Abstract
Prenatal exposures to chemical and psychosocial stressors can impact the developing brain, but few studies have examined their joint effects. We examined associations between prenatal phthalate exposures and child behavior, hypothesizing that prenatal stressful life events (PSLEs) may exacerbate risks. To do so, we harmonized data from three U.S. pregnancy cohorts comprising the ECHO-PATHWAYS consortium. Phthalate metabolites were measured in single mid-pregnancy urine samples. When children were ages 4–6 years, mothers completed the Child Behavior Checklist (CBCL), from which a Total Problems score was calculated. Mothers additionally provided recall on their exposure to 14 PSLEs during pregnancy. Primary models examined problem behaviors in relation to: (1) phthalate mixtures calculated through weighted quantile sums regression with permutation test-derived p-values; and (2) joint exposure to phthalate mixtures and PSLEs (counts) using interaction terms. We subsequently refitted models stratified by child sex. Secondarily, we fit linear and logistic regression models examining individual phthalate metabolites. In our main, fully adjusted models (n = 1536 mother–child dyads), we observed some evidence of weak main effects of phthalate mixtures on problem behaviors in the full cohort and stratified by child sex. Interaction models revealed unexpected relationships whereby greater gestational exposure to PSLEs predicted reduced associations between some phthalates (e.g., the metabolites of di-2-ethylhexyl phthalate, di-n-octyl phthalate, di-iso-nonyl phthalate) and problem behaviors, particularly in males. Few associations were observed in females. Additional research is needed to replicate results and examine potential mechanisms.
Keywords: Phthalates, Stressful life events, Child behavior, Child mental health, Pregnancy, Prenatal stress, EDCs
1. Introduction
Phthalates are synthetic chemicals that are found in personal care products, packaged foods, medical equipment, and consumer goods. Increasingly evidence has linked gestational phthalate exposure to adverse pregnancy outcomes including preterm birth and pregnancy complications such as pre-eclampsia and gestational diabetes (Bedell et al., 2021; Cantonwine et al., 2016; Ferguson et al., 2019b; James-Todd et al., 2016; Shaffer et al., 2019; Welch et al., 2022; Yan et al., 2022). Because phthalates can cross the placenta (and alter the development and function of the placenta itself), they also pose a risk to fetal development (Bräuner et al., 2022; Jensen et al., 2015; Seymore et al., 2022). Epidemiological studies have reported associations between prenatal phthalate exposure and a range of child outcomes including birth size and postnatal growth trajectories, reproductive development, and respiratory health, among others (Adgent et al., 2020; Bornehag et al., 2015; Ferguson et al., 2022; Shu et al., 2018; Swan et al., 2015; van den Dries et al., 2021b).
The literature on prenatal phthalate exposure and child behavior, in particular, has been both extensive and equivocal. Several recent reviews have evaluated the strength of the literature, generally noting inconsistent patterns of results across studies as well as considerable variation in methods (Ejaredar et al., 2015; Jankowska et al., 2021; Lee et al., 2018; Minatoya and Kishi, 2021; Radke et al., 2020). Notably, many studies on prenatal phthalate exposure and child behavior were based on relatively small samples (<400 participants) and may therefore have been underpowered, particularly to detect the sex-specific associations that often occur in relation to prenatal exposures to endocrine disruptors (Daniel et al., 2020; Doherty et al., 2017; England-Mason et al., 2020; Huang et al., 2019; Hyland et al., 2019; Kim et al., 2018; Li et al., 2020; Lien et al., 2015). The majority of studies, moreover, considered each phthalate metabolite individually, with only the most recent studies examining mixtures of phthalate metabolites that more closely approximate the impact of real-world simultaneous exposures to multiple phthalates (e.g., Daniel et al., 2020; Day et al., 2021; Guilbert et al., 2021; van den Dries et al., 2021a).
Perhaps the most noteworthy limitation of the current literature is the lack of consideration of the potentially powerful role of early life exposure to non-chemical stressors in programming children’s development, particularly mental health. Children born to mothers who experienced more stressful life events during pregnancy (PSLEs) have been found to exhibit more behavioral problems and fearfulness, as well as reduced self-regulation, and these changes can persist into adolescence (Bergman et al., 2007; Bush et al., 2017; Bush et al., 2023; MacKinnon et al., 2018; Noroña-Zhou et al., 2023; Tearne et al., 2015). Notably, chemical and non-chemical stressors often co-occur, operate through the same mechanistic pathways (e.g., oxidative stress, endocrine disruption, inflammation), and influence the same endpoints (e.g., growth, neurodevelopment, airway health) (Barrett and Padula 2019). As a result, it is increasingly apparent that non-chemical stressors (such as PSLEs) may interact with chemical exposures (such as phthalates) to impact child development. To that point, a small number of studies have demonstrated that prenatal exposures to phthalates and non-chemical stressors may interact to heighten risk of preterm birth (Eatman et al., 2023; Ferguson et al., 2019a), while other studies in this area have examined joint impacts on maternal hormones of pregnancy (Hlisníková et al., 2022) and children’s reproductive development (Arbuckle et al., 2019; Barrett et al., 2016). Despite the strong evidence that prenatal stressors affect children’s brain development and behavior, to our knowledge, no study to date has examined phthalate-stress interactions in the context of child behavioral outcomes.
The current analysis was therefore motivated by two aims. Our first aim was to conduct the largest study to date on prenatal phthalate exposure and child behavior based on a diverse U.S. sample comprised of participants from three cohorts in the ECHO PATHWAYS consortium, utilizing mixtures models and secondarily, individual metabolite models. Our second aim was to evaluate phthalate-stress interactions to assess whether exposure to PSLEs exacerbates the impact of phthalates on child behavior (if any). Across both aims, we additionally examined evidence of sex-specific associations.
2. Methods
2.1. Study population and overview
As part of the NIH’s Environmental Influences on Child Health Outcomes (ECHO) study, mother–child dyads from three prospective pregnancy cohorts (The Infant Development and Environment Study [TIDES], Conditions Affecting Neurocognitive Development and Learning in Early Childhood [CANDLE], Global Alliance to Prevent Prematurity and Stillbirth [GAPPS]) were invited to participate in the ECHO-PATHWAYS Consortium, a large, diverse, harmonized study of prenatal exposures and child outcomes (LeWinn et al., 2022). The current analysis includes ECHO-PATHWAYS participants from these cohorts who provided prenatal urine samples for phthalate metabolite analysis, completed a questionnaire on exposure to PSLEs that occurred during the index pregnancy, and completed the Child Behavior Checklist (CBCL) when the index child was age 4–6 years.
From 2010 to 2012, TIDES participants were recruited from four academic medical centers: the University of San Francisco, California (San Francisco, CA), the University of Rochester Medical Center (Rochester, NY), the University of Minnesota (Minneapolis, MN), and the University of Washington/Seattle Children’s Hospital (Seattle, WA) (Barrett et al., 2014). Participants were recruited in their first trimester based on the following eligibility criteria: less than 13 weeks pregnant, singleton pregnancy, English speaking, age 18 or over, no serious threat to the pregnancy, and planning to deliver at a study hospital. Participants completed study visits in each trimester including urine collection for phthalate analysis. In total, 749 women participated across pregnancy and gave birth in the study; 551 (74%) TIDES mother–child dyads participated in a study visit at age 6.
The CANDLE study, based at the University of Tennessee Health Science Center (Memphis, TN), recruited participants from 2006 to 2011 primarily through community and urban obstetric clinics (Sontag-Padilla et al., 2015). Participants were enrolled during the second trimester of pregnancy based on eligibility criteria including: age 16–40, a resident of Shelby County, TN, speaking English, having a low-risk, singleton pregnancy at the time of enrollment, and planning to deliver at a participating study hospital. CANDLE participants completed two prenatal study visits, corresponding roughly to the second and third trimesters, that included questionnaires and biospecimen collection (e. g., urine, blood). Overall, 1503 participants enrolled in CANDLE during pregnancy and of those, 1143 (76%) completed a study visit when the index children were age 4–6 years.
GAPPS was initially established as a biorepository to facilitate research on adverse birth outcomes (GAPPS, 2023). General eligibility criteria for GAPPS included: age 18 or older, English-speaking, and planning to deliver at the participating hospital where the participant enrolled. At prenatal recruitment, participants were given the option to be contacted about future research studies, such as the present one. For the purposes of the ECHO-PATHWAYS study, additional eligibility criteria included planned delivery at one of three participating hospitals (University of Washington [Seattle, WA], Swedish Hospital [Seattle, WA], or Yakima Valley Memorial Hospital [Yakima, WA], consent to contact for future research, at least one prenatal urine sample, pregnancy questionnaire data collected, and the index child being age 4–6 years at the time of follow-up. GAPPS dyads with children born from 2011 to 2015 were consented into ECHO-PATHWAYS and completed an age 4–6 visit (n = 439).
Study activities were approved by the Institutional Review Boards at all participating sites as well as the ECHO-PATHWAYS Coordinating Center at the University of Washington. Mothers provided informed consent for study participation on behalf of themselves and their children. Upon enrollment to ECHO-PATHWAYS, existing and prospective data were harmonized across sites and cohorts.
2.2. Phthalate metabolite measurement
Across all cohorts, during the second trimester of pregnancy, participants provided single spot urine samples in phthalate-free cups. Specific gravity (SpG) was measured at the time of collection using a handheld refractometer. Urine samples were subsequently stored at −80° until they were shipped to the Wadsworth laboratory at New York State Department of Health for phthalate metabolite analysis. Phthalate metabolite analysis consisted of enzymatic deconjugation followed by automated solid phase extraction and analysis using high-performance liquid chromatography (HPLC) interfaced with tandem mass spectrometry (MS/MS). For quality assurance, all assay runs included procedural and instrument blanks. A more detailed description of the analytic methods has been provided elsewhere (Asimakopoulos et al., 2016; Guo et al., 2014; Kannan et al., 2021; Rocha et al., 2017). Seventeen phthalate metabolites were included this analysis: phthalic acid (PA), monoethyl phthalate (MEP), monomethyl phthalate (MMP), monobutyl phthalate (MBP), monoisobutyl phthalate (MIBP), monobenzyl phthalate (MBZP), monohexyl phthalate (MHXP), monoheptyl phthalate (MHPP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-carboxymethylhexyl) phthalate (MCMHP), mono(3-carboxypropyl) phthalate (MCPP), monoisononyl phthalate (MINP), monocarboxyisooctyl phthalate (MCIOP), and monocarboxyisononyl phthalate (MCINP). Per convention, values below the LOD were imputed as LOD/<mi> (Hornung and Reed, 1989). Metabolite concentrations were adjusted for urinary specific gravity using the Boeninger equation: Pc = P[(SpGmedian-1)/(SpG-1)] where Pc represents the SpG-corrected value (in ng/mL), P is the observed concentration for the sample, SpGmedian is the median SpG for all samples, and SpG is the individual sample’s specific gravity (Boeniger et al., 1993).
2.3. Maternal Pregnancy Stressful Life Events (PSLEs)
PSLEs were reported retrospectively at the postnatal visit using an adapted 14-item version of the Centers for Disease Control (CDC)’s Pregnancy Risk Assessment Monitoring System (PRAMS) survey (Whitehead et al., 2003) (Supplemental Table 1). Participants reported whether each PSLE had occurred during the index pregnancy and the total number of affirmative scores was tallied to create a summary score ranging from 0 to 14. Retrospective measures of PSLEs have been found to be both valid and robust to bias (Ramos et al., 2020; Reuben et al., 2016).
2.4. Child Behavior Checklist (CBCL)
Child behavior was assessed at child ages 4–6 using the Child Behavior Checklist (CBCL), which asks caregivers to rate the frequency of child emotions and behaviors over the last two months (for children ages 1.5–5) or six months (for children ages 6–18). For each item, the caregiver responds as: “Not True (0)”, “Somewhat or Sometimes True (1)”, or “Very True or Often True (2)”. The preschool version (ages 1.5–5) and school-age version (ages 6–18) include 99 and 112 items, respectively (Achenbach, 2011). In our analysis, the types and counts of behaviors reported across the two forms were comparable, indicating the similar developmental stages of participating children across the narrow age span of 4–6 years, and the measure was harmonized across both form types as has been done previously (Bush et al., 2023). Our outcome measure was the raw Total Problems score, calculated as the sum of all maternal-reported behaviors related to aggression, inattention, depression, anxiety, and hyperactivity, among others. In our primary analyses, we modeled this sum continuously, with higher scores indicating more problem behaviors. There were three reasons to focus on this single overall metric of problem behavior: we reduced the number of statistical tests; conceptually it is aligned with evidence of co-occurrence of internalizing and externalizing symptoms in young children (Gilliom and Shaw 2004; Ip et al., 2019; Willner et al., 2016); and it has a high reliability at this age (Rescorla et al., 2011). Additionally, we examined the scores dichotomously with cutoffs based on form-specific normalized total problem scores at or above borderline clinical (84th percentile) and clinical (90th percentile) thresholds.
2.5. Covariates
Covariate data were obtained through maternal report at each timepoint as well as through medical record abstraction. Maternal-reported data collected during pregnancy included pre-pregnancy body mass index (BMI), highest level of education, gravidity, household size, and smoking during pregnancy. Data on pregnancy complications (gestational diabetes, gestational hypertension/pre-eclampsia), child sex, year of birth, gestational age at birth, and birth weight were abstracted from the medical record by trained staff. Mothers additionally reported the race and ethnicity of their children. At the time of the age 4–6 visit, mothers reported their breastfeeding history related to the index child and child’s current secondhand smoke exposure. At that visit, mothers completed the PROMIS depression scale, from which continuous T-scores were derived, and household income, which was then adjusted for household size, geographic region, and inflation rates.
2.6. Statistical analysis
We calculated descriptive statistics (geometric mean, standard deviation, median, min, max, % <LOD, percentages, frequency, missingness) for the full study population as well as by child sex and by cohort. In our first aim, we examined associations between phthalate metabolites and CBCL Total Problems scores. In our second aim, we focused on the interaction between phthalate exposures and PSLEs. Across both aims, our main models considered Total Problems scores continuously, and secondarily, we examined those scores dichotomized at the 84% and 90% percentiles, corresponding to borderline and clinically relevant Total Problems, respectively. Our primary models used weighted quantile sums (WQS) regression to examine phthalate metabolite mixtures and secondarily, we fitted linear and logistic regression models to examine exposures to individual phthalate metabolites. Across all models, we considered the metabolites of each parent compound separately, rather than as a sum (i.e., MEHP, MEOHP, MEHHP, MECHPP, MCMHP, rather than ΣDEHP), in light of the evidence of their varying toxicity (Janer et al., 2008).
Informed by prior literature, we adopted a staged approach to all our linear models, fitting three sets of multivariable models for each aim: minimally adjusted, fully adjusted, and extended. Minimally adjusted models included a set of basic analytic covariates including urine specific gravity, data collection site (Memphis [CANDLE]; San Francisco [TIDES]; Minneapolis [TIDES]; Rochester [TIDES]; Seattle-TIDES; Seattle-GAPPS; Yakima [GAPPS]), phthalate batch, and CBCL form type used (pre-school [ages 1.5–5 years] or school age [ages 6–18 years]). Urine specific gravity was included as a covariate in the secondary linear and logistic regressions, whereas in the primary WQS regressions, specific gravity was not adjusted as a covariate. Instead, phthalates were adjusted for specific gravity using the Levine-Fahy equation and then log-transformed prior to inclusion in the WQS regression exposure mixture (Levine and Fahy, 1945). We then fitted our primary, fully adjusted models which included all covariates from the minimally adjusted model as well as maternal age (continuous), smoking during pregnancy (binary: non-smoker, smoker), gravidity (counts), pre-pregnancy body mass index (continuous), maternal education (ordinal: less than high school, high school diploma or GED, vocational school, college degree, graduate degree), child’s year of birth (categorical: 2007–2015), child sex (binary: male or female), child’s age at outcome study visit (continuous), and log-transformed family income (adjusted for household size, region of the country, and inflation; continuous). We additionally included child race (categorical: White, Black/African American, Asian, Native Hawaiian/Other Pacific Islander, American Indian/Alaska Native, other, multiple races), and ethnicity (binary: Hispanic/Latinx or not Hispanic/Latinx) in primary models as proxies for systemic racism that may be associated with differential exposure to environmental hazards and rates of child mental health and behavior problems. Finally, in extended models we included all covariates from prior models as well as a set of covariates potentially on the pathway between exposure and outcome: gestational diabetes (binary), gestational hypertension (binary), gestational age at birth (continuous), birthweight (continuous), breastfeeding history (binary: any or none), postnatal secondhand smoke exposure (binary: any or none), and maternal self-reported depression at the time of the child visit (PROMISE T Score; continuous).
To model the joint effect of all phthalate metabolites on problem behaviors and to examine their relative contributions to that mixture, we used WQS regression as our primary approach (Carrico et al., 2015). In WQS regression, a weighted quantile sum (WQS) index representing the exposure mixture is calculated as the sum of quantile-transformed exposures each multiplied by a model-derived weight. That WQS index is then used as a predictor in a generalized linear model. We evaluated WQS separately in the positive and negative directions, thereby independently estimating the mixture association and weights contributing to an effect in each direction. We utilized linear and logistic forms of the WQS regression to associate decile-transformed, specific gravity-adjusted phthalate metabolites with continuous CBCL total score and secondarily, dichotomous indicators for CBCL total scores exceeding the 84th or 90th percentiles, respectively. For each WQS regression, we used 1000 bootstraps to derive stable weight estimates (Carrico et al., 2015). To avoid the substantial loss of power that is common to WQS analyses that rely on using separate training and validation data sets, we used the entire dataset for both training and validation steps. We used “HC0” Huber-White heteroskedasticity-consistent standard error sandwich estimators to calculate full sample 95% confidence intervals (CIs) (White, 1980). Because these full sample standard errors for the WQS index coefficient are likely biased in an anticonservative direction (Borovicka et al., 2012), we used a novel permutation test to estimate accurate WQS index coefficient p-values (Curtin et al., 2021; Day et al., 2022). We only applied permutation tests to the models in which the full sample 95% confidence intervals did not include the null, as the permutation test p-values provide a more conservative and better calibrated assessment of statistical significance than full sample 95% confidence intervals. We note that the permutation test only provides more accurate p-values and does not influence the WQS index coefficient point estimate or full sample 95% CIs. Based on the extensive literature suggesting that prenatal exposure to phthalates may influence development in offspring in sex-specific ways, we additionally fitted WQS models stratified by child sex.
In secondary models, we used a more traditional approach of separately evaluating associations between Total Problems scores and individual log10-transformed, specific gravity-adjusted phthalate metabolite concentrations in linear and logistic regression models to facilitate comparisons to other published studies. We used Huber-White standard error estimators to calculate 95% CIs and fitted models examining the entire cohort as well as models stratified by child sex.
In Aim 2, we additionally considered effect modification by maternal PSLE count. Interaction terms multiplying PSLE count with either the WQS regression-derived WQS index or individual phthalate metabolites were included in linear and logistic regressions. To visualize the impact of PSLE count on the associations between phthalates and CBCL total score, we plotted predicted WQS index or individual phthalate coefficients over PSLE count values when interaction term p-values were significant (p < 0.05) or near significant (p < 0.10). We rely on standard full sample p-values to assess statistical significance of interactions since the WQS optimization is not looking for mixtures weights that give rise to interactions with PSLEs. Analyses were performed using R version 4.1.3 (R Development Core Team 2022) and the R packages gWQS (version 3.0.4) (Renzetti et al., 2021) and wqspt (version 1.0.1) (Day et al., 2023).
3. Results
3.1. Descriptive statistics
In total, 1694 mother–child dyads had data on second trimester phthalate exposure, PSLEs, and CBCL scores and were therefore eligible for this analysis. Of those, 1536 had complete covariate data for fully adjusted (primary) models and 1467 had the additional data needed for extended models. Mothers were on average 33.8 ± 6.3 years old with a pre-pregnancy BMI of 27.1 ± 7.5 kg/m2 (Table 1). Highest level of maternal educational attainment was heterogenous with 3.8% having less than a high school education, 25.8% completing high school, 15.8% completing vocational school, 27.9% having a college degree, and 26.7% having a graduate degree. Only 5.9% of mothers smoked during pregnancy and children were born at 38.9 ± 2.0 weeks gestation, on average. At the time of CBCL assessment, children were on average 5.0 ± 1.0 years old and 51.1% were female. Of the participating children, 46.0% were White, 42.5% were Black, 1.8% were Asian, 0.6% were American Indian/Alaska Native, 2.4% identified as being of multiple races, and 6.8% endorsed belonging to another racial group. In addition, 7.4% reported Hispanic/Latino ethnicity. The largest group of participants was from the CANDLE study (59.5%), followed by TIDES (27.5%) and GAPPS (13%; Supplemental Table 1).
Table 1.
Characteristics of the study population overall and by child sex1.
| Continuous characteristics (mean ± SD) | Total (n = 1536) | Males (n = 750) | Females (n = 786) |
|---|---|---|---|
|
| |||
| Maternal age at delivery (yrs) | 33.8 ± 6.3 | 33.5 ± 6.3 | 34.0 ± 6.2 |
| Pre-pregnancy BMI (kg/m2) | 27.1 ± 7.5 | 27.2 ± 7.3 | 27.1 ± 7.6 |
| Gravidity | 2.5 ± 1.6 | 2.5 ± 1.6 | 2.5 ± 1.6 |
| Adjusted income ($)2 | 65,099.3 ± 53,313.6 | 64,129.8 ± 53,325.0 | 66,024.4 ± 53,320.1 |
| Gestational age at urine collection (wks) | 22.0 ± 3.4 | 22.2 ± 3.4 | 21.8 ± 3.4 |
| Prenatal stressful life events (PSLEs; count) | 1.5 ± 1.8 | 1.5 ± 1.8 | 1.5 ± 1.8 |
| Gestational age at birth (wks)3 | 38.9 ± 2.0 | 38.9 ± 2.0 | 39.0 ± 2.0 |
| Birthweight (kg)3 | 3.3 ± 0.6 | 3.3 ± 0.6 | 3.2 ± 0.6 |
| Child age at visit (yrs) | 5.0 ± 1.0 | 5.0 ± 1.0 | 5.0 ± 1.0 |
| PROMIS T-score3 | 48.6 ± 7.3 | 48.6 ± 7.2 | 48.6 ± 7.4 |
| CBCL Child Total Problems Score | 22.5 ± 18.0 | 23.7 ± 18.6 | 21.3 ± 17.4 |
| Categorical characteristics (n [%]) | |||
| Maternal education | |||
| Less than high school | 59 [3.8] | 25 [3.3] | 34 [4.3] |
| High school diploma or GED | 396 [25.8] | 213 [28.4] | 183 [23.3] |
| Vocational school | 243 [15.8] | 102 [13.6] | 141 [17.9] |
| College degree | 428 [27.9] | 210 [28.0] | 218 [27.7] |
| Graduate degree | 410 [26.7] | 200 [26.7] | 210 [26.7] |
| Child race | |||
| American Indian/Alaska Native | 9 [0.6] | 4 [0.5] | 5 [0.6] |
| Asian | 28 [1.8] | 14 [1.87] | 14 [1.78] |
| Black | 652 [42.5] | 325 [43.3] | 327 [41.6] |
| Mixed | 37 [2.4] | 23 [3.1] | 14 [1.8] |
| Other | 104 [6.8] | 46 [6.1] | 58 [7.4] |
| White | 706 [46.0] | 338 [45.1] | 368 [46.8] |
| Child ethnicity- Hispanic/Latino | 114 [7.4] | 57 [7.6] | 57 [7.3] |
| Smoking during pregnancy (any) | 90 [5.9] | 39 [5.2] | 51 [6.5] |
| Gestational diabetes (yes) 3 | 102 [6.7] | 57 [7.6] | 45 [5.8] |
| Gestational hypertension (yes) 3 | 166 [10.9] | 74 [10.0] | 92 [11.8] |
| Breastfeeding (any) 3 | 1183 [77.2] | 581 [77.6] | 602 [76.9] |
| Postnatal secondhand smoke (any) 3 | 286 [19.1] | 150 [20.4] | 136 [17.9] |
| Study site | |||
| Minneapolis, MN | 132 [8.6] | 71 [9.5] | 61 [7.8] |
| Rochester, NY | 104 [6.8] | 49 [6.5] | 55 [7.0] |
| San Francisco, CA | 118 [7.7] | 54 [7.2] | 64 [8.1] |
| Seattle, WA | 268 [17.4] | 124 [16.5] | 144 [19.2] |
| Memphis, TN | 914 [59.5] | 452 [60.3] | 462 [58.8] |
| Year of birth | |||
| 2007 | 55 [3.6] | 24 [3.2] | 31 [3.9] |
| 2008 | 149 [9.7] | 75 [10.0] | 74 [9.4] |
| 2009 | 236 [15.4] | 120 [16.0] | 116 [14.8] |
| 2010 | 269 [17.5] | 138 [18.4] | 131 [16.7] |
| 2011 | 362 [23.6] | 165 [22.0] | 197 [25.1] |
| 2012 | 301 [19.6] | 159 [21.2] | 142 [18.1] |
| 2013 | 112 [7.3] | 47 [6.3] | 65 [8.3] |
| 2014 | 50 [3.3] | 20 [2.7] | 30 [3.8] |
| 2015 | 2 [0.1] | 2 [0.3] | 0 [0.0] |
| CBCL Total Problems score at-or-above borderline threshold (84%) | 131 [8.5] | 68 [9.1] | 63[8.0] |
| CBCL Total Problems score at-or-above clinical threshold (90%) | 93 [6.1] | 45 [6.0] | 48 [6.1] |
| CBCL version | |||
| Age 1–5 | 1171 [69.1] | 529 [70.5] | 543 [69.1] |
| Age 6–18 | 523 [30.9] | 221 [29.5] | 243 [30.9] |
BMI = body mass index; CBCL = Child Behavior Checklist; GED = general education development test; SD = standard deviation.
Characteristics are reported for the analytic sample included in the primary, fully adjusted models unless otherwise noted.
Household annual income is regionally and inflation-adjusted and reported as median [IQR].
n = 1467.
Most phthalate metabolites were detectable in the vast majority of participants (Table 2). The metabolites most often below the LOD were MMP, MHXP, and MINP (36.6%, 23.5%, and 29.2% below LOD, respectively). Concentrations of MEP were the highest, with median levels of 70.8 ng/mL, ranging up to 9081 ng/mL, while concentrations of MHXP were the lowest at median 0.2 ng/mL, ranging up to 35.5 ng/mL. Mothers reported mean 1.5 ± 1.8 PSLEs and the most frequently reported PSLEs were moving to a new address, arguing with partner more than usual, and problems paying the rent/mortgage/bills, which were reported by 24.5%, 17.1%, and 14.6% of participants, respectively (Supplemental Table 2). Spearman correlation coefficients between all phthalate metabolites and PSLE counts are shown in Supplemental Figure 1. PSLEs were uncorrelated with phthalates, and among the phthalate metabolites, the highest correlations were between the DEHP metabolites.
Table 2.
Maternal urinary specific-gravity adjusted phthalate metabolite concentrations (ng/mL) among ECHO-PATHWAYS participants (n = 1536).
| Parent compound | Metabolite(s) | % below LOD | Mean (SD) | Median (Range) |
|---|---|---|---|---|
|
| ||||
| All phthalates | Phthalic acid (PA)1 | 2.7 | 73.3 (104.8) | 38.4 (0.02–1431.4) |
| Dimethyl phthalate (DMP) | Monomethyl phthalate (MMP) | 36.6 | 6.0 (11.0) | 2.1 (0.02–224.2) |
| Diethyl phthalate (DEP) | Monoethyl phthalate (MEP) | 0.2 | 214.4 (615.8) | 70.8 (0.1–9081.0) |
| Dibutyl phthalate (DBP) | Monobutyl phthalate (MBP) | 0.1 | 30.9 (57.5) | 17.9 (0.05–1229.0) |
| Diisobutyl phthalate (DIBP) | Monoisobutyl phthalate (MIBP) | 0.4 | 13.9 (20.9) | 8.3 (0.01–435.9) |
| Butylbenzyl phthalate (BBZP) | Monobenzyl phthalate (MBZP) | 0.8 | 27.8 (61.7) | 12.4 (0.02–1125.6) |
| Dihexyl phthalate (DHXP) | Monohexyl phthalate (MHXP) | 23.5 | 0.4 (1.0) | 0.2 (0.01–35.5) |
| Diheptyl phthalate (DHPP) | Monoheptyl phthalate (MHPP) | 6.3 | 1.4 (2.1) | 0.9 (0.01–33.7) |
| Di(2-ethylhexyl) phthalate (DEHP) | Mono(2-ethylhexyl) phthalate (MEHP) | 8.7 | 13.0 (52.0) | 4.1 (0.01–905.1) |
| Mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) | 0.1 | 19.5 (72.0) | 8.1 (0.03–1075.2) | |
| Mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) | 0.1 | 39.3 (141.5) | 15.5 (0.01–2701.6) | |
| Mono(2-ethyl-5-carboxypentyl) phthalate (MECPP) | 0.1 | 24.6 (61.6) | 12.4 (0.01–1428.5) | |
| Mono(2- carboxymethylhexyl) phthalate (MCMHP) | 0.4 | 23.9 (44.4) | 11.9 (0.1–830.0) | |
| Dioctyl phthalate (DOP) | Mono(3-carboxypropyl) phthalate (MCPP)2 | 2.0 | 6.0 (49.4) | 1.8 (0.1–1450.0) |
| Diisononyl phthalate (DINP) | Monoisononyl phthalate (MINP) | 29.2 | 2.1 (5.1) | 0.4 (0.01–80.2) |
| Monocarboxyisooctyl phthalate (MCIOP) | 0.4 | 29.2 (69.8) | 8.3 (0.02–824.4) | |
| Diisodecyl phthalate (DIDP) | Monocarboxyisononyl phthalate (MCINP) | 3.7 | 5.1 (20.5) | 2.4 (0.01–709.0) |
Phthalic acid is a non-specific metabolite of multiple parent phthalate esters.
MCPP is primarily a metabolite of DOP, but it can also be a minor metabolite of DBP and other higher molecular weight phthalates.
The average CBCL Total Problems score was 22.6 ± 18.0 and overall, 8.5% and 6.1% of children had borderline and clinically significant Total Problems scores, respectively. Females tended to have lower scores (21.3 ± 17.4) than males (23.7 ± 18.6) on average. The percentage of children with Total Problems scores that were of borderline or clinical significance was similar among females (borderline: 8.0%; clinically relevant: 6.1%) and males (borderline: 9.1%; clinically relevant: 6.0%). (Table 1).
3.2. Aim 1 mixtures analyses (Primary)
In our primary, fully adjusted models examining the full cohort, we observed no significant associations between phthalate metabolite mixtures and continuous Total Problems scores (Fig. 1, Supplemental Table 3).
Fig. 1.
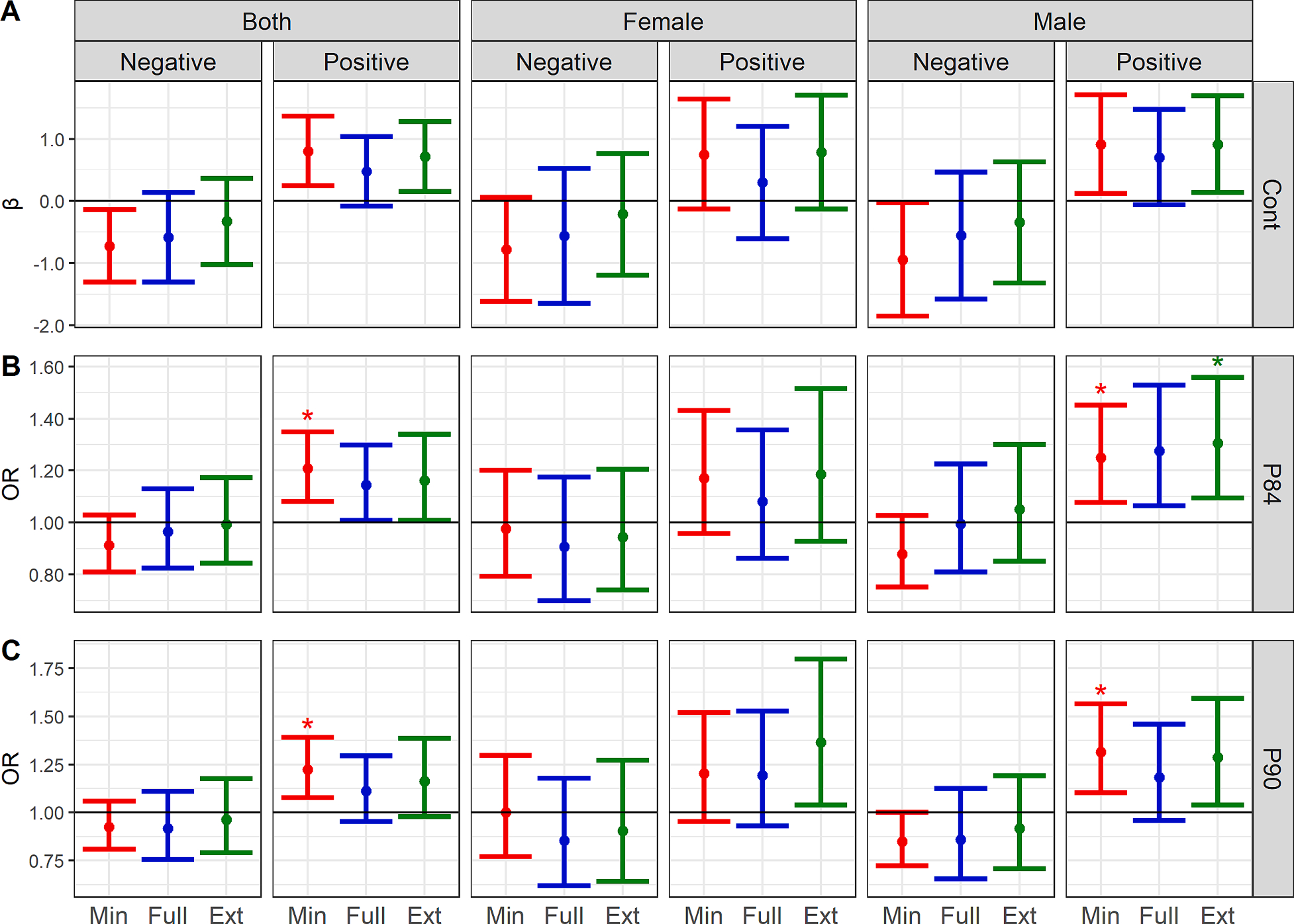
WQS regression coefficients or odds ratio (and 95% CIs) for associations between specific gravity-adjusted prenatal phthalate mixtures and CBCL Total Problems scores (A: continuous; B: dichotomized at the 84th percentile; C: dichotomized at the 90th percentile). Associations are presented for the whole cohort as well as stratified by child sex1. 1 Minimally adjusted models (red) include gestational age at urine collection, study site, CBCL version, and phthalate batch (n = 1694). Fully adjusted models (blue) include the minimal covariates plus log-adjusted income, maternal age, gravidity, pre-pregnancy BMI, child age, smoking during pregnancy, maternal education, year of birth (categorical), child sex, child race, and child ethnicity (n = 1536). Extended models (green) include all minimal and full covariates as well as gestational age at birth, birth weight, PROMIS T score, gestational diabetes, breastfeeding, postnatal secondhand smoke, and gestational hypertension (n = 1467).* Permutation test p-value (PTp) < 0.05. Note that CIs represent the default, anticonservative CIs calculated by the WQS regression. These are provided as additional information, but the PTp should be considered the most valid measure of statistical significance. Plot rows denote the form of the CBCL total problems score: A. continuous (Cont), B. dichotomized at the 84th percentile (P84), or C. dichotomized at the 90th percentile (P90). Plot columns first show the population included in each model, either both sexes combined or sex-stratified, as well as the direction of the WQS regression models.
When we examined the dichotomized outcome measures, in minimally adjusted models, higher exposure to the phthalate mixture was associated with increased odds of borderline Total Problems scores (≥84th percentile) in the positive direction (OR = 1.21, 95% CI:1.08, 1.35; PTp: 0.01) however results were somewhat attenuated in the fully adjusted model (OR = 1.14, 95% CI: 1.01, 1.30; PTp: 0.08). In that fully adjusted model, the metabolites with the highest weights in the mixture were MHXP (0.26), MCINP (0.18), and MEHP (0.11). Associations with clinically relevant dichotomized scores (≥90th percentile) were similar, but generally lesser in magnitude.
As in models evaluating the full cohort, few associations between the phthalate mixtures and Total Problems scores were observed in models stratified by sex. When we examined the dichotomized outcomes, we observed increased odds of borderline problem behaviors in males in minimally adjusted (OR = 1.25, 95% CI: 1.08, 1.45; PTp: 0.04), fully adjusted (OR = 1.28, 95% CI: 1.06, 1.53; PTp: 0.05), and extended models (OR = 1.31, 95% CI: 1.09, 1.56; PTp: 0.04) (Fig. 1, Supplemental Table 3). There was some variation in the most heavily weighted metabolites across staged models, however in the primary, fully adjusted models, MHXP (0.20), MEHP (0.19), and PA (0.12) had the highest weights. WQS mixtures results were similarly positive for the dichotomized clinically relevant Total Problems scores, however only results for the minimally adjusted model were statistically significant (OR = 1.31, 95% CI: 1.10, 1.57; PTp = 0.045) with the highest weights being for MBZP (0.36), MHXP (0.26), and PA (0.11). Our results suggested null associations between phthalate mixtures and problem behaviors in females.
3.3. Aim 1 individual metabolite models (secondary)
Although a small number of metabolites (e.g., MHXP, MBZP) were positively associated with Total Problems scores in minimally adjusted linear and logistic regression models examining individual phthalate metabolites in the entire cohort, results were largely attenuated in the main, fully adjusted models. One exception was a significant association between MHXP and dichotomized borderline Total Problems scores (≥84th percentile) (OR = 1.52, 95% CI: 1.02, 2.31) (Supplemental Figure 2). Inverse associations between MCIOP and Total Problems scores were also observed in minimally adjusted models (β = −2.16, 95% CI: −3.73, −0.59), but not adjusted models.
In models stratified by child sex, few significant associations were observed, however for many metabolites, associations tended to be stronger in males (Supplemental Figure 3A; Supplemental Table 4). For example, in fully adjusted models, estimates for the DEHP metabolites MEOHP, MEHHP, and MECPP (MEOHP: β: 1.98; 95% CI: −0.81, 4.76; MEHHP: β: 2.34; 95% CI: −0.28, 4.97); MECPP: β: 2.06; 95% CI: −0.75, 4.87) showed positive associations with continuous Total Problems scores in males, while in females, the estimates were weakly negative (Supplemental Figure 3B; Supplemental Table 4). Similarly, in fully adjusted models examining the dichotomized outcomes, MHXP was associated with increased odds of borderline (OR: 1.83; 95% CI: 1.00, 3.36) and clinically significant (OR: 1.29; 95% CI: 0.69, 2.39) problem behaviors in males, while associations in females were more modest.
3.4. Aim 2 mixtures analyses including PSLE interactions (Primary)
In Aim 2, we built upon phthalate analyses in Aim 1 to now include an interaction term (e.g., WQS index * PSLE count); aim 2 coefficients or odds ratios for WQS index by PSLE count interaction terms are shown in Fig. 2. These interaction coefficients are interpreted as the additive change (continuous) or multiplicative change (odds ratio) in mixture coefficient per one count higher total number of PSLEs, and that mixture coefficient is interpreted as the change in Total Problems score associated with a one decile higher level of all phthalate metabolites in the weighted mixture.
Fig. 2.
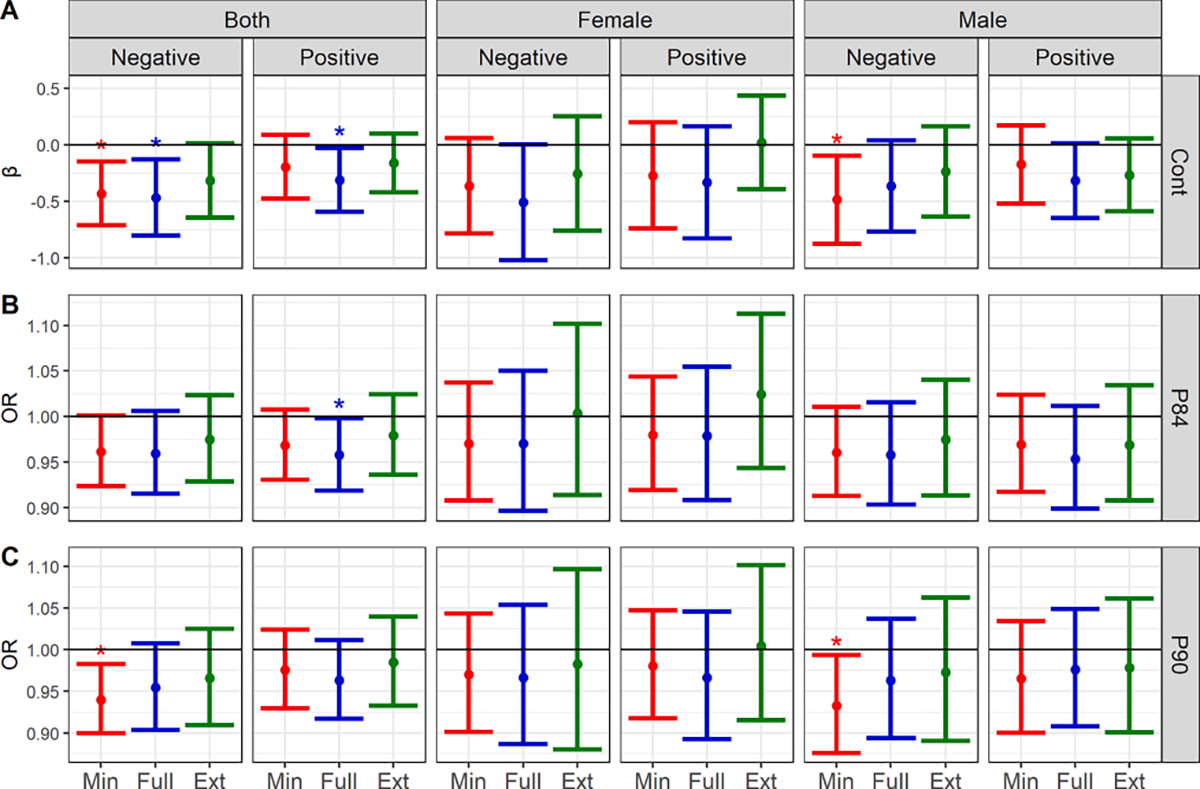
Estimates and 95% CIs for the interaction coefficient or odds ratio between prenatal phthalate mixtures and prenatal stressful life events (PSLEs) in relation to CBCL Total Problems scores (A: continuous; B: dichotomized at the 84th percentile; C: dichotomized at the 90th percentile) in the whole ECHO PATHWAYS cohort as well as stratified by child sex1.1 Minimally adjusted models include specific gravity, gestational age at urine collection, study site, CBCL version, and phthalate batch (n = 1694). Fully adjusted models include the minimal covariates plus log-adjusted income, maternal age, gravidity, pre-pregnancy BMI, child age, smoking during pregnancy, maternal education, year of birth (categorical), child sex, child race, and child ethnicity (n = 1536). Extended models include all minimal and full covariates as well as gestational age at birth, birth weight, PROMIS T score, gestational diabetes, breastfeeding, postnatal secondhand smoke, and gestational hypertension (n = 1467). All models additionally include an interaction term between the WQS mixture coefficient and prenatal stressful life events. * Statistically significant results (p < 0.05); Cont = continuous Total Problems score; P84 = 84th percentile of Total Problems scores; P90 = 90th percentile of Total Problems scores. Interaction term coefficients are represented for the Cont plot, while interaction odds ratios (i.e., the exponentiated interaction term coefficient) are presented for the P84 and P90 plots. The interpretation of the interaction odds ratios is that for each 1 event higher PSLE count, the odds ratio for the odds of being above a certain Total Problems score percentile in association with a 1-decile increase in all components of the phthalate mixture will change X-fold. For example, if the interaction odds ratio is 0.8 and the odds ratio for being above the 84th percentile of Total Problems scores is 2 in association with a 1-decile higher phthalate mixture when the PSLE count is zero, that odds ratio would be 1.6 when the PSLE count is one, 1.28 when the PSLE count is two, and so on.
Overall, interactions that were statistically significant were observed for seven models (out of 48), three from fully adjusted models. Specifically, in fully adjusted models evaluating the entire cohort, we observed negative (i.e., antagonistic) interactions between a weighted phthalate mixture positively associated with the outcome and both (1) continuous Total Problems scores (β = −0.31, 95% CI: −0.59, −0.03) and (2) Total Problems scores dichotomized at the 84th percentile (OR = −0.04, 95% CI: −0.08, −0.002). In addition, we observed negative interactions between a weighted phthalate mixture negatively associated with the outcome and continuous Total Problems scores (β = −0.43, 95% CI: −0.71, −0.15). We explored the statistically significant fully adjusted associations further by plotting the WQS index-associated coefficients or odds ratios over counts of PSLEs (Fig. 3).
Fig. 3.
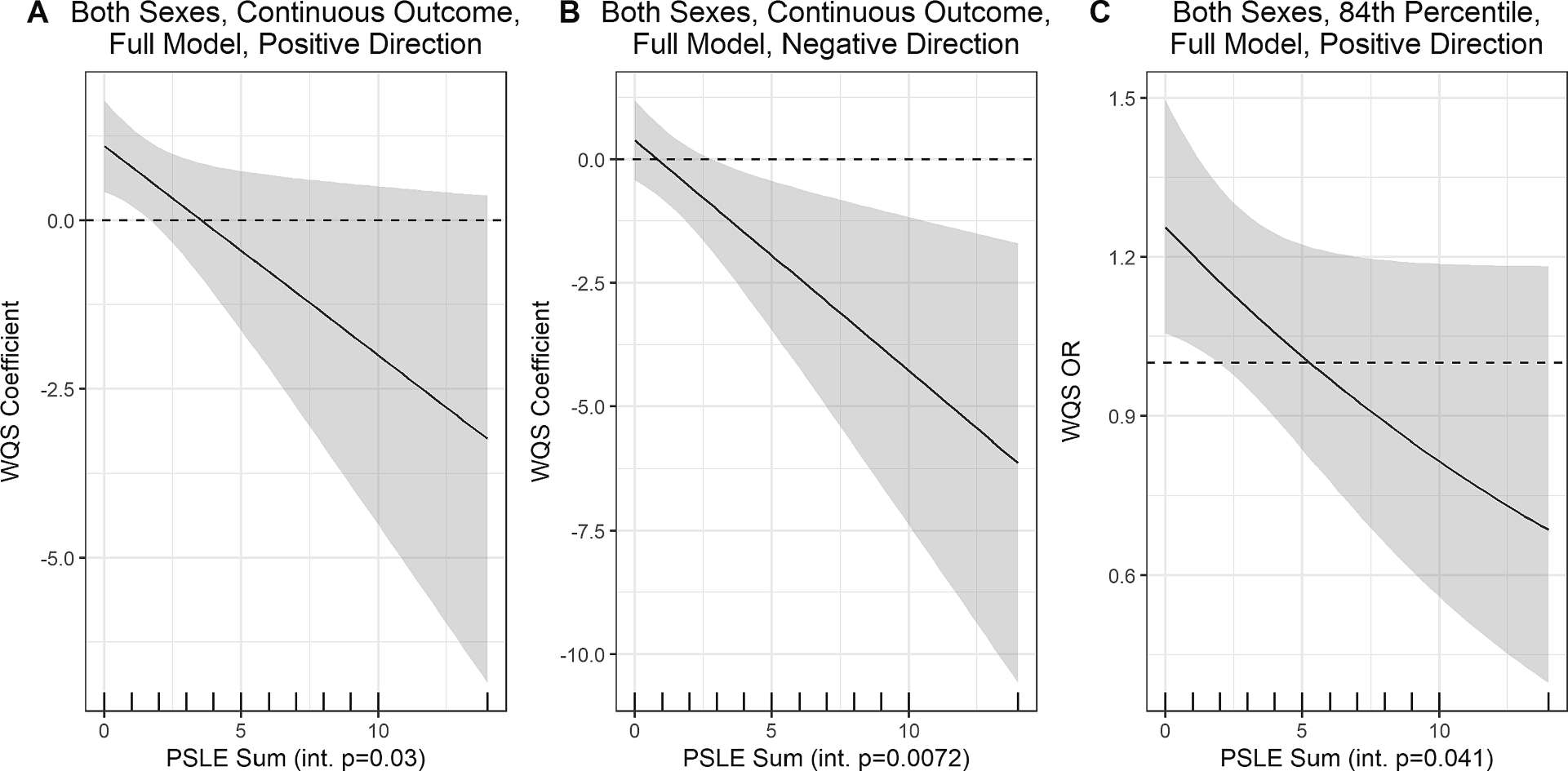
Predicted WQS coefficients (and 95% CIs) in association with Total Problems scores over counts of prenatal stressful life events (PSLEs) in interaction models1. 1 Plots are only shown for fully adjusted models evaluating both sexes combined in the full sample in which the interaction term was statistically significant (p < 0.05). Plot A shows predicted WQS coefficients for the model in the positive direction in association with the continuous Total Problems score, and Plot B shows the predicted WQS coefficients for the same outcome but in the negative direction. Plot C shows predicted WQS coefficients in the positive direction in association with the Total Problems score dichotomized at the 84th percentile. The rug plot on the x-axis shows observed PSLE count values, and the shaded region shows the 95% CIs of the WQS coefficients. Int p. = interaction term p-value.
Overall, all predictions of mixture coefficients across PSLE counts for the significant interactions suggested increasingly negative coefficients for the phthalate mixture as the number of PSLEs increased. As an example, in the fully adjusted model examining the continuous outcome in both sexes in the positive direction (Fig. 3A), at PSLE scores below 2 (66.5% of women in this sample), a phthalate mixture with high weights for MCINP (mixture weight = 0.29), MHXP (0.17), and MBZP (0.16) was associated with higher problem behaviors, however at higher exposure to prenatal PSLEs, the association was imprecise with an attenuated point estimate that trended toward being negative. At the same time, we observed a significant interaction in the analogous model with WQS weights selected to focus on overall negative associations between phthalates and continuous Total Problems, dominated by a different set of phthalate metabolites (Fig. 3B). Specifically, at higher levels of PSLEs (>3; 20.5% of women in this sample), there was an association between a phthalate mixture with high weights for MMP (0.30), MEP (0.21), and MINP (0.14) and Total Problems scores in the negative direction, however the association was null at lower levels of PSLE exposure. Similar to the associations with the continuous Total Problems score in the positive direction, a phthalate mixture with high weights for MHXP (0.26), MCINP (0.18), and MEHP (0.11) was positively associated with the odds of being above the 84th percentile of Total Problems scores, but only at PSLE counts below 3 (Fig. 3C).
3.5. Aim 2 Models examining interactions between individual phthalate metabolites and PSLEs (Secondary)
Secondarily, we extended the linear regression models to additionally include cross-product terms for the interactions between individual phthalate metabolites and PSLEs (Supplemental Figure 4). In the whole cohort, few interactions were observed. The interaction between MCIOP and PSLEs was significant in all minimally and fully adjusted models; limited evidence of interactions with MHPP and MCINP and PSLEs was observed as well. When we fitted sex-stratified models, we observed numerous interactions in the models restricted to males, but few interactions among females (Supplemental Figure 5). For instance, among males, across all models examining the continuous Total Problems score as the outcome, interactions were observed between PSLEs and numerous phthalate metabolites including MEOHP, MEHHP, MECPP, MCMHP, MHPP, MCPP, MCIOP, and MCINP (Supplemental Figure 5A). Results were similar in models examining the dichotomized outcomes. When we explored the interactions with the continuous outcomes in males further, we observed a general pattern whereby at higher exposure to PSLEs, coefficients for the phthalate metabolites were more strongly negative (Supplemental Figure 6). For example, at low numbers of PSLEs (~0–2), there were weak positive associations between the DEHP metabolites and continuous Total Problems scores in males. However, when the number of PSLEs was higher, there was a strong inverse association whereby higher phthalate metabolite concentrations were associated with lower problem behaviors scores. These associations were particularly notable for MCMHP, MCPP, MCIOP, and MHPP, although notably the 95% CIs widened considerably as the number of PSLEs increased due to sparsity of the data. No notable interactions between phthalate metabolites and PSLEs were observed in fully adjusted models restricted to females (Supplemental Figure 5B).
4. Discussion
Overall, we observed some evidence of weak associations between prenatal phthalate exposures and problem behaviors at age 4–6 in mixtures models as well as in individual phthalate metabolite models. When we considered moderation by PSLEs, few interactions were observed. In fully adjusted models where significant interactions were noted, a phthalate mixture dominated by MCINP, MHXP, and MBZP was positively associated with problem behaviors when exposure to PSLEs was low, whereas a different phthalate mixture dominated by MMP, MEP, and MINP was inversely associated with problem behaviors when exposure to PSLE was higher. Results of individual metabolite models further indicated that among male children, adverse associations between exposure to the DEHP metabolites (as well as MHPP, MCIOP, and MCINP) and more problem behaviors were most evident at low levels of PSLEs, and vice versa. Across all models, very few associations were observed in females. Of note, concentrations of phthalate metabolites in maternal urine in the current study tended to be lower than those reported in some international pregnancy cohorts (Lien et al., 2015), but similar to or slightly higher than other North American cohorts (e.g., Daniel et al., 2020; England-Mason et al., 2020; Kobrosly et al., 2014).
These results add to a sizeable and inconsistent literature on prenatal phthalate exposure and child behavior, including at least eight prior studies (representing six different cohorts) that used the CBCL to assess behavior in children ages 1–18 (Chen et al., 2019; Daniel et al., 2020; England-Mason et al., 2020; Guilbert et al., 2021; Huang et al., 2019; Kim et al., 2018; Kobrosly et al., 2014; Lien et al., 2015). Even within that subset of studies, results have varied considerably. For example, while maternal DEHP and/or DBP metabolites in late pregnancy were associated with child externalizing behaviors in one cohort (Huang et al., 2019; Lien et al., 2015), in other studies, those results have not been replicated (Kim et al., 2018) or have been observed in only one sex (Kobrosly et al., 2014). Indeed, in some studies, child’s sex appears to be an important moderator of phthalate-behavior associations. Of these, some studies have reported stronger associations in males, consistent with what we observed in the current study. For instance, Kobrosly et al., (2014) observed that higher maternal MIBP was associated with aggressive behavior as well as overall externalizing behaviors and total problem behaviors in males at age 6–10 years, while few associations were observed in females (Kobrosly et al., 2014). Other studies have reported associations in both sexes, but with differing patterns. For example, Daniel et al. (2020) reported inverse associations between third trimester DEHP metabolite concentrations and hyperactivity in females, but not males at age 7. Maternal MBZP and MIBP concentrations were additionally associated with more anxious-shy behavior in males (Daniel et al., 2020). Guilbert et al (2021) observed associations between a phthalate-phenol mixture and both internalizing and externalizing scores in both females and males at age 2, with 2–3-fold stronger associations in females and sex-dependent differences in terms of which metabolites were implicated as “bad actors” (Guilbert et al., 2021). If there are indeed sex differences in the association between prenatal phthalate exposure and child behavior, the underlying mechanisms remain unclear. While disruption of sex steroid activity is an obvious candidate given phthalates’ impacts on androgen and estrogen activity, there may be other pathways to consider, including thyroid hormones, fatty acid metabolism, and oxidative stress. Consideration of placental mechanisms is also important given increasing evidence of the importance of sex differences in placental in structure and function (Meakin et al., 2021; Rosenfeld 2015).
Of the studies on gestational phthalate exposure and child behavior to date, a small number have employed mixtures analyses to complement individual metabolite models, frequently using WQS, as we did in the current study (Daniel et al., 2020; Day et al., 2021; Guilbert et al., 2021). In a prior analysis in the TIDES cohort, gestational exposure to a phthalate mixture was associated with higher social impairment (as measured by the Social Responsiveness Scale) in both sexes at age 4–5 as well as higher externalizing scores in males (as measured by the Behavioral Assessment System for Children (BASC-2) (Day et al., 2021). By contrast, Daniel et al. (2020) observed no associations between a mixture of phthalate metabolites and internalizing or externalizing behaviors at age 7 measured using the Connors’ Parent Rating Scale. However, when models were refitted with mixtures limited to the DEHP metabolites, associations were observed with social problems in males as well as anxious-shy problems in females and emotional lability in the combined cohort. Although CBCL scores were considered in single metabolite models, they were not included in mixtures analyses. In the Guilbert et al. (2021) study, a phthalate-phenol mixture was associated with externalizing and internalizing behaviors in both sexes, with stronger associations observed in females, however comparisons to the current cohort are difficult given the inclusion of multiple phenols, which were often heavily weighted as the “bad actors” in their mixture, as well as the young age of the children (mean 2 years) and the variation in CBCL outcome scales considered.
In the current analysis, we focused on a single summary outcome measure, Total Problems, to evaluate children’s overall levels of emotional and behavior problems, which have been found in our prior work within this harmonized cohort to be associated with exposure to PSLEs (Bush et al., 2023). Our focus on a single outcome measure is in contrast to prior studies which have generally fitted models examining numerous narrowband behavior syndrome “DSM-oriented” sub-scales. That approach, while comprehensive, may compound multiple comparisons problems, particularly when phthalate metabolites are considered individually rather than in a mixture. Of the prior studies, several analyzed Total Problems scores, as we did here, in addition to the narrowband CBCL scores (England-Mason et al., 2020; Kobrosly et al., 2014). In a small, multi-site U.S. cohort, positive associations between maternal MIBP concentrations (at mean gestational age 26 weeks) and Total Problems scores were observed in males at mean age 8.5, while associations in females, in the combined cohort, and for ΣDEHP, MBP, MBZP, and MEP were null (Kobrosly et al., 2014). In a Canadian study, in adjusted models, no phthalate metabolite was associated with clinically relevant dichotomized Total Problems scores in the full cohort, and few associations were observed for CBCL sub-scales as well (England-Mason et al., 2020). When Total Problems were considered continuously, maternal MMP was associated with lower Total Problems scores in females and the sum of the low molecular weight phthalates was associated with higher Total Problems scores in males, however after adjustment for multiple comparisons, neither remained statistically significant. Overall, this literature (including the current study) may be interpreted as suggestive of weak positive associations between phthalate exposures and problem behaviors, particularly in male children.
Given that our prior work in this combined cohort found that prenatal PSLEs were associated with higher Total Problems scores as well as higher odds of clinically relevant problem behaviors at age 4–6 (Bush et al., 2023), echoing results from other cohorts showing the adverse impacts of prenatal stressful life events on child development (Bergman et al., 2007; Bush et al., 2017; MacKinnon et al., 2018; Tearne et al., 2015), we had anticipated that PSLEs would exacerbate the impacts of phthalates alone on neurodevelopment (if any). The apparent “antagonistic” interactions between phthalate exposures and PSLEs on problem behaviors in males observed here were unexpected, and there is a very limited literature to help contextualize those results. Of note, in one of the participating cohorts, TIDES, we previously examined maternal exposure to phthalates and a separate measure of PSLEs than was used here in relation to newborn anogenital distance (AGD), a measure of the prenatal androgen environment (Barrett et al., 2016). In that analysis, we observed that the expected associations between prenatal exposures to DEHP and DBP metabolites and reduced (less androgenized) AGD in newborn males were only observed when there was no exposure to PSLEs. In fact, parallel to findings here, estimates were in the opposite, positive direction among mothers who reported PSLEs during pregnancy. Those results were consistent, moreover, with our findings from a prior, smaller cohort (Barrett and Swan, 2015), however conflicted with those of a Canadian cohort in which positive associations were observed between phthalate concentrations and AGD in male infants regardless of maternal PSLEs (Arbuckle et al., 2019). Interestingly, in a small cross-sectional study in early pregnancy, multiple phthalate metabolites were positively associated with levels of maternal luteinizing hormone (LH, which stimulates sex steroid production) in women reporting high perceived stress, while inverse associations were observed in those reporting lower stress (Hlisníková et al., 2022). While synthesizing that body of work is not straight-forward, findings overall may indicate that prenatal exposure to stressors disrupts phthalate-induced suppression of testosterone production, potentially by activating gonadotropin, glucocorticoid, and/or adrenal androgen pathways (Barrett and Swan, 2015). The involvement of an androgen-related pathway linking exposures to phthalates and PSLEs with adverse childhood behaviors is further supported by findings from a prior TIDES analysis in which we additionally observed that higher maternal free and total testosterone in early pregnancy was associated with significantly higher behavioral symptoms scores (as measured by the BASC-2) at age 4–5, particularly among males (Day et al., 2020). This is an important pathway to consider given the importance of sex steroid hormones for brain development (Roselli et al., 2009; Wilson and Davies, 2007).
To our knowledge, this study is the first to examine phthalate-stress interactions in association with child neurodevelopment. Moreover, with over 1500 mother–child dyads, it is the largest study of prenatal phthalates and child behavior in the literature, with greater power to detect associations than prior studies that, with few exceptions, have included fewer than 500 dyads. Beyond the large size, a notable strength of this multi-site U.S. sample is the inclusion of participants from a range of sociodemographic backgrounds and geographic areas, which contrasts with many pregnancy cohorts that have focused on White, middle-class participants from a single geographic region. The diversity of our cohort is important given the known sociodemographic disparities in exposure to some phthalates as well as the disproportionate exposure to non-environmental stressors among non-White populations in the U.S. Additionally, we capitalized on widely used, validated scales to measure PSLEs and child problem behaviors. Finally, the inclusion of advanced mixtures models allowed us to estimate the effects of combined exposure to a panel of phthalates, while individual metabolite models facilitated comparisons to the prior literature.
At the same time, we note some important limitations of the current research. Phthalates have a short half-life in the body and for some metabolites (including MBZP and the DEHP metabolites) concentrations measured in a single urine sample may not be representative of exposures over longer periods (Braun et al., 2012; Fromme et al., 2007). Relatedly, brain development occurs over a long period in utero as well as postnatally. While numerous studies, including ours, have focused on mid-pregnancy phthalate exposure in relation to behavioral outcomes, it is not clear that this is the most critical period during which such exposures might most impact child behavior and future work examining exposure during multiple pre- and postnatal windows is warranted, as some studies have started to do in the context of other neurodevelopmental domains (Huang et al., 2019; Jankowska et al., 2019; Kim et al., 2018). In this work, we additionally focused on a single measure of stress, PSLEs, which was reported retrospectively. While prospective data collection is certainly preferable, due to the nature of this domain (which focuses on impactful, memorable events such as deaths and job loss), research suggests there is minimal recall bias associated with retrospective reports of PSLEs (Ramos et al., 2020; Reuben et al., 2016). Importantly, we did not capture participants’ subjective response to these life events, and while we evaluated maternal depression at the time of the child visit, we did not assess maternal mood in pregnancy. A more comprehensive measure of maternal stressors and affective symptoms during pregnancy could be useful in future work, and some studies have begun to adopt this multi-faceted approach to characterizing prenatal stressors (Eatman et al., 2023). As in any observational study, there is the potential for residual confounding and while we adjusted for a broad array of covariates in a staged manner, there may be other important factors that were not accounted for in these analyses. Finally, we fitted a large number of models and it is possible that some results are due to chance. We did not adjust for multiple comparisons, instead electing to focus on the patterns of association across the analysis and in light of concerns about type II error after adjustment for multiple comparisons (Rothman, 1990).
In summary, we observed some evidence of weak associations between prenatal phthalate exposure and problem behaviors in childhood, however there was some indication that prenatal exposures to phthalates and PSLEs may act antagonistically in the context of child behavioral outcomes, particularly in male offspring. Our unexpected results on phthalate-stress interactions warrant replication in other cohorts, and future work should examine additional child neurodevelopmental domains such as cognitive function and general development. As the cohort ages, moreover, and mental health outcomes become more differentiated during the peri-pubertal period, we will be better equipped to examine associations between phthalate exposures, stressors, and more specific outcome measures, such as internalizing and externalizing behaviors. In light of growing evidence that prenatal exposures to both synthetic chemicals, such as phthalates, and non-chemical exposures, such as PSLEs, may impact the developing brain, future work should focus on preventing exposures and identifying protective factors that may promote resilience among children and families with higher burden of exposure.
Supplementary Material
Acknowledgements
We thank the study staff and participants who made CANDLE, TIDES, GAPPS, and the ECHO-PATHWAYS consortium possible.
Study funding/competing interest(s)
This study was supported by the NIH ECHO program (UG3/UH3OD023271, UG3/UH3OD023305), NIEHS (R01ES25169, R01ES016863, P30ES007033, P30ES005022), and the Urban Child Institute. The content is the sole responsibility of the authors and does not represent the official views of the National Institutes of Health.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Emily S. Barrett: Conceptualization, Investigation, Supervision, Writing – original draft. Drew B. Day: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. Adam Szpiro: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. James Peng: Formal analysis, Writing – review & editing. Christine T. Loftus: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. Ugne Ziausyte: Investigation, Writing – original draft. Kurunthachalam Kannan: Data curation, Methodology, Resources, Writing – review & editing. Leonardo Trasande: Funding acquisition, Data curation, Project administration, Supervision, Writing – review & editing. Qi Zhao: Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. Ruby H.N. Nguyen: Investigation, Project administration, Writing – review & editing. Shanna Swan: Funding acquisition, Investigation, Project administration, Writing – review & editing. Catherine J. Karr: Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. Kaja Z. LeWinn: Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. Sheela Sathyanarayana: Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing. Nicole R. Bush: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2024.108425.
Data availability
Data will be made available on request.
Data availability
ECHO-PATHWAYS shares data through the NIH ECHO consortium, which can be used for approved ECHO analysis proposals using ECHO-wide data. Policies for use of ECHO data can be obtained through the ECHO coordinating center, echocc@duke.edu. ECHO data are also available through the publicly NICHD DASH repository (https://dash.nichd.nih.gov/). De-identified data from this particular analysis may be available upon reasonable request, subject to IRB review, provision of an appropriate analysis plan, and a formal data use agreement.
References
- Achenbach T 2011. Child behavior checklist. In: Encyclopedia of clinical neuropsychology, (Kreutzer J, DeLuca J, Caplan B, eds). New York, NY:Springer, 546–552. [Google Scholar]
- Adgent MA, Carroll KN, Hazlehurst MF, Loftus CT, Szpiro AA, Karr CJ, et al. , 2020. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ. Int 143, 105970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, MacPherson S, Barrett E, Muckle G, Séguin JR, Foster WG, et al. , 2019. Do stressful life events during pregnancy modify associations between phthalates and anogenital distance in newborns? Environ. Res 177, 108593. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Xue J, De Carvalho BP, Iyer A, Abualnaja KO, Yaghmoor SS, et al. , 2016. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in jeddah, saudi arabia. Environ. Res 150, 573–581. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Padula AM, 2019. Joint impact of synthetic chemical and non-chemical stressors on children’s health. Curr. Environ. Health. Rep 6, 225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RH, Kobrosly R, et al. , 2014. Environmental health attitudes and behaviors: Findings from a large pregnancy cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol 176, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Swan SH, 2015. Stress and androgen activity during fetal development. Endocrinology 156, 3435–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Sathyanarayana S, Redmon JB, Nguyen RH, Swan SH, 2016. Prenatal stress as a modifier of associations between phthalate exposure and reproductive development: Results from a multicentre pregnancy cohort study. Paediatr. Perinat. Epidemiol 30, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell SM, Lyden GR, Sathyanarayana S, Barrett ES, Ferguson KK, Santilli A, et al. , 2021. First- and third-trimester urinary phthalate metabolites in the development of hypertensive diseases of pregnancy. Int. J. Environ. Res. Public. Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman K, Sarkar P, O’Connor TG, Modi N, Glover V, 2007. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J. Am. Acad. Child. Adolesc. Psychiatry 46, 1454–1463. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J, 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am. Ind. Hyg. Assoc. J 54, 615–627. [DOI] [PubMed] [Google Scholar]
- Bornehag CG, Carlstedt F, Jönsson BA, Lindh CH, Jensen TK, Bodin A, et al. , 2015. Prenatal phthalate exposures and anogenital distance in swedish boys. Environ. Health. Perspect 123, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovicka T, Jirina M Jr, Kordik P, Jirina M 2012. Selecting representative data sets. Advances in data mining knowledge discovery and applications 12:43–70. [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, et al. , 2012. Variability of urinary phthalate metabolite and bisphenol a concentrations before and during pregnancy. Environ. Health. Perspect 120, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bräuner EV, Uldbjerg CS, Lim YH, Gregersen LS, Krause M, Frederiksen H,et al. , 2022. Presence of parabens, phenols and phthalates in paired maternal serum, urine and amniotic fluid. Environ. Int 158, 106987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, et al. , 2017. Effects of pre- and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Dev. Psychopathol 29, 1553–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Noroña-Zhou A, Coccia M, Rudd KL, Ahmad SI, Loftus CT, et al. , 2023. Intergenerational transmission of stress: Multi-domain stressors from maternal childhood and pregnancy predict children’s mental health in a racially and socioeconomically diverse, multi-site cohort. Soc. Psychiatry. Psychiatr. Epidemiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Meeker JD, Ferguson KK, Mukherjee B, Hauser R, McElrath TF, 2016. Urinary concentrations of bisphenol a and phthalate metabolites measured during pregnancy and risk of preeclampsia. Environ. Health. Perspect 124, 1651–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, Factor-Litvak P, 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat 20, 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Wang YH, Chen WJ, Hsiung CA, Leon Guo YL, Julie Wang SL, 2019. A benchmark dose study of prenatal exposure to di(2-ethylhexyl) phthalate and behavioral problems in children. Int. J. Hyg. Environ. Health 222, 971–980. [DOI] [PubMed] [Google Scholar]
- Curtin P, Kellogg J, Cech N, Gennings C, 2021. A random subset implementation of weighted quantile sum (wqsrs) regression for analysis of high-dimensional mixtures. Commun. Statistics-Simulat. Comput 50, 1119–1134. [Google Scholar]
- Daniel S, Balalian AA, Insel BJ, Liu X, Whyatt RM, Calafat AM, et al. , 2020. Prenatal and early childhood exposure to phthalates and childhood behavior at age 7 years. Environ. Int 143, 105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DB, Collett BR, Barrett ES, Bush NR, Swan SH, Wang C, et al. , 2020. Prenatal sex hormones and behavioral outcomes in children. Psychoneuroendocrinology 113, 104547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day DB, Collett BR, Barrett ES, Bush NR, Swan SH, Nguyen RHN, et al. , 2021. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ. Int 147, 106330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D, Peng J, Szpiro AA, 2023. Wqspt: Permutation test for weighted quantile sum regression. R package version 1, 1. [Google Scholar]
- Day DB, Sathyanarayana S, LeWinn KZ, Karr CJ, Mason WA, Szpiro AA, 2022. A permutation test-based approach to strengthening inference on the effects of environmental mixtures: Comparison between single-index analytic methods. Environ. Health. Perspect 130, 87010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, Wolff MS, 2017. Prenatal phthalate biomarker concentrations and performance on the bayley scales of infant development-ii in a population of young urban children. Environ. Res 152, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatman JA, Dunlop AL, Barr DB, Corwin EJ, Hill CC, Brennan PA, et al. , 2023. Exposure to phthalate metabolites, bisphenol a, and psychosocial stress mixtures and pregnancy outcomes in the atlanta african american maternal-child cohort. Environ. Res 233, 116464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaredar M, Nyanza EC, Ten Eycke K, Dewey D, 2015. Phthalate exposure and childrens neurodevelopment: A systematic review. Environ. Res 142, 51–60. [DOI] [PubMed] [Google Scholar]
- England-Mason G, Martin JW, MacDonald A, Kinniburgh D, Giesbrecht GF, Letourneau N, et al. , 2020. Similar names, different results: Consistency of the associations between prenatal exposure to phthalates and parent-ratings of behavior problems in preschool children. Environ. Int 142, 105892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Rosen EM, Barrett ES, Nguyen RHN, Bush N, McElrath TF, et al. , 2019a. Joint impact of phthalate exposure and stressful life events in pregnancy on preterm birth. Environ. Int 133, 105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Rosen EM, Rosario Z, Feric Z, Calafat AM, McElrath TF, et al. , 2019b. Environmental phthalate exposure and preterm birth in the protect birth cohort. Environ. Int 132, 105099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson KK, Bommarito PA, Arogbokun O, Rosen EM, Keil AP, Zhao S, et al. , 2022. Prenatal phthalate exposure and child weight and adiposity from in utero to 6 years of age. Environ. Health. Perspect 130, 47006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, et al. , 2007. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int. J. Hyg. Environ. Health 210, 21–33. [DOI] [PubMed] [Google Scholar]
- GAPPS. 2023. Gapps-global alliance to prevent prematurity and stillbirth. Available: https://www.gapps.org/ [accessed August 15 2023].
- Gilliom M, Shaw DS, 2004. Codevelopment of externalizing and internalizing problems in early childhood. Dev. Psychopathol 16, 313–333. [DOI] [PubMed] [Google Scholar]
- Guilbert A, Rolland M, Pin I, Thomsen C, Sakhi AK, Sabaredzovic A, et al. , 2021. Associations between a mixture of phenols and phthalates and child behaviour in a french mother-child cohort with repeated assessment of exposure. Environ. Int 156, 106697. [DOI] [PubMed] [Google Scholar]
- Guo Y, Weck J, Sundaram R, Goldstone AE, Louis GB, Kannan K, 2014. Urinary concentrations of phthalates in couples planning pregnancy and its association with 8-hydroxy-2’-deoxyguanosine, a biomarker of oxidative stress: Longitudinal investigation of fertility and the environment study. Environ. Sci. Technol 48, 9804–9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlisníková H, Nagyová M, Kolena B, Mlynček M, Trnovec T, Petrovičová I 2022. The joint effect of perceived psychosocial stress and phthalate exposure on hormonal concentrations during the early stage of pregnancy: A cross-sectional study. Children (Basel) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung R, Reed L, 1989. Estimation of average concentration in the presence of nondetectable values. Appl. Occupat. Environ. Hygiene 5, 46–51. [Google Scholar]
- Huang HB, Kuo PH, Su PH, Sun CW, Chen WJ, Wang SL, 2019. Prenatal and childhood exposure to phthalate diesters and neurobehavioral development in a 15-year follow-up birth cohort study. Environ. Res 172, 569–577. [DOI] [PubMed] [Google Scholar]
- Hyland C, Mora AM, Kogut K, Calafat AM, Harley K, Deardorff J, et al. , 2019. Prenatal exposure to phthalates and neurodevelopment in the chamacos cohort. Environ. Health. Perspect 127, 107010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip KI, Jester JM, Sameroff A, Olson SL, 2019. Linking research domain criteria (rdoc) constructs to developmental psychopathology: The role of self-regulation and emotion knowledge in the development of internalizing and externalizing growth trajectories from ages 3 to 10. Dev. Psychopathol 31, 1557–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, et al. , 2016. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int 96, 118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janer G, Verhoef A, Gilsing HD, Piersma AH, 2008. Use of the rat postimplantation embryo culture to assess the embryotoxic potency within a chemical category and to identify toxic metabolites. Toxicol. In. Vitro 22, 1797–1805. [DOI] [PubMed] [Google Scholar]
- Jankowska A, Polańska K, Hanke W, Wes ołowska E, Ligocka D, Waszkowska M, et al. , 2019. Prenatal and early postnatal phthalate exposure and child neurodevelopment at age of 7 years - polish mother and child cohort. Environ. Res 177, 108626. [DOI] [PubMed] [Google Scholar]
- Jankowska A, Nazareth L, Kaleta D, Polanska K, 2021. Review of the existing evidence for sex-specific relationships between prenatal phthalate exposure and children’s neurodevelopment. Int. J. Environ. Res. Public. Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, Jönsson BA, Bonde JP, Hougaard DM, et al. , 2015. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology 26, 91–99. [DOI] [PubMed] [Google Scholar]
- Kannan K, Stathis A, Mazzella MJ, Andra SS, Barr DB, Hecht SS, et al. , 2021. Quality assurance and harmonization for targeted biomonitoring measurements of environmental organic chemicals across the children’s health exposure analysis resource laboratory network. Int. J. Hyg. Environ. Health 234, 113741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, et al. , 2018. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age-check cohort study. Sci. Total. Environ 624, 377–384. [DOI] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, et al. , 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ. Health. Perspect 122, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Kim MS, Lim YH, Lee N, Hong YC, 2018. Prenatal and postnatal exposure to di-(2-ethylhexyl) phthalate and neurodevelopmental outcomes: A systematic review and meta-analysis. Environ. Res 167, 558–566. [DOI] [PubMed] [Google Scholar]
- Levine L, Fahy J, 1945. Evaluation of urinary lead concentrations. I. The significance of the specific gravity. J. Ind. Hyg. Toxicol 27, 217–223. [Google Scholar]
- LeWinn KZ, Karr CJ, Hazlehurst M, Carroll K, Loftus C, Nguyen R, et al. , 2022. Cohort profile: The echo prenatal and early childhood pathways to health consortium (echo-pathways). BMJ. Open 12, e064288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Papandonatos GD, Calafat AM, Yolton K, Lanphear BP, Chen A, et al. , 2020. Gestational and childhood exposure to phthalates and child behavior. Environ. Int 144, 106036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, et al. , 2015. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan maternal and infant cohort study. Environ. Health. Perspect 123, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon N, Kingsbury M, Mahedy L, Evans J, Colman I, 2018. The association between prenatal stress and externalizing symptoms in childhood: Evidence from the avon longitudinal study of parents and children. Biol. Psychiatry 83, 100–108. [DOI] [PubMed] [Google Scholar]
- Meakin AS, Cuffe JSM, Darby JRT, Morrison JL, Clifton VL, 2021. Let’s talk about placental sex, baby: Understanding mechanisms that drive female- and male-specific fetal growth and developmental outcomes. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatoya M, Kishi R, 2021. A review of recent studies on bisphenol a and phthalate exposures and child neurodevelopment. Int. J. Environ. Res. Public. Health 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroña-Zhou A, Coccia M, Sullivan A, O’Connor TG, Collett BR, Derefinko K, et al. , 2023. A multi-cohort examination of the independent contributions of maternal childhood adversity and pregnancy stressors to the prediction of children’s anxiety and depression. Res. Child. Adolesc. Psychopathol 51, 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Nachman RM, Cooper GS, 2020. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int 137, 105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos AM, Marceau K, Neiderhiser JM, De Araujo-Greecher M, Natsuaki MN, Leve LD, 2020. Maternal consistency in recalling prenatal experiences at 6 months and 8 years postnatal. J. Dev. Behav. Pediatr 41, 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Curtin P, Just A, Bello G, Gennings C. 2021. Gwqs. R package version 3.0.4. Available: https://cran.r-project.org/web/packages/gWQS/index.html. [Google Scholar]
- Rescorla LA, Achenbach TM, Ivanova MY, Harder VS, Otten L, Bilenberg N, et al. , 2011. International comparisons of behavioral and emotional problems in preschool children: Parents’ reports from 24 societies. J. Clin. Child. Adolesc. Psychol 40, 456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, et al. , 2016. Lest we forget: Comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J. Child. Psychol. Psychiatry 57, 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha BA, Asimakopoulos AG, Barbosa F Jr., Kannan K, 2017. Urinary concentrations of 25 phthalate metabolites in brazilian children and their association with oxidative DNA damage. Sci. Total. Environ 586, 152–162. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Liu M, Hurn PD, 2009. Brain aromatization: Classic roles and new perspectives. Semin. Reprod. Med 27, 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, 2015. Sex-specific placental responses in fetal development. Endocrinology 156, 3422–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology 1, 43–46. [PubMed] [Google Scholar]
- Seymore TN, Rivera-Núñez Z, Stapleton PA, Adibi JJ, Barrett ES, 2022. Phthalate exposures and placental health in animal models and humans: A systematic review. Toxicol. Sci 188, 153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer RM, Ferguson KK, Sheppard L, James-Todd T, Butts S, Chandrasekaran S, et al. , 2019. Maternal urinary phthalate metabolites in relation to gestational diabetes and glucose intolerance during pregnancy. Environ. Int 123, 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H, Wikstrom S, Jönsson BAG, Lindh CH, Svensson Å, Nånberg E, et al. ,¨ 2018. Prenatal phthalate exposure was associated with croup in swedish infants. Acta. Paediatr 107, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burnms R, Shih R, Griffin B, Martin L, Chandra A, et al. 2015. The urban child institute candle study: Methodological overview and baseline sample description.RAND Corporation. [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, Janssen S, Liu F, Nguyen RH, et al. , 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod 30, 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tearne JE, Allen KL, Herbison CE, Lawrence D, Whitehouse AJ, Sawyer MG, et al. , 2015. The association between prenatal environment and children’s mental health trajectories from 2 to 14 years. Eur. Child. Adolesc. Psychiatry 24, 1015–1024. [DOI] [PubMed] [Google Scholar]
- van den Dries MA, Ferguson KK, Keil AP, Pronk A, Spaan S, Ghassabian A, et al. , 2021a. Prenatal exposure to nonpersistent chemical mixtures and offspring iq and emotional and behavioral problems. Environ. Sci. Technol 55, 16502–16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Dries MA, Keil AP, Tiemeier H, Pronk A, Spaan S, Santos S, et al. , 2021b. Prenatal exposure to nonpersistent chemical mixtures and fetal growth: A population-based study. Environ. Health. Perspect 129, 117008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch BM, Keil AP, Buckley JP, Calafat AM, Christenbury KE, Engel SM, et al. , 2022. Associations between prenatal urinary biomarkers of phthalate exposure and preterm birth: A pooled study of 16 us cohorts. JAMA. Pediatr 176, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H, 1980. A heteroskdasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica 48, 817–838. [Google Scholar]
- Whitehead NS, Brogan DJ, Blackmore-Prince C, Hill HA, 2003. Correlates of experiencing life events just before or during pregnancy. J. Psychosom. Obstet. Gynaecol 24, 77–86. [DOI] [PubMed] [Google Scholar]
- Willner CJ, Gatzke-Kopp LM, Bray BC, 2016. The dynamics of internalizing and externalizing comorbidity across the early school years. Dev. Psychopathol 28, 1033–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Davies DC, 2007. The control of sexual differentiation of the reproductive system and brain. Reproduction 133, 331–359. [DOI] [PubMed] [Google Scholar]
- Yan D, Jiao Y, Yan H, Liu T, Yan H, Yuan J, 2022. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: A systematic review and meta-analysis. Environ. Health 21, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.
ECHO-PATHWAYS shares data through the NIH ECHO consortium, which can be used for approved ECHO analysis proposals using ECHO-wide data. Policies for use of ECHO data can be obtained through the ECHO coordinating center, echocc@duke.edu. ECHO data are also available through the publicly NICHD DASH repository (https://dash.nichd.nih.gov/). De-identified data from this particular analysis may be available upon reasonable request, subject to IRB review, provision of an appropriate analysis plan, and a formal data use agreement.


