Abstract
Cell-mediated immune (CMI) responses play a major role in protection as well as pathogenesis of many intracellular bacterial infections. In this study, we evaluated the infection kinetics and assessed histologically the lymphoid reactions and local, in vitro-restimulated CMI responses in lungs of BALB/c mice, during both primary infection and reinfection with Chlamydia pneumoniae. The primary challenge resulted in a self-restricted infection with elimination of culturable bacteria by day 27 after challenge. A mild lymphoid reaction characterized the pathology in the lungs. In vitro CMI responses consisted of a weak proliferative response and no secretion of gamma interferon (IFN-γ). The number of lung-derived mononuclear cells increased substantially during the primary infection; the largest relative increase was observed in B cells (B220+). After reinfection, the number of lung-derived mononuclear cells increased further, and the response consisted mainly of T cells. The reinfection was characterized in vivo by significant protection from infection (fewer cultivable bacteria in the lungs for a shorter period of time) but increased local lymphoid reaction at the infection site. In vitro, as opposed to the response in naive mice, acquired immunity was characterized by a strongly Th1-biased (IFN-γ) CMI response. These results suggest that repeated infections with C. pneumoniae may induce Th1-type responses with similar associated tissue reactions, as shown in C. trachomatis infection models.
Chlamydia pneumoniae is a frequent cause of acute respiratory infection and the most common species of Chlamydia in humans (17). Primary infection by C. pneumoniae may cause symptoms ranging from severe to mild or even be asymptomatic. Interestingly, C. pneumoniae infection has been recently associated with coronary artery disease, based on seroepidemiological studies and on C. pneumoniae demonstration in atheromatous lesions by PCR, immunohistochemistry, or electron microscopy (reviewed in reference 6). More recently, C. pneumoniae infection in apolipoprotein E-deficient transgenic mice and in rabbits has been shown to lead to inflammatory changes in the aorta (9, 19, 21). In addition, two preliminary antibiotic intervention studies support a role of C. pneumoniae in human atherosclerosis (11, 12).
The tendency to severe sequelae is common to infections caused by chlamydiae: infection by C. trachomatis may lead to development of blinding trachoma or pelvic inflammatory disease, with its complications of ectopic pregnancy and tubal infertility. The pathogenesis of these serious consequences is still largely unknown. Inflammatory responses to repeated or persistent infections seem to play a significant role (3); repeated infections tend to cause partial protection with fewer cultivable bacteria, but at the same time they lead to more severe local inflammation reactions. The manifestations of many other chronic intracellular infections are likewise often due to the host’s defense mechanisms rather than microbial virulence factors (14).
The purpose of this study was to analyze infection kinetics, local cell-mediated immune (CMI) responses, and the presence and development of the lymphoid reaction in the lungs during C. pneumoniae primary infection and reinfection in BALB/c mice. We used an experimental mouse model for C. pneumoniae infection, characterized by a mild and self-restricted acute respiratory infection (15). Although prior infection induces partial protection against infection in this model, demonstrated by fewer cultivable bacteria, it does not induce protection against inflammation reactions (16). In this study, we demonstrate that a Th1-type immune response with gamma interferon (IFN-γ) production and an increased lymphoid reaction are characteristic of reinfection. The results suggest that strong Th1-type CMI and IFN-γ production may play an important role in both the clearance and pathogenesis of C. pneumoniae infection in mice.
MATERIALS AND METHODS
Mice.
Female inbred BALB/c mice were obtained from the Laboratory Animal Centre, University of Helsinki, Helsinki, Finland. The mice were given food and water ad libitum and were kept in ventilated containers (Scantainer; Scanbur A/S, Køge, Denmark). This study was approved by the institutional animal care and use committee, which acts under the provincial board.
Chlamydia preparation.
C. pneumoniae isolate Kajaani 6 (7) was obtained from P. Saikku, National Public Health Institute, Oulu, Finland. It was propagated in HL (human cell line) cells; infected cells were harvested with sterile glass beads and ultrasonically disrupted. Cell-grown organisms were partially purified by one cycle of low-speed centrifugation (500 × g, 10 min) and stored aliquoted in sucrose-phosphate-glutamate solution (SPG) at −70°C. The disrupted HL cells, used as a control, did not induce any lymphoid reaction. For in vitro assays, the organism was purified by Nycodens (Nycomed Pharma AS, Oslo, Norway) gradient separation and inactivated with formalin (0.5%, 20 min); the protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.) (1 μg corresponded to approximately 106 inclusion-forming units [IFU]).
Experimental infection and culture of C. pneumoniae from the lungs.
The infection model was essentially the same as that developed by Kaukoranta-Tolvanen et al. (15). The 6- to 8-week-old mice were inoculated intranasally with 106 IFU of C. pneumoniae in 40 μl of SPG under light carbon dioxide anesthesia. The same dose was given in the same way as rechallenge 4 to 8 weeks after the primary challenge. At predetermined days after inoculation, mice were sacrificed by using carbon dioxide, and the lungs were dissected and mechanically homogenized (Stomacher 80; Seward Medical Limited, London, United Kingdom). The lung supernatants were cultured in several dilutions on Vero cell monolayers that had been grown on coverslips in 24-well plates (Grainer, Frickenhausen, Germany). The plates were centrifuged (500 × g, 1 h), and the cell monolayers were incubated for 48 h in Dulbecco modified Eagle medium (National Public Health Institute, Helsinki, Finland) containing 5% fetal calf serum (Integro bv, Zaandam, The Netherlands), l-glutamine (0.3 mg/ml; Fluka, Buchs, Switzerland), streptomycin (25 μg/ml; Sigma, St. Louis, Mo.), and cycloheximide (0.5 μg/ml; Sigma) in a 35°C, 5% CO2-saturated, humidified incubator. After incubation, the cells were fixed with methanol (Riedel-de Haen, Sleeze, Germany) and stained with fluorescein isothiocyanate-conjugated Chlamydia-specific antibodies (Kallestad, Chaska, Minn.). Intracellular inclusions were counted under a UV microscope. The results are expressed as logarithmic values of IFU/lung. After the dilution factors were taken into account, one inclusion seen by microscopy corresponded to a logarithmic value of 1.3 IFU/lung (the detection limit). If no inclusions were detected, an arbitrary value of half of log10 was used for calculating means and statistics.
Histopathological evaluation.
In one set of primary infection and reinfection experiments, the left lung was fixed in 10% buffered formalin, cut transversely at equidistance into three parts representing the cranial, central, and caudal parts of the left lung lobe, processed routinely, and stained with hematoxylin and eosin. The sections were examined under light microscopy. The whole area of each of the three cross sections was evaluated. The magnitude of perivascular and peribronchial lymphocyte and plasma cell infiltration and nodular hyperplasia, referred to here as the lymphoid reaction, was assigned an arbitrary score of 0, 1, 2, 3, or 4, corresponding to minimal, mild, moderate, marked, or severe, respectively. A nearly diffuse perivascular and peribronchiolar lymphoid reaction with thick cuffs affecting all three sections was scored as severe and a multifocal scattered change with thin cuffs was considered mild. Detailed description of the scoring system will be published separately (1).
Isolation of pulmonary mononuclear cells.
In one set of primary infection and reinfection experiments, one half of a lung or a whole lung was mechanically homogenized by using a plunger and a metal grid, and the tissue debris was passed through a 70-μm-pore-size filter (Becton Dickinson, Franklin Lakes, N.J.). The erythrocytes were lysed with a short hypotonic shock with H2O, and mononuclear cells were counted under a light microscope. The cells were suspended in complete growth medium containing RPMI 1640 (Sigma), 10% fetal calf serum, 10 mM HEPES (Sigma), l-glutamine (0.3 mg/ml; Gibco BRL, Life Technologies Ltd., Paisley, Scotland, United Kingdom), penicillin (10 U/ml; Sigma), streptomycin (10 μg/ml; Sigma), and 50 μM 2-mercaptoethanol (Sigma).
Lymphoproliferation assay.
Freshly isolated pulmonary mononuclear cells were plated into round-bottom 96-well plates at 0.2 × 106 cells per well. Formalin-inactivated C. pneumoniae was added at 1 μg of protein/ml, and the final volume was adjusted to 200 μl with complete growth medium. Control wells received medium alone (negative control) or 5 μg of concanavalin A (ConA; Sigma) per ml (positive control). The proliferative response was measured by incorporation of 1 μCi of 3H-labeled thymidine (Amersham, Aylesbury, United Kingdom) per well over the last 16 to 20 h of a 2-day culture period at 37°C in a 5% CO2 atmosphere. The proliferation index was calculated as (C. pneumoniae-induced proliferation − background proliferation)/background proliferation.
Cytokine EIA.
Freshly isolated pulmonary mononuclear cells were plated into 24-well plates (Grainer) at 2 × 106 cells per well. Formalin-inactivated C. pneumoniae was added at 1 μg of protein/ml, and the final volume was adjusted to 1 ml with complete growth medium. Control wells received medium alone (negative control) or 5 μg of ConA per ml (positive control). The cells were incubated at 37°C in a 5% CO2 atmosphere for 72 h, after which the supernatants were collected, frozen, and later analyzed for IFN-γ, interleukin 10 (IL-10), and IL-4 by enzyme-linked immunosorbent assay (EIA).
In EIA, 1 μg of anti-mouse IFN-γ (R4-6A2, rat immunoglobulin G (IgG); PharMingen, San Diego, Calif.) per ml, 5 μg of anti-mouse IL-10 (JES-5A2, rat IgG; a generous gift from R. L. Coffman, DNAX Research Institute, Palo Alto, Calif.) per ml, or 2 μg of anti-mouse IL-4 (BVD4-1D11, rat IgG; PharMingen) per ml was used as first antibody in round-bottom 96-well plates (Labsystems, Helsinki, Finland). The samples were plated in semilogarithmic dilutions and incubated overnight at 4°C. Biotinylated anti-mouse IFN-γ (XMG1.2, rat IgG; PharMingen) at 2 μg/ml, anti-mouse IL-10 (SXC-1, rat IgM; PharMingen) at 2 μg/ml, or anti-mouse IL-4 (BVD6-24G2, rat IgG; PharMingen) at 1 μg/ml was used as second antibody. ConA-induced cell culture supernatant of a Th1-type T-cell line that had been previously standardized against recombinant IFN-γ (a generous gift from R. L. Coffman), recombinant mouse IL-10 (PharMingen), and recombinant mouse IL-4 (C-236; a generous gift from R. L. Coffman) were used as standards. Detection was done with streptavidin peroxidase (Zymed Laboratories Inc., South San Francisco, Calif.) and o-phenylediamine dihydrochloride (Sigma).
The sensitivities of the cytokine EIAs, in our hands, were typically 0.5 ng/ml for IFN-γ, 0.2 ng/ml for IL-10, and 0.07 ng/ml for IL-4. The results are expressed as C. pneumoniae-induced cytokine secretion after subtraction of the background secretion.
Flow cytometric analysis.
Freshly isolated pulmonary mononuclear cells (0.4 × 106 for each test) were stained with 5 μl of each antibody: phycoerythrin-conjugated anti-rat IgG2b (for controlling of nonspecific binding) (Caltag, South San Francisco, Calif.), anti-CD4 (YTS 191.1; Caltag), anti-B220 (RA3-6B2; Caltag), and fluorescein isothiocyanate-conjugated anti-CD8 (α-chain specific, CT-CD8a; Caltag). After 30 min of incubation, the cells were washed with phosphate-buffered saline and fixed with 1% paraformaldehyde (Sigma). Unstained cells were used for adjustment of FACScan (Becton Dickinson, San Jose, Calif.); gating of lymphocytes was done by size. Data were typically collected from 10,000 gated events.
Statistical methods.
The culture results were calculated as arithmetic means from logarithmic values. If no inclusions were seen by microscope, an arbitrary value of 0.33 inclusion was used. All statistical significances of the differences were assessed by a nonparametric Mann-Whitney U test.
RESULTS
Infection kinetics and histopathology in the lungs.
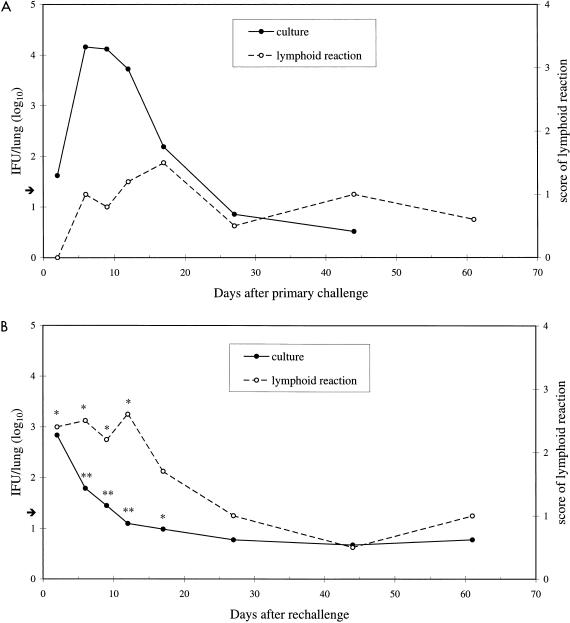
An intranasal challenge of 106 IFU of C. pneumoniae isolate Kajaani 6 was used in four independent sets of experimental primary infections and in three sets of reinfections. The primary challenge resulted in a self-restricted infection, and the number of bacteria recovered from the lungs peaked during the first 2 weeks at approximately 104 IFU per mouse (Fig. 1A). Thereafter, the number of bacteria gradually decreased, and by day 27 practically none could be cultivated from the lungs. Significant protection against C. pneumoniae was seen when the mice were reinfected with the same dose 4 to 8 weeks after the primary challenge (Fig. 1B). The numbers of bacteria recovered from the lungs were approximately 100-fold less than in primary infection at each time point from 6 to 17 days, and the reinfection was restricted in less than 2 weeks. On the second day after reinfection, the number of bacteria was higher than that after primary infection; however, the difference was not statistically significant (P = 0.06).
FIG. 1.
C. pneumoniae was cultured from the supernatants of homogenized lung samples obtained at several time points after intranasal primary challenge (A) and rechallenge (B) (given 4 to 8 weeks after primary challenge) of 106 IFU per mouse. The means were calculated from logarithmic data from four individual primary infection experiments and three individual reinfection experiments, with 4 to 10 mice per time point in each experiment. The arrow shows the detection limit for culture (1.3 IFU/lung). The magnitude of the pulmonary lymphoid reaction, evaluated by light microscopy from formalin-fixed lungs from one set of primary infection and reinfection experiments with four to five mice per time point, was scored as 0 (minimal), 1 (mild), 2 (moderate), 3 (marked), or 4 (severe). The asterisks in panel B indicate values that differ significantly between primary infection and reinfection (∗, P < 0.01; ∗∗, P < 0.001).
The magnitude of the lymphoid reaction was assessed by light microscopy from one set of primary infection and reinfection experiments. A mild peribronchial and perivascular lymphoid infiltration and nodular hyperplasia with a mean score of 0.8 characterized the primary infection in the lungs (Fig. 1A). Reinfection resulted in a significant increase in the magnitude of the lymphoid reaction (maximum score of 2.6) seen on each of days 2 to 12 after rechallenge (Fig. 1B).
Lymphocyte types at the site of infection.
The mean number of mononuclear cells isolated from lungs of uninfected mice was 1.9 × 106/mouse. It increased on average threefold after primary challenge with C. pneumoniae and fivefold after rechallenge.
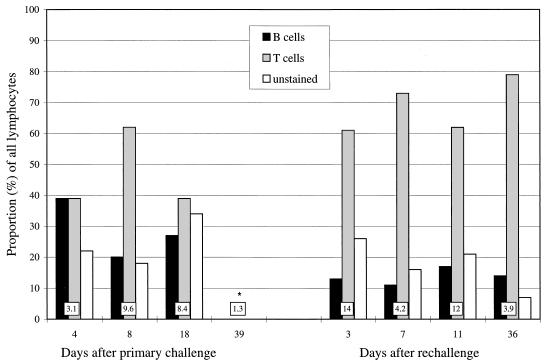
The cells isolated from uninfected mice consisted of 17% B cells (B220+), 73% T cells (CD4+ and/or CD8+), and 10% unstained cells (B220−, CD4−, and CD8−). During primary infection, the proportion of B cells increased to an average of 29% of lymphocytes (Fig. 2). During reinfection, the proportions of all cell types were similar to those in uninfected mice. The difference between the proportion of B cells during primary infection (days 4 to 18) and reinfection (days 3 to 36) was significant (P < 0.05). However, because of the larger total number of cells during reinfection, results can also be described by saying that the numbers of B cells remained the same as during primary infection but the number of T cells increased dramatically. In addition to CD4+ and CD8+ single-positive cells, a new CD4+ CD8+ double-positive cell population appeared among the pulmonary lymphocytes (ranges of 0 to 10% after primary challenge and 4 to 20% after rechallenge); these cells are characterized in more detail in reference 27. The proportions of CD4+ (range of 69 to 79% of all T cells during primary infection and 49 to 71% during reinfection), CD8+ (ranges of 15 to 26% and 21 to 25%, respectively), and CD4+ CD8+ cells (ranges of 0 to 16% and 5 to 28%, respectively) were not significantly different between primary infection and reinfection.
FIG. 2.
Proportions of B cells (B220+), T cells (CD4+ and/or CD8+), and unstained cells (CD4−, CD8−, and B220−) of all lung-derived lymphocytes isolated from C. pneumoniae-infected mice were assessed by flow cytometric analysis. All data are from one primary infection and reinfection experiment where the reinfection was given 49 days after primary infection to BALB/c mice, and lungs of four to five mice were pooled at each time point. The total number of mononuclear cells (106) isolated per lung at each time point is shown in a box at the base of each bar. As a comparison, approximately 1.9 × 106 mononuclear cells per lung were isolated from uninfected mice, and the proportions of B, T, and unstained cells of pulmonary lymphocytes (not shown) were 17, 73, and 10%, respectively. ∗, no data on proportions of different lymphocytes at this time point.
In vitro restimulation of lung-derived cells.
Mononuclear cells isolated from the lungs were restimulated in vitro with inactivated C. pneumoniae, and the capacity of the cells to respond either by proliferation or by secretion of cytokines (IFN-γ, IL-10, and IL-4) was evaluated.
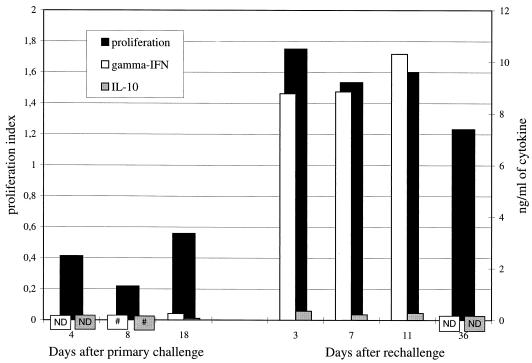
The mononuclear cells isolated from uninfected BALB/c mice did not respond by proliferation when stimulated with C. pneumoniae. At the peak of the primary infection, only a close-to-background proliferative response to C. pneumoniae was detected (mean proliferation index of 0.4), although the mitogen ConA induced abundant proliferation (mean index of 82.4). However, during reinfection, the in vitro C. pneumoniae-stimulated proliferative response increased approximately fourfold above the response during primary infection, to a mean proliferation index of 1.6 (P < 0.05), and remained elevated even 5 weeks after rechallenge (Fig. 3).
FIG. 3.
Proliferation induced by C. pneumoniae (formalin-inactivated antigen) in a 2-day culture of lung-derived pulmonary mononuclear cells was detected by incorporation of [3H]thymidine. The results are shown as proliferation indices [(C. pneumoniae-induced proliferation − background proliferation)/background proliferation], calculated from the arithmetic means of triplicate measurements. Secretion of IFN-γ and IL-10 was determined by EIA from the culture supernatants of the cells, 72 h after in vitro stimulation with C. pneumoniae. The results are shown as C. pneumoniae-induced cytokine secretion after subtraction of background secretion. Data are from the same experiment as that represented in Fig. 2. As a comparison, similarly measured proliferation index of cells of uninfected mice was −0.5, secretion of IFN-γ was 0.03 ng/ml, and secretion of IL-10 was zero. #, zero; ND, not determined.
Like the proliferative response, secretion of both IFN-γ and IL-10 in response to the inactivated C. pneumoniae was negligible during primary infection (Fig. 3). The cells were, however, capable of secreting both IFN-γ and IL-10, since ConA induced the secretion of an average of 52.4 ng of IFN-γ per ml and 1.1 ng of IL-10 per ml. After rechallenge, secretion of IFN-γ increased to a mean level of 9.3 ng/ml, while the concentration of IL-10 remained low (mean of 0.3 ng/ml) (Fig. 3). As a comparison, lung-derived cells from uninfected BALB/c mice when stimulated with C. pneumoniae secreted 0.03 ng of IFN-γ per ml and no IL-10.
In addition to IFN-γ and IL-10 secretion, C. pneumoniae-induced secretion of IL-4 was determined; none (<0.07 ng/ml) was detected in the culture media of cells isolated from either the infected or the uninfected mice at any of the studied time points. Consistent with this observation, histopathological evaluation revealed no marked eosinophilia in the lungs.
DISCUSSION
Significant protection was induced in the BALB/c mouse model by C. pneumoniae infection, since after rechallenge fewer bacteria could be cultivated from the lungs of the infected mice than after primary challenge. These results are similar to those of a previously published study of C. pneumoniae infection in a different mouse strain (outbred NIH/S) (16). In the present study, there was also a significantly increased lymphoid reaction in the lungs after rechallenge. Overall, a lymphoid reaction remained detectable up to 61 days after primary challenge and rechallenge. The phenomenon of more severe and longer-lasting pathology after repeated infections is well established in C. trachomatis infection models. Although fewer bacteria can be cultivated, the severity of, for example, salpingitis caused by C. trachomatis is more profound after reinfection than after primary infection in mice (31). Restimulation of immune cells by reinfection with C. pneumoniae has been postulated to exacerbate inflammation in atheromatous lesions.
In the present study, we show that reinfection by C. pneumoniae in mice significantly increases the local lymphoid reaction in the lungs. A similar phenomenon can be detected with lower doses of C. pneumoniae (1). The possible involvement of killed C. pneumoniae in the observed lymphoid reaction cannot be completely ruled out. However, we believe that this reaction could not have been induced mainly by noninfectious C. pneumoniae, since it has been shown that intranasally administered inactivated C. pneumoniae is quickly (within minutes) eliminated from the lungs (22).
Consistent with the magnitude of the elevated lymphoid reaction, almost twice as many mononuclear cells could be isolated from the lungs of mice during reinfection than during primary infection. Furthermore, the immunity induced by primary infection included a C. pneumoniae-specific proliferative response as well as a strongly Th1-biased cytokine profile (predominantly IFN-γ). Based mostly on studies of C. trachomatis, it has been hypothesized that IFN-γ has a dual role in chlamydial infections in vivo, associated both with the pathogenesis and the clearance of the bacteria (33). For example, a local Th1-type cytokine response has been detected after repeated experimental C. trachomatis infections that finally lead to tissue damage (25, 32). Harmful effects can also be seen at a distance from the infection site; Chlamydia-specific IFN-γ secretion of synovial T-cell clones in chlamydial arthritis has been reported (29). Among the beneficial effects, IFN-γ has been shown to be important for the elimination of cultivable bacteria in many in vivo C. trachomatis infection models using in vivo depletion, genetic deletion, or administration of IFN-γ (4, 13, 36).
In vitro experiments indicate that the local concentration of IFN-γ in vivo may be decisive for the outcome of the infection: in vitro high concentrations of IFN-γ can completely inhibit the growth of C. trachomatis, while lower concentrations may convert the infection into a latent/chronic state. The latent infection is characterized by noninfectious aberrant forms of the bacteria with continuous production of a putative inflammation-inducing antigen, Hsp60 (2, 23, 24, 26). In vivo, a latent/prolonged infection may sustain inflammation that is exacerbated by repeated infections. There is some evidence of latent infection in vivo in mice, in which reactivation of Chlamydia from a culture-negative state has been accomplished by immune suppression (5, 18, 20, 35).
The cell type(s) that secretes IFN-γ cannot be identified with the experimental design used in this study. In addition to conventional T cells, γδ and NK cells are known to be able to secrete IFN-γ in response to infections, and they are thought to have an important role especially in innate immunity (1a, 28, 34). In addition, macrophages have been reported to secrete IFN-γ in response to, e.g., lipopolysaccharide stimulation (10), a possibility that cannot be dismissed when one is dealing with gram-negative bacteria such as chlamydiae. Moreover, in a recent study, in vitro infection of human alveolar macrophages with Mycobacterium tuberculosis resulted in release of IFN-γ (8). In vivo depletion studies of different cell types might be a way to identify the cells that secrete IFN-γ during C. pneumoniae infection in the mouse model.
The mild lymphoid reaction during primary infection and apparent unresponsiveness of pulmonary cells to C. pneumoniae antigen are also interesting. It has been recently suggested that a new kind of dichotomy of T cells (IFN-γ producing versus transforming growth factor β producing) would regulate mucosal inflammatory reactions, with the former being proinflammatory and the latter anti-inflammatory (30). In our model, putative transforming growth factor β responses (a suppressor cytokine) during primary infection could explain the unresponsiveness detected in the in vitro assays and also the mild lymphoid reactions in vivo. The local unresponsiveness or slower response of the BALB/c mice during primary infection was different from the findings for infection in C57BL/6 or NIH/S outbred mice, which both responded by secreting IFN-γ after primary challenge with C. pneumoniae (27a). In BALB/c mice, a similar response was seen only after rechallenge, when the acquired immunity was dominated by a Th1-type response with significant protection on one hand but increased local lymphoid reaction on the other.
ACKNOWLEDGMENTS
This work was partially supported by Academy of Finland (grant 8400) and contract BIO4-CT96-0152 of the Biotechnology Programme of the Commission of the European Union.
We thank R. L. Coffman, DNAX Research Institute, Palo Alto, Calif., for providing reagents for cytokine EIAs. We are grateful for the skillful technical assistance of Irene Viinikangas, Outi Rautio, Carola Andersson-Parkkonen, and Maijastiina Voutilainen.
REFERENCES
- 1. Anttila, M., J. M. Penttilä, N. Rautonen, M. Puolakkainen, and P. H. Mäkelä. Pulmonary pathology of experimental C. pneumoniae infection in two mouse strains. Submitted for publication.
- 1a.Bancroft G J, Schreiber D, Unanue E R. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 2.Beatty W L, Byrne G, Morrison R P. Morphologic and antigenic characterization of interferon γ-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Byrne G I, Morrison R P. Repeated and persistent infection with Chlamydia and the development of chronic inflammation and disease. Trends Microbiol. 1994;2:94–98. doi: 10.1016/0966-842x(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 4.Byrne G I, Grubbs B, Dickey T J, Schachter J, Williams D M. Interferon in recovery from pneumonia due to Chlamydia trachomatis in the mouse. J Infect Dis. 1987;156:993–996. doi: 10.1093/infdis/156.6.993. [DOI] [PubMed] [Google Scholar]
- 5.Cotter T W, Miranpuri G S, Ramsey K H, Poulsen C E, Byrne G I. Reactivation of chlamydial genital tract infection in mice. Infect Immun. 1997;65:2067–2073. doi: 10.1128/iai.65.6.2067-2073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danesh J, Collins R, Peto R. Chronic infections and coronary disease: is there a link? Lancet. 1997;350:430–436. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 7.Ekman M-R, Grayston J T, Visakorpi R, Kleemola M, Kuo C-C, Saikku P. An epidemic of infections due to Chlamydia pneumoniae in military conscripts. Clin Infect Dis. 1993;17:420–425. doi: 10.1093/clinids/17.3.420. [DOI] [PubMed] [Google Scholar]
- 8.Fenton M J, Vermeulen M W, Kim S, Burdick M, Strieter R M, Kornfeld H. Induction of gamma interferon production in human alveolar macrophages by Mycobacterium tuberculosis. Infect Immun. 1997;65:5149–5156. doi: 10.1128/iai.65.12.5149-5156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong I W, Chiu B, Viira E, Fong M F, Jang D, Mahony J. Rabbit model for Chlamydia pneumoniae infection. J Clin Microbiol. 1997;35:48–52. doi: 10.1128/jcm.35.1.48-52.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fultz M J, Barber S A, Dieffenbach C W, Vogel S N. Induction of IFN-γ in macrophages by lipopolysaccharide. Int Immunol. 1993;5:1383–1392. doi: 10.1093/intimm/5.11.1383. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Leatham E W, Carrington D, Mendall M A, Kaski J C, Camm A J. Elevated Chlamydia pneumoniae antibodies, cardiovascular events, and azithromycin in male survivors of myocardial infarction. Circulation. 1997;96:404–407. doi: 10.1161/01.cir.96.2.404. [DOI] [PubMed] [Google Scholar]
- 12.Gurfinkel E, Bozovich G, Daroca A, Beck E, Mautner B for the ROXIS Study Group. Randomized trial of roxithromycin in non-Q-wave coronary syndromes: ROXIS pilot study. Lancet. 1997;350:404–407. doi: 10.1016/s0140-6736(97)07201-2. [DOI] [PubMed] [Google Scholar]
- 13.Johansson M, Schön K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 15.Kaukoranta-Tolvanen S-S, Laurila A L, Saikku P, Leinonen M, Liesirova L, Laitinen K. Experimental infection of Chlamydia pneumoniae in mice. Microb Pathog. 1993;15:293–302. doi: 10.1006/mpat.1993.1079. [DOI] [PubMed] [Google Scholar]
- 16.Kaukoranta-Tolvanen S-S E, Laurila A L, Saikku P, Leinonen M, Laitinen K. Experimental Chlamydia pneumoniae infection in mice: effect of reinfection and passive immunization. Microb Pathog. 1995;18:279–288. doi: 10.1016/s0882-4010(05)80004-x. [DOI] [PubMed] [Google Scholar]
- 17.Kuo C-C, Jackson L A, Campbell L A, Grayston J T. Chlamydia pneumoniae (TWAR) Clin Microbiol Rev. 1995;8:451–461. doi: 10.1128/cmr.8.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laitinen K, Laurila A L, Leinonen M, Saikku P. Reactivation of Chlamydia pneumoniae infection in mice by cortisone treatment. Infect Immun. 1996;64:1488–1490. doi: 10.1128/iai.64.4.1488-1490.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laitinen K, Laurila A, Pyhälä L, Leinonen M, Saikku P. Chlamydia pneumoniae infection induces inflammatory changes in the aortas of rabbits. Infect Immun. 1997;65:4832–4835. doi: 10.1128/iai.65.11.4832-4835.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malinverni R, Kuo C-C, Campbell L A, Grayston J T. Reactivation of Chlamydia pneumoniae lung infection in mice by cortisone. J Infect Dis. 1995;172:593–594. doi: 10.1093/infdis/172.2.593. [DOI] [PubMed] [Google Scholar]
- 21.Moazed T C, Kuo C-C, Grayston J T, Campbell L A. Murine models of Chlamydia pneumoniae infection and atherosclerosis. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 22.Moazed T C, Kuo C-C, Grayston J T, Campbell L A. Evidence of systemic dissemination of Chlamydia pneumoniae via macrophages in the mouse. J Infect Dis. 1998;177:1322–1325. doi: 10.1086/515280. [DOI] [PubMed] [Google Scholar]
- 23.Morrison R P, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989a;169:663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrison R P, Belland R J, Lyng K, Caldwell H D. Chlamydial disease pathogenesis: the 57 kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989b;170:1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patton D L, Kuo C-C, Wang S-P, Halbert S A. Distal tubal obstruction induced by repeated Chlamydia trachomatis salpingeal infections in pig-tailed macaques. J Infect Dis. 1987;155:1292–1299. doi: 10.1093/infdis/155.6.1292. [DOI] [PubMed] [Google Scholar]
- 26.Peeling R W, Bailey R L, Conway D J, Holland M J, Campbell A E, Jallow O, Whittle H C, Mabey D C W. Antibody response to the 60-kDa chlamydial heat-shock protein is associated with scarring trachoma. J Infect Dis. 1998;177:256–259. doi: 10.1086/517367. [DOI] [PubMed] [Google Scholar]
- 27.Penttilä J M, Pyhälä R, Sarvas M, Rautonen N. Expansion of a novel pulmonary CD3− CD4+ CD8+ cell population in mice during Chlamydia pneumoniae infection. Infect Immun. 1998;66:3290–3294. doi: 10.1128/iai.66.7.3290-3294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Penttilä, J. M., et al. Unpublished data.
- 28.Scott P, Kaufmann S H E. The role of T-cell subsets and cytokines in the regulation of infection. Immunol Today. 1991;12:346–348. doi: 10.1016/0167-5699(91)90063-Y. [DOI] [PubMed] [Google Scholar]
- 29.Simon A K, Seipelt E, Wu P, Wenzel B, Braun J, Sieper J. Analysis of cytokine profiles in synovial T cell clones from chlamydial reactive arthritis patients: predominance of the Th1 subset. Clin Exp Immunol. 1993;94:122–126. doi: 10.1111/j.1365-2249.1993.tb05988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strober W, Kelsall B, Fuss I, Marth T, Ludviksson B, Ehrhardt R, Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosal inflammation. Immunol Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- 31.Tuffrey M, Alexander F, Taylor-Robinson D. Severity of salpingitis in mice after primary and repeated inoculation with a human strain of Chlamydia trachomatis. J Exp Pathol. 1990;71:403–410. [PMC free article] [PubMed] [Google Scholar]
- 32.Van Voorhis W C, Barrett L K, Cosgrove Sweeney Y T, Kuo C-C, Patton D L. Repeated Chlamydia trachomatis infection of Macaca nemestrina fallopian tubes produces a Th1-like cytokine response associated with fibrosis and scarring. Infect Immun. 1997;65:2175–2182. doi: 10.1128/iai.65.6.2175-2182.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward M E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 34.Williams D M, Grubbs B G, Schachter J, Magee D M. Gamma interferon levels during Chlamydia trachomatis pneumonia in mice. Infect Immun. 1993;61:3556–3558. doi: 10.1128/iai.61.8.3556-3558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y-S, Kuo C-C, Chen W-J. Reactivation of Chlamydia trachomatis lung infection in mice by cortisone. Infect Immun. 1983;39:655–658. doi: 10.1128/iai.39.2.655-658.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong G, Peterson E M, Czarniecki C W, De La Maza L M. Recombinant murine gamma interferon inhibits Chlamydia trachomatis serovar L1 in vivo. Infect Immun. 1988;56:283–286. doi: 10.1128/iai.56.1.283-286.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]