Abstract
Background
Atrial fibrillation (AF) progression is closely related to heart failure occurrence, and catheter ablation carries a beneficial effect for heart failure prevention. Recently, particular attention has been given to left atrial (LA) function and functional reserve in the pathogenesis linking AF and heart failure, although its significance and reversibility is not well studied.
Methods and Results
We prospectively investigated 164 patients with AF with normal left ventricular systolic function and free from heart failure who underwent first catheter ablation and pre‐/postprocedural echocardiography. Conventional and speckle‐tracking echocardiography were performed at rest and during passive leg lifting to assess LA size, LA reservoir strain (LARS), and functional reserve calculated as passive leg lifting‐LARS – rest‐LARS. Patients were categorized into 3 AF subtypes: paroxysmal AF (N=95), persistent AF (PeAF; N=50), and long‐standing persistent AF (LS‐PeAF; N=19). The PeAF and LS‐PeAF groups had larger LA size and reduced LARS compared with the paroxysmal AF group (all P<0.05). LA functional reserve was significantly impaired in the LS‐PeAF group (P=0.003). In multivariable analysis, LS‐PeAF and advanced age were significantly associated with impaired LA functional reserve. Among 149 patients with sinus rhythm 1 to 2 days after catheter ablation, LARS was significantly improved in both PeAF and LS‐PeAF groups but was still lower than that in the paroxysmal AF group. Sinus rhythm restoration also led to amelioration of LA functional reserve in patients with LS‐PeAF.
Conclusions
AF progression was related to impaired LARS and LA functional reserve, and restoration of sinus rhythm might contribute to early LA reverse remodeling.
Keywords: atrial fibrillation, left atrial functional reserve, left atrial reservoir strain, speckle‐tracking echocardiography
Subject Categories: Atrial Fibrillation, Echocardiography
Nonstandard Abbreviations and Acronyms
- LARS
left atrial reservoir strain
- LS‐PeAF
long‐standing persistent atrial fibrillation
- PAF
paroxysmal atrial fibrillation
- PeAF
persistent atrial fibrillation
- PLL
passive leg lifting
Clinical Perspective.
What Is New?
Patients with persistent atrial fibrillation and those with long‐standing persistent atrial fibrillation exhibited lower left atrial (LA) reservoir strain compared with patients with paroxysmal atrial fibrillation, and LA functional reserve corresponding to acute volume overload was significantly impaired in the long‐standing persistent atrial fibrillation group.
Long‐standing persistent atrial fibrillation and advanced age were significantly associated with abnormal LA functional reserve.
Restoration of sinus rhythm resulted in early improvement of LA mechanics.
What Are the Clinical Implications?
LA reservoir strain and LA functional reserve reflecting LA distensibility to acute volume stress decreased with the progression of atrial fibrillation, which might be involved in the mechanisms linking the severity of atrial fibrillation and heart failure.
Resumption of sinus rhythm was associated with early improvement of LA function, which might modify heart failure risk.
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in daily practice, affecting a growing number of people worldwide owing to the aging of populations. 1 AF carries significant risk for heart failure (HF), particularly HF with preserved left ventricular ejection fraction, with a 7‐fold elevated risk, 2 and the incidence of HF increases with AF progression. 3 During the past 3 decades, catheter ablation of AF has evolved from an investigational procedure to an established therapy for restoring sinus rhythm, 4 and recent studies demonstrated its beneficial effect for HF prevention. 5 , 6 Left atrial (LA) functional alteration plays a key role in the incidence and maintenance of AF as well as subsequent HF occurrence. 7 , 8 , 9 LA reserves blood from the pulmonary vein and modifies left ventricular (LV) filling in response to the variation of preload. Impaired LA reservoir strain (LARS) assessed by speckle‐tracking echocardiography reflects reduced LA compliance, which precedes LA enlargement and serves as a sensitive and reliable marker of AF recurrence and adverse cardiovascular events in patients with AF. 10 , 11 More recently, particular attention has been given to LA functional reserve that reflects LA distensibility corresponding to increased preload in patients with HF, 12 , 13 and LARS response to passive leg lifting (PLL), a simple and noninvasive maneuver that rapidly increases venous return to the central circulation, corresponds to LA functional reserve. 14 However, the relationship between progression of AF and LA functional remodeling is not well described. In addition, the acute effect of restoring sinus rhythm by catheter ablation on LA mechanics is unknown. We therefore hypothesized that (1) LA function and functional reserve might be impaired with advancing AF stages, and (2) catheter ablation could ameliorate LA functional impairment. Accordingly, the aims of the present study were to evaluate the LA function/functional reserve and their changes after catheter ablation in patients with AF with normal LV systolic function and free from HF who underwent first catheter ablation.
Methods
The authors declare that all supporting data are available from the corresponding author upon reasonable request.
Study Population
This prospective study included 198 consecutive patients with AF who underwent a first catheter ablation and pre‐/postprocedural echocardiographic examination (1–4 days before and 1–2 days after catheter ablation) for the evaluation of LA mechanics from May 2019 to March 2022 at the University of Tokyo Hospital. The exclusion criteria were as follows: (1) congenital heart disease, (2) moderate or severe valvular disease, (3) dilated or hypertrophic cardiomyopathy, (4) history of cardiothoracic surgery within 3 months, (5) history of pacemaker implantation, and (6) renal insufficiency on hemodialysis. Among the 198 patients, those with a history of HF (N=11), LV ejection fraction <50% (N=18), and inadequate image quality (N=5) were then excluded from the analysis (Figure 1). Thus, the final study population included 164 patients who were categorized into 3 groups according to AF severity: paroxysmal AF (PAF, N=95), persistent AF (PeAF, N=50), and long‐standing persistent AF (LS‐PeAF, N=19). PAF was defined as AF that terminated spontaneously within 7 days of onset; PeAF as continuous AF for >7 days; and LS‐PeAF as continuous AF of >12 months' duration with AF rhythm. 4 All patients underwent clinical examination and laboratory tests, including metabolic profile, renal function, C‐reactive protein level, and B‐type natriuretic peptide level before catheter ablation. Written informed consent was collected from all study participants. The study was performed according to the principles of the Declaration of Helsinki, and the institutional review board of the University of Tokyo approved the study protocol (2018120NI).
Figure 1. Study flowchart.
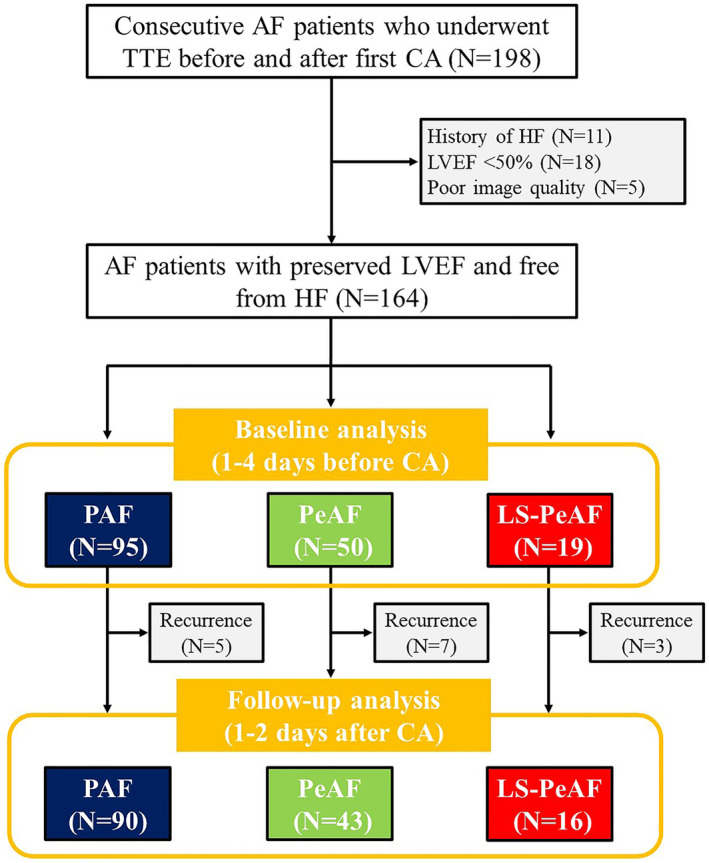
AF indicates atrial fibrillation; CA, catheter ablation; HF, heart failure; LS‐PeAF, long‐standing persistent atrial fibrillation; LVEF, left ventricular ejection fraction; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; and TTE, transthoracic echocardiography.
Echocardiography
Conventional Echocardiography
Transthoracic echocardiography was performed using a commercially available system, Vivid E95 (GE Vingmed Ultrasound, Horten, Norway) or EPIQ 7 (Koninklijke Philips N.V., the Netherlands), 1 to 4 days before and 1 to 2 days after catheter ablation. All images were recorded by experienced and registered cardiologists. Linear measurements of the cardiac chamber were performed in the standard manner. 15 LV mass was calculated as follows: LV mass=0.8×1.04×[(LV end‐diastolic dimension+posterior wall thickness+interventricular septum thickness)3 − (LV end‐diastolic dimension)3]+0.6. 15 LV ejection fraction and LA volume were evaluated by the biplane Simpson rule. 15 LV mass and LA volume were normalized for body surface area. Peak early (E) diastolic velocity was measured using transmitral inflow signals. Peak early diastolic velocity (e′) of the septal and lateral mitral annulus was measured from tissue Doppler imaging and averaged. E/e′ was then calculated. 16
Speckle‐Tracking Echocardiography
Speckle‐tracking analysis was performed using vendor‐independent commercial software (2‐dimensional Cardiac Performance Analysis; Tomtec Imaging System, Germany). The LA endocardial border was semiautomatically traced and tracked throughout the cardiac cycle, and was manually corrected in case of inadequate endocardial detection. LARS was assessed with the average of 6 peak LA segmental strains from the apical 2‐ and 4‐ chamber images. 17 LA serves as a reservoir that collects blood from the pulmonary vein during LV systole and ejects the blood into LV in diastole. In the reservoir phase, LA filling with longitudinal stretching corresponds to positive LA strain curve, and its peak before mitral valve opening is measured as LARS (Figure 2). 17 , 18 , 19
Figure 2. Assessment of LA reservoir strain and LA functional reserve.
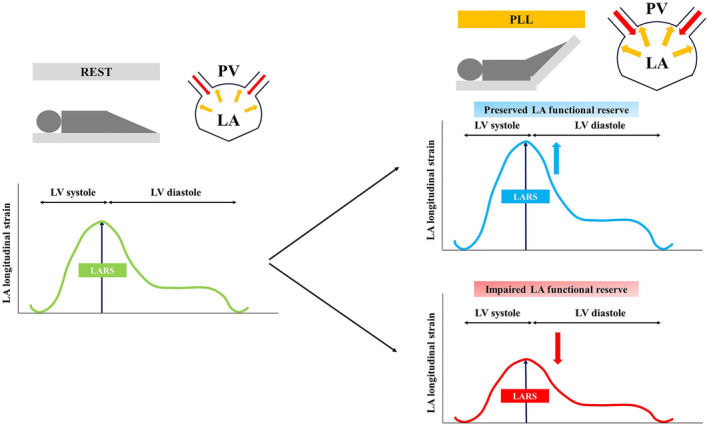
LA indicates left atrial; LARS, left atrial reservoir strain; LV, left ventricular; PLL, passive leg lifting; and PV, pulmonary vein.
Assessment of LA Functional Reserve
After baseline echocardiographic examination, echocardiographic measurements were repeated during PLL as previously described. 20 Briefly, each patient's legs were passively elevated to a 45° angle and maintained in that position for 3 minutes, and imaging data were obtained during leg raises. LA functional reserve was defined as change of LARS through PLL, calculated as PLL‐LARS – rest‐LARS. 14 Impaired LA functional reserve was defined as the change of LARS through PLL <0%, indicating lack of LA distensibility in response to increased preload (Figure 2).
Catheter Ablation Procedure
Catheter ablation was performed under sedation. All patients underwent pulmonary vein isolation by point‐by‐point radiofrequency energy or the balloon technique to restore sinus rhythm, with an end point of bidirectional block between the LA and the inside of the circumferential pulmonary vein isolation area. Additional procedures including cavotricuspid isthmus ablation, superior vena cava isolation, and roof line and mitral isthmus line ablation were performed according to the physician's discretion.
Statistical Analysis
Continuous variables were expressed as mean±standard deviation or median (interquartile range) and compared using analysis of variance with Tukey‐Kramer post hoc analysis or a Kruskal‐Wallis test with the posttest Dunn correction. Categorical variables were described as numbers and proportions, and analyzed using the χ2 test or Fisher exact test. Changes of echocardiographic parameters before and after PLL, and pre‐ and postprocedure were compared by the paired t test. Univariable and multivariable logistic regression analysis was performed to identify the variables that were statistically associated with impaired LA functional reserve (change of LARS through PLL <0%). Interobserver variability for LARS was assessed using intraclass correlation coefficient in 15 randomly selected patients by 2 independent and blinded observers. A P value of <0.05 was considered statistically significant. All analyses were performed with JMP Pro 15 statistical software (SAS Institute, Cary, NC).
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. Mean age was 64±11 years and 116 (70.7%) patients were men. Patients were categorized into 3 AF subtypes according to the AF severity (Figure 1): PAF (N=95), PeAF (N=50), and LS‐PeAF (N=19). The LS‐PeAF group had a greater proportion of men and higher heart rate but no significant differences in other patient demographics. Laboratory tests demonstrated that the circulating B‐type natriuretic peptide level was significantly higher in the PeAF and LS‐PeAF groups than in the PAF group (both P<0.05).
Table 1.
Baseline Characteristics According to the Atrial Fibrillation Types
| Characteristic | PAF (N=95) | PeAF (N=50) | LS‐PeAF (N=19) | P value |
|---|---|---|---|---|
| Age, y | 64±11 | 65±10 | 65±10 | 0.958 |
| Men, n (%) | 61 (64.2) | 37 (74.0) | 18 (94.7) | 0.016 |
| Body mass index, kg/m2 | 24.3±3.4 | 25.0±3.4 | 24.9±2.5 | 0.279 |
| Systolic blood pressure, mm Hg | 123±16 | 121±18 | 115±14 | 0.134 |
| Diastolic blood pressure, mm Hg | 68±10 | 70±14 | 65±10 | 0.335 |
| Heart rate, bpm | 71±12 | 76±11 | 84±12* , † | <0.001 |
| Current smoking, n (%) | 8 (8.4) | 4 (8.0) | 4 (21.1) | 0.217 |
| Hypertension, n (%) | 49 (51.6) | 27 (54.0) | 8 (42.1) | 0.673 |
| Diabetes, n (%) | 16 (16.8) | 15 (30.0) | 4 (21.1) | 0.187 |
| Dyslipidemia, n (%) | 42 (44.2) | 22 (44.0) | 6 (31.6) | 0.582 |
| CHA2DS2‐VASc score | 2 (1–3) | 2 (1–3) | 1 (0–2) | 0.304 |
| Medications | ||||
| β‐Blocker, n (%) | 29 (30.5) | 27 (54.0) | 5 (26.3) | 0.012 |
| RAAS blocker, n (%) | 27 (28.4) | 17 (34.0) | 7 (36.8) | 0.668 |
| Calcium channel blocker, n (%) | 33 (34.7) | 19 (38.0) | 5 (26.3) | 0.661 |
| Statin, n (%) | 28 (29.5) | 15 (30.0) | 4 (21.1) | 0.796 |
| Oral antidiabetic drug, n (%) | 13 (13.7) | 13 (26.0) | 2 (10.5) | 0.137 |
| Antiarrhythmic drug, n (%) | 22 (23.2) | 19 (38.0) | 9 (47.4) | 0.043 |
| Laboratory data | ||||
| LDL cholesterol, mg/dL | 115±29 | 113±35 | 125±29 | 0.332 |
| HDL cholesterol, mg/dL | 61±16 | 58±14 | 61±21 | 0.759 |
| Triglyceride, mg/dL | 100 (66–149) | 95 (69–129) | 112 (72–190) | 0.376 |
| Fasting blood glucose, mg/dL | 102±27 | 114±47* | 106±35 | 0.041 |
| eGFR, mL/min per 1.73 m2 | 67.4±14.2 | 67.2±16.6 | 63.6±13.6 | 0.594 |
| C‐reactive protein, mg/dL | 0.06 (0.03–0.10) | 0.07 (0.03–0.20) | 0.09 (0.06–0.21) | 0.089 |
| BNP, pg/mL | 33 (15–58) | 94 (60–177)* | 95 (43–186)* | <0.001 |
| Ablation procedure characteristics | ||||
| Total procedure time, min | 159±40 | 174±42 | 193±51* | 0.006 |
| PVI, n (%) | 95 (100.0) | 50 (100.0) | 19 (100.0) | N/A |
| Radiofrequency ablation, n (%) | 35 (36.8) | 42 (84.0) | 18 (94.7) | <0.001 |
| Cryoballoon ablation, n (%) | 60 (63.2) | 8 (16.0) | 1 (5.3) | <0.001 |
| Extensive LA ablation, n (%) | 3 (3.2) | 0 (0.0) | 0 (0.0) | 0.691 |
| SVC isolation, n (%) | 1 (1.1) | 0 (0.0) | 1 (5.3) | 0.311 |
| Cavotricuspid isthmus, n (%) | 21 (22.1) | 10 (20.0) | 6 (31.6) | 0.582 |
Data shown as mean±SD, n (percentage), or median (interquartile range). BNP indicates B‐type natriuretic peptide; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LA, left atrial; LDL, low‐density lipoprotein; LS‐PeAF, long‐standing persistent atrial fibrillation; N/A, not applicable; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; PVI, pulmonary vein isolation; RAAS, renin‐angiotensin‐aldosterone system; and SVC, superior vena cava.
P<0.05 vs PAF group.
P<0.05 vs PeAF group.
Preprocedural Echocardiographic Parameters at Rest and During PLL
All patients underwent echocardiographic examination 1 to 4 days before catheter ablation at rest and during PLL (Table 2). At rest, LV size, LV mass index, and E/e′ ratio were similar across the 3 AF groups. LV ejection fraction was lower in the PeAF and LS‐PeAF groups compared with the PAF group, whereas all patients had preserved LV ejection fraction (>50%) by virtue of the study design. As for LA parameters, patients with PeAF and LS‐PeAF had larger LA size (both P<0.05) and reduced LARS (both P<0.05; Figure 3A).
Table 2.
Preprocedural Echocardiographic Parameters at Rest and During PLL
| Parameter | PAF (N=95) | PeAF (N=50) | LS‐PeAF (N=19) | P value (rest) | P value (PLL) | |||
|---|---|---|---|---|---|---|---|---|
| Rest | PLL | Rest | PLL | Rest | PLL | |||
| AF rhythm, n (%) | 9 (9.5) | … | 36 (72.0) | … | 19 (100.0) | … | <0.001 | … |
| LV end‐diastolic volume index, mL/m2 | 41.5±11.1 | 43.7±10.6† | 39.5±10.9 | 41.0±11.7 | 37.6±12.1 | 39.0±12.9 | 0.154 | 0.049 |
| LV end‐systolic volume index, mL/m2 | 14.6±5.0 | 15.7±5.3† | 15.0±5.3 | 15.4±4.3 | 15.6±6.3 | 15.7±6.5 | 0.842 | 1.000 |
| LV ejection fraction, % | 65.0±5.9 | 64.5±6.2 | 62.3±6.2* | 62.0±4.9‡ | 59.5±6.7* | 59.8±7.3‡ | <0.001 | 0.002 |
| LV mass index, g/m2 | 82.9±16.9 | … | 90.9±24.0 | … | 85.1±19.8 | … | 0.211 | … |
| E wave, cm/s | 66.8±15.3 | 73.6±16.2† | 79.6±19.2* | 85.8±18.4† , ‡ | 86.3±18.5* | 93.9±16.6‡ , † | <0.001 | <0.001 |
| e′ Wave, cm/s | 8.3±2.4 | 9.4±2.5† | 8.9±2.1 | 9.8±2.4† | 10.7±2.5* , || | 12.1±3.0† , ‡ , § | <0.001 | 0.001 |
| E/e′ ratio | 8.5±2.6 | 8.2±2.2 | 9.3±3.0 | 9.3±3.0 | 8.3±1.9 | 8.0±1.7 | 0.216 | 0.120 |
| LA volume index, mL/m2 | 30.8±9.5 | 31.6±10.1 | 43.5±15.2* | 44.5±15.8‡ | 42.1±11.4* | 44.7±13.6† , ‡ | <0.001 | <0.001 |
| LARS, % | 29.1±8.8 | 31.1±8.5† | 17.6±6.8* | 19.1±7.6† , ‡ | 15.9±5.2* | 15.0±5.5‡ | <0.001 | <0.001 |
Data shown as mean±SD or n (percentage). AF indicates atrial fibrillation; E, early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; LA, left atrial; LARS, left atrial reservoir strain; LS‐PeAF, long‐standing persistent atrial fibrillation; LV, left ventricular; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; and PLL, passive leg lifting.
P<0.05 vs PAF group at rest.
P<0.05 vs parameters at rest.
P<0.05 vs PAF group during PLL.
P<0.05 vs PeAF group during PLL.
P<0.05 vs PeAF group at rest.
Figure 3. Baseline LA reservoir strain (A) and LA functional reserve (B) according to AF severity.
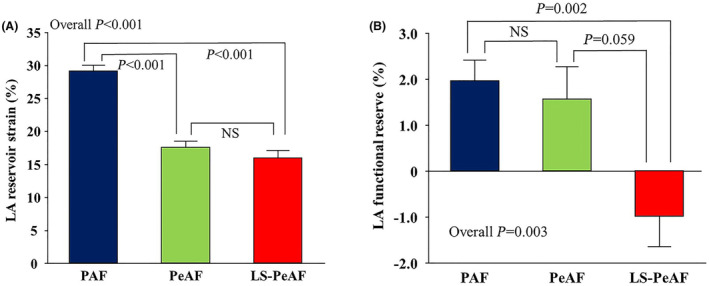
Data are mean and standard error. AF indicates atrial fibrillation; LA, left atrial; LS‐PeAF, long‐standing persistent atrial fibrillation; PAF, paroxysmal atrial fibrillation; and PeAF, persistent atrial fibrillation.
PLL led to increased preload as reflected in LV enlargement and accelerated mitral inflow (Table 2). Similarly, LA volume index tended to increase with PLL. Of note, patients with PAF and PeAF showed a significant increase in LARS during PLL (29.1±8.8% to 31.1±8.5%, P<0.001 for PAF; 17.6±6.8% to 19.1±7.6%, P=0.032 for PeAF; Table 2), but this was not the case in patients with LS‐PeAF. Figure 3B represents the change of LARS after PLL in the 3 AF subgroups. LA functional reserve was significantly impaired in the LS‐PeAF group (P=0.003; Table S1). Among the 164 patients included in the analysis, preserved LA functional reserve (change of LARS through PLL ≥0%) was observed in 104 (63.4%) patients, and the prevalence was greatest in the PAF (69.5%) group, followed by the PeAF (62.0%) and LS‐PeAF (36.8%; overall P=0.026) groups. Comparison of baseline characteristics and echocardiographic parameters stratified by the LA functional reserve are shown in Tables S2 and S3.
Determinant of LA Functional Reserve
Univariable and multivariable logistic regression analyses were conducted to investigate determinants of impaired LA functional reserve (change of LARS through PLL <0%) in patients with AF who underwent their first catheter ablation. In a multivariable model, LS‐PeAF (adjusted odds ratio [OR], 3.89; P=0.012) and advanced age (adjusted OR, 1.04; P=0.034) were significantly associated with increased risk of abnormal LA functional reserve (Table 3). On the other hand, there was no significant relationship between the mean LA pressure, which was measured immediately after transseptal puncture and LA functional reserve (r=−0.05, P=0.582).
Table 3.
Univariable and Multivariable Analysis for the Determinant of Impaired LA Functional Reserve
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
| LS‐PeAF (vs PAF) | 3.90 (1.39–10.92) | 0.010 | 3.89 (1.34–11.28) | 0.012 |
| PeAF (vs PAF) | 1.39 (0.68–2.86) | 0.364 | 1.34 (0.64–2.81) | 0.441 |
| Age, y | 1.04 (1.01–1.07) | 0.020 | 1.04 (1.002–1.07) | 0.034 |
| Men | 1.60 (0.77–3.30) | 0.206 | ||
| Body mass index, kg/m2 | 1.00 (0.91–1.10) | 0.978 | ||
| Systolic blood pressure, mm Hg | 0.99 (0.97–1.01) | 0.246 | ||
| Diastolic blood pressure, mm Hg | 0.99 (0.96–1.02) | 0.450 | ||
| Heart rate, bpm | 1.02 (0.99–1.05) | 0.158 | ||
| Current smoking | 1.04 (0.36–3.03) | 0.936 | ||
| Hypertension | 1.27 (0.67–2.40) | 0.462 | ||
| Diabetes | 1.40 (0.65–3.00) | 0.386 | ||
| Dyslipidemia | 1.04 (0.55–1.98) | 0.898 | ||
| β‐Blocker | 1.08 (0.56–2.08) | 0.819 | ||
| RAAS blocker | 2.14 (1.09–4.22) | 0.028 | 1.93 (0.95–3.91) | 0.068 |
| Calcium channel blocker | 1.14 (0.59–2.22) | 0.696 | ||
| Statin | 0.98 (0.48–1.97) | 0.944 | ||
| Oral antidiabetic drug | 1.15 (0.50–2.65) | 0.745 | ||
| Antiarrhythmic drug | 1.09 (0.55–2.17) | 0.803 | ||
| eGFR, mL/min per 1.73 m2 | 0.99 (0.96–1.01) | 0.197 | ||
| C‐reactive protein, mg/dL | 1.71 (0.86–3.40) | 0.128 | ||
| BNP, pg/mL | 1.54 (0.78–3.05) | 0.214 | ||
| LV end‐diastolic volume index, mL/m2 | 0.98 (0.95–1.01) | 0.234 | ||
| LV end‐systolic volume index, mL/m2 | 0.99 (0.93–1.05) | 0.750 | ||
| LV ejection fraction, % | 0.98 (0.93–1.03) | 0.398 | ||
| LV mass index, g/m2 | 1.00 (0.99–1.02) | 0.738 | ||
| E wave, cm/s | 1.00 (0.98–1.01) | 0.744 | ||
| e′ Wave, cm/s | 1.03 (0.90–1.17) | 0.683 | ||
| E/e′ ratio | 0.98 (0.86–1.10) | 0.697 | ||
| LA volume index, mL/m2 | 1.01 (0.98–1.03) | 0.526 | ||
| LA reservoir strain at rest, % | 1.00 (0.97–1.03) | 0.984 | ||
BNP indicates B‐type natriuretic peptide; E, early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; LA, left atrial; LS‐PeAF, long‐standing persistent atrial fibrillation; LV, left ventricular; OR, odds ratio; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; and RAAS, renin‐angiotensin‐aldosterone system.
LA Functional Reserve After Catheter Ablation
Within the total study population, 149 (90.9%) patients exhibited sinus rhythm at the postprocedural echocardiographic examination (1–2 days after catheter ablation), comprising 90 (94.7%) patients with PAF, 43 (86.0%) with PeAF, and 16 (84.2%) with LS‐PeAF (Figure 1). LARS was significantly improved just after catheter ablation in the PeAF (P=0.006) and LS‐PeAF groups (P=0.002) but was still lower than in the PAF group (both P<0.05; Figure 4). Postprocedural echocardiographic parameters at rest and during PLL are shown in Table 4. Patients with PAF and PeAF showed significant increases in LARS during PLL (29.6±7.3%–30.5±7.4%, P=0.028 for PAF; 19.8±6.4%–21.4±7.0%, P=0.014 for PeAF), and those with LS‐PeAF also exhibited a similar tendency, although not to a level that reached statistical significance (20.7±6.0%–21.8±5.4%, P=0.223). Change of LARS through PLL was comparable among the AF groups (P=0.618; Table S4), and the prevalence of preserved LA functional reserve (LARS change through PLL ≥0%) did not differ across the AF severity (63.3% in the PAF, 67.4% in the PeAF, and 62.5% in the LS‐PeAF groups, respectively [P=0.885]). Figure 5 shows 3 representative cases. Before catheter ablation, impaired LA functional reserve was observed in a patient with LS‐PeAF (8.1%–6.8% through PLL), whereas patients with PAF and PeAF exhibited preserved LA functional reserve (30.9%–41.9% in PAF and 10.0%–12.9% in PeAF through PLL). With recovery to sinus rhythm after catheter ablation, a patient with LS‐PeAF experienced an amelioration of LARS at rest and LA functional reserve (15.0%–16.6% through PLL; Figure 5). In subgroup analysis including patients presenting sinus rhythm at baseline echocardiography (N=100; 86 in PAF group and 14 in PeAF group), preprocedural echocardiography showed significantly reduced LARS in the PeAF group, and significant increase in LARS during PLL in both groups (P<0.05), which are in line with the results derived from the entire population. There were no significant changes in LA functional measures after catheter ablation, which may indicate that the improvement of LA mechanics in the acute phase may be mainly attributed to the restoration of sinus rhythm.
Figure 4. LA reservoir strain stratified by AF stages before and after CA.
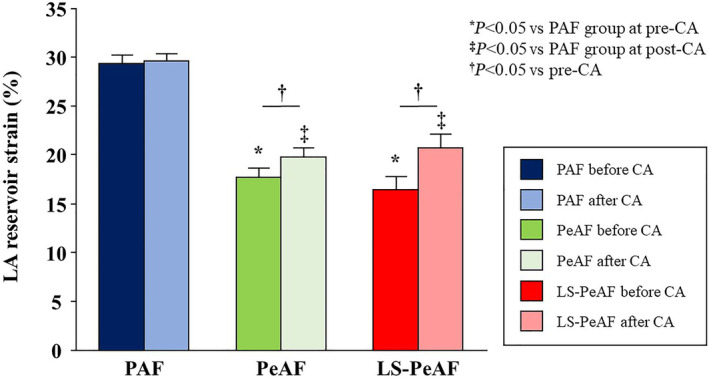
Data are mean and standard error. *P<0.05 vs PAF group at pre‐CA. ‡ P<0.05 vs PAF group at post‐CA. † P<0.05 vs parameters at pre‐CA. AF indicates atrial fibrillation; CA, catheter ablation; LA, left atrial; LS‐PeAF, long‐standing persistent atrial fibrillation; PAF, paroxysmal atrial fibrillation; and PeAF, persistent atrial fibrillation.
Table 4.
Echocardiographic Parameters at Rest and During PLL After Catheter Ablation in Patients Exhibiting Sinus Rhythm
| Parameter | PAF (N=90) | PeAF (N=43) | LS‐PeAF (N=16) | P value (rest) | P value (PLL) | |||
|---|---|---|---|---|---|---|---|---|
| Rest | PLL | Rest | PLL | Rest | PLL | |||
| LV end‐diastolic volume index, mL/m2 | 39.7±12.2 | 41.6±11.0† | 44.0±11.3 | 44.9±11.9 | 42.3±13.6 | 44.9±12.6 | 0.122 | 0.204 |
| LV end‐systolic volume index, mL/m2 | 14.7±5.9 | 15.4±5.1 | 17.3±5.5* | 17.5±5.4 | 17.1±6.8 | 17.9±5.9 | 0.028 | 0.052 |
| LV ejection fraction, % | 63.7±5.9 | 63.4±5.5 | 60.8±6.3* | 61.2±6.0 | 59.9±5.9 | 60.7±4.3 | 0.009 | 0.038 |
| E wave, cm/s | 73.9±17.1 | 79.6±16.9† | 84.0±18.5* | 89.7±17.2‡ , † | 82.0±17.5 | 87.1±17.2† | 0.006 | 0.005 |
| e′ Wave, cm/s | 8.1±2.2 | 8.7±2.2† | 7.9±2.0 | 8.6±1.7† | 9.2±2.1 | 9.8±2.3 | 0.104 | 0.179 |
| E/e′ ratio | 9.5±2.8 | 9.6±2.7 | 11.2±3.1* | 10.9±2.9‡ | 9.1±2.1 | 9.2±2.4 | 0.005 | 0.026 |
| LA volume index, mL/m2 | 32.6±8.9 | 34.2±9.4† | 43.0±13.7* | 44.6±14.9‡ | 46.4±14.8* | 48.8±15.2‡ | <0.001 | <0.001 |
| LARS, % | 29.6±7.3 | 30.5±7.4† | 19.8±6.4* | 21.4±7.0† , ‡ | 20.7±6.0* | 21.8±5.4‡ | <0.001 | <0.001 |
Data shown as mean±SD. E indicates early diastolic transmitral flow velocity; e′, early diastolic mitral annular velocity; LA, left atrial; LARS, left atrial reservoir strain; LS‐PeAF, long‐standing persistent atrial fibrillation; LV, left ventricular; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; and PLL, passive leg lifting.
P<0.05 vs PAF group at rest.
P<0.05 vs parameters at rest.
P<0.05 vs PAF group during PLL.
Figure 5. Representative images of LA functional reserve before and after catheter ablation in 3 AF subtypes of PAF, PeAF, and LS‐PeAF.
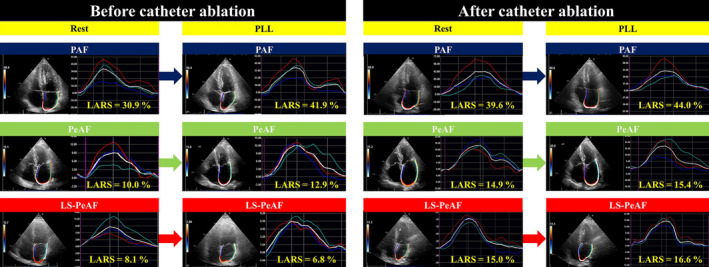
The white curve shows the average strain. AF indicates atrial fibrillation; LA, left atrial; LARS, left atrial reservoir strain; LS‐PeAF, long‐standing persistent atrial fibrillation; PAF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; and PLL, passive leg lifting.
Reproducibility Analysis
An excellent correlation was observed in the analysis of interobserver variability of LARS (intraclass correlation coefficient=0.93).
Discussion
The main findings of the present study were as follows: (1) Patients with PeAF and those with LS‐PeAF exhibited lower LARS compared with patients with PAF. (2) LA functional reserve corresponding to acute volume overload was significantly impaired in the LS‐PeAF group. (3) LS‐PeAF and advanced age were significantly associated with abnormal LA functional reserve. (4) Restoration of sinus rhythm resulted in early improvement of LA function at rest in patients with PeAF and LS‐PeAF, although it did not reach a similar level in patients with PAF. (5) Successful catheter ablation also tended to have a favorable effect on LA functional reserve in the LS‐PeAF group.
Association Between AF Stage and LA Functional Remodeling
The present study showed that LARS declined with the progression of AF from the paroxysmal to persistent phase. In patients with AF, loss of atrial myocytes, with increased collagen and extracellular matrix, and interstitial fibrosis contribute to LA electrical, structural, and functional remodeling, which in turn promotes perpetuation of AF and begets more advanced atrial myopathy. 21 , 22 The extent of LA fibrosis is enhanced with increasing AF burden, 23 and is inversely correlated with LARS. 24 , 25 Recent studies have revealed LA functional decay in PeAF relative to PAF, 7 , 8 which are in line with our results.
Interestingly, the present study found that patients with LS‐PeAF had a significantly reduced LA functional reserve in response to PLL‐induced acute volume stress. PLL is a simple and noninvasive method to produce a rapid increase in preload by raising lower extremities. 20 A change of LARS through PLL reflects LA functional reserve, which has been shown to deteriorate in the setting of HF. 14 However, no study has investigated the LA functional reserve in the setting of AF. The present study demonstrated for the first time that LS‐PeAF was involved in greater impairment of LA functional reserve, irrespective of resting LARS. In addition, we also identified that LS‐PeAF and advanced age were significantly related to impaired LA functional reserve. Age‐related inadequate LA distensibility to volume stress may reflect progressive LA myopathy with advancing age and partially account for an increasing burden of AF in the elderly. 26 , 27 , 28 Furthermore, an interaction between LS‐PeAF and LA functional reserve could partially explain the relationship between the AF progression and HF burden. 3 , 29
Acute Effect of Sinus Recovery With Catheter Ablation on LA Function
We showed that successful AF ablation could lead to LA functional improvement even just after the procedure. With advancement in techniques and technologies, catheter ablation has been shown to be more effective than antiarrhythmic agents for maintaining sinus rhythm, 30 emerging as a first‐line rhythm control therapy. 4 Moreover, recent studies demonstrated its beneficial effect for HF prevention. 5 , 6 Kirchhof et al showed that catheter ablation was associated with a lower risk of 5‐year adverse cardiovascular events including HF hospitalization than usual care in 2789 patients with AF. 5 Yang et al also reported that ablative intervention reduced the 4‐year HF admission risk compared with medical therapy in ≈200 000 patients with newly diagnosed AF. 6 Prior studies reported that maintenance of sinus rhythm with catheter ablation had a favorable effect on LA function. 31 , 32 However, these studies were mainly conducted for patients with PAF and focused on long‐term LA reverse remodeling after catheter ablation, and limited data were available on the efficacy of AF ablation on LA performance in the acute phase. In the present study, although still lower than the PAF group, LA functional recovery was achieved just after restoring sinus rhythm in both the PeAF and LS‐PeAF groups. Furthermore, postprocedural LA functional reserve tended to be ameliorated in the patients with LS‐PeAF. These findings might in part account for the preventive effect of AF ablation on HF occurrence.
Clinical Implications
The present study demonstrated that LARS and LA functional reserve reflecting LA distensibility to acute volume stress decreased with the progression of AF. Our results suggest that long persistence of AF could be associated with reduction in LA compliance with steeper elevation of LA pressure corresponding to acute increase in preload, which might play a key role in the pathogenesis of developing HF. 33 We also identified that resting echocardiographic parameters including LARS were similar in patients with and without abnormal LA functional reserve (Table S3). PLL enables volume challenge to be produced simply and less invasively, which could be a promising tool to evaluate LA diastolic extensibility to enhanced preload in patients with AF, and may help to identify patients with AF susceptible to HF because LA functional reserve plays a key role in the pathogenesis of HF. 14 , 20 Furthermore, we found that resumption of sinus rhythm was associated with early improvement of LA function, which might modify HF risk. 34 Future studies are required to investigate the effect of catheter ablation on long‐term LA functional restoration in patients with AF and its benefit on subsequent HF prevention.
Limitations
Several limitations of the present study should be noted. First, postprocedural echocardiography was performed 1 to 2 days after catheter ablation, and therefore, we could not evaluate the long‐term effect of ablation on LA functional properties and its association with HF occurrence. Second, some differences in baseline characteristics among 3 AF subgroups might be underestimated due to the relatively small number of the study population. Third, the information of LA bipolar voltage is not uniformly available in the present study, although recent studies demonstrated that patients with larger low‐voltage zones had reduced LA strain. 35 , 36 Fourth, because we included patients with AF who underwent first catheter ablation, only 3 patients received extensive LA line ablation such as roof line and mitral isthmus, and no patients received posterior wall isolation. Therefore, we cannot evaluate the impact of extensive ablation on LA function and functional reserve. Furthermore, there might be a certain association between number of radiofrequency (RF) lesions or RF‐on time and the change of LA function/functional reserve, which cannot be addressed in the present study. Finally, we do not have the data on the impact of cardioversion on LA function and functional reserve, which may provide valuable information to elucidate whether restoration of sinus rhythm is sufficient or whether catheter ablation provides some other benefits to improve LA mechanics beyond restoring sinus rhythm.
Conclusions
The progression of AF was related to impaired LA function including LARS at rest and LA functional reserve. Restoration of sinus rhythm could contribute to beneficial LA reverse remodeling even in a long‐standing persistent phase.
Sources of Funding
This work was supported in part by Grants‐in‐Aid for Scientific Research 19K20707 and 22K12859.
Disclosures
None.
Supporting information
Tables S1–S4
Acknowledgments
The authors thank Drs Hirokawa, Ishiwata, and Sawada for their general support. K.H. and K.N. contributed to the conception and design of the work. K.H., K.N., M.D., K.I., Y.Yamamoto, Y.M., Y.Yoshida, T.N., T.O., T.M., Y.S., G.O., T.K., E.H., and K.F. contributed to the acquisition, analysis, and interpretation of the data. K.H. drafted the article. K.N., M.D., K.I., Y.Yamamoto, Y.M., Y.Yoshida, T.N., T.O., T.M., Y.S., G.O., T.K., E.H., K.F., H.M., and I.K. critically revised the article. All authors provided final approval and agreed to be accountable for all aspects of the work, ensuring its integrity and accuracy.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032215
This article was sent to Luciano A. Sposato, MD, MBA, FRCPC, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 11.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990‐2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, Van Gilst WH, Van Gelder IC, Rienstra M. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community‐based study from The Netherlands. J Am Coll Cardiol. 2015;66:1000–1007. doi: 10.1016/j.jacc.2015.06.1314 [DOI] [PubMed] [Google Scholar]
- 3. Pandey A, Kim S, Moore C, Thomas L, Gersh B, Allen LA, Kowey PR, Mahaffey KW, Hylek E, Peterson ED, et al. Predictors and prognostic implications of incident heart failure in patients with prevalent atrial fibrillation. JACC Heart Fail. 2017;5:44–52. doi: 10.1016/j.jchf.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2018;20:157–208. doi: 10.1093/europace/eux275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 6. Yang PS, Sung JH, Jang E, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B. Catheter ablation improves mortality and other outcomes in real‐world patients with atrial fibrillation. J Am Heart Assoc. 2020;9:e015740. doi: 10.1161/JAHA.119.015740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung M, Abou R, van Rosendael PJ, van der Bijl P, van Wijngaarden SE, Regeer MV, Podlesnikar T, Ajmone Marsan N, Leung DY, Delgado V, et al. Relation of echocardiographic markers of left atrial fibrosis to atrial fibrillation burden. Am J Cardiol. 2018;122:584–591. doi: 10.1016/j.amjcard.2018.04.047 [DOI] [PubMed] [Google Scholar]
- 8. Olsen FJ, Darkner S, Chen X, Pehrson S, Johannessen A, Hansen J, Gislason G, Svendsen JH, Biering‐Sorensen T. Left atrial structure and function among different subtypes of atrial fibrillation: an echocardiographic substudy of the AMIO‐CAT trial. Eur Heart J Cardiovasc Imaging. 2020;21:1386–1394. doi: 10.1093/ehjci/jeaa222 [DOI] [PubMed] [Google Scholar]
- 9. Potter EL, Ramkumar S, Kawakami H, Yang H, Wright L, Negishi T, Marwick TH. Association of asymptomatic diastolic dysfunction assessed by left atrial strain with incident heart failure. JACC Cardiovasc Imaging. 2020;13:2316–2326. doi: 10.1016/j.jcmg.2020.04.028 [DOI] [PubMed] [Google Scholar]
- 10. Leung M, van Rosendael PJ, Abou R, Ajmone Marsan N, Leung DY, Delgado V, Bax JJ. Left atrial function to identify patients with atrial fibrillation at high risk of stroke: new insights from a large registry. Eur Heart J. 2018;39:1416–1425. doi: 10.1093/eurheartj/ehx736 [DOI] [PubMed] [Google Scholar]
- 11. Khan HR, Yakupoglu HY, Kralj‐Hans I, Haldar S, Bahrami T, Clague J, De Souza A, Hussain W, Jarman J, Jones DG, et al. Left atrial function predicts atrial arrhythmia recurrence following ablation of long‐standing persistent atrial fibrillation. Circ Cardiovasc Imaging. 2023;16:e015352. doi: 10.1161/CIRCIMAGING.123.015352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van de Bovenkamp AA, Wijkstra N, Oosterveer FPT, Vonk Noordegraaf A, Bogaard HJ, van Rossum AC, de Man FS, Borlaug BA, Handoko ML. The value of passive leg raise during right heart catheterization in diagnosing heart failure with preserved ejection fraction. Circ Heart Fail. 2022;15:e008935. doi: 10.1161/CIRCHEARTFAILURE.121.008935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, Tange S, Arai M, Kurabayashi M. Incremental diagnostic value of LA strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6:749–758. doi: 10.1016/j.jcmg.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 16. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 17. Thomas L, Muraru D, Popescu BA, Sitges M, Rosca M, Pedrizzetti G, Henein MY, Donal E, Badano LP. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. 2020;33:934–952. doi: 10.1016/j.echo.2020.03.021 [DOI] [PubMed] [Google Scholar]
- 18. Gan GCH, Bhat A, Chen HHL, Gu KH, Fernandez F, Kadappu KK, Byth K, Eshoo S, Thomas L. Left atrial reservoir strain by speckle tracking echocardiography: association with exercise capacity in chronic kidney disease. J Am Heart Assoc. 2021;10:e017840. doi: 10.1161/JAHA.120.017840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smiseth OA, Baron T, Marino PN, Marwick TH, Flachskampf FA. Imaging of the left atrium: pathophysiology insights and clinical utility. Eur Heart J Cardiovasc Imaging. 2021;23:2–13. doi: 10.1093/ehjci/jeab191 [DOI] [PubMed] [Google Scholar]
- 20. Abe Y, Akamatsu K, Furukawa A, Ito K, Matsumura Y, Haze K, Naruko T, Yoshiyama M, Yoshikawa J. Pre‐load‐induced changes in forward LV stroke and functional mitral regurgitation: echocardiographic detection of the descending limb of Starling's curve. JACC Cardiovasc Imaging. 2017;10:611–618. doi: 10.1016/j.jcmg.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 21. Xu J, Cui G, Esmailian F, Plunkett M, Marelli D, Ardehali A, Odim J, Laks H, Sen L. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52 [DOI] [PubMed] [Google Scholar]
- 22. Chung MK, Refaat M, Shen WK, Kutyifa V, Cha YM, Di Biase L, Baranchuk A, Lampert R, Natale A, Fisher J, et al. Atrial fibrillation: JACC council perspectives. J Am Coll Cardiol. 2020;75:1689–1713. doi: 10.1016/j.jacc.2020.02.025 [DOI] [PubMed] [Google Scholar]
- 23. Platonov PG, Mitrofanova LB, Orshanskaya V, Ho SY. Structural abnormalities in atrial walls are associated with presence and persistency of atrial fibrillation but not with age. J Am Coll Cardiol. 2011;58:2225–2232. doi: 10.1016/j.jacc.2011.05.061 [DOI] [PubMed] [Google Scholar]
- 24. Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, Rao SN, Blauer J, Fish EN, Dibella EV, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed‐enhancement MRI. Circ Cardiovasc Imaging. 2010;3:231–239. doi: 10.1161/CIRCIMAGING.109.865683 [DOI] [PubMed] [Google Scholar]
- 25. Habibi M, Lima JA, Khurram IM, Zimmerman SL, Zipunnikov V, Fukumoto K, Spragg D, Ashikaga H, Rickard J, Marine JE, et al. Association of left atrial function and left atrial enhancement in patients with atrial fibrillation: cardiac magnetic resonance study. Circ Cardiovasc Imaging. 2015;8:e002769. doi: 10.1161/CIRCIMAGING.114.002769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton‐Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yoshida Y, Nakanishi K, Daimon M, Ishiwata J, Sawada N, Hirokawa M, Kaneko H, Nakao T, Mizuno Y, Morita H, et al. Alteration of cardiac performance and serum B‐type natriuretic peptide level in healthy aging. J Am Coll Cardiol. 2019;74:1789–1800. doi: 10.1016/j.jacc.2019.07.080 [DOI] [PubMed] [Google Scholar]
- 28. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore‐Mensah Y, et al. Heart disease and stroke statistics‐2022 update: a report from the American Heart Association. Circulation. 2022;145:e153–e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 29. Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Lip GY. Predictors and prognostic implications of incident heart failure following the first diagnosis of atrial fibrillation in patients with structurally normal hearts: the Belgrade Atrial Fibrillation Study. Eur J Heart Fail. 2013;15:415–424. doi: 10.1093/eurjhf/hft004 [DOI] [PubMed] [Google Scholar]
- 30. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, Niebauer M, Makati K, Halperin B, Gauri A, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554 [DOI] [PubMed] [Google Scholar]
- 31. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, Zeppenfeld K, Holman E, Schalij MJ, Bax JJ. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2011;57:324–331. doi: 10.1016/j.jacc.2010.05.063 [DOI] [PubMed] [Google Scholar]
- 32. Walters TE, Nisbet A, Morris GM, Tan G, Mearns M, Teo E, Lewis N, Ng A, Gould P, Lee G, et al. Progression of atrial remodeling in patients with high‐burden atrial fibrillation: implications for early ablative intervention. Heart Rhythm. 2016;13:331–339. doi: 10.1016/j.hrthm.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 33. Khan MS, Memon MM, Murad MH, Vaduganathan M, Greene SJ, Hall M, Triposkiadis F, Lam CSP, Shah AM, Butler J, et al. Left atrial function in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail. 2020;22:472–485. doi: 10.1002/ejhf.1643 [DOI] [PubMed] [Google Scholar]
- 34. Inciardi RM, Bonelli A, Biering‐Sorensen T, Cameli M, Pagnesi M, Lombardi CM, Solomon SD, Metra M. Left atrial disease and left atrial reverse remodelling across different stages of heart failure development and progression: a new target for prevention and treatment. Eur J Heart Fail. 2022;24:959–975. doi: 10.1002/ejhf.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hohendanner F, Romero I, Blaschke F, Heinzel FR, Pieske B, Boldt LH, Parwani AS. Extent and magnitude of low‐voltage areas assessed by ultra‐high‐density electroanatomical mapping correlate with left atrial function. Int J Cardiol. 2018;272:108–112. doi: 10.1016/j.ijcard.2018.07.048 [DOI] [PubMed] [Google Scholar]
- 36. Laish‐Farkash A, Perelshtein Brezinov O, Valdman A, Tam D, Rahkovich M, Kogan Y, Marincheva G. Evaluation of left atrial remodeling by 2D‐speckle‐tracking echocardiography versus by high‐density voltage mapping in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2021;32:305–315. doi: 10.1111/jce.14837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


