Abstract
Incidental prostate carcinoma (iPC) is a subject of debate concerning its definition, incidence, biology, diagnosis, staging, and treatment. The present study aimed to assess the incidence and main clinical-morphological characteristics of iPC identified in radical cystoprostatectomy (RCP) specimens over a 5-year period. Using the database of the Urology and Pathology Departments, we identified all patients with bladder carcinomas (BCs) who underwent RCP within a 5-year frame time. We selected only those patients with synchronous BC and prostate carcinoma (PC). The following parameters were analyzed for these patients: age, type of bladder and prostate tumor, degree of differentiation, pathological stage, and other prognostic parameters. We identified 91 men with bladder tumors treated by RCP among whom 43, aged between 53 and 84 years (mean age: 69.2 years), presented synchronous PC. iPC was more prevalent in older individuals (>65 years: 30 patients, 69.8%), with only six out of the 43 (12.8%) patients with iPC being aged ≤60 years. All iPC cases were conventional adenocarcinoma. Well-differentiated prostate adenocarcinomas (grade group 1) predominated (65.1%). Among the 43 iPCs, 16 (37.2%) were clinically significant PCs. iPC is frequently identified in patients with BC when inclusion and evaluation of all or most of the prostate tissue are performed. Although more than half of iPCs were well-differentiated tumors confined to the prostate, a significant number of cases met the criteria of clinically significant PC. All men over the age of 50 who are candidates for RCP, should undergo evaluation through serum prostate specific antigen determination.
Keywords: incidental prostate carcinoma , radical cystoprostatectomy , Gleason score , grade group , prostate specific antigen
Introduction
Prostate carcinoma (PC) is the second most common cancer in men, but it is only the fifth leading cause of cancer-related death in this population, following lung, liver, colorectal, and gastric cancer [1]. This raises the following hypotheses: (i) many tumors are detected early; (ii) some forms of PC have an indolent evolution and rarely lead to the death of patients; (iii) existing therapeutic methods are extremely effective.
Incidental prostate carcinoma (iPC) remains a poorly understood and controversial entity [2, 3] concerning its definition, incidence, diagnosis, staging, and treatment. Considering that most iPCs are clinically insignificant tumors [4, 5, 6, 7], this form of PC becomes particularly attractive from the perspective of immunohistochemical (IHC) and molecular profiling, which might differ from that of clinically significant carcinomas and could require a different therapeutic approach.
Aim
The present study aimed to evaluate the incidence and the main clinical-morphological characteristics of incidentally discovered PC in the specimens of radical cystoprostatectomy (RCP) performed over a 5-year period at a reference hospital in Western Romania.
Materials and Methods
Ethical approval for this study was obtained from the Ethics Committee (Approval No. 64/30.09/2022) of the Victor Babeş University of Medicine and Pharmacy, Timişoara, Romania. It had a retrospective, observational character, and the histopathological evaluation of the RCP specimens was conducted, throughout the analyzed time frame by four different pathologists with expertise in genitourinary pathology. They examined standard Hematoxylin–Eosin (HE) stained slides, supplemented by IHC stains when required.
From the database of the Urology and the Pathology Departments, all patients with radical cystectomy performed for bladder carcinoma (BC) within a 5-year period (January 31, 2017–January 31, 2022) were initially identified. Next, only male patients who had undergone RCP were selected. Patients with synchronous prostate adenocarcinoma were then extracted from this group, excluding those with prostate involvement from BC or other types of tumors (secondary/metastatic, primary nonepithelial), as well as those with a known history of PC. The following parameters were analyzed for these patients: age, histological type of prostate tumor, Gleason score, World Health Organization (WHO)/International Society of Urological Pathology (ISUP) grade group and stage, histological type of bladder tumor, degree of differentiation, and stage according to the WHO Classification of Tumors of Urinary and Male Genital Tumors, 4th Edition, 2016 [8], the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 8th Edition [9], and the recommendations of the 2014 and 2019 ISUP Consensus Conference on Grading of Prostatic Carcinoma [10, 11]. PCs were considered clinically significant if they were classified as ≥pT3 (or with extracapsular extension), or had lymph node metastasis/es, or Gleason score ≥7 (or more than 6/or with Gleason pattern 4 or 5), or positive surgical margins. This approach is similar to the criteria used by others [4, 12, 13, 14]. A surgical margin was considered positive if the tumor was present on the inked surface.
The processing of the prostate from the RCP specimen was carried out similarly to the radical prostatectomy specimens for PC: fixation in 10% neutral buffered formalin for a period of 24–72 hours, inking the external surface with different colors, the same for the prostate lobe, the seminal vesicle, and the vas deferens on the same side. Then the conization of the apex was performed, followed by the sectioning of the prostate into slices with a thickness of 3–4 mm, made perpendicular to the prostate urethra, starting from the apex and ending at the base of the prostate, with the inclusion of all or almost all of the prostate tissue in paraffin blocks. For cases that were difficult to diagnose on the usual HE-stained slides, we used IHC stains to identify basal cells [with anti-p63, anti-high molecular weight cytokeratin (HMWCK), anti-cytokeratin 5 (CK5) antibodies] and alpha-methylacyl-coenzyme A racemase (AMACR) (Table 1). Visualization was performed using a polymeric detection system (Novolink, Novocastra), with 3,3’-Diaminobenzidine (DAB). Finally, the slides were counterstained with Hematoxylin and mounted using Entellan.
Table 1.
Characteristics of antibodies used for the IHC evaluation of incidentally discovered PC
|
Primary antibody |
Clone |
Provider |
Dilution |
Retrieval method |
|
HMWCK |
34BE12 |
Dako/Agilent |
RTU |
HIER, pH 9 |
|
CK5 |
XM26 |
Novocastra |
RTU |
HIER, pH 9 |
|
p63 |
7JUL |
Novocastra |
RTU |
HIER, pH 9 |
|
AMACR |
13H4 |
DAKO/Agilent |
RTU |
HIER, pH 9 |
AMACR: Alpha-methylacyl-coenzyme A racemase; CK: Cytokeratin; HIER: Heat-induced epitope retrieval; HMWCK: High molecular weight cytokeratin; IHC: Immunohistochemical; PC: Prostate carcinoma; RTU: Ready-to-use
Descriptive statistics were performed using Pearson’s χ2 (chi-squared) test and Mann–Whitney test.
Results
During the specified time frame, 97 patients with BC who underwent radical cystectomy were identified. After excluding female patients, we obtained a group of 91 men, aged between 43 and 84 years (mean age 67 years), who were treated by RCP.
Within the same time frame, 950 patients with PC were diagnosed at the Pathology Department of the hospital, aged between 47 and 93 years (mean age 70 years).
In 47 of the 91 (51.6%) patients, PC was detected in the RCP specimen performed for BC. Four out of the 47 patients were excluded from the study group because three of them had previously been diagnosed with PC by core needle biopsy (CNB) or transurethral resection of the prostate (TURP). The fourth patient was suspected to have PC based on a serum prostate specific antigen (PSA) value of 21 ng/mL. Thus, 43 of the 91 (47.3%) patients had PC incidentally discovered in the RCP specimen. These patients were aged between 53 and 84 years (mean age 69.2 years).
The distribution of cases by age groups showed that iPCa had an increasing incidence with advancing age, with most cases identified in the 70–79 age group (Figure 1).
Figure 1.
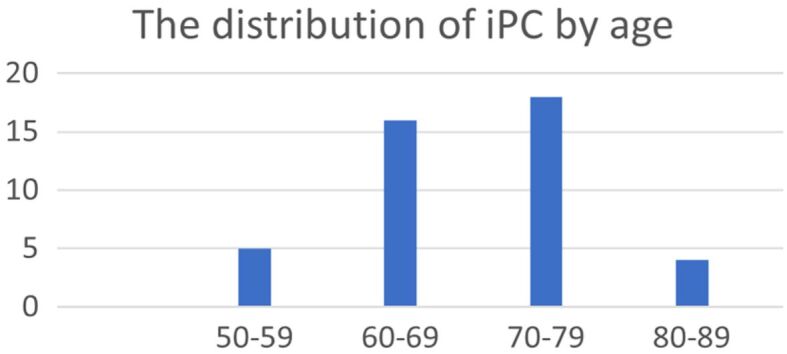
The distribution of cases by age group (n=43). iPC: Incidental prostate carcinoma
Most of the patients with iPC (30/43; 69.8%) were over 65 years old, with only six (14%) being 60 years old or younger. Among these younger patients, five out of six (83.3%) had tumors with Gleason score 6 (3+3), grade group 1, while only one case was diagnosed with Gleason score 7 (3+4), grade group 2 tumor. All iPC cases were conventional adenocarcinomas. In only one tumor with a Gleason score of 9, intraductal carcinoma was associated with invasive carcinoma (Figures 2, 3).
Figure 2.
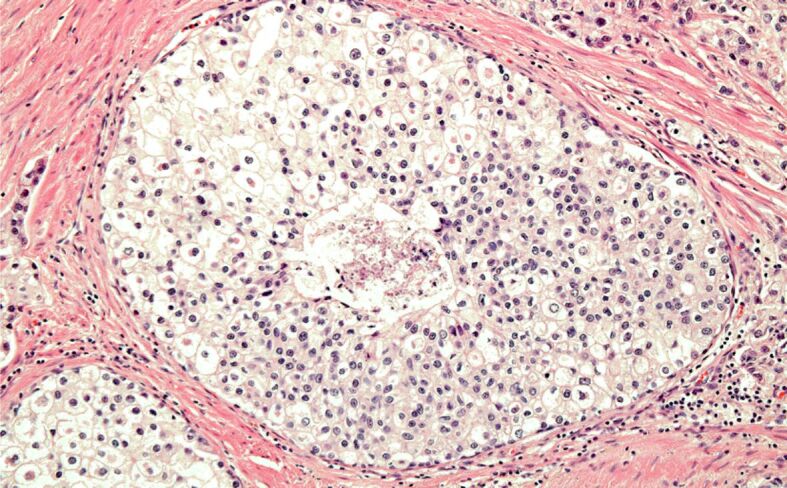
Intraductal PC: minute focus of comedonecrosis with calcification. HE staining, ×200. HE: Hematoxylin–Eosin; PC: Prostate carcinoma
Figure 3.
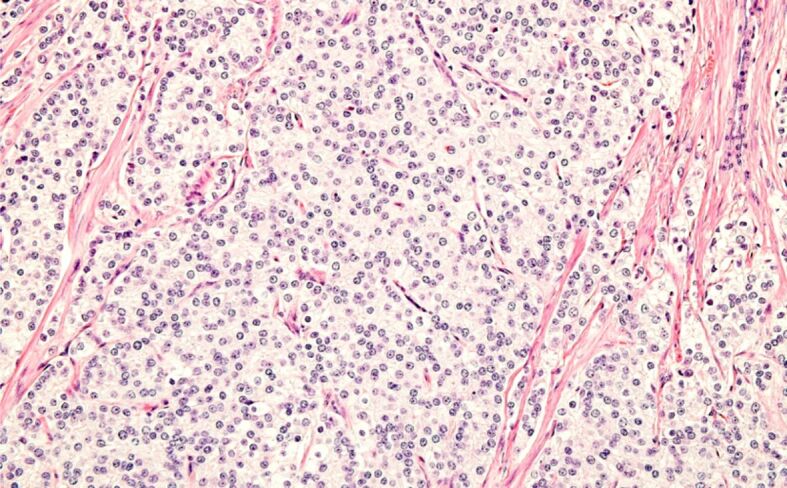
Incidental PC, Gleason score 9. HE staining, ×200
The distribution of iPC cases according to prognostic grade groups (Figure 4) was as follows: grade group 1 – 28 (65.1%) cases (Figure 5), grade group 2 – nine (20.9%) cases (Figure 6), grade group 3 – four (9.3%) cases, grade group 4 – one case (2.3%), grade group 5 – one case (2.3%).
Figure 4.
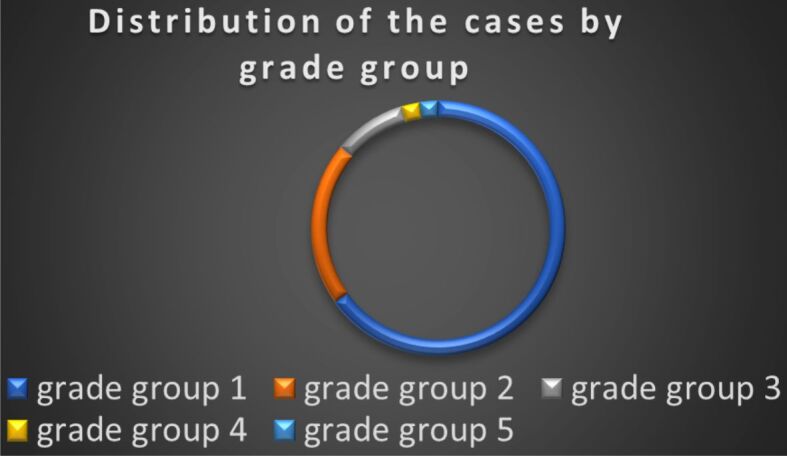
Distribution of cases by grade group of the PC (n=43).
Figure 5.
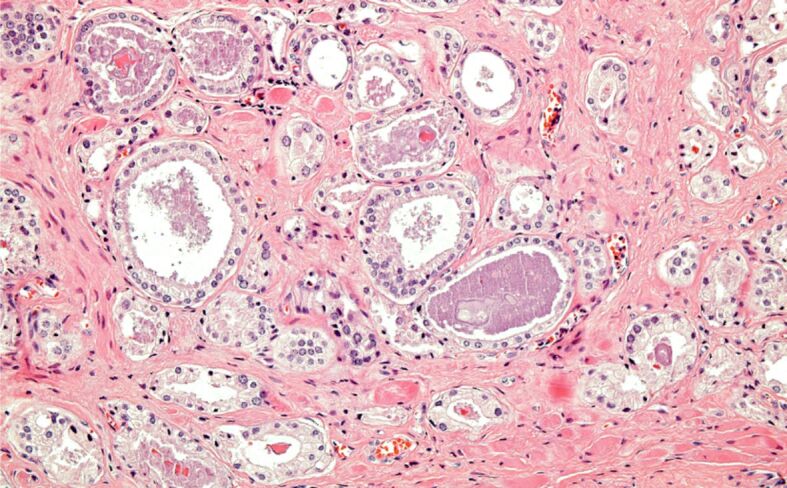
Incidental PC, Gleason score 6 (3+3). HE staining, ×200
Figure 6.
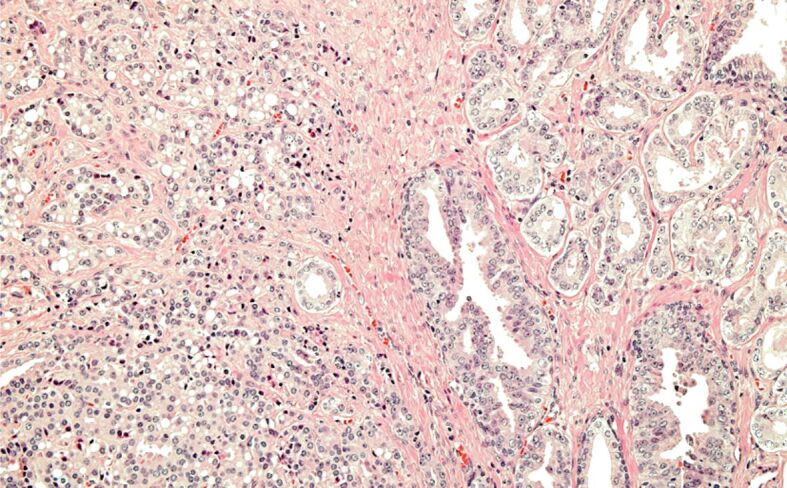
Incidental PC, grade group 2. HE staining, ×100
Regarding the extent of the primary tumor (pT category) (Figure 7), the incidentally discovered PC were pathologically classified as follows: pT2 – 35 (81.4%) cases, pT3a – six (14%) cases (Figure 8) and pT3b – two (4.6%) cases (Figure 9).
Figure 7.
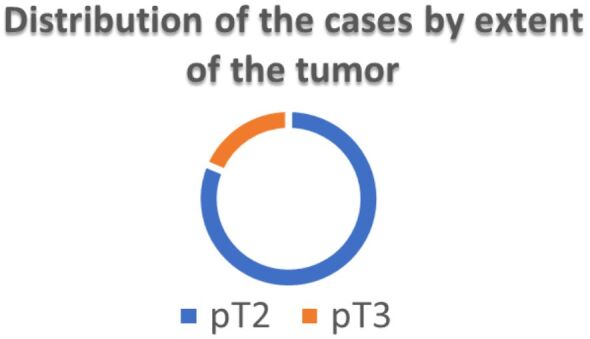
Distribution of cases by extent of the PC (n=43)
Figure 8.
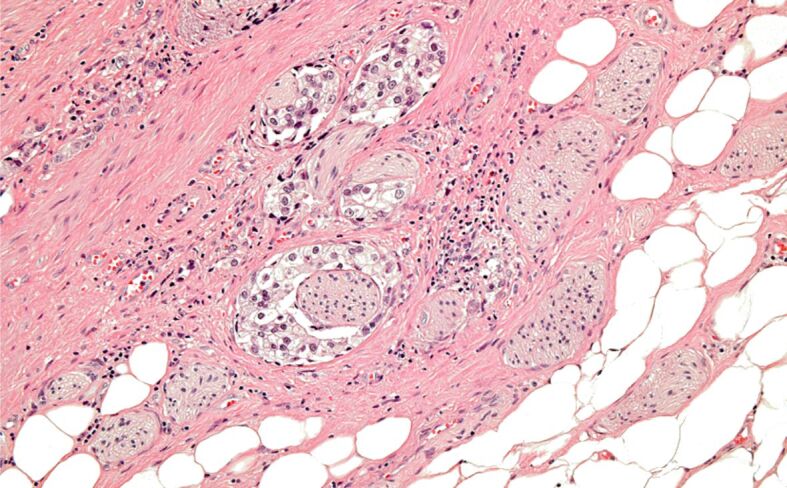
Incidental PC with extraprostatic extension – pT3a. HE staining, ×100
Figure 9.
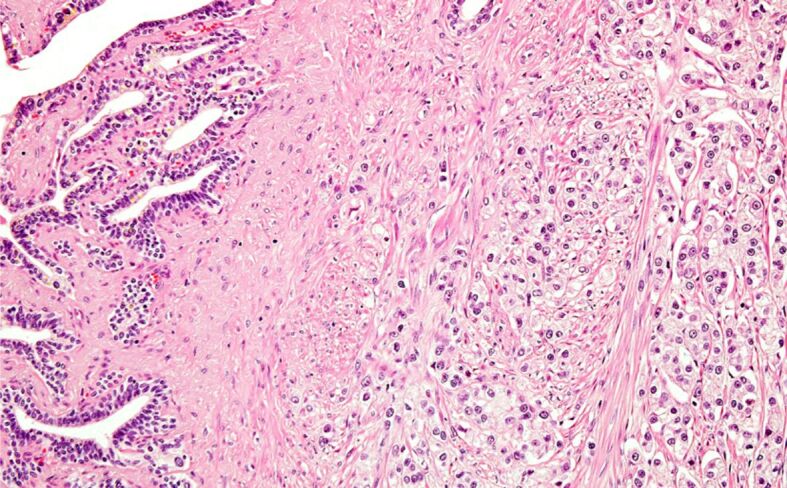
Incidental PC with seminal vesicle invasion – pT3b. HE staining, ×200
Out of the 43 cases of iPC, 19 (44.2%) had perineural invasion (PNI), while none had lymphovascular invasion. Among the 43 iPC cases, five (11.6%) tumors had positive resection margins (R1), all of which exhibited extraprostatic extension – pT3 (including four cases of pT3a and one case of pT3b).
Of all iPC, 16 (37.2%) cases had a Gleason score ≥7 and/or were ≥pT3 tumors, and/or had positive margins. Therefore, according to the definition, they were clinically significant PC. The remaining 27 (62.8%) cases were considered clinically insignificant PC.
The characteristics of bladder tumors for which RCP was performed were as follows: histological type – urothelial carcinoma (21 cases – 48.8%) (Figure 10), urothelial carcinoma with divergent differentiation (squamous and/or glandular) (20 cases – 46.5%), squamous carcinoma and sarcomatoid carcinoma (one case each – 2.3%). Regarding the depth of invasion in the bladder wall, the bladder tumors were categorized as follows: eight (18.6%) cases were non-muscle invasive BC, and 35 (81.4%) cases were muscle invasive BC with three (7%) cases classified as pT2, 19 (44.2%) cases as pT3 (including two cases of pT3a – 4.7%, and 17 cases of pT3b – 39.5%), and 13 (30.2%) cases as pT4. Out of the 38 cases in which regional lymph nodes dissection was performed, the regional lymph node status was: pN0 in 23 cases, and pN+ in 15 cases (Figure 11) (pN1 in five cases, and pN2 in 10 cases).
Figure 10.
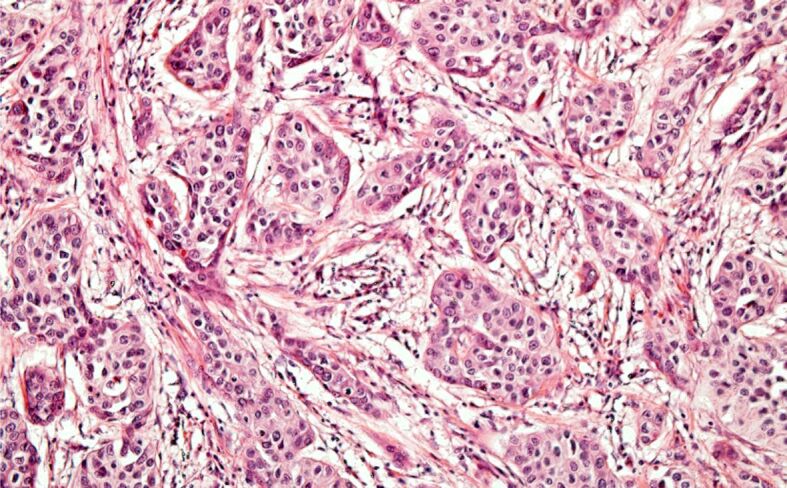
Urothelial carcinoma of the urinary bladder. HE staining, ×200
Figure 11.
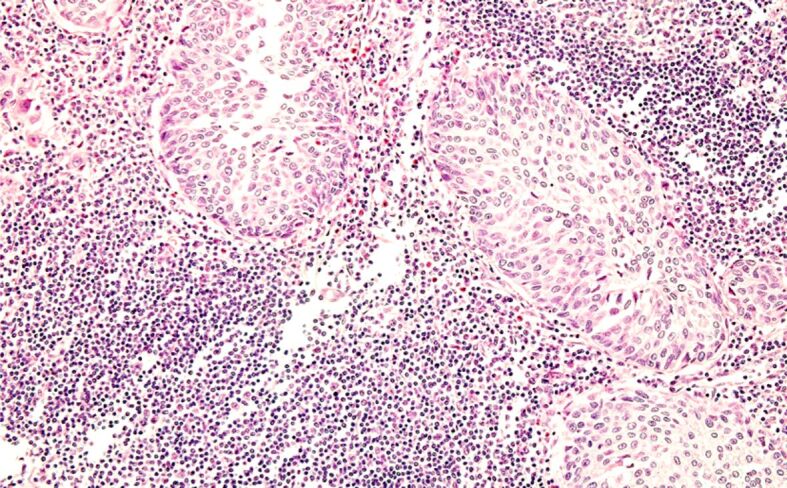
Lymph node metastases from urothelial carcinoma (pN+). HE staining, ×200
Discussions
PC can manifest itself in four major clinical forms: clinically manifest PC, which causes clinical signs and is confirmed microscopically; latent PC, defined as a tumor discovered during autopsy, in a man with no history of PC and no clinical signs of disease; iPC, discovered after a surgical intervention performed on patients with no clinical, imaging and serological suspicion of PC; and occult PC or with metastatic onset, detected during the investigation of a metastasis in a patient not previously known to have PC [3, 15, 16].
To the best of our knowledge, there are no data on iPC detected in TURP/adenomectomy or RCP specimens in men from Romania, a country where PC screening has never been implemented.
In this paper, we only analyzed the category of iPC detected in RCP specimens, considering that the presence of PC in the TURP/adenomectomy resection specimen may be the result of the extension of a PC from the peripheral area, indicating an advanced PC.
Detecting iPC in RCP specimen remains a challenge for urologists and pathologists in terms of definition, incidence, diagnosis, staging, and especially therapeutic implications.
The coexistence of BC and PC is not surprising considering that both types of tumors predominantly affect older patients, as our data also shows, with an average age of 69 years, identical to that reported by Bruins et al. [4] for patients with PC detected in men with BC treated by RCP. However, unlike their study, where over one-third of the iPC patients were younger than 60 years and had PSA<2.5 ng/mL, our study found iPC in a significantly lower percentage of patients younger than 60 years (12.76%). Literature evidence indicates that the incidence of iPC in RCP rises as age progresses [17, 18], as confirmed by our data, and with increasing serum PSA levels [6].
The reported incidence of iPC varies considerably (1–60%), depending on how iPC is defined, the geographic area and race, the amount of prostate tissue sampled and examined, and the diagnostic methods used (usual staining ± IHC techniques), etc. [4, 5, 7].
For most authors, iPC refers to those prostate neoplasms discovered by chance in patients treated for benign prostate hyperplasia (BPH) by TURP or open prostatectomy, with no clinical and paraclinical examination – digital rectal examination (DRE), transrectal ultrasound scan (TRUS), serum PSA, PSA density – raising suspicion of malignant prostate tumor [19, 20]. However, some authors include in this category also PC detected in the specimens of RCP performed for a BC [21] or only this category of PC [15]. Others use the term iPC for cases of prostate neoplasms detected at autopsy [22].
Studies have shown that the incidence of iPC can vary significantly across different regions or counties. Factors contributing to these variations may include differences in healthcare practices, screening programs, diagnostic procedures, and lifestyle factors [23]. As in the case of TURP specimens, the greater the amount of processed and examined prostate tissue from the RCP specimen, the higher the frequency with which iPC is detected [6, 7, 19], as shown in our study as well. Guidelines recommending the amount of tissue that must be examined to detect iCP refer especially to TURP specimens [7, 24], but less so for PC specimens where examining the entire prostate would be ideal, though not always feasible due to economic reasons in many laboratories. Newmann et al.’s study on TURP specimens demonstrated that the complete inclusion of prostate tissue increases the detection rate of iPC, including clinically significant iPC, leading them to recommend the inclusion and examination of the entire resected prostate tissue which is extremely difficult to achieve in practice [25].
We must not forget that the high incidence of iPC in our study could also be explained by the fact that for the group of patients with BC, we did not have serum PSA values available, somewhat related to the absence of PSA screening in our country. The symptomatology determined by PC, in cases with clinically significant tumors, could have been masked by the manifestations of muscle-invasive BC, so those cases of iPC from our study could actually represent clinically manifest PC. As a natural conclusion, all men over the age of 50, candidates for RCP, should be evaluated by determining serum PSA.
From the imaging perspective, iPC is difficult to differentiate from BPH using magnetic resonance imaging (MRI) techniques in use, including three-dimensional proton magnetic resonance spectroscopy (MRS) and diffusion-weighted imaging (DWI) features [20].
Incidental PC reported in the literature are usually conventional PC, in most cases well differentiated (Gleason score 6, grade I group), as our data also show.
Epidemiological data suggest that only 8% of PC are clinically apparent [5]. PC detected in the RCP specimen is usually clinically insignificant, does not negatively influence survival, and does not lead to the patient’s death [5, 26]. However, the definition of clinically insignificant PC differs, as follows: some consider it a tumor with volume <0.5 cm3, without extraprostatic extension, and with Gleason score ≤6 [13], while others define it as a tumor without extraprostatic extension, lymph node metastases, positive margins, and with Gleason score <7 [4, 14]. Trpkov et al. [27] exclude from this category the tumors with a tertiary pattern. Dell’Atti [5] add multifocality (with ≥ 3 tumors), and Sebo et al. [28] include the presence of PNI (a marker of tumor aggressiveness) as parameters defining clinically significant PC. Based on the Gleason score, extraprostatic extension, lymph node metastases, and resection margin status, 37.12% of iPC cases in our study were clinically significant tumors, a higher percentage compared to the 22% reported by Bruins et al. [4] or 27.3% reported by Dell’Atti [5].
While some studies show no differences in the mortality rate between patients with BC and synchronous PC compared to patients who only have BC [26], more recent data suggest a negative impact of this tumor association on the prognosis of the respective patients [29]. Jønck et al. [30] showed that only 0.9% of patients with iPC were treated for PC and that it is unlikely that they benefit from follow-up for PC. In general, the prognosis of these patients is influenced by the bladder tumor.
The tissue material from patients with clinically insignificant iPC might be used in the future for molecular studies that could indicate a distinct molecular profile of indolent PC from aggressive PC which threatens the patient’s life.
Data on the incidence and morphological characteristics of iPC are much more important from the perspective of therapy. In this sense, prostate sparing cystectomy (preserving the apex of the prostate, the capsule of the prostate, or the entire gland) has emerged as an alternative [31] to classical RCP, which is the “golden standard” treatment for muscle-invasive BC [32], but burdened by complications that are difficult to accept: urinary incontinence and the impairment of sexual function, which significantly affect the quality of life of those patients [5, 6, 33]. The advantages of prostate sparing cystectomy must be critically evaluated, considering the major disadvantages represented by the inability to confirm the invasion of the prostate by BC and/or the presence of a clinically significant/potentially aggressive iPC. Patients considered for this type of intervention must be carefully selected, considering the possibility of synchronous prostate tumor, through DRE, serum PSA level, TRUS, and eventually prostate biopsy [6, 26], to avoid over- or undertreatment of PC. Advanced age, elevated serum PSA level, BC with multifocal character, advanced stage or localization at the bladder neck, or association with carcinoma in situ (CIS) lesions would be contraindications for prostate sparing cystectomy [6, 34]. Furthermore, TURP or adenomectomy should precede prostate-sparing cystectomy to exclude from this procedure patients with prostate involvement by urothelial carcinoma or PC [35, 36]. For patients with high-risk PC, adjuvant PC treatment [5] should be considered.
Study limitations
The limitations of the current study are related to its retrospective nature, which might have influenced the composition of the patient group. Moreover, only iPCs from the RCP specimens were analyzed, and these were evaluated by four different pathologists, being known as the interobserver variability in establishing the Gleason score [37]. Other limitations of the study are related to the small number of analyzed cases and the lack of data regarding tumor volume and serum PSA values for those patients.
The observations of this study need to be confirmed by larger studies.
Conclusions
iPC is frequently identified in patients with BC when complete or almost complete embedding and evaluation of the prostate tissue are carried out. Although more than half of iPCs were well differentiated tumors confined to the prostate, a significant number of cases met the criteria for clinically significant PC. All men over the age of 50, candidates for RCP, should undergo evaluation of serum PSA levels.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Markiewicz D, Hanks GE. Therapeutic options in the management of incidental carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 1991;20(1):153–167. doi: 10.1016/0360-3016(91)90152-t. [DOI] [PubMed] [Google Scholar]
- 3. Faul P . In: Incidental carcinoma of the prostate . Altwein JE , et al., editors. Berlin-Heidelberg, Germany : Springer-Verlag ; 1991 . Problems and clinical significance of incidental carcinoma of the prostate ; pp. 1 – 9 . [Google Scholar]
- 4.Bruins HM, Djaladat H, Ahmadi H, Sherrod A, Cai J, Miranda G, Skinner EC, Daneshmand S. Incidental prostate cancer in patients with bladder urothelial carcinoma: comprehensive analysis of 1,476 radical cystoprostatectomy specimens. J Urol. 2013;190(5):1704–1709. doi: 10.1016/j.juro.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Dell’Atti L. Relevance of prostate cancer in patients with synchronous invasive bladder urothelial carcinoma: a monocentric retrospective analysis. Arch Ital Urol Androl. 2015;87(1):76–79. doi: 10.4081/aiua.2015.1.76. [DOI] [PubMed] [Google Scholar]
- 6.Yang X, Monn MF, Liu L, Liu Y, Su J, Lyu T, Gong Y, Wang L, Davidson DD, Cheng L. Incidental prostate cancer in Asian men: high prevalence of incidental prostatic adenocarcinoma in Chinese patients undergoing radical cystoprostatectomy for treatment of bladder cancer and selection of candidates for prostate-sparing cystectomy. Prostate. 2015;75(8):845–854. doi: 10.1002/pros.22966. [DOI] [PubMed] [Google Scholar]
- 7.Köllermann J, Hoeh B, Ruppel D, Smith K, Reis H, Wenzel M, Preisser F, Kosiba M, Mandel P, Karakiewicz PI, Becker A, Chun FKH, Wild P, Kluth LA. The significance of the extent of tissue embedding for the detection of incidental prostate carcinoma on transurethral prostate resection material: the more, the better. Virchows Arch. 2022;481(3):387–396. doi: 10.1007/s00428-022-03331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the urinary system and male genital organs-Part A: Renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 9. Amin MB , et al., editors. AJCC Cancer Staging Manual . 8th . Springer International Publishing ; 2017 . pp. 723 – 773 . [Google Scholar]
- 10.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA; The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 11.Iczkowski KA, van Leenders, van der. The 2019 International Society of Urological Pathology (ISUP) Consensus Conference on grading of prostatic carcinoma. Am J Surg Pathol. 2021;45(7):1005–1007. doi: 10.1097/PAS.0000000000001678. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–374. [PubMed] [Google Scholar]
- 13.Cheng L, Poulos CK, Pan CX, Jones TD, Daggy JK, Eble JN, Koch MO. Preoperative prediction of small volume cancer (less than 0.5 mL) in radical prostatectomy specimens. J Urol. 2005;174(3):898–902. doi: 10.1097/01.ju.0000169134.28610.66. [DOI] [PubMed] [Google Scholar]
- 14.Mazzucchelli R, Barbisan F, Scarpelli M, Lopez-Beltran A, van der, Cheng L, Montironi R. Is incidentally detected prostate cancer in patients undergoing radical cystoprostatectomy clinically significant. Am J Clin Pathol. 2009;131(2):279–283. doi: 10.1309/AJCP4OCYZBAN9TJU. [DOI] [PubMed] [Google Scholar]
- 15.Inaba H, Kimura T, Onuma H, Sato S, Kido M, Yamamoto T, Fukuda Y, Takahashi H, Egawa S. Tumor location and pathological features of latent and incidental prostate cancer in contemporary Japanese men. J Urol. 2020;204(2):267–272. doi: 10.1097/JU.0000000000000804. [DOI] [PubMed] [Google Scholar]
- 16. Ali A . In: Challenging cases in urological surgery . Pang KH , et al., editors. Oxford, UK : Oxford University Press ; 2023 . Case 10 Newly diagnosed metastatic prostate cancer ; pp. 10 – 104 . [Google Scholar]
- 17.Buse S, Höfner T, Müller SC, Hermann E, Wieland WF, May M, Stief CG, Bastian PJ, Hohenfellner M, Haferkamp A. Characterization and risk stratification of prostate cancer in patients undergoing radical cystoprostatectomy. Int J Urol. 2013;20(9):866–871. doi: 10.1111/iju.12073. [DOI] [PubMed] [Google Scholar]
- 18.Janjua TK, Yousuf MA, Iqbal MT, Memon SM, Abdullah A, Faridi N, Irfan M. Incidental finding of prostate cancer in transurethral resection of prostate (TURP) specimens: a retrospective analysis from a Tertiary Care Hospital in Pakistan. Pan Afr Med J. 2021;39:20–20. doi: 10.11604/pamj.2021.39.20.26931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Andel, Vleeming R, Kurth K, de Reijke. Incidental carcinoma of the prostate. Semin Surg Oncol. 1995;11(1):36–45. doi: 10.1002/ssu.2980110106. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XQ, Yu XR, Du ZL, Miao XF, Lu J, Zhou Q. Three-dimensional proton magnetic resonance spectroscopy and diffusion-weighted imaging in the differentiation of incidental prostate carcinoma from benign prostate hyperplasia. Oncol Lett. 2018;15(5):6541–6546. doi: 10.3892/ol.2018.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan J, Xue W, Sha J, Yang H, Xu F, Xuan H, Li D, Huang Y. Incidental prostate cancer at the time of cystectomy: the incidence and clinicopathological features in Chinese patients. PLoS One. 2014;9(4):e94490–e94490. doi: 10.1371/journal.pone.0094490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell KJL, Del Mar, Wright G, Dickinson J, Glasziou P. Prevalence of incidental prostate cancer: a systematic review of autopsy studies. Int J Cancer. 2015;137(7):1749–1757. doi: 10.1002/ijc.29538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 24.Srigley JR, Amin MB, Epstein JI, Grignon DJ, Humphrey PA, Renshaw AA, Wheeler TM;, College of. Updated protocol for the examination of specimens from patients with carcinomas of the prostate gland. Arch Pathol Lab Med. 2006;130(7):936–946. doi: 10.5858/2006-130-936-UPFTEO. [DOI] [PubMed] [Google Scholar]
- 25.Newman AJ, Graham MA, Carlton CE, Lieman S. Incidental carcinoma of the prostate at the time of transurethral resection: importance of evaluating every chip. J Urol. 1982;128(5):948–950. doi: 10.1016/s0022-5347(17)53293-0. [DOI] [PubMed] [Google Scholar]
- 26.Damiano R, Di Lorenzo, Cantiello F, De Sio, Perdonà S, D’Armiento M, Autorino R. Clinicopathologic features of prostate adenocarcinoma incidentally discovered at the time of radical cystectomy: an evidence-based analysis. Eur Urol. 2007;52(3):648–657. doi: 10.1016/j.eururo.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Trpkov K, Yilmaz A, Bismar TA, Montironi R. Insignificant’ prostate cancer on prostatectomy and cystoprostatectomy: variation on a theme ‘low-volume/low-grade’ prostate cancer. BJU Int. 2010;106(3):304–315. doi: 10.1111/j.1464-410X.2010.09499.x. [DOI] [PubMed] [Google Scholar]
- 28.Sebo TJ, Cheville JC, Riehle DL, Lohse CM, Pankratz VS, Myers RP, Blute ML, Zincke H. Perineural invasion and MIB-1 positivity in addition to Gleason score are significant preoperative predictors of progression after radical retropubic prostatectomy for prostate cancer. Am J Surg Pathol. 2002;26(4):431–439. doi: 10.1097/00000478-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Fahmy O, Khairul-Asri MG, Schubert T, Renninger M, Stenzl A, Gakis G. Clinicopathological features and prognostic value of incidental prostatic adenocarcinoma in radical cystoprostatectomy specimens: a systematic review and meta-analysis of 13,140 patients. J Urol. 2017;197(2):385–390. doi: 10.1016/j.juro.2016.08.088. [DOI] [PubMed] [Google Scholar]
- 30.Jønck S, Helgstrand JT, Røder MA, Klemann N, Grønkaer Toft, Brasso K. The prognostic impact of incidental prostate cancer following radical cystoprostatectomy: a nationwide analysis. Scand J Urol. 2018;52(5-6):358–363. doi: 10.1080/21681805.2018.1534885. [DOI] [PubMed] [Google Scholar]
- 31.Voskuilen CS, Fransen van, Pérez-Reggeti JI, van Werkhoven, Mertens LS, van Rhijn, Saad M, Bex A, Cathelineau X, van der, Horenblas S, Sanchez-Salas R, Meijer RP. Prostate sparing cystectomy for bladder cancer: a two-center study. Eur J Surg Oncol. 2018;44(9):1446–1452. doi: 10.1016/j.ejso.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 32.Mak RH, Hunt D, Shipley WU, Efstathiou JA, Tester WJ, Hagan MP, Kaufman DS, Heney NM, Zietman AL. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32(34):3801–3809. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novara G, Ficarra V, Minja A, De Marco, Artibani W. Functional results following vescica ileale Padovana (VIP) neobladder: midterm follow-up analysis with validated questionnaires. Eur Urol. 2010;57(6):1045–1051. doi: 10.1016/j.eururo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Arce J, Gaya JM, Huguet J, Rodriguez O, Palou J, Villavicencio H. Can we identify those patients who will benefit from prostate-sparing surgery? Predictive factors for invasive prostatic involvement by transitional cell carcinoma. Can J Urol. 2011;18(1):5529–5536. [PubMed] [Google Scholar]
- 35.Muto G, Bardari F, D’Urso L, Giona C. Seminal sparing cystectomy and ileocapsuloplasty: long-term followup results. J Urol. 2004;172(1):76–80. doi: 10.1097/01.ju.0000132130.64727.b6. [DOI] [PubMed] [Google Scholar]
- 36.Autorino R, Di Lorenzo, Damiano R, Giannarini G, De Sio, Cheng L, Montironi R. Pathology of the prostate in radical cystectomy specimens: a critical review. Surg Oncol. 2009;18(1):73–84. doi: 10.1016/j.suronc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Ozkan TA, Eruyar AT, Cebeci OO, Memik O, Ozcan L, Kuskonmaz I. Interobserver variability in Gleason histological grading of prostate cancer. Scand J Urol. 2016;50(6):420–424. doi: 10.1080/21681805.2016.1206619. [DOI] [PubMed] [Google Scholar]


