Abstract
Background
Health concordance within couples presents a promising opportunity to design interventions for disease management, including hypertension. We compared the concordance of prevalent hypertension within middle‐aged and older heterosexual couples in the United States, England, China, and India.
Methods and Results
Cross‐sectional dyadic data on heterosexual couples were used from contemporaneous waves of the HRS (US Health and Retirement Study, 2016/17, n=3989 couples), ELSA (English Longitudinal Study on Aging, 2016/17, n=1086), CHARLS (China Health and Retirement Longitudinal Study, 2015/16, n=6514), and LASI (Longitudinal Aging Study in India, 2017/19, n=22 389). Concordant hypertension was defined as both husband and wife in a couple having hypertension. The prevalence of concordant hypertension within couples was 37.9% (95% CI, 35.8–40.0) in the United States, 47.1% (95% CI, 43.2–50.9) in England, 20.8% (95% CI, 19.6–21.9) in China, and 19.8% (95% CI, 19.0–20.5) in India. Compared with wives married to husbands without hypertension, wives married to husbands with hypertension were more likely to have hypertension in the United States (prevalence ratio, 1.09 [95% CI, 1.01– 1.17), England (prevalence ratio, 1.09, 95% CI, 0.98–1.21), China (prevalence ratio, 1.26 [95% CI, 1.17–1.35), and India (prevalence ratio, 1.19 [95% CI, 1.15–1.24]). Within each country, similar associations were observed for husbands. Across countries, associations in the United States and England were similar, whereas they were slightly larger in China and India.
Conclusions
Concordance of hypertension within heterosexual couples was consistently observed across these 4 socially and economically diverse countries. Couple‐centered interventions may be an efficient strategy to prevent and manage hypertension in these countries.
Keywords: cross‐national study, hypertension, middle‐aged and older population, spousal concordance
Subject Categories: Epidemiology, Lifestyle, Risk Factors, Women, Aging
Nonstandard Abbreviations and Acronyms
- CHARLS
China Health and Retirement Longitudinal Study
- ELSA
English Longitudinal Study on Aging
- HRS
Health and Retirement Study
- LASI
Longitudinal Aging Study in India
Clinical Perspective.
What Is New?
This cross‐sectional study shows a high prevalence of concordant hypertension ranging from 20% to >40% among middle‐aged and older heterosexual couples in the United States, England, China, and India.
Positive associations of hypertension status within couples were observed across these 4 countries, with slightly stronger associations in China and India than in the United States and England.
What Are the Clinical Implications?
The high prevalence of concordant hypertension within heterosexual couples suggests that a large proportion of middle‐aged and older adults with hypertension could benefit from couple‐centered strategies to improve hypertension diagnosis and management.
Poor diagnosis and management of hypertension is a major public health concern worldwide. 1 , 2 , 3 This issue is particularly emerging in low‐ and middle‐income countries. For example, an analysis of 44 low‐ and middle‐income countries reported that 3 out of 5 individuals with hypertension are undiagnosed, and 9 out of 10 did not have their blood pressure under control. 4 Considering that hypertension is both preventable and treatable, early and improved diagnosis and management of hypertension can have significant implications to reduce disease burden. However, most prior work has focused on behavioral factors at the individual level. Spousal concordance of health, defined as similar health status within married or partnered couples, presents a promising opportunity to design interventions for hypertension identification and management at the couple level. 5 , 6 , 7 , 8 , 9 Compared with studies that identified individual‐ and community‐level risk factors for hypertension, spousal concordance studies quantify interpersonal risk within couples and could point toward screening, prevention, and management interventions aimed at households.
Previous studies of spousal concordance of hypertension reported inconsistent findings. 10 , 11 , 12 , 13 , 14 This inconsistency may be due to differential spousal influences of husbands on wives versus influences of wives on husbands, because there are sex differences in marriage experiences and behavioral norms. 7 , 15 , 16 It remains unclear whether sex differences exist in the spousal concordance of hypertension. Moreover, previous studies on spousal concordance of hypertension have only used regional or community‐based studies with limited sample sizes. This limits the generalizability of their findings because different economic and cultural settings may influence how couples may share health behaviors and outcomes. Therefore, cross‐national comparison studies using large, population‐representative samples, stratified by sex, may improve the precision of estimates and the generalizability of findings.
To address these research gaps, we investigated and compared the spousal concordance of hypertension among heterosexual couples in population‐representative studies of middle‐aged and older adults in the United States, England, China, and India. 17 , 18 , 19 , 20 These countries have a high prevalence of hypertension, ranging from ≈30% to >50% among adults. 2 , 21 Because our study used data from cohorts mainly consisting of middle‐aged and older adults, the prevalence of hypertension was higher than in younger adults. This is because hypertension prevalence increases with age. These 4 countries also differ in social, economic, cultural, and health care contexts, and are at different stages of the hypertension epidemiological transition, all of which may influence spousal concordance of hypertension. 22 In this study, we examined (1) the prevalence of concordant hypertension within heterosexual couples across countries; (2) the associations of hypertension status within couples for wives and husbands separately within each country; (3) the difference in the strength of associations comparing husbands to wives within each country; and (4) the difference in the strength of associations across countries.
METHODS
Ethics Approval and Consent to Participate
All participants gave written informed consent before participation. We were exempted from ethical approval for the secondary data analysis from the institutional review board of Emory University.
Data Availability Statement
All data sets used in this analysis are available at https://g2aging.org/downloads. The code for the analysis is available at https://github.com/jvargh7/g2aging_family.
Data and Sample
Cross‐sectional data from population‐representative studies of aging were used, including the 2016 to 2017 HRS (Health and Retirement Study) in the United States, 19 the 2016 to 2017 ELSA (English Longitudinal Study on Aging), 18 the 2015 to 2016 CHARLS (China Health and Retirement Longitudinal Study), 17 and the 2017 to 2019 LASI (Longitudinal Aging Study in India). 20 These 4 studies have harmonized design and measures, because they are all International Partner Studies of the US HRS. They all adopted a household survey design by first recruiting a primary participant meeting age eligibility and then inviting his or her spouse or partner if available to participate. The HRS and ELSA surveyed the American and British populations, respectively, both aged 50 years and above and their spouses regardless of age. 18 , 19 The CHARLS and LASI surveyed adults in China and India, respectively, both aged 45 years and older and their spouses regardless of age. 17 , 20 Therefore, while all primary respondents were middle‐aged and older populations, their spouses could be younger.
Additional details of each study, including sampling and survey methods, eligibility criteria of participants, data accessibility, data used in the current study, and references are summarized in Table S1. 17 , 18 , 19 , 20 To facilitate cross‐national comparison studies, the Gateway to the Global Aging Data team at the University of Southern California harmonizes the data by unifying the variables' names and measures coding levels across waves and studies. 23 The harmonized data contain ready‐to‐use measures that are comparable between the studies of different countries. This study primarily used harmonized data to conduct data analysis. Table S2 summarizes variables from each study used for harmonization. A flowchart for our analytic sample selection is provided in Figure 1. We henceforth refer to each survey by the country because all surveys are nationally representative for middle‐aged and older adults and their spouses, the United States for HRS, England for ELSA, China for CHARLS, and India for LASI.
Figure 1. Flowchart for analytic sample.
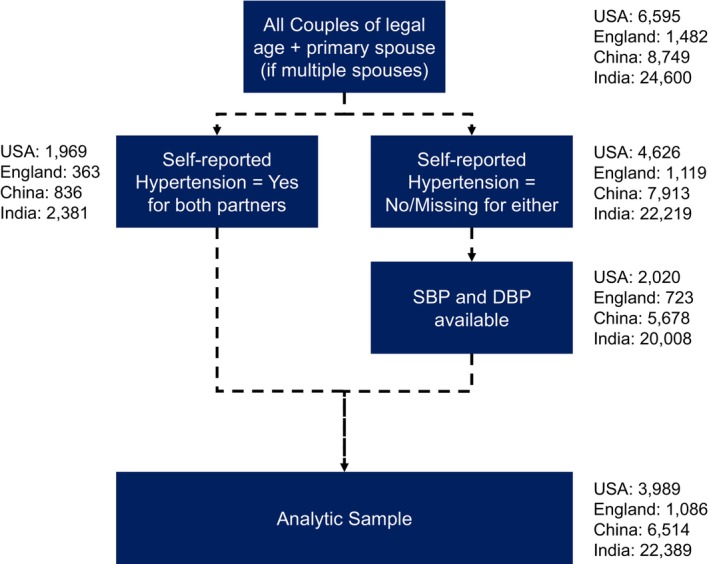
DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Eligible Couples
Couples were defined as heterosexual participants living in the same household who reported to be either married or partnered to one another, and those who were older than the legal age for marriage at the time of the survey (18 years and older for men and women in the United States; any age in England; 22 years and older for men and 20 years and older for women in China; 21 years and older for men and 18 years and older for women in India). In ELSA, survey weights are given for core members only, who were also respondents of the Health Survey for England. 18 Therefore, we excluded those who were not ELSA core members, resulting in different numbers of spousal pairs when conducting the analysis for husbands and wives. Homosexual couples were excluded because they were few or none across studies.
Measures
Hypertension
Although clinical guidelines recommend screening at least twice, 1 to 4 weeks apart, hypertension was defined based on blood pressure measurements at 1 time point in this study. 24 Blood pressure was measured 3 times by investigators using validated instruments, including the Omron HEM‐780 Intellisense Automated blood pressure monitor with ComFit cuff in the United States, the Omron HEM‐907 in England, the HEM‐7112 electronic monitor in China, and the Omron Blood Pressure monitor in India. For each participant, the mean of 3 available measures was used as estimates of systolic blood pressure and diastolic blood pressure. Participants were considered as having hypertension if they had 1 of the following indicators: systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or a history of high blood pressure (ie, if a health care provider had ever told participants they had high blood pressure or hypertension) (Table S3). 25 , 26 Medication status was asked only to those participants who self‐reported high blood pressure or hypertension. Spousal concordance of hypertension was defined as both partners in a couple having hypertension.
Individual Characteristics
Individual‐level sociodemographic and health characteristics that are considered traditional risk factors for hypertension were considered. 27 , 28 The sociodemographic characteristics of participants included age at the time of survey (in years), highest educational attainment according to the 1997 International Standard Classification of Education 23 (less than lower secondary, upper secondary or vocational training, or tertiary), and wage employment status (no wages or salaries, wage work, or retired). The classification of wage work was used to define employment categories because formal wage employment is tied to Social Security benefits, while informal wage employment and unorganized employment may lack access to these. 29
Health‐related characteristics of patients included body mass index (BMI), heavy alcohol consumption, and moderate or vigorous physical activity. 27 , 28 For BMI, height and weight were measured using validated instruments or interviews. Specifically, height was measured using a tape measure while the respondent was standing against the wall without shoes in the United States, a SecaTM213 stadiometer in China, and a standardized stadiometer in India. Weight was measured using a Healthometer 830KL scale in the United States, a digital weighing scale in England, an Omron HN‐286 scale in China, and a SECA 803 digital weighing scale in India. Measured height was obtained from previous waves in England. BMI (unit: kg/m2) was calculated as weight divided by height in square meters. Smoking history and current smoking status (current, former, never) and heavy alcohol consumption (>3 drinks per day) were self‐reported. Physical activity was measured as self‐reported days of either moderate or vigorous physical activity per week.
Household Characteristics
Household characteristics shared by couples that are likely to confound potential spousal concordance in health were included in our models: wealth, expenditures, number of household members, number of children, length of marriage, and region of residence. 15 , 30 Household wealth was assessed using questionnaires that enumerated total wealth based on assets and savings in all countries. Both household wealth and household expenditures were categorized as within‐country quintiles to indicate the relative position of financial status within each country. Number of children counted as the couple's number of children or stepchildren. The length of the current marriage (in years) was self‐reported. The region of residence was categorized as urban or rural based on the US Census Region/Division, the National Bureau of Statistics in China, and Census 2011 in India. Household expenditure was not available for all respondents in the United States; length of current marriage and region of residence were not available in England.
Statistical Analysis
Multiple analytical steps were conducted. First, missingness patterns were examined, and multiple imputation was performed separately by country. Second, spousal concordance of hypertension was separately examined for wives and husbands in each country. Third, data were pooled for wives and husbands for each country to examine sex differences in spousal concordance. Last, data were pooled across 4 countries, separately for wives and husbands, to examine whether country differences exist. All analyses were carried out using R version 4.2.0 using survey (version 4.1), and geepack package (version 1.3.9). Analyses incorporated the complex survey designs and applied survey weights provided by Gateway to Global Aging Data harmonized data sets for HRS and its international partner studies.
Missing Data and Multiple Imputation
Information on missingness is provided for each variable by country in Table S4. Assuming the data were missing at random, multiple imputation was performed with predictive mean matching (10 data sets, 50 iterations) separately by country. We used variables such as household income and history of health/disease in imputation as auxiliary variables. We included the outcome variable (hypertension) as well as auxiliary variables in imputing the covariates. Auxiliary variables that were included in imputing covariates but not in analysis differed by country (United States: history of diabetes, measured waist circumference, household income tertile; England: self‐reported diagnosis and treatment of diabetes, household income tertile; China: history of diabetes, measured waist circumference, household income tertile; India: self‐reported diagnosis and treatment of diabetes, measured waist circumference, measured hip circumference, household income tertile). We prespecified all interaction terms in the imputation step for obtaining unbiased estimates of association within the categories of exposure and effect modifier with the outcome. Therefore, our analytic sample consists only of those couples for whom both partners had data on hypertension.
Association of Hypertension Status Between Couples in Each Country
For each country, we estimated the association of hypertension status between couples (coefficient β1 in Equation 1a and 1b) using Poisson regression with logarithmic link function and robust standard errors as prevalence ratios (PR). This was done separately for wives and husbands. We conducted analysis with and without adjustment for individual and household characteristics. Because the results were consistent, we presented findings based on adjusted models.
| (1a) |
| (1b) |
where HTN is hypertension.
We also stratified analysis by age group (aged under 65 versus 65 years and over), education level (less than lower secondary, upper secondary and vocational training, and tertiary), urbanicity of residence (urban versus rural), household wealth quintile, and length of marriage (<10 versus ≥10 years). 25 , 27 , 28 , 31 , 32 , 33 , 34 , 35 We further included individual characteristics specific to each country's context for sensitivity analysis in adjusted models. These covariates included religion (Protestant, Catholic, Jewish, Others/None/No preference), and race/ethnicity (non‐Hispanic [NH] White, NH Black, NH Other, Hispanic) for United States 31 , 33 ; religion (Christian, None, Others) and race/ethnicity (White, Others) for England 35 ; Hukou registration status (Agricultural, non‐Agricultural) for China 25 , 34 ; and caste (General or None, Other Backward Caste, Scheduled Caste, Scheduled Tribe) and religion (Hindu, Muslim, Others) for India. 32 The 'Others' category for religion and race/ethnicity across these countries indicates the presence of various minority religions or races/ethnicities, or it signifies unreported information. These groups had a small number of observations and were thus combined for analysis. We repeated the analysis after adjusting for individual characteristics alone, and for individual, household, and spousal characteristics.
Sex Difference in Associations Comparing Wives and Husbands
We pooled data for wives and husbands in each country and examined whether there were sex differences in the strengths of associations. We used generalized estimating equations under a Poisson distribution with a logarithmic link and robust standard errors. We clustered each couple as per their unique couple identifier. We assessed sex differences in associations based on the magnitude and confidence intervals of the interaction (coefficient δ in Equation 2). In the below equation, the term “Male” refers to husbands, with wives as the reference category.
| (2) |
Country Difference in Associations for Wives and Husbands
We conducted a pooled analysis across countries stratified by sex. We excluded covariates that could not be harmonized across countries and added statistical interactions (coefficient δ i in Equation 3a and 3b) between spouse's hypertension and country‐fixed effects. We first normalized the sample weight for each country by dividing the weight of each observation by the sum of sample weights. Next, we inverse‐weighted this normalized sample weight with proportion of participants in pooled data from the country to account for different analytic sample sizes by country.
| (3a) |
| (3b) |
RESULTS
Our analytic sample consisted of 3989 couples from the United States, 1086 couples from England, 6514 couples from China, and 22 389 couples from India. Table 1 shows household and individual characteristics for wives and husbands separately by country. Major variations in these household and individual characteristics across countries were observed. The mean age ranged from 51.1 (India) to 72.5 (England) among wives and 57.2 (India) to 74.2 (England) among husbands. Across countries, the prevalence of hypertension among wives was lower than that among husbands, with the largest difference being within couples in the United States (54.5% for wives versus 64.5% for husbands). The prevalence of concordant hypertension was 37.9% (95% CI, 35.8–40) in the United States, 47.1% (43.2–50.9) in England, 20.8% (19.6–21.9) in China, and 19.8% (19.0–20.5) in India, respectively. Distributions of systolic and diastolic blood pressure among wives and husbands by country are presented in Figure S1.
Table 1.
Summary of Household‐ and Individual‐Level Characteristics Across Countries
| United States | England | China | India | |||||
|---|---|---|---|---|---|---|---|---|
| Wives | Husbands | Wives | Husbands | Wives | Husbands | Wives | Husbands | |
| Sample size (n for couples) | 3989 | 1086 | 6514 | 22 389 | ||||
| Shared household characteristics | ||||||||
| Residence (Rural, %) | 26 (22.4–29.6) | … | 78 (76.5– 79.5) | 69.2 (66.9–71.4) | ||||
| Wealth quintile of household | ||||||||
| 1 (Poorest) | 20.2 (18–22.5) | 14.1 (7.4–20.8) | 20.9 (19.4–22.4) | 20.1 (19.2–20.9) | ||||
| 2 (%) | 20.3 (18.4–22.2) | 20.6 (10.1–31.1) | 20.8 (19.3–22.3) | 20.4 (19.6–21.2) | ||||
| 3 (%) | 19.9 (18.1–21.7) | 20.9 (18.1–23.6) | 20.7 (19.2–22.2) | 20.1 (19.3–20.9) | ||||
| 4 (%) | 19.8 (17.7–21.9) | 22.2 (15.9–28.5) | 19.7 (18.2–21.1) | 19.7 (18.9–20.5) | ||||
| 5 (Wealthiest) (%) | 19.8 (17.5–22) | 22.2 (12.8–31.5) | 18 (16.1, 19.9) | 19.7 (18.8, 20.6) | ||||
| Length of current marriage, y | 33.6 (32.7–34.6) | 45.7 (44.2–47.3) | 37.7 (37.3–38) | 34.7 (34.5–34.9) | ||||
| Current marriage length ≥10 y | 91.0 (89–93) | 99.9 (99.6–99.9) | 99.0 (98.7–99.3) | 99.6 (99.5–99.7) | ||||
| Number of people in the same household | 2.6 (2.5–2.6) | 2.0 (2.0–2.0) | 3.2 (3.2–3.3) | 5.2 (5.2–5.3) | ||||
| Number of children | 3.0 (2.9–3) | 2.4 (2.3–2.5) | 2.7 (2.7– 2.7) | 3.7 (3.6–3.7) | ||||
| Sociodemographic characteristics | ||||||||
| Age, y | 62.9 (62.3–63.4) | 65.7 (65.1–66.3) | 72.5 (71.8–73.3) | 74.2 (73.4–75) | 59.2 (58.9–59.5) | 61.5 (61.2–61.8) | 51.1 (50.9–51.3) | 57.2 (57.1–57.4) |
| Education level | ||||||||
| Less than lower secondary (%) | 8.5 (6.6–10.4) | 11.1 (9.2–12.9) | 32 (25.8–38.1) | 26.3 (20–32.6) | 92.5 (91.7–93.4) | 84.5 (83.3–85.7) | 77.3 (76.1–78.4) | 62.3 (61.1–63.6) |
| Upper secondary and vocational training (%) | 59.9 (57.1–62.6) | 54.3 (51.5–57.2) | 43.1 (37.1–49) | 49.4 (45.2–53.7) | 6.4 (5.7–7.1) | 12.8 (11.7–14) | 19.3 (18.4–20.2) | 30.2 (29.2–31.2) |
| Tertiary (%) | 31.6 (28.7–34.6) | 34.5 (31.3–37.7) | 13.5 (9.8–17.1) | 19.4 (15.8–23.1) | 1.1 (0.5–1.7) | 2.7 (2.2–3.1) | 3.5 (3–3.9) | 7.4 (6.8–8.1) |
| Employment status | ||||||||
| No wage employment (%) | 10.1 (8.6–11.5) | 3.6 (2.7–4.4) | 8.8 (6.2–11.5) | 2.2 (1.5–2.8) | 2.7 (2.3–3.1) | 1.1 (0.6–1.7) | 65.3 (64.1–66.6) | 22.9 (22.1–23.7) |
| Wage employment (%) | 38.7 (36.3–41.1) | 42.2 (39.4–45) | 7.8 (6.3–9.4) | 10.2 (7.8–12.5) | 64.2 (62.8–65.6) | 74 (72.7–75.4) | 33.9 (32.6–35.2) | 70.4 (69.5–71.3) |
| Retired (%) | 50.6 (47.9–53.3) | 53.4 (50.6–56.3) | 83.3 (79.6–87) | 87.6 (84.9–90.2) | 33.1 (31.7–34.5) | 24.8 (23.5–26.2) | 0.8 (0.6–0.9) | 6.7 (6.2–7.1) |
| Health behaviors and self‐reported health outcomes | ||||||||
| Smoking | ||||||||
| Never (%) | 53.8 (51.7–55.9) | 40.3 (38.1–42.6) | 42.4 (39–45.8) | 25.8 (21–30.5) | 92.8 (92.1–93.4) | 18.5 (17.3–19.7) | 97.3 (96.9–97.7) | 65.8 (64.6–66.9) |
| Former (%) | 35.3 (33.3–37.4) | 47.6 (45.4–49.8) | 51.3 (48.5–54.1) | 68.4 (64.3–72.5) | 3.1 (2.6–3.5) | 29.1 (27.7–30.5) | 0.5 (0.4–0.6) | 7.1 (6.7–7.6) |
| Current (%) | 9.9 (8.7–11.1) | 11 (9.7–12.4) | 6.3 (4–8.6) | 5.8 (4.8–6.8) | 4.2 (3.7–4.7) | 52.4 (50.9–53.9) | 2.2 (1.8–2.5) | 27.1 (26–28.2) |
| Heavy drinking (Yes, %) | 19.7 (17.6–21.9) | 26.9 (24.9–29) | 14.5 (11.2–17.8) | 13.6 (10.9–16.2) | 3.1 (2.9–3.3) | 11.2 (10.8–11.6) | 0.6 (0.5–0.8) | 10 (9.3–10.6) |
| Moderate physical activity (d/wk) | 2.5 (2.4–2.5) | 2.6 (2.5–2.7) | 0.6 (0.5–0.6) | 0.6 (0.6–0.7) | 3.5 (3.4–3.7) | 2.8 (2.7–2.9) | 3.8 (3.8–3.8) | 3.1 (3–3.1) |
| Vigorous Physical activity (d/wk) | 1.1 (1–1.2) | 1.5 (1.4–1.6) | 0.1 (0.1–0.2) | 0.2 (0.2–0.2) | 1.6 (1.5–1.8) | 2.3 (2.1–2.4) | 1.9 (1.8–1.9) | 2.7 (2.7–2.7) |
| Self‐reported diagnosis of hypertension (%) | 48.5 (46.3–50.7) | 56 (53.8–58.2) | 52.6 (48.4–56.8) | 56.1 (51.2–61) | 36.8 (35.2–38.4) | 35.7 (34.2–37.2) | 24.4 (23.6–25.3) | 22.6 (21.8–23.4) |
| Self‐reported diagnosis of diabetes (%) | 20.1 (18.6–21.7) | 25.6 (23.8–27.3) | 11.7 (9.8–13.7) | 16.9 (14.1–19.7) | 10.8 (9.9–11.7) | 8.6 (7.8–9.5) | 9.3 (8.8–9.8) | 12.4 (11.8–13) |
| Clinical measures | ||||||||
| Body mass index, kg/m2 | 28.7 (28.4–29) | 29.1 (28.9–29.3) | 28.1 (27.6–28.6) | 28.1 (27.6–28.6) | 25.3 (24.4–26.2) | 24 (23.5–24.4) | 23.5 (23.4–23.7) | 22.2 (22.1–22.3) |
| Waist circumference, cm | 99.1 (98.2–100.1) | 107.3 (106.4–108.2) | 86.1 (85.8–86.5) | 85.9 (85.5–86.3) | 84.7 (84.3–85) | 85.9 (85.6–86.2) | ||
| Systolic blood pressure, mm Hg | 121.9 (121.3–122.6) | 129.3 (128.6–130) | 131.5 (129.7–133.3) | 131.5 (129.7–133.3) | 126.1 (125.5–126.8) | 128.5 (127.9–129.2) | 122.5 (122.2–122.9) | 126.4 (126–126.8) |
| Diastolic blood pressure, mm Hg | 77.6 (77.2–78) | 78.4 (77.9–78.8) | 70.4 (68.9–71.9) | 70.4 (68.9–71.9) | 74.1 (73.8–74.5) | 76.3 (75.9–76.8) | 80.1 (79.9–80.3) | 82.2 (82–82.4) |
| Hypertension*, % | 54.6 (52.3–56.9) | 64.5 (62.5–66.6) | 64.5 (61.0–67.9) | 67.8 (64.9–70.7) | 46.5 (44.9–48.1) | 48.1 (46.5–49.7) | 38.7 (37.8–39.7) | 42.8 (41.9–43.8) |
| Concordant hypertension, % | 37.9 (35.8–40) | 47.1 (43.2–50.9) | 20.8 (19.6–21.9) | 19.8 (19–20.5) | ||||
All values are survey‐weighted means or percentages with 95% CIs.
Defined as self‐reported diagnosis of hypertension by a doctor or physician (=yes) or high blood pressure (>140/90 mm Hg).
Positive associations of hypertension status within couples were observed for both wives and husbands in each country (Figure 2A). Compared with wives who were married to husbands without hypertension, wives who had husbands with hypertension were more likely to have hypertension in each of the following: the United States (PR, 1.09 [95% CI, 1.01–1.17]), England (PR, 1.09 [95% CI, 0.98–1.21]), China (PR, 1.26 [95% CI, 1.17–1.35]), and India (PR, 1.19 [95% CI, 1.15–1.24) (Table S5). Compared with husbands who had wives without hypertension, husbands who had wives with hypertension were more likely to have hypertension in each of the following: the United States (PR, 1.06 [95% CI, 1.00–1.13]), England (PR, 1.05 [95% CI, 0.96–1.16]), China (PR, 1.26 [95% CI, 1.18–1.35]), and India (PR, 1.20 [95% CI, 1.12–1.28]) (Table S5). The results did not change when adjusting for additional individual, spousal, and household characteristics, including spousal BMI, age, and other factors (Table S6). We did not observe differences in analyses stratified by residence, household wealth quintile, length of marriage, age groups, and education levels (Table S7).
Figure 2. Associations of hypertension status between couples and sex differences within each country.
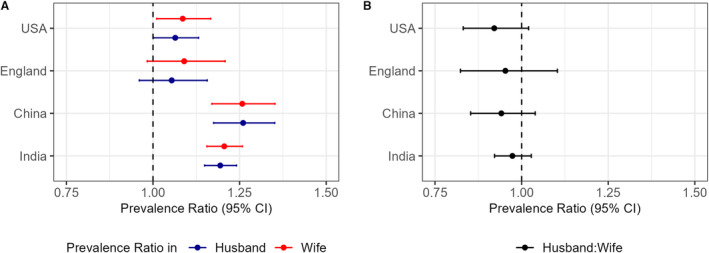
A, Association of hypertension status between couples (Table S4, Equation 1a and 1b). B, Sex difference in associations comparing husbands and wives (Table S4; Equation 2). All values are survey‐weighted prevalence ratios with 95% robust CIs, after adjusting for individual characteristics and household characteristics.
A potential sex difference in association of hypertension status within couples was examined by pooling data for wives and husbands together in each country. No sex differences were observed in the magnitude of associations for hypertension when comparing husbands and wives (Figure 2B). Comparing husbands to wives, the difference in association (in multiplicative scale; exponent of interaction term) was 0.92 (95% CI, 0.83–1.02) in the United States, 0.95 (95% CI, 0.82–1.10) in England, 0.94 (95% CI, 0.85–1.04) in China, and 0.97 (95% CI, 0.92–1.03) in India (Table S5).
A pooled analysis across 4 countries was further conducted to examine country‐level differences in the associations of hypertension status within couples for both wives and husbands separately (Figure 3A and 3B). While associations were comparable between the United States and England, associations were slightly larger in China and India. Among wives, the difference in association, compared with the United States, was 0.97 (95% CI, 0.88–1.11) in England, 1.13 (95% CI, 1.01–1.26) in China, and 1.19 (95% CI, 1.08–1.31) in India; among husbands, the difference in association was 0.98 (95% CI, 0.88–1.11) in England, 1.18 (95% CI, 1.07–1.29) in China, and 1.20 (95% CI, 1.11–1.30) in India (Table S8).
Figure 3. Country differences in association of hypertension status between couples across countries.
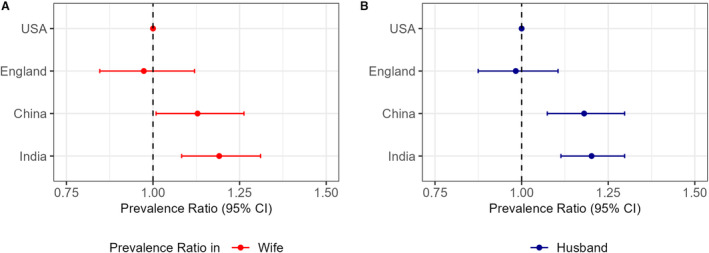
A, Statistical interaction for each country (relative to the United States) in pooled analysis of wives (Table S5; Equation 3a). B, Statistical interaction for each country (relative to the United States) in pooled analysis of husbands (Table S5; Equation (3b). Estimates of association may be numerically, but not statistically, different between models used (eg, between survey‐weighted Poisson regression of analysis separately by country and in pooled country data set) due to differences in estimation method (maximum likelihood with survey design vs generalized estimating equations), confounding adjustment, etc.
DISCUSSION
Our study examined the spousal concordance of prevalent hypertension using nationally representative surveys of middle‐aged and older adults from 4 countries: the United States, England, China, and India. Across countries, we observed a high prevalence of hypertension, ranging from 40% to 65% for both wives and husbands separately, suggesting a heavy public health burden. 25 , 26 , 36 We also observed a high prevalence of spousal concordant hypertension, ranging from 20% to over 40%, and consistent associations of hypertension status within couples in all these countries. The observed spousal concordance remained consistent when we stratified analyses across various socioeconomic subgroups (eg, residence, education, wealth). These results suggest that around half of all hypertension cases in these populous countries are concordant within couples. Considering the high prevalence of hypertension and the observed spousal concordance, our findings highlight the potential utility of couple‐based interventions for hypertension diagnosis and management, such as couple‐based screening, skills training, or joint participation in programs. 8 , 37
Within each country, we observed similar strengths of associations of spousal concordance of hypertension when comparing husbands and wives, suggesting no sex differences. This did not follow the existing hypothesis that strengths of spousal effects may differ for wives and husbands according to the sex differences in marital relationship dynamics highlighted in prior literature. 7 , 16 One explanation is that couples' health behaviors become similar over time. According to the social control theory, while wives may be more vulnerable to their husbands' health condition due to the traditional caretaker role, 7 they may also actively attempt to change their husbands' health behaviors. 16 As such, the couples' health influence on each other may converge over time. This theory is especially germane for these findings, considering the couples studied here had been married for a mean of 30 years and over. This aligns with several prior studies that did not find sex differences in the spousal associations of other health measures, such as the development of functional limitation among Chinese middle‐aged and older couples, 30 and physical activity among American couples. 38
Across countries, we observed that spousal concordance was slightly stronger in China and India (PR ranging from 1.19 to 1.26) than in the United States and England (PR ranging from 1.05 to 1.09). These differences require further investigation and are likely to be multifactorial. They could be attributed to cultural differences between countries. In Asian cultural contexts, collectivism is enshrined, and family members, including spouses, are encouraged to depend on each other, producing a stronger interpersonal relationship. 39 , 40 In contrast, individualism emphasized by Western cultures promotes mutual independence and freedom. As such, couples in India and China may exhibit a stronger spousal concordance than those in the United States and England. Additionally, there might be genetic, environmental, or gene–environment interactions that may also explain the differences in the magnitude of association. 41
In general, our findings align with previous studies that reported spousal concordance of hypertension within couples, although the magnitudes of prior observed association vary. 10 , 11 , 12 , 13 , 14 A meta‐analysis of 8 studies (range of reported odds ratios: 1.15–2.23) comprising 81 928 spouse pairs (20–94 years old) concluded that “spouses of individuals with hypertension had 1.41 higher pooled odds (95% CI, 1.21–1.64) of having hypertension themselves.” 14 The reported estimates of association in the present study are similar to those from prior research; minor differences may be explained by inflation of relative risk when using odds ratios, differences in study design, study population, analytic sample, and inclusion of a large case–control study from the United Kingdom that reported a high odds ratio (2.23, 95% CI, 1.75–2.72). 42 Additionally, our study used nationally representative, harmonized data. While the associations observed in this study were small in magnitude, they can still have important public health implications at a population level. As population aging is accelerating around the globe, hypertension among older adults is an increasingly important public health concern. As such, despite the small associations, considering the growing prevalence of hypertension among older adults in these countries, the potential number of people showing spousal concordance in hypertension would likely be very large. Given that hypertension is preventable and treatable, our study findings imply that we may promote cardiovascular health in old age through incremental changes such as designing couple‐based interventions.
The observed positive association of concordant hypertension within couples may operate through the mechanisms of assortative mating or cohabitation. 4 , 5 Assortative mating leads individuals to choose a partner with similar demographic characteristics, socioeconomic position (such as education and employment), and health behaviors (eg, alcohol and tobacco use, physical activity). 18 While our cross‐sectional study did not include genetic measures, premarital historical variables such as birth weight or other early life health characteristics that may be associated with later‐life hypertension, examination of this mechanism presents additional research opportunities. In contrast, cohabitation emphasizes the shared characteristics of the couple after marriage. Specifically, as couples live together, they share the same environment and resources, and cope with common life events and stress together, which in turn shapes their health behaviors and outcomes over time. 16 However, adjusting for multiple spousal characteristics correlated with these factors did not change our results. More broadly, psychosocial and cultural factors such as stress, family intimacy, or cultural beliefs may affect the extent of health concordance within the couple. For example, couples with greater intimacy or who live in a society that emphasizes interpersonal dependence may have higher concordance of health.
We note that the lack of sex differences in spousal concordance and the differences between countries might also be a statistical artifact due to a similar marginal prevalence of hypertension among spouses and between countries. For example, when exploring sex differences, if the marginal prevalence of the outcome is similar, prevalence ratios in the overall analytic sample for both husbands and wives may be similar (Data S1). Also, the similarities in the marginal prevalence of hypertension between countries may lead to similar estimates of association in the absence of strong genetic or environmental modifiers at the country level. At higher marginal prevalence, in order to observe similar magnitudes of spousal concordance, there should be a greater difference between expected and observed concordant hypertension (Data S1). In summary, given a fixed marginal prevalence of hypertension among husbands and wives, one will observe a higher prevalence ratio at a higher prevalence of concordant hypertension.
Our findings suggest that screening married couples and designing couple‐based interventions around the family unit may improve hypertension diagnosis and management. 24 , 25 According to the Theory of Dyadic Illness Management, chronic disease is a shared stressor for married couples. Couples may therefore act as an interdependent team to appraise the problem, change their behavior, and manage the illness together. 43 For example, the diagnosis of 1 partner may be associated with higher participation in health‐related behavior change interventions. 44 Couple‐based interventions for chronic conditions may also be more effective in improving health behaviors than individual‐focused intervention, although a review noted that further assessment of effectiveness is required. 8 This may therefore improve hypertension diagnosis at the population level, especially in countries where it is an emerging public health issue; 1 in 4 and 1 in 3 of those with hypertension were unaware of their status in China and India, respectively. As such, in our study context, instead of focusing on individuals, health professionals may invite patients and their spouses/partners to screen for hypertension similar to sexually transmitted infections, develop a joint treatment plan for both partners, and encourage them to manage the diseases together as a unit. 45 Such couple‐based strategies may achieve better diagnostic and treatment outcomes.
Our study has several major strengths. We used harmonized, nationally representative data sets of couples in 4 countries at different stages of the epidemiological transition. The HRS International Partner Studies have harmonized designs and study measures, allowing us to conduct a pooled analysis that collected consistent key sociodemographic covariates across countries. For those who did not self‐report a history of hypertension, we used blood pressure measurements that were obtained by trained enumerators using a validated protocol. Given the rich health data these 4 studies have, future research may conduct cross‐national studies examining the spousal concordance in health behaviors (eg, smoking, drinking, physical activity) and other diseases (eg, diabetes and obesity). Our analysis was restricted to older adults because hypertension in those <35 years of age may be secondary to another disease process (eg, hyperthyroidism, hyperaldosteronism, etc). Our analytic sample would therefore predominantly consist of those with primary hypertension related to lifestyle.
Our study also has some limitations. First, it is a cross‐sectional design because only 1 wave of data was available for LASI. As additional waves of these cohorts are completed, we will be able to examine whether spousal concordance changes over time. Future research may also investigate potential mechanisms for the observed positive concordance of hypertension status within couples to address the research question of how spouses influence each other's hypertension status. Second, we classified participants as having hypertension based on blood pressure measurements at 1 time point. Clinical guidelines recommend screening at least twice, 1 to 4 weeks apart. 46 However, an analysis of the National Health and Nutrition Examination Survey in 2013 to 2014 that corrected for the probability of observing elevated blood pressure on 2 separate occasions did not observe differences in prevalence before and after correction (36.3% versus 34.2%). 47
In summary, our study found positive concordance of hypertension status within heterosexual couples across 4 socially and economically diverse countries using data from large, population‐representative studies. Associations were of similar magnitude for husbands and wives within each country. The spousal concordance of hypertension was slightly stronger in China and India than in the United States or England. Approximately half of all hypertension cases are concordant within spouses, implying that up to half of middle‐aged and older adults with hypertension could benefit from the couple‐centered strategy to improve hypertension diagnosis and management.
Sources of Funding
Funding for this manuscript was provided by Emory Global Diabetes Research Center of Woodruff Health Sciences Center and Emory University. L.C. Kobayashi was supported by the National Institute on Aging (NIA) R01AG070953. S.A. Patel and M.K. Ali were supported by the National Heart, Lung, and Blood Institute P01HL154996‐01A1. C. Li was supported by NIA R01AG070953 and R01AG075719.
Disclosures
None.
Supporting information
Data S1
Tables S1–S8
Figure S1
Acknowledgments
We thank the participants and enumerators of the Health and Retirement Study, English Longitudinal Study on Aging, China Health and Retirement Longitudinal Study, and Longitudinal Aging Study in India. We thank the Gateway to Global Aging Data Team for preparing the harmonized data. Author contributions: All authors contributed to study conceptualization, data analysis, writing, interpretation of results, and reviewing the final draft.
This manuscript was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030765
For Sources of Funding and Disclosures, see page 11.
References
- 1. Chow CK, Teo KK, Rangarajan S, Islam S, Gupta R, Avezum A, Bahonar A, Chifamba J, Dagenais G, Diaz R, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959–968. doi: 10.1001/jama.2013.184182 [DOI] [PubMed] [Google Scholar]
- 2. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 . To 2019: a pooled analysis of 1201 population‐representative studies with 104 million participants. Lancet. 2021;398(10304):957–980. doi: 10.1016/S0140-6736(21)01330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burnier M, Egan BM. Adherence in hypertension. Circ Res. 2019;124:1124–1140. doi: 10.1161/CIRCRESAHA.118.313220 [DOI] [PubMed] [Google Scholar]
- 4. Geldsetzer P, Manne‐Goehler J, Marcus ME, Ebert C, Zhumadilov Z, Wesseh CS, Tsabedze L, Supiyev A, Sturua L, Bahendeka SK, et al. The state of hypertension care in 44 low‐income and middle‐income countries: a cross‐sectional study of nationally representative individual‐level data from 1.1 million adults. Lancet. 2019;394:652–662. doi: 10.1016/S0140-6736(19)30955-9 [DOI] [PubMed] [Google Scholar]
- 5. Di Castelnuovo A, Quacquaruccio G, Donati MB, de Gaetano G, Iacoviello L. Spousal concordance for major coronary risk factors: a systematic review and meta‐analysis. Am J Epidemiol. 2009;169:1–8. doi: 10.1093/aje/kwn234 [DOI] [PubMed] [Google Scholar]
- 6. Meyler D, Stimpson JP, Peek MK. Health concordance within couples: a systematic review. Soc Sci Med. 2007;64:2297–2310. doi: 10.1016/j.socscimed.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 7. Walker RB, Luszcz MA. The health and relationship dynamics of late‐life couples: a systematic review of the literature. Ageing Soc. 2009;29:455–480. doi: 10.1017/S0144686X08007903 [DOI] [Google Scholar]
- 8. Arden‐Close E, McGrath N. Health behaviour change interventions for couples: a systematic review. Br J Health Psychol. 2017;22:215–237. doi: 10.1111/bjhp.12227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carr RM, Prestwich A, Kwasnicka D, Thøgersen‐Ntoumani C, Gucciardi DF, Quested E, Hall LH, Ntoumanis N. Dyadic interventions to promote physical activity and reduce sedentary behaviour: systematic review and meta‐analysis. Health Psychol Rev. 2019;13:91–109. doi: 10.1080/17437199.2018.1532312 [DOI] [PubMed] [Google Scholar]
- 10. Bloch KV, Klein CH, de Souza e Silva NA, Nogueira Ada R, Salis LH. Socioeconomic aspects of spousal concordance for hypertension, obesity, and smoking in a community of Rio de Janeiro, Brazil. Arq Bras Cardiol. 2003;80:179–186. doi: 10.1590/S0066-782X2003000200006 [DOI] [PubMed] [Google Scholar]
- 11. Peek MK, Markides KS. Blood pressure concordance in older married Mexican‐American couples. J Am Geriatr Soc. 2003;51:1655–1659. doi: 10.1046/j.1532-5415.2003.51520.x [DOI] [PubMed] [Google Scholar]
- 12. Speers MA, Kasl SV, Freeman DH Jr, Ostfeld AM. Blood pressure concordance between spouses. Am J Epidemiol. 1986;123:818–829. doi: 10.1093/oxfordjournals.aje.a114311 [DOI] [PubMed] [Google Scholar]
- 13. Suarez L, Criqui MH, Barrett‐Connor E. Spouse concordance for systolic and diastolic blood pressure. Am J Epidemiol. 1983;118:345–351. doi: 10.1093/oxfordjournals.aje.a113641 [DOI] [PubMed] [Google Scholar]
- 14. Wang Z, Ji W, Song Y, Li J, Shen Y, Zheng H, Ding Y. Spousal concordance for hypertension: a meta‐analysis of observational studies. J Clin Hypertens (Greenwich). 2017;19:1088–1095. doi: 10.1111/jch.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiecolt‐Glaser JK, Wilson SJ. Lovesick: how couples' relationships influence health. Annu Rev Clin Psychol. 2017;13:421–443. doi: 10.1146/annurev-clinpsy-032816-045111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis MA, McBride CM, Pollak KI, Puleo E, Butterfield RM, Emmons KM. Understanding health behavior change among couples: an interdependence and communal coping approach. Soc Sci Med. 2006;62:1369–1380. doi: 10.1016/j.socscimed.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 17. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China health and retirement longitudinal study (CHARLS). Int J Epidemiol. 2014;43:61–68. doi: 10.1093/ije/dys203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42:1640–1648. doi: 10.1093/ije/dys168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the health and retirement study (HRS). Int J Epidemiol. 2014;43:576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perianayagam A, Bloom D, Lee J, Parasuraman S, Sekher TV, Mohanty SK, Chattopadhyay A, Govil D, Pedgaonkar S, Gupta S, et al. Cohort profile: the longitudinal ageing study in India (LASI). Int J Epidemiol. 2022;51:e167–e176. doi: 10.1093/ije/dyab266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliveros E, Patel H, Kyung S, Fugar S, Goldberg A, Madan N, Williams KA. Hypertension in older adults: assessment, management, and challenges. Clin Cardiol. 2020;43:99–107. doi: 10.1002/clc.23303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Monden C. Partners in health? Exploring resemblance in health between partners in married and cohabiting couples. Sociol Health Illn. 2007;29:391–411. doi: 10.1111/j.1467-9566.2007.01003.x [DOI] [PubMed] [Google Scholar]
- 23. Lee J, Phillips D, Wilkens J. Gateway to global aging data: resources for cross‐national comparisons of family, social environment, and healthy aging. J Gerontol B Psychol Sci Soc Sci. 2021;76:S5–S16. doi: 10.1093/geronb/gbab050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whelton PK, Carey RM, Mancia G, Kreutz R, Bundy JD, Williams B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/hypertension guidelines: comparisons, reflections, and recommendations. J Am Coll Cardiol. 2022;80:1192–1201. doi: 10.1016/j.jacc.2022.07.005 [DOI] [PubMed] [Google Scholar]
- 25. Li C, Lumey LH. Impact of disease screening on awareness and management of hypertension and diabetes between 2011 and 2015: results from the China health and retirement longitudinal study. BMC Public Health. 2019;19:421. doi: 10.1186/s12889-019-6753-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee J, Wilkens J, Meijer E, Sekher TV, Bloom DE, Hu P. Hypertension awareness, treatment, and control and their association with healthcare access in the middle‐aged and older Indian population: a nationwide cohort study. PLoS Med. 2022;19:e1003855. doi: 10.1371/journal.pmed.1003855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18:785–802. doi: 10.1038/s41569-021-00559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–237. doi: 10.1038/s41581-019-0244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rothboeck S, Kring T. Promoting transition towards formalization: selected good practices in four sectors. ILO DWT for South Asia and Country Office for India International Labour Office (ILO); 2014. [Google Scholar]
- 30. Wang J, Wang Q, Hou XY, Chen S, Guo Z, Du W, Fan L. Spousal concordance in the development of functional limitations among married adults in China. JAMA Netw Open. 2021;4:e2125577. doi: 10.1001/jamanetworkopen.2021.25577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bell CN, Thorpe RJ Jr, Laveist TA. Race/ethnicity and hypertension: the role of social support. Am J Hypertens. 2010;23:534–540. doi: 10.1038/ajh.2010.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Corsi DJ, Subramanian SV. Socioeconomic gradients and distribution of diabetes, hypertension, and obesity in India. JAMA Netw Open. 2019;2:e190411. doi: 10.1001/jamanetworkopen.2019.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levin JS, Vanderpool HY. Is religion therapeutically significant for hypertension? Soc Sci Med. 1989;29:69–78. doi: 10.1016/0277-9536(89)90129-9 [DOI] [PubMed] [Google Scholar]
- 34. Li J, Shi L, Li S, Xu L, Qin W, Wang H. Urban‐rural disparities in hypertension prevalence, detection, and medication use among Chinese adults from 1993 to 2011. Int J Equity Health. 2017;16:50. doi: 10.1186/s12939-017-0545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schofield P, Saka O, Ashworth M. Ethnic differences in blood pressure monitoring and control in south east London. Br J Gen Pract. 2011;61:190–196. doi: 10.3399/bjgp11X567126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li C, Zhu Y, Ma Y, Hua R, Zhong B, Xie W. Association of cumulative blood pressure with cognitive decline, dementia, and mortality. J Am Coll Cardiol. 2022;79:1321–1335. doi: 10.1016/j.jacc.2022.01.045 [DOI] [PubMed] [Google Scholar]
- 37. Jeemon P, Harikrishnan S, Sanjay G, Sivasubramonian S, Lekha TR, Padmanabhan S, Tandon N, Prabhakaran D. A PROgramme of lifestyle intervention in families for cardiovascular risk reduction (PROLIFIC study): design and rationale of a family based randomized controlled trial in individuals with family history of premature coronary heart disease. BMC Public Health. 2017;17:10. doi: 10.1186/s12889-016-3928-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cobb LK, Godino JG, Selvin E, Kucharska‐Newton A, Coresh J, Koton S. Spousal influence on physical activity in middle‐aged and older adults: the ARIC study. Am J Epidemiol. 2016;183:444–451. doi: 10.1093/aje/kwv104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leong S, Eom K, Ishii K, Aichberger MC, Fetz K, Müller TS, Kim HS, Sherman DK. Individual costs and community benefits: collectivism and individuals' compliance with public health interventions. PLoS One. 2022;17:e0275388. doi: 10.1371/journal.pone.0275388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao J, Zhang J, Xie J, Gu J. Gender specificity of spousal concordance in the development of chronic disease among middle‐aged and older Chinese couples: a prospective dyadic analysis. Int J Environ Res Public Health. 2021;18:18. doi: 10.3390/ijerph18062886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rosenberg NA, Edge MD, Pritchard JK, Feldman MW. Interpreting polygenic scores, polygenic adaptation, and human phenotypic differences. Evol Med Public Health. 2019;2019:26–34. doi: 10.1093/emph/eoy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hippisley‐Cox J, Pringle M. Are spouses of patients with hypertension at increased risk of having hypertension? A population‐based case‐control study. Br J Gen Pract. 1998;48:1580–1583. [PMC free article] [PubMed] [Google Scholar]
- 43. Lyons KS, Lee CS. The theory of dyadic illness management. J Fam Nurs. 2018;24:8–28. doi: 10.1177/1074840717745669 [DOI] [PubMed] [Google Scholar]
- 44. Schmittdiel JA, Cunningham SA, Adams SR, Nielsen J, Ali MK. Influence of a new diabetes diagnosis on the health behaviors of the patient's partner. Ann Fam Med. 2018;16:290–295. doi: 10.1370/afm.2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alam N, Chamot E, Vermund SH, Streatfield K, Kristensen S. Partner notification for sexually transmitted infections in developing countries: a systematic review. BMC Public Health. 2010;10:19. doi: 10.1186/1471-2458-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, Ramirez A, Schlaich M, Stergiou GS, Tomaszewski M, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026 [DOI] [PubMed] [Google Scholar]
- 47. Lamprea‐Montealegre JA, Zelnick LR, Hall YN, Bansal N, de Boer IH. Prevalence of hypertension and cardiovascular risk according to blood pressure thresholds used for diagnosis. Hypertension. 2018;72:602–609. doi: 10.1161/HYPERTENSIONAHA.118.11609 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S8
Figure S1
Data Availability Statement
All data sets used in this analysis are available at https://g2aging.org/downloads. The code for the analysis is available at https://github.com/jvargh7/g2aging_family.


