Abstract
NAD kinase was exclusively found in the soluble portion of the cell components. The enzyme was purified about 100-fold from sucrose extracts of spinach leaves.
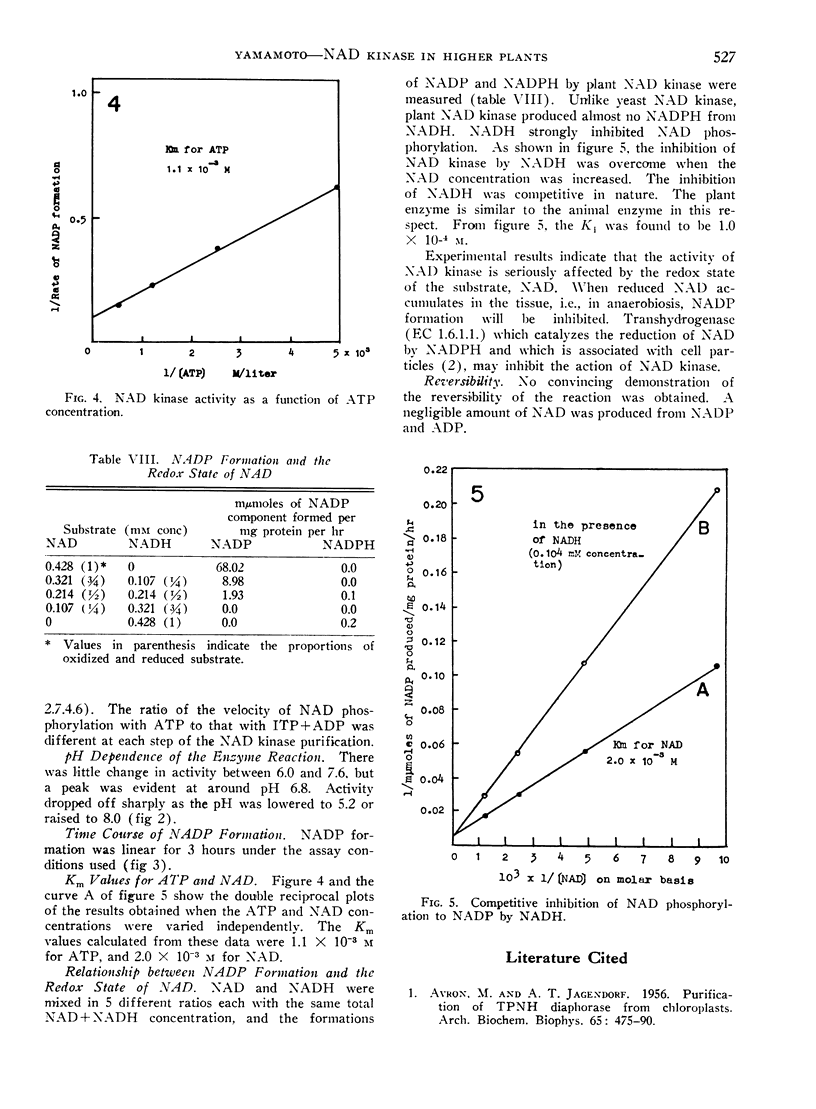
Plant NAD kinase catalyzes formation of NADP from NAD, ATP, and Mg++, but scarcely any formation of NADPH from NADH. NADH was a very potent competitive inhibitor of NAD phosphorylation by plant NAD kinase. The Ki was 1.0 × 10−4 m.
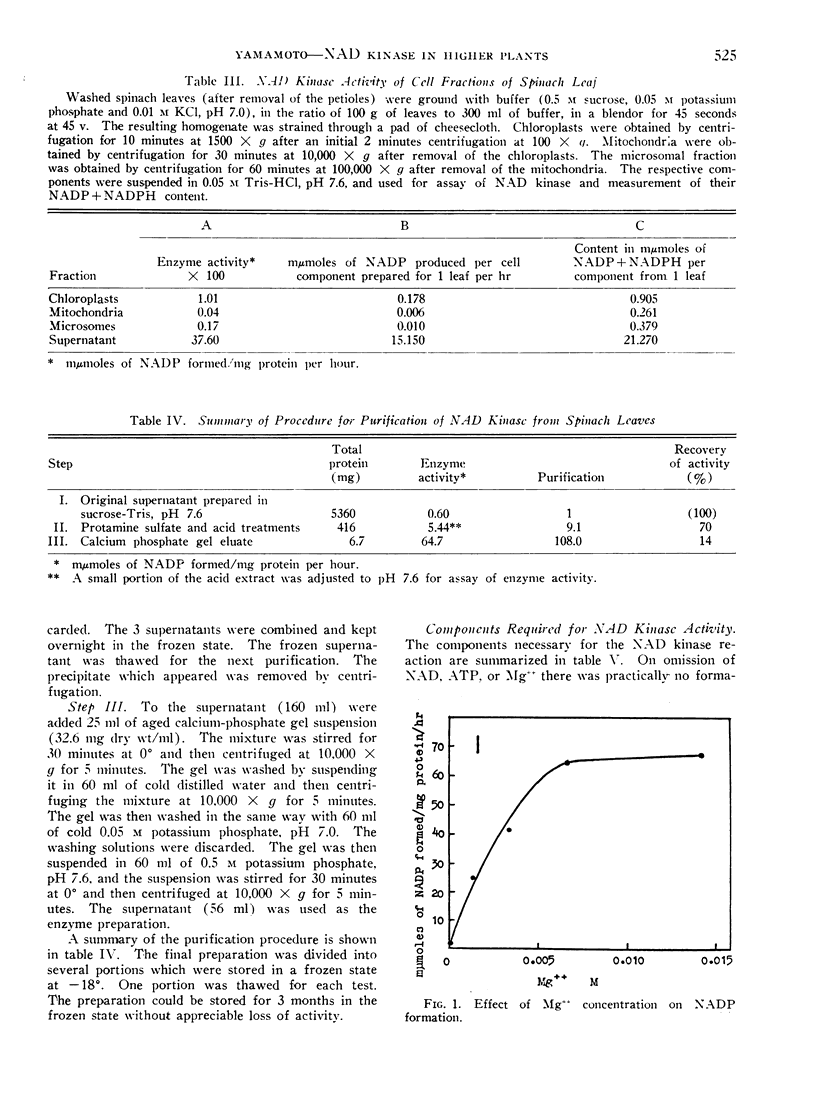
The concentration of Mg++ required to produce maximal activity was 10−2m. Co++ or Mn++ could replace Mg++ in the system.
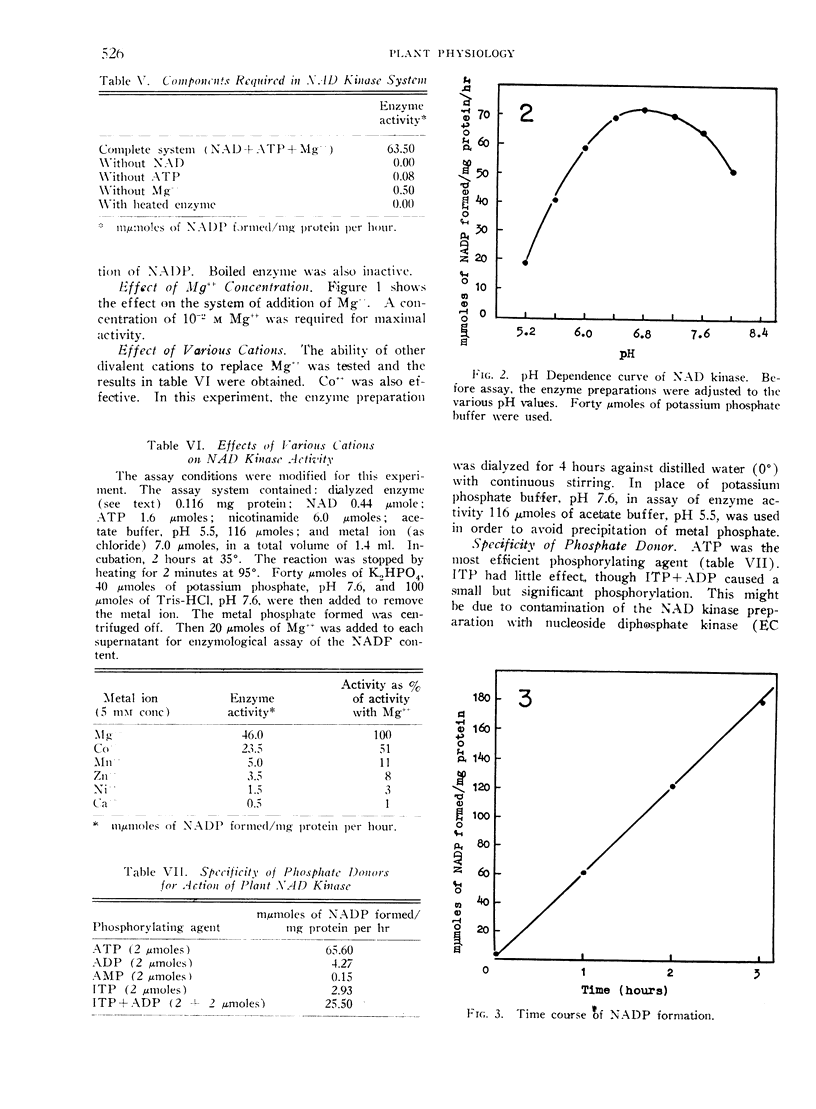
The pH optimum was 6.8. The Km for NAD was 2.0 × 10−3 m and that for ATP was 1.1 × 10−3 m. No convincing demonstration of the reversibility of the reaction was obtained.
It was inferred from above properties of the enzyme that NADP formation ceases in plant tissues in which reduced NAD is accumulated, or in anaerobic tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M., JAGENDORF A. T. A TPNH diaphorase from chloroplasts. Arch Biochem Biophys. 1956 Dec;65(2):475–490. doi: 10.1016/0003-9861(56)90207-7. [DOI] [PubMed] [Google Scholar]
- WANG T. P., KAPLAN N. O. Kinases for the synthesis of coenzyme A and triphosphopyridine nucleotide. J Biol Chem. 1954 Jan;206(1):311–325. [PubMed] [Google Scholar]
- Yamamoto Y. Pyridine Nucleotide Content in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1963 Jan;38(1):45–54. doi: 10.1104/pp.38.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Variation of nicotinamide adenine dinucleotide phosphate level in bean hypocotyls in relation to o(2) concentration. Plant Physiol. 1966 Mar;41(3):519–522. doi: 10.1104/pp.41.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


