Abstract
In an earlier paper we described the transcriptionally regulated differential levels of expression of two lipoproteins of Borrelia burgdorferi, P35 and P7.5, during growth of the spirochetes in culture from logarithmic phase to stationary phase (K. J. Indest, R. Ramamoorthy, M. Solé, R. D. Gilmore, B. J. B. Johnson, and M. T. Philipp, Infect. Immun. 65:1165–1171, 1997). Here we further assess this phenomenon by investigating whether the expression of other antigens of B. burgdorferi, including some well-characterized ones, are also regulated in a growth-phase-dependent manner in vitro. These studies revealed 13 additional antigens, including OspC, BmpD, and GroEL, that were upregulated 2- to 66-fold and a 28-kDa protein that was downregulated 2- to 10-fold, during the interval between the logarithmic- and stationary-growth phases. Unlike with these in vitro-regulated proteins, the levels of expression of OspA, OspB, P72, flagellin, and BmpA remained unchanged throughout growth of the spirochetes in culture. Furthermore, ospAB, bmpAB, groEL, and fla all exhibited similar mRNA profiles, which is consistent with the constitutive expression of these genes. By contrast, the mRNA and protein profiles of ospC and bmpD indicated regulated expression of these genes. While bmpD exhibited a spike in mRNA expression in early stationary phase, ospC maintained a relatively higher level of mRNA throughout culture. These findings demonstrate that there are additional genes besides P7.5 and P35 whose regulated expression can be investigated in vitro and which may thus serve as models to facilitate the study of regulatory mechanisms in an organism that cycles between an arthropod and a vertebrate host.
Borrelia burgdorferi, the spirochete that causes Lyme disease, is a remarkably adaptable bacterium. During its life cycle, it is able to survive in the midguts of vector ticks (of the Ixodes ricinus complex) both in the presence and absence of host blood and in the ticks’ saliva. In addition, after withstanding the transit between a poikilothermal and a homeothermal host (usually a rodent) (16), it will colonize practically every organ of the latter. It stands to reason that numerous changes must take place on the spirochetal surface in anticipation of, or after transfer to, a new host or host environment. Several such changes have been observed, yet their functional significance remains unknown. For example, following a tick blood meal, there is a switch in the major surface protein from OspA to OspC (5, 6, 23). Another antigen, P21, appears to be selectively expressed in the mammalian host and not in ticks (3, 26). However, little else is known about other adaptive changes, especially the ones that do not involve antigens, primarily due to the difficulty in harvesting a sufficient number of spirochetes from their hosts for direct examination.
In a recent publication, we showed that the expression of two plasmid-borne genes that encode the lipoproteins P35 (7, 8) and P7.5 (11) of B. burgdorferi is upregulated in the post-logarithmic and stationary phases during growth of the spirochetes in culture (9). We demonstrated further that the upregulation of expression of these genes correlated with higher levels of their mRNA during the later stages of in vitro growth. The phenomenon of growth phase regulation of genes in B. burgdorferi is of biological relevance because the spirochetal population experiences alternating periods of rapid growth and quiescence during its transit through the ticks (4, 19). We therefore decided to investigate more thoroughly this phenomenon with the long-term objective of developing models for understanding the life cycle of the spirochete within a tick. As a result of this study, we have now identified additional candidate proteins that are differentially expressed or upregulated in B. burgdorferi during growth in vitro from logarithmic to stationary phase. We present the results of our study in this paper.
MATERIALS AND METHODS
Bacterial strains.
The JD1 strain of B. burgdorferi originally isolated from an Ixodes dammini (scapularis) nymph (18) was used for this study. The Escherichia coli strain used was XL1 Blue (Stratagene, La Jolla, Calif.).
In vitro culture conditions.
B. burgdorferi JD1 (passage 5) was cultured in BSK-H medium (Sigma Chemical Company, St. Louis, Mo.) as described previously (20) with the following modifications. Briefly, 750 ml of fresh BSK-H medium was inoculated with 1.5 ml of a frozen stock of the JD1 strain of B. burgdorferi to yield a starting cell density of 105 spirochetes/ml. The cell density was monitored daily by counting spirochetes under dark-field microscopy. Cells were harvested on days 3 (at a cell density of 4 × 106 spirochetes per ml), 4 (2 × 107 per ml), 6 (8 × 107 per ml), and 8 (1 × 108 per ml), and whole-cell lysates for protein analysis and RNA for Northern blot analysis were prepared. Sample volumes were adjusted for the various cell densities (see below). An aliquot of cells in the stationary phase (day 8) was reinoculated into fresh BSK-H medium at an initial density of 105 spirochetes per ml as described above and allowed to progress to stationary phase, at which point the cells were subcultured for one final growth cycle. Cells in the log phase of this last subculture, i.e., of passage 8, hereinafter designated “logrev” for log revertant, were also processed for protein and RNA analyses as mentioned above.
Antibodies.
Mouse monoclonal antibodies H5332 and H9724, specific for the B. burgdorferi proteins OspA and flagellin, respectively, were purchased from the University of Texas Health Sciences Center (San Antonio). Additional mouse monoclonal antibodies that are specific to B. burgdorferi OspB, BmpA (P39), GroEL, and a 72-kDa protein were kindly provided by Barbara Johnson, Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention, Ft. Collins, Colo., and an anti-OspC monoclonal antibody (L221F8) (28) was provided by Bettina Wilske, Max von Pettenkofer Institut, Munich, Germany. Finally, the P35-specific monoclonal antibody has been described previously (9). A mouse monospecific polyclonal antibody directed against the BmpD protein of B. burgdorferi, was generated as follows. The mature BmpD polypeptide starting at Cys17 was expressed with a leader peptide, Met-Arg-Gly-Ser-His6-Gly-Ser-Cys, in which the cysteine residue represents the first residue of the mature BmpD sequence. This His6 affinity-peptide-tagged BmpD protein was purified with a nickel-nitrilotriacetic acid-agarose affinity column as per the instructions of the manufacturer (Stratagene). C57BL/10.j mice were intraperitoneally immunized with 15 μg of the fusion protein emulsified in complete Freund’s adjuvant (Sigma). Thereafter, the mice received one of two more injections of the same antigen in Freund’s incomplete adjuvant (Sigma) at 1- and 2-months intervals and were bled 2 weeks after the last immunization.
Preparation of protein samples, SDS-PAGE, and Western blotting.
Cultures (100 ml for sample 1 [4 × 106/ml] [see Fig. 1A] and 50 ml for the rest of the samples) were spun down at 5,000 × g at 4°C for 10 min in a tabletop centrifuge and washed with 1 ml of a wash buffer (20 mM HEPES [pH 7.6], 10 mM NaCl) (2). The washed spirochetes were then resuspended in 100 μl of the wash buffer, and the optical density at 600 nm (OD600) of a 1:100 dilution of this was measured in a spectrophotometer. The remainder of the cells was adjusted with wash buffer and 3× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) lysis buffer such that the final lysate concentration was equivalent to an OD600 of 1, thus normalizing each sample by cell mass.
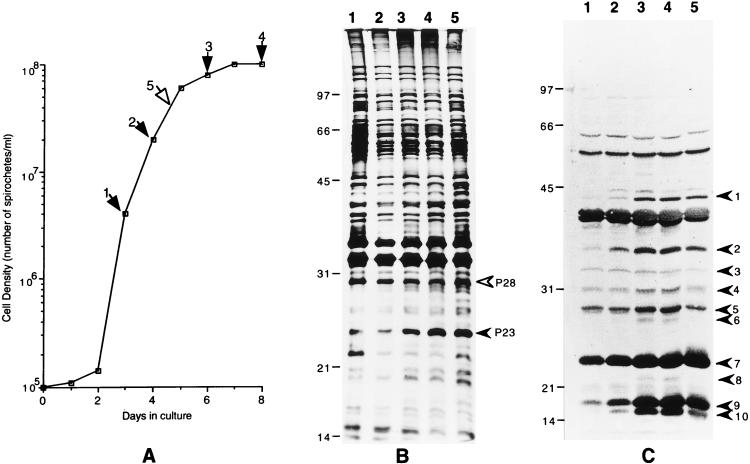
FIG. 1.
(A) Growth curve of B. burgdorferi JD1 (passage 6) in vitro. The arrows indicate the cell densities at which spirochetes were harvested and processed for Western and Northern blotting analyses. Numbers above the arrows designate each sample. The open arrow depicts the passage 8 logrev sampling point. (B) Silver-stained gel showing the profiles of proteins expressed at different times during culture. The filled arrowhead indicates OspC, and the open arrowhead indicates P28. The lane numbers correspond to the numbers in panel A and designate the time point at which each sample was collected. (C) Western blot developed with serum from a monkey with an acute infection of B. burgdorferi JD1 (22). Arrowheads indicate antigens whose levels of expression steadily increased with the increasing age of the culture. Lane numbers are as described for panel B.
The protein samples (10 μl/lane) were electrophoresed through a 12.5% polyacrylamide gel and electroblotted onto nitrocellulose paper (Schleicher & Schuell, Keene, N.H.). The transferred proteins were reacted with B. burgdorferi-infected rhesus monkey serum (K216, 6 weeks postinfection) (22) or with monoclonal and polyclonal antibody reagents specific for several well-characterized B. burgdorferi antigens by a procedure described previously (1). For staining of proteins with silver, lysate samples were diluted 10-fold in sample buffer to a concentration equivalent to an OD600 of 0.1 and processed according to the protocol of the manufacturer (Bio-Rad, Hercules, Calif.).
RNA isolation and Northern blotting.
RNA isolation and Northern blotting of spirochetal samples taken at specific time points along the growth curve were performed essentially as described previously (21). Equal amounts (3 μg/lane) of the RNA samples were electrophoresed through a 1.4% agarose gel in the presence of 2.2 M formaldehyde. In addition to spectrophotometrically quantifying RNA, we stained one blot with methylene blue prior to hybridization to confirm equal loadings of the RNA samples that were obtained at the different time points.
The DNA probes used for hybridization either were restriction fragments from cloned genes or were generated by PCR. PCR primers were designed to amplify only the coding sequences of the relevant genes. The probes were radiolabeled with [α-32P]dATP and the Klenow fragment of DNA polymerase I (Prime-a-Gene kit from Promega Biotech, Madison, Wis.). Reaction mixtures were primed with either random hexamers or gene-specific primers complementary to the RNA sequence. All Northern blots were washed as described previously (21) between 50 and 60°C and exposed to X-ray film.
Quantification of bands on Western and Northern blots.
The blots were digitized with a scanner (Hewlett-Packard Scanjet 4c), and individual bands in the digitized images were quantified with Digital Science 1D software, version 2.02 (Eastman Kodak Company, Rochester, N.Y.).
RESULTS
In a recent paper we described the cell density-dependent expression of B. burgdorferi lipoproteins P7.5 and P35, during growth of the spirochetes in culture (9). In this study we explored the possibility that other B. burgdorferi proteins were similarly differentially expressed during growth in vitro. Samples collected at time points along the growth curve that corresponded to the logarithmic (days 3 and 4), postlogarithmic (day 6), and stationary (day 8) phases were processed for protein and RNA analysis (Fig. 1A). In order to distinguish between proteins that are truly differentially expressed versus those whose expression may be irreversibly affected for unknown reasons, we also analyzed spirochetes from the same batch after they had undergone two additional cycles of growth. Accordingly, we have designated this sample of spirochetes harvested at a cell density similar to that of the parental day 4 logarithmic phase culture “logrev” to indicate the cells’ phenotypic reversion to their predecessors’ logarithmic-phase phenotype.
Profile of protein expression in B. burgdorferi during growth from log to stationary phase in vitro.
Initially, we analyzed the protein samples from the different time points along the growth curve by one-dimensional SDS-PAGE followed by silver staining. This method identified two proteins, 23 and 28 kDa in size, that were differentially expressed against a background of several bands that remained fairly constant in intensity (Fig. 1B). The level of expression of the 28-kDa protein was 2-fold higher in the experiment shown in Fig. 1B (Table 1) and 10-fold higher in a previous experiment (data not shown) early during the logarithmic phase than its level of expression in the stationary phase. In contrast, increasing amounts of the 23-kDa protein accumulated within the cell during growth from logarithmic to stationary phase (Table 1). The 23-kDa band corresponded to the lipoprotein OspC (discussed later). The other proteins that are also known to be differentially expressed, such as P35 and P7.5 (9) and the antigens newly identified in this study by Western blotting (see below), are not apparent in the silver-stained gel, perhaps due to their poor overall levels of expression (P35) or, in the case of P7.5, because they were run off the gel.
TABLE 1.
Relative levels of the antigens identified in Fig. 1
| Method of detection | Protein | Band on blotb | Relative level of antigen in samplea:
|
||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Silver staining of gel | P28 | 1 | 0.8 | 0.6 | 0.4 | 0.8 | |
| P23 | 1 | 1 | 3.5 | 6.5 | 7 | ||
| Western blotting | P43.5 | 1 | 1 | 2.4 | 20 | 19 | 17 |
| P35 | 2 | 1 | 4.4 | 12 | 11 | 7 | |
| P34 | 3 | 1 | 1.5 | 2.3 | 1.5 | 0.9 | |
| P31 | 4 | 1 | 1.5 | 2.8 | 2.8 | 2 | |
| P29 | 5 | 1 | 1.1 | 1.9 | 2.0 | 1.1 | |
| P28 | 6 | ND | 1 | 55 | 50 | 18 | |
| P23 | 7 | 1 | 1.6 | 2.0 | 2.4 | 2.4 | |
| P22 | 8 | ND | 1 | 7 | 10 | 0.7 | |
| P16.5 | 9 | 1 | 3.2 | 7.6 | 8.7 | 7.4 | |
| P15 | 10 | 1 | 11 | 48 | 59 | 41 | |
The sample numbers are the same as those in Fig. 1A. The densitometry values for each protein were normalized to the estimated value of the same protein in sample 1 or, in those cases in which a band was not visible in sample 1, in sample 2. ND, no band detected.
Band numbers are the same as those in Fig. 1C.
In addition to silver staining, the same set of protein samples was also examined by Western blotting with serum from a rhesus monkey with an acute infection with spirochetes from the JD1 strain of B. burgdorferi in a search for antigens that are expressed early in infection and are differentially regulated in vitro. This serum recognized at least 18 distinct bands in the Western blot of the whole-cell lysate from strain JD1 (Fig. 1C). Ten of these bands, which correspond to proteins of 15, 16.5, 22, 23, 28, 29, 31, 34, 35, and 43.5 kDa, were upregulated between 2- to 50-fold during growth from logarithmic phase to stationary phase (Fig. 1C; Table 1). More specifically, the levels of expression of all 10 proteins steadily increased between days 3 (logarithmic phase) and 8 (stationary phase) of culture, an expression pattern similar to those described for P35 and P7.5 (9). It is very likely that the 23- and the 35-kDa bands are OspC and P35, respectively. The levels of expression of a majority of the 10 proteins in the logrev sample were between the levels found on days 4 and 6, as was expected from the intermediate position of the logrev sampling point on the growth curve. Apart from the changes noted for these 10 bands, there were no significant changes in the intensities of the remaining bands among the samples from days 3, 4, 6, 8, and logrev.
Examination by Western and Northern blotting of the expression of several well-characterized antigens during in vitro growth of B. burgdorferi.
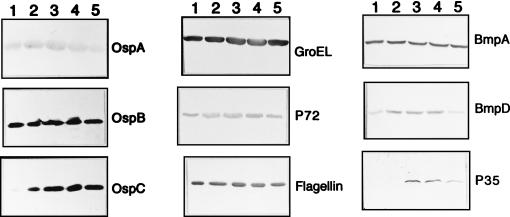
Next, we determined whether the expression of some of the well-characterized borrelial antigens was affected by the growth phase of the spirochetes. Western blots were analyzed with monoclonal antibodies to BmpD, GroEL, flagellin, OspC, and P35 (Fig. 2), as well as to OspA, OspB, BmpA (P39), and P72 (not shown). P35 was included as a control for a growth-phase-regulated protein because in a previous study we had demonstrated that its expression was upregulated during the postexponential stages of growth (9).
FIG. 2.
Western blots showing the patterns of expression of specific B. burgdorferi proteins during growth of the spirochetes in vitro. The blots were developed with monoclonal or monospecific polyclonal antibodies specific for the following proteins: OspA, OspB, OspC, GroEL, P72, flagellin, BmpA, BmpD, and P35. Lanes 1 to 5 contain samples of the initial culture taken on days 3, 4, 6, and 8 and of the second subculture taken on day 5, respectively.
The Western blots did not reveal any significant changes in the levels of OspA, OspB, BmpA, flagellin, and P72 during in vitro growth of B. burgdorferi JD1, but they did reveal average increases of four- and twofold in the levels of BmpD and GroEL, respectively, between the samples drawn on days 3 and 8 (Fig. 2; Table 2). In contrast, the level of OspC increased up to 66-fold between the day 3 (logarithmic phase) and day 8 (postlogarithmic phase) time points (Fig. 2; Table 2), which was consistent with the pattern observed for the 23-kDa protein in the silver-stained gel and the Western blot developed with the infected monkey serum. As expected, the expression of the control protein P35 was discernible only in the postlogarithmic and stationary phases (Fig. 2).
TABLE 2.
Relative levels of well-characterized B. burgdorferi proteins during in vitro growth
| Protein | Relative level of proteina
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expt 1, sample:
|
Expt 2, sample:
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| OspA | 1 | 0.9 | 0.8 | 1.0 | 1.3 | 1 | 1.0 | 1.2 | 0.7 | 0.4 |
| OspB | 1 | 1.0 | 1.0 | 0.9 | 1.1 | 1 | 0.9 | 1.2 | 1.2 | 1.0 |
| OspC | 1 | 1.1 | 1.1 | 1.1 | NE | 1 | 25 | 66 | 64 | 62 |
| BmpA | 1 | 1.0 | 1.0 | 1.1 | 1.1 | 1 | 1.0 | 0.9 | 1.0 | 0.9 |
| BmpD | 1 | 4.7 | 5.2 | 5.1 | 1.8 | 1 | 2.4 | 2.6 | 2.7 | 1.2 |
| Flagellin | 1 | 1.2 | 1.5 | 1.6 | 1.4 | 1 | 1.3 | 1.3 | 1.2 | 1.0 |
| GroEL | 1 | 2.6 | 1.5 | 1.9 | 1.0 | 1 | 1.3 | 2.0 | 1.5 | 1.2 |
| P35 | ND | 1 | 2.3 | 7.4 | ND | ND | 1 | 1.8 | 3.6 | 1.0 |
| P72 | 1 | 1.3 | 1.1 | 1.1 | 1.3 | 1 | 1.4 | 1.4 | 1.2 | 1.1 |
The sample numbers for the two experiments are the same as those in Fig. 1A. The densitometry values for each protein have been normalized to the estimated value of the same protein in sample 1 or, in the case of P35, in sample 2. NE, not estimated (the intensity of the band was below the range of estimation); ND, no band detected.
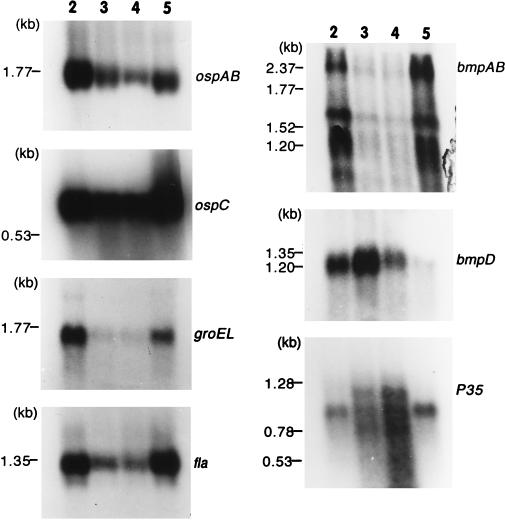
In order to correlate the protein expression with mRNA expression for the above-described antigens of B. burgdorferi during growth in culture, their steady-state mRNA profiles were visualized by Northern blotting and probing with gene-specific sequences. Except with bmpA and P35, where the probe hybridized to multiple transcripts, in all other instances the gene-specific probes detected a single transcript. The measured sizes of the transcripts for the examined genes were as follows: 1.8 kb for ospAB; 0.9 kb for ospC; 2.4, 1.6, and 1.1 kb for bmpA; 1.3 kb for bmpD; 1.75 kb for groEL; 1.35 kb for fla; and 0.8, 1.0, and 1.2 kb for P35 (Fig. 3).
FIG. 3.
Northern blots showing the mRNA profiles for ospAB, ospC, groEL, fla, bmpAB, bmpD, and P35. It should be noted that lanes 2 to 5 contain samples of the initial culture taken on days 4, 6, and 8 of the second subculture taken on day 5, respectively.
The changes in the mRNA profiles of ospAB, fla, groEL, and bmpA (all three detected transcripts) were nearly indistinguishable from each other (Fig. 3). In each case, there was a progressive decline in the level of mRNA between the level found on day 4 and the level found on day 8 (Table 3). This finding is not surprising and is in fact expected of most genes, given the gradual deceleration in the various metabolic processes, including transcription, that occurs in cells between the log and stationary phases. The bmpD mRNA steady-state profile was overall similar to those of the above-described genes except that the level peaked on day 6 (Fig. 3; Table 3), which can be best described, based on the growth curve in Fig. 1A, as an early-stationary-phase time point. We also probed one Northern blot with the P35 sequence to verify that the results obtained were not a consequence of unequal levels of loading of the RNA samples. The P35 probe hybridized to three transcripts of 0.8, 1.0, and 1.2 kb. This is not surprising because the recent sequence analysis of the B. burgdorferi genome revealed the presence of multiple homologs of this gene (7), and therefore, the three transcripts may correspond to three different P35 homologs. Nevertheless, there was a steady increase in the cumulative level of the three transcripts as the spirochetes progressed to the stationary phase. This was consistent with the steady increase in the level of the P35 protein during the same time period (Table 3).
TABLE 3.
Relative levels of mRNAs
| mRNA | Relative level of mRNAa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Expt 1, sample:
|
Expt 2, sample:
|
||||||||
| 1 | 2 | 3 | 4 | 5 | 2 | 3 | 4 | 5 | |
| ospAB | 1 | 0.6 | 0.2 | 0.1 | 0.9 | 1 | 0.4 | 0.2 | 0.5 |
| ospC | 1 | 0.4 | 0.2 | 0.2 | 0.1 | 1 | 0.9 | 0.8 | 1.4 |
| bmpABb | 1 | 0.4 | 0.1 | 0.1 | 0.9 | 1 | 0.2 | 0.1 | 1.1 |
| bmpD | 1 | 2.4 | 0.4 | 0.1 | 0.5 | 1 | 3.1 | 0.7 | 0.4 |
| groEL | 1 | 0.6 | 0.03 | 0.02 | 0.7 | 1 | 0.01 | 0.01 | 0.6 |
| fla | 1 | 0.2 | 0.04 | 0.01 | 0.5 | 1 | 0.3 | 0.2 | 1.1 |
| P35b | 1 | 3 | 7 | 19 | 1.4 | 1 | 2 | 4 | 1.4 |
The sample numbers are the same as those in Fig. 1A.
The results are the sums of the values obtained for each of the three transcripts of these genes.
DISCUSSION
In this paper we have extended our earlier study of the phenomenon of the regulated expression of genes in B. burgdorferi during growth in vitro. We generally searched for proteins that might be differentially expressed during in vitro growth of these spirochetes and also specifically evaluated some of these organisms’ best-characterized antigens. We have so far identified 13 proteins whose expression levels are affected in cultured spirochetes during growth from the logarithmic to the stationary phase. These proteins include the previously described P35 and P7.5 (9) and, from this study, P15, P16.5, P22, P23 (OspC), P27, P28, P29, P31, and P43.5, based on an estimation of their apparent molecular weights as determined by SDS-PAGE. With the exception of P28, the expression of the other seven newly identified proteins, including OspC, increased steadily during growth from logarithmic to stationary phase, a pattern of expression similar to that of P35 and P7.5. P28, on the other hand, was expressed at a relatively higher level in the logarithmic phase than in the stationary phase.
Among the previously characterized proteins individually tested with specific monoclonal antibodies, OspC, GroEL, and BmpD displayed differential levels of expression during growth of the spirochetes in culture. The expression of OspA, OspB, BmpA, flagellin, and P72 remained largely constant throughout the culture period. There was an excellent correlation between the mRNA and protein levels for ospAB, bmpA, and fla. With groEL, the marginal increase in the protein level during the later stages of growth did not correspond to its mRNA levels at these time points. Nevertheless, the overall good correlation between the protein and the mRNA levels is strong evidence that the expression of these genes, including groEL, is predominantly controlled at the transcriptional level during in vitro growth of spirochetes in BSK-H medium. Furthermore, the close resemblance of the steady-state protein and RNA profiles of ospAB, bmpAB, groEL, and fla may mean that these genes are regulated (or unregulated) similarly at the transcriptional level. However, this may not always be true of ospAB and fla. ospAB expression has been found to be quite varied at the RNA level among strains and between the different passages of a given strain (10, 13). In addition, a DNA-binding activity that binds to a region upstream of the ospAB operon has been demonstrated recently in stationary-phase spirochetes (14). Based on the 5′ flanking DNA sequence, it has been speculated that the expression of the fla gene may be under the control of an alternative sigma factor and hence may be regulated (17).
The expression of OspC increased steadily during the transition from logarithmic to stationary phase. However, the expression of OspC in the logrev sample was not always as expected. In one experiment, there was very little expression of OspC in the logrev sample (data not shown), whereas its level of expression in a repeat experiment was consistent with the pattern of a steady increase with the increasing age of the culture (Fig. 1B and C). Several other studies have also demonstrated the variable expression of OspC in cultured spirochetes (12, 15, 25, 28). More importantly, OspC has been shown to be upregulated by a shift in culture temperature from 24 to 37°C (24) and this induction occurs at the transcriptional level (27). It is interesting to speculate, based on the results from this study, that ospC expression may also be regulated transcriptionally and translationally during growth in culture. First, the level of ospC mRNA was relatively higher than those of ospAB, bmpAB, groEL, and fla throughout the culture period, suggesting that ospC transcription may be active during both the logarithmic and postlogarithmic phases, although the persistence of the ospC mRNA may also reflect increased messenger stability. Second, while the ospC mRNA level peaked on day 4 (late logarithmic phase) the protein level reached a maximum only on day 6 (early stationary phase) (Fig. 1B and 2). By contrast, as mentioned above, the levels of expression of other proteins, such as OspA, OspB, flagellin, BmpA, and GroEL, correlated very well with their levels of mRNA. This result hints at the possibility of additional translational control of ospC expression.
The expression of bmpD also appears to be regulated in vitro, but the pattern of RNA expression suggests an entirely different mechanism from that of ospC or P35 RNA expression. The levels of bmpD mRNA and protein peaked at the onset of the stationary phase, unlike those of the genes that are expressed early in logarithmic phase and of the 11 antigens, including P7.5, P35, and ospC, whose expression continued to increase late in growth. The notion of regulated expression of bmpD is supported by the presence of a 13-bp inverted repeat sequence upstream of the gene and overlapping the putative −35 region (21). Furthermore, this overlap of the inverted repeat with the −35 region and the lack of a consensus −35 sequence suggest that bmpD may be positively regulated, being turned on or induced by the binding of a transcriptional activator to the inverted-repeat region.
Interestingly, in all our Western blots developed either with infected-monkey serum or with specific monoclonal antibodies we saw no evidence for a decrease in the levels of antigens during the culture period. In other words, once these antigens were synthesized, their levels were maintained by the bacterial cell throughout the culture period. Included in this group of antigens are some of the well-characterized lipoproteins of B. burgdorferi such as OspA, OspB, OspC, BmpA, and BmpD and the nonlipidated flagellin. Therefore, the diminished expression of proteins such as OspA and OspB that has been observed in tick-borne spirochetes after a tick blood meal (23) may reflect a combination of transcriptional repression of the OspAB operon and selective loss of these proteins during rapid spirochetal multiplication.
The 18 proteins, including OspC, that are recognized by the serum of the B. burgdorferi-infected rhesus monkey correspond to antigens that are present in the spirochete during the early stages of infection. However, OspC expression is known to be turned on in the spirochetes within the tick gut during the course of a blood meal and to continue following infection of the mammalian host. It is tempting then to speculate that the other nine antigens that are upregulated like OspC during growth from logarithmic to stationary phase in vitro may also be similarly turned on within the tick during the course of the blood meal prior to invasion of the mammalian host. Surprisingly, the same serum failed to bind to the abundantly expressed and logarithmic-phase-specific protein P28. This may simply mean that P28 is not immunogenic or that it is absent in spirochetes during the early stages of invasion of the mammalian host.
To summarize, in our earlier paper we suggested that the lipoprotein P35, whose expression is transcriptionally regulated in vitro, might be used as a model to dissect mechanisms of gene expression in B. burgdorferi. We have now identified other proteins, including BmpD, OspC, and P28, all of which also may be used for this purpose.
ACKNOWLEDGMENTS
This work was supported by grants AI 35027 and RR00164 from the National Institutes of Health.
We thank Laura Povinelli for help with the production of mouse anti-BmpD antiserum and Barbara J. B. Johnson (Centers for Disease Control and Prevention, Fort Collins, Colo.) and Bettina Wilske (Max von Pettenkofer Institut) for kindly providing monoclonal antibodies. The excellent secretarial help of Christie Trew and the photographic skill of Murphy Dowouis are acknowledged with thanks.
REFERENCES
- 1.Aydintug M, Gu Y, Philipp M T. Borrelia burgdorferi antigens that are targeted by antibody-dependent, complement-mediated killing in the rhesus monkey. Infect Immun. 1994;62:4929–4937. doi: 10.1128/iai.62.11.4929-4937.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bledsoe H A, Carrol J A, Whelchel T R, Farmer M A, Dorward D W, Gherardini F C. Isolation and partial characterization of Borrelia burgdorferi inner and outer membranes by using isopycnic centrifugation. J Bacteriol. 1994;176:7447–7455. doi: 10.1128/jb.176.24.7447-7455.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das S, Barthold S W, Giles S S, Montgomery R R, Telford III S R, Fikrig E. Temporal pattern of Borrelia burgdorferi p21 expression in ticks and the mammalian host. J Clin Investig. 1997;99:987–995. doi: 10.1172/JCI119264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Silva A M, Fikrig E. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am J Trop Med Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 5.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fingerle V, Hauser U, Liegl G, Petko B, Preac-Mursic V, Wilske B. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J Clin Microbiol. 1995;33:1867–1869. doi: 10.1128/jcm.33.7.1867-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J-F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Weidman J, Utterback T, Whattey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton M D, Horst K, Roberts K, Hatch B, Smith H O, Venter J C. Genome sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 8.Gilmore R D, Jr, Kappel K J, Johnson B J. Molecular characterization of a 35-kilodalton protein of Borrelia burgdorferi, an antigen of diagnostic importance in early Lyme disease. J Clin Microbiol. 1997;35:86–91. doi: 10.1128/jcm.35.1.86-91.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indest K J, Ramamoorthy R, Solé M, Gilmore R D, Johnson B J B, Philipp M T. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson M, Bergstrom S. Transcriptional and translational regulation of the expression of the major outer surface proteins in Lyme disease Borrelia strains. Microbiology. 1995;141:1321–1329. doi: 10.1099/13500872-141-6-1321. [DOI] [PubMed] [Google Scholar]
- 11.Lahdenne P, Porcella S F, Hagman K E, Akins D R, Popova T G, Cox D L, Katona L I, Radolf J D, Norgard M V. Molecular characterization of a 6.6-kilodalton Borrelia burgdorferi outer membrane-associated lipoprotein (lp6.6) which appears to be downregulated during mammalian infection. Infect Immun. 1997;65:412–421. doi: 10.1128/iai.65.2.412-421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marconi R T, Samuels D S, Garon C F. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol. 1993;175:926–932. doi: 10.1128/jb.175.4.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margolis N, Rosa P A. Regulation of expression of major outer surface proteins in Borrelia burgdorferi. Infect Immun. 1993;61:2207–2210. doi: 10.1128/iai.61.5.2207-2210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis N, Samuels D S. Proteins binding to the promoter region of the operon encoding the major outer surface proteins OspA and OspB of Borrelia burgdorferi. Mol Biol Rep. 1995;21:159–164. doi: 10.1007/BF00997238. [DOI] [PubMed] [Google Scholar]
- 15.Masuzawa T, Kurita T, Kawabata H, Yanagihara Y. Relationship between infectivity and OspC expression in Lyme disease Borrelia. FEMS Microbiol Lett. 1994;123:319–324. doi: 10.1111/j.1574-6968.1994.tb07242.x. [DOI] [PubMed] [Google Scholar]
- 16.Mather T N, Wilson M L, Moore S I, Ribeiro J M C, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete. Am J Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- 17.Noppa L, Burman N, Sadziene A, Barbour A G, Bergstrom S. Expression of the flagellin gene in Borrelia is controlled by an alternative sigma factor. Microbiology. 1995;141:85–93. doi: 10.1099/00221287-141-1-85. [DOI] [PubMed] [Google Scholar]
- 18.Piesman J, Mather T N, Sinsky R, Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987;25:557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piesman J, Oliver J R, Sinsky R J. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini) Am J Trop Med Hyg. 1990;42:352–357. doi: 10.4269/ajtmh.1990.42.352. [DOI] [PubMed] [Google Scholar]
- 20.Pollack R J, Telford III S R, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramamoorthy R, Povinelli L, Philipp M T. Molecular characterization, genomic arrangement, and expression of bmpD, a new member of the bmp class of genes encoding membrane proteins of Borrelia burgdorferi. Infect Immun. 1996;64:1259–1264. doi: 10.1128/iai.64.4.1259-1264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts E D, Bohm R P, Jr, Cogswell F B, Lanners H N, Lowrie R C, Jr, Povinelli L, Piesman J, Philipp M T. Chronic Lyme disease in the rhesus monkey. Lab Investig. 1995;72:146–160. [PubMed] [Google Scholar]
- 23.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson B, Barthold S W. Expression and sequence of outer surface protein C among North American isolates of Borrelia burgdorferi. FEMS Microbiol Lett. 1994;124:367–372. doi: 10.1111/j.1574-6968.1994.tb07310.x. [DOI] [PubMed] [Google Scholar]
- 26.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilly K, Casjens S, Stevenson B, Bono J L, Samuels D S, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]