Abstract
Background
Intravenous thrombolysis (IVT) is an effective stroke therapy that remains underused. Currently, the use of IVT in patients with recent direct oral anticoagulant (DOAC) intake is not recommended. In this study we aim to investigate the safety and efficacy of IVT in patients with acute ischemic stroke and recent DOAC use.
Methods and Results
A systematic review and meta‐analysis of proportions evaluating IVT with recent DOAC use was conducted. Outcomes included symptomatic intracranial hemorrhage, any intracranial hemorrhage, serious systemic bleeding, and 90‐day functional independence (modified Rankin scale score 0–2). Additionally, rates were compared between patients receiving IVT using DOAC and non‐DOAC by a random effect meta‐analysis to calculate pooled odds ratios (OR) for each outcome. Finally, sensitivity analysis for idarucizumab, National Institutes of Health Stroke Scale, and timing of DOAC administration was completed. Fourteen studies with 247 079 patients were included (3610 in DOAC and 243 469 in non‐DOAC). The rates of IVT complications in the DOAC group were 3% (95% CI, 3–4) symptomatic intracranial hemorrhage, 12% (95% CI, 7–19) any ICH, and 0.7% (95%CI, 0–1) serious systemic bleeding, and 90‐day functional independence was achieved in 57% (95% CI, 43–70). The rates of symptomatic intracranial hemorrhage (3.4 versus 3.5%; OR, 0.95 [95% CI, 0.67–1.36]), any intracranial hemorrhage (17.7 versus 17.3%; OR, 1.23 [95% CI, 0.61–2.48]), serious systemic bleeding (0.7 versus 0.6%; OR, 1.27 [95% CI, 0.79–2.02]), and 90‐day modified Rankin scale score 0–2 (46.4 versus 56.8%; OR, 1.21 [95% CI, 0.400–3.67]) did not differ between DOAC and non‐DOAC groups. There was no difference in symptomatic intracranial hemorrhage rate based on idarucizumab administration.
Conclusions
Patients with acute ischemic stroke treated with IVT in recent DOAC versus non‐DOAC use have similar rates of hemorrhagic complications and functional independence. Further prospective randomized trials are warranted.
Keywords: acute ischemic stroke, coagulopathy, direct oral anticoagulants, idarucizumab, intravenous thrombolysis, safety, symptomatic intracranial hemorrhage
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- DOAC
direct oral anticoagulant
- ICH
intracranial hemorrhage
- IVT
intravenous thrombolysis
- sICH
symptomatic intracranial hemorrhage
- SSB
serious systemic bleeding
Clinical Perspective.
What Is New?
Despite multiple guidelines advising against the administration of intravenous thrombolysis to patients who have taken direct oral anticoagulants within 48 hours of experiencing a stroke, the results of this study indicate that intravenous thrombolysis does not lead to a higher occurrence of hemorrhagic complications in comparison to those not on direct oral anticoagulants, and the rates of achieving functional independence also appear similar between the 2 groups.
What Are the Clinical Implications?
Given the lack of prospective trials offering a more comprehensive evaluation of the risk‐to‐benefit ratio within this particular patients' subset, the consideration of intravenous thrombolysis could find validation in scenarios where the timeline of recent direct oral anticoagulants usage remains uncertain.
Additionally, the incidence of symptomatic intracranial hemorrhage demonstrated no significant variation between patients who had undergone idarucizumab administration (antidote) before intravenous thrombolysis and those who had not received it.
Stroke is the leading cause of acquired disability in adults and the second leading cause of mortality worldwide. 1 One‐quarter of ischemic strokes are due to cardioembolism, primarily caused by atrial fibrillation (AF), leading to more significant infarcts and severe neurological deficits. 2 , 3 Direct oral anticoagulants (DOAC) reduce the risk of ischemic stroke in patients with AF and are increasingly used for stroke prevention in these patients. 4 According to Navar et al, in a cohort of patients with AF, there was a significant increase in DOAC use from 4.7% to 47.9% between 2011 and 2020, as well as a remarkable decline of warfarin use from 52.4% to 17.7% during the same period of time. 5 Despite their efficacy in stroke prevention, the annual risk of ischemic stroke is 1% to 2% in patients taking DOAC. 6 A significant portion of those patients presents within the time window for acute reperfusion therapy, but the safety of thrombolytic administration in this population is unknown. 6 , 7 , 8
Intravenous thrombolysis (IVT) has been a standard of acute stroke treatment for over 3 decades. 9 Several studies have demonstrated the efficacy of IVT in appropriately selected patients. 9 , 10 Unfortunately, IVT remains underused in the community. 11 In recent years, the American Heart Association guidelines have eased the contraindications to IVT with the goal to increase the number of eligible patients that could benefit from its therapeutic effect. 12 Patients taking anticoagulants have been traditionally excluded from all IVT studies due to concern for an increased risk of major bleeding, including intracerebral hemorrhage. Thus, several guidelines recommend against the use of IVT in patients with DOAC intake within 48 hours of stroke onset (DOAC <48 hours) 12 , 13 , 14 , 15 or abnormal coagulation test results. 12 , 13 , 14 , 15 , 16 Emerging evidence from case series, hospital‐based cohorts, and stroke registries suggests IVT may be safe in patients with recent DOAC use. In addition, in the absence of readily available biomarkers, it is often unclear in practice if and when the patient last took a DOAC. Herein, we aim to evaluate the safety and efficacy of patients with acute ischemic stroke receiving IVT in the context of a recent DOAC therapy and compare them with a cohort that did not use DOAC before receiving IVT.
METHODS
This systematic review and meta‐analysis is reported following the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis guidelines. 17 The study is registered with International Prospective Register of Systematic Reviews, ID code: CRD42023400706. No institutional review board approval or informed consent was necessary for conducting this meta‐analysis. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Inclusion and Exclusion Criteria
Studies
Our systematic search included all the randomized controlled trials, cohort studies, cross‐sectional studies, case series (5 or more patients), and case–control studies of patients taking DOAC who received IVT for ischemic stroke. The control group consisted of a similar population not taking DOAC. Studies were included if they contained the intervention of interest or head‐to‐head comparisons and at least 1 of the outcomes of interest described herein. There was no restriction on language or country. We excluded case reports, abstracts, posters, review articles, studies in nonhumans, and studies discussing any of the following: (1) interventions other than IVT with alteplase or tenecteplase (for instance intra‐atrial thrombolytic therapy with tissue plasminogen activator [tPA]), (2) patients who were taking other oral anticoagulants such as warfarin before the ischemic stroke, (3) patients who were on any nonoral anticoagulants such as heparin or enoxaparin before the ischemic stroke, (4) coadministration of tPA and other anticoagulants (eg, argatroban), and (5) presence of obvious contraindications for thrombolysis rather than treatment with DOAC.
Participants
Participants were adults (≥18 years old) with recent use or nonuse of DOAC, presented with acute ischemic stroke and treated with IVT, with or without specific antidote before IVT, as well as with or without mechanical thrombectomy following IVT.
Interventions
IVT consisted of acute intravenous administration of either alteplase or tenecteplase. 12 , 13 , 18 The doses of alteplase used were 0.9 mg/kg, 0.6 mg/kg, or 0.7 mg/kg, depending on regional guidelines and practice. Tenecteplase was administered at doses of 0.4 mg/kg or 0.25 mg/kg. All doses were administered within 4.5 hours from the last known well time.
Primary and Secondary Outcomes
Our primary outcome was symptomatic intracranial hemorrhage (sICH), which was defined according to each study's criteria. The sICH definitions that were used in the included studies are presented in Table S1. 19
Secondary outcomes included any intracranial hemorrhage (ICH), serious systemic bleeding (SSB) (any severe or life‐threatening systemic hemorrhage requiring blood transfusion or causing hemodynamic impairment caused by administering IVT), and functional independence at 90 days. A modified Rankin scale score of 0 to 2 defined a favorable functional outcome. The modified Rankin scale is a categorical scale (0, no symptoms; 6, death) that reflects the degree of disability after a stroke. A score of 0 to 2 equates to independence in activities of daily living, and a score of 0 to 1 is considered as excellent functional outcome. 20
Search Strategy and Studies Selection
We performed a systematic electronic literature search by entries to PubMed, Embase, Web of Science, Scielo, and the Cochrane Library Central Register of Controlled Trials through March 2023. To identify further studies, we screened the reference list of relevant records. The complete search strategy is detailed in Table S2.
Two independent reviewers (F.K., A.A.Q.) preliminarily screened all identified records. Next, all titles and abstracts were reviewed using an online application for systematic reviews (https://rayyan.qcri.org/). All relevant studies were assessed then as full text by 2 reviewers (M.G., M.A.) independently, who identified studies for inclusion and recorded reasons for exclusion. Then, reviewers extracted data using a data collection tool designed as a table and cross‐checked the extracted data. Any disagreements were resolved through discussion with the senior reviewer (S.O.G.).
The data for the non‐DOAC cohort were directly extracted from the previously published systemic review by Shahjouei et al in 2019. 21 In addition, we used that systematic review to extract the sICH data for the DOAC cohort by Seiffge et al. 21 , 22 , 23 When 1 study used more than 1 sICH definition to calculate the event rate, we included in our analysis the data from the definition with higher event rates.
Baseline Data and Outcome Variables
We extracted the following data, if available: authors, year of publication, affiliated institutions, type of study, patient demographics and characteristics, including the number of participants, age, sex, comorbidities, smoking status, ischemic events, type of DOAC, stroke workflow time metrics, receiving antidote, blood glucose level, creatinine level, admission systolic blood pressure, medications received during an intervention, type of intervention, time of follow‐up, complications including sICH, any ICH and SSB, as well as functional independence at 90 days.
Risk of Bias Assessment
Two independent reviewers (M.G., M.D.) evaluated the quality of the studies according to the study's design. The included studies consisted of cohorts with and without control groups. Thus, we used the Risk of Bias in Non‐randomized Studies of Interventions, 24 with the overall risk of bias categorized as low, moderate, serious, and critical. 24 , 25
Statistical Analysis
Measures of Intervention Effect
For single‐arm studies, we pooled rates and obtained a weighted overall proportion with 95% CI. We used generalized linear mixed models when 1 or more studies had small sample size (<50) or events (<10), and when the overall number of events was either too small or too big (<10%, >90%); all of our meta‐analyses of proportions met these criteria, thus only the generalized linear mixed models approach was used. 26 , 27
To pool studies with pairwise comparisons, we performed meta‐analysis of binary outcome data. We used the Mantel–Haenszel method to calculate the weighted estimate of intervention effect and reported odds ratios (ORs) and 95% CIs.
Our primary approach was to use a random‐effects model for all the meta‐analyses. Sensitivity analyses with the fixed effects method were conducted for the meta‐analyses of binary outcome data. To account for inaccuracies from the application of the generalized linear mixed models approach, we also conducted fixed‐effects analyses using the Clopper–Pearson method for the meta‐analyses of proportions. If these results were similar to the random‐effects approach, we reported only the latest.
Heterogeneity and Publication Bias
To evaluate between‐study variability, we calculated the tau‐squared (Tau2) using the Paul–Mandel and the Q‐profile methods for analyses of binary outcome data and the maximum‐likelihood estimator for analyses of proportions. 28 Additionally, we reported prediction intervals for all the meta‐analyses. 29 For the primary outcome, we performed subgroup meta‐analyses to explore the sources of heterogeneity and differences between subgroups. Publication bias was assessed when there were 10 studies or more for a given outcome. This was done using Egger's test and through the visual inspection of asymmetry in funnel plots.
Sensitivity and Subgroup Analyses
To further account for statistical heterogeneity, we conducted analyses in prespecified subpopulations: (1) patients taking DOAC who received idarucizumab, (2) patients who did not receive idarucizumab, (3) patients who ingested DOAC in the prior 48 hours, and (4) patients who ingested DOAC in the prior 48 hours and did not receive idarucizumab. We used subgroup meta‐analysis methods and observed the effect on calculated heterogeneity.
We performed further analysis for patients with recent DOAC use and treated with IVT for sICH outcome based on the National Institutes of Health Stroke Scale (NIHSS) score (≤10 versus >10), and idarucizumab administration before IVT (received versus not), using subgroup meta‐analysis methods. 28 Additionally, for the outcome “mortality at 90 days,” we generated Baujat plots 29 to identify outliers and conducted an influence analysis, which consisted of the repetition of the analysis multiple time, taking out 1 study at a time to evaluate the differences in effect size. 30
All statistical analyses and graphs included in this study were performed using R Statistical Software (version 4.1.3) and R Studio. A 2‐tailed P value of 0.05 or less was considered statistically significant.
Certainty of the Evidence
Following Cochrane recommendations, 2 reviewers (M.G., M.G.C.) assessed the quality of the body of evidence using the grading of recommendation, assessment, development, and evaluation approach. For each outcome, we downgraded the evidence for serious study limitations in risk of bias, indirectness of evidence, inconsistency, imprecision of effect estimates, or publication bias, and analyses where only pooled proportions were calculated. It is reported in Table S3 as a summary of the findings, performed in the the grading of recommendation, assessment, development, and evaluation online tool (http://gradepro.org). 31
RESULTS
Study Selection
We identified 4903 titles and abstracts, of which 3484 were eligible for screening (Figure S1). In the full‐text evaluation, we excluded 65 documents (Table S4) and included 14 studies.
Study Characteristics
Eight nonrandomized comparative cohorts were included with 246 933 participants, 3464 in the DOAC arm and 243 469 in non‐DOAC arm. Six single‐arm cohorts were included, with 146 patients taking DOAC who received IVT (Table 1). The data for the non‐DOAC cohort of 2 studies (Suzuki et al and Shahjouei et al) were directly extracted from the previously published systematic review by Shahjouei et al in 2019. 21 In addition, we used that systematic review to extract the sICH data for the DOAC cohort by Seiffge et al. 22 , 23
Table 1.
Patient Baseline Characteristics
| Study | No. of patients | Age, y, mean (SD) | Female sex(%) | Atrial fibrillation (%) | Stroke or transient ischemic attack (%) | Hypertension (%) | Diabetes (%) | Hyperlipidemia (%) | Smoking (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | |
| Seiffge et al 2017 22 , 23 | 78 | 8938 | 76 (68–81)* | 71 (60–79)* | 49.3 | 43.9 | 87.2 | 24.3 | 25.4 | 14 | 87.1 | 63.2 | 24.3 | 16.8 | 49.2 | 41.4 | … | … |
| Meinel et al 2023 32 | 832 | 32 375 | 78 (10) | 71 (13) | 42.6 | 43.5 | 73 | 12.3 | … | … | 67.9 | 61.9 | 20.7 | 19.5 | 38.7 | 37.3 | 11.4 | 17.9 |
| Kam et al 2022 33 | 2207 | 160 831 | 74 (13) | 70 (17) | 46.2 | 49.1 | 73.1 | 14.6 | 38.1 | 29.4 | 79.4 | 71.9 | 32.5 | 29.2 | 49.8 | 44.8 | 10.9 | 18 |
| Okada et al 2022 34 | 40 | 753 | 80 (10) | 76 (12) | 27.5 | 37 | 90 | 31.3 | 27.5 | 13.6 | 82.5 | 71.3 | 20 | 20.4 | 52.5 | 52.8 | 27.5 | 26.1 |
| Frol et al 2021 35 | 22 | 182 | 75 (10) | 75 (14) | 50 | 47.8 | … | … | 9 | 7.7 | 72.7 | 67 | 4.5 | 20.3 | … | … | … | … |
| Xian et al 2017 36 | 251 | 41 136 | 74 (12) | 71 (17) | 51.3 | 50 | 78 | 18 | 30.6 | 25.6 | 78.9 | 73.2 | 25.5 | 27.2 | 42.2 | 43.5 | 7.5 | 17.8 |
| Beharry et al 2020 37 | 13 | 78 (13) | 30.7 | 100 | 38.5 | 53.8 | 15.4 | … | … | |||||||||
| Fang et al 2019 38 | 10 | 71(8) | 10 | 100 | 70 | 100 | 10 | … | … | |||||||||
| Kikule et al 2022 39 | 9 | 76 (9) | 22.2 | 100 | … | … | … | … | … | |||||||||
| Kermer et al 2020 40 | 80 | 76 (11) | 36.2 | 88.7 | … | … | … | … | … | |||||||||
| Šaňák et al 2018 41 | 13 | 70 (9) | 46.1 | 84.6 | … | 92.3 | 30.7 | 46.1 | … | |||||||||
| Tse et al 2017 42 | 6 | 68 (11) | 66.6 | 100 | … | … | … | … | … | |||||||||
| Suzuki et al 2017 43 | 71 16† | 77 (10)‡ and 75 (7)§ | 43.6 | … | 100 | … | 50.7 | … | 71.8 | … | 18.3 | … | 35.2 | … | 31 | … | ||
| Shahjouei et al 2015 44 | 5728† | 70 (11) | … | 40 | … | 60 | … | 20 | … | 100 | … | 60 | … | 40 | … | … | ||
AC indicates anticoagulation group; CTRL, control group; and tPA, tissue plasminogen activator.
Interquartile range.
Data obtained from a previous meta‐analysis (Shahjouei et al, 2020) 21 for our outcomes of interest; baseline characteristics are not reported in original studies.
Patients who received only tPA.
Patients who received tPA and underwent thrombectomy.
In the pooled sample of DOAC patients (3610 patients), the mean age ranged from 68 to 80 years and 45% were women. Comorbidities were present at the following rates: hypertension 77% (1620/3577), diabetes 28% (991/3482), AF 75% (2662/3555), hyperlipidemia 46% (1591/3437), previous stroke/transitory ischemic attack 37% (986/2637), coronary artery disease 28% (729/2597), heart failure 19% (473/2526), chronic kidney disease 8% (180/2247), and smoking 11% (389/3419). Additional studies' characteristics are presented in Table S5. A total of 28% (946/3343) of patients took antiplatelet agents before the onset of stroke. Among the DOAC group with known medication, 46% (593/1299) of patients were prescribed dabigatran, 16% (214/1299) apixaban, 32% (413/1299) rivaroxaban, and 6% (78/1299) edoxaban. Of note, in 64% (2311/3610) of patients, the DOAC was not specified. About 29% (1038/3610) of patients received DOAC with reported last dose within 48 hours of stroke onset, about 48% of them (500/1038), the reported DOAC last use was <24 hours of stroke onset. The rest of the patients had nonspecific last dose ingestion reported (reported to be within 7 days from stroke onset). In addition, 11% (407/3610) received idarucizumab before IVT administration. Additional details regarding DOAC and antidote variables are presented in Table S6.
The median NIHSS score at presentation ranged from 6 to 21. About 22% (748/3326) of patients underwent mechanical thrombectomy. Alteplase was used in all patients except 2% (64/3610) who received tenecteplase. The dose of alteplase was 0.9 mg/kg in all patients except in 5% (189/3546) of patients who were treated with 0.6 mg/kg according to the Japanese national guidelines and 0.08% (3/3546) of patients who were given alteplase 0.7 mg/kg. The tenecteplase dose in 2 of the 64 patients was 0.4 mg/kg, and the remainder (62 patients) received 0.25 mg/kg. Finally, 0.2% (7/3546) of patients were treated with IVT outside the standard 4.5‐hour time window (Table 2).
Table 2.
Details on Stroke Evaluation and Treatment
| Study | National Institutes of Health Stroke Scale score, median (IQR) | Onset to intravenous thrombolysis (min) median (IQR) | Any antiplatelets (%) | Alteplase (%) | Tenecteplase (%) | Endovascular therapy (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | AC | CTRL | |
| Seiffge et al 2017 22 , 23 | 13 (7–19) | 37 (30–60)* | 16.6 | 100 | 0 | 0 | ||||||
| Meinel et al 2023 32 † | 11 (6–17) | 9 (5–16) | 153 (110–210) | 138 (98–190) | 10.6 | 32 | 93.9 | 96.2 | 6.1 | 3.8 | 34.2 | 18.8 |
| Kam et al 2022 33 | 10 (5–17) | 7 (4–14) | 122 (89–168) | 123 (91–168) | 34.8 | 45.6 | 100 | 100 | 0 | 0 | 18.8 | 11.5 |
| Okada et al 2022 34 | 15 (5–24) | 9 (4–17) | 148 (103–190) | 122 (90–173) | 17.5 | 27.3 | 100 | 100 | 0 | 0 | 40 | 25.7 |
| Frol et al 2021 35 | 10.5 (18) | 8 (27) | 140 (−)‡ | … | … | … | 100 | 100 | 0 | 0 | 0 | 0 |
| Xian et al 201736 § | 12 (6–18) | 9 (5–15) | … | … | 30.3 | 47.8 | 100 | 100 | 0 | 0 | … | … |
| Beharry et al 2020 37 | 6 (4–21) | … | 7.7 | 0 | 100 | 38.4 | ||||||
| Fang et al 2019 38 | 14 (12–23) | 11.1 (4.9)ǁ , ‡ | … | 100 | 0 | 10 | ||||||
| Kikule et al 2022 39 | 9 (6–16) | … | … | 100 | 0 | 0 | ||||||
| Kermer et al 2020 40 | 9 (−) | … | … | 100 | 0 | 7.5 | ||||||
| Šaňák et al 2018 41 | 7 (3–24) | 22 (18)§ , ‡ | … | 100 | 0 | 7.7 | ||||||
| Tse et al 2017 42 | 21 (6–22) | 211.5 (185–220) | … | 100 | 0 | 16.6 | ||||||
| Suzuki et al 2017 43 | 13 (8–18)¶, 21 (18–27)# | 159 (48)¶ , #, 148 (51)# , ‡ | 2.8 | 100 | 0 | 21.2 | ||||||
| Shahjouei et al 2015 44 | 10 (2–15) | 115 (29)‡ | … | 100 | 0 | 0 | ||||||
AC indicates anticoagulation group; CTRL, control group; IQR, interquartile range; and tPA, tissue plasminogen activator.
Door to needle time.
Tenecteplase 0.25 mg/kg was given to 51 and 1225 patients of the AC and CTRL groups, respectively.
Mean (SD).
The alteplase dose was 0.6 mg/kg.
Time from the end of idarucizumab injection to intravenous tPA.
Patients who received only tPA.
Patients who received tPA and underwent thrombectomy.
Risk of Bias Among Studies
The results of the quality assessment using Risk of Bias in Non‐randomized Studies of Interventions are shown in Figure S2. Seven studies had a moderate risk of overall bias, and the remaining studies had a serious risk of overall bias. For sICH, funnel plot was considered visually asymmetric (Figure S3). For the rest of outcomes, the test for funnel plot asymmetry was not performed because of the small number of studies included. Egger's tests were not suggestive of publication bias (Table S7).
Measurements of Effects and Sensitivity Analysis
Symptomatic Intracranial Hemorrhage
A meta‐analysis of proportions from 14 studies was performed on 3610 participants who were treated with IVT and were already taking DOAC. 22 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 The sICH rate was 3% (95% CI, 3–4; moderate‐certainty evidence, Tau2=0.00) (Figure 1A). Sensitivity analysis and meta‐analysis of proportions all yielded comparable results or lower odds of sICH (Table S8). Data on sICH were available in a total of 246 933 participants from 8 comparative studies. 22 , 32 , 33 , 34 , 35 , 36 , 43 , 44 There were no differences after IVT between the DOAC arm and the non‐DOAC arm in sICH (3.4% versus 3.5%; OR, 0.95 [95% CI, 0.67–1.36]; moderate‐certainty evidence, Tau2=0.02 [95% CI, 0.00–1.62]) (Figure 1B). Pairwise comparison of studies in which DOAC <48 hours of stroke onset yielded similar results (2.7% versus 3.4%; OR, 0.79 [95% CI, 0.45–1.37], Tau2=0.05 [95% CI, 0.00–5.79]) (Table S9). Studies in which DOAC <48 hours of stroke onset without giving idarucizumab before IVT also yielded to similar results (3.2% versus 3.4%; OR, 0.83 [95% CI, 0.55–1.25], Tau2=0.08 [95% CI, 0.25–3.19]).
Figure 1. Forest plot for IVT in patients were taking DOAC for symptomatic intracranial hemorrhage.
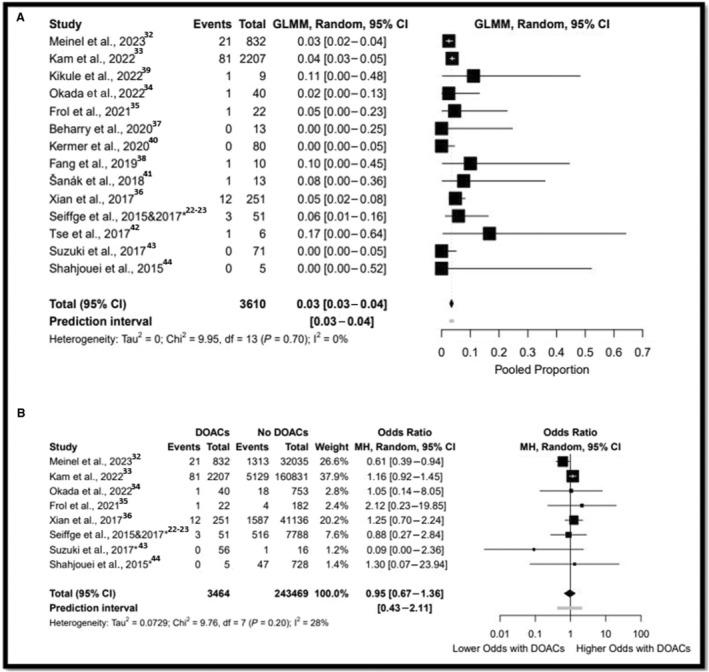
A, Refers to the meta‐analysis of proportions. B, Refers to the comparative analysis. *: direct communication with the authors. DOAC indicates direct oral anticoagulant; GLMM, generalized linear mixed model; IVT, intravenous thrombolysis; and MH, Mantel–Haenszel.
Any Intracranial Hemorrhage
A meta‐analysis of proportions was performed with a total of 1062 participants from 11 studies. 22 , 32 , 34 , 35 , 37 , 38 , 40 , 41 , 42 , 43 , 44 Any ICH rate of the DOAC group who received IVT was 12% (Tau2=0.42 [95% CI, 7–19]) (Figure 2A)]. Sensitivity analysis and meta‐analysis of proportions yielded similar results (Table S8). Data on any ICH were available from 33 816 participants from 3 comparative studies. 32 , 34 , 35 There were no differences after IVT between the DOAC arm and non‐DOAC arm in any ICH (17.7% versus 17.3%; OR, 1.23 [95% CI, 0.61–2.48], Tau2=0.42 [95% CI, 0.00–29.36]) (Figure 2B).
Figure 2. Forest plot for IVT in patients were taking DOAC for any ICH.
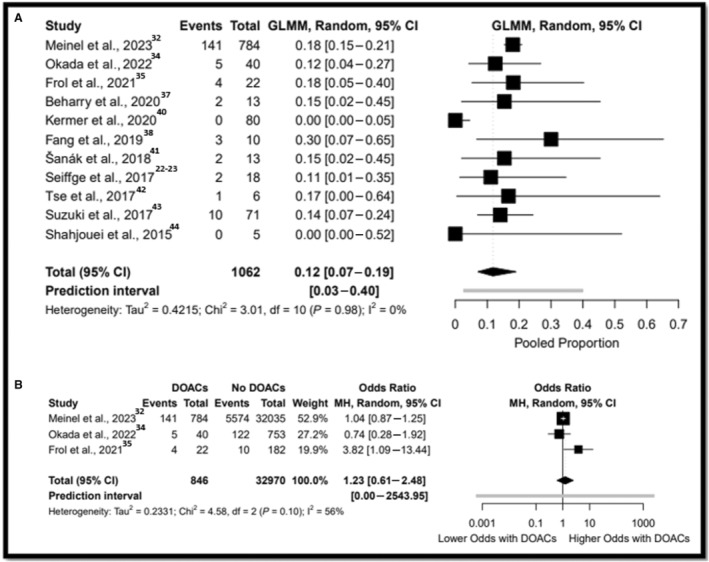
A, Refers to the meta‐analysis of proportions. B, Refers to the comparative analysis. DOAC indicates direct oral anticoagulant; GLMM, generalized linear mixed model; ICH, intracranial hemorrhage; IVT, intravenous thrombolysis; and MH, Mantel–Haenszel.
Serious Systemic Bleeding
A meta‐analysis of proportions was performed in a total of 2602 participants from 7 studies. 33 , 34 , 36 , 40 , 41 , 42 , 44 SSB rate was 0.7% (Tau2=0.00 [95% CI, 0–1]) (Figure 3A). A sensitivity analysis and meta‐analysis of proportions yielded similar results (Table S8). Data on SSB were available in a total of 205 218 participants from 3 comparative studies. 32 , 34 , 35 There were no differences after IVT between the DOAC arm and non‐DOAC arm in SSB (0.7% versus 0.6%; OR, 1.27 [95% CI, 0.79–2.02]; Tau2=0.00 [95% CI, 0.00–25.58]) (Figure 3B).
Figure 3. Forest plot for IVT in patients were taking DOAC for serious systemic bleeding.
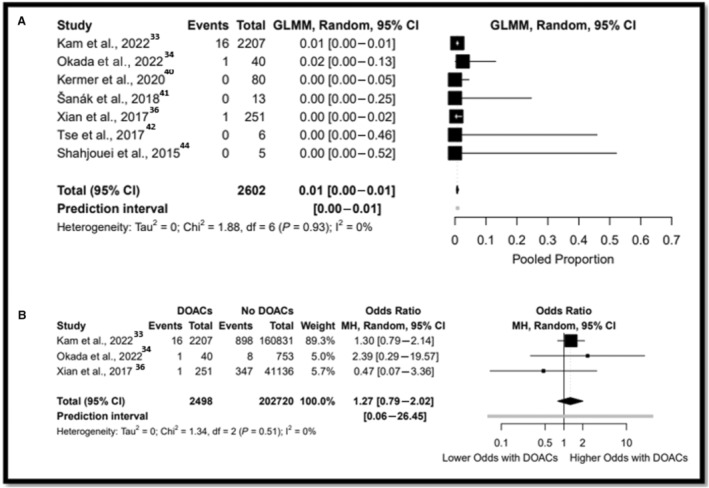
A, Refers to the meta‐analysis of proportions. B, Refers to the comparative analysis. DOAC indicates direct oral anticoagulant; GLMM, generalized linear mixed model; IVT, intravenous thrombolysis; and MH, Mantel–Haenszel.
Functional Independence and Excellent Outcome at 90 Days
A meta‐analysis of proportions was performed in a total of 776 participants from 7 studies. 22 , 32 , 34 , 35 , 37 , 41 , 42 The rate of functional independence at 90 days for the DOAC group who received IVT was 57% (Tau2=0.34% [95% CI, 43–70]) (Figure S4). Sensitivity analysis and meta‐analysis of proportions yielded comparable results (Table S8). Data for favorable functional outcomes were available in a total of 30 687 participants from 3 comparative studies. 32 , 34 , 35 There were no differences after IVT between the DOAC arm and the control arm in achieving independent functional status (46.4% versus 56.8%; OR, 1.215 [95% CI, 0.400–3.67], Tau2=0.93 [95% CI, 0.08–47.13]) (Figure S4). A meta‐analysis of proportion of excellent outcome at 90 days for the DOAC group who received IVT in a total of 1542 participants from 3 studies revealed a pooled rate of 27% (Tau2=0% [95% CI, 25–29]) (Figure S5).
Mortality
A meta‐analysis of proportion of mortality for the DOAC group who received IVT in a total of 786 participants from 8 studies revealed a pooled rate of 17% (Tau2=0.00 [95% CI, 15–20]) (Figure S6). The results of sensitivity analysis and the Baujat plot for this outcome are presented in Figures S7 and S8. Data for mortality were available in a total of 30 687 participants from 3 comparative studies. 32 , 34 , 35 There was a significant increase in mortality in the DOAC arm as compared with the control arm (17% versus 13; OR, 1.43 [95% CI, 1.18–1.74], Tau2=0.00 [95% CI, 0.00–5.66]) (Figure S9). Omitting Meinel et al 32 rendered this relationship and resulted in no significant difference between the DOAC arm and the control arm (OR, 1.33 [95% CI, 0.50–3.55]) (Figure S10). Furthermore, the Baujat plot identified Meinel et al 32 as an outlier study with the biggest influence on the overall results (Figure S11).
Subgroup Analysis in Patients With Recent DOAC Use and Treated With IVT
Subgroup analysis for sICH between patients (Table 3) based on the NIHSS score (≤10 versus >10) and idarucizumab administration before IVT (received versus not) did not reveal any significant difference between the subgroups (Figure S12).
Table 3.
Subgroup Analysis for Symptomatic Intracranial Hemorrhage
| Subgroup | No. of studies | No. of participants | Pooled rate (95% CI) | P value of subgroup testing |
|---|---|---|---|---|
| Median National Institutes of Health Stroke Scale score | ||||
| >10 | 8 | 1250 | 3% (2%–4%) | 0.46 |
| ≤10 | 6 | 2327 | 4% (3%–4%) | |
| Idarucizumab | ||||
| Yes | 8 | 405 | 3% (1%–7%) | 0.54 |
| No | 6 | 3150 | 4% (3%–4%) | |
Synthesis of Results
The effects of interventions with the certainty assessment are presented on the summary of findings tables (Table S10). A summary of the tau‐squared of each analysis is showed in Table S11.
DISCUSSION
Our systematic review and meta‐analysis evaluate the safety and efficacy of using IVT in a diverse multinational large population of patients who are taking DOAC therapy. In this study, we observed no significant difference in rates of sICH, SSB, any ICH, and independent functional status between patients with recent DOAC use and those not taking DOAC following the administration of IVT. Furthermore, there was no significant difference in sICH rate among those groups when we selectively studied IVT in patients with DOAC use within 48 hours before stroke onset or based on reception of idarucizumab before IVT (received versus not), as well as in higher NIHSS score (>10 versus ≤10). These results further add to the growing evidence that supports the safety and feasibility of using IVT in selective patients with ischemic stroke and recent DOAC use.
These findings align with previous observations. Shahjouei et al conducted a systematic review to examine the safety of IVT in patients who had recently used DOAC. 21 Their study indicated that recent DOAC use did not significantly increase the risk of sICH following IVT administration. However, the study had limitations, including a small sample size (only 366 patients in the DOAC arm), which affected its statistical power. Additionally, the study by Shahjouei et al 21 did not investigate other complications associated with IVT, such as systemic bleeding. Furthermore, the analysis did not assess functional independence at 90 days and excluded patients who had received idarucizumab before IVT. Given these factors, the need for an updated and comprehensive meta‐analysis has arisen, considering recent publications. 11 , 33
Our study examined the occurrence of sICH in patients who received IVT after taking DOAC. The results of our study demonstrated that the rate of sICH in the DOAC group was comparable to the historical rates observed in ischemic stroke patients treated with IVT, as reported in previous studies. 9 , 10 , 45 Moreover, our findings align well with individual reports that have investigated the outcomes of IVT in patients with prior DOAC ingestion. We performed subgroup analyses based on different factors such as ingestion time (within 48 hours versus >48 hours), prior administration of idarucizumab (received versus not), and higher NIHSS scores (>10 versus ≤10). These subgroup analyses did not reveal any significant differences in sICH rates, indicating that these factors did not influence the occurrence of sICH in DOAC‐treated patients undergoing IVT. Additionally, our study compared the incidence of any ICH between patients who received DOAC and those who did not, both of whom underwent IVT. Interestingly, our results indicated no significant difference in the overall incidence of any ICH between the DOAC and non‐DOAC groups. Notably, in our pooled analysis of 1062 DOAC‐treated patients who received IVT, the rate of any ICH was found to be 12%, which is lower than the previously reported rates in the literature. 11
We examined the association between recent use of DOAC and the risk of systemic bleeding in patients treated with IVT. Like the findings in the National Institute of Neurological Disorders and Stroke recombinant tPA trial, we found that recent use of DOAC was not associated with an increased risk of systemic bleeding. 9 , 41 The annual risk of systemic bleeding associated with DOAC administration in patients with AF has been reported to range from 2.13% to 3.16%. 6 , 7 , 8 However, our pooled analysis revealed a lower rate of systemic bleeding. This difference may be attributed to several factors observed in the included studies. First, 4 of the 7 studies administered idarucizumab, an agent that effectively reverses coagulopathy caused by dabigatran. 32 , 38 , 39 , 40 This may have contributed to a reduced risk of bleeding in patients with recent DOAC intake. Additionally, 3 studies administered low dose alteplase, which has been associated with a lower risk of sICH. 32 , 36 , 44 In summary, our results support the safety of tPA administration in patients with recent DOAC intake.
We found no significant difference in favorable 3‐month functional outcomes occurred in DOAC patients receiving IVT compared with non‐DOAC patients. Although the concomitant use of anticoagulation could have theoretically resulted in more efficient recanalization, and lower rates of reocclusion, after using IVT, it did not translate in an increase rate of functional independence. 46 Moreover, these results seem to be consistent with the multicenter cohort study by Seiffge et al. 23 Additional information is presented in Figure S4.
In this study, we observed a significantly higher rates of mortality in the DOAC patients who were treated with IVT as compared with the controls. However, this effect was mainly due to Meinel et al, 32 and the estimated effect became nonsignificant after omitting this study from the analysis. It is important to note that in the study by Meinel et al, even though there was a significant increase in mortality in the DOAC group as compared with the control group in the univariable analysis, this difference was not statistically significant after adjusting for confounders. Therefore, although our overall unadjusted analysis suggests that mortality may be higher in the DOAC group, the effect may be negligible if adjusted analyses were performed. Therefore, we conclude that the unadjusted increased rates in mortality are likely the result of confounding.
The strength of our work lies in the compilation of the largest sample size of DOAC patients who were treated with IVT for acute ischemic stroke in the literature. The breadth of the meta‐analysis and systemic review provides additional information regarding the safety and efficacy of IVT in this diverse multinational patient population. Furthermore, our work suggests the urgent need for a randomized control study. To design this trial, several factors including the type of antithrombotic, type of DOAC, and dosage of the selected antithrombotic will need to be considered. Additionally, certain inclusion criteria such as the timing of the last DOAC intake and whether a reversal agent will be provided or not will need to be defined.
We still recognize the limitations, which mostly stem from the quality of the studies included. All these studies were observational, and most were retrospective (>80%). This raises the concern for biases and confounding factors that could produce heterogeneity and impact the reported outcomes. Methodological heterogeneity was observed among studies on sICH and functional independence. Seven studies were assessed as at serious risk of bias due to missing data because they reported missing data for the prioritized outcomes of this meta‐analysis. 34 , 35 , 37 , 39 , 41 , 42 , 44 Additionally, the complete clinical data that may influence the risk of IVT complications including sICH were not available. This is not surprising, and it is very possible that patients were not randomly selected to receive IVT while on DOAC. In order to address confounding factors and other biases, we performed sensitivity and subgroup meta‐analyses with different approaches for defining study eligibility criteria as well as qualitative methods (Risk of Bias in Non‐randomized Studies of Interventions tool and the grading of recommendation, assessment, development, and evaluation assessment of certainty) to address the methodological issues that could affect the validity of our results, as recommended when meta‐analyzing nonrandomized studies. 47 Moreover, in this meta‐analysis, we included all studies including those in which the duration because DOAC ingestion was not specified. This could have resulted in misclassification biases because some of those patients may have not been truly anticoagulated if ingested more than 48 hours before the stroke onset. This is always a challenge in the acute stroke scenario, where the patients often cannot provide a good history regarding adherence. Therefore, we performed sensitivity analysis and subgroup analysis to include only patients who were known to have received DOAC within 48 hours of stroke onset. Finally other limitations included the significant difference between the intervention (DOAC) and control arm (non‐DOAC) (3464 versus 243 469), which might affect the power of the study. In addition, the IVT dose was slightly different between populations, which might be a source of confounding. And finally, the conventional stroke assessment scales (modified Rankin scale, NIHSS) may not adequately measure the patient's clinical deficits and response to treatment.
CONCLUSIONS
In patients presenting with acute ischemic stroke who were taking DOAC, IVT administration was not associated with an increase in hemorrhagic complications compared with patients not taking DOAC, including symptomatic intracranial hemorrhage, any intracranial hemorrhage, or serious systemic bleeding. In addition, there was no significant difference in functional independence at 90 days in patients using the DOAC before IVT administration when compared with non‐DOAC cohorts. The sICH rate did not differ for patients who received idaracizumab before IVT versus those who did not. In the absence of prospective trials to provide a more rigorous assessment of the risk/benefits in this specific population, the use of IVT may be justified in cases where the recent use of DOAC is unclear.
Source of Funding
None.
Disclosures
Dr Ortega‐Gutierrez is a consultant for Medtronic and Stryker. Dr Samaniego is a consultant for Medtronic and Rapid Medical. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S11
Figures S1–S12
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031669
For Sources of Funding and Disclosures, see page 11.
This manuscript was sent to Kori S. Zachrison, MD, MSc, Associate Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1. Organization WH . The Global Burden of Disease: 2004 Update. World Health Organization; 2008. [Google Scholar]
- 2. Callaly E, Ni Chroinin D, Hannon N, Marnane M, Akijian L, Sheehan O, Merwick A, Hayden D, Horgan G, Duggan J, et al. Rates, predictors, and outcomes of early and late recurrence after stroke: the North Dublin Population Stroke study. Stroke. 2016;47:244–246. doi: 10.1161/STROKEAHA.115.011248 [DOI] [PubMed] [Google Scholar]
- 3. Perera KS, Vanassche T, Bosch J, Swaminathan B, Mundl H, Giruparajah M, Barboza MA, O'Donnell MJ, Gomez‐Schneider M, Hankey GJ, et al. Global survey of the frequency of atrial fibrillation‐associated stroke: embolic stroke of undetermined source global registry. Stroke. 2016;47:2197–2202. doi: 10.1161/STROKEAHA.116.013378 [DOI] [PubMed] [Google Scholar]
- 4. Seiffge DJ, Polymeris AA, Fladt J, Lyrer PA, Engelter ST, De Marchis GM. Management of patients with stroke treated with direct oral anticoagulants. J Neurol. 2018;265:3022–3033. doi: 10.1007/s00415-018-9061-y [DOI] [PubMed] [Google Scholar]
- 5. Navar AM, Kolkailah AA, Overton R, Shah NP, Rousseau JF, Flaker GC, Pignone MP, Peterson ED. Trends in oral anticoagulant use among 436 864 patients with atrial fibrillation in community practice, 2011 to 2020. J Am Heart Assoc. 2022;11:e026723. doi: 10.1161/JAHA.122.026723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 7. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 8. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 9. National Institute of Neurological D, Stroke rt PASSG . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 10. Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 11. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 12. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 13. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I–LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toyoda K, Yamagami H, Koga M. Consensus guides on stroke thrombolysis for anticoagulated patients from Japan: application to other populations. J Stroke. 2018;20:321–331. doi: 10.5853/jos.2018.01788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Toyoda K, Koga M, Iguchi Y, Itabashi R, Inoue M, Okada Y, Ogasawara K, Tsujino A, Hasegawa Y, Hatano T, et al. Guidelines for intravenous thrombolysis (recombinant tissue‐type plasminogen activator), the third edition, march 2019: a guideline from the Japan stroke society. Neurol Med Chir (Tokyo). 2019;59:449–491. doi: 10.2176/nmc.st.2019-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyamoto S, Ogasawara K, Kuroda S, Itabashi R, Toyoda K, Itoh Y, Iguchi Y, Shiokawa Y, Takagi Y, Ohtsuki T, et al. Japan Stroke Society guideline 2021 for the treatment of stroke. Int J Stroke. 2022;17:1039–1049. doi: 10.1177/17474930221090347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao NM, Levine SR, Gornbein JA, Saver JL. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue‐type plasminogen activator trials. Stroke. 2014;45:2728–2733. doi: 10.1161/STROKEAHA.114.005135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604 [DOI] [PubMed] [Google Scholar]
- 21. Shahjouei S, Tsivgoulis G, Goyal N, Sadighi A, Mowla A, Wang M, Seiffge DJ, Zand R. Safety of intravenous thrombolysis among patients taking direct oral anticoagulants: a systematic review and meta‐analysis. Stroke. 2020;51:533–541. doi: 10.1161/STROKEAHA.119.026426 [DOI] [PubMed] [Google Scholar]
- 22. Seiffge DJ, Traenka C, Polymeris AA, Thilemann S, Wagner B, Hert L, Muller MD, Gensicke H, Peters N, Nickel CH, et al. Intravenous thrombolysis in patients with stroke taking rivaroxaban using drug specific plasma levels: experience with a standard operation procedure in clinical practice. J Stroke. 2017;19:347–355. doi: 10.5853/jos.2017.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seiffge DJ, Hooff RJ, Nolte CH, Bejot Y, Turc G, Ikenberg B, Berge E, Persike M, Dequatre‐Ponchelle N, Strbian D, et al. Recanalization therapies in acute ischemic stroke patients: impact of prior treatment with novel oral anticoagulants on bleeding complications and outcome. Circulation. 2015;132:1261–1269. doi: 10.1161/CIRCULATIONAHA.115.015484 [DOI] [PubMed] [Google Scholar]
- 24. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. doi: 10.1002/9781119536604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwarzer G, Chemaitelly H, Abu‐Raddad LJ, Rucker G. Seriously misleading results using inverse of Freeman‐Tukey double arcsine transformation in meta‐analysis of single proportions. Res Synth Methods. 2019;10:476–483. doi: 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin L, Xu C, Chu H. Empirical comparisons of 12 meta‐analysis methods for synthesizing proportions of binary outcomes. J Gen Intern Med. 2022;37:308–317. doi: 10.1007/s11606-021-07098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, Kuss O, Higgins JP, Langan D, Salanti G. Methods to estimate the between‐study variance and its uncertainty in meta‐analysis. Res Synth Methods. 2016;7:55–79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Borenstein M. In a meta‐analysis, the I‐squared statistic does not tell us how much the effect size varies. J Clin Epidemiol. 2022;152:281–284. doi: 10.1016/j.jclinepi.2022.10.003 [DOI] [PubMed] [Google Scholar]
- 30. Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta‐analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 31. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck‐Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 32. Meinel TR, Wilson D, Gensicke H, Scheitz JF, Ringleb P, Goganau I, Kaesmacher J, Bae HJ, Kim DY, Kermer P, et al. Intravenous thrombolysis in patients with ischemic stroke and recent ingestion of direct Oral anticoagulants. JAMA Neurol. 2023;80:233–243. doi: 10.1001/jamaneurol.2022.4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kam W, Holmes DN, Hernandez AF, Saver JL, Fonarow GC, Smith EE, Bhatt DL, Schwamm LH, Reeves MJ, Matsouaka RA, et al. Association of Recent use of non‐vitamin K antagonist Oral anticoagulants with intracranial hemorrhage among patients with acute ischemic stroke treated with alteplase. JAMA. 2022;327:760–771. doi: 10.1001/jama.2022.0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okada T, Yoshimoto T, Wada S, Yoshimura S, Chiba T, Egashira S, Kimura S, Shiozawa M, Inoue M, Ihara M, et al. Intravenous thrombolysis with alteplase at 0.6 mg/kg in patients with ischemic stroke taking direct oral anticoagulants. J Am Heart Assoc. 2022;11:e025809. doi: 10.1161/JAHA.122.025809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frol S, Sernec LP, Hudnik LK, Sabovic M, Oblak JP. Idarucizumab reversal of dabigatran in patients with acute ischemic stroke and intracranial hemorrhage: comparison with non‐idarucizumab‐treated patients. CNS Drugs. 2021;35:233–242. doi: 10.1007/s40263-021-00792-2 [DOI] [PubMed] [Google Scholar]
- 36. Xian Y, Federspiel JJ, Hernandez AF, Laskowitz DT, Schwamm LH, Bhatt DL, Smith EE, Fonarow GC, Peterson ED. Use of intravenous recombinant tissue plasminogen activator in patients with acute ischemic stroke who take non‐vitamin K antagonist oral anticoagulants before stroke. Circulation. 2017;135:1024–1035. doi: 10.1161/CIRCULATIONAHA.116.023940 [DOI] [PubMed] [Google Scholar]
- 37. Beharry J, Waters MJ, Drew R, Fink JN, Wilson D, Campbell BCV, Parsons MW, Kleinig TJ, Wu TY. Dabigatran reversal before intravenous tenecteplase in acute ischemic stroke. Stroke. 2020;51:1616–1619. doi: 10.1161/STROKEAHA.119.028327 [DOI] [PubMed] [Google Scholar]
- 38. Fang CW, Tsai YT, Chou PC, Chen HM, Lu CM, Tsao CR, Chen CL, Sun MC, Shih YS, Hsieh CY, et al. Intravenous thrombolysis in acute ischemic stroke after Idarucizumab reversal of dabigatran effect: analysis of the cases from Taiwan. J Stroke Cerebrovasc Dis. 2019;28:815–820. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 39. Kikule I, Baborikina A, Haritoncenko I, Karelis G. Idarucizumab in dabigatran‐treated patients with acute ischemic stroke receiving thrombolytic therapy. Medicina (Kaunas). 2022;58:58. doi: 10.3390/medicina58101355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kermer P, Eschenfelder CC, Diener HC, Grond M, Abdalla Y, Abraham A, Althaus K, Becks G, Berrouschot J, Berthel J, et al. Antagonizing dabigatran by idarucizumab in cases of ischemic stroke or intracranial hemorrhage in Germany‐updated series of 120 cases. Int J Stroke. 2020;15:609–618. doi: 10.1177/1747493019895654 [DOI] [PubMed] [Google Scholar]
- 41. Sanak D, Jakubicek S, Cernik D, Herzig R, Kunas Z, Mikulik R, Ostry S, Reif M, Rohan V, Tomek A, et al. Intravenous thrombolysis in patients with acute ischemic stroke after a reversal of dabigatran anticoagulation with Idarucizumab: a real‐world clinical experience. J Stroke Cerebrovasc Dis. 2018;27:2479–2483. doi: 10.1016/j.jstrokecerebrovasdis.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 42. Tse DM, Young L, Ranta A, Barber PA. Intravenous alteplase and endovascular clot retrieval following reversal of dabigatran with idarucizumab. J Neurol Neurosurg Psychiatry. 2018;89:549–550. doi: 10.1136/jnnp-2017-316449 [DOI] [PubMed] [Google Scholar]
- 43. Suzuki K, Aoki J, Sakamoto Y, Abe A, Suda S, Okubo S, Nagao T, Kimura K. Low risk of ICH after reperfusion therapy in acute stroke patients treated with direct oral anti‐coagulant. J Neurol Sci. 2017;379:207–211. doi: 10.1016/j.jns.2017.06.004 [DOI] [PubMed] [Google Scholar]
- 44. Shahjouei S, Tsivgoulis G, Bavarsad Shahripour R, Jones GM, Alexandrov AV, Zand R. Safety of intravenous thrombolysis among stroke patients taking new Oral anticoagulants–case series and systematic review of reported cases. J Stroke Cerebrovasc Dis. 2015;24:2685–2693. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.021 [DOI] [PubMed] [Google Scholar]
- 45. Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta‐analysis. Lancet Neurol. 2016;15:925–933. doi: 10.1016/S1474-4422(16)30076-X [DOI] [PubMed] [Google Scholar]
- 46. Deeds SI, Barreto A, Elm J, Derdeyn CP, Berry S, Khatri P, Moy C, Janis S, Broderick J, Grotta J, et al. The multiarm optimization of stroke thrombolysis phase 3 acute stroke randomized clinical trial: rationale and methods. Int J Stroke. 2021;16:873–880. doi: 10.1177/1747493020978345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mathur MB, VanderWeele TJ. Methods to address confounding and other biases in meta‐analyses: review and recommendations. Annu Rev Public Health. 2022;43:19–35. doi: 10.1146/annurev-publhealth-051920-114020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S11
Figures S1–S12


