Abstract
Background
Peripheral artery disease (PAD) and microvascular disease (MVD) are highly prevalent conditions that share common risk factors. This observational study aimed to characterize patients with both conditions and determine the impact of comorbid PAD/MVD on outcomes.
Methods and Results
Patients admitted across 31 states January 2011 through December 2018 with a primary or secondary diagnosis of PAD or MVD were included from the National Readmissions Database and weighted to approximate a national sample. Those age <18 years or with nonatherosclerotic leg injuries were excluded. Patients were divided into 3 groups: PAD‐only, MVD‐only, or comorbid PAD/MVD. Multiple logistic regression was used to evaluate associations with major and minor amputations, major adverse cardiac events, and in‐hospital mortality. Cox regression was used to evaluate associations with readmission within 1 year. The PAD group was used as reference. The final cohort included 33 972 772 admissions: 9.1 million with PAD, 21.3 million with MVD, and 3.6 million with both. Annual admissions for PAD/MVD increased to >500 000 in 2018. Major and minor amputations increased ≈50% for PAD/MVD between 2011 and 2018. Compared with PAD‐only, PAD/MVD was associated with a higher risk for major amputation (odds ratio [OR], 1.30 [95% CI, 1.28–1.32]), minor amputation (OR, 2.15 [95% CI, 2.12–2.18]), major adverse cardiac events (OR, 1.04 [95% CI, 1.03–1.04]), in‐hospital mortality (OR, 1.07 [95% CI, 1.05–1.09]), and readmission (hazard ratio, 1.02 [95% CI, 1.02–1.02]) after adjustment for baseline factors.
Conclusions
Comorbid MVD is present in a large and growing number of patients with PAD and is associated with augmented risk for adverse outcomes. Further prospective research is merited to understand this vulnerable population.
Keywords: amputations, microvascular disease, mortality, peripheral artery disease
Subject Categories: Peripheral Vascular Disease
Nonstandard Abbreviations and Acronyms
- CLI
critical limb ischemia
- MACE
major adverse cardiac events
- MVD
microvascular disease
- NRD
National Readmissions Database
Clinical Perspective.
What Is New?
Over one‐quarter of patients admitted with peripheral artery disease have comorbid microvascular disease, and major and minor amputation rates rose between 2011 and 2018 in patients with both conditions.
After controlling for patient characteristics and comorbidities, comorbid microvascular disease was found to be associated with higher risk for major and minor amputation, major adverse cardiac events, in‐hospital mortality, and readmission for patients with underlying peripheral artery disease.
What Are the Clinical Implications?
Increased emphasis should be placed on assessing for concurrent microvascular disease in addition to traditional cardiovascular risk factors for patients with peripheral artery disease.
Lower extremity peripheral artery disease (PAD), or atherosclerosis restricting blood flow to the lower extremities, affects an estimated 8.5 million individuals in the United States, over 230 million individuals worldwide, and places significant financial strain on the health care system. 1 , 2 , 3 As the third most common manifestation of atherosclerosis behind coronary artery disease and stroke, PAD is associated with an increased risk of lower limb amputations, major adverse cardiovascular events (MACE), and mortalty. 4 , 5 , 6 Microvascular disease (MVD, “small vessel disease”) refers to a family of disorders with a shared pathophysiology of smooth muscle and endothelial wall dysfunction impacting small vessels (diameter <150 μm) such as arterioles, capillaries, and venules. 7 MVD has various clinical manifestations including retinopathy, neuropathy, and nephropathy that are increasingly thought of as markers of a systemic process causing capillary bed dysfunction throughout the body. 8 , 9 PAD and MVD are often coprevalent due to the abundance of shared risk factors such as diabetes, obesity, and chronic kidney disease. 10 , 11 , 12
The interplay between macro‐ and microvascular disease in the legs is an area of active investigation. In patients with type 2 diabetes, for instance, MVD was found to be associated with development of PAD at 5 years, whereas preexisting macrovascular disease (eg, coronary disease or stroke) was not. 13 Furthermore, a recent study in the Veterans Affairs health care system demonstrated that a diagnosis of MVD among Veterans was associated with a 3.7‐fold increased risk for lower limb amputation after controlling for traditional atherosclerotic risk factors. When combined with comorbid PAD, a dramatically increased risk of amputation was observed. 14 However, the generalizability of these findings to the greater US health care system remains poorly understood, as does the impact of comorbid PAD/MVD on other important outcomes such as in‐hospital mortality and risk of readmission.
Using nationally representative data available through the National Readmissions Database (NRD), this study aims to (1) quantify and characterize patients admitted to US hospitals with PAD, MVD, or comorbid PAD/MVD; (2) describe temporal trends in admissions, major and minor amputations, MACE, and in‐hospital mortality in these populations; and (3) determine the association between comorbid PAD/MVD and these outcomes. We hypothesize that concurrent MVD augments risk for major or minor amputations, MACE, in‐hospital mortality, and readmission for patients with PAD. Insights from this study could provide clinicians with an additional tool to identify patients at the highest risk for adverse events.
METHODS
Data Source
The specific data supporting this study's findings are available from the NRD and can be accessed at www.hcup‐us.ahrq.gov/nrdoverview.jsp. 15 Code for statistical analysis is available upon reasonable request to the corresponding author. The NRD is a database sponsored by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project. The NRD includes information associated with approximately 17 million admissions across 31 states annually, which are weighted using several criteria including hospital teaching status, hospital size, and location to estimate ≈32 million national admissions each year. Weighted admissions are derived from stratum variables and are used to derive national estimates. Data are deidentified but remain linked to individual patients for up to 1 calendar year to allow for capture of readmissions. 15 This study was ruled exempt by the Yale University institutional review board because it utilized data from a deidentified, publicly accessible national database. One author (M.A.) had full access to the data and takes responsibility for its integrity and analysis.
Study Population
Patients admitted from January 2011 through December 2018 with a primary or secondary diagnosis of PAD or MVD were included in the study. Admissions were weighted as previously described to approximate national estimates. 15 Those (1) <18 years of age or (2) with nonatherosclerotic causes of lower extremity injury were excluded. Sociodemographic factors including age, sex, insurance status, and median household income based upon hospitalization zip code were extracted. Comorbidities were captured, including hypertension, diabetes, dyslipidemia, obesity, coronary artery disease, heart failure, valvular disease, chronic lung disease, renal failure, anemia, hypothyroidism, smoking status, depression, schizophrenia, bipolar disorder, generalized anxiety disorder, and other stress‐related disorders. History of prior amputation and current episode of critical limb ischemia (CLI) were collected as proxies of PAD disease severity. 16 The type of admission (elective versus emergency department visit) was also noted.
Study Exposures
Patients were separated into 3 mutually exclusive groups: those diagnosed with PAD‐only, MVD‐only, or comorbid PAD/MVD. Diagnoses of PAD and MVD were defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes before October 1, 2015, after which the United States transitioned to the International Classification of Diseases, Tenth Revision, Clinical Modification and Procedure Coding System (ICD‐10‐CM/PCS). All ICD‐9‐CM and ICD‐10‐CM/PCS codes for both conditions are in line with previously published work and were independently reviewed by 2 authors (M.A., P.L.). 14 , 17 , 18 , 19 , 20 All codes used in this study are available in Table S1 or were obtained as distinct NRD data elements. 15
Study Outcomes
The primary outcomes for this study included major and minor amputations, MACE, in‐hospital all‐cause mortality, and readmission (Table S1). Major amputations were defined as amputations occurring during index admission above the level of the ankle joint. 21 Minor amputations were considered at or below this level. MACE was defined as instances of ST‐segment elevation myocardial infarction, non–ST‐segment elevation myocardial infarction, or ischemic stroke during index admission. In‐hospital mortality data were available as an NRD data element. Readmissions for any cause were considered within 1 calendar year or up to death, the maximum available timeframe within the NRD that subsequent admissions could be linked to the same patient within state inpatient databases. Secondary outcomes of the study included receiving a lower extremity endovascular or surgical revascularization procedure, median length‐of‐stay, and costs associated with the index admission.
Statistical Analysis
Categorical variables were reported as counts with percentages, and continuous variables were reported as medians with first and third quartiles (Q1; Q3). Pairwise comparisons were made between disease groups using standardized mean differences (Cohen's d). Pairwise differences were reported beyond the threshold of |d|=0.2 (which corresponds to a “small” effect size). 22 , 23 The corresponding risk of comorbidities as well as outcomes in the PAD‐only, MVD‐only, and PAD/MVD were compared using relative risk (RR) ratios.
Multiple logistic regression was used to evaluate associations between disease group and major amputations, minor amputations, MACE, and in‐hospital mortality. The models were adjusted for age, sex, elective admission status, median household income quartile by zip code, insurance status, calendar year, and comorbidities including hypertension, valvular disease, diabetes, coronary artery disease, heart failure, chronic lung disease, renal failure, anemia, hypothyroidism, obesity, smoking status, dyslipidemia, and depression in line with prior work. 24 , 25
A Cox proportional hazard model was constructed to assess the association between readmission risk at 1 year and disease group. 26 The model was adjusted for all observed baseline characteristics and comorbidities listed above. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC) in accordance with recommended methodological standards. 27
RESULTS
Patient Characteristics
A total of 35 625 645 admissions with a primary or secondary diagnosis of PAD or MVD were identified from 2011 to 2018. Of those admissions, 107 896 were excluded for age <18 years and 1 544 977 for nonatherosclerotic lower extremity injury, leaving a final cohort of 33 972 772 (Figure 1). There were ≈9.1 million admissions with PAD only, 21.3 million with MVD only, and 3.6 million carrying both diagnoses (Table 1). The study cohort was 52.6% male with median ages for the PAD, MVD, and PAD/MVD groups of 72.9 (63.9; 81.4), 67.5 (56.0; 78.4), and 69.8 (60.4; 78.8) years, respectively, with no substantial differences between groups (all pairs |d|<0.2). Most patients received insurance through Medicare (71.2%), followed by private insurance (13.8%), Medicaid (10.1%), or another insurer (2.2%); 2.2% of admissions were paid out‐of‐pocket, 0.3% were free of charge, and 0.2% were missing insurance information (all pairs |d|<0.2). The median household income for the cohort fell predominantly into the lowest regional quartile (32.4%), followed by quartile 2 (26.2%), quartile 3 (22.6%), and quartile 4 (17.3%) with 0.2% missing this information (all pairs |d|<0.2).
Figure 1. Study cohort flow diagram.
MVD indicates microvascular disease; NRD, National Readmissions Database; and PAD, peripheral artery disease.
Table 1.
Baseline Sociodemographic Factors and Comorbidities Stratified by Disease Group
| Characteristic | PAD‐only | MVD‐only | PAD/MVD | Standardized difference (d) | ||
|---|---|---|---|---|---|---|
| N=9 133 257 | N=21 270 667 | N=3 568 848 | MVD vs PAD | PAD/MVD vs PAD | PAD/MVD vs MVD | |
| Age, y, median (Q1;Q3) | 72.9 (63.9; 81.4) | 67.5 (56.0; 78.4) | 69.8 (60.4; 78.8) | −0.035 | −0.027 | 0.017 |
| Sex, n (%) | −0.096 | 0.098 | 0.194 | |||
| Female | 4 119 612 (45.1) | 10 514 557 (49.4) | 1 454 239 (40.8) | |||
| Male | 5 013 645 (54.9) | 10 756 110 (50.6) | 2 114 609 (59.3) | |||
| Insurance status, n (%) | 0.189 | 0.004 | −0.183 | |||
| Medicare | 7 160 947 (78.4) | 14 288 520 (67.2) | 2 749 860 (77.1) | |||
| Medicaid | 593 648 (6.5) | 2 521 958 (11.9) | 302 835 (8.5) | |||
| Private insurance | 1 041 816 (11.4) | 3 246 396 (15.3) | 392 073 (11.0) | |||
| Self‐pay | 127 271 (1.4) | 576 199 (2.7) | 47 007 (1.3) | |||
| No charge | 18 314 (0.2) | 85 082 (0.4) | 7077 (0.2) | |||
| Other | 178 089 (2.0) | 518 043 (2.4) | 65 650 (1.8) | |||
| Missing | 13 172 (0.1) | 34 469 (0.2) | 4346 (0.1) | |||
| Median household income, n (%) | −0.061 | −0.025 | 0.036 | |||
| Quartile 1 (lowest) | 2 819 192 (30.9) | 7 053 533 (33.2) | 1 139 507 (31.9) | |||
| Quartile 2 | 2 387 633 (26.1) | 5 586 040 (26.3) | 929 205 (26.0) | |||
| Quartile 3 | 2 112 701 (23.1) | 4 761 195 (22.4) | 817 109 (22.9) | |||
| Quartile 4 (highest) | 1 689 388 (18.5) | 3 559 284 (16.7) | 634 755 (17.8) | |||
| Missing | 124 343 (1.4) | 310 615 (1.5) | 48 272 (1.4) | |||
| Hypertension, n (%) | 6 192 147 (67.8) | 14 152 606 (66.5) | 2 462 770 (69.0) | −0.032 | 0.031 | 0.062 |
| Valvular disease, n (%) | 1 016 443 (11.1) | 1 617 455 (7.6) | 359 229 (10.1) | −0.231* | −0.062 | 0.170 |
| Diabetes, n (%) | 3 524 172 (38.6) | 14 344 693 (67.4) | 2 868 663 (80.4) | 0.658* | 1.034* | 0.376* |
| Coronary artery disease, n (%) | 4 952 811 (54.2) | 8 075 151 (38.0) | 2 099 861 (58.8) | −0.364* | 0.104 | 0.468* |
| Heart failure, n (%) | 2 305 943 (25.3) | 5 278 608 (24.8) | 1 152 565 (32.3) | −0.013 | 0.190 | 0.203* |
| Chronic lung disease, n (%) | 3 149 123 (34.5) | 5 624 927 (26.4) | 1 078 629 (30.2) | −0.210* | −0.107 | 0.103 |
| Renal failure, n (%) | 2 290 239 (25.1) | 11 847 022 (55.7) | 2 238 468 (62.7) | 0.730* | 0.890* | 0.161 |
| Anemia, n (%) | 2 403 870 (26.3) | 6 312 107 (29.7) | 1 352 048 (37.9) | 0.092 | 0.295* | 0.203* |
| Hypothyroidism, n (%) | 1 358 972 (14.9) | 3 474 851 (16.3) | 544 186 (15.3) | 0.061 | 0.016 | −0.045 |
| Obesity, n (%) | 1 226 487 (13.4) | 4 920 327 (23.1) | 796 761 (22.3) | 0.365* | 0.340* | −0.025 |
| Smoking, n (%) | 4 238 635 (46.4) | 6 673 044 (31.4) | 1 373 138 (38.5) | −0.352* | −0.180 | 0.173 |
| Dyslipidemia, n (%) | 5 231 127 (57.3) | 10 292 693 (48.4) | 2 184 244 (61.2) | −0.197 | 0.090 | 0.287* |
| Prior amputation, n (%) | 695 561 (7.6) | 473 091 (2.2) | 576 967 (16.2) | −0.710* | 0.469* | 1.178* |
| CLI, n (%) | 1 401 848 (15.4) | 1 640 275 (7.7) | 1 063 810 (29.8) | −0.427* | 0.469* | 0.896* |
| Depression, n (%) | 1 143 387 (12.5) | 3 251 368 (15.3) | 506 776 (14.2) | 0.128 | 0.080 | −0.048 |
| Schizophrenia, n (%) | 57 955 (0.6) | 236 072 (1.1) | 21 777 (0.6) | 0.311* | −0.022 | −0.333* |
| Bipolar, n (%) | 121 435 (1.3) | 578 615 (2.7) | 52 997 (1.5) | 0.403* | 0.062 | −0.341* |
| Anxiety, n (%) | 970 254 (10.6) | 2 445 732 (11.5) | 343 336 (9.6) | 0.049 | −0.061 | −0.110 |
| Stress‐related disorders, n (%) | 43 241 (0.5) | 188 771 (0.9) | 16 540 (0.5) | 0.349* | −0.012 | −0.361* |
CLI indicates critical limb ischemia; MVD, microvascular disease; PAD, peripheral artery disease; Q1, first quartile; and Q3, third quartile.
Standardized differences (Cohen's d) correspond to at least a “small” effect size (|d| ≥ 0.2).
Compared with patients with PAD alone, those with PAD/MVD had higher rates of diabetes (RR=2.1, d=1.034), renal failure (RR=2.5, d=0.890), anemia (RR=1.4, d=0.295), and obesity (RR=1.7, d=0.340) at baseline. They also presented with more severe disease, as evidenced by more frequent prior amputations (RR=2.1, d=0.469) and current CLI (RR=1.9, d=0.469). For further between‐group comparisons see Table 1.
Primary and Secondary Outcomes by Disease Group
The PAD/MVD group had higher rates of major amputation compared with both the PAD (RR=1.8, d=0.324) and MVD groups (RR=25.2, d=1.805) (Table 2). Those with PAD/MVD also had higher rates of minor amputation than those with PAD (RR=3.0, d=0.637) or MVD (RR=11.5, d=1.388) alone. The difference in MACE was negligible between the PAD/MVD and PAD‐only groups (10.6% versus 9.9%, d=0.046), although higher compared with the MVD‐only group (10.6% versus 7.4%, d=0.216). In‐hospital mortality did not substantially differ across groups (all pairs |d|<0.2).
Table 2.
Breakdown of Amputations, Revascularizations, MACE, In‐Hospital Mortality, Length of Stay, and Total Charges of Admission Stratified by Disease Group
| PAD‐only | MVD‐only | PAD/MVD | Standardized difference (|d|) | |||
|---|---|---|---|---|---|---|
| Outcome | N=9 133 257 | N=21 270 667 | N=3 568 848 | MVD vs PAD | PAD/MVD vs PAD | PAD/MVD vs MVD |
| Minor amputation, n (%) | 223 241 (2.4) | 135 477 (0.6) | 262 930 (7.4) | −0.752* | 0.637* | 1.388* |
| Major amputation, n (%) | 235 183 (2.6) | 38 235 (0.2) | 162 009 (4.5) | −1.481* | 0.324* | 1.805* |
| Any amputation, n (%) | 443 994 (4.9) | 171 075 (0.8) | 407 237 (11.4) | −1.015* | 0.510* | 1.525* |
| Endovascular Revascularization, n (%) | 658 121 (7.2) | 52 961 (0.2) | 235 075 (6.6) | −1.895* | −0.053 | 1.842* |
| Surgical revascularization, n (%) | 500 990 (5.5) | 7545 (0.0) | 113 631 (3.2) | −2.810* | −0.313* | 2.497* |
| Any revascularization, n (%) | 1 020 386 (11.2) | 59 157 (0.3) | 315 356 (8.8) | −2.100* | −0.144 | 1.956* |
| STEMI, n (%) | 368 267 (4.0) | 613 832 (2.9) | 149 809 (4.2) | −0.191 | 0.023 | 0.214* |
| NSTEMI, n (%) | 640 087 (7.0) | 1 085 322 (5.1) | 294 116 (8.2) | −0.186 | 0.097 | 0.283* |
| Ischemic stroke, n (%) | 175 518 (1.9) | 345 737 (1.6) | 60 412 (1.7) | −0.094 | −0.071 | 0.023 |
| MACE, n (%) | 902 192 (9.9) | 1 584 635 (7.4) | 379 912 (10.6) | −0.170 | 0.046 | 0.216* |
| In‐hospital mortality, n (%) | 368 142 (4.0) | 694 932 (3.3) | 130 858 (3.7) | −0.120 | −0.054 | 0.066 |
| LOS, d, median (Q1;Q3) | 3.5 (1.7, 6.7) | 3.5 (1.9, 6.5) | 4.4 (2.2, 8.3) | −0.001 | 0.023 | 0.020 |
| Total charges, $, median (Q1; Q3) | 41 496 (21 684; 82 186) | 34 433 (18 457; 67 591) | 46 333 (23 795; 93 405) | −0.005 | 0.006 | 0.009 |
LOS indicates length of stay; MACE, major adverse cardiac events; MVD, microvascular disease; NSTEMI, non–ST‐segment–elevation myocardial infarction; PAD, peripheral artery disease; Q1, first quartile; Q3, third quartile; and STEMI, ST‐segment–elevation myocardial infarction.
Standardized Differences (Cohen's d) correspond to at least a “small” effect size (|d|≥0.2).
With regard to secondary outcomes, rates of receiving either surgical or endovascular revascularization were lower in the MVD group than in either the PAD or PAD/MVD groups (0.3% versus 11.2%, 8.8%, d=−2.100, −1.956, respectively). Median length of stay fell between 3 and 5 days for all groups with negligible differences between groups (|d|<0.2 for all pairs). Median charges associated with admissions fell between $30 000 to $50 000 and again displayed no substantial differences between groups (|d|<0.2 for all pairs).
Trends in Annual Admissions, Amputations, MACE, and In‐Hospital Mortality 2011 to 2018
From 2011 to 2018, annual admissions with PAD increased from 1 028 521 to 1 353 793 (+31.6%), annual admissions with MVD increased from 2 478 490 to 2 968 604 (+19.8%), and annual admissions with PAD/MVD increased from 397 326 to 507 404 (+27.7%). During this period, major amputation rates remained relatively stable for patients with PAD alone (1.3% to 1.3%) or MVD alone (0.1% to 0.1%) but increased from 1.9% to 2.5% in patients with PAD/MVD (Figure 2A). Although minor amputation rates increased slightly for the PAD‐only and MVD‐only groups, the jump was most pronounced for those with PAD/MVD (from 2.6% to 4.7%) (Figure 2B). During this timeframe, MACE rates decreased across all groups: from 12.4% to 5.8% for PAD‐only, 8.9% to 4.9% for MVD‐only, and 12.7% to 6.9% for PAD/MVD (Figure 2C). In‐hospital mortality remained relatively stable across groups (≈3%–4%) during the study period (Figure 2D).
Figure 2. Trends in adverse outcomes among patients with PAD, MVD, or Comorbid PAD/MVD from 2011 to 2018.
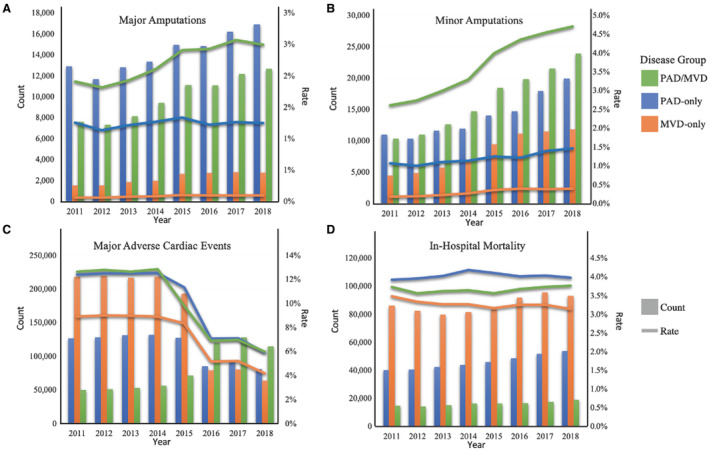
A, Trends in major amputation counts and rates. B, Trends in minor amputation counts and rates. C, Trends in major adverse cardiac event counts and rates. D, Trends in‐hospital mortality counts and rates. MVD indicates microvascular disease; and PAD, peripheral artery disease.
Impact of Disease Group on Amputations, MACE, In‐Hospital Mortality, and Readmissions
Even after adjustment for patient‐ and hospital‐level characteristics, a concurrent diagnosis of MVD with PAD was more strongly associated with both major amputations (odds ratio [OR], 1.30 [95% CI, 1.28–1.32]) and minor amputations (OR, 2.15 [2.12–2.18]) compared with PAD alone (Table 3). The same trend was observed for both MACE (OR, 1.04 [1.03–1.04]) and in‐hospital mortality (OR, 1.07 [1.05–1.09]), although the magnitude of the effect was less pronounced. The 1‐year adjusted readmission risk was higher in PAD/MVD relative to PAD‐only (hazard ratio, 1.02 [1.02–1.02]) (Table 4).
Table 3.
Multiple Logistic Regression Models for Amputations, MACE, and In‐Hospital Mortality by Disease Group
| Disease group | Minor amputation | Major amputation | MACE | In‐hospital mortality | ||||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||
| Unadjusted models | ||||||||||||
| PAD‐only (reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| MVD‐only | 0.26 | 0.25 | 0.26 | 0.07 | 0.07 | 0.07 | 0.73 | 0.73 | 0.74 | 0.80 | 0.80 | 0.81 |
| PAD/MVD | 3.18 | 3.13 | 3.22 | 1.80 | 1.77 | 1.83 | 1.09 | 1.08 | 1.10 | 1.08 | 1.07 | 1.09 |
| Adjusted models* | ||||||||||||
| PAD‐only (Reference) | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| MVD‐only | 0.15 | 0.14 | 0.15 | 0.05 | 0.05 | 0.05 | 0.66 | 0.61 | 0.70 | 0.73 | 0.73 | 0.74 |
| PAD/MVD | 2.15 | 2.12 | 2.18 | 1.30 | 1.28 | 1.32 | 1.04 | 1.03 | 1.04 | 1.07 | 1.05 | 1.09 |
MACE indicates major adverse cardiac events; MVD, microvascular disease; OR, odds ratio; and PAD, peripheral artery disease.
Adjusted for age, sex, emergency department visit, elective admission status, median household income, insurance status, categorical calendar year, and comorbidities including hypertension, valvular disease, diabetes, coronary artery disease, heart failure, chronic lung disease, renal failure, anemia, hypothyroidism, obesity, smoking status, dyslipidemia, and depression.
Table 4.
Cox Proportional Hazard Models for 1‐Year Readmission Risk
| Disease group | Hazard ratio | 95% CI | P value | |
|---|---|---|---|---|
| Unadjusted Model | ||||
| PAD‐only | 1 [Reference] | |||
| MVD‐only | 0.97 | 0.97 | 0.97 | <0.0001 |
| PAD/MVD | 1.03 | 1.03 | 1.03 | <0.0001 |
| Adjusted model* | ||||
| PAD‐only | 1 [Reference] | |||
| MVD‐only | 0.95 | 0.95 | 0.95 | <0.0001 |
| PAD/MVD | 1.02 | 1.02 | 1.02 | <0.0001 |
MVD, microvascular disease; and PAD, peripheral artery disease.
Adjusted for age, sex, emergency department visit, elective admission status, median household income, insurance status, categorical calendar year, and comorbidities including hypertension, valvular disease, diabetes, coronary artery disease, heart failure, chronic lung disease, renal failure, anemia, hypothyroidism, obesity, smoking status, dyslipidemia, and depression.
DISCUSSION
This study using nationally representative admissions data found comorbid MVD was present in 28.1% of all patients presenting to hospitals with PAD and that these patients have been increasing in number over time. Patients with comorbid PAD/MVD had higher rates of diabetes, chronic kidney disease, anemia, and obesity than their counterparts with PAD alone and presented with more severe PAD as evidenced by more frequent CLI and prior amputations. Compared with the PAD group, the PAD/MVD group had a 76% higher major amputation rate, a 202% higher minor amputation rate, with similar levels of MACE and in‐hospital mortality. After adjustment for patient characteristics and comorbidities, MVD was found to significantly potentiate risk for major and minor amputation, MACE, in‐hospital mortality, and readmission.
The observation of an interactive effect between PAD and MVD on risk of amputation complements prior work by Beckman et al within a veteran population, replicating their findings in a nationally representative and more demographically diverse cohort. 14 While the precise mechanism for this interaction remains unknown, it has been postulated that poor wound healing, impaired angiogenesis, and serial ischemic–reperfusion injuries contribute to chronic inflammation in patients with PAD/MVD, which has been found to predict amputations in those with CLI. 28 , 29 Our study demonstrates that the same pattern of potentiated risk extends to, but is less pronounced for, MACE, in‐hospital mortality, and readmissions. Our findings also bring into sharp focus the magnitude and temporality of the issue within the US hospital system. Annual admissions with concomitant PAD/MVD numbered over half a million in 2018 and rose 27.7% over the study period: a growth rate 5 times that of the US census over the same time period. 30 The staggering size and steady expansion of the population with comorbid PAD/MVD is concerning but not unexpected given the abundance and growth of shared risk factors such as advanced age, diabetes, obesity, and chronic kidney disease within the US population. 11 , 31 , 32 Given that the prevalence of conditions such as obesity are predicted to continue rising over the next 20 years and some forecast the prevalence of diabetes to as much as double by the year 2060, it is likely the clinical footprint of comorbid PAD/MVD will continue to expand. 33 , 34
The rise in admissions with PAD/MVD is even more alarming contextualized by trends in amputations within this subpopulation. While major and minor amputation rates remained relatively stable from 2011 to 2018 for those with PAD alone, both rates increased ≈50% for those with comorbid MVD. This growth mirrors sharp uptrends in CLI‐related admissions between 2011 and 2017 previously described by Harris et al primarily driven by younger patients with higher burdens of obesity and diabetes. 35 Although the cause for increasing amputations among those admitted with PAD/MVD is likely multifactorial, a partial explanation might stem from the observation that nontraumatic amputation rates in young and middle‐aged men with diabetes rose from 2000 to 2015. 36 Some attribute this trend to the skyrocketing of insulin prices in the early 2000s. 37 For context, the cost of a vial of Humalog (Eli Lilly and Company, Indianapolis, Indiana) rose 1000% between 1999 and 2019 to $332 per vial. 38 Given the majority of patients in this study fell into the lowest quartile of household income of their area, a nonzero proportion of admissions were paid out‐of‐pocket, and an average admission costs tens of thousands of dollars, it is probable this subpopulation faced substantial financial barriers to accessing medications that could have otherwise prevented progression of their microvascular disease.
Of note, we also found that this vulnerable subpopulation received invasive treatment less often than those with PAD alone. Surgical and endovascular revascularization rates were >20% lower for patients with PAD and MVD compared with those without MVD, despite these patients presenting more frequently with CLI and thus an indication for invasive treatment under the most recent America Heart Association guidelines. 39 The observed gap in revascularization therapy is likely compounded by patients with diabetes and chronic kidney disease responding less durably to revascularization of femoropopliteal disease. 40 One possible explanation for this difference in treatment might be that those with conditions such as diabetic neuropathy are less likely to notice foot wounds or infection early and are more likely to present with more extensive or irreversible tissue damage that would necessitate amputation without first undergoing revascularization. 41 It is also possible these patients more frequently present with few favorable targets for revascularization therapy, which would not have been captured by this study.
There is increasing recognition of MVD as a systemic process of widespread microvascular dysfunction. 9 Our finding that MVD is independently associated with adverse outcomes in PAD after controlling for diseases such as renal failure and diabetes further supports this paradigm shift, underscoring the finding that this newly recognized population is at a remarkably elevated risk for adverse outcomes upon hospitalization, particularly amputation. The high morbidity and mortality associated with PAD has prompted movements such as the PAD National Action Plan spearheaded by the American Heart Association aimed at enhancing PAD prevention, diagnosis, and treatment. 42 We contend that screening for MVD alongside established comorbidities such as chronic kidney disease and diabetes in patients presenting with PAD could advance these goals by helping risk‐stratify during the shared decision‐making process. Although noninvasive macrovascular tests such as ankle brachial index and pulse volume recording remain a cornerstone of PAD care, providers should consider placing increasing emphasis on toe brachial indexes and transcutaneous oxygen pressures as proxies of microvascular health while assessing patients. While current guidelines recommend evaluating toe brachial indexes in the setting of noncompressible ankle brachial index or in suspected CLI, we contend wider screening may improve risk stratification for mortality, amputations, and readmission within the PAD population. 39 Patients and providers alike would benefit from further education and prospective research into the synergistic role of MVD in PAD. Additionally, exploration of interactions between MVD and other emerging risk factors in PAD, such as frailty and malnutrition, would also enhance our understanding of what drives adverse outcomes in this population.
Limitations
This study had several limitations. The first are those inherent to retrospective study design such as lack of randomization and the potential for confounding. To minimize the chance of confounding, models were adjusted for observed patient factors and comorbidities. Secondly, although the NRD is a powerful research tool, admissions records are not linked to individuals across states or calendar years. Therefore, our readmissions rates are likely underestimates and accurately measuring longer‐term outcomes is not feasible. Thirdly, due to MVD's novelty as a subject of outcomes research, there is not yet an established set of diagnostic codes used to capture the disease. To address this issue, the study derived PAD and MVD codes from previous studies. 4 , 14 , 19 Although these codes encompass MVD in the peripheral nervous system, retina, and nephron, they fail to capture microvascular dysfunction elsewhere in the body. Also as pointed out by Beckman et al, missing MVD diagnoses due to omitted codes would likely be distributed evenly across groups and thus bias any results towards the null. 14
CONCLUSIONS
Our study found more than one quarter of all patients admitted with PAD had comorbid MVD. Between 2011 and 2018, PAD/MVD admissions increased in number and their major and minor amputation rates rose substantially. After controlling for patient factors and other comorbidities, comorbid MVD in patients with PAD was associated with significantly increased risk for major and minor amputation, and to a lesser degree MACE, in‐hospital mortality, and readmission. Increased emphasis on assessing for MVD in addition to other comorbidities during PAD care might aid providers in risk stratification. Further prospective research is needed to phenotype this vulnerable population and their responsiveness to different treatment modalities. Focus should also be placed on uncovering mechanisms behind the increased risk of adverse events in the PAD/MVD population.
Sources of Funding
None.
Disclosures
K.G. Smolderen reports unrestricted grant funding from Johnson & Johnson, Cardiva, Shockwave, and Merck and she is a consultant for Optum Labs, Abbott, and Cook Medical. Dr Mena‐Hurtado is a consultant for Abbott & Cook and has unrestricted grant funding from Abbott, Cardiva, Shockwave, and Merck & Philips. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supporting information
Table S1
This manuscript was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.030710
For Sources of Funding and Disclosures, see page 9.
References
- 1. Song P, Rudan D, Zhu Y, Fowkes FJI, Rahimi K, Fowkes FGR, Rudan I. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. Lancet Glob Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4 [DOI] [PubMed] [Google Scholar]
- 2. Hirsch AT, Hartman L, Town RJ, Virnig BA. National health care costs of peripheral arterial disease in the Medicare population. Vasc Med. 2008;13:209–215. doi: 10.1177/1358863X08089277 [DOI] [PubMed] [Google Scholar]
- 3. Aday AW, Matsushita K. Epidemiology of peripheral artery disease and polyvascular disease. Circ Res. 2021;128:1818–1832. doi: 10.1161/CIRCRESAHA.121.318535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anand SS, Caron F, Eikelboom JW, Bosch J, Dyal L, Aboyans V, Abola MT, Branch KRH, Keltai K, Bhatt DL, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS trial. J Am Coll Cardiol. 2018;71:2306–2315. doi: 10.1016/j.jacc.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 5. Agnelli G, Belch JJF, Baumgartner I, Giovas P, Hoffmann U. Morbidity and mortality associated with atherosclerotic peripheral artery disease: a systematic review. Atherosclerosis. 2020;293:94–100. doi: 10.1016/j.atherosclerosis.2019.09.012 [DOI] [PubMed] [Google Scholar]
- 6. Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0 [DOI] [PubMed] [Google Scholar]
- 7. Climie RE, van Sloten TT, Bruno RM, Taddei S, Empana JP, Stehouwer CDA, Sharman JE, Boutouyrie P, Laurent S. Macrovasculature and microvasculature at the crossroads between type 2 diabetes mellitus and hypertension. Hypertension. 2019;73:1138–1149. doi: 10.1161/HYPERTENSIONAHA.118.11769 [DOI] [PubMed] [Google Scholar]
- 8. Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med. 2020;9:2880. doi: 10.3390/jcm9092880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feuer DS, Handberg EM, Mehrad B, Wei J, Bairey Merz CN, Pepine CJ, Keeley EC. Microvascular dysfunction as a systemic disease: a review of the evidence. Am J Med. 2022;135:1059–1068. doi: 10.1016/j.amjmed.2022.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faselis C, Katsimardou A, Imprialos K, Deligkaris P, Kallistratos M, Dimitriadis K. Microvascular complications of type 2 diabetes mellitus. Curr Vasc Pharmacol. 2020;18:117–124. doi: 10.2174/1570161117666190502103733 [DOI] [PubMed] [Google Scholar]
- 11. Gao S, Zhang H, Long C, Xing Z. Association between obesity and microvascular diseases in patients with type 2 diabetes mellitus. Front Endocrinol (Lausanne). 2021;12:719515. doi: 10.3389/fendo.2021.719515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Querfeld U, Mak RH, Pries AR. Microvascular disease in chronic kidney disease: the base of the iceberg in cardiovascular comorbidity. Clin Sci (Lond). 2020;134:1333–1356. doi: 10.1042/CS20200279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Williams B, Lisheng L, Rodgers A, Mancia G, Neal B, Harrap S, et al. Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care. 2016;39:1796–1803. doi: 10.2337/dc16-0588 [DOI] [PubMed] [Google Scholar]
- 14. Beckman JA, Duncan MS, Damrauer SM, Wells QS, Barnett JV, Wasserman DH, Bedimo RJ, Butt AA, Marconi VC, Sico JJ, et al. Microvascular disease, peripheral artery disease, and amputation. Circulation. 2019;140:449–458. doi: 10.1161/CIRCULATIONAHA.119.040672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agency for Healthcare Research and Quality, Rockville, MD. Overview of the Nationwide Readmissions Database (NRD). Healthcare Cost and Utilization Project: Services DoHH. 2022. Accessed November 9, 2023. https://hcup‐us.ahrq.gov/nrdoverview.jsp.
- 16. Lo RC, Bensley RP, Dahlberg SE, Matyal R, Hamdan AD, Wyers M, Chaikof EL, Schermerhorn ML. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–418.e3. doi: 10.1016/j.jvs.2013.07.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agarwal S, Sud K, Shishehbor MH. Nationwide trends of hospital admission and outcomes among critical limb ischemia patients: from 2003–2011. J Am Coll Cardiol. 2016;67:1901–1913. doi: 10.1016/j.jacc.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 18. Altibi AM, Prousi G, Agarwal M, Shah M, Tripathi B, Ram P, Patel B. Readmission‐free period and in‐hospital mortality at the time of first readmission in acute heart failure patients‐NRD‐based analysis of 40,000 heart failure readmissions. Heart Fail Rev. 2021;26:57–64. doi: 10.1007/s10741-019-09912-z [DOI] [PubMed] [Google Scholar]
- 19. Anantha‐Narayanan M, Doshi RP, Patel K, Sheikh AB, Llanos‐Chea F, Abbott JD, Shishehbor MH, Guzman RJ, Hiatt WR, Duval S, et al. Contemporary trends in hospital admissions and outcomes in patients with critical limb ischemia: an analysis from the National Inpatient Sample Database. Circ Cardiovasc Qual Outcomes. 2021;14:e007539. doi: 10.1161/CIRCOUTCOMES.120.007539 [DOI] [PubMed] [Google Scholar]
- 20. Patel B, Prousi G, Shah M, Secheresiu P, Garg L, Agarwal M, Patil S, Gupta R, Feldman B. Thirty‐day readmission rate in acute heart failure patients discharged against medical advice in a matched cohort study. Mayo Clin Proc. 2018;93:1397–1403. doi: 10.1016/j.mayocp.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 21. Ratliff HT, Shibuya N, Jupiter DC. Minor vs. major leg amputation in adults with diabetes: six‐month readmissions, reamputations, and complications. J Diabetes Complications. 2021;35:107886. doi: 10.1016/j.jdiacomp.2021.107886 [DOI] [PubMed] [Google Scholar]
- 22. Sullivan GM, Feinn R. Using effect size‐or why the P value is not enough. J Grad Med Educ. 2012;4:279–282. doi: 10.4300/JGME-D-12-00156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen J. Statistical power analysis for the behavioral sciences. Vol 2. Routledge; 1988:1–597. doi: 10.4324/9780203771587 [DOI] [Google Scholar]
- 24. Hinks T, Zhou X, Staples K, Dimitrov B, Manta A, Petrossian T, Lum P, Smith C, Ward J, Howarth P, et al. Multidimensional endotypes of asthma: topological data analysis of cross‐sectional clinical, pathological, and immunological data. Lancet. 2015;385(suppl 1):S42. doi: 10.1016/S0140-6736(15)60357-9 [DOI] [PubMed] [Google Scholar]
- 25. Patel N, Chakraborty S, Bandyopadhyay D, Amgai B, Hajra A, Atti V, Das A, Ghosh RK, Deedwania PC, Aronow WS, et al. Association between depression and readmission of heart failure: a national representative database study. Prog Cardiovasc Dis. 2020;63:585–590. doi: 10.1016/j.pcad.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 26. Harrell FE. Cox Proportional Hazards Regression Model. Regression Modeling Strategies: with Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer New York; 2001:465–507. doi: 10.1007/978-1-4757-3462-1_19 [DOI] [Google Scholar]
- 27. Khera R, Angraal S, Couch T, Welsh JW, Nallamothu BK, Girotra S, Chan PS, Krumholz HM. Adherence to methodological standards in research using the National Inpatient Sample. JAMA. 2017;318:2011–2018. doi: 10.1001/jama.2017.17653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Behroozian A, Beckman JA. Microvascular disease increases amputation in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40:534–540. doi: 10.1161/ATVBAHA.119.312859 [DOI] [PubMed] [Google Scholar]
- 29. Mätzke S, Biancari F, Ihlberg L, Albäck A, Kantonen I, Railo M, Lepäntalo M. Increased preoperative c‐reactive protein level as a prognostic factor for postoperative amputation after femoropopliteal bypass surgery for CLI. Ann Chir Gynaecol. 2001;90:19–22. [PubMed] [Google Scholar]
- 30. United States Census Bureau . U.S. Population Up 5.96% Since 2010. Census Interactive Gallery. 2018. Accessed November 9, 2023. https://www.census.gov/library/visualizations/interactive/population‐increase‐2018.html.
- 31. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849 [DOI] [PubMed] [Google Scholar]
- 32. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12:7–11. doi: 10.1016/j.kisu.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lin J, Thompson TJ, Cheng YJ, Zhuo X, Zhang P, Gregg E, Rolka DB. Projection of the future diabetes burden in the United States through 2060. Popul Health Metr. 2018;16:9. doi: 10.1186/s12963-018-0166-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, et al. IDF diabetes atlas: global, regional and country‐level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harris KM, Mena‐Hurtado C, Arham A, Burg MM, Freedland KE, Sinha R, Alabi O, Smolderen KG. Increasing prevalence of critical limb ischemia hospitalizations with distinct mental health burden among younger adults. J Am Coll Cardiol. 2021;78:2126–2128. doi: 10.1016/j.jacc.2021.09.025 [DOI] [PubMed] [Google Scholar]
- 36. Geiss LS, Li Y, Hora I, Albright A, Rolka D, Gregg EW. Resurgence of diabetes‐related nontraumatic lower‐extremity amputation in the young and middle‐aged adult U.S. population. Diabetes Care. 2019;42:50–54. doi: 10.2337/dc18-1380 [DOI] [PubMed] [Google Scholar]
- 37. Caffrey M. Diabetic Amputations May be Rising in the United States. Managed Care & Healthcare Communications; 2018. [Google Scholar]
- 38. Rajkumar SV. The high cost of insulin in the United States: an urgent call to action. Mayo Clin Proc. 2020;95:22–28. doi: 10.1016/j.mayocp.2019.11.013 [DOI] [PubMed] [Google Scholar]
- 39. Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, et al. 2016 AHA/ACC guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty. Factors influencing long‐term success. Circulation. 1991;83:I70–I80. [PubMed] [Google Scholar]
- 41. Pickwell K, Siersma V, Kars M, Apelqvist J, Bakker K, Edmonds M, Holstein P, Jirkovska A, Jude E, Mauricio D, et al. Predictors of lower‐extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852–857. doi: 10.2337/dc14-1598 [DOI] [PubMed] [Google Scholar]
- 42. American Heart Association, Inc. PAD National Action Plan. 2022. Accessed November 9, 2023. Heart.org. https://professional.heart.org/en/science‐news/pad‐national‐action‐plan.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


