Abstract
Kaposi’s sarcoma (KS) represents a type of cancer that usually arises on the skin and very rarely in other organs. KS-associated herpesvirus (KSHV), also known as human herpesvirus-8 (HHV-8) commonly arises in patients with acquired immunodeficiency syndrome (AIDS). Laryngeal involvement of KS is very rare. Our study comprised of three cases with laryngeal KS. All cases were solved through surgical excision of the tumor. Histopathological and immunohistochemistry examinations revealed laryngeal KS. Laryngeal KS should be managed through surgical resection, followed by oncological treatment.
Keywords: Kaposi’s sarcoma , larynx , surgical management , histopathology , immunohistochemistry
Introduction
Kaposi’s sarcoma (KS) represents an uncommon type of cancer that can arise in the skin or in other organs (liver, spleen, lungs, intestine, mouth, lymph nodes, etc.), forming various tumor masses. It is an angioproliferative tumor, usually developing in the lower extremities, and rarely in other organs. First described in 1872 by Moritz Kaposi, as a rare, idiopathic, multi-pigmented skin sarcoma affecting especially the Mediterranean population [1, 2, 3], it occurs more frequently in people with low immunity. It was later suggested that KS might be of viral etiology. It is now known that the disease is caused by an infection with KS-associated herpesvirus (KSHV), also called human herpesvirus-8 (HHV-8). KS develops most frequently in people infected with the human immunodeficiency virus (HIV) [3, 4]; it is also a common type of cancer arising in patients with acquired immunodeficiency syndrome (AIDS) [4, 5, 6]. KSHV may also cause primary effusion lymphoma or inflammatory cytokine syndrome [6, 7].
KS has a male to female ratio of 17:1 and is usually encountered in patients over the age of 50 years old, mostly of Mediterranean or Eastern European origin [7, 8, 9].
Laryngeal involvement of KS is rare and even less common in patients that are not immunocompromised [10, 11]. Its manifestations within the larynx represent a diagnostic challenge, since KS tends to appear under the form of a highly vascularized lesion that grows sufficiently enough to determine airway obstruction. Symptoms usually include hoarseness as the presenting symptom, followed by dysphagia, stridor, or even complete airway obstruction, depending on the local extension [12].
Aim
The paper is aimed at presenting three cases of KS with laryngeal localization, all encountered in non-immunocompromised patients.
Case No. 1
Our first case is that of a 65-year-old female patient, sent to the Ear, Nose and Throat (ENT) Clinic of the Emergency County Clinical Hospital of Craiova, Romania, who was admitted for continuous hoarseness over the last three months, accompanied by progressive inspiratory dyspnea within the last week.
Patient had a medical history of HHV-8-positive KS situated on the thorax and members, diagnosed two years prior, for which she underwent concurrent chemotherapy and immunotherapy, with a favorable evolution.
The patient also had a history of cardiac diseases: grade I mitral regurgitation, systolic hypertension, Class I New York Heart Association (NYHA) chronic heart failure for which she was receiving ongoing treatment.
The patient was HIV-negative and had no history of immune suppression.
A physical examination revealed no remaining lesions from the previous KS, following her treatment.
Flexible white light endoscopy (WLE) revealed a large, lobulated, violaceous, highly vascularized tumor mass of approximately 2 cm in diameter, originating from the superior part of the left vocal fold, partially involving Morgagni’s ventricle, and occupying most of the glottic space, diminishing the flow of air through the glottic passage.
Narrow-band imaging (NBI) endoscopy was performed, using different optical filters, confirming the highly vascularized nature of the laryngeal tumor (Figure 1A, 1B).
Figure 1.
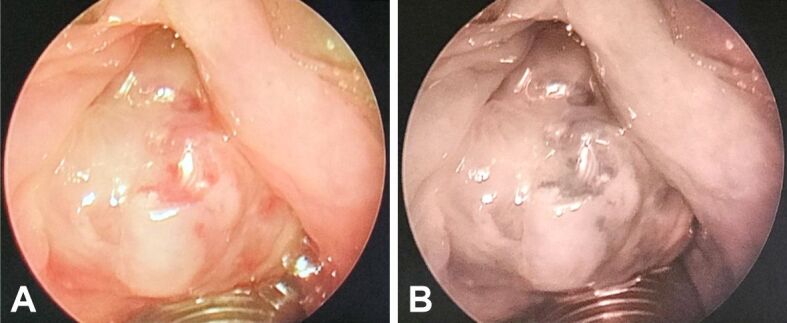
(A and B) NBI endoscopy revealing a laryngeal tumor inserted upon the left vocal fold. NBI: Narrow-band imaging.
It was decided patient undergo suspended laryngoscopy under general anesthesia with orotracheal intubation in order to perform an excisional biopsy of the left vocal fold tumor (Figure 2). There was no need to perform a tracheostomy. The surgery was successful, with minimal bleeding followed by local hemostasis.
Figure 2.
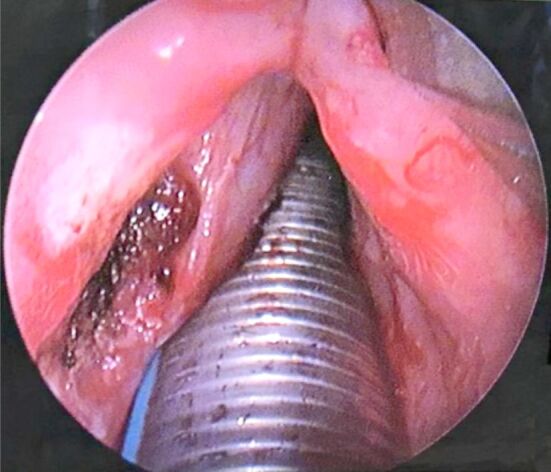
Intraoperative image taken after tumor resection and cauterization of insertion area
Case No. 2
Our second case is that of a 54-year-old patient that presented to the ENT Clinic of the Emergency County Clinical Hospital of Craiova for long term dysphonia and progressive inspiratory dyspnea over the past few weeks. The patient was admitted for further tests and treatment.
The patient was HIV-negative and had no history of immune suppression.
A flexible WLE was performed, revealing a large, lobulated, violaceous tumoral mass of 1 cm in diameter, situated within the anterior commissure (Figure 3). NBI confirmed its vascular nature.
Figure 3.
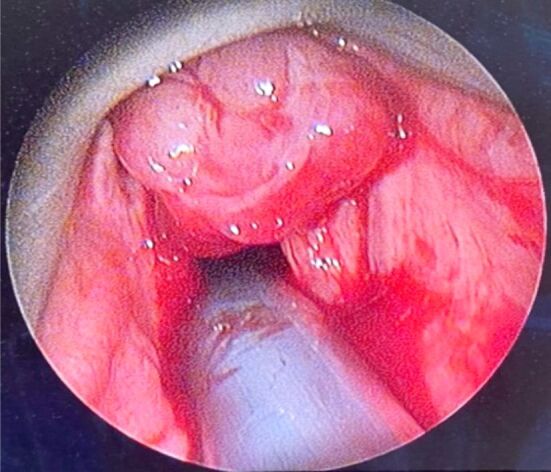
Tumoral mass in direct suspension laryngoscopy, situated within the anterior commissure
The patient underwent cold-steel excisional biopsy through direct laryngoscopy, under general anesthesia and orotracheal intubation, without the need for a tracheostomy. The vascular and friable nature of the tumor mass was observed during the excisional maneuvers. Local hemostasis was mandatory. Postoperative recovery was favorable.
Patient underwent oncological treatment consisting in chemotherapy, with favorable results.
Over the past two years regular follow-up consultations were performed, with no sign of relapse.
Case No. 3
Our third case is that of a 68-year-old patient that presented to the ENT Clinic of the Emergency County Clinical Hospital of Craiova, for long term dysphonia (hoarseness) and acute inspiratory dyspnea.
The patient was HIV-negative and had no history of immune suppression.
A flexible WLE was performed, revealing a massive, vegetating, pink-purple in color, multilobulated, fragile tumoral mass of approximately 4 cm in diameter, with an elongated pedicle inserted on the right vocal cord, manifesting a ball valve effect, with a severe reduction in airflow. The tumor would descend and block the subglottic space during inspiration and subsequently rise during expiration, hiding the vocal folds from view (Figure 4). NBI confirmed its vascular nature. No cervical lymphadenopathies were observed.
Figure 4.
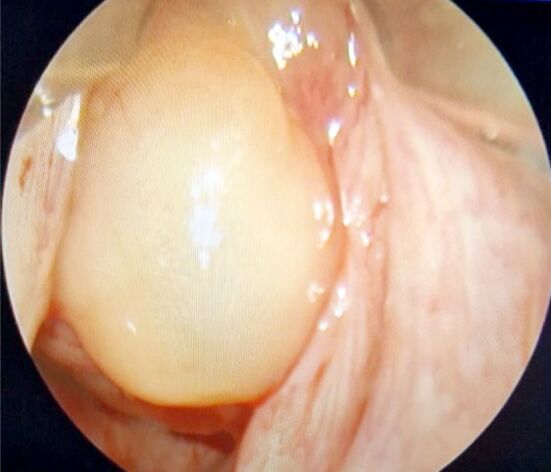
Large laryngeal tumoral mass large implanted on right vocal cord
Given the airway glottic insufficiency, we were required to perform an emergency tracheostomy in order to secure the patients airway. Tracheostomy was followed by cold-steel excisional biopsy through direct laryngoscopy with the removal of the large and friable tumor mass. Upon excision, the rest of the laryngeal mucosal integrity was ascertained. Hemostasis was performed. After surgery, integrity of the airway, local hemostasis, and resumption of vocal fold mobility were observed and upon attaining all, the tracheostomy was closed. Postoperative evolution was favorable.
The biopsy piece was represented entirely by a pseudo-nodular tumor proliferation.
Histopathological analysis
The excised tumors were sent to the anatomical pathology laboratory, where the histopathological (HP) examination revealed fragments of the laryngeal mucosa, covered with non-keratinizing stratified squamous epithelium, partially ulcerated, with hyperkeratosis. In the subepithelial connective tissue, a significant vascular proliferation was evident, showing thin-walled vessels delimited by tumor cells (Figure 5). Tumor cells were medium-sized fusiform, with eosinophilic cytoplasm without clear boundaries, with hypochromic oval nuclei, with loose chromatin and visible nucleoli. The fusiform cells were arranged in crossed bundles that formed frequent vascular slits, in which blood vessels (arterioles, venules, capillaries) and lymphatics were identified (Figure 6). Many of the tumor cells showed mitoses. In the tumor stroma, interstitial microhemorrhages, siderophages, areas with lymphocytic and plasma cells inflammatory infiltrates and sometimes interstitial edema were highlighted (Figure 7). The spaces created by the intersection of tumor cell bundles were of varied sizes and non-homogeneously distributed in the tumor (Figure 8).
Figure 5.
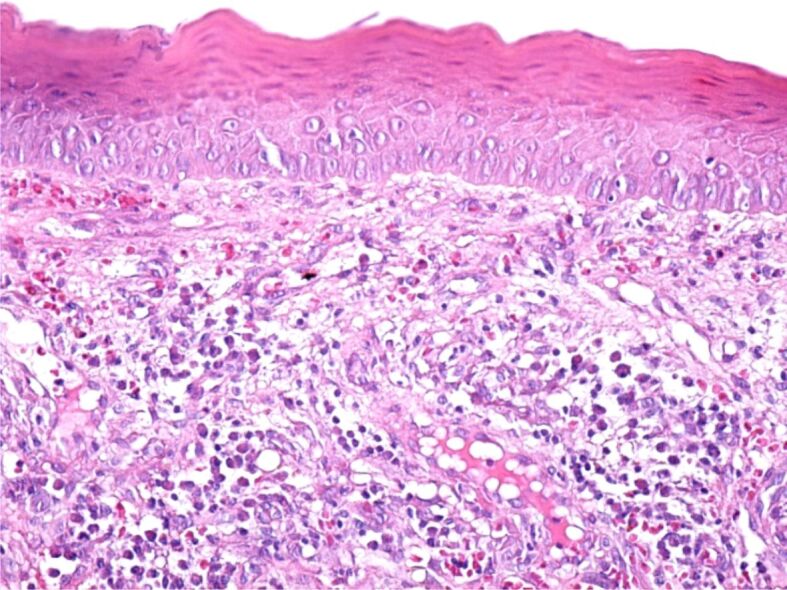
Tumor fragment covered by a stratified squamous epithelium, non-keratinized, with areas of hyperkeratosis. In the structure of the tumor, we can identify tumor cells with a fusiform aspect, numerous blood vessels, siderophages and a moderate inflammatory infiltrate consisting of lymphocytes, plasma cells and macrophages. Hematoxylin–Eosin (HE) staining, ×200
Figure 6.
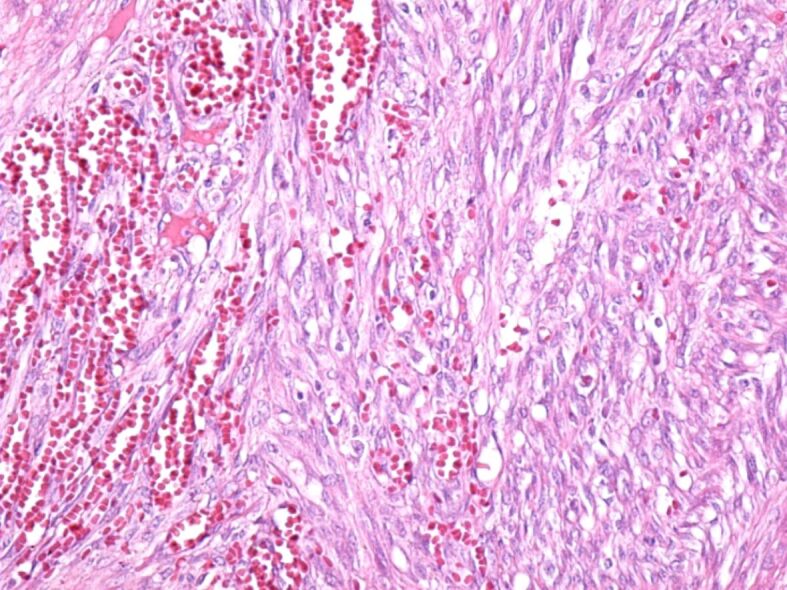
Overall appearance of the tumor in which the microscopic structure is highlighted, consisting of bundles of fusiform cells, which intersect and form numerous slits in which there are blood and lymphatic vessels. HE staining, ×200
Figure 7.
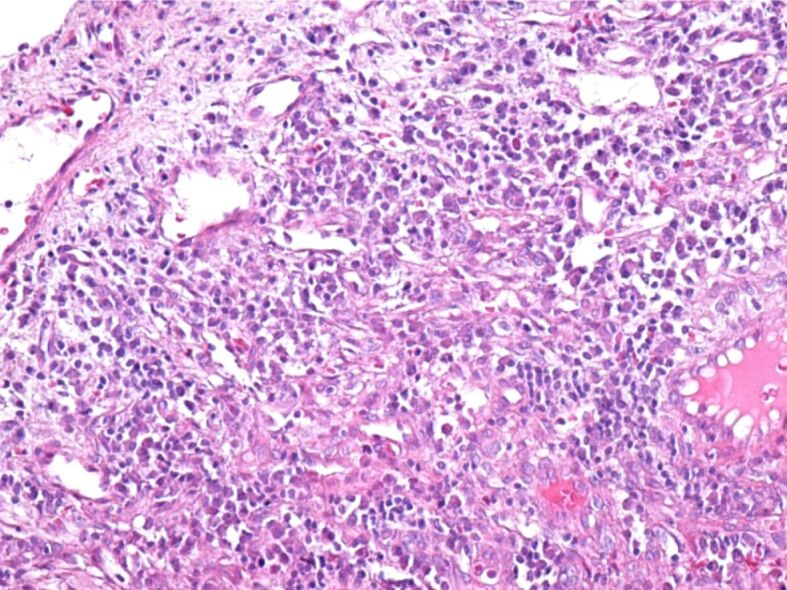
Area of Kaposi’s tumor containing a well-developed lymphoplasmacytic inflammatory infiltrate in the stroma. HE staining, ×200
Figure 8.
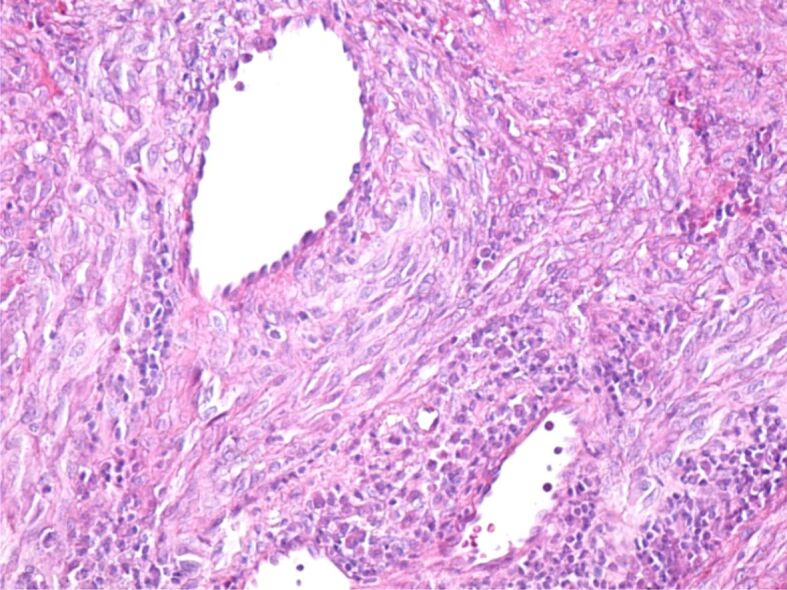
Blood vessels of various sizes, created by the intersection of bundles of tumor cells. HE staining, ×200
Immunohistochemical examination
For a more accurate and differential diagnosis, we used immunohistochemistry techniques.
The following antibodies were used: anti-cluster of differentiation (CD)31 (monoclonal mouse anti-human CD31, endothelial cell, clone JC70A, 1/50 dilution, Dako); anti-D2-40 (monoclonal mouse anti-human D2-40, clone D2-40, 1/50 dilution, Dako); anti-CD34 (monoclonal mouse anti-human CD34 Class II, clone QBEnd/10, 1/50 dilution, Dako); anti-cytokeratin (CK) AE1AE3 (monoclonal mouse anti-human CK, clone AE1/AE3, 1/100 dilution, Dako); anti-alpha-smooth muscle actin (α-SMA) (monoclonal mouse anti-human SMA, clone 1A4, 1/100 dilution, Dako); anti-p53 (monoclonal mouse anti-human p53 protein, clone DO-7, 1/100 dilution, Dako); anti-human melanoma black 45 (HMB45) (monoclonal mouse anti-human melanosome, clone HMB45, 1/50 dilution, Dako); anti-Ki67 (monoclonal mouse anti-human Ki67, clone MIB-1, 1/50 dilution, Dako).
Immunohistochemical (IHC) examinations showed that tumor cells were moderately positive for the anti-CD31 and anti-D2-40 antibodies, intensely positive for the anti-CD34 antibody (Figures 9, 10, 11), and partially positive for the anti-α-SMA antibody (Figure 12). Tumor cells were negative for anti-CK AE1/AE3, anti-p53 and anti-HMB45 antibodies (Figures 13, 14, 15). Proliferative capacity exploration by anti-Ki67 antibody showed that tumor cells were positive between 30% and 65% (Figure 16).
Figure 9.
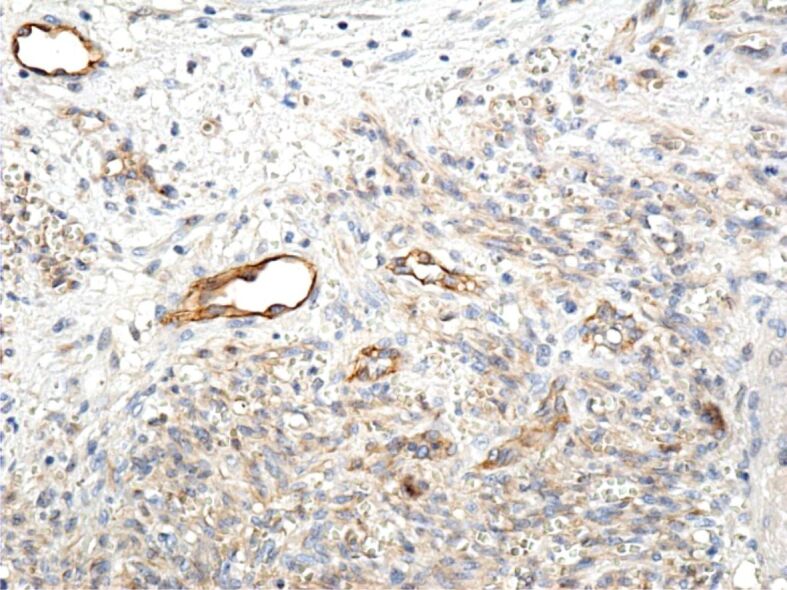
Moderate reaction of the tumor cells to the anti-CD31 antibody; intensely positive reaction of vascular endothelial cells to the same antibody. Immunomarking with anti-CD31 antibody, ×200. CD31: Cluster of differentiation 31.
Figure 10.
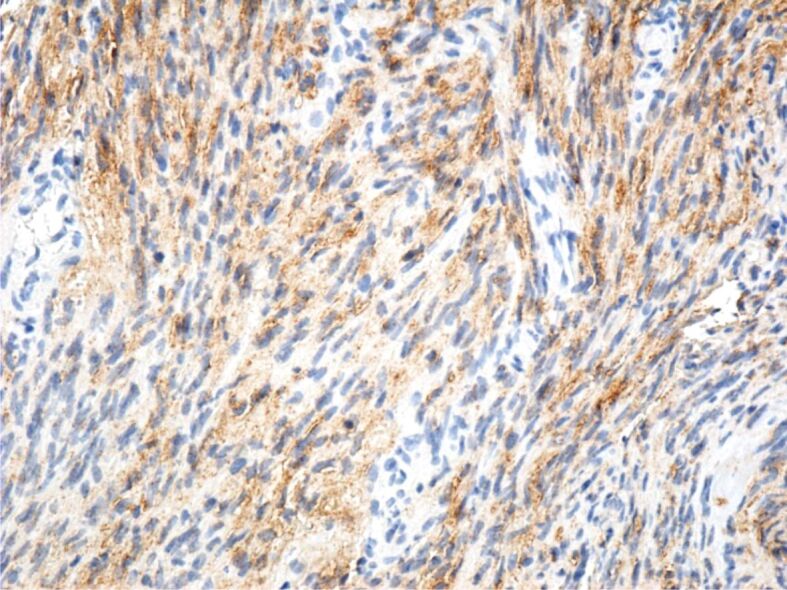
Tumor cells with a moderate immunohistochemical reaction to the D2-40 antibody. Immunomarking with anti-D2-40 antibody, ×200
Figure 11.
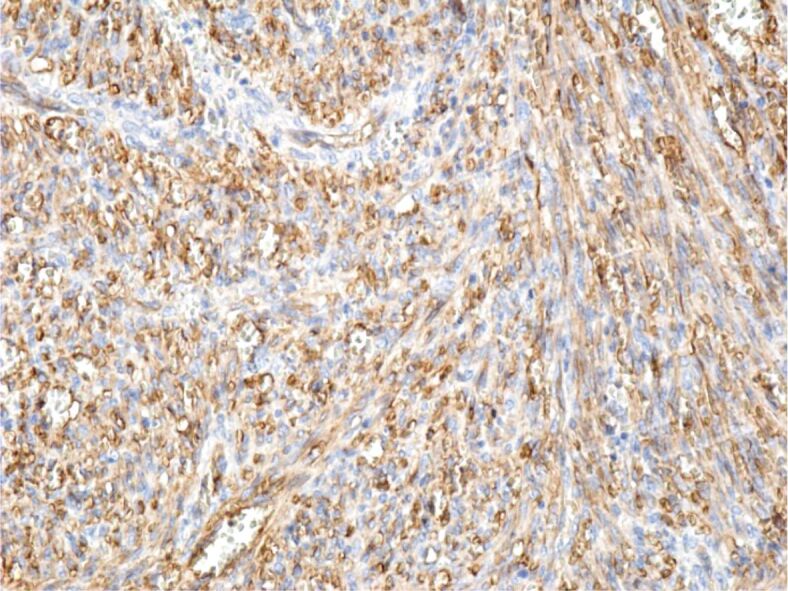
Tumor and vascular endothelial cells with intensely positive reaction to the anti-CD34 antibody. Immunomarking with anti-CD34 antibody, ×200. CD34: Cluster of differentiation 34
Figure 12.
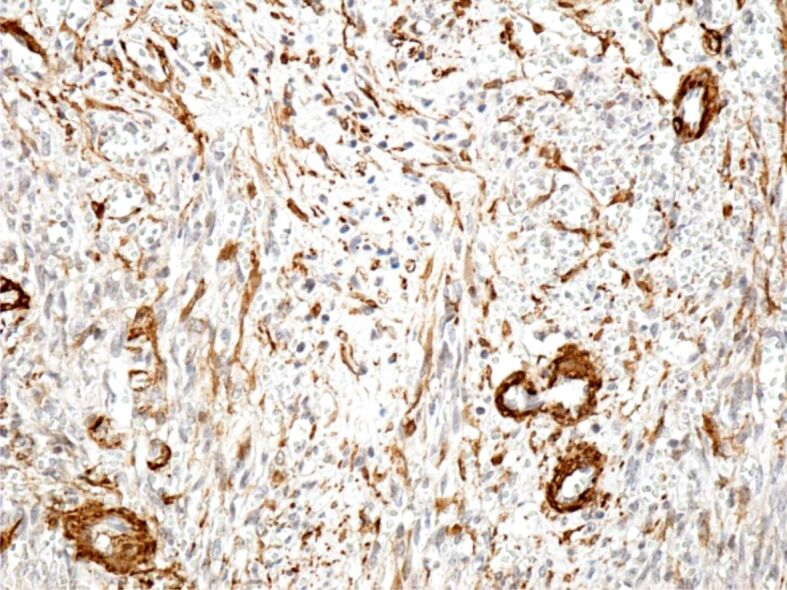
Intensely positive reaction of some tumor cells to the anti-α-SMA antibody. Immunomarking with anti-α-SMA antibody, ×200. α-SMA: Alpha-smooth muscle actin
Figure 13.
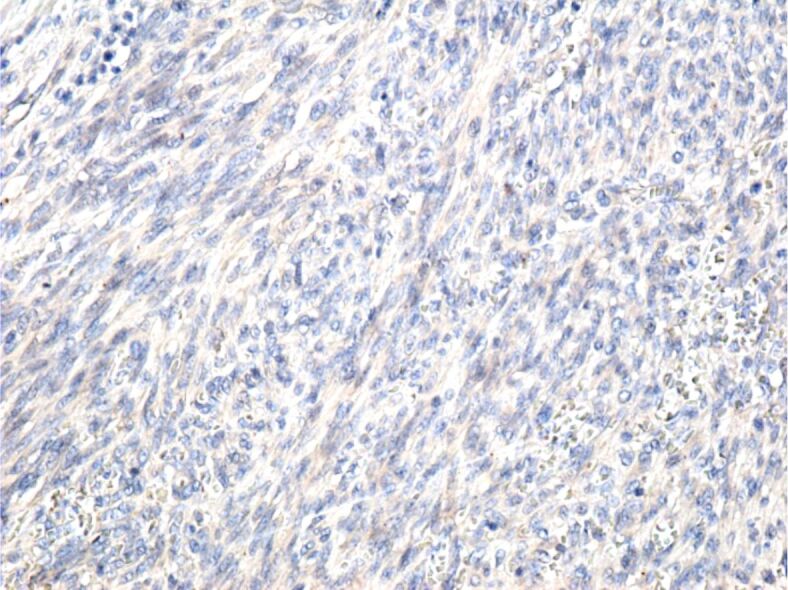
Negative reaction of tumor cells to the anti-CK AE1/AE3. Immunomarking with anti-CK AE1/AE3 antibody, ×200. CK: Cytokeratin
Figure 14.
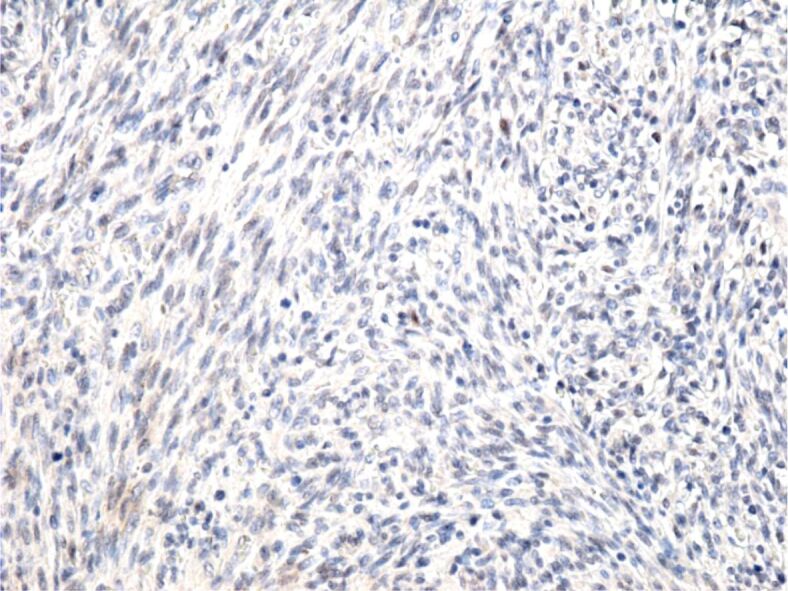
Tumor cells with a negative reaction to the anti-p53 antibody. Immunomarking with anti-p53 antibody, ×200.
Figure 15.
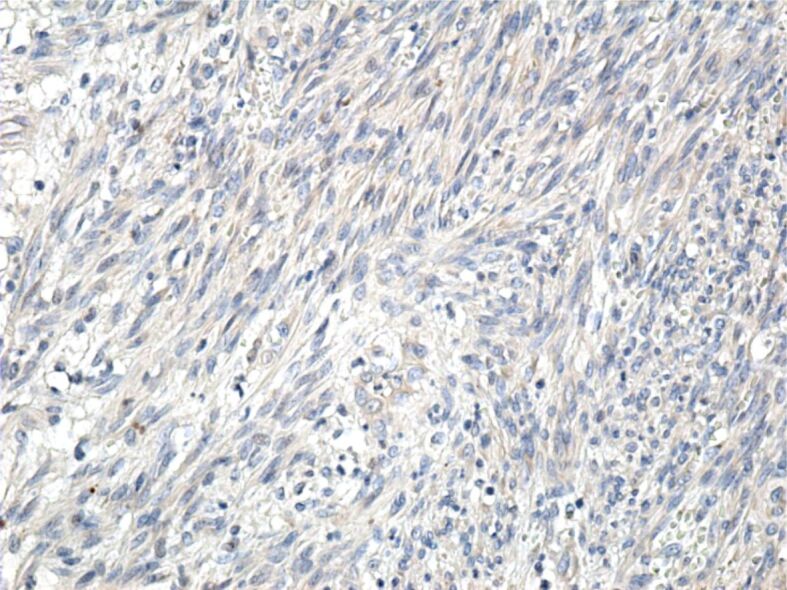
Tumor cells with a negative reaction to the anti-HMB45 antibody. Immunomarking with anti-HMB45 antibody, ×200. HMB45: Human melanoma black 45
Figure 16.
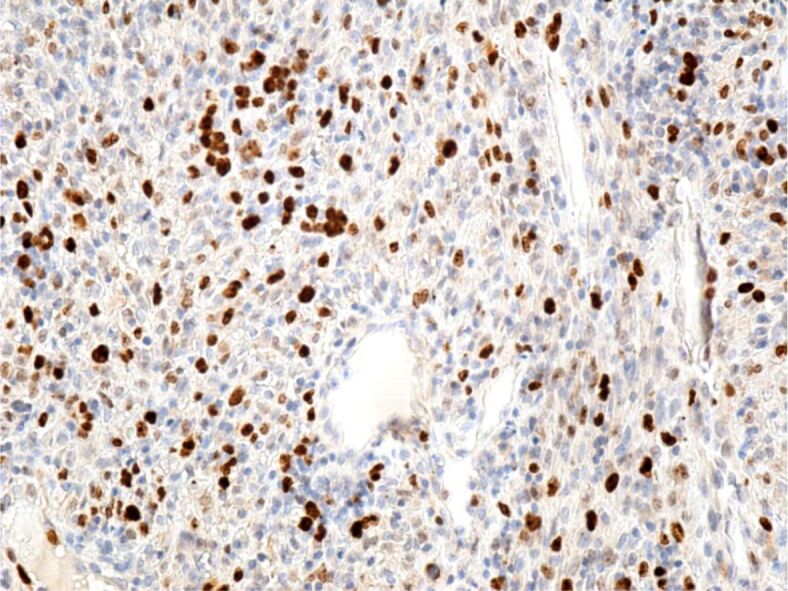
Intense reaction of tumor cells to the cell proliferation anti-Ki67 antibody. Immunomarking with anti-Ki67 antibody, ×200
HP and IHC examinations showed that the laryngeal tumor lesions were KSs.
Discussions
KS is regarded as a rare angioproliferative tumor that may be encountered more frequently in immunocompromised patients (AIDS, HIV). It is uncommonly encountered in non-immunocompromised patients, and there are few reported cases of laryngeal involvement [13]. A recent study reported 77 published cases of laryngeal KSs worldwide [14].
KS emerges from a primary infection of an endothelial cell or a progenitor cell by KSHV (HHV-8). However, most KS cases arise when infected patients additionally suffer from a concurrent form of altered immune function, ensuring suitable conditions for tumor growth [15].
Symptoms range from hoarseness in the early stages, to dysphagia, stridor in more locally advanced stages of the disease, all the way to complete airway obstruction [12]. Although all cases treated in our Clinic were admitted in rather advanced stages of the disease, with dysphonia and inspiratory dyspnea, only one tracheostomy was performed.
And while it is recommended patient undergo tracheostomy before biopsy, given the vascular nature of the tumor and the intraoperatory risk of bleeding [16, 17, 18], we opted for excisional biopsies, performing a tracheostomy in a single case of severe airway obstruction.
White light nasopharyngeal endoscopy is mandatory and should be followed, if possible, by a NBI endoscopy, given the highly vascular nature of the lesion [19].
Regarding treatment options, we opted for excisional biopsy all the way into healthy tissue (surgical safety margins), followed by cauterization of the tumor insertion point. Several other treatment options are available, such as combined antiretroviral therapy (cART), cryotherapy, low dose radiotherapy, chemotherapy, or immunotherapy. Most of these treatment options consist in shrinking the tumor as well as preventing its growth and dissemination. Out of the afore mentioned treatment options, cART is showing promising results by reducing disease incidence as well as improving survival rates of AIDS-related KSs. However, clinical monitoring is mandatory during cART given the risk of KS flare ensuing from immune reconstitution inflammatory syndrome (IRIS) [20, 21, 22, 23].
KSs with laryngeal localization is a rare tumor, slightly aggressive and has a low potential of malignancy. It is most often associated with similar skin lesions, or it develops as a result of HIV infection. Untreated, KS of the larynx can cause severe airway obstruction [23, 24].
There are studies suggesting that head and neck cancer patients present a higher risk of weight loss and malnutrition caused by difficulties swallowing secondary to mucosal injury, chemotherapy, or radiotherapy, leading to prolonged hospitalization and a reduced compliance with treatment. In such cases, enteral feeding through percutaneous endoscopic gastrostomy (PEG tubes) is recommended; however, in our cases, given the placement within the anterior commissure and the successful excision of the tumor, with no destruction of the surrounding mucosa or anatomical structures, there was no need for PEG tubes [25, 26].
The microscopic examination is of major importance for the diagnosis of tumor lesions, regardless of whether they are malignant or benign [27, 28, 29]. At the level of the larynx, various tumor formations can develop, primary or secondary, malignant, or benign. The most common tumors of the larynx are squamous carcinomas, which end up representing 90% of all tumors. Rarely, melanomas, leiomyosarcomas, chondrosarcomas, KSs or neuroendocrine tumors develop in this area [30, 31, 32].
The cases presented by us are cases of classical KS, composed of spindle-shaped tumor cells arranged in bundles that intersected and formed numerous slits occupied by blood or lymphatic vessels. Similar to other authors [10, 33, 34], we showed that the tumor cells were positive for anti-CD31, anti-CD34 and anti-D2-40 antibodies, which suggests their vascular origin. The absence of the IHC reaction to the anti-CK AE1/AE3 antibody excludes the epithelial nature of the tumor cells, and the absence of the anti-HMB45 antibody reaction excludes the presence of a melanoma.
Follow-up is mandatory since KS has a high recurrence rate, and as shown in one of our cases, it can be multifocal. This implies fiberoptic examination (WLE and NBI, if available) as well as general examination. While there is no consensus regarding follow-up frequency or duration, we reviewed the patients at one month, two months and three months, then every six months over the first two years, similar to other cases found in the literature [18].
Conclusions
KS is regarded as a rare angioproliferative entity. KS is commonly encountered in immunocompromised patients (AIDS, HIV) and less encountered in non-immunocompromised patients. Laryngeal proliferation of KS is exceptionally rare. KS should be taken into consideration as a differential diagnosis in cases presenting with violaceous lesions of the larynx, particularly in patients with a medical history of immunosuppressive diseases (AIDS) or of previous KS. When encountered, the current consensus is that the tumors should be excised, not biopsied, as they are highly vascularized. Follow-up upon surgical resection and oncological treatment is mandatory.
Conflict of interests
The authors declare no conflict of interests.
Author contribution
Mircea Sorin Ciolofan and Florin Anghelina equally contributed to this article.
References
- 1.Kaposi undefined. Idiopathisches multiples Pigmentsarkom der Haut. Arch Dermatol Syphilis. 1872;4:265–273. [Google Scholar]
- 2.Sternbach G, Varon J. Moritz Kaposi: idiopathic pigmented sarcoma of the skin. J Emerg Med. 1995;13(5):671–674. doi: 10.1016/0736-4679(95)00077-n. [DOI] [PubMed] [Google Scholar]
- 3.Stănescu L, Foarfă C, Georgescu AC, Georgescu I. Kaposi’s sarcoma associated with AIDS. Rom J Morphol Embryol. 2007;48(2):181–187. [PubMed] [Google Scholar]
- 4.Flore O, Rafii S, Ely S, O’Leary JJ, Hyjek EM, Cesarman E. Transformation of primary human endothelial cells by Kaposi’s sarcoma-associated herpesvirus. Nature. 1998;394(6693):588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 5.Kemény L, Gyulai R, Kiss M, Nagy F, Dobozy A. Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8: a new virus in human pathology. J Am Acad Dermatol. 1997;37(1):107–113. doi: 10.1016/s0190-9622(97)70220-2. [DOI] [PubMed] [Google Scholar]
- 6.Bisceglia M, Minenna E, Altobella A, Sanguedolce F, Panniello G, Bisceglia S, Ben-Dor DJ. Anaplastic Kaposi’s sarcoma of the adrenal in an HIV-negative patient with literature review. Adv Anat Pathol. 2019;26(2):133–149. doi: 10.1097/PAP.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 7.Bishop BN, Lynch DT. Kaposi sarcoma. StatPearls. 2023 Jan; [PubMed] [Google Scholar]
- 8.Fatahzadeh M. Kaposi sarcoma: review and medical management update. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(1):2–16. doi: 10.1016/j.tripleo.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi sarcoma: epidemiology and risk factors. Cancer. 2000;88(3):500–517. [PubMed] [Google Scholar]
- 10.Server EA, Durna YM, Yigit O, Bozkurt ER. Supraglottic Kaposi’s sarcoma in HIV-negative patients: case report and literature review. Case Rep Otolaryngol. 2016;2016:1818304–1818304. doi: 10.1155/2016/1818304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ares C, Allal AS. Long-term complete remission of laryngeal Kaposi’s sarcoma after palliative radiotherapy. Nat Clin Pract Oncol. 2005;2(9):473–477; quiz 1 p following 477. doi: 10.1038/ncponc0294. [DOI] [PubMed] [Google Scholar]
- 12.Mohd Tahir, Gopalan KN, Marina MB, Primuharsa Putra. A rare case of laryngeal Kaposi’s sarcoma. Bangladesh J Med Sci. 2010;9(2):107–109. [Google Scholar]
- 13.Schiff NF, Woo P, Annino DJ, Shapshay SM. Kaposi’s sarcoma of the larynx. Ann Otol Rhinol Laryngol. 1997;106(7 Pt 1):563–567. doi: 10.1177/000348949710600706. [DOI] [PubMed] [Google Scholar]
- 14.Barron K, Omiunu A, Celidonio J, Cruz-Mullane A, Din-Lovinescu C, Chemas-Velez MM, Baredes S, Eloy JA, Fang CH. Kaposi sarcoma of the larynx: a systematic review. Otolaryngol Head Neck Surg. 2023;168(3):269–281. doi: 10.1177/01945998221105059. [DOI] [PubMed] [Google Scholar]
- 15.Douglas JL, Gustin JK, Moses AV, Dezube BJ, Pantanowitz L. Kaposi sarcoma pathogenesis: a triad of viral infection, oncogenesis and chronic inflammation. Transl Biomed. 2010;1(2):172–172. [PMC free article] [PubMed] [Google Scholar]
- 16.Mochloulis G, Irving RM, Grant HR, Miller RF. Laryngeal Kaposi’s sarcoma in patients with AIDS. J Laryngol Otol. 1996;110(11):1034–1037. doi: 10.1017/s0022215100135698. [DOI] [PubMed] [Google Scholar]
- 17.Osei N, Fletcher G, Showunmi A, Ahluwalia M. A case of non-cutaneous Kaposi sarcoma. Cureus. 2022;14(12):e32394–e32394. doi: 10.7759/cureus.32394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chemas-Velez MM, Rodríguez-Ruiz MT, Vargas MJ, Jimenez Fandiño. Kaposi’s sarcoma of the larynx: an unusual presentation. Case report. Otolaryngol Case Rep. 2020;17:100237–100237. [Google Scholar]
- 19.Popescu B, Oancea ALA, Androne RG, Arjoca EM, Berteşteanu SVG. Rare laryngeal Kaposi’s sarcoma: case report and innovative surgical approach. Ars Medica Tomitana. 2019;25(4):207–213. [Google Scholar]
- 20.Grabar S, Abraham B, Mahamat A, Del Giudice, Rosenthal E, Costagliola D. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. J Clin Oncol. 2006;24(21):3408–3414. doi: 10.1200/JCO.2005.05.4072. [DOI] [PubMed] [Google Scholar]
- 21.Dumic I, Radovanovic M, Igandan O, Savic I, Nordstrom CW, Jevtic D, Subramanian A, Ramanan P. A fatal case of Kaposi sarcoma immune reconstitution syndrome (KS-IRIS) complicated by Kaposi sarcoma inflammatory cytokine syndrome (KICS) or multicentric Castleman disease (MCD): a case report and review. Am J Case Rep. 2020;21:e926433–e926433. doi: 10.12659/AJCR.926433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190–190. doi: 10.1186/1471-2407-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naimi Z, Mahjoubi K, Adouni O, Abidi R, Driss M, Nasr C. Kaposi’s sarcoma of the larynx: an unusual location in an HIV-negative patient (a case report) Pan Afr Med J. 2020;37:206–206. doi: 10.11604/pamj.2020.37.206.26175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angouridakis N, Constantinidis J, Karkavelas G, Vlachtsis K, Mpouras K, Daniilidis J. Classic (Mediterranean) Kaposi’s sarcoma of the true vocal cord: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2006;263(6):537–540. doi: 10.1007/s00405-006-0007-0. [DOI] [PubMed] [Google Scholar]
- 25.Madhoun MF, Blankenship MM, Blankenship DM, Krempl GA, Tierney WM. Prophylactic PEG placement in head and neck cancer: how many feeding tubes are unused (and unnecessary) World J Gastroenterol. 2011;17(8):1004–1008. doi: 10.3748/wjg.v17.i8.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anwander T, Bergé S, Appel T, von Lindern, Martini M, Mommsen J, Kipnowski J, Niederhagen B. Percutaneous endoscopic gastrostomy for long-term feeding of patients with oropharyngeal tumors. Nutr Cancer. 2004;50(1):40–45. doi: 10.1207/s15327914nc5001_6. [DOI] [PubMed] [Google Scholar]
- 27.Nyeko R, Geriga F, Angom R, Kambugu JB. Oral-visceral iatrogenic Kaposi sarcoma following treatment for acute lymphoblastic leukemia: a case report and review of the literature. J Med Case Rep. 2022;16(1):405–405. doi: 10.1186/s13256-022-03620-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Văduva CC, Constantinescu C, Radu MM, Văduva AR, Pănuş A, Ţenovici M, Diţescu D, Albu DF. Pregnancy resulting from IMSI after testicular biopsy in a patient with obstructive azoospermia. Rom J Morphol Embryol. 2016;57(2 Suppl):879–883. [PubMed] [Google Scholar]
- 29.Ciolofan MS, Mogoantă CA, Ioniţă I, Mitroi MR, Mitroi GF, Anghelina F, Vlăescu AN, Căpitănescu AN, Vîlcea AM, Mitroi GG, Ică OM, Stoica LE. Cutaneous malignant melanoma metastatic to the larynx and trachea: a case report and review of the literature. Life (Basel) 2023;13(7):1452–1452. doi: 10.3390/life13071452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nocini R, Molteni G, Mattiuzzi C, Lippi G. Updates on larynx cancer epidemiology. Chin J Cancer Res. 2020;32(1):18–25. doi: 10.21147/j.issn.1000-9604.2020.01.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iovănescu G, Bîrsăşteanu F, Borugă VM, Apostol A, Ştefănescu EH, Budu VA, Baderca F, Trifu SC, Mogoantă CA, Bonţe DC, Ivan MV. Clinical, ultrasound and histopathological correlation of clinically N0 neck nodes in patients with cancers of the pharynx and larynx. Rom J Morphol Embryol. 2020;61(2):433–439. doi: 10.47162/RJME.61.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liberale C, Soloperto D, Marchioni A, Monzani D, Sacchetto L. Updates on larynx cancer: risk factors and oncogenesis. Int J Mol Sci. 2023;24(16):12913–12913. doi: 10.3390/ijms241612913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato H, Yanagisawa N, Morioka H, Sasaki S, Sekiya N, Suganuma A, Imamura A, Ajisawa A. Laryngeal Kaposi’s sarcoma complicated by the immune reconstitution inflammatory syndrome in an HIV-infected patient. Intern Med. 2016;55(8):1001–1005. doi: 10.2169/internalmedicine.55.5813. [DOI] [PubMed] [Google Scholar]
- 34.Xiang P, Liu M, Lu X, Tang W, Liu J. Primary Kaposi’s sarcoma of the nasal cavity: clinical experience and review of the literature. Ear Nose Throat J. 2022;27:1455613221111734–1455613221111734. doi: 10.1177/01455613221111734. [DOI] [PubMed] [Google Scholar]


