Abstract
Background
Rest‐activity rhythms (RARs), a measure of circadian rhythmicity in the free‐living setting, are related to mortality risk, but evidence is limited on associations with cardiovascular disease (CVD) and its risk factors.
Methods and Results
Participants included 4521 adults from the 2013 to 2014 National Health and Nutrition Examination Survey physical activity monitoring examination. Wrist‐worn ActiGraph GT3X+ data were used to estimate RARs. Multivariable logistic models evaluated associations of RARs with prevalent CVD, hypertension, obesity, and central adiposity. Participants (mean age, 49 years) in the highest versus lowest tertile of relative amplitude (greater circadian rhythmicity) had 39% to 62% lower odds of prevalent CVD, hypertension, obesity, and central adiposity. A more active wake period was associated with 19% to 72% lower CVD, hypertension, obesity, and central adiposity odds. Higher interdaily stability (regular sleep‐wake and rest‐activity patterns) was related to 52% and 23% lower CVD and obesity odds, respectively. In contrast, participants in the highest versus lowest tertile of intradaily variability (fragmented RAR and inefficient sleep) had >3‐fold and 24% higher CVD and obesity odds, respectively. A later and less restful sleep period was associated with 36% to 2‐fold higher CVD, hypertension, obesity, and central adiposity odds. A statistically significant linear trend was observed for all associations (P‐trend<0.05).
Conclusions
A robust, stable, and less fragmented RAR, an active wake period, and an earlier and more restful sleep period are associated with lower prevalent CVD, hypertension, obesity, and central adiposity, with evidence of a dose‐response relationship. The magnitude, timing, and regularity of sleep‐wake and rest‐activity patterns may be important targets for reducing cardiovascular risk.
Keywords: cardiovascular disease, central adiposity, circadian rhythmicity, hypertension, obesity, rest‐activity rhythms, sleep
Subject Categories: Cardiovascular Disease; Diabetes, Type 2; Epidemiology; Lifestyle; Risk Factors
Nonstandard Abbreviations and Acronyms
- L5
least active 5‐hour period
- M10
most active 10‐hour period
- NHANES
National Health and Nutrition Examination Survey
- RAR
rest‐activity rhythm
Research Perspective.
What Is New?
It is known that circadian misalignment in highly controlled laboratory settings alters energy homeostasis, promotes weight gain, and increases blood pressure, but there are limited data on how circadian disruption at the population level, which occurs beyond just shift work, is related to cardiovascular risk; the estimation of rest‐activity rhythm characteristics from wrist actigraphy, reflecting sleep‐wake cycles, physical activity levels, and circadian influences, provides insight into circadian rhythmicity and multidimensional sleep‐wake and rest‐activity patterns in the free‐living community setting.
We demonstrate that rest‐activity rhythms indicative of greater overall circadian rhythmicity, a more active wake period, less variability in sleep‐wake and rest‐activity patterns across days, and an earlier, more restful, and efficient sleep period were associated with lower prevalent cardiovascular disease, hypertension, obesity, and central adiposity in US adults, with evidence of a dose‐response relationship.
What Question Should Be Addressed Next?
Studies of intervention programs to improve circadian rhythmicity through modifications of lifestyle, behavioral, and environmental factors should be conducted to shed light on the causal links between circadian disruption, cardiometabolic risk, and cardiovascular disease.
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in the United States and worldwide, with hypertension and excess adiposity being the strongest risk factors contributing to the CVD burden at the population level. 1 , 2 , 3 , 4 Indeed, approximately half of US adults have hypertension, and ≈40% present with obesity. 4 , 5 Furthermore, the prevalence of central adiposity has been steadily increasing over the past 2 decades and is projected to reach ≈56% in men and ≈80% in women by 2030. 6 Lifestyle modification has been the cornerstone of CVD prevention approaches, with lifestyle recommendations, and consequently interventions, traditionally focused on consumption of a healthy diet, achievement of the physical activity guidelines, and, more recently, having a sufficient sleep duration of ≥7 hours and <9 hours. 7 However, the global increase in CVD and its risk factors over the past 3 decades 2 highlights the importance of identifying and targeting novel modifiable risk factors that could enhance existing behavioral prevention approaches.
Emerging data suggest that the alignment of lifestyle behaviors, including sleep‐wake, rest‐activity, and eating‐fasting patterns, with innate circadian rhythms may also play an important role in preserving cardiometabolic health and preventing CVD. 8 , 9 Intervention studies demonstrate that circadian misalignment in highly controlled laboratory settings increases blood pressure (BP) and impacts energy homeostasis, predisposing to weight gain and thereby elevating CVD risk. 9 , 10 , 11 Nevertheless, circadian disruption can also occur in a real‐world setting when lifestyle behaviors are misaligned with innate circadian rhythms. 12 For instance, shift work, particularly night and rotating shift work, represents an extreme model of circadian misalignment in the real world and has been linked to higher risk for CVD, hypertension, and obesity. 13 , 14 , 15 However, circadian disruption at the population level occurs beyond just shift work; nighttime eating and irregular eating timing patterns, later and irregular sleep‐wake patterns, and being active or working later in the day because of individuals' choices or their social constraints have become characteristic of modern society. 16 The impact of circadian misalignment at the population level on CVD and its risk factors is much less studied.
The collection of objective activity and sleep data in population‐based cohorts and the application of rhythmometric statistical approaches to minute‐level wrist actigraphy data collected over multiple consecutive days enables the estimation of 24‐hour rest‐activity patterns and sleep‐wake cycles related to circadian phase. 17 , 18 The resulting rest‐activity rhythm (RAR) variables, representing an innovative measure of circadian rhythmicity in the free‐living setting, enable the investigation of how characteristics of behavioral circadian rhythms contribute to the CVD burden at the population level. 18 , 19 RARs, characterizing the magnitude, timing, and regularity of sleep‐wake and rest‐activity patterns, have not been comprehensively evaluated in relation to CVD and its risk factors, including in nationally representative samples of adults. Thus, the purpose of the present study was to investigate nonparametric RAR variables in relation to odds of prevalent CVD, hypertension, obesity, and central adiposity in a nationally representative cohort of racially and ethnically diverse US adults. We focused on nonparametric RAR variables given that they provide a measure of behavioral circadian rhythms that is translational for public health guidance and lifestyle modification approaches. We hypothesized that RAR characteristics indicative of greater overall circadian rhythmicity would be related to lower odds of CVD and its risk factors.
Methods
Study Design and Population
This was a cross‐sectional study of adults from the 2013 to 2014 National Health and Nutrition Examination Survey (NHANES), who participated in the accelerometry‐based physical activity monitoring examination. 20 NHANES data are available in a public, open access repository: https://www.cdc.gov/nchs/nhanes/index.htm. NHANES, which was conducted by the National Center for Health Statistics of the US Centers for Disease Control and Prevention, is nationally representative, as recruitment is accomplished through a complex, stratified, multistage probability sampling design (counties, segments, households, and individuals) to ensure external validity. 20 In addition to completing the physical activity monitoring, participants completed both in‐home interviews and in‐person mobile examination center visits to provide sociodemographic, lifestyle, and medical history data, a blood sample, and complete anthropometric and BP assessments. Of the total participants in the NHANES 2013 to 2014 physical activity monitoring examination (n=7732), we excluded, in the following order: (1) those aged <20 years (n=2910), (2) those with <3 days of valid accelerometer data to compute the RAR variables (n=235), and (3) pregnant women (n=66). The final analytic sample included 4521 men and women. NHANES is approved by the National Center for Health Statistics Research Ethics Review Board (protocol number 2011‐17). All NHANES participants provide written informed consent before commencing any data collection procedures. 20
Assessment of RARs
Participants in the physical activity monitoring examination wore the ActiGraph GT3X+ accelerometer (Pensacola, FL) on their nondominant wrist continuously across the 24‐hour period for 7 consecutive days. 21 The first and last days of accelerometry data were excluded for each participant because of incomplete 24‐hour periods (from midnight to midnight). Furthermore, those with <3 days of valid accelerometer data were excluded; we considered days with >1 hour of nonwear time during sleep and <10 hours of wear time during wakefulness to be invalid. 22 The actigraph was programmed to record acceleration data on the x, y, or z axis with 80‐Hz sampling frequency. Acceleration measurements from all 3 axes were summed for each minute; these summary measures, specified in Monitor Independent Movement Summary units, a nonproprietary, open‐source, device‐independent universal summary metric, were used. 23
We estimated RAR variables, describing strength, timing, and regularity of sleep and physical activity patterns and representing in part a behavioral manifestation of the circadian function in response to environmental cycles. 18 , 19 , 24 RAR metrics serve as reliable estimates of 24‐hour rest‐activity patterns and sleep‐wake cycles and circadian influences that are generalizable to the US population. Nonparametric rhythmometric analysis was used to estimate RAR variables, 25 , 26 given that nonparametric approaches do not make distributional or functional assumptions on the rest‐activity data and may therefore be superior to the traditionally used cosinor approach in characterizing 24‐hour RARs. 27 The following RAR variables with detailed definitions and interpretation shown in Table 1 were calculated by nonparametric methods and identified pre hoc for the analysis:
Interdaily stability: a measure of day‐to‐day stability in sleep‐wake and rest‐activity patterns, with higher values indicating greater stability across days.
Intradaily variability: a measure of RAR fragmentation across the 24‐hour day, with higher values representing greater rhythm fragmentation typical of frequent napping and inefficient sleep.
Most active 10‐hour period (M10) counts and midpoint: a measure of timing and level of physical activity, with higher M10 counts representing a more active wake period and a later midpoint indicating that activity occurs later on in the day.
Least active 5‐hour period (L5) counts and timing: a measure of restfulness and timing of the sleep period, given that this metric coincides with sleep, with higher L5 counts indicating less restful sleep and a later midpoint indicating a later sleep period.
Relative amplitude: a summary measure of the robustness of the overall RAR, computed from M10 and L5 counts and timing, with higher values indicating greater circadian rhythmicity.
Table 1.
Definitions and Interpretation of Nonparametric 24‐Hour Rest‐Activity Rhythm Variables
| Rest‐activity rhythm variable | Definition | Interpretation |
|---|---|---|
| IS | Measure of sleep‐wake and rest‐activity patterns' regularity across days and synchronization to 24‐h light/dark cycles and environmental cues |
0≤IS≤1 Higher IS indicates less day‐to‐day variability in sleep‐wake and rest‐activity cycles and better synchronization to 24‐h light/dark cycles and other environmental cues that regulate the biological clock (assuming that an individual is awake during the daytime and asleep during the nighttime). |
| IV | Measure of rhythm fragmentation (frequency and extent of transitions between rest and activity) and sleep efficiency |
IV≥0 (IV=0 for perfect sine wave and IV≅2 for Gaussian noise). For example, higher IVs are typically observed among those who nap during the daytime and have more frequent awakenings during the night. |
| M10 timing and counts | Measure of wake time activity and circadian phase |
Higher M10 counts indicate a more active wake period. Higher M10 timing indicates that an individual is active later on in the day. |
| L5 timing and counts | Measure of restfulness of sleep and circadian phase |
Higher L5 counts indicate less restful sleep. Higher L5 timing indicates that bedtime occurs later in the day. |
| Relative amplitude | Measure of 24‐h rhythm robustness and overall circadian rhythmicity derived from timing and activity counts of M10 and L5 | Higher values indicate a more robust 24‐h rest‐activity rhythm and greater overall circadian rhythmicity (ie, higher activity when awake and lower activity at night). |
IS indicates interdaily stability; IV, intradaily variability; L5, least active 5‐hour period; and M10, most active 10‐hour period.
Ascertainment of CVD
A composite CVD outcome that included heart failure, coronary heart disease, angina/angina pectoris, heart attack, and stroke, obtained by self‐report on the medical conditions questionnaire, was created. 28
Assessment of Hypertension
During the mobile examination center medical examination, 4 BP measures were obtained on participants' right arm after they had been resting in a seated position for ≈5 minutes. The average of the first 3 measurements was used for the present analysis, consistent with prior work in NHANES. 28 Hypertension was defined as having systolic BP ≥130 mm Hg or diastolic BP ≥80 mm Hg or self‐reported use of any prescribed drugs to control BP, consistent with the 2017 American College of Cardiology/American Heart Association guidelines. 4
Assessment of Adiposity
Anthropometric measures were collected during the mobile examination center medical examination by trained health technicians. Height was measured using a stadiometer, and weight was assessed using a digital weight scale to calculate body mass index (BMI; kg/m2). Obesity was defined by having a BMI ≥30 kg/m2. Waist circumference measurements were obtained using a measuring tape wrapped just above the iliac crest and rounded to the nearest 0.1 cm. Waist circumference values ≥102 cm for men and ≥88 cm for women were considered to be indicative of central adiposity. 29
Statistical Analysis
Participants' sociodemographic, lifestyle, and clinical characteristics were described using mean±SD for continuous variables and frequencies for categorical variables for the overall sample and by categories of relative amplitude, given that relative amplitude serves as a summary measure of overall RAR robustness (ie, circadian rhythmicity). t‐Tests and χ2 tests were used to determine whether participant characteristics varied significantly among those with relative amplitude greater than versus below the median value. Survey‐weighted univariate and multivariable logistic regression models were used to evaluate tertiles of the RAR variables in relation to odds of prevalent CVD, hypertension, obesity, and central adiposity. The test for linear contrast was used to compute P‐trend values for the detection of a linear trend across the tertiles of RAR variables.
We present, for all associations, results from 3 models, a univariate model (model 1) and 2 multivariable models (model 2 and model 3). Model 2 was adjusted for the following a priori selected covariates: age, sex, race and ethnicity, marital status, education, smoking status, and alcohol use. We additionally considered a model 3, adjusted for all model 2 covariates plus self‐reported sleep disorders. Model 2 was the primary model given that sleep disorders were not objectively assessed, but rather self‐reported in response to the question: “Ever told by doctor have sleep disorder?,” making it unclear what type of sleep disorder the participant is reporting and introducing some measurement error. Furthermore, disrupted RARs may be a consequence or a manifestation of sleep disorders and would thus likely represent partial mediators of the relation between sleep disorders and cardiovascular risk. 30 P<0.05 was considered statistically significant. Statistical analyses were performed using R, version 3.6.3, and all analyses accounted for the complex stratified survey design of NHANES.
Results
Study Population Characteristics
The weighted sociodemographic, lifestyle, clinical, and RAR characteristics of the study population are shown in Table 2. The average age of participants was 49±17 years, 53% were women, and 64% reported being married or living with a partner and an education level equivalent to some college and above. In terms of the racial and ethnic distribution, 5% were Asian, 11% were Black, 15% were Hispanic, and 67% were non‐Hispanic White. The prevalence of CVD and hypertension in the overall sample was 10% and 43%, respectively, whereas that of obesity and central adiposity was 39% and 57%, respectively.
Table 2.
Descriptive Characteristics of the Overall Study Population and by RA Categories
| Characteristics | Overall sample (n=4521) | RA below median (n=2260) | RA above median (n=2261) | P value |
|---|---|---|---|---|
| Age, y | 48.7 (16.7) | 49.6 (17.6) | 47.0 (16.4) | <0.001 |
| Female sex, % | 52.9 | 51.0 | 54.5 | 0.096 |
| Race and ethnicity, % | ||||
| Asian | 4.8 | 4.8 | 4.8 | <0.001 |
| Black | 11 | 16.5 | 6.7 | |
| Hispanic | 14.5 | 12.6 | 16.2 | |
| White | 66.8 | 62.3 | 70.2 | |
| Married/living with partner, % | 63.9 | 56.3 | 68.6 | <0.001 |
| Education level of some college and above, % | 63.6 | 61.9 | 64.5 | 0.288 |
| Current smoker, % | 19.3 | 24 | 15.8 | <0.001 |
| Alcohol, No. of drinks on drinking days | 2.5 (2.1) | 2.6 (2.2) | 2.5 (2.1) | 0.250 |
| Self‐reported sleep disorder, % | 10.8 | 12 | 9.6 | 0.092 |
| BMI, kg/m2 | 29.4 (7.1) | 30.5 (7.7) | 28.4 (6.4) | <0.001 |
| Obesity, % | 39.2 | 45.2 | 33.5 | <0.001 |
| Waist circumference, cm | 100.1 (16.6) | 103.1 (17.8) | 97.2 (15.1) | <0.001 |
| Central adiposity, % | 56.8 | 62.4 | 50.8 | <0.001 |
| Systolic blood pressure, mm Hg | 121.3 (16.4) | 123.4 (17.3) | 119.3 (15.1) | <0.001 |
| Diastolic blood pressure, mm Hg | 69.5 (11.9) | 69.6 (13.1) | 69.1 (11.1) | 0.211 |
| Hypertension, % | 43.3 | 49.6 | 36.7 | <0.001 |
| Cardiovascular disease, % | 9.5 | 13.4 | 5.9 | <0.001 |
| Interdaily stability | 0.64 (0.16) | 0.58 (0.16) | 0.68 (0.15) | <0.001 |
| Intradaily variability | 0.69 (0.25) | 0.76 (0.25) | 0.63 (0.24) | <0.001 |
| M10 midpoint | 14.16 (2.72) | 14.48 (2.99) | 13.92 (2.47) | <0.001 |
| M10 counts | 497.17 (169.59) | 455.74 (153.98) | 532.67 (174.95) | <0.001 |
| L5 midpoint | 3.89 (3.67) | 4.41 (4.04) | 3.47 (3.23) | <0.001 |
| L5 counts | 36.82 (37.39) | 59.75 (45.35) | 17.97 (9.5) | <0.001 |
| RA | 0.86 (0.12) | 0.77 (0.13) | 0.94 (0.03) | <0.001 |
Data are given as mean (SD) unless otherwise indicated. RA median value was 0.89. χ2 Tests were used to compare frequencies for categorical variables, and t‐tests were used to compare means for continuous variables. BMI indicates body mass index; L5, least active 5‐hour period; M10, most active 10‐hour period; and RA, relative amplitude.
When participant characteristics were compared by categories of relative amplitude, those with relative amplitude greater than the median value (0.89), indicative of greater circadian rhythmicity, were more likely to be younger, married, and nonsmokers and more likely to report Hispanic or non‐Hispanic White race and ethnicity. Furthermore, participants with relative amplitude greater than the median value had significantly lower prevalence of CVD (6% versus 13%; P<0.001), hypertension (37% versus 50%; P<0.001), obesity (34% versus 45%; P<0.001), and central adiposity (51% versus 62%; P<0.001). Notably, participants with relative amplitude above the median also had a more favorable overall RAR profile characterized by higher interdaily stability and M10 counts, lower intradaily variability and L5 counts, and an earlier timing of the M10 and L5 periods in the 24‐hour day.
RARs and CVD
In multivariable models (model 2), those in the highest versus lowest tertile of interdaily stability, M10 counts, and relative amplitude, indicative of greater day‐to‐day stability in sleep‐wake and rest‐activity patterns, a more active wake period, and greater circadian rhythmicity, had 52%, 72%, and 62% lower odds of prevalent CVD, respectively (Figure). In contrast, participants in the highest versus lowest tertile of intradaily variability, indicative of a more fragmented RAR and inefficient sleep, had >3‐fold greater odds of CVD. Furthermore, those with a later L5 midpoint and higher L5 counts, representing a later, less restful sleep period, had 70% and 59% higher odds of CVD, respectively. A statistically significant linear trend demonstrating a dose‐response relationship was observed across tertiles of these RAR characteristics (P<0.05). Null associations were observed for the M10 midpoint in relation to CVD. Additional adjustment for sleep disorders (model 3) did not alter any of these associations (Table S1).
Figure . Multivariable associations of 24‐hour rest‐activity rhythms and cardiovascular disease (n=4521).
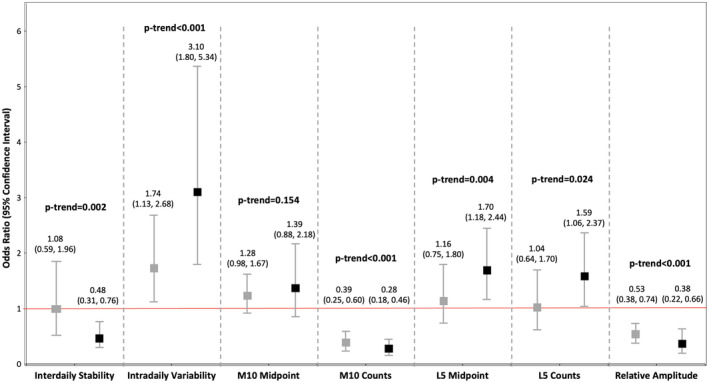
This figure shows multivariable associations of rest‐activity rhythm characteristics with prevalent cardiovascular disease. Tertiles of nonparametric rest‐activity rhythm variables were evaluated in relation to odds of cardiovascular disease. Gray plots represent odds ratio (95% CI) of participants in tertile 2 vs tertile 1, whereas black plots represent odds ratio (95% CI) of participants in tertile 3 vs tertile 1. Logistic regression models were adjusted for age, sex, race and ethnicity, marital status, education, smoking, and alcohol use. Additional adjustment for self‐reported sleep disorders did not alter statistical significance of any associations (Table S1). P‐trend was computed using the linear contrast method, and P‐trend<0.05 was considered indicative of a dose‐response relationship across the tertile categories of rest‐activity rhythm variables. L5 indicates least active 5‐hour period; and M10, most active 10‐hour period.
RARs and Hypertension
Being in the highest versus lowest tertile of M10 counts and relative amplitude was associated with 19% and 39% lower odds of hypertension, respectively (P‐trend=0.032 and P‐trend=0.002, respectively). In contrast, being in the highest versus lowest tertile of L5 counts was associated with 59% higher odds of hypertension (P‐trend=0.007) (Table 3). Null associations were observed for all other RAR characteristics in multivariable models. Associations were similar after additional adjustment for sleep disorders.
Table 3.
Multivariable Associations of 24‐Hour RAR Variables and Hypertension (n=4521)
| 24‐h Rest‐activity rhythm variables | Model 1 OR (95% CI)* | Model 2 OR (95% CI)† | Model 3 OR (95% CI)‡ |
|---|---|---|---|
| Interdaily stability | |||
| <0.58 | 1.00 | 1.00 | 1.00 |
| 0.58–0.73 | 1.16 (1.0–1.34) | 0.92 (0.78–1.09) | 0.92 (0.78–1.09) |
| >0.73 | 1.19 (1.07–1.33) | 0.93 (0.72–1.19) | 0.94 (0.73–1.21) |
| P‐trend | 0.002 | 0.565 | 0.613 |
| Intradaily variability | |||
| <0.56 | 1.00 | 1.00 | 1.00 |
| 0.56–0.77 | 1.25 (1.01–1.54) | 1.10 (0.87–1.39) | 1.09 (0.85–1.38) |
| >0.77 | 1.5 (1.19–1.89) | 0.98 (0.69–1.40) | 0.94 (0.66–1.35) |
| P‐trend | <0.001 | 0.921 | 0.754 |
| M10 midpoint | |||
| <12.82 | 1.00 | 1.00 | 1.00 |
| 12.82–15.15 | 1.02 (0.87–1.20) | 1.07 (0.83–1.39) | 1.08 (0.84–1.4) |
| >15.15 | 0.55 (0.48–0.63) | 0.97 (0.81–1.17) | 0.96 (0.80–1.16) |
| P‐trend | <0.001 | 0.782 | 0.699 |
| M10 counts | |||
| <413 | 1.00 | 1.00 | 1.00 |
| 413–557 | 0.71 (0.6–0.84) | 1.08 (0.88–1.32) | 1.09 (0.89–1.35) |
| >557 | 0.45 (0.40–0.51) | 0.81 (0.66–0.98) | 0.83 (0.69–1.00) |
| P‐trend | <0.001 | 0.032 | 0.055 |
| L5 midpoint | |||
| <2.6 | 1.00 | 1.00 | 1.00 |
| 2.6–3.98 | 0.83 (0.72–0.96) | 0.88 (0.75–1.03) | 0.89 (0.75–1.04) |
| >3.98 | 0.85 (0.69–1.04) | 1.16 (0.93–1.45) | 1.14 (0.90–1.43) |
| P‐trend | 0.117 | 0.189 | 0.280 |
| L5 counts | |||
| <21 | 1.00 | 1.00 | 1.00 |
| 21–39 | 1.25 (1.05–1.49) | 1.39 (1.04–1.84) | 1.38 (1.03–1.85) |
| >39 | 1.43 (1.17–1.74) | 1.59 (1.13–2.24) | 1.57 (1.10–2.24) |
| P‐trend | <0.001 | 0.007 | 0.013 |
| Relative amplitude | |||
| <0.85 | 1.00 | 1.00 | 1.00 |
| 0.85–0.92 | 0.80 (0.71–0.92) | 0.87 (0.65–1.15) | 0.88 (0.65–1.19) |
| >0.92 | 0.50 (0.43–0.58) | 0.61 (0.44–0.84) | 0.62 (0.44–0.88) |
| P‐trend | <0.001 | 0.002 | 0.007 |
Risk estimates are shown from logistic regression models, comparing odds of hypertension among those in the highest vs lowest tertile of each RAR variable. L5 indicates least active 5‐hour period; M10, most active 10‐hour period; OR, odds ratio; and RAR, rest‐activity rhythm.
Model 1 represents univariate associations.
Model 2 is adjusted for age, sex, marital status, education, race and ethnicity, smoking, and alcohol use.
Model 3 is adjusted for model 2 covariates and additionally for sleep disorders.
RARs and Adiposity
In multivariable models (model 2), being in the highest versus lowest tertile of relative amplitude (ie, greater overall circadian rhythmicity) was associated with 55% and 53% lower odds of obesity and central adiposity, respectively (Tables 4 and 5). Similarly, participants in the highest versus lowest tertile of M10 counts (more active wake period) had 58% and 45% lower odds of these outcomes, respectively, whereas those in the highest versus lowest tertile of interdaily stability (ie, greater invariability across days) had 23% lower odds of obesity only. A statistically significant linear trend was observed for all these associations (P‐trend<0.001).
Table 4.
Multivariable Associations of 24‐Hour RAR Variables and Obesity (n=4521)
| 24‐h Rest‐activity rhythm variables | Model 1 OR (95% CI)* | Model 2 OR (95% CI)† | Model 3 OR (95% CI)‡ |
|---|---|---|---|
| Interdaily stability | |||
| <0.58 | 1.00 | 1.00 | 1.00 |
| 0.58–0.73 | 0.95 (0.83–1.09) | 0.93 (0.73–1.17) | 0.93 (0.76–1.14) |
| >0.73 | 0.85 (0.73–0.98) | 0.77 (0.67–0.88) | 0.79 (0.69–0.90) |
| P‐trend | 0.026 | <0.001 | <0.001 |
| Intradaily variability | |||
| <0.56 | 1.00 | 1.00 | 1.00 |
| 0.56–0.77 | 0.95 (0.79–1.15) | 0.94 (0.75–1.17) | 0.9 (0.72–1.13) |
| >0.77 | 1.16 (0.99–1.36) | 1.24 (1.05–1.47) | 1.12 (0.93–1.34) |
| P‐trend | 0.063 | 0.014 | 0.234 |
| M10 midpoint | |||
| <12.82 | 1.00 | 1.00 | 1.00 |
| 12.82–15.15 | 0.81 (0.67–0.99) | 0.90 (0.69–1.17) | 0.92 (0.7–1.21) |
| >15.15 | 0.87 (0.73–1.05) | 1.08 (0.82–1.42) | 1.05 (0.81–1.35) |
| P‐trend | 0.151 | 0.579 | 0.737 |
| M10 counts | |||
| <413 | 1.00 | 1.00 | 1.00 |
| 413–5.57 | 0.89 (0.78–1.02) | 0.80 (0.65–0.99) | 0.84 (0.67–1.05) |
| >557 | 0.54 (0.47–0.63) | 0.42 (0.34–0.52) | 0.46 (0.36–0.58) |
| P‐trend | <0.001 | <0.001 | <0.001 |
| L5 midpoint | |||
| <2.6 | 1.00 | 1.00 | 1.00 |
| 2.6–3.98 | 1.04 (0.88–1.24) | 1.07 (0.87–1.31) | 1.08 (0.87–1.33) |
| >3.98 | 1.12 (0.97–1.29) | 1.36 (1.11–1.66) | 1.28 (1.02–1.60) |
| P‐trend | 0.135 | 0.003 | 0.032 |
| L5 counts | |||
| <21 | 1.00 | 1.00 | 1.00 |
| 21–39 | 1.33 (1.09–1.62) | 1.37 (1.12–1.67) | 1.39 (1.14–1.68) |
| >39 | 1.64 (1.44–1.86) | 1.72 (1.35–2.18) | 1.67 (1.33–2.08) |
| P‐trend | <0.001 | <0.001 | <0.001 |
| Relative amplitude | |||
| <0.85 | 1.00 | 1.00 | 1.00 |
| 0.85–0.92 | 0.74 (0.61–0.91) | 0.71 (0.55–0.92) | 0.74 (0.57–0.94) |
| >0.92 | 0.51 (0.42–0.62) | 0.45 (0.34–0.60) | 0.47 (0.35–0.63) |
| P‐trend | <0.001 | <0.001 | <0.001 |
Risk estimates are shown from logistic regression models, comparing odds of obesity among those in the highest vs lowest tertile of each RAR variable. L5 indicates least active 5‐hour period; M10, most active 10‐hour period; OR, odds ratio; and RAR, rest‐activity rhythm.
Model 1 represents univariate associations.
Model 2 is adjusted for age, sex, marital status, education, race and ethnicity, smoking, and alcohol use.
Model 3 is adjusted for model 2 covariates and additionally for sleep disorders.
Table 5.
Multivariable Associations of 24‐Hour RAR Variables and Central Adiposity (n=4521)
| 24‐h Rest‐activity rhythm variables | Model 1 OR (95% CI)* | Model 2 OR (95% CI)† | Model 3 OR (95% CI)‡ |
|---|---|---|---|
| Interdaily stability | |||
| <0.58 | 1.00 | 1.00 | 1.00 |
| 0.58–0.73 | 1.20 (0.97–1.49) | 1.00 (0.74–1.34) | 1.01 (0.75–1.35) |
| >0.73 | 1.17 (1.02–1.35) | 0.92 (0.78–1.09) | 0.94 (0.81–1.10) |
| P‐trend | 0.027 | 0.348 | 0.473 |
| Intradaily variability | |||
| <0.56 | 1.00 | 1.00 | 1.00 |
| 0.56–0.77 | 1.21 (0.97–1.50) | 1.08 (0.81–1.44) | 1.06 (0.79–1.42) |
| >0.77 | 1.17 (0.99–1.37) | 1.05 (0.87–1.27) | 0.98 (0.8–1.18) |
| P‐trend | 0.059 | 0.618 | 0.799 |
| M10 midpoint | |||
| <12.82 | 1.00 | 1.00 | 1.00 |
| 12.82–15.15 | 1.00 (0.85–1.19) | 1.08 (0.85–1.38) | 1.10 (0.86–1.42) |
| >15.15 | 0.80 (0.66–0.97) | 1.02 (0.74–1.42) | 1.00 (0.73–1.36) |
| P‐trend | 0.024 | 0.890 | 0.988 |
| M10 counts | |||
| <413 | 1.00 | 1.00 | 1.00 |
| 413–557 | 0.90 (0.78–1.04) | 0.76 (0.59–0.97) | 0.78 (0.61–1.00) |
| >557 | 0.68 (0.59–0.78) | 0.55 (0.44–0.68) | 0.58 (0.47–0.72) |
| P‐trend | <0.001 | <0.001 | <0.001 |
| L5 midpoint | |||
| <2.6 | 1.00 | 1.00 | 1.00 |
| 2.6–3.98 | 0.94 (0.81–1.09) | 0.88 (0.73–1.05) | 0.88 (0.73–1.07) |
| >3.98 | 0.91 (0.76–1.09) | 1.18 (0.91–1.52) | 1.12 (0.86–1.47) |
| P‐trend | 0.306 | 0.210 | 0.395 |
| L5 counts | |||
| <21 | 1.00 | 1.00 | 1.00 |
| 21–39 | 1.48 (1.29–1.71) | 1.60 (1.33–1.93) | 1.61 (1.31–1.97) |
| >39 | 1.70 (1.38–2.08) | 2.00 (1.55–2.58) | 1.96 (1.53–2.50) |
| P‐trend | <0.001 | <0.001 | <0.001 |
| Relative amplitude | |||
| <0.85 | 1.00 | 1.00 | 1.00 |
| 0.85–0.92 | 0.95 (0.7–1.29) | 0.91 (0.63–1.31) | 0.94 (0.65–1.35) |
| >0.92 | 0.56 (0.45–0.70) | 0.47 (0.36–0.61) | 0.49 (0.37–0.63) |
| P‐trend | <0.001 | <0.001 | <0.001 |
Risk estimates are shown from logistic regression models, comparing odds of central adiposity among those in the highest vs lowest tertile of each RAR variable. L5 indicates least active 5‐hour period; M10, most active 10‐hour period; OR, odds ratio; and RAR, rest‐activity rhythm.
Model 1 represents univariate associations.
Model 2 is adjusted for age, sex, marital status, education, race and ethnicity, smoking, and alcohol use.
Model 3 is adjusted for model 2 covariates and additionally for sleep disorders.
In contrast, greater L5 counts were associated with 72% and 2‐fold greater odds of obesity and central adiposity, respectively (P‐trend<0.001). Higher intradaily variability and L5 midpoint, indicating greater rhythm fragmentation and a later sleep period, were additionally associated with 24% and 36% greater odds of obesity (P‐trend<0.05), respectively, but no associations were observed with central adiposity. M10 timing was not associated with either outcome. All associations persisted after adjustment for sleep disorders, with the exception of the association between intradaily variability and obesity, which was no longer statistically significant (P=0.234).
Discussion
In this sample of nationally representative US adults, greater overall circadian rhythmicity, an active wake period, invariability in sleep‐wake and rest‐activity patterns across days, and an earlier, restful, and more efficient sleep period were associated with lower prevalent CVD, hypertension, obesity, and central adiposity, with evidence of a dose‐response relationship. To our knowledge, this is the first study to systematically investigate nonparametric RARs, assessed in a real‐life setting using a large, representative sample of US adults, in relation to CVD and its risk factors. Although we cannot determine the directionality of the associations in this cross‐sectional study, these findings, in light of prior experimental work on circadian misalignment, suggest that reduced circadian rhythmicity may contribute to the CVD burden at the population level in US adults, and that RAR characteristics may play a role in the pathogenesis of CVD, hypertension, and excess adiposity.
There are no prior studies of nonparametric RAR variables in relation to CVD in a nationally representative cohort of US adults. However, 1 prior study investigated RAR variables from extended cosine models in relation to incident CVD in older US men (mean age, 76 years) who participated in the MrOS (Osteoporotic Fractures in Men) Study. 31 In the MrOS Study, decreased circadian activity rhythm robustness, represented by reduced amplitude and greater minimum activity, was associated with up to a 33% increased risk of CVD events. Furthermore, a dampened RAR, estimated from extended cosine models, has been linked to >2‐fold greater CVD mortality risk in older adults from the MrOS Study and the SOF (Study of Osteoporotic Fractures). 32 , 33 Collectively, these results are in line with our findings that reduced overall circadian rhythmicity and a dampened RAR are associated with higher odds of prevalent CVD, but we further demonstrate robust associations between other RAR characteristics and CVD.
There are limited studies on RARs in relation to hypertension and adiposity. 19 , 34 , 35 In the cross‐sectional Hispanic Community Health Study/Study of Latinos Sueno sleep ancillary study of ≈2000 adults, encompassing different life stages (mean age, 47 years), those with hypertension had lower interdaily stability, but associations of interdaily stability and sleep timing with hypertension prevalence and BMI were not significant in multivariable models. 34 In contrast, in the Rush Memory and Aging cohort of ≈1000 older adults (mean age, 82 years), having higher interdaily stability was associated with 22% and 27% lower odds of prevalent hypertension and obesity, respectively. 35 Furthermore, in a predominantly female (≈80%) sample of 578 community‐dwelling adults (mean age, 52 years), participants in the lowest versus highest quintile of relative amplitude had 3 kg/m2 higher BMI, but null results were reported for other RAR variables. 19 It is possible that we observed no association between interdaily stability and hypertension, consistent with the HCHS/SOL but not the Rush cohort, due to the similar age distribution, as associations of sleep regularity with BP may be more pronounced in older adults. 36 Our result that lower overall rhythmicity is related to prevalent obesity is consistent with the previous finding of an inverse association between relative amplitude and BMI. However, we additionally demonstrate that other RAR characteristics are related to obesity; the null associations in prior work may be ascribed to the modest sample size, resulting in limited statistical power to detect associations. 19
The association between RARs and CVD is biologically plausible, given the observed relations between disrupted RARs and individual CVD risk factors observed here. It is likely that excess adiposity and hypertension may lie in the causal pathway of the association between RARs and CVD. However, although all RAR characteristics, with the exception of M10 timing, were associated with CVD, differential associations were observed with hypertension and central adiposity. On the other hand, associations of RAR with obesity mirrored those with CVD, but additional adjustment of multivariable models on RARs in relation to CVD for BMI did not alter the statistical significance of findings (Table S1), suggesting that there are likely additional mechanisms at play.
In addition to being linked to excess adiposity and hypertension, dampened RARs are associated with metabolic syndrome, diabetes, and dyslipidemia, which are also CVD risk factors. 35 , 37 , 38 For instance, in adults without type 2 diabetes, who participated in the NHANES physical activity monitoring examination, lower relative amplitude and higher intradaily variability were related to impaired glucose tolerance. 38 Furthermore, those with the weakest rhythmicity based on cosine model analysis had >2 times greater odds of diabetes. 37 Finally, in the Rush Memory and Aging cohort, lower interdaily stability was linked to 24%, 31%, and 18% higher odds of diabetes, metabolic syndrome, and dyslipidemia, respectively, highlighting additional pathways through which RARs could influence CVD risk. 35
Lastly, our findings are consistent with intervention studies providing causal evidence that forced misalignment of behavioral and environmental cycles, including sleep‐wake cycles, with endogenous circadian rhythms increases blood pressure, blood glucose, and inflammatory markers (eg, interleukin‐6, CRP [C‐reactive protein], and tumor necrosis factor‐α) and reduces insulin sensitivity, contributing to greater risk of developing CVD and hypertension. 9 , 39 , 40 Circadian misalignment in experimental studies has also been linked to higher levels of ghrelin, reduced leptin, and increased appetite for energy‐dense foods predisposing to obesity. 40 , 41 Our finding that a more active wake period is related to lower CVD, hypertension, and obesity risk is also consistent with the health effects of interventions aimed at increasing daytime physical activity through implementing physical activity bouts into daily routines, reducing sedentary time, and increasing adherence to physical activity guidelines. 42 , 43 , 44 , 45 These interventions are effective at improving blood pressure, blood glucose, BMI, and low‐density lipoprotein, while also having positive effects on psychosocial CVD risk factors, such as stress and mood. 42 , 43 , 44 , 45
Strengths of this study include the population‐based nature of the study cohort, which was nationally representative and diverse in terms of age, race, and ethnicity, as the limited prior studies on RARs were conducted primarily in geographically restricted samples or with small sample sizes, limiting generalizability of findings. The use of objectively assessed sleep, rest, and activity data from accelerometry, high device‐wearing compliance by participants (>90% with at least 6 days of monitoring), and rigorous approach for assessing data validity reduces the chances of measurement error. Furthermore, the use of nonparametric rhythmometric methods to estimate RAR variables makes fewer assumptions about the distribution of the data, resulting in estimates of the amplitude of circadian oscillations that are biologically relevant to cardiovascular outcomes 19 and that are easier to interpret with translational applications for lifestyle guidelines and interventions in public health settings. Finally, weight, height, waist circumference, and BP were measured in mobile examination clinics by trained staff using gold standard approaches, minimizing the potential for measurement error for these outcomes. In sum, the high‐quality sampling and rigorous data collection procedures of NHANES enhance internal and external validity of our study's findings.
Nevertheless, our study has some limitations that should be considered when interpreting the results. First, the cross‐sectional study design prohibits the establishment of temporality and limits the ability to understand any potential causal associations between blunted RARs and CVD risk. Reverse causality is also possible but unlikely, given that several plausible biological mechanisms underlie the association of reduced circadian rhythmicity or circadian misalignment, poor sleep, and a less active wake period with cardiometabolic risk factors and CVD and the consistency of our findings with prior work, as outlined above. 9 , 39 , 40 Second, the composite CVD outcome was based on self‐report and is, therefore, prone to measurement error. Third, we did not adjust for type 1 error, so some findings may be attributable to chance; however, given the robustness of our findings, most associations would persist even after adjustment for multiple comparisons. Fourth, we did not have sufficient power for subgroup analyses, given that CVD was a primary outcome. Fifth, although we adjusted for relevant sociodemographic and behavioral factors, our results are prone to residual confounding, particularly by unmeasured environmental factors and aspects of endogenous circadian rhythms. Related to that, although RARs are a measure of circadian rhythmicity in the free‐living setting, they do not directly measure physiological circadian rhythms. Nevertheless, prior work has shown that 24‐hour RARs are relevant measures of circadian rhythmicity linked to the biological measures of chronodisruption, as fragmented RARs have been linked to declines in the amplitude of melatonin secretion. 46
Conclusions
We demonstrate that weakened RARs are associated with CVD and its major risk factors, hypertension, obesity, and central adiposity. These findings contribute novel evidence to a growing body of literature suggesting that RARs may play a key role in cardiometabolic health preservation and CVD prevention. Notably, the strongest associations were observed for relative amplitude, the summary measure of overall circadian rhythmicity, and for M10 counts, reflecting physical activity levels, whereas no association was observed for M10 timing, indicating that greater physical activity levels may lower CVD risk regardless of their timing in the 24‐hour day. Longitudinal observational studies are needed to illuminate the temporal sequence of circadian disruption, cardiometabolic dysfunction, and CVD and investigate their complex associations across the life course and by sex, race, and ethnicity. Furthermore, although investigation of RARs in relation to subclinical CVD (eg, coronary artery calcium and endothelial dysfunction) is needed to elucidate additional mechanisms underlying the RAR and CVD link, the magnitude, timing, and regularity of sleep‐wake and rest‐activity patterns may represent an important target for reducing cardiovascular risk in adults. Ultimately, studies of intervention programs to improve circadian rhythmicity through modifications of lifestyle, behavioral, and environmental factors should be conducted to shed light on the causal links between circadian disruption, cardiometabolic risk, and CVD.
Sources of Funding
N.M. is supported by National Heart, Lung, and Blood Institute grant R00‐HL148511, American Heart Association grant 855050, and National Institute on Minority Health and Health Disparities grant P50MD017341 (subproject identifier: 8126). A.S. is supported by National Heart, Lung, and Blood Institute grants R01HL141494, R01HL157341, and R01HL146911. The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Disclosures
None.
Supporting information
Table S1
This article was sent to Tiffany M. Powell‐Wiley, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.032073
For Sources of Funding and Disclosures, see page 11.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberliain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883 [DOI] [PubMed] [Google Scholar]
- 4. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 5. Hales C, Carroll M, Fryar C, Ogden C. Prevalence of obesity and severe obesity among adults: United States, 2017‐2018. NCHS Data Brief. 2020;360:1–8. [PubMed] [Google Scholar]
- 6. Wang Y, Beydoun MA, Min J, Xue H, Kaminsky LA, Cheskin LJ. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int J Epidemiol. 2020;49:810–823. doi: 10.1093/ije/dyz273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lloyd‐Jones DM, Allen NB, Anderson CA, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, et al. Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thosar SS, Butler MP, Shea SA. Role of the circadian system in cardiovascular disease. J Clin Invest. 2018;128:2157–2167. doi: 10.1172/JCI80590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci. 2016;113:E1402–E1411. doi: 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qian J, Morris CJ, Caputo R, Wang W, Garaulet M, Scheer FA. Sex differences in the circadian misalignment effects on energy regulation. Proc Natl Acad Sci USA. 2019;116:23806–23812. doi: 10.1073/pnas.1914003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHill AW, Melanson EL, Higgins J, Connick E, Moehlman TM, Stothard ER, Wright KP. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci USA. 2014;111:17302–17307. doi: 10.1073/pnas.1412021111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abbott SM, Malkani RG, Zee PC. Circadian disruption and human health: a bidirectional relationship. Eur J Neurosci. 2020;51:567–583. doi: 10.1111/ejn.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang D, Ruan W, Chen Z, Peng Y, Li W. Shift work and risk of cardiovascular disease morbidity and mortality: a dose–response meta‐analysis of cohort studies. Eur J Prev Cardiol. 2018;25:1293–1302. doi: 10.1177/2047487318783892 [DOI] [PubMed] [Google Scholar]
- 14. Makarem N, Alcántara C, Williams N, Bello NA, Abdalla M. Effect of sleep disturbances on blood pressure. Hypertension. 2021;77:1036–1046. doi: 10.1161/HYPERTENSIONAHA.120.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Q, Shi J, Duan P, Liu B, Li T, Wang C, Li H, Yang T, Gan Y, Wang X, et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta‐analysis. Int J Epidemiol. 2018;47:1956–1971. doi: 10.1093/ije/dyy079 [DOI] [PubMed] [Google Scholar]
- 16. Chellappa SL, Vujovic N, Williams JS, Scheer FA. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol Metab. 2019;30:767–779. doi: 10.1016/j.tem.2019.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ancoli‐Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 18. Mitchell JA, Quante M, Godbole S, James P, Hipp JA, Marinac CR, Mariani S, Cespedes Feliciano EM, Glanz K, Laden F, et al. Variation in actigraphy‐estimated rest‐activity patterns by demographic factors. Chronobiol Int. 2017;34:1042–1056. doi: 10.1080/07420528.2017.1337032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cespedes Feliciano EM, Quante M, Weng J, Mitchell JA, James P, Marinac CR, Mariani S, Redline S, Kerr J, Godbole S, et al. Actigraphy‐derived daily rest–activity patterns and body mass index in community‐dwelling adults. Sleep. 2017;40:zsx168. doi: 10.1093/sleep/zsx168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention . National Health and nutrition examination survey 2018. NHANES Questionnaires, Datasets, and Related Documentation.
- 21. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3 [DOI] [PubMed] [Google Scholar]
- 22. Jefferis BJ, Parsons TJ, Sartini C, Ash S, Lennon LT, Papacosta O, Morris RW, Wannamethee SG, Lee I, Whincup PH. Objectively measured physical activity, sedentary behaviour and all‐cause mortality in older men: does volume of activity matter more than pattern of accumulation? Br J Sports Med. 2019;53:1013–1020. doi: 10.1136/bjsports-2017-098733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. John D, Tang Q, Albinali F, Intille S. An open‐source monitor‐independent movement summary for accelerometer data processing. J Measur Phys Behav. 2019;2:268–281. doi: 10.1123/jmpb.2018-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, Somers VK, Lopez‐Jimenez F, Di J, Covassin N. Demographic characteristics associated with circadian rest‐activity rhythm patterns: a cross‐sectional study. Int J Behav Nutr Phys Act. 2021;18:107. doi: 10.1186/s12966-021-01174-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest‐activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int. 1999;16:505–518. doi: 10.3109/07420529908998724 [DOI] [PubMed] [Google Scholar]
- 26. Gonçalves BS, Cavalcanti PR, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: an update. Sleep Sci. 2014;7:158–164. doi: 10.1016/j.slsci.2014.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju Y‐ES. Circadian rest‐activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol. 2018;75:582–590. doi: 10.1001/jamaneurol.2017.4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Makarem N, Alcantara C, Musick S, Quesada O, Sears DD, Chen Z, Tehranifar P. Multidimensional sleep health is associated with cardiovascular disease prevalence and cardiometabolic health in US adults. Int J Environ Res Public Health. 2022;19:10749. doi: 10.3390/ijerph191710749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. National Heart, Lung and Blood Institute . Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks. 2022.
- 30. Roh HW, Choi SJ, Jo H, Kim D, Choi J, Son SJ, Joo EY. Associations of actigraphy derived rest activity patterns and circadian phase with clinical symptoms and polysomnographic parameters in chronic insomnia disorders. Sci Rep. 2022;12:1–9. doi: 10.1038/s41598-022-08899-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paudel ML, Taylor BC, Ancoli‐Israel S, Stone KL, Tranah G, Redline S, Barrett‐Connor E, Stefanick ML, Ensrud KE. Rest/activity rhythms and cardiovascular disease in older men. Chronobiol Int. 2011;28:258–266. doi: 10.3109/07420528.2011.553016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tranah GJ, Blackwell T, Ancoli‐Israel S, Paudel ML, Ensrud KE, Cauley JA, Redline S, Hillier TA, Cummings SR, Stone KL. Circadian activity rhythms and mortality: the study of osteoporotic fractures. J Am Geriatr Soc. 2010;58:282–291. doi: 10.1111/j.1532-5415.2009.02674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paudel ML, Taylor BC, Ancoli‐Israel S, Blackwell T, Stone KL, Tranah G, Redline S, Cummings SR, Ensrud KE. Rest/activity rhythms and mortality rates in older men: MrOS sleep study. Chronobiol Int. 2010;27:363–377. doi: 10.3109/07420520903419157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abbott SM, Weng J, Reid KJ, Daviglus ML, Gallo LC, Loredo JS, Nyenhuis SM, Ramos AR, Shah NA, Sotres‐Alvarez D, et al. Sleep timing, stability, and BP in the sueno ancillary study of the hispanic community health study/study of latinos. Chest. 2019;155:60–68. doi: 10.1016/j.chest.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sohail S, Yu L, Bennett DA, Buchman AS, Lim AS. Irregular 24‐hour activity rhythms and the metabolic syndrome in older adults. Chronobiol Int. 2015;32:802–813. doi: 10.3109/07420528.2015.1041597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Makarem N, Zuraikat FM, Aggarwal B, Jelic S, St‐Onge M‐P. Variability in sleep patterns: an emerging risk factor for hypertension. Curr Hypertens Rep. 2020;22:1–10. doi: 10.1007/s11906-020-1025-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiao Q, Matthews CE, Playdon M, Bauer C. The association between rest‐activity rhythms and glycemic markers: the US National Health and nutrition examination survey, 2011–2014. Sleep. 2022;45:zsab291. doi: 10.1093/sleep/zsab291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xu Y, Su S, McCall WV, Isales C, Snieder H, Wang X. Rest‐activity circadian rhythm and impaired glucose tolerance in adults: an analysis of NHANES 2011–2014. BMJ Open Diabetes Res Care. 2022;10:e002632. doi: 10.1136/bmjdrc-2021-002632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qian J, Dalla Man C, Morris CJ, Cobelli C, Scheer F. Differential effects of the circadian system and circadian misalignment on insulin sensitivity and insulin secretion in humans. Diabetes Obes Metab. 2018;20:2481–2485. doi: 10.1111/dom.13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qian J, Morris CJ, Caputo R, Garaulet M, Scheer F. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int J Obes. 2019;43:1644–1649. doi: 10.1038/s41366-018-0208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barr‐Anderson DJ, AuYoung M, Whitt‐Glover MC, Glenn BA, Yancey AK. Integration of short bouts of physical activity into organizational routine a systematic review of the literature. Am J Prev Med. 2011;40:76–93. doi: 10.1016/j.amepre.2010.09.033 [DOI] [PubMed] [Google Scholar]
- 43. Brierley ML, Chater AM, Smith LR, Bailey DP. The effectiveness of sedentary behaviour reduction workplace interventions on cardiometabolic risk markers: a systematic review. Sports Med. 2019;49:1739–1767. doi: 10.1007/s40279-019-01168-9 [DOI] [PubMed] [Google Scholar]
- 44. Hadgraft NT, Winkler E, Climie RE, Grace MS, Romero L, Owen N, Dunstan D, Healy G, Dempsey PC. Effects of sedentary behaviour interventions on biomarkers of cardiometabolic risk in adults: systematic review with meta‐analyses. Br J Sports Med. 2021;55:144–154. doi: 10.1136/bjsports-2019-101154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reed JL, Prince SA, Elliott CG, Mullen KA, Tulloch HE, Hiremath S, Cotie LM, Pipe AL, Reid RD. Impact of workplace physical activity interventions on physical activity and cardiometabolic health among working‐age women: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10:10. doi: 10.1161/CIRCOUTCOMES.116.003516 [DOI] [PubMed] [Google Scholar]
- 46. Corbalán‐Tutau M, Madrid J, Ordovás J, Smith C, Nicolás F, Garaulet M. Differences in daily rhythms of wrist temperature between obese and normal‐weight women: associations with metabolic syndrome features. Chronobiol Int. 2011;28:425–433. doi: 10.3109/07420528.2011.574766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


