Abstract
A member of the epidermal growth factor (EGF) family, the heparin-binding EGF (HB-EGF) is expressed in the uteri of both humans and mice during the implantation process. To study the effects of HB-EGF on adhesion stage, we developed an in vitro implantation model employing Ishikawa cell line and JAR cell line, which may attach to Ishikawa cells. For 1, 6, 12, and 24 hours, co-cultures of JAR spheroids grown on Ishikawa monolayers were treated with 1, 10, and 100 ng/mL doses of HB-EGF. Using immunocytochemistry and Western blot analysis, the effects of HB-EGF on the protein expressions of E-cadherin, Erb-B2 receptor tyrosine kinase 4 (ErbB4), and integrin ανβ3 in Ishikawa and JAR cells were examined semi-quantitatively and quantitatively. Ultrastructural changes of in vitro implantation model were investigated by transmission electron microscopy. We revealed that HB-EGF influenced trophoblast cell adhesion to endometrial cells by upregulating the expression of the proteins ErbB4 and trophoblastic integrin ανβ3. Decrease in trophoblastic E-cadherin expression and increase in endometrial E-cadherin expression were demonstrated accompanying morphological variations in cells required for the invasion. We discovered ultrastructurally that Ishikawa cells acquired uterodome-like appearance, including the organelles, when 10 and 100 ng/mL dosages of HB-EGF were administered for 12 and 24 hours. However, following additional hours of adhesion and invasion, their intercellular spaces enlarged. The trafficking of vesicular transport was enhanced by JAR spheroids. We therefore discovered that in this implantation paradigm, HB-EGF may enhance the receptivity of Ishikawa cells and the adherence of JAR cells.
Keywords: HB-EGF , Ishikawa and JAR cells , E-cadherin , ErbB4 , integrin ανβ3
Introduction
Implantation process includes a series of complex events separated into three different stages: apposition, adhesion, and invasion. In humans and other mammalians, several cytokines and growth factors participate in multiple complex signal pathways of implantation. The actions of factors like interleukin-1 (IL-1), leukemia inhibitory factor (LIF) and heparin-binding epidermal growth factor (HB-EGF) are the main focus of these signals [1, 2]. Molecules like integrins, E-cadherins, laminin, fibronectin, chemokine receptors and Erb-B2 receptor tyrosine kinase 4 (ErbB4) (one member of receptor tyrosine kinase family) have been identified in the trophectoderm of human implanted embryo [3].
E-cadherin is related to invasion and metastasis of many tumor types. In the placenta, E-cadherin mediates a strong intracellular interaction between adjacent trophoblast cells [4]. Integrin-dependent adhesion occurs in a very short time after apposition. One of the endometrial integrins, integrin ανβ3, emerges on apical surface of endometrial epithelium at 19–20th days of the menstrual cycle, at the beginning of implantation window and subsequently decreases until the new week [5]. Integrin ανβ3 is primarily regulated by EGF and HB-EGF in human endometrium [6]. Differentiating cells express the cofactor of HB-EGF, namely heparan sulfate proteoglycan (HSPG), in various ways. HB-EGF interacts with integrin α3β1 and cluster of differentiation 9 (CD9) among other transmembrane proteins to form complexes, although it is unknown how important these interactions are for implantation and placentation.
Ishikawa cells, an endometrial epithelial carcinoma cell line, have been chosen as a model for receptive endometrium. JAR cells, a trophoblastic choriocarcinoma cell line, show cytotrophoblast (CTB) features and have ability to attach in vitro to Ishikawa cells [7]. A co-culture model including Ishikawa cells with JAR spheroids has been used to investigate endometrial epithelial cell attachment [2].
In humans, autocrine and paracrine roles of HB-EGF, a member of EGF family, are distinctive in implantation and placentation. HB-EGF is expressed by uterine epithelium in implantation window and induces in vitro human blastocyst development [8].
Aim
The aim was to study the effects of HB-EGF on the adhesion phase in an in vitro implantation model created by co-cultures of Ishikawa and JAR spheroids. In this model, we intended to search the interaction between trophoblast–endometrial cells and the effects of HB-EGF on adhesion of these cells and adhesion molecules by using immunocytochemical, Western blot and transmission electron microscopy (TEM) techniques.
Materials and Methods
Cell culture procedures
In this study, JAR cell line (trophoblastic choriocarcinoma) and Ishikawa cell line (endometrial adenocarcinoma) were used, provided from American Type Culture Collection (ATCC).
As a medium for JAR cells, high glucose Dulbecco’s Modified Eagle Medium–Ham’s Nutrient Mixture F-12 (DMEM/F-12) containing 10% fetal bovine serum (FBS) and Penicillin and Streptomycin (100 μg/mL) was used. As a medium for Ishikawa cells, normal DMEM/F-12 medium containing 10% FBS and Penicillin and Streptomycin (100 μg/mL) was used. Cell cultures were sustained in a humidified incubator pumping 5% carbon dioxide (CO2) at 37°C.
To create an implantation model with co-cultures [9, 10], approximately 300 000 JAR cells were cultured on 1% agar and harvested spheroids reached to desired size were co-cultured with Ishikawa monolayers. For experimental groups, 1, 10 and 100 ng/mL HB-EGF (R&D System; 259-HE) were applied to co-cultures for 1, 6, 12 and 24 hours in line with the literature [6]. For control group, HB-EGF was not applied, and co-cultures were incubated at given hours only with cell medium. Harvested cell clusters were fixed and embedded with egg white, and then routine histological techniques were applied to obtain paraffin blocks. 4–5 μm thick sections were prepared from these cell blocks by microtome and stained with Hematoxylin–Eosin (HE).
Immunocytochemical analysis
Ishikawa cells cultured on cover slips were incubated for 24 hours, subsequently three different doses of HB-EGF applied. After six hours incubation, JAR spheroids were implanted on Ishikawa cells to create the implantation model defined above. Three repetitions of the experiment were conducted, ending at predetermined intervals. After fixing the cells in Methanol for five minutes at -20°C, the cells were immunostained for E-cadherin, ErbB4, and integrin ανβ3 using the indirect Streptavidin immunoperoxidase technique using an anti-polyvalent Horseradish peroxidase (HRP) Kit (SensiTek, ScyTek Laboratories, Utah, USA). The samples on the cover slips were incubated overnight at 4°C using the anti-E-cadherin (Santa Cruz Biotechnology, Texas, USA; sc-7870), anti-ErbB4 (Santa Cruz Biotechnology, Texas, USA; sc-53280) and anti-integrin ανβ3 (Biorbyt, Cambridge, UK; orb-10927) primary antibodies according to their protocols (1:100 dilution). The 3-Amino-9-Ethylcarbazole (AEC) Substrate Detection System (ScyTek) was then used to visualize the antigen–antibody complex. Histoscore (H-score) analysis was used to assess the intensity of immunoreactivity semi-quantitatively in accordance with the literature [11].
Western blot analysis
For the analysis of protein levels of E-cadherin, ErbB4 and integrin ανβ3, two different sets of Ishikawa and JAR cells and their nuclear extracts were diluted with sample buffer containing 5% Sodium Dodecyl Sulfate (SDS) and 20% Glycerol prepared in 0.4 M Tris (pH 6.8) with 0.02% Bromophenol Blue and then run in 8% Polyacrylamide gel electrophoresis (PAGE) according to the methods described in literature [12]. Proteins (50 μg/lane) were electrophoretically separated by the SDS–PAGE and blotted onto 0.45 μm Nitrocellulose membranes (Thermo Scientific; 88018). By soaking the Nitrocellulose membranes in Tris-buffered saline (TBS) containing 5% bovine serum albumin (BSA) (HyClone; SH 40015-11) for one hour, nonspecific binding sites were prevented. Subsequently, the membranes underwent four rounds of washing in TBS containing 0.75% Tween 20. They were then incubated with antibodies (1:200 dilution) against E-cadherin (Santa Cruz; sc-7870), ErbB4 (Santa Cruz; sc-53280), and integrin ανβ3 (Biorbyt; orb-10927) prepared in TBS containing 0.01% Tween 20 for one hour. After that, the membranes were rinsed with TBS that contained 0.75% Tween 20. They were then incubated for one hour in 0.5% BSA in TBS that contained 0.01% Tween 20, along with goat anti-rabbit and anti-mouse secondary antibodies conjugated with HRP (Santa Cruz; sc-2030 and sc-2005). Integrin ανβ3, ErbB4, and E-cadherin were expressed as a percentage of control. We utilized the housekeeping protein beta-actin (Santa Cruz; sc-47778) levels for standardization.
Transmission electron microscopy analysis
After harvesting co-cultures, 2.5% Glutaraldehyde in 0.1 M Millonig phosphate buffer was used to fix them. The co-cultures were then post-fixed for one hour at 4°C in 1% Osmium Tetroxide in the same buffer [13]. Cells were incubated in 1% Uranyl Acetate for one hour at 4°C, dehydrated in a graded Propylene Oxide series (Merck Millipore; 8.07027.1000) and embedded in Araldite (Fluka Analytical; 10953). Samples were cut using an ultramicrotome in 0.5 μm semi-thin sections, stained by Toluidine Blue to check the presence of cells. Next, 70 nm-thick ultrathin sections were cut and were counterstained with Reynolds’ Lead Citrate and stained with 5% Uranyl Acetate. Sections were photographed and analyzed under TEM (JEOL JEM-1011).
Statistical analysis
All the semi-quantitative and quantitative data of groups were statistically assessed using the GraphPad InStat ver. 3.06 software (GraphPad Inc., CA, USA). One-way analysis of variance (ANOVA) was used to compare the means of the continuous variables, and the Tukey–Kramer multiple comparison test was used to examine the differences between the groups. P-values less than 0.001 were regarded as statistically significant.
Results
JAR spheroids were successfully obtained after 24-hour incubation on agar (Figure 1A) and at the same time, Ishikawa cells were incubated for 24 hours to obtain monolayers for co-cultures (Figure 1B). Shortly thereafter, JAR spheroids were cultured on Ishikawa monolayers for a certain time (Figure 1C, 1D, 1E, 1F). At the end of 1-hour incubation, JAR spheroids started to adhere to Ishikawa cells (Figure 1C), at the end of six hours, partially adhered JAR spheroids with underlying Ishikawa cells were observed (Figure 1D). 12-hour incubation led to totally adhered spheroids onto Ishikawa cells (Figure 1E). After 24 hours, spheroids were shown to start to invade through the Ishikawa cells (Figure 1F).
Figure 1.
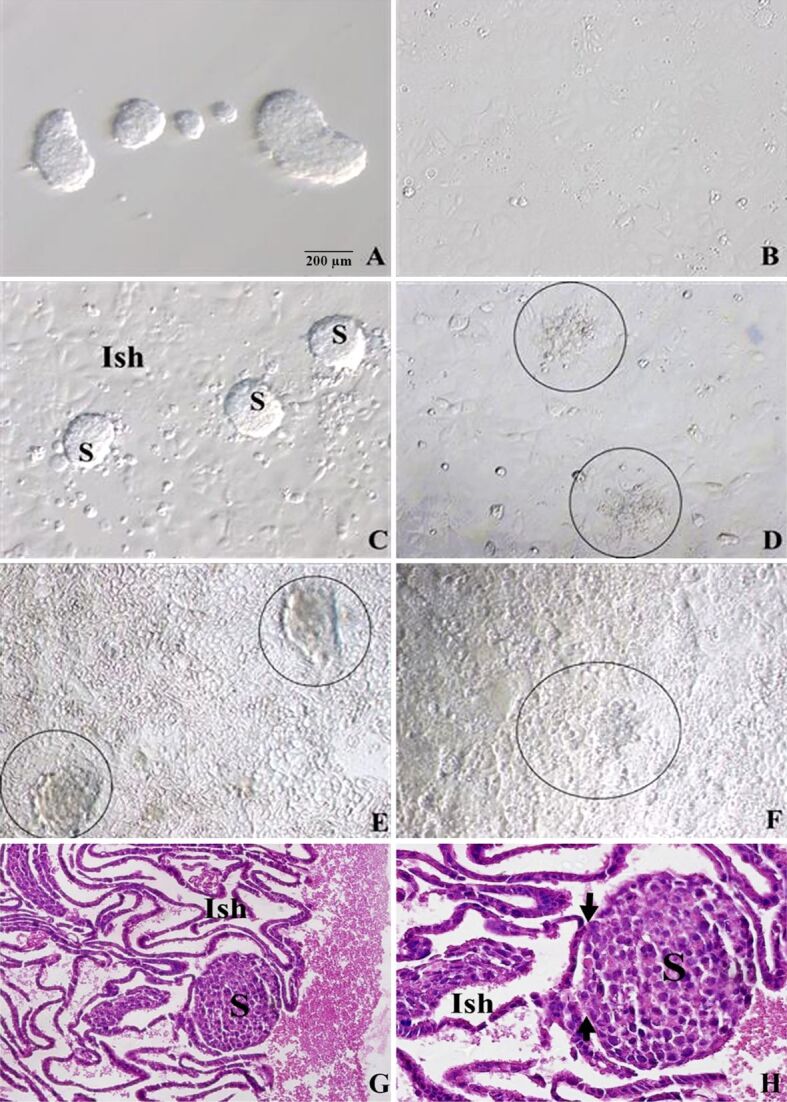
Inverted microscopic images of co-cultures of S on the Ish monolayers: (A) S incubated for 24 hours; (B) Ish monolayers incubated for 24 hours before co-cultures; (C–F) Co-cultures incubated for given hours, showing S on the Ish monolayers: (C) 1-hour incubation, (D) 6-hour incubated S (circle) adhered partially onto Ish cells, (E) 12-hour incubated S (circle) attached totally onto Ish cells, (F) 24-hour incubated, barely definable S (circle) started to invade through Ish cells; (G and H) HE-stained images of co-cultures of S on the Ish monolayers; successful focal attachment of S was observed on the Ish cells (arrow). (A–G) ×200; (H) ×400. HE: Hematoxylin–Eosin; Ish: Ishikawa; S: JAR spheroids
There were JAR spheroids which have not invaded onto Ishikawa cells yet in the control group, while attached spheroids were identified in 1 ng/mL HB-EGF group (Figure 2A, 2B). 10 ng/mL HB-EGF group had tightly adhered spheroids on Ishikawa cells, while 100 ng/mL HB-EGF group had same tightly adhered spheroids (Figure 2C, 2D).
Figure 2.
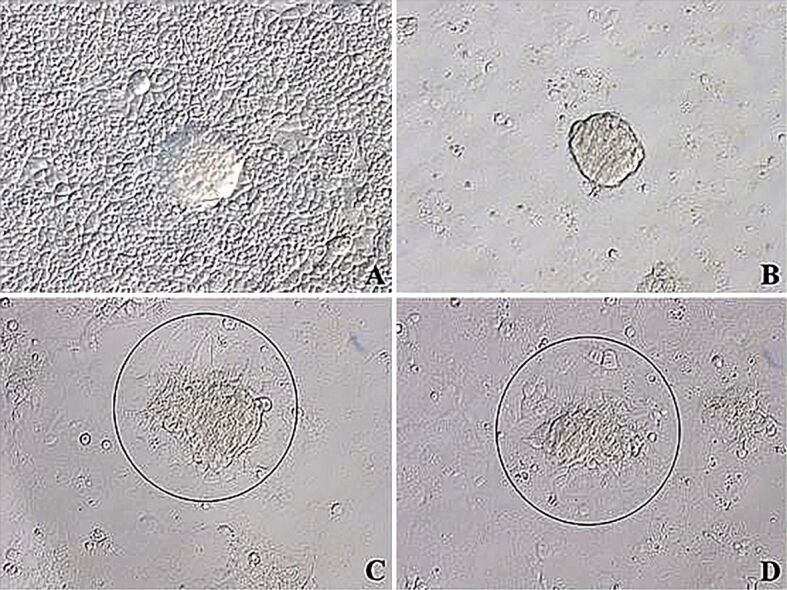
Inverted microscopic images of co-cultures of JAR spheroids (circle) on the Ishikawa monolayers incubated with given doses of HB-EGF for six hours: (A) Control group; (B) 1 ng/mL HB-EGF group showed attached JAR spheroids onto Ishikawa monolayers; (C) 10 ng/mL HB-EGF group showed tightly adhered spheroids on monolayers; (D) 100 ng/mL HB-EGF group showed tightly adhered spheroids on monolayers. (A–D) ×200. HB-EGF: Heparin-binding epidermal growth factor
HE-stained sections were given in Figure 1G, 1H, establishing light microscopic proofs for the success of implantation model of JAR spheroids incubated on Ishikawa cells.
Immunocytochemical findings
Regarding E-cadherin immunoreactivities in Ishikawa cells in implantation region, 1-hour incubation with any dose of HB-EGF did not lead to any statistical difference between groups but 6-hour incubation resulted in a significant immunoreactivity in control, 10 and 100 ng/mL HB-EGF groups, compared to 1 ng/mL HB-EGF group (p<0.001) (Figure 3A, 3B). After 12-hour incubation, there was significant decrease in E-cadherin immunoreactivity of 1 ng/mL HB-EGF group and a significant increase in 10 ng/mL HB-EGF group, compared to the control group (p<0.001), and there was not any significant difference between 100 ng/mL HB-EGF group and control group. Moreover, 24-hour incubation also did not cause any significant difference between groups, in terms of E-cadherin immunoreactivity (Figure 3A, 3B).
Figure 3.
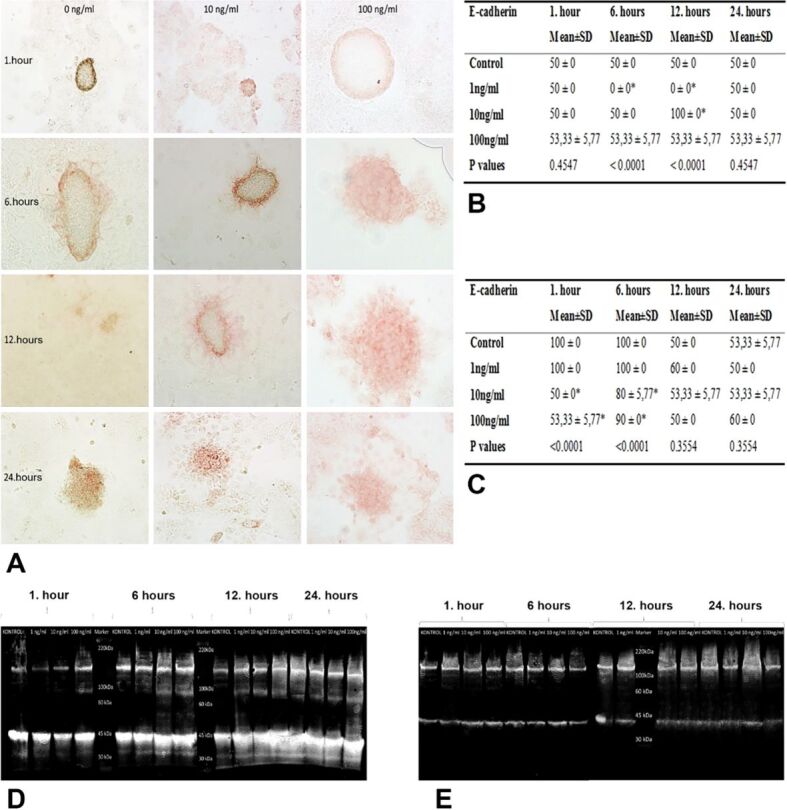
(A) E-cadherin immunoreactivities of Ishikawa and JAR cells from co-cultures incubated with 1, 10 and 100 ng/mL HB-EGF for 1, 6, 12 and 24 hours; (B) H-score table of E-cadherin immunopositive Ishikawa cells; (C) H-score table of E-cadherin immunopositive JAR cells (*p<0.001 vs control group); (D and E) Western blot images of E-cadherin expression in Ishikawa (D) and JAR (E) cells applied 1, 10 and 100 ng/mL HB-EGF for 1, 6, 12 and 24 hours. H-score: Histoscore; HB-EGF: Heparin-binding epidermal growth factor; SD: Standard deviation
Analyzing E-cadherin immunoreactivity of JAR cells, incubations with 10 and 100 ng/mL HB-EGF for 1 and 6 hours revealed significant declines in comparison with other groups (p<0.001) (Figure 3A, 3C). On the other hand, there was not any significance in E-cadherin immunoreactivity between different doses of 12- and 24-hour groups (Figure 3A, 3C). But the immunoreactivity of these 12- and 24-hour groups decreased significantly for all doses, compared to 1- and 6-hour groups (p<0.001) (Figure 3A, 3C).
There was not any immunoreactivity for ErbB4 in JAR cells in implantation regions, incubated with 1, 10 and 100 ng/mL HB-EGF for 1 and 6 hours. Therefore, semi-quantitative evaluation did not give any significance. However, 12- and 24-hour incubations significantly increased ErbB4 immunoreactivity of 10 ng/mL HB-EGF group, compared to other groups (p<0.001) (Figure 4A, 4B, 4C).
Figure 4.
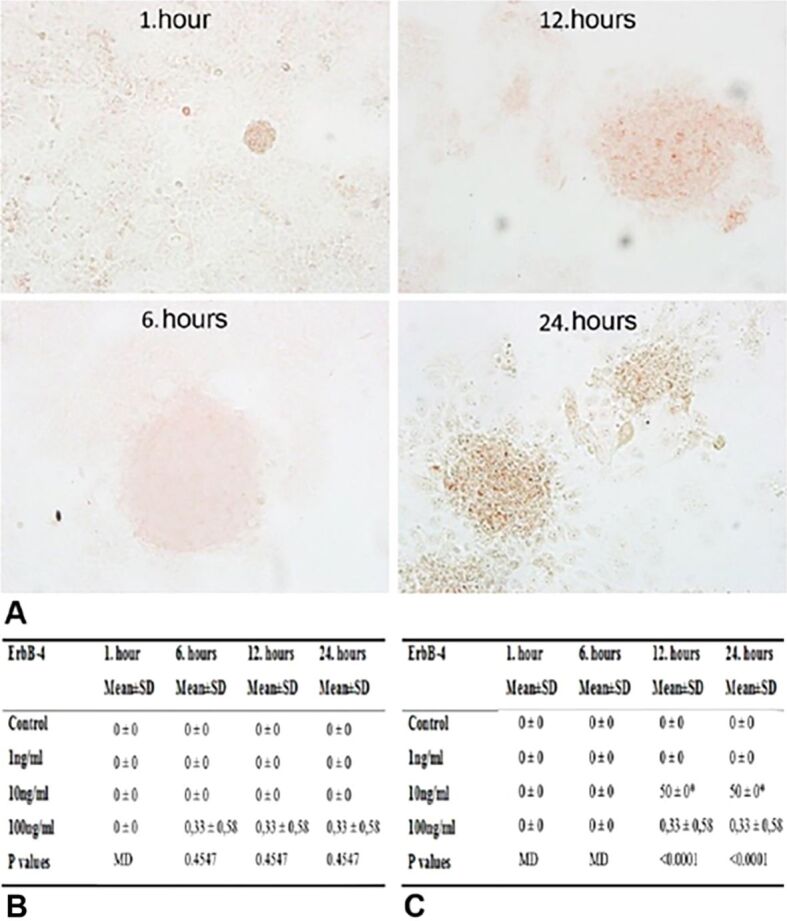
(A) ErbB4 immunoreactivities of JAR cells from co-cultures incubated with 1, 10 and 100 ng/mL HB-EGF for 1, 6, 12 and 24 hours; (B) H-score table of ErbB4 immunopositive Ishikawa cells; (C) H-score table of ErbB4 immunopositive JAR cells (*p<0.001 vs control group). ErbB4: Erb-B2 receptor tyrosine kinase 4; H-score: Histoscore; HB-EGF: Heparin-binding epidermal growth factor; SD: Standard deviation
Evaluating integrin αvβ3 immunoreactivities of JAR cells, 1-hour group had no significant differences between different doses of HB-EGF, whereas immunoreactivity of 6-hour group for all doses increased significantly in comparison with the control group (p<0.001) (Figure 5A, 5B, 5C). However, 12-hour incubation with 1 ng/mL dose of HB-EGF resulted in a significant increase in integrin αvβ3 immunoreactivity of JAR cells (p<0.001). Moreover, 24-hour incubation with 10 and 100 ng/mL doses of HB-EGF increased the immunoreactivity significantly, compared to the control and 1 ng/mL HB-EGF groups (p<0.001) (Figure 5A, 5B, 5C).
Figure 5.
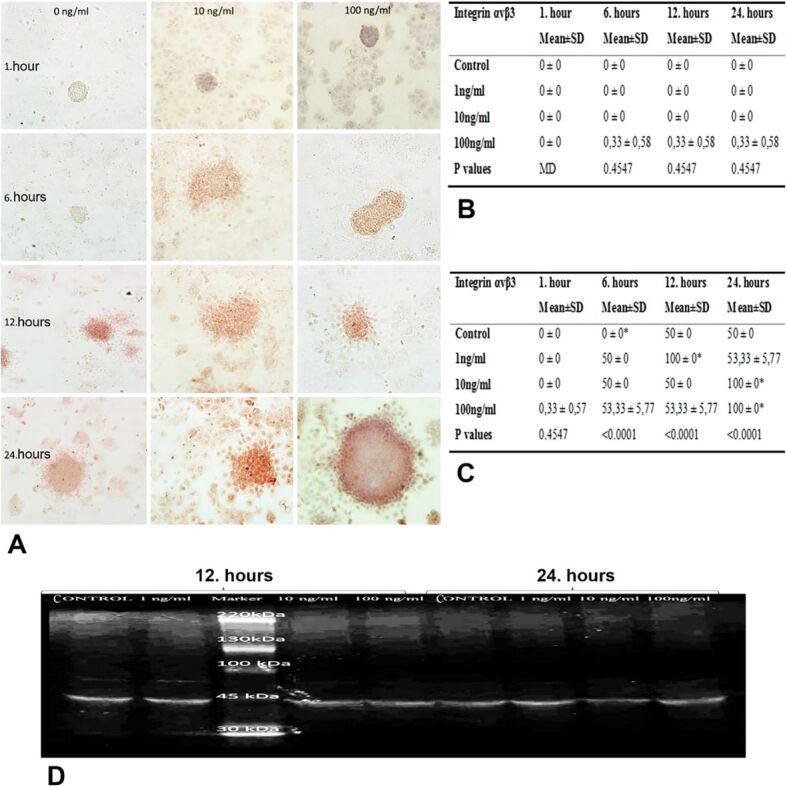
(A) Integrin ανβ3 immunoreactivities of Ishikawa and JAR cells from co-cultures incubated with 1, 10 and 100 ng/mL HB-EGF for 1, 6, 12 and 24 hours; (B) H-score table of integrin ανβ3 immunopositive Ishikawa cells; (C) H-score table of integrin ανβ3 immunopositive JAR cells (*p<0.001 vs control group); (D) Western blot image of integrin ανβ3 expression in JAR cells applied 1, 10 and 100 ng/mL HB-EGF
Western blot results
Examining E-cadherin expression levels of JAR monolayer cells, we observed time- and dose-dependent reduction depending on growth factor applied to the cells (Figure 3E). In particular, groups of 1- and 6-hour incubations with 10 and 100 ng/mL HB-EGF had decreased expression of E-cadherin compared to control and 1 ng/mL HB-EGF group, whereas 12- and 24-hour incubations with all doses of HB-EGF resulted in decreased levels in comparison with 1- and 6-hour incubations (Figure 3E).
In E-cadherin expression levels of Ishikawa monolayer cells, we observed time- and dose-dependent elevations depending on growth factor applied to the cells (Figure 3D). In particular, groups of 6-, 12- and 24-hour incubations resulted in significantly increased expression of E-cadherin compared to 1 ng/mL HB-EGF group (Figure 3D).
Examining the levels of integrin ανβ3 expression in JAR monolayer cells, we detected time- and dose-dependent increases in 12- and 24-hour incubations groups, compared with 1- and 6-hour incubations (Figure 5D). In particular, groups of 24-hour incubation with 10 ng/mL HB-EGF showed remarkable increased expression, compared to other groups (Figure 5D).
We could not detect any expression of ErbB4 protein in any of JAR and Ishikawa cells for all given doses and hours.
Transmission electron microscopy results
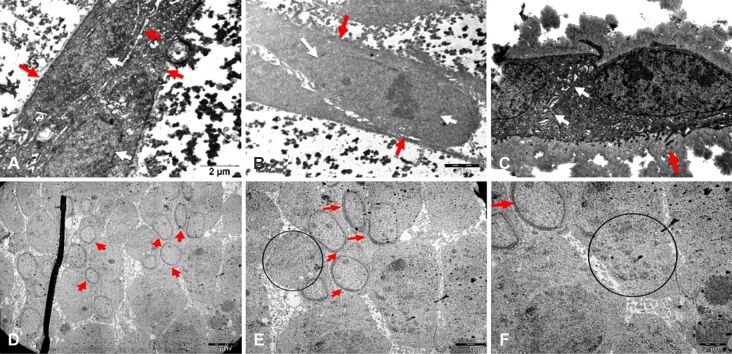
Ishikawa cells of 1-hour incubation group applied 10 ng/mL HB-EGF showed normal ultrastructure of cells with regular surfaces and their adhesion to the culture surface. Moreover, TEM images of co-cultures of 1-hour incubation with 100 ng/mL HB-EGF showed mitotic cells in some regions (Figure 6C).
Figure 6.
Electron micrographs of Ishikawa cells: (A) 10 ng/mL HB-EGF for six hours, showing regular nuclei (red star) with loose chromatin and compact nucleoli (white star); (B) 10 ng/mL HB-EGF for 12 hours, showing locally shortened microvilli (red arrow) and enlarged intercellular spaces; (C) 100 ng/mL HB-EGF for one hour, showing mitotic cells (red arrow); (D) 100 ng/mL HB-EGF for six hours, showing normal structure of cell membrane (red arrow) and nuclei (red star); (E) JAR cells incubated with 10 ng/mL HB-EGF group for one hour, showing local thickening of nuclear membrane (red arrow) and intracytoplasmic vesicles peripherally aligned in chain-like series (circle); (F) JAR cells incubated with 10 ng/mL HB-EGF group for six hours, showing local thickening of nuclear membrane (red arrow) and intracytoplasmic vesicles peripherally aligned in chain-like series (circle). Scale bars: (A–C and F) 2 μm; (D and E) 5 μm. HB-EGF: Heparin-binding epidermal growth factor
After 6-hour incubation with 10 ng/mL HB-EGF, Ishikawa cells revealed an intact cell membrane and nuclear membrane (Figure 6A). In addition to regular epithelial cell morphology, the cell nuclei with loose chromatin and compact nucleoli were remarkable. Similarly, cells of the group of 6-hour incubation with 100 ng/mL HB-EGF had also regular ultrastructure of cell membrane and nuclei located in parallel to the long axis. Moreover, microvilli with normal ultrastructure were notable in TEM images. Healthy cells were observed to interact with each other through their extensions (Figure 6D).
TEM images of Ishikawa cells incubated with 10 ng/mL HB-EGF for 12 hours demonstrated dome-shaped cells with intact cell membrane and nuclear membrane having regular ultrastructure, as well as curving and interdigitating lateral plasma membrane, enlarged intercellular spaces and decreased number of locally shortened microvilli (Figure 6B).
In TEM images of JAR spheroids incubated with 10 ng/mL HB-EGF for one hour, accumulation of intracytoplasmic vesicles was notable in adjacent to the membrane. Some cells had an interesting locally thickened nuclear membrane (Figure 6E). Particularly in these cells, intracytoplasmic vesicles aligned in chain-like series remarkably dispersed through the periphery of cytoplasm (Figure 6F).
Discussions
Many studies are needed to define the complex process of embryo implantation, examining the local molecules functioning in early embryo–uterus interaction. Since there are limitations in availability of trophoblast cell adhesion to the endometrial epithelium of materials derived directly from human [14], although there are many studies with decidual cells, it is possible to conduct the research with adenocarcinoma cell lines to examine the adhesion stage [15, 16]. In this study, we aimed to create an in vitro implantation model to investigate the effects of HB-EGF on adhesion stage of implantation. For this purpose, we used Ishikawa cell line because of its compatibility with receptive endometrium and JAR cell line reflecting the features of trophoblastic cell line [17], and we created the implantation model with co-culture of these two cell lines containing monolayer of Ishikawa cells underlying JAR spheroids, allowing to examine the interaction between Ishikawa and JAR cells and to determine possible effects of HB-EGF on adhesion and adhesion molecules in this interaction. Moreover, we determined significant differences for localization and expression levels of E-cadherin, integrin ανβ3 and ErbB4 proteins in co-cultured cells incubated with 1, 10 and 100 ng/mL doses of HB-EGF for 1, 6, 12 and 24 hours by using immunocytochemistry and Western blot analysis.
HB-EGF was shown to have roles in ovarian and uterine functions, expressed particularly in attachment site before blastocyst attachment, therefore, to be effective in embryo implantation [18, 19]. In the present study evaluating the effects of different doses of HB-EGF on implantation of JAR spheroids on Ishikawa cells, we found that JAR spheroids only attached onto Ishikawa cells in control group without HB-EGF and in 1 ng/mL HB-EGF group; whereas JAR cells adhered tightly and even started to invade into Ishikawa cells in 10 and 100 ng/mL HB-EGF groups especially after 6-hour incubation. These findings were essentially consistent with the data reporting the effects of HB-EGF on implantation window.
HB-EGF, having a vital role in embryonic implantation to induce invasiveness through endometrium, attracts attention in implantation model studies. Thus, we searched the effect of HB-EGF on invasiveness of JAR spheroids in the endometrial model created by Ishikawa monolayers and detected significant decrease in E-cadherin levels, and significant elevations in integrin ανβ3 and ErbB4 levels of JAR cells especially in 12- and 24-hour incubation groups. Once more, these findings revealed the importance of HB-EGF protein in implantation process.
HB-EGF is originally expressed in villous and extravillous CTBs of normal placenta. Differentiating into extravillous and endovascular phenotypes, trophoblasts enter the process of integrin switching, allowing variations in expression of adhesion molecules [20, 21]. Different integrins control attachment, invasion, and vascular remodeling processes. Primary CTB cultures have been found to have integrin switching during extravillous differentiation on Matrigel basal membrane of placenta in first trimester [22]. However, there are few numbers of in vitro studies reporting the role of integrin ανβ3 in attachment and invasion of trophoblasts to the endometrium. In this study, we demonstrated that trophoblastic JAR cells had increased levels of integrin ανβ3 expression due to incubation with HB-EGF for 12 and 24 hours, showing a marked increase in especially 10 ng/mL and 100 ng/mL HB-EGF dose groups incubated for 24 hours. This result suggested that integrin ανβ3 may mediate the effect of HB-EGF on trophoblastic attachment and invasion.
Schmitz et al. determined the presence of integrin ανβ3 in both Ishikawa and JAR cells by using immunocytochemical and immunofluorescence techniques [23]. However, we could not detect any integrin expression by immunocytochemical analysis of Ishikawa cells incubated with HB-EGF. This issue leads to the need for more in vitro experiments including several molecular analyses like Western blot and reverse transcription polymerase chain reaction (RT–PCR) methods to disclose the role of integrin ανβ3, which is different from structural endometrial integrins, in this implantation model.
HB-EGF expression was detected in the CTB and syncytiotrophoblasts of the chorionic villi during early pregnancy in the pathology specimens that were archived from pregnancy terminations that occurred between six and eight weeks [24]. Based on these findings, it appears that HB-EGF promotes trophoblast invasion and mitogenesis in the human endometrium. In an in vitro study employing cultivated endometrial stromal cells, the mitogenic potential of HB-EGF on the human endometrium was further shown. EGF receptor (EGFR; ErbB1), ErbB4, and transmembrane HB-EGF (HB-EGFTM) are expressed by these cells, and HB-EGF addition to culture medium results in increased deoxyribonucleic acid (DNA) synthesis [25]. When Chinese hamster ovary (CHO) cells expressing HB-EGFTM were used, the same outcomes were observed. The trophectoderm in human blastocysts showed a high concentration of ErbB4, suggesting that the human implantation is mediated by HB-EGF expressed in the luminal epithelium of the endometrium interacting with blastocyst ErbB4 [26]. Our study could not show the ErbB4 immunoreactivitiy in 1, 10 and 100 ng/mL HB-EGF groups of Ishikawa cells incubated for 1, 6, 12 and 24 hours, especially on the implantation site of JAR spheroids. Also, we could not find any ErbB4 immunoreactivitiy in 1, 10 and 100 ng/mL HB-EGF groups of JAR cells incubated for one and six hours. However, longer incubations with 10 ng/mL HB-EGF (12- and 24-hour groups) resulted in notable reactivity of ErbB4 for JAR spheroids, even in the implantation sites. This result suggested that even a moderate amount of HB-EGF may be effective on invasion rather than adhesion of trophoblasts during blastocyst implantation. However, the fact that there was no immunoreactivity for ErbB4 protein in Ishikawa cells.
In the prenatal trophoblastic diseases including the choriocarcinoma and total hydatidiform mole, which are characterized by invasive trophoblastic behavior, the trophoblastic E-cadherin levels drop during the first trimester of the placenta [27]. Studies showing that heparins increase trophoblastic invasiveness by expressing E-cadherin may provide insight into how heparin may improve trophoblast differentiation and motility [28]. In the present implantation model, we found that E-cadherin immunoreactivity and expression was significantly higher in JAR cells during adhesion to the implantation site, while then the levels of E-cadherin decreased in subsequent stage of invasion. At high doses of HB-EGF applied for one and six hours, we observed that E-cadherin immunoreactivity and expression levels were lower than the control and low dose groups; however, this difference disappeared after 12- and 24-hour incubations and E-cadherin levels of all groups remained at basal levels. In consistent with rare data from the literature, E-cadherin level was high on the first hour when HB-EGF may initially support trophoblastic adhesion, but then the levels significantly decreased during the shift to invasion process stage and continue to invasion. The study by Gonzalez et al. investigating the co-cultures created by human trophoblastic spheroids and decidualized endometrial stromal cells can be given as a proof for this issue [29].
In an epigenetic study by Rahnama et al., E-cadherin was shown to be regulated epigenetically in choriocarcinoma cells, suggesting the uterine epithelia gain adhesives qualities by increasing E-cadherin expression during the receptive phase [30]. In this study, we detected that HB-EGF incubation in monolayer cultures with Ishikawa cells resulted in time- and dose-dependent elevations of E-cadherin expression, especially after 6-, 12- and 24-hour incubations compared to 1-hour incubation. Although we could not detect any E-cadherin immunocytochemical reactivity and only mono-layer cultures incubated with the growth factor until 24 hours had increased levels of E-cadherin, it would appear that many studies comparing the co-cultures with and without spheroids and evaluating the long-term effects of HB-EGF are still needed.
During implantation window, the lateral plasma membrane transformation is fully completed under the control of maternal ovarian hormones, while the adherens junction and its associated terminal web are completely lost from the membrane, and their dissolution at the time of endometrial receptivity does not depend on the presence of a blastocyst [31]. Cellular vesicles and other organelles that had previously been kept out of the submembranous cytoplasm, most likely by the dense terminal web of filaments, were discovered to be able to approach the apical plasma membrane when the terminal web was removed [32]. The distinctively large apical vesicles of progesterone-regulated uterine epithelial cells play a significant role in these activities. As the terminal web is gone, these vesicles approach and integrate with the apical membrane surrounding the receptive phase for attachment [32]. The reasons of shortening and declining of microvilli of Ishikawa cells treated with HB-EGF for 12 and 24 hours and of elevation in vesicular traffic in JAR spheroids in this report may be plasma membrane transformation during shift from adhesion to the invasion stages and cytoskeletal transformations. Moreover, in consistent with the fact that adherens junctions on lateral plasma membrane modify during endometrial receptivity, we reported that Ishikawa cells protect ultrastructure of their intercellular junctions but the distance between intercellular spaces enlarged by next hours of adhesion and invasion. This confirms the phenomenon of these intraepithelial junctions needed to be unfastened for trophoblastic invasion.
In vitro interactions between the human blastocyst and endometrial epithelia take place especially at the lateral plasma membrane and may be part of the process of penetration, allowing induction of lysis of interepithelial apical junctional complexes and desmosomes [33]. The involvement of trophoblastic cells facilitates the regeneration of these connections. By means of invasive penetration, human blastocysts enter the endometrial surface epithelium. In our study, we observed distinctive intracytoplasmic vesicle accumulations located adjacent to the cell membrane of JAR cells in 10 ng/mL HB-EGF group treated for one hour. Some cells had interesting focal thickening of their nuclear membranes. Especially in these cells, notable peripherally distributed intracytoplasmic vesicles forming chain-like series suggest that JAR spheroids secrete these secretory vesicles to induce the disjunction of interepithelial apical junctional complexes and desmosomes.
Conclusions
Our in vitro implantation model exhibited the effects of HB-EGF on trophoblastic adhesion and invasion into endometrial cells through increasing the expression of trophoblastic integrin ανβ3 and ErbB4, decreasing the expression of trophoblastic E-cadherin and increasing the expression of endometrial E-cadherin, also accompanying by morphological changes needed for the invasion. Variations in HB-EGF function among the tissues may be caused by the milieu around the targeted cells. Further research is required to fully comprehend the genes that HB-EGF induces in reproductive organs as well as the molecular relationships that control HB-EGF expression. Lastly, since the number of in vitro and in vivo studies reporting expression of E-cadherin, integrin ανβ3 and ErbB4 together is limited in the literature, it is readily apparent that the genes of these proteins induced by HB-EGF are needed to be investigated particularly in vivo reproductive tissues to understand the molecular pathways in the expression of these proteins.
Conflict of interests
The authors declare that they have no conflict of interests.
Acknowledgments
This study was funded by the Scientific Research Projects Coordination Unit of Istanbul University. Project number: 35703.
References
- 1.Ai Z, Jing W, Fang L. Cytokine-like protein 1 (Cytl1): a potential molecular mediator in embryo implantation. PLoS One. 2016;11(1):e0147424–e0147424. doi: 10.1371/journal.pone.0147424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weimar CHE, Uiterweer EDP, Teklenburg G, Heijnen CJ, Macklon NS. In-vitro model systems for the study of human embryo-endometrium interactions. Reprod Biomed Online. 2013;27(5):461–476. doi: 10.1016/j.rbmo.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Mardon H, Grewal S, Mills K. Experimental models for investigating implantation of the human embryo. Semin Reprod Med. 2007;25(6):410–417. doi: 10.1055/s-2007-991038. [DOI] [PubMed] [Google Scholar]
- 4.Zhao HB, Wang C, Li RX, Tang CL, Li MQ, Du MR, Hou XF, Li DJ. E-cadherin, as a negative regulator of invasive behavior of human trophoblast cells, is down-regulated by cyclosporin A via epidermal growth factor/extracellular signal-regulated protein kinase signaling pathway. Biol Reprod. 2010;83(3):370–376. doi: 10.1095/biolreprod.110.083402. [DOI] [PubMed] [Google Scholar]
- 5.Johnson GA, Burghardt RC, Bazer FW, Seo H, Cain JW. Integrins and their potential roles in mammalian pregnancy. J Anim Sci Biotechnol. 2023;14(1):115–115. doi: 10.1186/s40104-023-00918-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim JJ, Lee DR, Song HS, Kim KS, Yoon TK, Gye MC, Kim MK. Heparin-binding epidermal growth factor (HB-EGF) may improve embryonic development and implantation by increasing vitronectin receptor (integrin ανβ3) expression in peri-implantation mouse embryos. J Assist Reprod Genet. 2006;23(3):111–119. doi: 10.1007/s10815-006-9021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Kodithuwakku SP, Ng PY, Chai J, Ng EHY, Yeung WSB, Ho PC, Lee KF. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod. 2010;25(2):479–490. doi: 10.1093/humrep/dep429. [DOI] [PubMed] [Google Scholar]
- 8.Armant DR. Blastocysts don’t go it alone. Extrinsic signals fine-tune the intrinsic developmental program of trophoblast cells. Dev Biol. 2005;280(2):260–280. doi: 10.1016/j.ydbio.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shirane A, Wada-Hiraike O, Tanikawa M, Seiki T, Hiraike H, Miyamoto Y, Sone K, Hirano M, Oishi H, Oda K, Kawana K, Nakagawa S, Osuga Y, Fujii T, Yano T, Kozuma S, Taketani Y. Regulation of SIRT1 determines initial step of endometrial receptivity by controlling E-cadherin expression. Biochem Biophys Res Commun. 2012;424(3):604–610. doi: 10.1016/j.bbrc.2012.06.160. [DOI] [PubMed] [Google Scholar]
- 10.Xiong T, Zhao Y, Hu D, Meng J, Wang R, Yang X, Ai J, Qian K, Zhang H. Administration of calcitonin promotes blastocyst implantation in mice by up-regulating integrin β3 expression in endometrial epithelial cells. Hum Reprod. 2012;27(12):3540–3551. doi: 10.1093/humrep/des330. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, Takizawa T, Sunamori M, Handa M, Kondo T, Sasano H. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab. 2003;88(5):2309–2317. doi: 10.1210/jc.2002-021353. [DOI] [PubMed] [Google Scholar]
- 12.Harduf H, Goldman S, Shalev E. Progesterone receptor A and c-Met mediates spheroids-endometrium attachment. Reprod Biol Endocrinol. 2009;7:14–14. doi: 10.1186/1477-7827-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilir A, Erguven M, Ermis E, Sencan M, Yazihan N. Combination of imatinib mesylate with lithium chloride and medroxyprogesterone acetate is highly active in Ishikawa endometrial carcinoma in vitro. J Gynecol Oncol. 2011;22(4):225–232. doi: 10.3802/jgo.2011.22.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hohn HP, Linke M, Denker HW. Adhesion of trophoblast to uterine epithelium as related to the state of trophoblast differentiation: in vitro studies using cell lines. Mol Reprod Dev. 2000;57(2):135–145. doi: 10.1002/1098-2795(200010)57:2<135::AID-MRD4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Uchida H, Maruyama T, Ohta K, Ono M, Arase T, Kagami M, Oda H, Kajitani T, Asada H, Yoshimura Y. Histone deacetylase inhibitor-induced glycodelin enhances the initial step of implantation. Hum Reprod. 2007;22(10):2615–2622. doi: 10.1093/humrep/dem263. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Pilla F, Anderson S, Martínez-Escribano S, Herrer I, Moreno-Moya JM, Musti S, Bocca S, Oehninger S, Horcajadas JA. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol Hum Reprod. 2012;18(1):33–43. doi: 10.1093/molehr/gar064. [DOI] [PubMed] [Google Scholar]
- 17.Heneweer C, Schmidt M, Denker HW, Thie M. Molecular mechanisms in uterine epithelium during trophoblast binding: the role of small GTPase RhoA in human uterine Ishikawa cells. J Exp Clin Assist Reprod. 2005;2(1):4–4. doi: 10.1186/1743-1050-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development. 1994;120(5):1071–1083. doi: 10.1242/dev.120.5.1071. [DOI] [PubMed] [Google Scholar]
- 19.Xie H, Wang H, Tranguch S, Iwamoto R, Mekada E, DeMayo FJ, Lydon JP, Das SK, Dey SK. Maternal heparin-binding-EGF deficiency limits pregnancy success in mice. Proc Natl Acad Sci U S A. 2007;104(46):18315–18320. doi: 10.1073/pnas.0707909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome. J Clin Invest. 1997;99(9):2152–2164. doi: 10.1172/JCI119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate. A strategy for successful endovascular invasion. J Clin Invest. 1997;99(9):2139–2151. doi: 10.1172/JCI119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120(12):3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz C, Yu L, Bocca S, Anderson S, Cunha-Filho JS, Rhavi BS, Oehninger S. Role for the endometrial epithelial protein MFG-E8 and its receptor integrin ανβ3 in human implantation: results of an in vitro trophoblast attachment study using established human cell lines. Fertil Steril. 2014;101(3):874–882. doi: 10.1016/j.fertnstert.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84(9):3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- 25.Stavreus-Evers A, Aghajanova L, Brismar H, Eriksson H, Landgren BM, Hovatta O. Co-existence of heparin-binding epidermal growth factor-like growth factor and pinopodes in human endometrium at the time of implantation. Mol Hum Reprod. 2002;8(8):765–769. doi: 10.1093/molehr/8.8.765. [DOI] [PubMed] [Google Scholar]
- 26.Chobotova K, Spyropoulou I, Carver J, Manek S, Heath JK, Gullick WJ, Barlow DH, Sargent IL, Mardon HJ. Heparin-binding epidermal growth factor and its receptor ErbB4 mediate implantation of the human blastocyst. Mech Dev. 2002;119(2):137–144. doi: 10.1016/s0925-4773(02)00342-8. [DOI] [PubMed] [Google Scholar]
- 27.Simopoulou M, Nikolopoulou E, Dimakakos A, Charalabopoulos K, Koutsilieris M. Cell adhesion molecules and in vitro fertilization. In Vivo. 2014;28(5):683–690. [PubMed] [Google Scholar]
- 28. Holcberg G , Segal D , Bashiri A . In: Recurrent pregnancy loss: evidence-based evaluation, diagnosis and treatment . Bashiri A , et al., editors. Cham, Switzerland : Springer ; 2016 . Implantation, physiology of placentation ; pp. 19 – 34 . [Google Scholar]
- 29.Gonzalez M, Neufeld J, Reimann K, Wittmann S, Samalecos A, Wolf A, Bamberger AM, Gellersen B. Expansion of human trophoblastic spheroids is promoted by decidualized endometrial stromal cells and enhanced by heparin-binding epidermal growth factor-like growth factor and interleukin-1β. Mol Hum Reprod. 2011;17(7):421–433. doi: 10.1093/molehr/gar015. [DOI] [PubMed] [Google Scholar]
- 30.Rahnama F, Thompson B, Steiner M, Shafiei F, Lobie PE, Mitchell MD. Epigenetic regulation of E-cadherin controls endometrial receptivity. Endocrinology. 2009;150(3):1466–1472. doi: 10.1210/en.2008-1142. [DOI] [PubMed] [Google Scholar]
- 31.Murphy CR. Commonality within diversity: the plasma membrane transformation of uterine epithelial cells during early placentation. J Assist Reprod Genet. 1998;15(4):179–183. doi: 10.1023/A:1023092100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luxford KA, Murphy CR. Reorganization of the apical cytoskeleton of uterine epithelial cells during early pregnancy in the rat: a study with myosin subfragment 1. Biol Cell. 1992;74(2):195–202. doi: 10.1016/0248-4900(92)90025-v. [DOI] [PubMed] [Google Scholar]
- 33.Bentin-Ley U, Horn T, Sjögren A, Sorensen S, Falck Larsen, Hamberger L. Ultrastructure of human blastocyst-endometrial interactions in vitro. J Reprod Fertil. 2000;120(2):337–350. doi: 10.1530/jrf.0.1200337. [DOI] [PubMed] [Google Scholar]