.64 Kirabo et al65 also have shown that a high salt intake is associated with changes in the gut microbiome, as well as higher blood pressures in humans. Further, blood pressures in salt-treated mice were elevated when the mice received subpressor doses of Ang II (angiotensin II).65 HSD was associated with changes in an increase in the genera Firmicutes, Proteobacteria, and Prevotella bacteria and a decrease in the genera of lactic acid–producing bacteria.65 Adaptive fecal microbial transfer from mice fed an HSD predisposes germ-free recipients to inflammation and hypertension.65 Further evidence for microbiota-dependent effects of an HSD on inflammation is the observation that an HSD promotes experimental DSS- and dinitrobenzenesulfonic acid–induced colitis in mice by reducing the presence of intestinal Lactobacilli and butyrate production. This effect was not observed in germ-free mice.62
SODIUM BLUNTS ANTI-INFLAMMATORY REGULATORY T-CELL FUNCTION
Regulatory FOXP3+ (forkhead box P3) T cells (Tregs) play an essential role for the maintenance of peripheral tolerance and immune cell homeostasis.66,67 Depending on the microenvironment and tissue, Tregs show the ability to suppress innate and adaptive immune cells by the secretion of anti-inflammatory cytokines, such as IL-10, or by cell-cell contact-dependent mechanism involving costimulatory receptors like cytotoxic T-lymphocyte–associated protein 4.66,67 In humans, mutations in the FOXP3 gene, a master regulator of Tregs, lead to dysfunctional Tregs with fatal autoimmunity. Mutations have been linked to multiple sclerosis, type 1 diabetes, systemic lupus erythematosus, chronic infections, or inflammation like rheumatoid arthritis.66–69 Further, differential expression of FOXP3 splice variants related to Treg function was associated with unstable plaques in patients with atherosclerosis.70 Experimental and clinical studies confirmed that Tregs are important mediators to contain chronic inflammation in CVD such as atherosclerosis,71 hypertensive target organ damage,72 or wound healing after myocardial infarction.73 However, importantly, reduced Treg numbers and a dysfunctional Treg phenotype similar to autoimmunity have been reported in atherosclerosis, heart failure, and myocardial infarction and are associated with progression of disease.74–77 Furthermore, HSD also negatively affected the regulatory balance of T cells in transplantation and precipitates rejection.78 Of note, the deleterious effect of an HSD in the absence of SGK1 on CD4+ T cells in transplanted recipients was diminished.78
Mechanistically, dysfunctional Tregs found in several inflammatory diseases are characterized by a proinflammatory Th1-like phenotype with high expression levels of IFN-γ (interferon-γ) and lower levels of IL-10.66,79–81 Several reports suggested that SGK1 not only plays a pivotal role in high salt–induced Th17 cell polarization29,42,43 but also in Tregs.50,78,79,82–84 In vitro and in vivo, salt-SGK1 signaling axis enables Treg cells to acquire a Th17-like phenotype, thus establishing salt as a nonimmune factor that affects Treg functional adaptation.85 In vitro, high salt was sufficient to induce RORγt expression in both thymic Treg and inducible Tregs without IL-17A production.85 A moderate decrease in FOXP3 expression in thymic high salt–induced RORγt+ Treg cells was not associated with a loss of Treg identity and function most likely due to still sufficient FOXP3 levels. In contrast, IL-2384 or IL-1β86 can readily induce IL-17A+RORgt+FOXP3+ inducible Treg cells into inflammatory Th17-like Treg cells. These data suggest that salt-induced Treg dysfunction could accelerate not only the pathogenesis of autoimmune disease but also the progression of CVD.
SODIUM INHIBITS MITOCHONDRIAL FUNCTION
As mentioned above, sodium is the most abundant cation in the extracellular space, kept in low concentrations intracellularly. This asymmetrical distribution builds up a high electrochemical gradient across the plasma membrane and allows for a fast and substantial sodium influx. The latter is mediated by different channels and transporters, such as the NHE (sodium-proton exchanger), the NKCC (sodium-potassium-chloride cotransporter), the NCLX, the TRPM4 (transient receptor potential melastatin 4), and the amiloride-sensitive sodium channel.87 Sodium influx and efflux not only affect cell membrane potential, excitation, and electrochemical conductivity but also intracellular pH and concentration of other ions and metabolites.
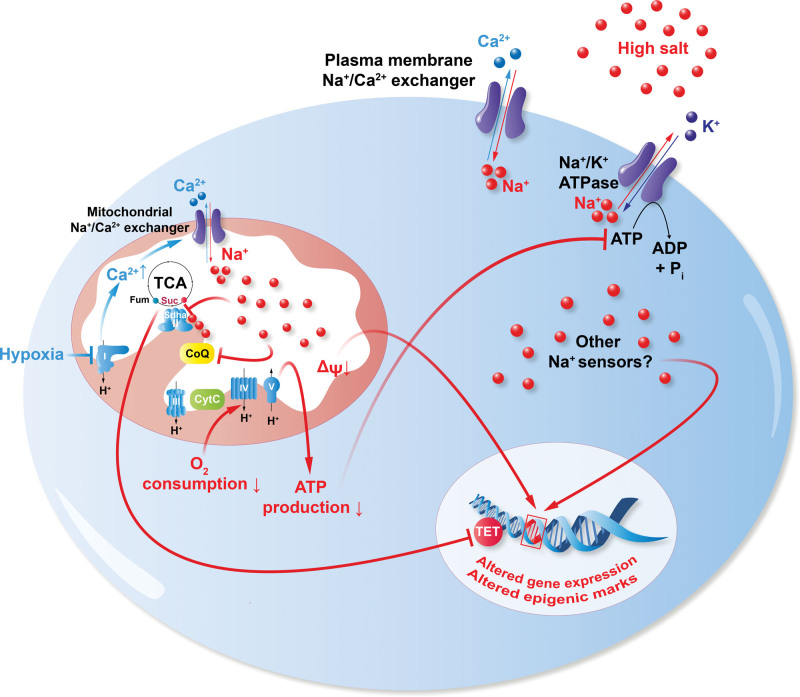
Interestingly, during ischemia, sodium not only accumulates in myocardial tissue but was also shown to invade the intracellular space and accumulate in subcellular compartments, more concretely, in the mitochondria. The contraction of healthy cardiac myocytes consumes large quantities of ATP, which is produced by mitochondrial oxidative phosphorylation.88 During cardiac ischemia, intracellular and mitochondrial Na+ levels increase along with a reduction in mitochondrial respiration with reduced ATP production (Figure 2), which might, in part, explain the imbalance of the energy demand in the failing heart.88 Also studies in patients with type 2 diabetes have shown that the myocardium under these conditions display reduced mitochondrial respiration and higher oxidative stress, further linking mitochondrial dysfunction with the pathophysiology of heart failure.89 Besides its effect on oxidative phosphorylation, the Na+ overload can cause an imbalance in Ca2+ homeostasis by reverting the calcium-sodium exchange process and thus accelerate heart failure.90,91 These studies highlight how HSD and imbalances in the ionic microenvironment can be detrimental to a plethora of different diseases and how cellular metabolic shifts are key in regulating cellular function and overall homeostasis.
Figure 2.
Sodium and mitochondrial metabolism. Pictogram of a cell under hypoxic or hypertonic saline conditions. Sodium enters the cell and mitochondria (at least, in part) via the NCLX (Na+/Ca2+ exchanger) and inhibits the electron transfer chain at the level of succinate dehydrogenase complex flavoprotein subunit A complex II (Sdha, II)/ubiquinone (CoQ). Subsequently, mitochondrial respiration, membrane potential (ΔΨ), and ATP production are reduced. In several immune cells, a proinflammatory gene expression is observed. CytC indicates cytochomre C; Fum, fumarate; Suc, succinate; TCA, tricarboxylic acid cycle; and TET, ten-eleven translocation.
It was shown that sodium could impact circulating and tissue-invading monocytes, M1/M2 macrophages, dendritic cells,87 and as outlined before in detail, Th17 cells and Tregs, as well as vascular endothelial cell stiffness.34 Interestingly, many of these phenotypes are mediated by NCLX, both at the plasma and the inner mitochondrial membrane. With these findings, there has been a strong focus on how sodium affects cellular bioenergetics.
Cellular metabolism and specifically its plasticity are undoubtedly of outmost importance for cell function and adaptation. Metabolic reprogramming is not just a hallmark of cancer but also emerged as critical regulator of immune cell activation.92,93 Sodium was shown to have several metabolic targets. In ischemic myocardial tissue, mononuclear phagocytes, and Tregs, sodium reduces mitochondrial oxidative phosphorylation. Sodium could enter the mitochondrial matrix via NCLX and interacts with phospholipids in the inner mitochondrial membrane, reducing the membrane fluidity and the diffusion of ubiquinone between complex II and complex III of the electron transport chain.94 In M1/M2 macrophages38 and Tregs,95 salt directly inhibits complex II/III of the electron transport chain. Treg cell–specific ablation of mitochondrial respiratory chain complex III in mice resulted in loss of function and subsequent development of fatal inflammatory disease early in life, without altering Treg cell proliferation and survival.96 Recent data demonstrated that high salt mirrored the metabolic and gene expression signatures and functional phenotype observed after complex III inhibition.95 The loss of mitochondrial function after short-term engagement in high-salt environments in vitro provoked a long-term loss of function of human and murine Tregs in vivo.95 Xenogeneic graft versus host disease in immunodeficient mice is a model for in vivo analysis of human Treg functionality.83,97,98 Adoptive transfer of high salt–treated or complex III inhibited human Tregs in xenogeneic graft versus host disease or murine high salt–treated Tregs to EAE similarly have long-term consequences in vivo.95 Interestingly, inhibition of CII/III is accompanied by an accumulation of succinate, which in turn inhibits TET2-mediated DNA demethylation (Figure 2). This accumulation could be a potential mechanism by which HSD induces altered epigenetic markers and thereby produces long-term effects despite a relatively short-term high-salt stimulation. Besides electron transport chain complex II/III, in cancer and macrophage cell lines, salt induces aerobic glycolysis via a pyruvate dehydrogenase kinase–mediated activation of pyruvate dehydrogenase, with reduced tricarboxylic acid cycling and oxidative phosphorylation. Interestingly, sodium was recently also identified as a regulator of the liquidity of intracellular condensates, affecting protein-protein interactions (at least partially by electrostatic shielding of the proteins) and thus protein aggregation under hypertonic stress.99 However, the exact extend of salt-induced metabolic remodeling in various different cell types remains ill defined. And there are so far only limited data available in respect to time resolution of salt-induced metabolic remodeling in different cell types under different metabolic states. The integration and investigation of in-parallel–occurring signaling events will be crucial to understand these processes in more detail.
OUTLOOK
Accumulating evidence suggests that the blood pressure–centric definition of salt sensitivity could be broadened to cellular and metabolic sodium sensitivity. While the actions of other cations such as Ca2+ as important intracellular messengers are widely recognized, evidence continues to accumulate highlighting various unexpected roles of sodium in the regulation of cellular function. Overall, it is becoming clearer that sodium modulates broader bodily functions besides fluid homeostasis and regulates various cell functions, particularly in cells of the innate and adaptive immune system. Given the relevance of immune function for CVD and cardiometabolic disease, it is tempting to speculate that these findings may have important implications and are not only relevant for autoimmunity. However, it remains elusive as to where, why, and how sodium is compartmentalized, both on a tissue/supracellular and organelle/subcellular level and whether all cells are similarly sodium responsive. More research is needed to understand how tissue or cellular salinization is linked to health or disease states, age, and gender, as well as nutrition, and how metabolic plasticity on both the cellular and body level is affected.
Recent investigations (and studies well back in the 20th century) showed that the substitution of Na+ with K+ (in terms of intake) showed significantly lower rates of stroke, major adverse cardiovascular events, and death from any cause.100 The driving force for this potassium-protection idea were the seminal studies and observations from the Tobian group.101 With potassium augmentation, better systolic blood pressure lowering was observed,102 compared with those subjects who received a regular salt intake. Whether or not salt (NaCl) reduction or salt substitution by KCl can affect the tissue/supracellular and organelle/subcellular micromilieu, signaling, and immunometabolism should be addressed in future studies. By understanding the dependencies for these microenvironmental changes and the corresponding environmental sensing mechanisms, we envision targeted approaches to fine-tune immune cell, myocardial, fibroblast, and endothelial cell function and thus better control inflammation and CVD.
ARTICLE INFORMATION
Sources of Funding
H. Miyauchi is supported by a Postdoctoral Fellowship from the Alexander von Humboldt Foundation and Takeda Science Foundation, S. Geisberger by the Bundesministerium für Bildung und Forschung funding Multimodal Clinical Mass Spectrometry to Target Treatment Resistance (MSTARS), and D.N. Müller and N. Wilck by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation; SFB 1470; Projektnummer 437531118 A06 and A10). D.N. Müller is supported by the Deutsches Zentrum für Herz-Kreislauf-Forschung (81Z0100110). N. Wilck and M. Kleinewietfeld are supported by the European Research Council under the European Union Horizon 2020 research and innovation program grant (852796, N. Wilck; 640116, M. Kleinewietfeld) and by the Corona Foundation in the German Stifter-Verband. J. Jantsch received funding from the DFG (JA1993/6-1) and DFG TRR 374 grant (project No. 509149993, TP B05). M. Kleinewietfeld was supported by a Strategisch Actieplan Limburg in het Kwadraat (SALK) grant from the government of Flanders and by an Odysseus grant of the Research Foundation Flanders, Belgium.
Disclosures
M. Kleinewietfeld is listed inventor on a pending patent related to mitochondrial metabolism and immunomodulation.
Nonstandard Abbreviations and Acronyms
- CVD
- cardiovascular disease
- EAE
- experimental autoimmune encephalomyelitis
- HSD
- high-salt diet
- IL
- interleukin
- NCLX
- Na+/Ca2+ exchanger
- Th17
- T helper 17
- VEGF-C
- vascular endothelial growth factor C
This manuscript is part of the Salt Review Series.
H. Miyauchi and S. Geisberger contributed equally.
For Sources of Funding and Disclosures, see page 433.
REFERENCES
- 1.Pitts RF. Physiology of the Kidney and Body Fluids. Year Book Medical Publishers Inc; 1974;3:11–34. [Google Scholar]
- 2.Andersson B. Regulation of body fluids. Annu Rev Physiol. 1977;39:185–200. doi: 10.1146/annurev.ph.39.030177.001153 [DOI] [PubMed] [Google Scholar]
- 3.Edelman IS, Leibman J, O’Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958;37:1236–1256. doi: 10.1172/JCI103712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkenfeld LW, Leibman J, O’Meara MP, Edelman IS. Total exchangeable sodium, total exchangeable potassium, and total body water in edematous patients with cirrhosis of the liver and congestive heart failure. J Clin Invest. 1958;37:687–698. doi: 10.1172/JCI103655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titze J, Maillet A, Lang R, Gunga HC, Johannes B, Gauquelin-Koch G, Kihm E, Larina I, Gharib C, Kirsch KA. Long-term sodium balance in humans in a terrestrial space station simulation study. Am J Kidney Dis. 2002;40:508–516. doi: 10.1053/ajkd.2002.34908 [DOI] [PubMed] [Google Scholar]
- 6.Titze J, Lang R, Ilies C, Schwind KH, Kirsch KA, Dietsch P, Luft FC, Hilgers KF. Osmotically inactive skin Na+ storage in rats. Am J Physiol Renal Physiol. 2003;285:F1108–F1117. doi: 10.1152/ajprenal.00200.2003 [DOI] [PubMed] [Google Scholar]
- 7.Titze J, Shakibaei M, Schafflhuber M, Schulze-Tanzil G, Porst M, Schwind KH, Dietsch P, Hilgers KF. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004;287:H203–H208. doi: 10.1152/ajpheart.01237.2003 [DOI] [PubMed] [Google Scholar]
- 8.Schafflhuber M, Volpi N, Dahlmann A, Hilgers KF, Maccari F, Dietsch P, Wagner H, Luft FC, Eckardt KU, Titze J. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am J Physiol Renal Physiol. 2007;292:F1490–F1500. doi: 10.1152/ajprenal.00300.2006 [DOI] [PubMed] [Google Scholar]
- 9.Thowsen IM, Reikvam T, Skogstrand T, Samuelsson AM, Muller DN, Tenstad O, Alitalo K, Karlsen T, Wiig H. Genetic engineering of lymphangiogenesis in skin does not affect blood pressure in mouse models of salt-sensitive hypertension. Hypertension. 2022;79:2451–2462. doi: 10.1161/HYPERTENSIONAHA.122.19777 [DOI] [PubMed] [Google Scholar]
- 10.Thowsen IM, Karlsen TV, Nikpey E, Haslene-Hox H, Skogstrand T, Randolph GJ, Zinselmeyer BH, Tenstad O, Wiig H. Na(+) is shifted from the extracellular to the intracellular compartment and is not inactivated by glycosaminoglycans during high salt conditions in rats. J Physiol. 2022;600:2293–2309. doi: 10.1113/JP282715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp C, Linz P, Wachsmuth L, Dahlmann A, Horbach T, Schofl C, Renz W, Santoro D, Niendorf T, Muller DN, et al. (23)Na magnetic resonance imaging of tissue sodium. Hypertension. 2012;59:167–172. doi: 10.1161/HYPERTENSIONAHA.111.183517 [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Deger MS, Kang H, Ikizler TA, Titze J, Gore JC. Sex differences in sodium deposition in human muscle and skin. Magn Reson Imaging. 2017;36:93–97. doi: 10.1016/j.mri.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maifeld A, Wild J, Karlsen TV, Rakova N, Wistorf E, Linz P, Jung R, Birukov A, Gimenez-Rivera VA, Wilck N, et al. Skin sodium accumulates in psoriasis and reflects disease severity. J Invest Dermatol. 2022;142:166–178.e8. doi: 10.1016/j.jid.2021.06.013 [DOI] [PubMed] [Google Scholar]
- 14.Karg MV, Bosch A, Kannenkeril D, Striepe K, Ott C, Schneider MP, Boemke-Zelch F, Linz P, Nagel AM, Titze J, et al. SGLT-2-inhibition with dapagliflozin reduces tissue sodium content: a randomised controlled trial. Cardiovasc Diabetol. 2018;17:5. doi: 10.1186/s12933-017-0654-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammon M, Grossmann S, Linz P, Kopp C, Dahlmann A, Garlichs C, Janka R, Cavallaro A, Luft FC, Uder M, et al. 23Na magnetic resonance imaging of the lower leg of acute heart failure patients during diuretic treatment. PLoS One. 2015;10:e0141336. doi: 10.1371/journal.pone.0141336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlmann A, Dorfelt K, Eicher F, Linz P, Kopp C, Mossinger I, Horn S, Buschges-Seraphin B, Wabel P, Hammon M, et al. Magnetic resonance-determined sodium removal from tissue stores in hemodialysis patients. Kidney Int. 2015;87:434–441. doi: 10.1038/ki.2014.269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dahlmann A, Linz P, Zucker I, Haag V, Jantsch J, Dienemann T, Nagel AM, Neubert P, Rosenhauer D, Rauh M, et al. Reduction of tissue Na(+) accumulation after renal transplantation. Kidney Int Rep. 2021;6:2338–2347. doi: 10.1016/j.ekir.2021.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvarajah V, Maki-Petaja KM, Pedro L, Bruggraber SFA, Burling K, Goodhart AK, Brown MJ, McEniery CM, Wilkinson IB. Novel mechanism for buffering dietary salt in humans: effects of salt loading on skin sodium, vascular endothelial growth factor C, and blood pressure. Hypertension. 2017;70:930–937. doi: 10.1161/HYPERTENSIONAHA.117.10003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rakova N, Juttner K, Dahlmann A, Schroder A, Linz P, Kopp C, Rauh M, Goller U, Beck L, Agureev A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–131. doi: 10.1016/j.cmet.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 20.Bie P. Mechanisms of sodium balance: total body sodium, surrogate variables, and renal sodium excretion. Am J Physiol Regul Integr Comp Physiol. 2018;315:R945–R962. doi: 10.1152/ajpregu.00363.2017 [DOI] [PubMed] [Google Scholar]
- 21.Ellison DH, Welling P. Insights into salt handling and blood pressure. Reply. N Engl J Med. 2022;386:e18. doi: 10.1056/NEJMc2119480 [DOI] [PubMed] [Google Scholar]
- 22.Miyakawa H, Woo SK, Dahl SC, Handler JS, Kwon HM. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc Natl Acad Sci USA. 1999;96:2538–2542. doi: 10.1073/pnas.96.5.2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Muller DN, Derer W, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–552. doi: 10.1038/nm.1960 [DOI] [PubMed] [Google Scholar]
- 24.Zhang MZ, Yao B, Wang Y, Yang S, Wang S, Fan X, Harris RC. Inhibition of cyclooxygenase-2 in hematopoietic cells results in salt-sensitive hypertension. J Clin Invest. 2015;125:4281–4294. doi: 10.1172/JCI81550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenstedt EF, Verberk SG, Kroon J, Neele AE, Baardman J, Claessen N, Pasaoglu OT, Rademaker E, Schrooten EM, Wouda RD, et al. Salt increases monocyte CCR2 expression and inflammatory responses in humans. JCI Insight. 2019;4:e130508. doi: 10.1172/jci.insight.130508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiig H, Schroder A, Neuhofer W, Jantsch J, Kopp C, Karlsen TV, Boschmann M, Goss J, Bry M, Rakova N, et al. Immune cells control skin lymphatic electrolyte homeostasis and blood pressure. J Clin Invest. 2013;123:2803–2815. doi: 10.1172/JCI60113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silhavy J, Mlejnek P, Simakova M, Liska F, Kubovciak J, Sticova E, Pravenec M. Sodium accumulation and blood capillary rarefaction in the skin predispose spontaneously hypertensive rats to salt sensitive hypertension. Biomedicines. 2022;10:376. doi: 10.3390/biomedicines10020376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jantsch J, Schatz V, Friedrich D, Schroder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, et al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab. 2015;21:493–501. doi: 10.1016/j.cmet.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kopp C, Beyer C, Linz P, Dahlmann A, Hammon M, Jantsch J, Neubert P, Rosenhauer D, Muller DN, Cavallaro A, et al. Na+ deposition in the fibrotic skin of systemic sclerosis patients detected by 23Na-magnetic resonance imaging. Rheumatology (Oxford). 2017;56:556–560. doi: 10.1093/rheumatology/kew371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthias J, Maul J, Noster R, Meinl H, Chao YY, Gerstenberg H, Jeschke F, Gasparoni G, Welle A, Walter J, et al. Sodium chloride is an ionic checkpoint for human T(H)2 cells and shapes the atopic skin microenvironment. Sci Transl Med. 2019;11:eaau0683. doi: 10.1126/scitranslmed.aau0683 [DOI] [PubMed] [Google Scholar]
- 31.Ertuglu LA, Sahinoz M, Alsouqi A, Deger SM, Guide A, Stewart TG, Pike M, Robinson-Cohen C, Akwo E, Pridmore M, et al. High tissue-sodium associates with systemic inflammation and insulin resistance in obese individuals. Nutr Metab Cardiovasc Dis. 2023;33:1398–1406. doi: 10.1016/j.numecd.2023.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossitto G, Mary S, Chen JY, Boder P, Chew KS, Neves KB, Alves RL, Montezano AC, Welsh P, Petrie MC, et al. Tissue sodium excess is not hypertonic and reflects extracellular volume expansion. Nat Commun. 2020;11:4222. doi: 10.1038/s41467-020-17820-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro L, Dinarello CA. Osmotic regulation of cytokine synthesis in vitro. Proc Natl Acad Sci U S A. 1995;92:12230–12234. doi: 10.1073/pnas.92.26.12230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller DN, Wilck N, Haase S, Kleinewietfeld M, Linker RA. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol. 2019;19:243–254. doi: 10.1038/s41577-018-0113-4 [DOI] [PubMed] [Google Scholar]
- 35.Jobin K, Muller DN, Jantsch J, Kurts C. Sodium and its manifold impact on our immune system. Trends Immunol. 2021;42:469–479. doi: 10.1016/j.it.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 36.Neubert P, Homann A, Wendelborn D, Bar AL, Krampert L, Trum M, Schroder A, Ebner S, Weichselbaum A, Schatz V, et al. NCX1 represents an ionic Na+ sensing mechanism in macrophages. PLoS Biol. 2020;18:e3000722. doi: 10.1371/journal.pbio.3000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neubert P, Weichselbaum A, Reitinger C, Schatz V, Schroder A, Ferdinand JR, Simon M, Bar AL, Brochhausen C, Gerlach RG, et al. HIF1A and NFAT5 coordinate Na(+)-boosted antibacterial defense via enhanced autophagy and autolysosomal targeting. Autophagy. 2019;15:1899–1916. doi: 10.1080/15548627.2019.1596483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geisberger S, Bartolomaeus H, Neubert P, Willebrand R, Zasada C, Bartolomaeus T, McParland V, Swinnen D, Geuzens A, Maifeld A, et al. Salt transiently inhibits mitochondrial energetics in mononuclear phagocytes. Circulation. 2021;144:144–158. doi: 10.1161/CIRCULATIONAHA.120.052788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, et al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest. 2015;125:4223–4238. doi: 10.1172/JCI80919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry MR, Mathews RJ, Ferdinand JR, Jing C, Loudon KW, Wlodek E, Dennison TW, Kuper C, Neuhofer W, Clatworthy MR. Renal sodium gradient orchestrates a dynamic antibacterial defense zone. Cell. 2017;170:860–874.e19. doi: 10.1016/j.cell.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 41.Evans RDR, Antonelou M, Sathiananthamoorthy S, Rega M, Henderson S, Ceron-Gutierrez L, Barcenas-Morales G, Muller CA, Doffinger R, Walsh SB, et al. Inherited salt-losing tubulopathies are associated with immunodeficiency due to impaired IL-17 responses. Nat Commun. 2020;11:4368. doi: 10.1038/s41467-020-18184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–517. doi: 10.1038/nature11984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jorg S, Kissel J, Manzel A, Kleinewietfeld M, Haghikia A, Gold R, Muller DN, Linker RA. High salt drives Th17 responses in experimental autoimmune encephalomyelitis without impacting myeloid dendritic cells. Exp Neurol. 2016;279:212–222. doi: 10.1016/j.expneurol.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 45.Yao Y, Jiang Q, Jiang L, Wu J, Zhang Q, Wang J, Feng H, Zang P. Lnc-SGK1 induced by Helicobacter pylori infection and highsalt diet promote Th2 and Th17 differentiation in human gastric cancer by SGK1/Jun B signaling. Oncotarget. 2016;7:20549–20560. doi: 10.18632/oncotarget.7823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Beusecum JP, Barbaro NR, McDowell Z, Aden LA, Xiao L, Pandey AK, Itani HA, Himmel LE, Harrison DG, Kirabo A. High salt activates CD11c(+) antigen-presenting cells via SGK (serum glucocorticoid kinase) 1 to promote renal inflammation and salt-sensitive hypertension. Hypertension. 2019;74:555–563. doi: 10.1161/HYPERTENSIONAHA.119.12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo T, Ji WJ, Yuan F, Guo ZZ, Li YX, Dong Y, Ma YQ, Zhou X, Li YM. Th17/Treg imbalance induced by dietary salt variation indicates inflammation of target organs in humans. Sci Rep. 2016;6:26767. doi: 10.1038/srep26767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mahler A, Balogh A, Marko L, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wen W, Wan Z, Ren K, Zhou D, Gao Q, Wu Y, Wang L, Yuan Z, Zhou J. Potassium supplementation inhibits IL-17A production induced by salt loading in human T lymphocytes via p38/MAPK-SGK1 pathway. Exp Mol Pathol. 2016;100:370–377. doi: 10.1016/j.yexmp.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 50.Matthias J, Heink S, Picard F, Zeitrag J, Kolz A, Chao YY, Soll D, de Almeida GP, Glasmacher E, Jacobsen ID, et al. Salt generates antiinflammatory Th17 cells but amplifies pathogenicity in proinflammatory cytokine microenvironments. J Clin Invest. 2020;130:4587–4600. doi: 10.1172/JCI137786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, Tselios AL, Metsios GS, Elisaf MS, Kitas GD. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford). 2007;46:1477–1482. doi: 10.1093/rheumatology/kem169 [DOI] [PubMed] [Google Scholar]
- 53.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–835. doi: 10.1016/j.jaad.2006.08.040 [DOI] [PubMed] [Google Scholar]
- 54.Czesnikiewicz-Guzik M, Osmenda G, Siedlinski M, Nosalski R, Pelka P, Nowakowski D, Wilk G, Mikolajczyk TP, Schramm-Luc A, Furtak A, et al. Causal association between periodontitis and hypertension: evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur Heart J. 2019;40:3459–3470. doi: 10.1093/eurheartj/ehz646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faraco G, Brea D, Garcia-Bonilla L, Wang G, Racchumi G, Chang H, Buendia I, Santisteban MM, Segarra SG, Koizumi K, et al. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat Neurosci. 2018;21:240–249. doi: 10.1038/s41593-017-0059-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang X, Yao G, Chen W, Tang X, Feng X, Sun L. Exacerbation of lupus nephritis by high sodium chloride related to activation of SGK1 pathway. Int Immunopharmacol. 2015;29:568–573. doi: 10.1016/j.intimp.2015.09.027 [DOI] [PubMed] [Google Scholar]
- 57.Wu H, Huang X, Qiu H, Zhao M, Liao W, Yuan S, Xie Y, Dai Y, Chang C, Yoshimura A, et al. High salt promotes autoimmunity by TET2-induced DNA demethylation and driving the differentiation of Tfh cells. Sci Rep. 2016;6:28065. doi: 10.1038/srep28065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teixeira DE, Peruchetti DB, Souza MC, das Gracas Henriques MG, Pinheiro AAS, Caruso-Neves C. A high salt diet induces tubular damage associated with a pro-inflammatory and pro-fibrotic response in a hypertension-independent manner. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165907. doi: 10.1016/j.bbadis.2020.165907 [DOI] [PubMed] [Google Scholar]
- 59.Monteleone I, Marafini I, Dinallo V, Di Fusco D, Troncone E, Zorzi F, Laudisi F, Monteleone G. Sodium chloride-enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J Crohns Colitis. 2017;11:237–245. doi: 10.1093/ecco-jcc/jjw139 [DOI] [PubMed] [Google Scholar]
- 60.Aguiar SLF, Miranda MCG, Guimaraes MAF, Santiago HC, Queiroz CP, Cunha PDS, Cara DC, Foureaux G, Ferreira AJ, Cardoso VN, et al. High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front Immunol. 2017;8:1969. doi: 10.3389/fimmu.2017.01969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang Z, Zhao J, Tian C, Chen X, Li H, Wei X, Lin W, Zheng N, Jiang A, Feng R, et al. Targeting the gut microbiota to investigate the mechanism of lactulose in negating the effects of a high-salt diet on hypertension. Mol Nutr Food Res. 2019;63:e1800941. doi: 10.1002/mnfr.201800941 [DOI] [PubMed] [Google Scholar]
- 62.Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JL, Verdu EF, Collins SM, Bercik P. High salt diet exacerbates colitis in mice by decreasing Lactobacillus levels and butyrate production. Microbiome. 2018;6:57. doi: 10.1186/s40168-018-0433-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamad I, Cardilli A, Corte-Real BF, Dyczko A, Vangronsveld J, Kleinewietfeld M. High-salt diet induces depletion of lactic acid-producing bacteria in murine gut. Nutrients. 2022;14:1171. doi: 10.3390/nu14061171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469 [DOI] [PubMed] [Google Scholar]
- 65.Ferguson JF, Aden LA, Barbaro NR, Van Beusecum JP, Xiao L, Simmons AJ, Warden C, Pasic L, Himmel LE, Washington MK, et al. High dietary salt-induced dendritic cell activation underlies microbial dysbiosis-associated hypertension. JCI Insight. 2019;5:e126241. doi: 10.1172/jci.insight.126241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259:231–244. doi: 10.1111/imr.12169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785 [DOI] [PubMed] [Google Scholar]
- 68.Arroyo Hornero R, Hamad I, Côrte-Real B, Kleinewietfeld M. The impact of dietary components on regulatory T cells and disease. Front Immunol. 2020;11:253. doi: 10.3389/fimmu.2020.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleinewietfeld M, Hafler DA. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin Immunol. 2013;25:305–312. doi: 10.1016/j.smim.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Joly AL, Seitz C, Liu S, Kuznetsov NV, Gertow K, Westerberg LS, Paulsson-Berne G, Hansson GK, Andersson J. Alternative splicing of FOXP3 controls regulatory T cell effector functions and is associated with human atherosclerotic plaque stability. Circ Res. 2018;122:1385–1394. doi: 10.1161/CIRCRESAHA.117.312340 [DOI] [PubMed] [Google Scholar]
- 71.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343 [DOI] [PubMed] [Google Scholar]
- 72.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation. 2009;119:2904–2912. doi: 10.1161/CIRCULATIONAHA.108.832782 [DOI] [PubMed] [Google Scholar]
- 73.Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895 [DOI] [PubMed] [Google Scholar]
- 74.Meng X, Yang J, Dong M, Zhang K, Tu E, Gao Q, Chen W, Zhang C, Zhang Y. Regulatory T cells in cardiovascular diseases. Nat Rev Cardiol. 2016;13:167–179. doi: 10.1038/nrcardio.2015.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baardman J, Lutgens E. Regulatory T cell metabolism in atherosclerosis. Metabolites. 2020;10:279. doi: 10.3390/metabo10070279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saigusa R, Winkels H, Ley K. T cell subsets and functions in atherosclerosis. Nat Rev Cardiol. 2020;17:387–401. doi: 10.1038/s41569-020-0352-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang TT, Ding YJ, Liao YH, Yu X, Xiao H, Xie JJ, Yuan J, Zhou ZH, Liao MY, Yao R, et al. Defective circulating CD4CD25+Foxp3+CD127(low) regulatory T-cells in patients with chronic heart failure. Cell Physiol Biochem. 2010;25:451–458. doi: 10.1159/000303050 [DOI] [PubMed] [Google Scholar]
- 78.Safa K, Ohori S, Borges TJ, Uehara M, Batal I, Shimizu T, Magee CN, Belizaire R, Abdi R, Wu C, et al. Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory T cells. J Am Soc Nephrol. 2015;26:2341–2347. doi: 10.1681/ASN.2014090914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sumida T, Lincoln MR, Ukeje CM, Rodriguez DM, Akazawa H, Noda T, Naito AT, Komuro I, Dominguez-Villar M, Hafler DA. Activated β-catenin in Foxp3(+) regulatory T cells links inflammatory environments to autoimmunity. Nat Immunol. 2018;19:1391–1402. doi: 10.1038/s41590-018-0236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19:665–673. doi: 10.1038/s41590-018-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation. 2019;139:206–221. doi: 10.1161/CIRCULATIONAHA.118.036065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo Y, Xue Y, Wang J, Dang J, Fang Q, Huang G, Olsen N, Zheng SG. Negligible effect of sodium chloride on the development and function of TGF-beta-induced CD4(+) Foxp3(+) regulatory T cells. Cell Rep. 2019;26:1869–1879.e3. doi: 10.1016/j.celrep.2019.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu N, Deng S, Herold KC, Kuchroo VK, Kleinewietfeld M, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125:4212–4222. doi: 10.1172/JCI81151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu C, Chen Z, Xiao S, Thalhamer T, Madi A, Han T, Kuchroo V. SGK1 governs the reciprocal development of Th17 and regulatory T cells. Cell Rep. 2018;22:653–665. doi: 10.1016/j.celrep.2017.12.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang YH, Istomine R, Alvarez F, Al-Aubodah TA, Shi XQ, Takano T, Thornton AM, Shevach EM, Zhang J, Piccirillo CA. Salt sensing by serum/glucocorticoid-regulated kinase 1 promotes Th17-like inflammatory adaptation of Foxp3(+) regulatory T cells. Cell Rep. 2020;30:1515–1529.e4. doi: 10.1016/j.celrep.2020.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Alvarez F, Istomine R, Shourian M, Pavey N, Al-Aubodah TA, Qureshi S, Fritz JH, Piccirillo CA. The alarmins IL-1 and IL-33 differentially regulate the functional specialisation of Foxp3(+) regulatory T cells during mucosal inflammation. Mucosal Immunol. 2019;12:746–760. doi: 10.1038/s41385-019-0153-5 [DOI] [PubMed] [Google Scholar]
- 87.Barbaro NR, Foss JD, Kryshtal DO, Tsyba N, Kumaresan S, Xiao L, Mernaugh RL, Itani HA, Loperena R, Chen W, et al. Dendritic cell amiloride-sensitive channels mediate sodium-induced inflammation and hypertension. Cell Rep. 2017;21:1009–1020. doi: 10.1016/j.celrep.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tian R, Colucci WS, Arany Z, Bachschmid MM, Ballinger SW, Boudina S, Bruce JE, Busija DW, Dikalov S, Dorn GW, II, et al. Unlocking the secrets of mitochondria in the cardiovascular system: path to a cure in heart failure-a report from the 2018 National Heart, Lung, and Blood Institute Workshop. Circulation. 2019;140:1205–1216. doi: 10.1161/CIRCULATIONAHA.119.040551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554–564. doi: 10.1161/CIRCULATIONAHA.113.008476 [DOI] [PubMed] [Google Scholar]
- 90.Mattiello JA, Margulies KB, Jeevanandam V, Houser SR. Contribution of reverse-mode sodium-calcium exchange to contractions in failing human left ventricular myocytes. Cardiovasc Res. 1998;37:424–431. doi: 10.1016/s0008-6363(97)00271-x [DOI] [PubMed] [Google Scholar]
- 91.Lai L, Qiu H. The physiological and pathological roles of mitochondrial calcium uptake in heart. Int J Mol Sci. 2020;21:7689. doi: 10.3390/ijms21207689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Teijlingen Bakker N, Pearce EJ. Cell-intrinsic metabolic regulation of mononuclear phagocyte activation: findings from the tip of the iceberg. Immunol Rev. 2020;295:54–67. doi: 10.1111/imr.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geltink RIK, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–488. doi: 10.1146/annurev-immunol-042617-053019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hernansanz-Agustin P, Choya-Foces C, Carregal-Romero S, Ramos E, Oliva T, Villa-Pina T, Moreno L, Izquierdo-Alvarez A, Cabrera-Garcia JD, Cortes A, et al. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature. 2020;586:287–291. doi: 10.1038/s41586-020-2551-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corte-Real BF, Hamad I, Arroyo Hornero R, Geisberger S, Roels J, Van Zeebroeck L, Dyczko A, van Gisbergen MW, Kurniawan H, Wagner A, et al. Sodium perturbs mitochondrial respiration and induces dysfunctional Tregs. Cell Metab. 2023;35:299–315.e8. doi: 10.1016/j.cmet.2023.01.009 [DOI] [PubMed] [Google Scholar]
- 96.Weinberg SE, Singer BD, Steinert EM, Martinez CA, Mehta MM, Martinez-Reyes I, Gao P, Helmin KA, Abdala-Valencia H, Sena LA, et al. Mitochondrial complex III is essential for suppressive function of regulatory T cells. Nature. 2019;565:495–499. doi: 10.1038/s41586-018-0846-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hahn SA, Bellinghausen I, Trinschek B, Becker C. Translating Treg therapy in humanized mice. Front Immunol. 2015;6:623. doi: 10.3389/fimmu.2015.00623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kleinewietfeld M, Starke M, Di Mitri D, Borsellino G, Battistini L, Rotzschke O, Falk K. CD49d provides access to “untouched” human Foxp3+ Treg free of contaminating effector cells. Blood. 2009;113:827–836. doi: 10.1182/blood-2008-04-150524 [DOI] [PubMed] [Google Scholar]
- 99.Morishita K, Watanabe K, Naguro I, Ichijo H. Sodium ion influx regulates liquidity of biomolecular condensates in hyperosmotic stress response. Cell Rep. 2023;42:112315. doi: 10.1016/j.celrep.2023.112315 [DOI] [PubMed] [Google Scholar]
- 100.Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, Zhang J, Tian M, Huang L, Li Z, et al. Effect of salt substitution on cardiovascular events and death. N Engl J Med. 2021;385:1067–1077. doi: 10.1056/NEJMoa2105675 [DOI] [PubMed] [Google Scholar]
- 101.Tobian L. Protecting arteries against hypertensive injury. Clin Exp Hypertens A. 1992;14:35–43. doi: 10.3109/10641969209036169 [DOI] [PubMed] [Google Scholar]
- 102.Yuan Y, Jin A, Neal B, Feng X, Qiao Q, Wang H, Zhang R, Li J, Duan P, Cao L, et al. Salt substitution and salt-supply restriction for lowering blood pressure in elderly care facilities: a cluster-randomized trial. Nat Med. 2023;29:973–981. doi: 10.1038/s41591-023-02286-8 [DOI] [PubMed] [Google Scholar]