Abstract
Nonautotrophic CO2 metabolism in Opuntia echinocarpa roots was studied with techniques of manometry and radiometry. The roots were grown in a one-quarter strength nutrient solution for several days; the distal 2 cm was used for physiological studies. The roots assimilated significant quantities of 14CO2 and appeared to show a crassulacean-type acid metabolism with respect to quality and quantity. Most of the 14C activity was associated with the distal portion of the elongating root indicating correlation with metabolic activity. The 14CO2 assimilation was comparable to a crassulacean leaf succulent, but 3 times greater than that found for stem tissue of the same Opuntia species.
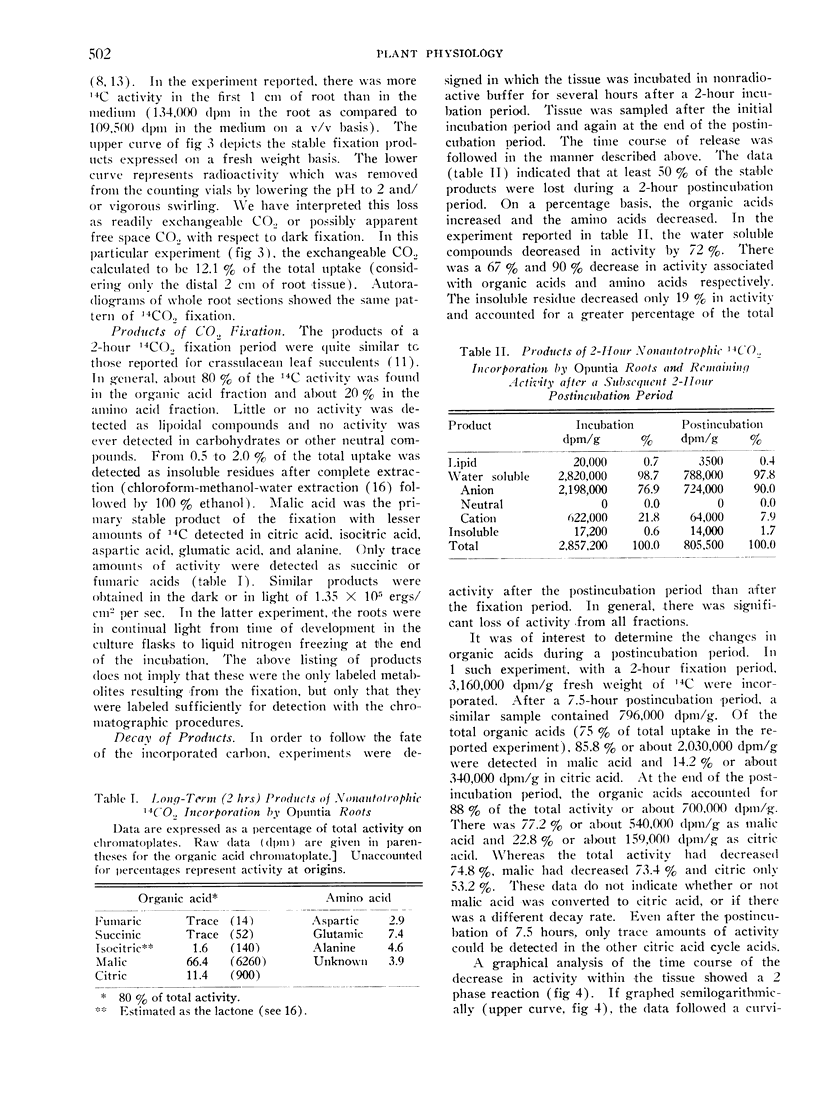
The rates of O2 and CO2 exchange and estimated CO2 fixation were 180, 123, and 57 μl/g per hour. A respiratory quotient of 0.66 was found.
The products of 14CO2 fixation were similar in most respects to reported experiments with leaf succulents. Equilibration of the predominant malic acid with isocitric, succinic, and fumaric acids was not evident. The latter observation was interpreted as metabolic isolation of the fixation products rather than poor citric acid cycle activity.
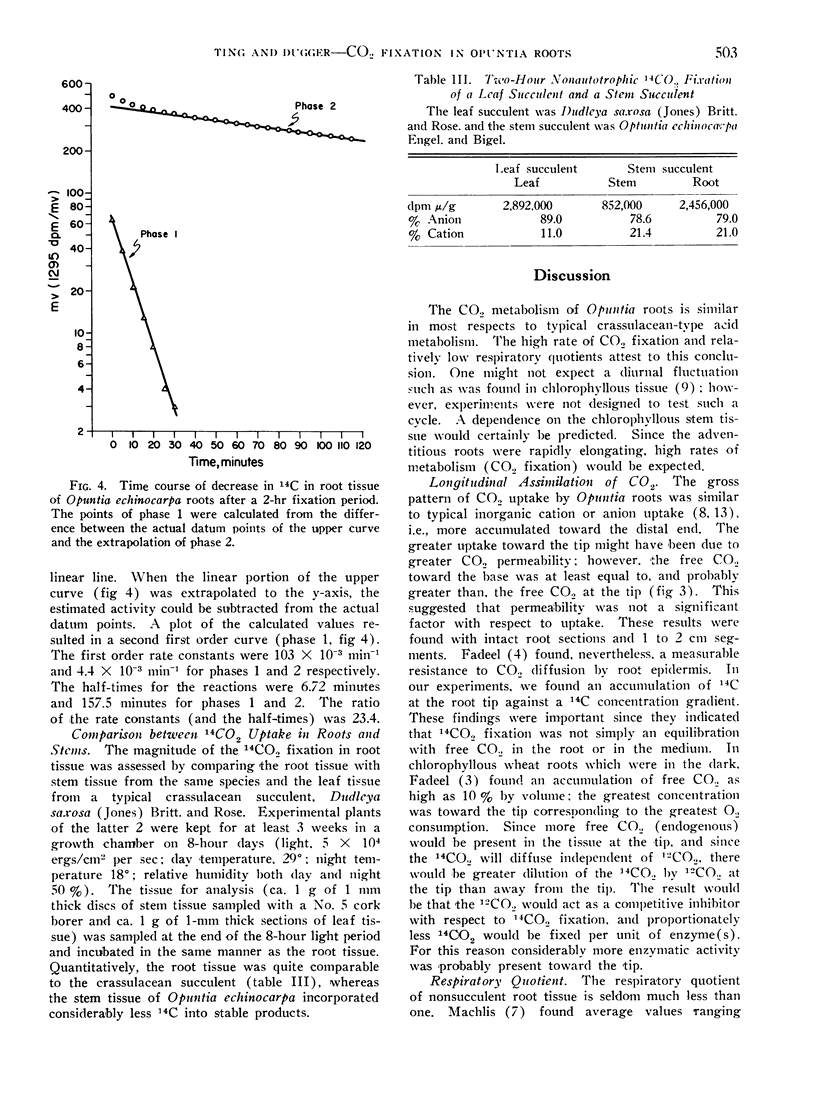
A rapid turnover of the fixed 14CO2 was measured by following decarboxlyation kinetics and by product analysis after a postincubation period. The first order rate constant for the steady state release was 4.4 × 10−3 min−1 with a half-time of 157.5 minutes. Amino acids decayed at a more rapid rate than organic acids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Carell E. F., Price C. A. Porphyrins and the iron requirement for chlorophyll formation in Euglena. Plant Physiol. 1965 Jan;40(1):1–7. doi: 10.1104/pp.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot P., Steward F. C. SALIENT FEATURES OF THE ROOT SYSTEM RELATIVE TO THE PROBLEM OF SALT ABSORPTION. Plant Physiol. 1936 Jul;11(3):509–534. doi: 10.1104/pp.11.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucher G. W., Vickery H. B., Abrahams M. D., Leavenworth C. S. STUDIES IN THE METABOLISM OF CRASSULACEAN PLANTS: DIURNAL VARIATION OF ORGANIC ACIDS AND STARCH IN EXCISED LEAVES OF BRYOPHYLLUM CALYCINUM. Plant Physiol. 1949 Oct;24(4):610–620. doi: 10.1104/pp.24.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltman P., Kunitake G., Spolter H., Stitts C. The Dark Fixation of CO(2) by Succulent Leaves: The First Products. Plant Physiol. 1956 Nov;31(6):464–468. doi: 10.1104/pp.31.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward F. C., Prevot P., Harrison J. A. ABSORPTION AND ACCUMULATION OF RUBIDIUM BROMIDE BY BARLEY PLANTS. LOCALIZATION IN THE ROOT OF CATION ACCUMULATION AND OF TRANSFER TO THE SHOOT. Plant Physiol. 1942 Jul;17(3):411–421. doi: 10.1104/pp.17.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz R. E., Burris R. H. PHOTOSYNTHESIS AND METABOLISM OF ORGANIC ACIDS IN HIGHER PLANTS. Plant Physiol. 1951 Apr;26(2):226–243. doi: 10.1104/pp.26.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Dugger W. M., Jr Separation and detection of organic acids on silica gel. Anal Biochem. 1965 Sep;12(3):571–578. doi: 10.1016/0003-2697(65)90224-1. [DOI] [PubMed] [Google Scholar]
- VARNER J. E., BURRELL R. C. Use of C14 in the study of the acid metabolism of Bryophyllum calycinum. Arch Biochem. 1950 Feb;25(2):280–287. [PubMed] [Google Scholar]



