Abstract
Background
Low‐grade systemic inflammation is a relevant pathogenic mechanism underlying the development of hypertension. In this study, we hypothesized that plasma calprotectin levels, as a biomarker of neutrophil‐mediated inflammation, is associated with developing new‐onset hypertension in the general population.
Methods and Results
Plasma calprotectin levels were determined in 3524 participants who participated in the PREVEND (Prevention of Renal and Vascular End‐Stage Disease) study, a prospective population‐based cohort study. Plasma calprotectin levels were studied for associations with the risk of new‐onset hypertension, defined as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or the first recorded use of antihypertensives. Participants with hypertension at baseline were excluded. Median plasma calprotectin levels were 0.48 (0.34–0.66) mg/L, and median systolic blood pressure was 117 (109–126) mm Hg. Plasma calprotectin levels were significantly associated with the risk of new‐onset hypertension (hazard ratio [HR], per doubling 1.30 [95% CI, 1.21–1.41]; P<0.001), also after adjustment for age and sex (HR, 1.26 [95% CI, 1.16–1.37]; P<0.001), but not after additional adjustment for potentially confounding factors, including baseline systolic blood pressure (HR, 1.00 [95% CI, 0.90–1.11]; P=0.996). Stratified analyses showed significant effect modification by sex (P interaction=0.023) and urinary albumin excretion (P interaction=0.004), with higher HRs in men (compared with women) and in individuals with higher urinary albumin excretion (>9.3 mg per 24 hours) compared with lower urinary albumin excretion (≤9.3 mg per 24 hours).
Conclusions
Higher plasma calprotectin levels are associated with an increased risk of new‐onset hypertension in the general population. This association is dependent on baseline systolic blood pressure and is particularly prominent in men compared with women.
Keywords: calprotectin, cardiovascular risk, hypertension, inflammation, population
Subject Categories: Hypertension, High Blood Pressure, Cardiovascular Disease, Epidemiology, Biomarkers
Nonstandard Abbreviations and Acronyms
- PREVEND
Prevention of Renal and Vascular End‐Stage Disease
- SBP
systolic blood pressure
- UAE
urinary albumin excretion
Clinical Perspective.
What Is New?
This study shows that high circulating levels of calprotectin, a neutrophil‐based biomarker reflecting low‐grade systemic inflammation, are associated with an increased risk of developing new‐onset hypertension in individuals derived from the general population.
What Are the Clinical Implications?
These findings support the potential usefulness of plasma calprotectin as a biomarker for the future development of hypertension, albeit validation and longitudinal assessments across various stages of hypertension are needed to further establish its definitive role in clinical practice.
Hypertension is one of the most common cardiovascular conditions and constitutes an important risk factor for cardiovascular diseases (CVDs) such as coronary heart disease and heart failure, as well as chronic kidney disease. 1 Hypertension is frequently considered in cardiovascular risk assessment, because it may be regarded as a strongly modifiable risk factor for CVDs. Hypertension often coincides with other established CVD risk factors, including smoking, alcohol consumption, obesity, and physical inactivity. 2 Inflammation is one of the key pathogenic drivers of hypertension, and like the development of atherosclerosis is marked by low‐grade systemic inflammation. 3 , 4 , 5 For example, hypertension has been associated with a variety of circulating inflammatory biomarkers like C‐reactive protein (CRP) and interleukins. 6 , 7 For instance, endothelial inflammation may give rise to endothelial dysfunction and vascular fibrosis, and calcification, which all contribute to the development of hypertension. 3 , 5
Calprotectin is a protein heterodimer that consists of S100A8/S100A9 complexes that are predominantly found in the cytosol of myeloid cells such as neutrophils and macrophages. 8 Under circumstances of systemic inflammation, calprotectin is actively released upon degranulation. After its release, calprotectin may act as an antibacterial component (eg, being part of neutrophil extracellular traps) or as an endogenous danger signaling molecule (alarmin). 9 , 10 Generally, calprotectin is considered an acute‐phase protein, and accumulating evidence suggests that it may be implicated in the pathogenesis of CVDs. For example, plasma calprotectin levels have previously been associated with the risk of new‐onset CVD in a population‐based cohort. 11 Other studies have also shown elevated calprotectin levels in atherosclerotic manifestations such as coronary artery disease. 12 , 13 Notably, fecal calprotectin levels are commonly used in the clinic as a biomarker of intestinal inflammation in patients with inflammatory bowel disease, and in this context they also associate with systemic inflammation. 14 Given the existing knowledge on the role of systemic inflammation in the development of hypertension, and in particular the role of vascular infiltration of myeloid cells, high circulating levels of calprotectin may potentially antedate the onset of hypertension.
Until now, the relationship between circulating calprotectin levels and the risk of new‐onset hypertension has not yet been systematically investigated in a population‐based cohort. Therefore, we hypothesized that plasma calprotectin levels, as a biomarker of systemic inflammation, may confer predictive potential with regard to the future development of hypertension. As such, we aimed to investigate the potential association between plasma calprotectin levels and the risk of new‐onset hypertension in the general population.
Methods
The data sets generated for this study are available upon reasonable request to the corresponding author.
Study Design and Study Population
This study featured data from the PREVEND (Prevention of Renal and Vascular End‐Stage Disease) study, which represents a prospective population‐based cohort study and is based in the city of Groningen, the Netherlands. 15 The PREVEND study was initiated in 1997 and was designed to investigate the potential role of urinary albumin excretion in the development of cardiovascular and renal diseases. Multiple health‐associated parameters, particularly those relevant to renal and cardiovascular diseases, were collected within this study. During the period of 1997 to 1998, individuals living in the city of Groningen aged 28 to 75 years were invited to participate in the study. Those who were willing to participate were requested to provide a first morning urine sample and complete a questionnaire containing questions about demographic parameters and their history of cardiovascular diseases. A total of 40 856 individuals responded to this request (Figure 1). Of these individuals, a total of 7786 individuals who had urinary albumin concentrations ≥10 mg/L and a randomly selected group of controls (n=3395) with urinary albumin concentrations <10 mg/L were invited to participate in a second screening visit at the outpatient clinic of the University Medical Center Groningen. Pregnant women and individuals with type 1 diabetes or insulin‐treated type 2 diabetes were excluded from participation. This second screening program was completed by a total of 8592 individuals, of whom 6000 had urinary albumin concentrations ≥10 mg/L, and 2592 had urinary albumin concentrations <10 mg/L. These 8592 individuals were subsequently followed‐up in future study investigations. During the period of 2001 to 2003, a second round of study investigations was performed, when additional blood samples from 6894 participants were collected. This second round was taken as the baseline for the current study. Of the total of 6894 participants, individuals with hypertension at baseline (n=2380), individuals without follow‐up data on the development of hypertension (n=578), and individuals with missing data on plasma calprotectin (n=412) were excluded. After checking the degree of overlap, this resulted in a total of 3524 participants who were eligible for the current study. After this second round of study investigations, participants visited the outpatient clinic of the University Medical Center Groningen for a medical examination at ~3‐year intervals. More specifically, participants attended 2 outpatient visits separated by 3 weeks during a third (2003–2006), fourth (2006–2008), and fifth (2009–2011) round of study investigations. The study follow‐up period eventually ended on January 1, 2011. The PREVEND study was approved by the institutional review board of the University Medical Center Groningen (full name in Dutch: Medisch Ethische Toetsingscommissie, institutional review board number 01/139). All participants provided written informed consent for study participation. The study was conducted according to the Declaration of Helsinki (2013). The description of this study conforms to the Enhancing the QUAlity and Transparency Of health Research guideline on the Strengthening of the Reporting of Observational Studies in Epidemiology. 16
Figure 1. Flowchart of the PREVEND study.
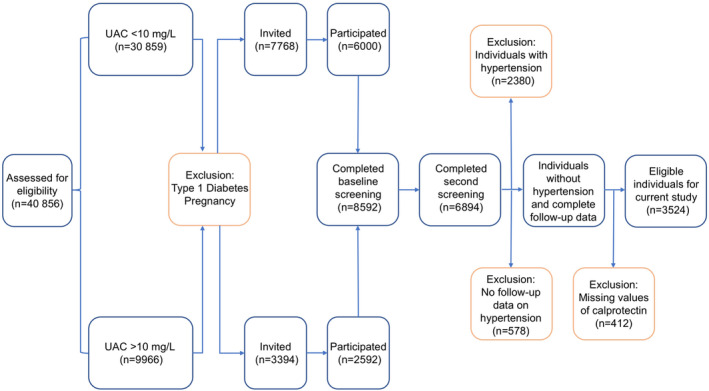
PREVEND study procedure showing the inclusion and exclusion of participants for the purposes of this study. PREVEND indicates Prevention of Renal and Vascular End‐Stage Disease; and UAC, urinary albumin concentration.
Data Collection
All study participants of the second round (baseline) completed a questionnaire detailing information about demographic variables (eg, age, sex, and race), medical history (eg, history of CVD and diabetes, medication use), and lifestyle (eg, smoking). Anthropometric measurements were performed as well, which are composed of measurements of length, weight, the subsequent body mass index (BMI), and waist circumference. The waist circumference was measured on the skin at the level of the natural indentation between the 10th rib and the iliac crest. Blood pressure was measured on the right arm in a supine position, measured every minute for 8 minutes using an automatic device (Dinamap XL Model 9300 series, Johnson & Johnson Medical, Tampa, FL), as described previously. 17 , 18 , 19 The final 2 blood pressure measurements were averaged, and this value was used for analysis, because this provided the most trustworthy values after blood pressure stabilization. Smoking behavior was noted as never, former, or current. Participants were instructed to remain fasted from 10:00 pm the day before visiting the outpatient research clinic.
Laboratory Measurements
Blood was obtained by venipuncture from an antecubital vein after 15 to 30 minutes of rest. All blood samples were taken during a morning outpatient visit, usually between 8:00 and 10:00 am. Ethylenediaminetetraacetic acid anticoagulated blood samples were collected on melting ice. Plasma was prepared by centrifugation at 1000g for 10 minutes at 4 °C. Shortly thereafter, plasma aliquots were stored at −80 °C. Urine samples were also collected and stored at −20 °C until analysis. Serum creatinine was measured through an enzymatic method (Roche Modular; Roche, Mannheim, Germany). Cystatin C was measured using the Gentian Cystatin C Immunoassay (Gentian AS, Moss, Norway) applied on a modular analyzer (Roche Diagnostics). Calibration standards for cystatin C were applied following the supplier's instructions and according to the International Federation of Clinical Chemistry Working Group for Standardization of Serum Cystatin C. 20 High‐sensitivity CRP (hs‐CRP) was measured by nephelometry (Dade Behring Diagnostics, Marburg, Germany). Total cholesterol levels were measured by dry chemistry (Eastman Kodak, Rochester, NY). Triglycerides were measured enzymatically, and low‐density lipoprotein cholesterol was determined by the Friedewald formula (if triglycerides ≤4.5 mmoL/L). Twenty‐four‐hour urine samples were produced twice by all participants, collected over 2 consecutive days after provision after both oral and written instructions. Urinary albumin excretion (UAE) and Na excretion were determined in these samples by nephelometry (Dade Behring Diagnostics). UAE and urinary Na excretion in both collections were averaged for statistical analysis. All laboratory measurements, including the measurements of circulating calprotectin levels, were performed in blood and urine samples that were collected during the second round of study investigations, as noted earlier.
Measurement of Plasma Calprotectin
Plasma calprotectin concentrations were measured on the Gentian Calprotectin turbidimetric immunoassay (Gentian, Moss, Norway), which was performed on a Mindray BS‐400 Analyzer (Mindray, Shenzhen, China). Total imprecision for all samples and controls with calprotectin concentrations >1 mg/L is <3%. The Gentian Calprotectin Immunoassay for serum and lithium‐heparin plasma samples is robust in frozen samples over at least 1 freeze and thaw cycle. A previous study that investigated the effect of freezing on stability of calprotectin could not report reduced stability over 9 freezing cycles in serum samples with concentrations around 2 to 3 mg/L. 21 Another study using purified antigen solution instead of native samples found that levels decreased by 14% after 1 freeze cycle but remained stable for the next 3 cycles, hence staying within ±20% recovery criteria. 22 In conclusion, the results from this study and the others argue that serum/plasma samples can be frozen for long‐term storage and that calprotectin can be measured in frozen samples after at least 1 cycle of freezing and thawing.
Study Outcomes and Definitions
The primary study outcome consisted of new‐onset hypertension, which was defined as the occurrence of either a systolic blood pressure (SBP) of at least 140 mm Hg, a diastolic blood pressure of at least 90 mm Hg, or the first recorded use of antihypertensive medications at subsequent study investigations performed after the baseline period. The use of antihypertensives was ascertained through a questionnaire that participants completed at subsequent study investigations, which was complemented with information obtained from a pharmacy‐dispensing registry. Use of antihypertensives included the following 5 second‐level Anatomical Therapeutic Chemical codes: C02 (antihypertensives [ie, α‐adrenergic blockers), C03 (diuretics), C07 (β‐blockers), C08 (calcium channel blockers), and C09 (renin‐angiotensin system inhibitors). 23 The first recorded use of antihypertensive medications according to the central pharmacy registry follow‐up data was complete as of January 1, 2011 (end of follow‐up), and yielded complete information on drug use of >90% of subjects in the PREVEND study. Estimated glomerular filtration rate was calculated using the combined creatinine cystatin C‐based Chronic Kidney Disease Epidemiology Collaboration equation. 24 Type 2 diabetes was defined as having the presence of a fasting glucose level ≥7.0 mmoL/L or the use of glucose‐lowering drugs according to the American Diabetes Association guidelines.
Statistical Analysis
Baseline study population characteristics were presented as mean (SD), or median (interquartile range [IQR]) for continuous variables, or as proportions with corresponding percentages in case of nominal variables. Normal distributions were assessed by visually inspecting histograms and normal probability (Q‐Q) plots. Baseline characteristics were presented for the total study cohort and shown separately by tertiles of plasma calprotectin levels. Differences in baseline characteristics across tertiles of plasma calprotectin levels were tested using 1‐way ANOVA or Kruskal‐Wallis tests in case of continuous variables, and χ2 tests or Fisher exact tests in case of nominal variables. Cox proportional hazards regression analyses were performed to evaluate associations between plasma calprotectin levels and the risk of new‐onset hypertension, for which results were expressed as hazard ratios (HRs) with 95% CI. For these analyses, plasma calprotectin levels were 2log‐transformed to facilitate interpretation of results (HRs expressed as per doubling in calprotectin levels). For each predictor, proportionality of hazards was checked to avoid violation of model assumptions. Furthermore, the potential existence of multicollinearity in the various models was checked to avoid variance overinflation. Crude Cox proportional hazards regression analyses were followed by multivariable Cox proportional hazards regression models allowing the adjustment for potentially confounding factors. Kaplan‐Meier survival curve analysis was performed to assess survival distributions across tertiles of plasma calprotectin levels, which were compared using the log‐rank test. Survival time was defined from baseline until the date of the last visit that participants attended, at the incidence of hypertension, death, or on January 1, 2011 (end of follow‐up). Additionally, restricted cubic splines were fitted using 3 knots to evaluate the potential existence of nonlinearity of the association observed in Cox regression analysis. Nonlinearity was tested by likelihood ratio tests, where nested models were compared using linear or linear and cubic spline terms. Stratified Cox proportional hazards regression analyses were performed to evaluate the association of plasma calprotectin levels with the risk of new‐onset hypertension across various relevant subgroups. Potential interactions for relevant variables were evaluated by calculating interaction terms, for which P interaction<0.05 was considered to indicate significant effect modification. Data analysis was performed using SPSS Statistics 28.0 (IBM, Armonk, NY) and R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria). Data visualization was performed using RStudio (version 1.2.1335; Boston, MA). Two‐sided P values ≤0.05 were considered statistically significant.
Selection and Rationale for Confounding Variables
Directed acyclic graphs were leveraged to establish a set of confounding variables to be incorporated in the analyses in which the outcome of interest, new‐onset hypertension, is estimated. 25 , 26 , 27 , 28 , 29 , 30 , 31 The directed acyclic graph represents a theoretical framework in which the hypothesized causal relationships believed to underlie the association under study are visualized (Figure S1). Here, depicted arrows represent the hypothesized causal effects between variables (direct effects), whereas the absence of arrows points to the assumption of no such effects. In this study, we focused on the relationship between plasma calprotectin levels, as surrogate of systemic inflammation and oxidative stress, and the risk of new‐onset hypertension. For this association, we rationalized a distinct set of potentially confounding factors for which conditioning was performed to establish an unconfounded effect estimate in statistical analysis. 32 , 33 , 34 , 35 , 36 , 37 Based on our assumptions and the directed acyclic graph framework, the following parameters were selected as potentially confounding factors in the analysis: age, sex, BMI, current smoking, diabetes history, total cholesterol (as surrogate for dyslipidemia), hs‐CRP, 24‐hour Na excretion, and baseline SBP.
Results
Baseline Study Population Characteristics
A total of 3524 individuals were included in the present study, of which baseline demographic, clinical, and laboratory data are presented in Table 1, both for the total cohort and grouped by tertiles of plasma calprotectin levels. Median plasma calprotectin levels were 0.48 (IQR, 0.34–0.66) mg/L. Median SBP was 117 (IQR, 109–126) mm Hg, and median diastolic blood pressure was 70 (IQR, 65–75] mm Hg. Mean age of the study population was 49.1 (SD, 10.2) years, and 1862 (52.8%) individuals were women. Study participants within the highest tertile of plasma calprotectin levels were generally older (P<0.001), more often men (P<0.001), had a higher BMI, waist circumference, SBP, and pulse rate (all P<0.001). Notably, participants within the highest tertile of plasma calprotectin levels smoked considerably more often than participants within the lowest tertile (41.7% versus 19.6%, P<0.001). Moreover, participants in the highest calprotectin tertile demonstrated higher circulating levels of total cholesterol, hs‐CRP, glucose, creatinine (all P<0.001), and had a higher UAE (P<0.001) and urinary Na excretion (P=0.005).
Table 1.
Baseline Demographic, Clinical, and Laboratory Characteristics of the Study Population
| Characteristic | Total | Tertile 1 | Tertile 2 | Tertile 3 | P value |
|---|---|---|---|---|---|
| n=3524 | n=1171 | n=1184 | n=1169 | ||
| Plasma calprotectin levels, mg/L | 0.48 [0.34–0.66] | 0.29 [0.24–0.34] | 0.48 [0.43–0.53] | 0.77 [0.66–0.97] | <0.001 |
| Demographics | |||||
| Age, y | 49.1 (10.2) | 48.2 (9.9) | 49.4 (10.3) | 49.9 (10.3) | <0.001 |
| Female sex, n (%) | 1862 (52.8) | 693 (59.2) | 608 (51.4) | 561 (48.0) | <0.001 |
| Race, n (%) | 3501 (99.3) | 1159 (99.0) | 1177 (99.4) | 1165 (99.7) | 0.192 |
| White, n (%) | 3362 (96.0) | 1100 (94.9) | 1132 (96.2) | 1130 (97.0) | |
| Black, n (%) | 25 (0.7) | 8 (0.7) | 9 (0.8) | 8 (0.7) | |
| Asian, n (%) | 74 (2.1) | 34 (2.9) | 24 (2.0) | 16 (1.4) | |
| Other, n (%) | 40 (1.1) | 17 (1.5) | 12 (1.0) | 11 (0.9) | |
| Anthropometrics | |||||
| BMI, kg/m2 | 25.1 [23.1–27.8] | 24.4 [22.4–26.9] | 25.2 [23.2–27.9] | 26.0 [23.7–28.6] | <0.001 |
| Waist circumference, cm | 88 [80–96] | 85 [77–93] | 89 [81–96] | 91 [84–99] | <0.001 |
| Blood pressure | |||||
| SBP, mm Hg | 117 [109–126] | 114 [107–123] | 118 [110–127] | 119 [111–128] | <0.001 |
| DBP, mm Hg | 70 [65–75] | 69 [64–74] | 70 [66–76] | 71 [66–77] | <0.001 |
| Heart rate, bpm | 67 [61–74] | 66 [60–72] | 67 [61–74] | 69 [63–76] | <0.001 |
| Smoking, n (%) | 3510 (99.6) | 1166 (99.6) | 1181 (99.7) | 1163 (99.5) | <0.001 |
| Never, n (%) | 1127 (32.1) | 441 (37.8) | 390 (33.0) | 296 (25.5) | |
| Former, n (%) | 1345 (32.8) | 496 (42.5) | 467 (39.5) | 382 (32.8) | |
| Current, n (%) | 1038 (29.6) | 229 (19.6) | 324 (27.4) | 485 (41.7) | |
| Comorbidities | |||||
| History of CVD, n (%) | 49 (1.4) | 11 (0.9) | 19 (1.6) | 19 (1.6) | 0.272 |
| History of diabetes, n (%) | 26 (0.7) | 3 (0.3) | 11 (0.9) | 12 (1.0) | 0.060 |
| Medication usage | |||||
| Lipid‐lowering drugs, n (%) | 99 (2.8) | 23 (2.0) | 35 (3.1) | 41 93.6) | 0.141 |
| Glucose‐lowering drugs, n (%) | 13 (0.4) | 2 (0.5) | 2 (0.5) | 6 (0.6) | 0.351 |
| Laboratory parameters | |||||
| Total cholesterol, mmol/L | 5.3 [4.7–6.1] | 5.2 [4.6–5.9] | 5.3 [4.6–6.0] | 5.4 [4.8–6.2] | <0.001 |
| LDL cholesterol, mmol/L | 3.4 [2.7–4.2] | 3.4 [2.7–4.0] | 3.1 [2.7–3.9] | 3.6 [2.7–4.3] | 0.184 |
| Triglycerides, mg/dL | 90.2 [66.3–129] | 79.9 [58.5–114] | 89.9 [67.1–126] | 102 [74.0–147] | <0.001 |
| hs‐CRP, mg/L | 1.0 [0.5–2.4] | 0.6 [0.3–1.3] | 1.0 [0.5–2.1] | 1.9 [0.9–4.1] | <0.001 |
| Glucose, mmol/L | 4.7 [4.4–5.1] | 4.6 [4.3–5.0] | 4.7 [4.4–5.1] | 4.7 [4.4–5.2] | <0.001 |
| Creatinine, μmol/L | 81.1 [72.9–90.9] | 80.1 [71.9–89.3] | 82.1 [72.9–91.4] | 82.1 [73.9–91.4] | <0.001 |
| Cystatin C, mg/dL | 0.8 [0.8–0.9] | 0.8 [0.8–0.9] | 0.8 [0.8–0.9] | 0.9 [0.8–1.0] | <0.001 |
| eGFR, mL/min per 1.73 m2 | 98.1 [87.9–107] | 100 [89.8–109] | 98.0 [87.2–107] | 96.6 [86.6–106] | <0.001 |
| UAE/24 h, mg/L | 7.6 [5.8–11.7] | 7.2 [5.7–10.7] | 7.6 [5.7–11.8] | 8.2 [6.0–12.8] | <0.001 |
| Urinary Na excretion/24 h, mmol/L | 88.8 [64.5–120] | 85.0 [61.5–116] | 90.0 [65.0–121] | 90.5 [66.5–122] | 0.005 |
| Outcome | |||||
| New‐onset hypertension, n (%) | 1000 (28.4) | 259 (22.1) | 329 (27.8) | 412 (35.2) | <0.001 |
Data are presented as mean (SD), median [IQR], or numbers with corresponding percentages (%). P values are derived from comparisons of baseline differences across tertiles of plasma calprotectin levels. Here, continuously distributed variables were compared using 1‐way ANOVA or Kruskal‐Wallis tests (in case of skewed variables), whereas categorical variables were compared using χ2 tests or Fisher exact tests (in case assumptions of the χ2 test could not be fulfilled). P values ≤0.05 were considered statistically significant. BMI indicates body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; IQR, interquartile range; LDL, low‐density lipoprotein; SBP, systolic blood pressure; and UAE, urinary albumin excretion.
Plasma Calprotectin Levels and the Risk of New‐Onset Hypertension
The overall median follow‐up of study participants was 7.1 (IQR, 3.6–7.6; full range: 0.9–10.5) years. During this period, a total of 1000 individuals (28.4%) developed new‐onset hypertension. The highest rate of new‐onset hypertension was observed in the highest tertile of plasma calprotectin levels (n=412, 35.2%) when compared with the second tertile (n=329, 27.8%) and lowest tertile (n=259, 22.1%) (P<0.001). Kaplan‐Meier survival curve analysis demonstrated significantly differential survival distributions across tertiles of plasma calprotectin levels (log‐rank test, P<0.001), showing the lowest hypertension‐free survival among participants within the highest tertile (T3) of plasma calprotectin levels (Figure 2A). Cox proportional hazards regression analyses demonstrated a significant association between plasma calprotectin levels and the risk of incident hypertension (Table 2, Model 1; HR per doubling, 1.30 [95% CI, 1.21–1.41]; P<0.001), also after age‐ and sex‐adjusted analysis (Table 2, Model 2; HR per doubling 1.26 [95% CI, 1.16–1.37]; P<0.001). However, when adjusting for BMI, current smoking, diabetes, total cholesterol, 24‐hour Na excretion, hs‐CRP, and baseline SBP, this association completely lost statistical significance (Model 4; HR per doubling 1.00 [95% CI, 0.90–1.11]; P=0.996). Restricted cubic spline analysis demonstrated no deviance from linear association with the risk of new‐onset hypertension for plasma calprotectin levels (P=0.061; Figure 2B). In sex‐stratified Cox proportional hazards regression analyses, the association between plasma calprotectin levels and the risk of incident hypertension appeared to be most prominent in men when compared with women (Table 2). However, in both sexes, the association between plasma calprotectin levels and the risk of incident hypertension lost statistical significance after adjustment for all potentially confounding factors (men: Model 4; HR per doubling 1.15 [95% CI, 0.98–1.35]; P=0.080; women: Model 4; HR per doubling 0.89 [95% CI, 0.77–1.03]; P=0.129).
Figure 2. Higher levels of plasma calprotectin are associated with an increased risk of developing hypertension.
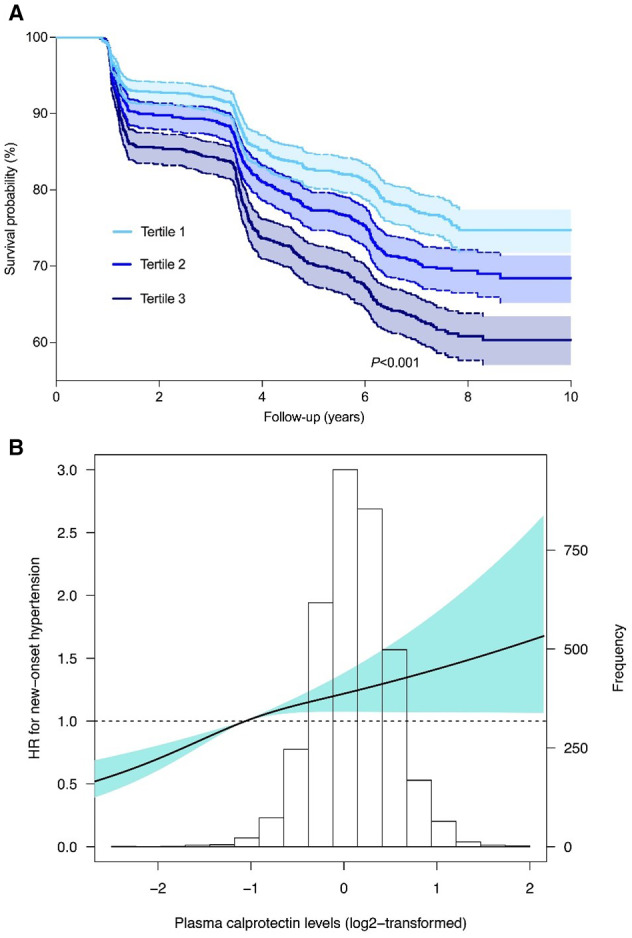
A, Kaplan‐Meier survival curves for tertiles of plasma calprotectin levels demonstrating that individuals within the highest tertile (T3, dark blue) of plasma calprotectin levels have the lowest hypertension‐free survival (log‐rank test, P<0.001). B, Restricted cubic spline curve showing the crude association between plasma calprotectin levels and the risk of new‐onset hypertension. Estimated association was based on Cox proportional hazards regression analysis with restricted cubic spline curve based on 3 knots (set at the 5th, 50th, and 95th percentiles). The likelihood ratio test for nonlinearity was not significant (P=0.061). The light blue‐shaded area represents the 95% CI. HR indicates hazard ratio.
Table 2.
Cox Proportional Hazards Regression Analyses for Associations Between Plasma Calprotectin Levels and the Risk of Incident Hypertension
| Variable | HR per doubling | Tertile 1 | Tertile 2 | Tertile 3 |
|---|---|---|---|---|
| Incident hypertension (n=1000, full cohort) | ||||
| Model 1 | 1.30 [1.21–1.41], P<0.001* | 1.00 (reference) | 1.33 [1.13–1.57], P<0.001* | 1.80 [1.54–2.10], P<0.001* |
| Model 2 | 1.26 [1.16–1.37], P<0.001* | 1.00 (reference) | 1.25 [1.07–1.48], P=0.006* | 1.63 [1.39–1.90], P<0.001* |
| Model 3 | 1.08 [0.98–1.20], P=0.136 | 1.00 (reference) | 1.17 [0.98–1.41], P=0.091 | 1.26 [1.04–1.53], P=0.017* |
| Model 4 | 1.00 [0.90–1.11], P=0.996 | 1.00 (reference) | 0.99 [0.82–1.19], P=0.917 | 1.04 [0.86–1.26], P=0.659 |
| Incident hypertension (n=540, men) | ||||
| Model 1 | 1.33 [1.19–1.48], P<0.001* | 1.00 (reference) | 1.21 [0.97–1.53], P=0.097 | 1.68 [1.36–2.09], P<0.001* |
| Model 2 | 1.32 [1.18–1.48], P<0.001* | 1.00 (reference) | 1.23 [0.98–1.54], P=0.082 | 1.62 [1.30–2.01], P<0.001* |
| Model 3 | 1.28 [1.09–1.49], P=0.002* | 1.00 (reference) | 1.21 [0.92–1.58], P=0.178 | 1.48 [1.12–1.94], P=0.005* |
| Model 4 | 1.15 [0.98–1.35], P=0.080 | 1.00 (reference) | 1.06 [0.81–1.39], P=0.674 | 1.27 [0.96–1.66], P=0.093 |
| Incident hypertension (n=460, women) | ||||
| Model 1 | 1.24 [1.10–1.39], P<0.001* | 1.00 (reference) | 1.40 [1.11–1.77], P=0.004* | 1.80 [1.44–2.26], P<0.001* |
| Model 2 | 1.18 [1.05–1.34], P=0.007* | 1.00 (reference) | 1.28 [1.02–1.62], P=0.037* | 1.63 [1.30–2.05], P<0.001* |
| Model 3 | 0.94 [0.81–1.09], P=0.405 | 1.00 (reference) | 1.14 [0.88–1.47], P=0.315 | 1.08 [0.83–1.42], P=0.566 |
| Model 4 | 0.89 [0.77–1.03], P=0.129 | 1.00 (reference) | 0.93 [0.72–1.21], P=0.595 | 0.88 [0.67–1.16], P=0.359 |
Model 1, crude model. Model 2, Model 1 with adjustment for age and sex. Model 3, Model 2 with adjustment for body mass index, current smoking, diabetes, total cholesterol, high‐sensitivity C‐reactive protein, and 24‐hour Na excretion. Model 4, Model 3 with adjustment for baseline systolic blood pressure. HR indicates hazard ratio.
P value indicates statistical significance.
Stratified Analyses for the Association Between Plasma Calprotectin Levels and Incident Hypertension
Next, stratified analyses were performed to study the association between plasma calprotectin levels and the risk of new‐onset hypertension across various relevant subgroups and to test for potential interactions (Figure 3, Table S1). Multivariable‐adjusted stratified analyses (with adjustment for factors as in Model 4, see Table 2) showed significant effect modification by sex (P interaction=0.023) and UAE (P interaction=0.004). In these analyses, HRs for the risk of new‐onset hypertension were higher in men and in participants with higher UAE (>9.3 mg per 24 hours) compared with women and participants with lower UAE (≤9.3 mg per 24 hours), respectively.
Figure 3. Stratified analyses for the association between plasma calprotectin levels and the risk of new‐onset hypertension across various subgroups.
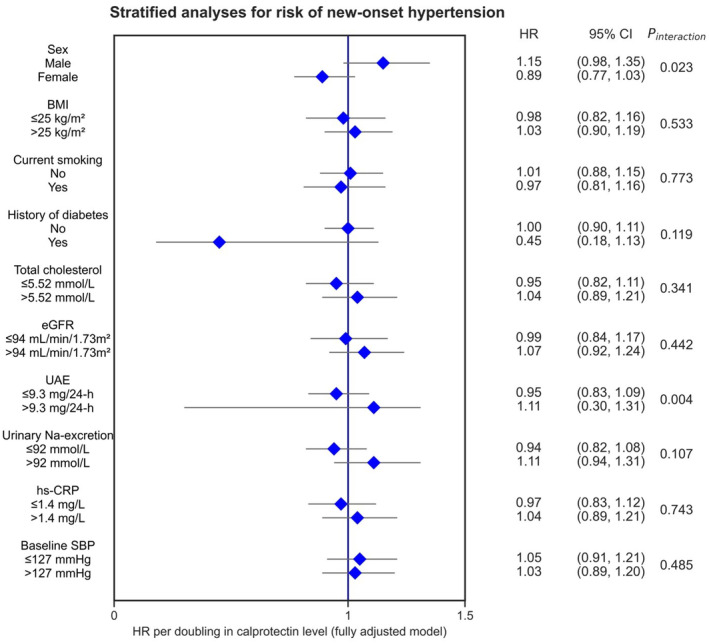
HRs are shown with corresponding 95% CIs. HRs were adjusted for potential confounding factors (based on the directed acyclic graph, Model 4), including age, sex, current smoking, diabetes, BMI, hs‐CRP, 24‐hour urinary Na excretion, total cholesterol levels, and SBP. BMI indicates body mass index; eGFR, estimated glomerular filtration rate; HR, hazard ratio; hs‐CRP, high‐sensitivity C‐reactive protein; SBP, systolic blood pressure; and UAE, urinary albumin excretion.
Discussion
In this study, we demonstrated that higher plasma calprotectin levels, reflecting higher low‐grade systemic inflammation, are associated with an increased risk of new‐onset hypertension in individuals derived from the general population. Notably, after adjustment for relevant covariates, including age, sex, BMI, current smoking, total cholesterol, hs‐CRP, history of diabetes, 24‐hour Na excretion, and baseline SBP, this association lost statistical significance, which may suggest the absence of a direct effect from calprotectin to new‐onset hypertension. The association between plasma calprotectin and the risk of new‐onset hypertension was particularly prominent in men (compared with women), which was significant after adjustment for all relevant covariates, except for baseline SBP. Our study emphasizes the importance of low‐grade systemic inflammation as an early operative pathogenic mechanism in the development of hypertension, as reflected by circulating calprotectin levels.
Calprotectin is an abundant group of cytosolic proteins in myeloid cells and is strongly associated with inflammation, which is also commonly implicated in the pathogenesis of atherosclerosis, to which hypertension is a key predisposing factor. 38 Pathophysiological events, such as endothelial and perivascular inflammation, as well as vascular fibrosis and calcification, are key underlying events of the development of hypertension, and may synergistically contribute to the phenomenon of vascular aging. 39 These pathophysiological changes are accompanied by endothelial dysfunction, which leads to disturbed vasorelaxation and may subsequently result in an impaired dilating capacity of arteries when this is physiologically required. 40 Given the fact that myeloid cells are the primary source of calprotectin, higher levels of calprotectin may be reflective of myeloid cell involvement in the pathogenesis of hypertension. Previous studies have shown that myeloid cells, such as neutrophils, may foster the formation of atherosclerotic plaques by activating the vascular endothelium, disrupting its integrity, and promoting apoptosis and the generation of reactive oxygen species. The latter may result in oxidative stress, as well as in oxidation of low‐density lipoproteins. 41 , 42 , 43 In addition to myeloid cell activation, plasma calprotectin levels may also contribute to activation of the receptor for advanced glycation end products, which in turn triggers a variety of inflammatory and thrombotic events and further contributes to the onset of atherosclerosis. 44 , 45 Although the precise role of calprotectin in the pathogenesis of hypertension remains incompletely understood, circulating calprotectin levels likely augment the inflammatory response by further stimulating immune cell recruitment to vascular tissue and by activating neighboring immune cells. Furthermore, as mentioned, the release of calprotectin is accompanied by an overproduction of reactive oxygen species, resulting in oxidative stress, and the secretion of proinflammatory cytokines, the latter of which is also considered proatherogenic and thus may in parallel contribute to hypertension development. 9 , 46
An important observation of this study was that the significance of the association between plasma calprotectin levels and the risk of new‐onset hypertension was abrogated after adjustment for relevant covariates, including age, sex, BMI, current smoking, total cholesterol, hs‐CRP, history of diabetes, 24‐hour Na excretion, and baseline SBP. As such, the direct effect between plasma calprotectin and new‐onset hypertension as hypothesized in the directed acyclic graph could not be demonstrated, which may suggest neutralization by another, unobserved effect or the presence of erroneously assumed causal effects. For instance, baseline SBP is related to new‐onset hypertension, and because plasma calprotectin is related to baseline SBP (Table 1), these results may also suggest that effects of calprotectin may be mediated through effects on baseline SBP, which does not obviate a role for calprotectin in mediating the occurrence of new‐onset hypertension. These alternative possibilities might be explained by considering hypertension primarily as a risk factor for various manifestations of CVDs, of which the pathogenesis is not solely driven by inflammation. Additional pathogenetic mechanisms are likely to be involved (eg, oxidative stress due to reactive oxygen species overproduction and metabolic disturbances such as lipid and cholesterol metabolic alterations). In any case, our study still highlights the importance of low‐grade systemic inflammation in the development of hypertension, which supports the ongoing investigations for accurate and reliable biomarkers in monitoring cardiovascular risk. In stratified analyses, we observed significant effect modification by sex and UAE for the association between plasma calprotectin levels and the risk of new‐onset hypertension. Generally, associations were stronger in men and in individuals with relatively higher UAE. The significant interaction with sex underlines the importance of sex‐specific cardiovascular risk assessment, and may suggest a more prominent role for (neutrophil‐mediated) systemic inflammation in the development of hypertension in men. Although we are uncertain about reasons for the significant interaction with UAE, this observation might be explained by the notion that the presence of albuminuria reflects more extensive endothelial dysfunction, resulting in a stronger proinflammatory response, and thus a stronger association between plasma calprotectin and the risk of new‐onset hypertension. 47 Finally, albeit no significant interaction was observed, a strong association between current smoking and plasma calprotectin levels was apparent, corroborating previously reported findings. 48
Several relevant strengths and limitations of this study warrant recognition. Major strengths include the large sample size consisting of >3000 individuals, concomitant with well‐documented health‐ and disease‐associated phenotypes, and the relatively long median follow‐up period of ~7 years, which allowed a comprehensive analysis of plasma calprotectin as prognostic biomarker for the development of hypertension. The availability of many health‐related parameters enabled detailed assessment of these associations, because we were able to adjust for a multitude of relevant potentially confounding factors in our analyses. On the other hand, several limitations need to be acknowledged. First, the PREVEND study is based on individuals living in the northern parts of the Netherlands with most participants being White. This limits the external generalizability of our findings to other races and geographical areas. Second, although this was a prospective observational cohort study, the assessment of calprotectin levels was performed cross‐sectionally, and not longitudinally, the latter of which would have allowed us to analyze the dynamics of calprotectin levels alongside various stages of hypertension development. The same principle applies to the determination of hypertension by multiple blood pressure measurements on the same day, whereas measurements on separate days would yield a more accurate estimation. Furthermore, because of this single determination of calprotectin and the correlative nature of the study, we cannot exclude the possibility of reverse causation, which greatly limits causal inference. No follow‐up samples were available to determine additional calprotectin measurements, which could have been used to study the longitudinal dynamics of this biomarker in relation to the development of hypertension. For instance, one could think of a follow‐up study in which plasma calprotectin levels are measured at various time points up until the occurrence of hypertension. Such an approach would not only yield insights into the dynamics of this biomarker, but may also provide more information on the pathophysiologic timespan as well as the usefulness of calprotectin as a biomarker or screening method for hypertension. Third, the exclusion of many participants due to limited availability of plasma samples (or insufficient volume of samples), which precluded the measurement of calprotectin in these samples, could have introduced some exclusion bias. Fourth, the first recorded use of antihypertensive medications may have complicated the definition of new‐onset hypertension, because some of these medications (eg, β‐blockers), are also prescribed for indications other than hypertension. Fifth, blood pressure measurements were performed while participants were in a supine position, which may elevate blood pressure and is not likely to be commonly used in the clinic. Finally, no additional serum or plasma samples were available to comparatively evaluate other inflammatory biomarkers (eg, using multiomics‐based platforms such as proteomics or metabolomics approaches). Such approaches could have been a valuable strategy to interrogate the key integrative inflammatory components driving the development of hypertension, resulting in a biomarker signature with an overall high predictive value. Furthermore, this multiomics strategy holds potential to yield more pathogenic insight into the development of hypertension, because it may shed light on other components that may interact with calprotectin and modify its relationship with the future occurrence of hypertension.
In summary, we conclude that higher circulating levels of calprotectin, considered a surrogate marker of low‐grade systemic inflammation, are associated with an increased risk of new‐onset hypertension in individuals from the general population. Although this association is not independent of other risk factors, in particular baseline SBP, it is particularly prominent in men. Our findings support the potential usefulness of plasma calprotectin measurement as a biomarker for the development of hypertension. Future studies are, however, warranted to validate the present findings and to longitudinally assess calprotectin levels across various stages of hypertension and other CVDs.
Sources of Funding
The PREVEND study has been made possible by grants from the Dutch Kidney Foundation (grant number E.013) and by a grant from the Netherlands Heart Foundation (grant number 2001.005).
Disclosures
None.
Supporting information
Table S1
Figure S1
Acknowledgments
The authors express their gratitude toward all participants of the PREVEND cohort study. A.R.B., M.F.B., R.T.G., S.J.L.B., H.v.G., and A.E.A. were involved in conceptualization and study design. R.T.G., S.J.L.B., and H.v.G. were responsible for funding acquisition and resources. R.T.G., S.J.L.B., and H.v.G. collected all study data. A.R.B. performed data analysis and data visualization. A.R.B. wrote the first draft of the article. All authors contributed to results interpretation, critically reviewed the article, contributed to article revision, and read and approved the final version of the article to be submitted for publication.
This article was sent to Tochukwu M. Okwuosa, DO, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031458
For Sources of Funding and Disclosures, see page 10.
References
- 1. Beaney T, Schutte AE, Stergiou GS, Borghi C, Burger D, Charchar F, Cro S, Diaz A, Damasceno A, Espeche W, et al. May measurement month 2019: the global blood pressure screening campaign of the International Society of Hypertension. Hypertension. 2020;76:333–341. doi: 10.1161/HYPERTENSIONAHA.120.14874 [DOI] [PubMed] [Google Scholar]
- 2. van Oort S, Beulens JWJ, van Ballegooijen AJ, Grobbee DE, Larsson SC. Association of cardiovascular risk factors and lifestyle behaviors with hypertension: a Mendelian randomization study. Hypertension. 2020;76:1971–1979. doi: 10.1161/HYPERTENSIONAHA.120.15761 [DOI] [PubMed] [Google Scholar]
- 3. Patrick DM, Van Beusecum JP, Kirabo A. The role of inflammation in hypertension: novel concepts. Curr Opin Physio. 2021;19:92–98. doi: 10.1016/j.cophys.2020.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 5. Harrison DG, Marvar PJ, Titze JM. Vascular inflammatory cells in hypertension. Front Physiol. 2012;3:128. doi: 10.3389/fphys.2012.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sesso HD, Buring JE, Rifai N, Blake GJ, Gaziano JM, Ridker PM. C‐reactive protein and the risk of developing hypertension. JAMA. 2003;290:2945–2951. doi: 10.1001/jama.290.22.2945 [DOI] [PubMed] [Google Scholar]
- 7. Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C‐reactive protein, interleukin‐6, and TNF‐alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785 [DOI] [PubMed] [Google Scholar]
- 8. Foell D, Wittkowski H, Roth J. Monitoring disease activity by stool analyses: from occult blood to molecular markers of intestinal inflammation and damage. Gut. 2009;58:859–868. doi: 10.1136/gut.2008.170019 [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teng TS, Ji AI, Ji XY, Li YZ. Neutrophils and immunity: from bactericidal action to being conquered. J Immunol Res. 2017;2017:9671604. doi: 10.1155/2017/9671604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kunutsor SK, Flores‐Guerrero JL, Kieneker LM, Nilsen T, Hidden C, Sundrehagen E, Seidu S, Dullaart RPF, Bakker SJL. Plasma calprotectin and risk of cardiovascular disease: findings from the PREVEND prospective cohort study. Atherosclerosis. 2018;275:205–213. doi: 10.1016/j.atherosclerosis.2018.06.817 [DOI] [PubMed] [Google Scholar]
- 12. Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, Roffi M, Sütsch G, Gay S, von Eckardstein A, et al. Myeloid‐related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28:941–948. doi: 10.1093/eurheartj/ehm078 [DOI] [PubMed] [Google Scholar]
- 13. Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH, Moll FL, de Vries JPM, Pasterkamp G, de Kleijn DPV. High levels of myeloid‐related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture‐prone lesions. Arterioscler Thromb Vasc Biol. 2009;29:1220–1227. doi: 10.1161/ATVBAHA.109.190314 [DOI] [PubMed] [Google Scholar]
- 14. Bourgonje AR, von Martels JZH, de Vos P, Faber KN, Dijkstra G. Increased fecal calprotectin levels in Crohn's disease correlate with elevated serum Th1‐ and Th17‐associated cytokines. PLoS One. 2018;13:e0193202. doi: 10.1371/journal.pone.0193202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hillege HL, Janssen WM, Bak AA, Diercks GF, Grobbee DE, Crijns HJ, Van Gilst WH, De Zeeuw D, De Jong PE; Prevend Study Group . Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J Intern Med. 2001;249:519–526. doi: 10.1046/j.1365-2796.2001.00833.x [DOI] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 17. Coppieters Y, Parent F, Berghmans L, Godin I, Levêque A. Blood pressure measurement in epidemiological investigations in teenagers. Eur J Epidemiol. 2001;17:901–906. doi: 10.1023/A:1016250827716 [DOI] [PubMed] [Google Scholar]
- 18. de Greeff A, Reggiori F, Shennan AH. Clinical assessment of the DINAMAP ProCare monitor in an adult population according to the British hypertension protocol. Blood Press Monit. 2007;12:51–55. doi: 10.1097/MBP.0b013e3280858b73 [DOI] [PubMed] [Google Scholar]
- 19. Post A, Kremer D, Swarte JC, Sokooti S, Vogelpohl FA, Groothof D, Kema IP, Garcia E, Connelly MA, Wallimann T, et al. Plasma creatine concentration is associated with incident hypertension in a cohort enriched for the presence of high urinary albumin concentration: the Prevention of Renal and Vascular Endstage Disease study. J Hypertens. 2021;40:229–239. doi: 10.1097/HJH.0000000000002996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grubb A, Blirup‐Jensen S, Lindström V, Schmidt C, Althaus H, Zegers I; IFCC Working Group on Standardisation of Cystatin C (WG‐SCC) . First certified reference material for cystatin C in human serum ERM‐DA471/IFCC. Clin Chem Lab Med. 2010;48:1619–1621. doi: 10.1515/CCLM.2010.318 [DOI] [PubMed] [Google Scholar]
- 21. Gao J, Ulvik A, McCann A, Ueland PM, Meyer K. Microheterogeneity and preanalytical stability of protein biomarkers of inflammation and renal function. Talanta. 2021;223:121774. doi: 10.1016/j.talanta.2020.121774 [DOI] [PubMed] [Google Scholar]
- 22. Nilsen T, Haugen SH, Larsson A. Extraction, isolation, and concentration of calprotectin antigen (S100A8/S100A9) from granulocytes. Health Sci Rep. 2018;1:e35. doi: 10.1002/hsr2.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Structure and principles ATC classification system. WHO Collaborating Center for Drug Statistics Methodology. Accessed June 19, 2023. https://www.whocc.no/atc/structure_and_principles/
- 24. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pearl J. Causality, Models, Reasoning, and Inference. Cambridge University Press; 2000. [Google Scholar]
- 26. La Bastide‐Van GS, van den Heuvel E. Exploring causal hypotheses: breaking with long‐standing research traditions. Dev Med Child Neurol. 2013;55:975–976. doi: 10.1111/dmcn.12269 [DOI] [PubMed] [Google Scholar]
- 27. Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, Ost DE, Punjabi NM, Schatz M, Smyth AR, et al. Control of confounding and reporting of results in causal inference studies. Guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS [DOI] [PubMed] [Google Scholar]
- 28. Erhardt L. Cigarette smoking: an undertreated risk factor for cardiovascular disease. Atherosclerosis. 2009;205:23–32. doi: 10.1016/j.atherosclerosis.2009.01.007 [DOI] [PubMed] [Google Scholar]
- 29. Tzoulaki I, Elliott P, Kontis V, Ezzati M. Worldwide exposures to cardiovascular risk factors and associated health effects: current knowledge and data gaps. Circulation. 2016;133:2314–2333. doi: 10.1161/CIRCULATIONAHA.115.008718 [DOI] [PubMed] [Google Scholar]
- 30. Pencina MJ, D'Agostino RB, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham heart study. Circulation. 2009;119:3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Salzano S, Checconi P, Hanschmann EM, Lillig CH, Bowler LD, Chan P, Vaudry D, Mengozzi M, Coppo L, Sacre S, et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin‐2, which acts as a danger signal. Proc Natl Acad Sci USA. 2014;111:12157–12162. doi: 10.1073/pnas.1401712111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lu Y, Lu M, Dai H, Yang P, Smith‐Gagen J, Miao R, Zhong Z, Chen R, Liu X, Huang Z, et al. Lifestyle and risk of hypertension: follow‐up of a young pre‐hypertensive cohort. Int J Med Sci. 2015;12:605–612. doi: 10.7150/ijms.12446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bao W, Srinivasan SR, Berenson GS. Persistent elevation of plasma insulin levels is associated with increased cardiovascular risk in children and young adults. The Bogalusa Heart Study. Circulation. 1996;93:54–59. doi: 10.1161/01.cir.93.1.54 [DOI] [PubMed] [Google Scholar]
- 34. Cox KL, Puddey IB, Morton AR, Burke V, Beilin LJ, McAleer M. Exercise and weight control in sedentary overweight men: effects on clinic and ambulatory blood pressure. J Hypertens. 1996;14:779–790. doi: 10.1097/00004872-199606000-00015 [DOI] [PubMed] [Google Scholar]
- 35. Groppelli A, Giorgi DM, Omboni S, Parati G, Mancia G. Persistent blood pressure increase induced by heavy smoking. J Hypertens. 1992;10:495–499. doi: 10.1097/00004872-199205000-00014 [DOI] [PubMed] [Google Scholar]
- 36. Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium intake and hypertension. Nutrients. 2019;11:1970. doi: 10.3390/nu11091970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsimihodimos V, Gonzalez‐Villalpando C, Meigs JB, Ferrannini E. Hypertension and diabetes mellitus: coprediction and time trajectories. Hypertension. 2018;71:422–428. doi: 10.1161/HYPERTENSIONAHA.117.10546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Libby R. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nilsson PM, Boutouyrie P, Laurent S. Vascular aging: a tale of EVA and ADAM in cardiovascular risk assessment and prevention. Hypertension. 2009;54:3–10. doi: 10.1161/HYPERTENSIONAHA.109.129114 [DOI] [PubMed] [Google Scholar]
- 41. Viemann D, Barczyk K, Vogl T, Fischer U, Sunderkötter C, Schulze‐Osthoff K, Roth J. MRP8/MRP14 impairs endothelial integrity and induces a caspase‐dependent and ‐independent cell death program. Blood. 2007;109:2453–2460. doi: 10.1182/blood-2006-08-040444 [DOI] [PubMed] [Google Scholar]
- 42. Della Bona R, Cardillo MT, Leo M, Biasillo G, Gustapane M, Trotta F, Biasucci LM. Polymorphonuclear neutrophils and instability of the atherosclerotic plaque: a causative role? Inflamm Res. 2013;62:537–550. doi: 10.1007/s00011-013-0617-0 [DOI] [PubMed] [Google Scholar]
- 43. Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535 [DOI] [PubMed] [Google Scholar]
- 44. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6 [DOI] [PubMed] [Google Scholar]
- 45. Nagareddy PR, Murphy AJ, Stirzaker RA, Hu Y, Yu S, Miller RG, Ramkhelawon B, Distel E, Westerterp M, Huang LS, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17:695–708. doi: 10.1016/j.cmet.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538 [DOI] [PubMed] [Google Scholar]
- 47. van Bussel BC, Schouten F, Henry RM, Schalkwijk CG, de Boer MR, Ferreira I, Smulders YM, Twisk JW, Stehouwer CD. Endothelial dysfunction and low‐grade inflammation are associated with greater arterial stiffness over a 6‐year period. Hypertension. 2011;58:588–595. doi: 10.1161/HYPERTENSIONAHA.111.174557 [DOI] [PubMed] [Google Scholar]
- 48. Otieno SB, Altahan A, Karri S, Kaweeta F, Lands L, Weir A. CIN or not: an approach to the evaluation and management of chronic idiopathic neutrophilia. Blood Rev. 2021;46:100739. doi: 10.1016/j.blre.2020.100739 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


